Abstract
Leishmania (Viannia) organisms are the most prevalent etiologic agents of human cutaneous leishmaniasis in the Americas. Nevertheless, our knowledge of the immunological mechanisms exploited by L. (Viannia) organisms remains limited and the mechanisms underlying disease are not well understood. Here, we report the development of a BALB/c mouse model of L. (V.) panamensis infection that is able to reproduce chronic disease, with persistent infection and clinically evident lesions for over 1 year. The immune response of the mouse resembles that found for L. (V.) panamensis-infected patients with chronic and recurrent lesions, presenting a mixed Th1/Th2 response with the presence of TNF-α, IFN-γ, IL-10 and IL-13. Using immunodeficient mice, the critical role for IL-13 and/or IL-4Rα in determining susceptibility to chronic infection was evident. With the induction of healing in the immunodeficient mice, increases in IFN-γ and IL-17 were found, concomitant with parasite control and elimination. Specifically, increases in CD4+ (but not CD8+) T cells producing IFN-γ were observed. These results suggest that IL-13 represents an important target for disease control of L. (V.) panamensis infection. This murine model should be useful to further understand the pathology associated with chronic disease and to develop methods for the treatment and prevention of leishmaniasis caused by L. (Viannia) parasites.
Keywords: Chronic infection, IL-13, Leishmaniasis, Leishmania (Viannia), Murine model
Introduction
The infection by organisms of the genus Leishmania in humans causes a broad spectrum of clinical manifestations ranging from asymptomatic and cutaneous to more serious, disfiguring and fatal conditions that can be classified as diffuse cutaneous, mucocutaneous and visceral leishmaniasis. The human disease is found throughout the world, affecting 21 nations in the Americas and 66 nations in Africa, Europe and Asia. The prevalence of the disease is approximately 12 million, with a population at risk of approximately 350 million (http://www.who.int/leishmaniasis/burden/magnitude/burden_magnitude/en/index.html) [1, 2].
Although organisms belonging to the Leishmania (Leishmania) and L. (Viannia) subgenus are the etiologic agents of human leishmaniasis, the latter is the most frequent cause of human disease in the Americas [3]. Further, the characteristic human immune response across the spectrum of infection with L. (Viannia) organisms is marked by mixed cytokine response (Th1/Th2) with concomitant production of IFN-γ, IL-13, IL-10 and TNF-α [4–8]. Despite the public health importance and the distinct immune response to infection by organisms of the L. (Viannia) subgenus, we have a limited understanding of the mechanisms of disease and disease-associated pathology. Currently, our knowledge and insights regarding the immunological mechanisms that potentially drive and sustain the infection are primarily based on the human studies [4–12]. These studies are essential to our understanding but are limited in terms of the ability to explore the early phases of infection and the impact of various cell populations and mediators on disease.
The apparently poor infectivity of L. (Viannia) species in mice has been a major factor limiting the use of a murine model, where a self-healing transient lesion has been consistently observed [13–16]. Despite this, several authors have successfully employed the murine infection model to explore mechanisms leading to disease exacerbation; using genetic approaches, several elements/mechanisms that confer immunological resistance, including IFN-γ, IL-12, STAT4, TNF-α and iNOS have been identified.
However, a mouse model of chronic disease, as can occur in humans, has remained elusive; nevertheless, the genetic and immunological tools available for the mouse make this an advantageous model. Previous studies by Neal and Hale [17], found that BALB/c mice are susceptible to a high inoculum (107) of L (V.) panamensis promastigotes. Therefore, we focused on the development of a chronic infection model for L. (V.) panamensis using numbers of parasites that would more closely approximate infection in nature. The development of infective promastigotes for infection was monitored using mAb that recognize Ag that are upregulated in the amastigote stage [18]; the inoculation of 5 × 104 organisms generates chronic lesions in BALB/c mice with persistent parasites at site of the infection and draining LN (>1 year). Throughout infection, a mixed cytokine response (IFN-γ, IL-13, IL-10 and TNF-α), consistent with cytokine profiles of infected individuals and representative of the spectrum of outcomes of infection (asymptomatic, chronic and recurrent) is observed. Our results indicate that IL-13 is critical to disease pathogenesis, as BALB/c mice genetically deficient in either IL-4Rα or IL-13 were resistant to infection. Increased IFN-γ and/or IL-17 was observed in with the onset of healing in the resistant mice, suggesting a role in healing. These findings support a pivotal role for IL-13 in the regulation of L. (V.) panamensis infection; consequently, IL-13 signaling may represent a target for immunotherapeutic intervention.
Results
Amastigote Ag expression of L. (V.) panamensis promastigotes is associated with infectivity
Parasites of the L. (Viannia) subgenus are known to be poorly infectious to most laboratory strains of mice [13–16]. In initial experiments, we were able to establish infection in BALB/c mice using the 1–2 × 107 stationary promastigotes of several strains of L. (V.) panamensis as reported previously [17]. However, only 25–30% of the mice developed chronic lesions (data not shown), suggesting an inconsistent infection and low levels of infective organisms. A number of parameters, previously shown to lead to the development of infective promastigotes for other Leishmania species, including parasite resistance to complement lysis and medium pH were evaluated. However, these parameters did not consistently correlate with persistent/chronic lesion development.
Consequently, an alternate approach was taken. Infective organisms (metacyclic) begin biochemically to resemble the amastigote stage, expressing molecules that ultimately allow for its survival within the mammalian host. Consequently, in order to assess the process of metacyclogenesis or development of infectivity of L. (V.) panamensis, we decided to utilize the expression of amastigote-specific Ag recognized by previously characterized mAb [18]. The mAb were used with L. (V.) panamensis organisms at distinct differentiation stages: (i) promastigotes from midlog phase, (ii) gradient-purified late stationary phase promastigotes and (iii) axenic amastigotes. A small fraction (1.9–4.1%) of gradient purified live promastigotes from late stationary phase and all axenic amastigotes (as expected) expressed the Ag (Supporting Information Fig. 1). Consistent with the observations of the development of L. (V.) panamensis promastigotes within the sand fly vector [19], the promastigotes recognized by the mAb did not appear to have a unique morphology. Optimal development of infective organisms occurred between 15 and 21 days in culture and correlated with the initiation of amastigote Ag expression.
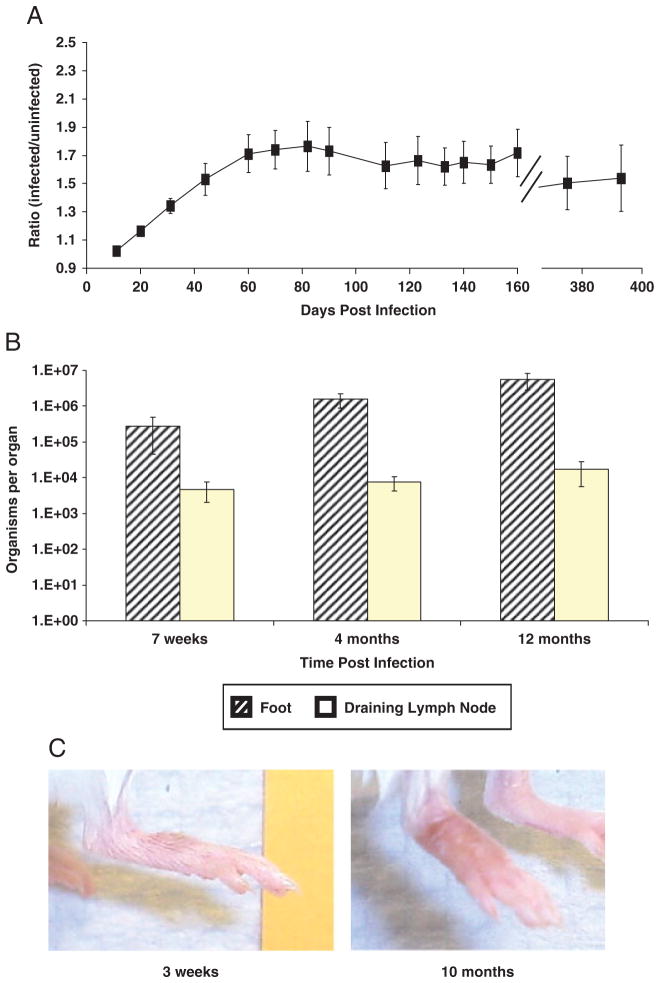
Therefore, for experimental infections, promastigotes (5 × 104) were isolated from percoll gradients [20] after 20 days in culture and used for infection. This procedure generated reproducible/consistent infections with development of chronic lesions lasting for more than a year (Fig. 1A), with amastigotes present at the site of infection and draining LN (Fig. 1B). Appearance of average lesions in the infected BALB/c mice at 3 wk and 10 months post-infection is shown in Fig. 1C. In contrast to the unrestrained cutaneous disease of BALB/c mice by other species of Leishmania, lesion development appeared to plateau (50–60 days post-infection; lesion ratio, 1.7:2.1) and was maintained (1 year post-infection). Further, lesions sizes contrast to those found in the BALB/c mouse with L. amazonensis or L. major [21, 22], which at 10 wk post-infection already have ratios of 1.9:2.6 and continue to progressively develop. Consequently, although parasites persist, chronic disease caused by L. (V.) panamensis appears self-limited.
Figure 1.
Stationary phase L. (V.) panamensis promastigotes generate chronic lesions in BALB/c mice. Mice were intradermally infected (5 × 104 late log phase percoll-purified organisms) on the top of the right rear hind foot. Course of infection was monitored by measuring (A) ratio of lesion development in BALB/c footpad compared with the contralateral uninfected foot (n = 10), and by (B) parasite burden analyses of feet (diagonal-stripped bars) or pooled draining popliteal and inguinal LN (open bars, n = 4–5). (C) Representative median lesions of infection at 3 wk or 10 months after infection. Data show mean ± SEM and are representative of at least two independent experiments.
Although infection could be established with tenfold fewer parasites, the course of lesion development was slower (Supporting Information Fig. 2); consequently, 5 × 104 parasites were routinely used for infection. Metastases to the spleen and liver were also detected; however, this occurred in a portion of the infected mice. This is consistent with the recent observations, indicating that L. (Viannia) parasites can be detected at sites distal from the lesions, with little to no overt pathology [23–25].
Therefore, these results appear to differ from findings for the species L. (V.) braziliensis in mice, where, in general, lesions have been found to resolve spontaneously. Further study is required, however, to determine whether these apparent differences of disease development in mice reflect species variation or alternately differences in culture and isolation of infective organisms.
Human response to L. (V.) panamensis infection
Natural infection with L. (V.) panamensis produces a wide range of clinical outcomes ranging from asymptomatic infection to recurrent or chronic lesions, including mucocutaneous leishmaniasis [5, 26]. PBMC stimulated with promastigote Ag secreted a mixed Th1/Th2 profile of cytokines with significantly increased production of IL-10, IL-13, IFN-γ and TNF-α in patients with chronic or recurrent leishmaniasis (Fig. 2A) compared with asymptomatically infected individuals and controls. However, IL-4 was not detected. Notably, these cytokines were also induced, though at a lower level, in PBMC from asymptomatic individuals. Hence, the mixed cytokine profile is found across the spectrum of outcomes of human infection by L. (V.) panamensis and is more pronounced in individuals who have had chronic and recurrent disease.
Figure 2.
Human natural infection presents a clinical spectrum (from chronic and recurrent lesions to asymptomatic infection) characterized by an immune response constituted by a mixed pro- and anti-inflammatory cytokine profile. (A) Cytokine production by PBMC from patients with active chronic (CH) and recurrent disease (RE), asymptomatically infected individuals (AS) and healthy controls (HC). ANOVA (p < 0.001 for all cytokines) and Duncan’s test for multiple comparison, *p < 0.01. (B) Regression analyses of cytokine responses across the spectrum of disease caused by L. (V.) panamensis. Regression analyses of cytokine production were conducted only in infected individuals (chronic, recurrent and asymptomatic) using Pearson correlation analysis. p-Values of less than 0.05 are considered significant.
Regression analysis showed that Th1 and Th2 cytokine production were positively correlated for infected individuals, Fig. 2B. However, the putative clinically susceptible (chronic and recurrent disease) and resistant (asymptomatically infected) individuals were distinctly distributed with susceptible individuals presenting concomitantly elevated Th1 and Th2 cytokines and a significant correlation between IFN-γ and IL-10 production. Overall, these results demonstrate concomitant increases in inflammatory–anti-inflammatory cytokines in response to L. (Viannia) infection.
Immune response of L. (V.) panamensis-infected mice parallels that found in human infection
To assess the immune response of the BALB/c mouse to infection, in vitro cytokine production to L. (V.) panamensis promastigote Ag (pLAg) stimulation was evaluated. The production of IL-10, IL-13 and IFN-γ was observed in both the acute phase and the chronic phase (Table 1) in accordance with what is observed for human infection. Notably, the IL-4 levels were low until late in infection (Fig. 3).
Table 1.
Cytokine responses of L. (V.) panamensis infected BALB/c Micea)
| Cytokine | 3 Wk post-infection | 4 Months post-infection | 8 Months post-infection |
|---|---|---|---|
| IFN-γ | 9.6 ± 0.3 ng/mL | 43.6 ± 3.1 ng/mL | 36.5 ± 0.02 ng/mL |
| IL-10 | 0.27 ± 0.04 ng/mL | 1.5 ± 0.4 ng/mL | 0.48 ± 0.02 ng/mL |
| IL-13 | 0.11 ± 0.03 ng/mL | 0.31 ± 0.06 ng/mL | 0.16 ± 0.01 ng/mL |
| TNF-α | Not determined | 0.24 ± 0.03 ng/mL | Not determined |
Lymphocytes (DLN) from L. (V) panamensis-infected BALB/c mice (three to five per group) were stimulated for 72 h with soluble leishmanial Ag and cytokine production was assessed by ELISA.
Figure 3.
L. (V.) panamensis infection in BALB/c does not require B cells. (A) Lesion development was monitored by lesion development of infected footpad in BALB/c WT (squares) or B-cell-deficient, JhD (circles) mice, infected with 5 × 104 stationary phase purified promastigotes, a minimum of ten mice of each group was analyzed for lesion development. Results are representative of three independent experiments. Parasite burden was determined at (B) 2 or (C) 4 months post-infection (n = 4/group/determination). Data show mean ± SEM, *p < 0.05 by unpaired Wilcoxon test.
Additionally, the changes in the LN cell populations in response to infection were examined, as lymphadenopathy is a common feature in the early stages of human disease caused by L. (Viannia) [27]. Chronic lesions were accompanied by lymphadenopathy in the draining (inguinal and popliteal) LN with an overall 3.4-fold increase in cell number by 3.5 wk post-infection. Analysis of the cell populations in the draining LN by flow cytometry indicated an expansion of CD4+, CD8+ and CD19+ cells in the draining LN (Table 2). However, overall the T/B ratio decreased from 3.3 to 1.3, indicating a preferential increase in the B-cell population. B-cell expansion continued throughout the chronic phase of the disease, with T/B ratio of 0.9 found at 4 months post-infection. These results are similar to what has been observed for LN cell populations in the late phase of human disease caused by L. (Viannia) [27] as well as in murine models of leishmaniasis [28, 29]. Interestingly, in the case of murine leishmaniasis, B cells have been found to contribute to disease exacerbation.
Table 2.
Draining LN cell populations in BALB/c WT or IL-13-deficient micea)
| Cell Population | WT BALB/c
|
Ratio: Infected/naïve | IL-13-deficient BALB/c
|
Ratio: Infected/naïve | ||
|---|---|---|---|---|---|---|
| Naïve | Infected | Naïve | Infected | |||
| CD3e+ | 1.45 ± 0.01 × 106 | 3.69 ± 0.01 × 106 | 2.54 | 1.36 ± 0.01 × 106 | 2.13 ± 0.01 × 106 | 1.57 |
| CD4+ | 9.70 ± 0.03 × 105 | 2.66 ± 0.03 × 106 | 2.74 | 9.93 ± 0.06 × 105 | 1.52 ± 0.05 × 106 | 1.53 |
| CD8+ | 4.19 ± 0.05 × 105 | 1.05 ± 0.01 × 106 | 2.50 | 3.52 ± 0.01 × 105 | 5.97 ± 0.02 × 105 | 1.70 |
| CD19+ | 4.37 ± 0.05 × 105 | 2.77 ± 0.03 × 106 | 6.45 | 2.68 ± 0.01 × 105 | 1.00 ± 0.01 × 106 | 3.73 |
| CD11b+ | 2.04 ± 0.01 × 104 | 5.00 ± 0.0 × 104 | 2.45 | 1.68 ± 0.04 × 104 | 4.00 ± 0.22 × 104 | 2.38 |
| CD4+FOXP3+ | 1.03 ± 0.02 × 105 | 2.68 ± 0.03 × 105 | 2.60 | 7.26 ± 0.29 × 104 | 1.72 ± 0.03 × 105 | 2.37 |
Values represent the absolute number of LN cells (average of six to ten pooled sets of popliteal and inguinal LN) at 3.5 wk post-infection that are positive for each cell marker (CD4, CD8, CD19, CD3e and CD11b) and FOXP3. Staining was performed in duplicate and the standard deviation for each measurement is presented. The experimental data are representative of two independent experiments.
Consequently, the chronic infection mouse model of L. (V.) panamensis appears to mimic the characteristic cytokine and cellular responses observed in human disease. Given the mixed cytokine response (IFN-γ and IL-13), and B-cell expansion during chronic lesion development, the regulation of chronic infection (cytokines, B cells) was examined.
B cells in murine L. (V.) panamensis infection
B cells have been shown to be important in the development of the disease caused by L. (L.) amazonensis, L. (L.) pifanoi, L. (L.) donovani and some strains of L. (L.) major [22, 30, 31]. As the draining LN CD19+ compartment expands during the L. (V.) panamensis infection, we further evaluated the role of B cells in the development of chronic infection using BALB/c JHD mice, which are deficient in B cells. Overall, in initial phase of infection (first month) the JHD mice had similar lesion development to that observed for WT BALB/c mice. Subsequently, lesion progression is slower in the JHD mice, with lesions being significantly smaller at 50 days post-infection, when lesion development plateaued in the WT BALB/c mice. Nevertheless, lesion development in the JHD mice continued to increase and by 4 months post-infection no significant difference was observed (Fig. 3A). Furthermore, parasite burdens although lower in the JHD mice at 50 days post-infection were not significantly different from the WT BALB/c mice (Fig. 3B). As expected, the parasite levels in lesions and LN at 4 months post-infection were indistinguishable between the two groups of mice (Fig. 3C). These results suggest that B cells can contribute to disease pathology (lesion development), but do not impact significantly on parasite burden. This differs from what has been observed in the murine model with other species of Leishmania, where B-cell Ig and Ag presentation foster disease exacerbation [22, 30, 31].
IL-13 and/or IL-4Rα play major roles in disease development and susceptibility
As IL-13 has been linked to susceptibility to Leishmania infection in mice [32–34] and appeared to be a major Th2-like cytokine produced in human infection (above), we investigated the role of IL-13 in the L. (V.) panamensis infection. BALB/c (WT or deficient in IL-13 or IL-4Rα) were infected and lesion development and immune responses monitored. The lesion development in both the IL-13−/− and the IL-4Rα−/− mice was significantly reduced when compared with WT BALB/c mice (Fig. 4A). At 9 wk post-infection, the lack of lesions reflected a complete elimination of the parasite (foot and DLN) of the IL-4Rα−/− mice. Parasites were significantly reduced in the IL-13−/− mice, with a 1.5 × 104-fold reduction in parasite numbers in comparison to WT mice (Fig. 4B and C); only 25% of the IL-13−/− mice examined had detectable levels of parasites.
Figure 4.
L. (V.) panamensis infection in BALB/c requires IL-13 and IL-4Rα. Course of infection was monitored by (A) footpad lesion development in WT (squares), and in IL-4Rα (triangles) or IL-13- (open circles) deficient BALB/c mice (data show at least four mice per group and are representative of three independent experiments). *(IL-13 KO) and +(IL-4Rα KO) indicate p < 0.001 by unpaired Wilcoxon test compared with WT controls. Parasite burden was determined at (B) 25 days and (C) 60 days post-infection in BALB/c WT (filled bars), IL-13 KO (diagonally stripped bars) and IL-4Rα KO (open bars). The numbers of mice with positive parasite burden in each group are indicated beneath the bars. *p < 0.03 by unpaired Wilcoxon test and error bars represent standard error of the mean (n = 4). Similar results were observed at 180 and 360 days post-infection.
Given the control of parasitemia observed, we analyzed the immune response of WT BALB/c as well as the IL-13−/− and IL-4Rα−/− mice (Fig. 5) at three time points: (i) early in infection (day 10 post-infection); (ii) when the parasite burdens are relatively similar but disease is beginning to resolve in the IL-13 and IL-4Rα-deficient mice (3.5 wk post-infection) and also upon (iii) resolution of disease (2 months post-infection). Lymphocytes from infected mice were stimulated in vitro with either concanavalin A or with pLAg. Interestingly, at 3.5 wk post-infection (beginning of disease resolution), the absence of IL-13 or IL-4Rα enhances IFN-γ or IL-17 production. No differences in the levels of TNF-α were observed. Although both CD4+ and CD8+ T cells were found to produce IFN-γ (Fig. 6) in both WT and immunodeficient mice in response to infection, the increased level of IFN-γ observed was due to a preferential expansion of CD4+IFN-γ+ T cells in the immunodeficient mice (p < 0.03); no similar increase in the relative levels of CD8+IFN-γ+ T cells was observed.
Figure 5.
Response to infection with L. (V.) panamensis of WT and IL-13 or IL-4Rα-deficient mice. Cytokine responses of WT, IL-13- or IL-4Rα-deficient BALB/c mice at different times post-infection, as indicated. ELISA analyses for cytokines were performed on supernatants of spleen-derived lymphocytic cells stimulated with Con A or pLAg (as indicated in the Materials and methods section). Data show mean ± SEM. *(IL-13 KO) and +(IL-4Rα KO) indicate p < 0.05 by Student’s T-test. Similar results were obtained in another independent experiment at 3.5 wk. The IL-4 production by lymphocytes of IL-4Rα-deficient mice is not shown. However, consistent with the previous observations [68], IL-4 levels were found to be >300 pg/mL at all time points.
Figure 6.
Lack of IL-13 signaling leads to increases in CD4+IFN-γ+ T cells. IFN-γ responses of WT or IL-13-deficient BALB/c mice at 3.5 wk post-infection. FACS analyses for IFN-γ were performed as indicated in the Materials and methods section (gating strategy is shown in Supporting Information Fig. 4) using spleen lymphocytes stimulated with pLAg. Lymphocyte populations (based on the side and forward scatter) were sorted for either surface (A) CD4 or (B) CD8 expression (as indicated) and examined for IFN-γ synthesis. Results are representative of two independent experiments (n = 4 mice/group). (C) Ratio of % IFN-γ+ cells divided by % IFN-γ+ cells in the WT group is shown (average of points from multiple experiments), error bars indicate SD, and *p < 0.03 by Wilcoxon test.
Notably, the levels of IL-10 also increased in the IL-13−/− and IL-4Rα−/− mice at 3.5 wk post-infection as healing is developing, with higher levels occurring than for the WT BALB/c mice. However, overall the ratios of IFN-γ/IL-10 were comparable in the nonhealing BALB/c and healing (IL-4Rα−/− and IL-13−/−) mice (IFN-γ ng/IL–10 pg of 0.54–0.48). At other times, post-infection, comparable levels IL-10 were observed for both the WT and the immunodeficient mice. Consequently, IL-10, in the absence of IL-13, does not appear to be sufficient for disease development and parasite persistence. Nevertheless, infection in IL-10-deficient BALB/c mice presented reduced parasite burden clearly, indicating that this cytokine also has a key role in disease outcome (Supporting Information Fig. 3). Consequently, although IL-10 alone is insufficient for disease, the absence of IL-10 can lead to healing. Interestingly, in the absence of IL-10, no changes in the IL-13 response were observed; however, increased levels of IL-17 were found. These results suggest that the lack of IL-10 or IL-13 and/or IL-4 signaling leads to increased level of the TH1/TH17 responses and disease resolution. Further, IL-13/IL-4Rα signaling in this context appears to prevent an expansion of CD4+IFN-γ+ T cells essential to generate disease resolution.
Discussion
Clinical studies indicate that the human response to infection with L. (Viannia) parasites [35] differs from that which occurs upon infection with members of the L. (Leishmania) subgenus [36–39]. Given the public health importance of disease caused by L. (Viannia) parasites and the facts that asymptomatic infection as well as persistence after treatment [23, 25] and disease reactivation are known to occur [40], an understanding of the immunological mechanisms underlying disease is essential. However, the lack of a suitable small mammalian model for the chronic form of disease caused by L. (Viannia) parasites has restricted experimental approaches to elucidating the mechanisms contributing to susceptibility to infection/disease. In this article, we report the development of a chronic infection model for L. (V.) panamensis that requires a relatively low-dose inoculum. Further, the immunological features were evaluated in the context of the main aspects of human L. (V.) panamensis infection to determine the general similarities and disparities between the model and the human disease and to understand mechanisms underlying susceptibility that could potentially guide improvements in treatment and/or vaccine development.
Development of chronic infection was dependent on the expression of amastigote-specific Ag [18] by stationary phase L. (V.) panamensis promastigotes. As metacyclic promastigotes differentiate from procyclic promastigotes and prepare to survive inside the mammalian host, the upregulation and expression of molecules that are highly selectively expressed in the amastigote stage have been observed [41, 42]. After 20 days in culture, a consistent but low percentage of the organisms (3.2 ± 1.1% or 1.6 ± 0.6 × 103 promastigotes/infection) expressed amastigote Ag. Consequently, it is possible that infection was established with fewer parasites. Whether these amastigote molecules are involved in the virulence of the parasite is currently not known, but will be of interest for future studies utilizing this model.
Infected mice display lymphadenopathy, with the number of amastigotes found in the draining LN proportional to the level found in the lesion. In human infection, lymphadenopathy is observed in the case of L. (V.) panamensis infection [43] and is one of the earliest (and sometimes the sole) clinical signs of infection caused by L. (V.) braziliensis [44, 45]. Accompanying the cellular expansion of the DLN upon infection, a selective expansion of B cells was observed, with an inversion of the T/B cell ratio. Although an expansion of the B-cell compartment upon infection is not unexpected, B-cell infiltration at the site of infection and increased Ab titers have been correlated with pathology development in the human infection with L. (V.) panamensis [46–49] and L. (V.) braziliensis [27]. Moreover, in murine models for leishmaniasis, B cells (Ab production and APC functions) have been demonstrated to be important in disease caused by infection with other Leishmania organisms [22, 30, 31]. Indeed, the absence of B cells resulted in delayed lesion development in the case of L. (V.) panamensis infection; however, the reduction in parasite numbers either at the site of infection or within the DLN was not significant. However, the plateau in lesion development observed during the chronic disease phase could mask the overall importance of the B-cell contribution to disease susceptibility (as clearly the lesion development is delayed in the B-cell-deficient mice). Overall, these results suggest that although B cells can contribute to disease pathology caused by L. (V.) panamensis, their role may not be as significant as found for other species of Leishmania.
Of major interest was the observation that the BALB/c immune response to infection with L. (V.) panamensis resembles the human immune response with a concomitant production of a mixed Th1 and Th2 cytokine response (primarily IL-10, IL-13, IFN-γ, TNF-α and low/absent IL-4). Our studies are consistent with the earlier studies [5, 50] of human patients, showing a mixed IL-10, IFN-γ response to L. (V.) panamensis infection. Interestingly, untreated active chronic patients have been found to present similar in vitro response (IFN-γ, IL-13, IL-10, IL-2 and TNF-α) to Leishmania parasites as those of treated chronic patients. On the contrary, treated historic recurrent patients produced significantly higher levels of IFN-γ and TNF-α than active recurrent patients (p < 0.05) [51]. Consequently, a similar cytokine profile is observed as a consequence of infection –before and after treatment. Given that drug treatment has been found not to eradicate parasites [52–54], this may reflect persistent infection.
The mixed Th1/Th2 cytokine profile is also common to human infection with other L. (Viannia) species [4–8]. Notably, both IL-10 and IL-13 have been implicated in the negative regulation of L. (Viannia) infection in human patients [8, 55]. The consistent (human and mouse) mixed cytokine response suggests common mechanisms to sustain infection for this subgenus are present in this murine model of chronic L. (V.) panamensis infection.
Although IL-4 production in response to leishmanial Ag is observed in some cases of human L. (V.) braziliensis infection [4, 27], IL-13 and IL-10 appear to be the predominant “Th2” cytokines [6, 8, 11] found. Notably, an IL-10 response occurred throughout infection in both the WT and the IL-13- and IL-4Rα-deficient mice, suggesting that IL-10 alone is insufficient to maintain disease and indicating a critical role for IL-13. IL-13 has been shown in murine models of cutaneous leishmaniasis to be important in the susceptibility and maintenance of nonhealing response to L. (L.) major and L. (L.) mexicana infection [32, 33, 56]. IL-4 and IL-13 signal through an overlapping network of receptors that shares a functional signaling receptor chain, namely IL-4Rα [57]. As IL-4Rα-deficient mice controlled infection more rapidly than IL-13-deficient mice, it is possible that both IL-4 and IL-13 may contribute to disease. However, deficiency in IL-13 alone closely approximates the effect seen in IL-4Rα-deficient mice, suggesting that IL-4 may play a relatively minor role in host susceptibility to L. (V.) panamensis infection. However, further experiments are required to verify this point.
The increased susceptibility to infection in BALB/c mice that is dependent on IL-13 and IL-4Rα correlates with an increase of the IFN-γ and IL-17 responses observed with the initiation of disease resolution in the absence of IL-13 or IL-4Rα. The importance of IL-13 in reducing the IFN-γ response has been previously observed in murine L. (L.) major and L. (L.) mexicana infection [32, 33]. The targeted cell populations and mechanisms by which IL-13 and IL-4Rα ensure host permissiveness are not explored in this study. However, in studies of murine L. (L.) major infection, IL-4Rα has been shown to be important for alternative activation of macrophages [58] and also to act through IL-4 on the development of CD4+ T cells [34]. Considering that IL-4 appears to have a minimal role in the regulation of L. (V.) panamensis infection and that IL-13 deficiency alone is sufficient to abrogate susceptibility, it is possible that IL-13 effects on macrophage activation are playing a role in determining infection outcome. In the case of L. (L.) mexicana infection, although infection in the absence of IL-13 led to partial disease amelioration, this was accompanied by an increase in IL-12Rβ2 expression and IFN-γ production. These results are consistent with the studies of L. (V.) braziliensis in mice [15, 59], where IL-12 deficiency leads to overt disease; notably, deficiency in IFN-γ led to a more severe disease [59], suggesting that the multiple IFN-γ-dependent processes might be critical to parasite containment. Interestingly, in the case of human leishmaniasis caused by L. (V.) guyanensis, IL-13 has been found to regulate the expression of IL-12Rβ2, although changes in the IFN-γ response were not noted [11]. Whether similar changes in IL-12 responsiveness occur in the case of L. (V.) panamensis remain to be determined and are of interest. However, the downregulation of IFN-γ production as a consequence of IL-13 (whether through changes in IL-12 signaling and/or macrophage function) is likely critical for disease containment.
Although an IL-17 response appears to be upregulated in the case of mucosal disease caused by L. (V.) braziliensis [10], IL-17 is also found in response to limited cutaneous disease. Hence, the role of IL-17 in human cutaneous leishmaniasis caused by L. (V.) braziliensis remains unclear. In the case of murine cutaneous leishmaniasis, IL-17 has been found to exacerbate murine leishmaniasis caused by L. (L.) major [60]. On the contrary, IL-17 has been detected in response to L. (V.) braziliensis infection of murine dendritic cells [61] and has been (together with IFN-γ) observed in mice infected with L. (V.) braziliensis [61] to correlate with healing in response. These results are consistent to those found here in IL-13- and IL-10-deficient mice and suggest that IL-17 together with IFN-γ may be important for disease resolution for disease caused by infection with L. (Viannia) parasites. Interestingly, Th17 cells have been shown to express IL-13 receptor and to be downregulated by IL-13 [62]. Further study is required to dissect the role of IL-17 in New World leishmaniasis.
In summary, we have demonstrated a key role for IL-13 in host permissiveness to L. (V.) panamensis infection. Our results suggest that IL-13 acts in part through downregulation of IFN-γ and/or IL-17, which are important for infection control and parasite elimination. Hence, the IL-13 signaling pathway appears, as for asthma, to represent a promising target for immunotherapy [63]. We believe that the L. (V.) panamensis murine model will be useful for further studies of the host–parasite interaction and will aid in understanding the immunological mechanisms underlying disease and healing.
Materials and methods
Mice
Female BALB/c mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA) or National Cancer Institute (Frederick, MD, USA). Mice were housed in the Yale University School of Medicine facilities, which are approved by the American Association for Accreditation of Laboratory Animal Care-facilities. The experiments were approved by Yale University Committee on Use and Care of Animals (Assurance number A3230-01). Breeding pairs of IL-4Rα−/− (BALB/c IL4rαtm1Sz/J) and IL-10−/− (C.129P2-IL-10tm1Cgn/J) mice were purchased from Jackson Laboratories. Breeding pairs of IL-13−/− and B-cell-deficient JHD mice BALB/c mice were the kind gifts from Dr. Marsha Wills-Karp (University of Cincinnati College of Medicine) and Dr. Mark Shlomchik (Yale University), respectively.
Indirect immunofluorescence assay
Indirect immunofluorescence of L. (V.) panamensis organisms was performed using standard methodology [18]. Digital images were taken in a Leitz Orthoplan 2 fluorescence microscope immunofluorescence microscope.
Leishmania culture, mouse infection and parasite burden analyses
L.( V.) panamensis (strain MHOM/CO/1995/1989), isolated from a human case of cutaneous leishmaniasis in Colombia, was used for the studies in mice. The parasites were routinely checked either by PCR or by mAb to verify that these were L. (V.) panamensis [64, 65].
L. (V.) panamensis was grown in Schneider’s Medium supplemented with 10 or 20% heat-inactivated FBS and 17.5 μg/mL gentamycin (GIBCO BRL®). Axenic amastigotes were maintained in the same medium at 33°C, and were obtained from promastigotes, as described previously [18].
Promastigotes were grown at 22°C. Promastigotes, recently transformed from amastigotes isolated from infected tissue, were used for infection. Late stationary phase (15–21 days in culture) promastigotes provided for optimal infectivity and consistent chronic lesion development; organisms harvested earlier (7–10 days), whereas infective, did not generally result in chronic disease. Infective promastigotes were purified using a step percoll gradient (Sigma Chemical, St. Louis, MO, USA; PBS containing 20 mM EDTA) that was a modification of the previously described method [20]. Briefly, infective promastigotes were purified from a 45/60% percoll interface; washed promastigotes were then employed for intradermal infection (10 μL, 5 × 104). At different time points after infection, four to five mice were sacrificed and the number of parasites in various tissue sites was determined by limiting dilution analysis, as described previously [20].
Immune response analyses – BALB/c mice
Single-cell suspensions were obtained from either spleen or LN tissues. Cells (5 × 106 cells/mL) were then incubated at 37°C/5% CO2 for 72 h alone or stimulated with either concanavalin A (5 μg/mL), or L. (V.) panamensis promastigote freeze–thaw lysates (Lag – equivalent to 1 or 5 × 106 organisms/mL). Supernatants were collected and stored at −80°C until used. Cytokine levels (TNF-α, IL-10, IL-4 and IFN-γ) were determined by sandwich ELISA, according to the manufacturer’s specifications, as described previously [20]. IL-17 was determined using a kit from eBiosciences (San Diego, CA, USA). For IL-13 measurements, paired Ab (MAB413 and BAF413 from R&D Systems, Minneapolis, MN, USA) and rmIL-13 (eBiosciences) were used.
For flow cytometric analyses, LN cells were directly employed for ex vivo analysis or, alternately, cells stimulated/or not with pLAg for 72 h (as indicated above) were cultured for 4 h in the presence of Brefeldin-A (BD Biosciences Pharmingen) according to manufacture’s instructions. The cells were then stained for surface markers (CD3ε (145-2C11), CD4 (L3T4), CD8α (Ly-2), CD19 (1D3) and CD11b (M1/70)), washed and fixed. Subsequently, cells were permeablized and stained for FOXP3 (FJK-16S, eBiosciences), or IFN-γ (XMG1.2). Controls were performed with the respective isotype controls: Hamster-IgG, Rat-IgG2a and Rat-IgG1 from eBiosciences; and Rat-IgG2a (R35-95), Rat-IgG2b (A95-1) from BD Biosciences Pharmingen. Data were acquired on a BD FACSCalibur (BD Biosciences), using software CellQuest™ Pro, and collected data were analyzed on FlowJo Software (Tree Star).
Human study population
The study population consisted of 57 individuals between 15 and 58 years of age, of either gender. They were classified into four groups: (i) Historical Chronic Disease, individuals who had previously presented parasitologically confirmed lesions of >6 months of evolution that had cured after treatment with Glucantime® (Specia Rhone-Poulenc Rorer, Paris, France); (ii) Historical Recurrent Disease, individuals who had presented a new leishmanial lesion after healing of a documented, parasitologically confirmed leishmaniasis. Neither historical groups presented active lesions at the time of the study; (iii) Asymptomatic Infection, subjects living in an endemic area of transmission of dermal leishmaniasis (Tumaco, Nariño, Colombia), without active or healed lesions or clinical history of leishmaniasis, having a positive Montenegro skin test [66] or in vitro proliferative response to Leishmania Ag. These participants were identified from the previous studies of incidence and prevalence of leishmaniasis [67] and (iv) Healthy Controls, laboratory personnel without history of exposure to areas of transmission of leishmaniasis and negative lymphoproliferation response to Leishmania Ag. All participants of the study were seronegative for HIV-1/HIV-2 and HTLV-1 virus determined by ELISA (Abbott Laboratories, Abbott Park, IL, USA).
This study was conducted in accordance with national (Resolution No. 008430, 1993, República de Colombia, Ministry of Health) and international (Declaration of Helsinki and amendments, World Medical Association, Edinburg, Scotland, October 2000) guidelines for protection of human subjects involved in research. The study was approved and monitored by the CIDEIM Institutional Review Board. Informed, signed consent was provided by each participant.
Isolation of human blood mononuclear cells and lymphoproliferation assays
PBMC were isolated from heparinized blood by centrifugation over Ficoll-Hypaque (Sigma Chemical). After washing, PBMC were cultured (1 × 106/mL) in RPMI 1640 (Nunc, St. Louis, MO, USA) containing 10% pooled human serum free of IL-10, in medium alone or in the presence of Ag from 2.5 × 104/mL lysed promastigotes of L. (V.) panamensis (MHOM/COL/1982/LS94), as described previously [46].
Human cytokine quantification
Cytokine levels were determined using culture supernatants of 2.0 × 106 PBMC/mL of human patients cultured in the absence and presence of 2.5 × 104/mL L. (V.) panamensis promastigotes. Based on the kinetic assays to determine the optimal time to evaluate each cytokine, IFN-γ, IL-10 and IL-13 were measured at 72 h and TNF-α at 18 h. ELISA immunoassays of IFN-γ, IL-10 and TNF-α were performed as described previously [5]. IL-13 (Pharmingen, San Diego, CA, USA) was determined in accordance with manufacturer’s instructions.
Statistical analysis
Statistical analyses of human PBMC’s cytokine production were carried out using SPSS software. Cytokine concentration was normalized by conversion to a base 10 logarithm. One-way ANOVA was performed to establish statistical differences among the clinical groups and Duncan procedure was used to determine multiple comparisons. Statistical analysis of the BALB/c data was performed in Kaleidagraph using Student’s T and/or unpaired Wilcoxon tests. Values of p less than 0.05 (p < 0.05) were considered as significant.
Supplementary Material
Acknowledgments
The authors thank Rafael Tovar for performing the statistical analyses of the human PBMC’s cytokine production data and patient data management, and Harold Golston for technical support. This work was supported through a grant from NIAID, U19 AI65866 (D. Mc. -P.).
Footnotes
Conflict of interest: The authors declare no financial or commercial conflict of interest.
Supporting Information available online
References
- 1.Murray HW, Flanders KC, Donaldson DD, Sypek JP, Gotwals PJ, Liu J, Ma X. Antagonizing deactivating cytokines to enhance host defense and chemotherapy in experimental visceral leishmaniasis. Infect Immun. 2005;73:3903–3911. doi: 10.1128/IAI.73.7.3903-3911.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. Magnitude of the Problem. 2009 http://www.who.int/leishmaniasis/burden/magnitude/burden_magnitude/en/index.html.
- 3.Grimaldi G, Jr, Tesh RB, McMahon-Pratt D. A review of the geographic distribution and epidemiology of leishmaniasis in the New World. Am J Trop Med Hyg. 1989;41:687–725. doi: 10.4269/ajtmh.1989.41.687. [DOI] [PubMed] [Google Scholar]
- 4.Pirmez C, Yamamura M, Uyemura K, Paes-Oliveira M, Conceicao-Silva F, Modlin RL. Cytokine patterns in the pathogenesis of human leishmaniasis. J Clin Invest. 1993;91:1390–1395. doi: 10.1172/JCI116341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bosque F, Saravia NG, Valderrama L, Milon G. Distinct innate and acquired immune responses to Leishmania in putative susceptible and resistant human populations endemically exposed to L. (Viannia) panamensis infection. Scand J Immunol. 2000;51:533–541. doi: 10.1046/j.1365-3083.2000.00724.x. [DOI] [PubMed] [Google Scholar]
- 6.Bourreau E, Prevot G, Gardon J, Pradinaud R, Launois P. High intralesional interleukin-10 messenger RNA expression in localized cutaneous leishmaniasis is associated with unresponsiveness to treatment. J Infect Dis. 2001;184:1628–1630. doi: 10.1086/324665. [DOI] [PubMed] [Google Scholar]
- 7.Gollob KJ, Antonelli LR, Faria DR, Keesen TS, Dutra WO. Immunoregulatory mechanisms and CD4-CD8- (double negative) T cell subpopulations in human cutaneous leishmaniasis: a balancing act between protection and pathology. Int Immunopharmacol. 2008;8:1338–1343. doi: 10.1016/j.intimp.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salhi A, Rodrigues V, Jr, Santoro F, Dessein H, Romano A, Castellano LR, Sertorio M, et al. Immunological and genetic evidence for a crucial role of IL-10 in cutaneous lesions in humans infected with Leishmania braziliensis. J Immunol. 2008;180:6139–6148. doi: 10.4049/jimmunol.180.9.6139. [DOI] [PubMed] [Google Scholar]
- 9.Bacellar O, Lessa H, Schriefer A, Machado P, Ribeiro de Jesus A, Dutra WO, Gollob KJ, Carvalho EM. Up-regulation of Th1-type responses in mucosal leishmaniasis patients. Infect Immun. 2002;70:6734–6740. doi: 10.1128/IAI.70.12.6734-6740.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bacellar O, Faria D, Nascimento M, Cardoso TM, Gollob KJ, Dutra WO, Scott P, Carvalho EM. Interleukin 17 production among patients with American cutaneous leishmaniasis. J Infect Dis. 2009;200:75–78. doi: 10.1086/599380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bourreau E, Prevot G, Pradinaud R, Launois P. Interleukin (IL)-13 is the predominant Th2 cytokine in localized cutaneous leishmaniasis lesions and renders specific CD4+T cells unresponsive to IL-12. J Infect Dis. 2001;183:953–959. doi: 10.1086/319249. [DOI] [PubMed] [Google Scholar]
- 12.Maurer-Cecchini A, Decuypere S, Chappuis F, Alexandrenne C, De Doncker S, Boelaert M, Dujardin JC, et al. Immunological determinants of clinical outcome in Peruvian patients with tegumentary leishmaniasis treated with pentavalent antimonials. Infect Immun. 2009;77:2022–2029. doi: 10.1128/IAI.01513-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samuelson J, Lerner E, Tesh R, Titus R. A mouse model of Leishmania braziliensis infection produced by coinjection with sand fly saliva. J Exp Med. 1991;173:49–54. doi: 10.1084/jem.173.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Moura TR, Novais FO, Oliveira F, Clarencio J, Noronha A, Barral A, Brodskyn C, de Oliveira CI. Toward a novel experimental model of infection to study American cutaneous leishmaniasis caused by Leishmania braziliensis. Infect Immun. 2005;73:5827–5834. doi: 10.1128/IAI.73.9.5827-5834.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rocha FJ, Schleicher U, Mattner J, Alber G, Bogdan C. Cytokines, signaling pathways, and effector molecules required for the control of Leishmania (Viannia) braziliensis in mice. Infect Immun. 2007;75:3823–3832. doi: 10.1128/IAI.01335-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vargas-Inchaustegui DA, Tai W, Xin L, Hogg AE, Corry DB, Soong L. Distinct roles for MyD88 and Toll-like receptor 2 during Leishmania braziliensis infection in mice. Infect Immun. 2009;77:2948–2956. doi: 10.1128/IAI.00154-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neal RA, Hale C. A comparative study of susceptibility of inbred and outbred mouse strains compared with hamsters to infection with New World cutaneous leishmaniases. Parasitology. 1983;87:7–13. doi: 10.1017/s0031182000052379. [DOI] [PubMed] [Google Scholar]
- 18.Eperon S, McMahon-Pratt D. Extracellular amastigote-like forms of Leishmania panamensis and L. braziliensis II. Stage- and sspecies-specific monoclonal antibodies. J Protozool. 1989;36:510–518. doi: 10.1111/j.1550-7408.1989.tb01087.x. [DOI] [PubMed] [Google Scholar]
- 19.Walters LL, Chaplin GL, Modi GB, Tesh RB. Ultrastructural biology of Leishmania (Viannia) panamensis ( = Leishmania braziliensis panamensis) in Lutzomyia gomezi (Diptera: Psychodidae): a natural host-parasite association. Am J Trop Med Hyg. 1989;40:19–39. doi: 10.4269/ajtmh.1989.40.19. [DOI] [PubMed] [Google Scholar]
- 20.Ahmed S, Colmenares M, Soong L, Goldsmith-Pestana K, Munstermann L, Molina R, McMahon-Pratt D. Intradermal infection model for pathogenesis and vaccine studies of murine visceral leishmaniasis. Infect Immun. 2003;71:401–410. doi: 10.1128/IAI.71.1.401-410.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colmenares M, Constant SL, Kima PE, McMahon-Pratt D. Leishmania pifanoi pathogenesis: selective lack of a local cutaneous response in the absence of circulating antibody. Infect Immun. 2002;70:6597–6605. doi: 10.1128/IAI.70.12.6597-6605.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kima PE, Constant SL, Hannum L, Colmenares M, Lee KS, Haberman AM, Shlomchik MJ, McMahon-Pratt D. Internalization of Leishmania mexicana complex amastigotes via the Fc receptor is required to sustain infection in murine cutaneous leishmaniasis. J Exp Med. 2000;191:1063–1068. doi: 10.1084/jem.191.6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Oliveira Camera P, Junger J, do Espirito Santo Silva Pires F, Mattos M, Oliveira-Neto MP, Fernandes O, Pirmez C. Haematogenous dissemination of Leishmania (Viannia) braziliensis in human American tegumentary leishmaniasis. Trans R Soc Trop Med Hyg. 2006;100:1112–1117. doi: 10.1016/j.trstmh.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 24.Fagundes A, Marzochi MC, Fernandes O, Perez MA, Schubach AO, Schubach TM, Amendoeira MR, et al. First encounter of subclinical human Leishmania (Viannia) infection in State of Rio Grande do Sul, Brazil. Mem Inst Oswaldo Cruz. 2007;102:1003–1005. doi: 10.1590/s0074-02762007000800018. [DOI] [PubMed] [Google Scholar]
- 25.Figueroa RA, Lozano LE, Romero IC, Cardona MT, Prager M, Pacheco R, Diaz YR, et al. Detection of Leishmania in unaffected mucosal tissues of patients with cutaneous leishmaniasis caused by Leishmania (Viannia) species. J Infect Dis. 2009;200:638–646. doi: 10.1086/600109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saravia NG, Holguin AF, McMahon-Pratt D, D’Alessandro A. Mucocutaneous leishmaniasis in Colombia: Leishmania braziliensis subspecies diversity. Am J Trop Med Hyg. 1985;34:714–720. doi: 10.4269/ajtmh.1985.34.714. [DOI] [PubMed] [Google Scholar]
- 27.Bomfim G, Andrade BB, Santos S, Clarencio J, Barral-Netto M, Barral A. Cellular analysis of cutaneous leishmaniasis lymphadenopathy: insights into the early phases of human disease. Am J Trop Med Hyg. 2007;77:854–859. [PubMed] [Google Scholar]
- 28.Deak E, Jayakumar A, Cho KW, Goldsmith-Pestana K, Dondji B, Lambris JD, McMahon-Pratt D. Murine visceral leishmaniasis: IgM and polyclonal B-cell activation lead to disease exacerbation. Eur J Immunol. 2010;40:1355–1368. doi: 10.1002/eji.200939455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsu AC, Scott P. Leishmania mexicana infection induces impaired lymph node expansion and Th1 cell differentiation despite normal T cell proliferation. J Immunol. 2007;179:8200–8207. doi: 10.4049/jimmunol.179.12.8200. [DOI] [PubMed] [Google Scholar]
- 30.Smelt SC, Cotterell SE, Engwerda CR, Kaye PM. B cell-deficient mice are highly resistant to Leishmania donovani infection, but develop neutrophil-mediated tissue pathology. J Immunol. 2000;164:3681–3688. doi: 10.4049/jimmunol.164.7.3681. [DOI] [PubMed] [Google Scholar]
- 31.Ronet C, Voigt H, Himmelrich H, Doucey MA, Hauyon-La Torre Y, Revaz-Breton M, et al. Leishmania major-specific B cells are necessary for Th2 cell development and susceptibility to L. major LV39 in BALB/c mice. J Immunol. 2008;180:4825–4835. doi: 10.4049/jimmunol.180.7.4825. [DOI] [PubMed] [Google Scholar]
- 32.Matthews DJ, Emson CL, McKenzie GJ, Jolin HE, Blackwell JM, McKenzie AN. IL-13 is a susceptibility factor for Leishmania major infection. J Immunol. 2000;164:1458–1462. doi: 10.4049/jimmunol.164.3.1458. [DOI] [PubMed] [Google Scholar]
- 33.Alexander J, Brombacher F, McGachy HA, McKenzie AN, Walker W, Carter KC. An essential role for IL-13 in maintaining a non-healing response following Leishmania mexicana infection. Eur J Immunol. 2002;32:2923–2933. doi: 10.1002/1521-4141(2002010)32:10<2923::AID-IMMU2923>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 34.Radwanska M, Cutler AJ, Hoving JC, Magez S, Holscher C, Bohms A, Arendse B, et al. Deletion of IL-4Ralpha on CD4 T cells renders BALB/c mice resistant to Leishmania major infection. PLoS Pathog. 2007;3:e68. doi: 10.1371/journal.ppat.0030068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silveira FT, Lainson R, De Castro Gomes CM, Laurenti MD, Corbett CE. Immunopathogenic competences of Leishmania (V.) braziliensis and L. (L.) amazonensis in American cutaneous leishmaniasis. Parasite Immunol. 2009;31:423–431. doi: 10.1111/j.1365-3024.2009.01116.x. [DOI] [PubMed] [Google Scholar]
- 36.Laskay T, Mariam HG, Berhane TY, Fehniger TE, Kiessling R. Immune reactivity to fractionated Leishmania aethiopica antigens during active human infection. J Clin Microbiol. 1991;29:757–763. doi: 10.1128/jcm.29.4.757-763.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ajdary S, Alimohammadian MH, Eslami MB, Kemp K, Kharazmi A. Comparison of the immune profile of nonhealing cutaneous leishmaniasis patients with those with active lesions and those who have recovered from infection. Infect Immun. 2000;68:1760–1764. doi: 10.1128/iai.68.4.1760-1764.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.el-Hassan AM, Zijlstra EE. Leishmaniasis in Sudan: cutaneous leishmaniasis. Trans R Soc Trop Med Hyg. 2001;95:S1–S17. doi: 10.1016/s0035-9203(01)90216-0. [DOI] [PubMed] [Google Scholar]
- 39.Melby PC, Andrade-Narvaez FJ, Darnell BJ, Valencia-Pacheco G, Tryon VV, Palomo-Cetina A. Increased expression of proinflammatory cytokines in chronic lesions of human cutaneous leishmaniasis. Infect Immun. 1994;62:837–842. doi: 10.1128/iai.62.3.837-842.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saravia NG, Weigle K, Segura I, Giannini SH, Pacheco R, Labrada LA, Goncalves A. Recurrent lesions in human Leishmania braziliensis infection – reactivation or reinfection? Lancet. 1990;336:398–402. doi: 10.1016/0140-6736(90)91945-7. [DOI] [PubMed] [Google Scholar]
- 41.Nugent PG, Karsani SA, Wait R, Tempero J, Smith DF. Proteomic analysis of Leishmania mexicana differentiation. Mol Biochem Parasitol. 2004;136:51–62. doi: 10.1016/j.molbiopara.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 42.Courret N, Frehel C, Prina E, Lang T, Antoine JC. Kinetics of the intracellular differentiation of Leishmania amazonensis and internalization of host MHC molecules by the intermediate parasite stages. Parasitology. 2001;122:263–279. doi: 10.1017/s0031182001007387. [DOI] [PubMed] [Google Scholar]
- 43.Gutierrez Y, Salinas GH, Palma G, Valderrama L, Santrich CV, Saravia NG. Correlation between histopathology, immune response, clinical presentation, and evolution in Leishmania braziliensis infection. Am J Trop Med Hyg. 1991;45:281–289. doi: 10.4269/ajtmh.1991.45.281. [DOI] [PubMed] [Google Scholar]
- 44.Barral A, Barral-Netto M, Almeida R, de Jesus AR, Grimaldi Junior G, Netto EM, et al. Lymphadenopathy associated with Leishmania braziliensis cutaneous infection. Am J Trop Med Hyg. 1992;47:587–592. doi: 10.4269/ajtmh.1992.47.587. [DOI] [PubMed] [Google Scholar]
- 45.Barral A, Guerreiro J, Bomfim G, Correia D, Barral-Netto M, Carvalho EM. Lymphadenopathy as the first sign of human cutaneous infection by Leishmania braziliensis. Am J Trop Med Hyg. 1995;53:256–259. doi: 10.4269/ajtmh.1995.53.256. [DOI] [PubMed] [Google Scholar]
- 46.Saravia NG, Valderrama L, Labrada M, Holguin AF, Navas C, Palma G, Weigle KA. The relationship of Leishmania braziliensis subspecies and immune response to disease expression in New World leishmaniasis. J Infect Dis. 1989;159:725–735. doi: 10.1093/infdis/159.4.725. [DOI] [PubMed] [Google Scholar]
- 47.Guarin N, Palma GI, Pirmez C, Valderrama L, Tovar R, Saravia NG. Comparative immunohistological analysis of the Montenegro skin test reaction in asymptomatic infection and in acute and chronic cutaneous leishmaniasis. Biomedica. 2006;26:38–48. [PubMed] [Google Scholar]
- 48.O’Neil CE, Labrada M, Saravia NG. Leishmania (Viannia) panamensis-specific IgE and IgA antibodies in relation to expression of human tegumentary leishmaniasis. Am J Trop Med Hyg. 1993;49:181–188. doi: 10.4269/ajtmh.1993.49.181. [DOI] [PubMed] [Google Scholar]
- 49.Palma GI, Saravia NG. In situ characterization of the human host response to Leishmania panamensis. Am J Dermatopathol. 1997;19:585–590. doi: 10.1097/00000372-199712000-00006. [DOI] [PubMed] [Google Scholar]
- 50.Trujillo CM, Robledo SM, Franco JL, Velez ID, Erb KJ, Patino PJ. Endemically exposed asymptomatic individuals show no increase in the specific Leishmania (Viannia) panamensis-Th1 immune response in comparison to patients with localized cutaneous leishmaniasis. Parasite Immunol. 2002;24:455–462. doi: 10.1046/j.1365-3024.2002.00488.x. [DOI] [PubMed] [Google Scholar]
- 51.Diaz YR, Rojas R, Valderrama L, Saravia NG. T-bet, GATA-3, and Foxp3 expression and Th1/Th2 cytokine production in the clinical outcome of human infection with Leishmania (Viannia) species. J Infect Dis. 2010;202:406–415. doi: 10.1086/653829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mendonca MG, de Brito ME, Rodrigues EH, Bandeira V, Jardim ML, Abath FG. Persistence of leishmania parasites in scars after clinical cure of American cutaneous leishmaniasis: is there a sterile cure? J Infect Dis. 2004;189:1018–1023. doi: 10.1086/382135. [DOI] [PubMed] [Google Scholar]
- 53.Coutinho SG, Pirmez C, Da-Cruz AM. Parasitological and immunological follow-up of American tegumentary leishmaniasis patients. Trans R Soc Trop Med Hyg. 2002;96:S173–S178. doi: 10.1016/s0035-9203(02)90072-6. [DOI] [PubMed] [Google Scholar]
- 54.Schubach A, Marzochi MC, Cuzzi-Maya T, Oliveira AV, Araujo ML, Oliveira AL, Pacheco RS, et al. Cutaneous scars in American tegumentary leishmaniasis patients: a site of Leishmania (Viannia) braziliensis persistence and viability eleven years after antimonial therapy and clinical cure. Am J Trop Med Hyg. 1998;58:824–827. doi: 10.4269/ajtmh.1998.58.824. [DOI] [PubMed] [Google Scholar]
- 55.Bourreau E, Ronet C, Darsissac E, Lise MC, Marie DS, Clity E, Tacchini-Cottier F, et al. In leishmaniasis due to Leishmania guyanensis infection, distinct intralesional interleukin-10 and Foxp3 mRNA expression are associated with unresponsiveness to treatment. J Infect Dis. 2009;199:576–579. doi: 10.1086/596508. [DOI] [PubMed] [Google Scholar]
- 56.Sosa MR, Rosas LE, McKenzie AN, Satoskar AR. IL-13 gene-deficient mice are susceptible to cutaneous L. mexicana infection. Eur J Immunol. 2001;31:3255–3260. doi: 10.1002/1521-4141(200111)31:11<3255::aid-immu3255>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 57.Wills-Karp M, Finkelman FD. Untangling the complex web of IL-4-and IL-13-mediated signaling pathways. Sci Signal. 2008;1:pe55. doi: 10.1126/scisignal.1.51.pe55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Holscher C, Arendse B, Schwegmann A, Myburgh E, Brombacher F. Impairment of alternative macrophage activation delays cutaneous leishmaniasis in nonhealing BALB/c mice. J Immunol. 2006;176:1115–1121. doi: 10.4049/jimmunol.176.2.1115. [DOI] [PubMed] [Google Scholar]
- 59.de Souza-Neto SM, Carneiro CM, Vieira LQ, Afonso LC. Leishmania braziliensis: partial control of experimental infection by interleukin-12 p40 deficient mice. Mem Inst Oswaldo Cruz. 2004;99:289–294. doi: 10.1590/s0074-02762004000300009. [DOI] [PubMed] [Google Scholar]
- 60.Lopez Kostka S, Dinges S, Griewank K, Iwakura Y, Udey MC, von Stebut E. IL-17 promotes progression of cutaneous leishmaniasis in susceptible mice. J Immunol. 2009;182:3039–3046. doi: 10.4049/jimmunol.0713598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vargas-Inchaustegui DA, Xin L, Soong L. Leishmania braziliensis infection induces dendritic cell activation, ISG15 transcription, and the generation of protective immune responses. J Immunol. 2008;180:7537–7545. doi: 10.4049/jimmunol.180.11.7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Newcomb DC, Zhou W, Moore ML, Goleniewska K, Hershey GK, Kolls JK, Peebles RS., Jr A functional IL-13 receptor is expressed on polarized murine CD4+Th17 cells and IL-13 signaling attenuates Th17 cytokine production. J Immunol. 2009;182:5317–5321. doi: 10.4049/jimmunol.0803868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McCusker CT, Wang Y, Shan J, Kinyanjui MW, Villeneuve A, Michael H, Fixman ED. Inhibition of experimental allergic airways disease by local application of a cell-penetrating dominant-negative STAT-6 peptide. J Immunol. 2007;179:2556–2564. doi: 10.4049/jimmunol.179.4.2556. [DOI] [PubMed] [Google Scholar]
- 64.Castilho TM, Camargo LM, McMahon-Pratt D, Shaw JJ, Floeter-Winter LM. A real-time polymerase chain reaction assay for the identification and quantification of American Leishmania species on the basis of glucose-6-phosphate dehydrogenase. Am J Trop Med Hyg. 2008;78:122–132. [PubMed] [Google Scholar]
- 65.McMahon-Pratt D, Bennett E, David JR. Monoclonal antibodies that distinguish subspecies of Leishmania braziliensis. J Immunol. 1982;129:926–927. [PubMed] [Google Scholar]
- 66.Weigle KA, Valderrama L, Arias AL, Santrich C, Saravia NG. Leishmanin skin test standardization and evaluation of safety, dose, storage, longevity of reaction and sensitization. Am J Trop Med Hyg. 1991;44:260–271. doi: 10.4269/ajtmh.1991.44.260. [DOI] [PubMed] [Google Scholar]
- 67.Weigle KA, Santrich C, Martinez F, Valderrama L, Saravia NG. Epidemiology of cutaneous leishmaniasis in Colombia: a longitudinal study of the natural history, prevalence, and incidence of infection and clinical manifestations. J Infect Dis. 1993;168:699–708. doi: 10.1093/infdis/168.3.699. [DOI] [PubMed] [Google Scholar]
- 68.Mohrs M, Holscher C, Brombacher F. Interleukin-4 receptor alpha-deficient BALB/c mice show an unimpaired T helper 2 polarization in response to Leishmania major infection. Infect Immun. 2000;68:1773–1780. doi: 10.1128/iai.68.4.1773-1780.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.