Abstract
Hard tissue is difficult to repair especially dental structures. Tooth enamel is incapable of self-repairing whereas dentin and cememtum can regenerate with limited capacity. Enamel and dentin are commonly under the attack by caries. Extensive forms of caries destroy enamel and dentin and can lead to dental pulp infection. Entire pulp amputation followed by the pulp space disinfection and filled with an artificial rubber-like material is employed to treat the infection --commonly known as root canal or endodontic therapy. Regeneration of dentin relies on having vital pulps; however, regeneration of pulp tissue has been difficult as the tissue is encased in dentin without collateral blood supply except from the root apical end. With the advent of modern tissue engineering concept and the discovery of dental stem cells, regeneration of pulp and dentin has been tested. This article will review the recent endeavor on pulp and dentin tissue engineering and regeneration. The prospective outcome of the current advancement and challenge in this line of research will be discussed.
Keywords: Tissue engineering, regeneration, enamel, dental pulp, dentin, stem cells, tooth regeneration, endodontics, periodotal ligement, dental pulp stem cells, stem cells from apical papilla, scaffold
2. INTRODUCTION
Tooth enamel is incapable of self-repairing whereas dentin and cememtum have limited capacity to regenerate. Tooth is the only organ that penetrates from the host internal tissue through the “oral integumentary layer” into the external environment – the oral cavity, which is a completely wet environment. Although the mucosal system is heavily protected by a strong immune defense mechanism, tooth is vulnerable to microbial invasion. When invasion occurs and manifests as a dental caries, the caries may become a gateway into the host internal tissues. Root canal infection develops if the caries is extensive and the infection often spreads into the jawbone. Before any microbial invasion is taken place, the root canal is occupied by dental pulp tissue whose main function is to produce dentin and maintain the biological and physiological vitality of the dentin. Additionally, it possesses highly responsive sensory nervous system that generates unbearable pain when tooth is inflicted by mechanical trauma, chemical irritation or microbial invasion. Due to the small volume of the pulp tissue encased in dentin with blood supply, only from one opening end at the root apex, when pulp tissue is infected, it is difficult for the immune system to eradicate the infection without collateral blood supply.
Tooth is built by cells originated from both ectoderm and mesoderm. Enamel is made by ameloblasts derived from ectoderm and these cells are no longer present after the hard tissue formation is complete. Dentin is produced by odontoblasts derived from ectomesenchyme and these cells continue to exist in the pulp throughout life span. Recent advances in tissue engineering have drawn scientists to test the possibility of regeneration a whole tooth or part of the tooth structure (1–4). Tooth is a complex organ consisting of highly organized structures with various but specific shapes. It develops within the jawbone and after the eruption its root(s) are firmly anchored in the alveolar bone proper. Between the tooth and bone, there is a thin layer of membrane termed periodontal ligament (PDL), which plays a pivotal role in providing multiple functions to support the tooth including serving as a shock absorber or a cushion to take up forces transmitted via the tooth. Losing the PDL will lead to ankylosis and the root will be replaced by bone (5). Whole tooth engineering and regeneration is difficult and the technology is still at its very infancy (6–10).
When tooth is damaged but still reparable, regeneration of parts of the tooth structure can prevent or delay the lost of the whole tooth. Partially removing the infected pulp, a procedure termed partial pulpectomy, has been proven ineffective that infection may still be left behind. Therefore, this approach has not been a popular clinical practice. When pulp is diagnosed with irreversible pulpitis, i.e., no treatment can reverse the situation, regardless of the amount of remaining normal pulp tissue, the entire pulp is amputated by pulpectomy. The pulp space is then disinfected and replaced with a rubber-like material - gutta percha. This treatment protocol commonly known as root canal or endodontic therapy has been a common clinical practice for decades.
Regeneration of dentin relies on having vital pulps; however, regeneration of pulp tissue has been difficult as the tissue is encased in dentin without collateral blood supply except from the root apical end. Attempts to regenerate pulp tissue have been a long quest. With the advent of modern tissue engineering concept and the discovery of dental stem cells, regeneration of pulp and dentin has been tested. Moony and Rutherford [6–8] led the first team that initiated the testing of pulp tissue engineering (11–13). This endeavor halted due to the lack of isolation and characterization of pulp stem cells that potentially may differentiate into odontoblasts. Regenerated pulp tissue should be functionally competent, e.g., capable of forming dentin to repair lost structure. Reports have shown that isolated pulp cells can be induced to differentiate into odontoblast-like cells and generate dentin-like mineral structure in vitro (14, 15). The in vivo evidence of pulp cells capable of generating dentin was demonstrated by Gronthos et al. (2000) that pieces of human pulp/dentin complex can be formed ectopically in immunocompromised mice (16). This discovery has promoted the investigation on the stem cell-based regenerating pulp/dentin for clinical applications.
3. THE DIFFICULTY OF ENGINEERING AND REGENERATION OF A WHOLE TOOTH
Losing teeth leads to multiple consequences, although it is not life threatening. In an advanced society, lacking quality of life such as caused by missing teeth is an undesirable situation. Artificial dentures, especially the removable type, are a primitive approach trying to solve the missing teeth problem. Currently, the advancement of dental implants has provided a great service to those with missing teeth. However, similar to any artificial prosthesis, dental implants can never replace the functions and physiological normalcy of natural teeth (17). The fundamental pitfall is the lack of a natural structural relationship with the alveolar bone, i.e., absence of PDL. In fact, it requires a direct integration with bone onto its surface, which is prerequisite for success, an unnatural relation with bone as compared to a natural tooth. Furthermore, the unnatural contours and its structural interaction with the supporting structures create other problems, such as severe food impaction and peri-implantitis (18).
The alternative is tooth regeneration. As mentioned, the complexity of the tooth in terms of its structure and anatomical location makes it difficult to engineer and regenerate. Tooth is in fact a continuous structure of the jaw alveolar bone during and after its formation. This continuity is evidenced by two pieces of tissues associated with the tooth: pulp and PDL. Extracting the tooth out of the socket severs the continuity, which cause a permanent damage to the pulp as well as various levels of damage to the PDL. The outcome of PDL healing depends on how quickly the tooth is replanted back to the socket. If replanting the tooth immediately back to the socket after extraction, the PDL is likely to recover. Any loss of viability of PDL due to drying or other damage, ankylosis will occur after replantation and the root will eventually be replaced by bone (replacement resorption). The blood and nerve supply of the pulp tissue is completely severed after extraction. If the tooth is matured with a small canal foramen at the apex, replantation is not likely to restore the vascularity or innervations of the pulp, therefore, the pulp will undergo necrosis. If the tooth is immature with a wide opening apex, there will have various extents of revascularization and reinnervation. However, the long-term recovery of the pulp tissue is usually poor and resulting in calcification. Because of these reasons, transplantation of a tooth from another site to replace a missing tooth cannot lead to a favorable outcome. These characteristics further indicate the inseparable structural nature between teeth and their supporting tissues.
Therefore, to engineer and regenerate a completely functional tooth with normal anatomic relationship to the alveolar bone, it needs to start from the very beginning of the tooth development – tooth germ or bud stage. Using isolated tooth bud cells and engineering technologies, tooth regeneration has been tested ectopically and orthotopically. In animal study models, cells isolated from tooth buds can be seeded onto scaffolds and form ectopic teeth in vivo (6, 19–21). Nakao et al reported engineering of teeth ectopically followed by transplantation into an othrotopic site in the mouse jaw (21). Tooth regeneration at orthotopic sites using larger animals such as dogs and swine has been tested (22, 23). The study in dog failed to demonstrate root formation (22), while the swine model was able to show root formation with a 33.3% success rate (23). To ensure that the regenerated tooth is integrated with bone, an approach using hybrid tooth-bone constructs to engineer and regenerated tooth was developed and tested but much improvement is needed before a clinical application can be foreseen (7, 24). To date, the largest animal model used for tooth regeneration is minipig. The whole tooth may be regenerated in minpig jaws and reaches eruption (unpublished data presented by Dr. T.-F. Kuo, Taipei, Taiwan, 2009). However, the direction of the eruption is uncontrollable, i.e., the regenerated tooth may become impacted. The most difficult part of tooth regeneration lies in the lack of the source of autologous tooth bud cells. Unless the tooth buds (3rd molars) are removed and stored beforehand, no autologous tooth bud cells can be available when tooth regeneration is needed.
4. PULP/DENTIN REGENERATION TO PREVENT TOOTH LOSS
The potential of pulp tissue to self-regenerate lost dentin is well known. When pulp tissue is exposed due to the lost of the overlaying dentin, direct pulp capping therapy can allow the pulp to form new dentin, which is termed dentin bridge. Using various cement-based materials for pulp capping such as calcium hydroxide and mineral trioxide aggregate (MTA) has been well documented and studied (25, 26). When the tooth is further damaged, self-regeneration of dentin becomes difficult, as it needs a healthy pulp, which may be compromised by the disease.
To remove the infection, first the entire pulp tissue has to be removed (pulpectomy). Subsequently, in order to thoroughly disinfect and fill the canal with gutta percha, the root canal space must be enlarged significantly causing further tooth structure loss (Fig. 1). Although studies have shown that the success rate of endodontic treatment is relatively high (78%–98%) (27–30), other ramifications are normally not taken into the consideration in measuring the success. For example, i) endodontic procedures are technically sensitive that mishaps occur, including blockage of the root canal space, breakage of instruments in the canals, perforations, etc. (31–36); ii) after endodontic treatment of immature tooth, no additional dentin tissue can be gained leaving behind a week tooth susceptible to fracture from traumatic injuries (37–41); iii) significant amount of tooth structure is lost from endodontic treatment and more so from the subsequent restorative procedures (Fig. 1) (42–44); and iv) pulpless teeth have no sensation to irritations, rendering caries progression unnoticed by patients. Long-term studies have shown that tooth loss is higher for endodontically treated teeth than non-treated due to secondary caries and complex restoration associated problems (45–48).
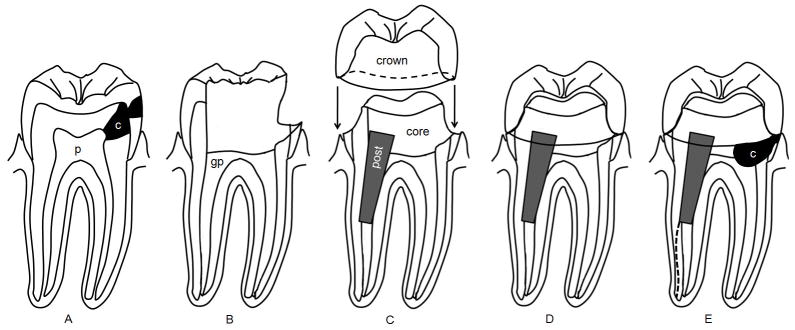
Fig. 1.
Tooth structure loss resulting from caries, root canal treatment and restoration. (A) Tooth is decayed (c) and causes irreversible pulpitis that requires root canal therapy. p, pulp. (B) Tooth decay is completely removed and the root canal space enlarged (cleaned and shaped) and filled with gutta percha (gp). (C) A post is placed in a canal and core material placed to fill the coronal space. The natural crown is then prepared/cut into a specific configuation and an artificial crown is manufactured for insertion. (D) the artificial crown is cemented onto the tooth as a final restoration and the tooth is back to its function. (E) If without good and more rigorous care, tooth decay can recur. Tooth may also undergo fracture (dashed line) due to loss of structure which weakens its capacity to withhold mechanical stress. In this condition, the tooth is not salvageable and to be extracted.
Therefore, if lost pulp and dentin can be regenerated via tissue engineering process as part of endodontic therapy, decayed tooth is likely to defer the fate of extraction. Depending on the clinical situations, two types of pulp regeneration can be considered: i) Partial pulp regeneration: It has been observed that pulpal infection and inflammation is compartmentalized until the entire pulp tissue undergoes necrosis (49, 50). Before the complete pulp necrosis, the remaining pulp tissue may be recoverable after disinfection and help regenerate the lost portion. To enhance the regeneration, engineered pulp tissues may be inserted into the pulp space to facilitate the entire recovery of pulp tissue and the generation of new dentin. ii) De novo synthesis of pulp: When the entire pulp tissue is lost, de novo synthesis of pulp must take place in order to regenerate the tissue. The volume of the mature pulp tissue is very small (~10–100 μ1), therefore, regeneration of pulp should be relatively simpler than that for larger organs or tissues. However, it has been considered a difficult task to engineer and regenerate the entire pulp and its product, dentin, due to the following situations: 1) unique anatomical location of the pulp tissue -encased within dentin having mainly one apical foramen to allow angiogenesis for the engineered tissue; 2) unique microstructure of pulp tissue, i.e., different types of cells (e.g., odontoblasts) in different layers or zones and complex innervation (Fig. 2); and 3) specific location of dentin located only peripherally of the pulp tissue and the highly organized dentin structure with well-aligned dentinal tubules (Fig. 2).
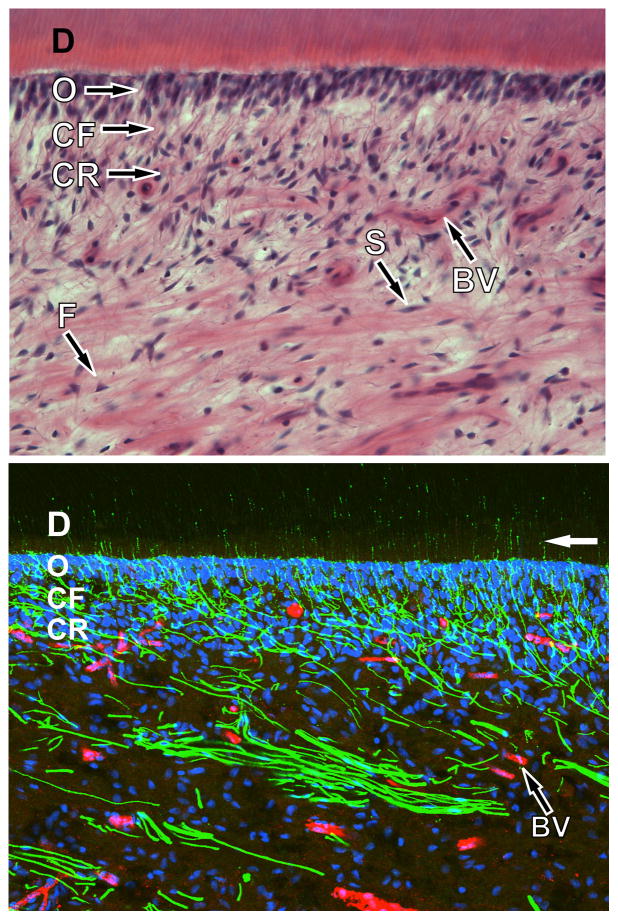
Fig. 2.
Micrographes of human dental pulp. (Top) Light micrograph of the pulpodentin complex from the pulp horn region of a decalcified human tooth following staining with hematoxylin and eosin. The pulpodentin complex consists of a highly differentiated tissue with a consistent morphological pattern that includes dentin and dentinal tubules (D), the odontoblast layer (O), the cell free zone (zone of Weil; CF), and the cell rich zone (CR). Other components of the pulp include Schwann cells (S) that enwrap nerve fibers, blood vessels (BV), and fibroblasts (F). (Bottom) Confocal micrograph of the pulpodentin complex from the pulp horn region of the same sample as seen above shows immunofluorescence for N52 (green; identifies nerve fibers) and von Willebrand factor (red; identifies endothelial cells associated with blood vessels), whereas cellular nuclei are stained with ToPro-3 (blue). The nerves fibers form an extensive plexus just below the odontoblasts. Some nerve fibers pass through the odontoblastic layer, where they enter and continue within dentinal tubules for about 100 microns (white arrow) before terminating. Both images courtesy of Dr. Michael Henry, University of Texas Health Science Center at San Antonio).
Fig. 3.
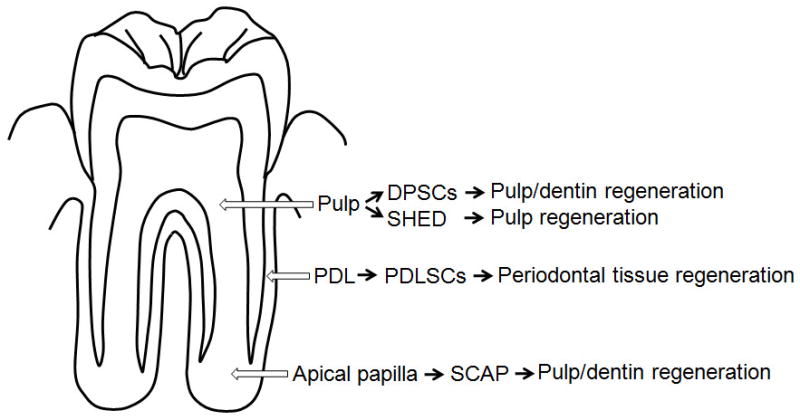
Source of dental stem cells and use for dental tissue regeneration. Note DPSCs are from pulp of permanent teeth, SHED from exfoliated primary teeth.
5. DISCIPLINE OF TISSUE ENGINEERING FOR PULP/DENTIN REGENERATION
Regeneration of any tissue back to its original condition has been a long quest in any medical disciplines. To enhance the regeneration, blood clot has been used as a rich source of growth factors to help tissue repair. Creating hemorrhage to fill into the surgical site has been a routine practice for certain conditions in surgery. In endodontics, this idea was tested by Ostby in the 1960s (51) and in the 1970s by another group (52). They observed limited tissue regeneration in the canal space, but not pulp tissue.
The discipline “tissue engineering” emerged in the late 1980s and one of the key components of this modern concept is the utilization of synthetic biocompatible/biodegradable polymers configured as scaffolds seeded with ex vivo expanded viable cells (53). The scaffold provides a three-dimensional environment for cells to attach and grow, therefore mimicking the in vivo condition. Additionally, these synthetic matrices can be fabricated such that it may form any desired shape and carry needed growth factors to guide the process of cell differentiation and tissue formation (54, 55). Generally, tissue-engineering technology involves generating tissue or organ constructs in vitro for subsequent implantation. The biodegradable material can be synthetic polymers, e.g., D, L-lactide and glycolide (PLG) or processed biological products, e.g., collagen matrix or gel (54–56).
Using this modern tissue engineering concepts, research groups led by Mooney and Rutherford tested the pulp regeneration by growing pulp cells onto a synthetic polymer scaffold of polyglycolic acid (PGA) and performed in vitro and in vivo analyses (11–13). Their approaches are a proof-of-principle to test whether cultured pulp cells can grow well and produce matrix on PGA, and whether the engineered pulp can be vascularized using in vivo study models. However, due to the lack of the technology to isolate stem cells in pulp tissues that can give rise to odontoblasts and make dentin in vivo, this line of research was discontinued until pulp stem cells were isolated and characterized.
A type of mesenchymal stem cell-like cells residing in human dental pulp was reported by a series of papers by Shi and colleagues (2000–2003). These cells were named postnatal dental pulp stem cells (DPSCs) and exhibited the ability to form pieces of ectopic human pulp-/dentin-like complex as well as to form scattered dentin-like structures on existing human dentin surface in immunocompromised mice. (16, 57, 58). Besides DPSCs, several other types of stem cells or progenitor cells from dental tissues have been isolated and characterized, these are stem cells from exfoliated deciduous teeth (SHED) (59), periodontal ligament stem cells (PDLSCs) (60), stem cells from apical papilla (SCAP) (10, 61) and dental follicle progenitor cells (DFPCs) (62). These different types of dental stem cells appear to be good cell sources for regenerating dental tissues (Fig. 3). In the field of orofacial regeneration, these stem cells have been tested in animal models on their potential for clinical applications (3). Particularly, they promoted a new discipline in clinical endodontics, the term regenerative endodontics which focuses on the understanding of these stem cells and their use for the regeneration of endodontic tissues including pulp and dentin (17, 63, 64).
5.1. Non-cell-based pulp/dentin tissue regeneration
For a sizable tissue defect, cell based therapy is inevitable for a complete regeneration of the lost tissue. Non cell-based approach is preferable if the tissue defect is not extensive because the utilization of cells, especially ex vivo expanded, as a therapeutic mode is much more complex. The source of cells is a major issue.
Application of recombinant growth factors to the injured site to enhance the regeneration of dentin has been investigated for repair of small amount of dentin lost (65). Utilizing growth factors to attract stem cells residing in the remaining pulp tissue to the defected site and to regenerate the lost part is considered a preferred approach. However, pulp tissue appears to lack the tendency to expand when there is space next to it. Our recent report showed that when pulp is partially damaged in an immature tooth with open apex, the remaining pulp tissue did not expand to the damaged site during healing process, instead, the site was filled in by periapical tissue, i.e., periodontal ligament and cementum (66) (Fig 4). This finding suggests that relying on attracting stem cells in the remaining healthy pulp tissue to the damaged site to regrow pulp using growth factors is unlikely to work. The damaged site was filled with blood clot, a rich source of growth factors, was not able to attract stem cells from the remaining pulp to the damaged site.
Fig 4.
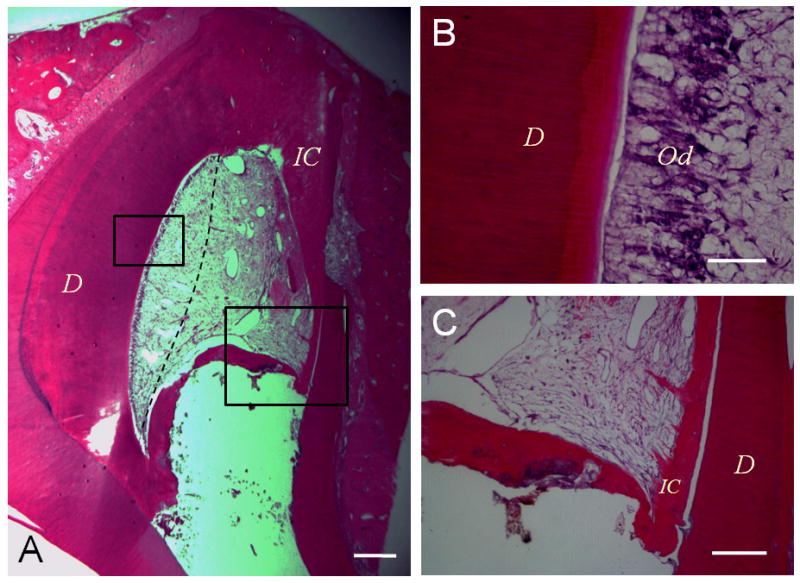
Lost portion of pulp is replaced by periapical tissues. A dog tooth was accessed and the pulp tissue partially removed and infected. The root canal was then disinfected and the space filled with blood clot. (A) Histologic view of the healed pulp tissue 3 months after the disinfection. The pulp tissue on the left side healed and right side of the pulp tissue was lost and the space filled in by periodontal tissue including soft connective soft tissue and intracanal cementum (IC). Dashed line separates the healed pulp tissue (left) and the ingrown periapical connective tissue (right). (B) Higher magnification view of the odontoblast layer (od) from the left boxed region in (A). (C) Magnified view of the right boxed region in (A). Pulp space is filled with soft connective tissue. The hard tissue IC extends from the dentinal wall toward the opposite of the canal forming a bridge. Scale bars: 500 μm (A); 50 μm (B); 200 μm (C). (66).
Evidence on more extensive regeneration using non-cell-based approach is lacking. As mentioned before, attempt to blood clot to regenerate totally lost pulp tissue was not successful. A recent paper utilized a set of growth factors to regenerate pulp tissue without the inclusion of cells yielded formation of vascularized tissues in the root canal space (67). However, the nature of the regenerated tissue is questionable and the quality is far from being comparable to the regenerated pulp tissue using stem cell-based described below.
5.2. Stem cell-based pulp/dentin tissue regeneration
Cell-based therapy is effective for repairing extensive size defect. Stem cell-based approach provides even more optimal results due to their potency in dividing and differentiating in response to microenvironmental cues. Because of this premise, stem cell biology has emerged as one of the fundamental underpinnings for regenerative medicine.
5.2.1. Suitable cell types for pulp/dentin engineering and regeneration
DPSCs, SHED and SCAP are potentially suitable cell sources for pulp/dentin regeneration because they are derived from pulp tissue (source of DPSCs and SHED), or the precursor of pulp (source of SCAP). DPSCs and SCAP can form pieces pulp/dentin complex when transplanted into immunocompromised mice (10, 16, 57) whereas SHED form mineralized tissue without distinct pulp/dentin complex (59). Whether other type of stem cells, such as BMMSCs, can differentiate into odontoblasts and make dentin is questionable. Hu et al showed that mouse crude bone marrow cells rarely give rise to dental cells and only c-kit+-enriched bone marrow cells can acquire the characteristics of odontoblasts. However, this phenomenon requires the interactions between oral epithelial cells and the enriched BM cells (68). Jin’s research team further investigated this issue using a rat model in the following two studies. First, they compared the odontogenic capability between BM mesenchymal stromal cells (BMSSCs) and DPSCs by co-culturing these cells with apical bud cells (ABCs). They found that recombined DPSCs/ABCs formed typical tooth-shaped tissues with balanced amelogenesis and dentinogenesis, whereas BMSSCs/ABC recombinants developed into atypical dentin –pulp complexes without enamel formation (69). The team next observed that multipotent dermal cells incubated with conditioned medium of embryonic tooth germ cells can behave similarly to DPSCs by undergoing odontogenic differentiation (70). Therefore, other sources of MSCs may be a source of dentinogenic cells when guided by an appropriate environment. Nevertheless, these findings indicate that utilizing other cell types to regenerate pulp/dentin is a less straightforward approach than using DPSCs, SCAP and SHED.
5.2.2. Stem cell source
The existing of stem/progenitor cells in pulp tissue that can give rise to newly differentiated pulp cells especially the highly specialized cells odontoblasts that produce dentin has been known (3). Dental stem cells are considered a population of MSC-like cells, therefore, markers that have been used for identifying MSCs are also used for dental stem cells, such as the positive markers: STRO-1, CD13, CD44, CD24, CD29, CD73, CD90, CD105, CD106, CD146, Oct4, Nanog, beta2 integrin etc and the negative markers: CD14, CD34, CD45, HLA-DR, etc (71–77). Like all MSCs, dental stem cells are also heterogeneous and various markers listed above may be expressed by subpopulations of these stem cells (57). Subpopulations expressing c-kit+/CD34+/STRO-1+ DPSCs or SHED were reported to be multipotent stem cells, although c-kit and CD34 are known to be markers for hematopoietic lineages of cells (78, 79). Side population cells exist in porcine dental pulp exhibiting stem cell properties with self-renewal and multipotency for dentinogenesis, chondrogenesis, adipogenesis, and neurogenesis (80). In a dog animal model, a subfraction of side population of pulp cells, CD31−/CD146− cells were found to regenerate partially removed pulp tissue in pulp chamber (81). The human counterparts of these subpopulations of DPSCs have also been reported (82).
Using STRO-1, CD146 and pericyte associated antigen (3G5) as markers, the DPSC niche in human dental pulp was found to be localized in the perivascular and perineural sheath regions (77). Isolated STRO-1+/CD146+ DPSCs form dentin-pulp-like complex in vivo, similar to the multiple colony-derived DPSCs. The STRO-1 positive region in the pulp of deciduous teeth is similar to that of permanent teeth, also in the perivascular regions. STRO-1 staining of apical papilla has shown that the positive stain is located in the perivascular region as well as other regions scattered in the tissue (10).
5.2.3. Partial regeneration of pulp
Nakashima & Akamine illustrated the possibility of generating a piece of pulp/dentin complex in vitro as a filling material (83). This approach appears difficult as engineering and generating a three-dimensional structure of pulp-dentin complex in vitro is very technically challenging. However, Nakashima’s research team was able to show partial regeneration of pulp using a dog model by two approaches: i) Autologous DPSCs was grown as a three-dimensional pellet treated with the growth factor BMP-2 and implanted into the space of partially amputated pulp chamber. This approach was able to stimulate reparative dentin formation by the newly differentiated odontoblasts (84). ii) A subfraction of side population of pulp cells (CD31−/CD146−) were mixed with a collagen scaffold and inserted into the pulp chamber space where tissues were removed by pulpotomy. Formation of regenerated pulp tissue with good vacularity and new dentin deposition was observed in the pulp chamber (81) (Fig. 5).
Fig. 5.
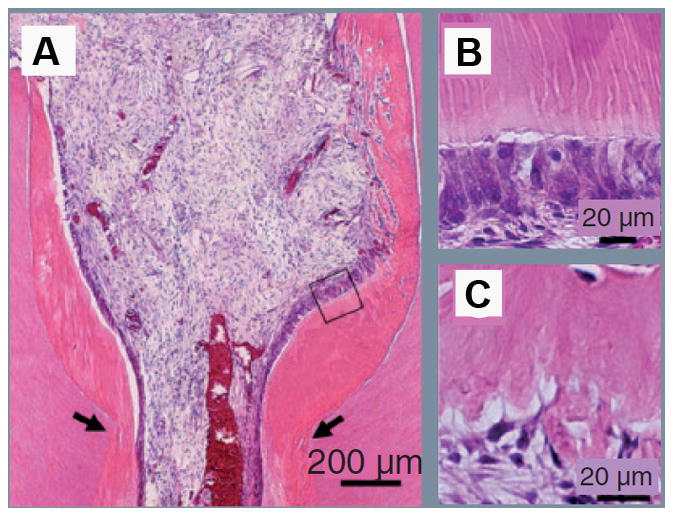
Partial pulp regeneration in dogs after autologous transplantation of CD31-/CD146- side population cells. (A) Regenerated pulp tissue in the cavity on the amputated sites (arrows). Note the tubular dentin and/or osteodentin only along the dentinal wall. (B) Tubular dentin formation along the dentinal wall in the cavity. Image originated from boxed area in (A). (C) Osteodentin formation at the top of the cavity under cement. (Adapted after permission (81)).
5.2.4. De novo regeneration of dental pulp and dentin
To regenerate pulp tissue from in an empty root canal space, pulp stem cells seeded into a scaffold and inserted into the canal space appears to be a clinically practical approach. To simulate the clinical situation, a tooth fragment model was employed by our team. A section of human tooth roots of ~ 6–8 mm in length was obtained. The canal content was totally removed and the space enlarged to 1–3 mm in diameter with one end of the canal opening sealed with a cement – MTA (Fig. 6, black and white illustration). PLG was utilized as a scaffold to carry heterogeneous population of SCAP or DPSCs, packed into the canal space and the constructs transplanted subcutaneously into SCID mice. Three-four months later, the emptied canal space was filled with a good quality of vascularized pulp-like tissue and more importantly, a uniformed thickness of a newly generated dentin-like layer was deposited onto the canal dentin wall as well as onto the MTA cement (Fig. 6) (85).
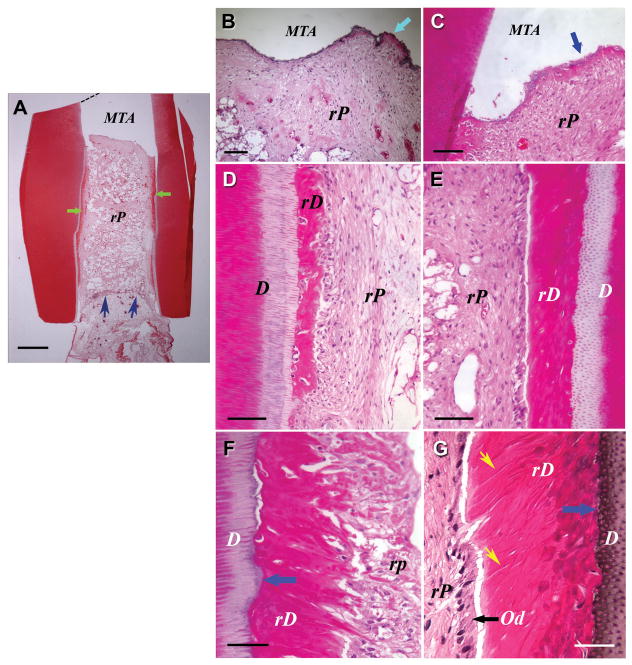
Fig. 6.
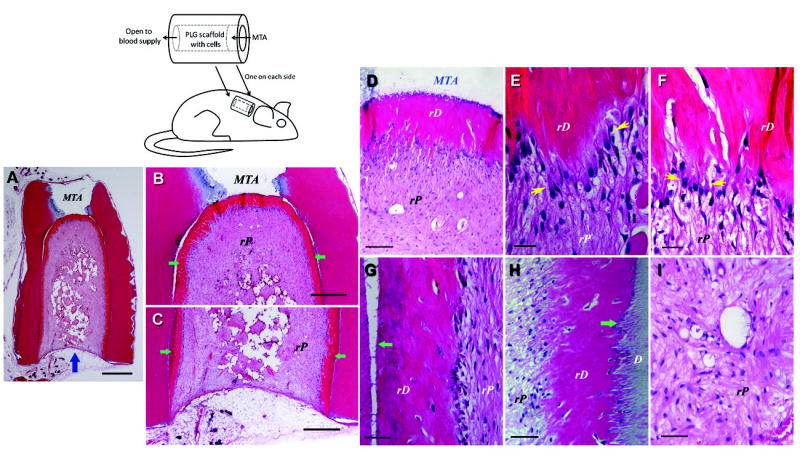
De novo regeneration of human dental pulp/dentin. Illustration at upper left depicts SCID mouse subcutaneous study model for pulp/dentin regeneration. The canal space of human tooth root fragments (~6–7 mm long) was enlarged to ~2.5 mm in diameter. One end of the canal opening was sealed with MTA cement. (A–I) Histological analysis of in vivo pulp/dentin regeneration using SCAP. A root fragment was prepared and the canal space inserted with SCAP/PLG and transplanted into a SCID mouse for 3 months. The sample was harvested and processed for H&E staining. D, original dentin; rD, regenerated dentin-like tissue; rP, regenerated pulp-like tissue. Blue arrow in (A) indicates the blood supply entrance; green arrows in (B&C) indicate continuous layer of uniformed thickness of rD; yellow arrows in (E&F) indicate the region of well-aligned odontoblast-like cells with polarized cell bodies; green arrows in (G&H) indicate junctions between D and rD. Scale bars: (A) 1 mm; (B& C) 500 μm. (D) 100 μm; (E & F) 20 μm; (G–I) 50 μm. (Adapted from (85) with permission)
Higher magnification observations show that a layer of odontoblast-like cells is lined against the mineralized dentin-like tissue. There are also scattered cells embedded within the dentin-like structure. Unlike the natural dentin, the pulp side of the regenerated dentin-like tissue is not smooth; instead, there are projections of the structures into the pulp side. The odontoblast-as if cells are not well organized, nor well aligned as the natural counterparts, and it is difficult to observe these typical characteristics of natural odontoblasts possess (e.g., polarized cell bodies). Nonetheless, some regions of the odontoblast-like cells are of somewhat typical odontoblast characteristics. Groups of odontoblast-like cells are well aligned, such that each cell is spatially juxtaposed to adjacent cells with overt polarized morphology (Fig. 6E&F and Fig. 7G). The regenerated dentin-like tissue did not form observable well-organized dentinal tubules, except in a few regions where odontoblast-like cells were better aligned (Fig. 7G). No different cell or structural zones like the natural pulp tissue are seen. Nonetheless, these well-organized cell and structural zone are normally seen in the pulp chamber of the natural teeth, less so or none in the pulp of the root. Therefore, the data presented in Figs 6 &7 represent a real possibility that pulp and dentin can be de novo regenerated using a cell-based engineering approach.
Fig. 7.
Histological analysis of in vivo pulp/dentin regeneration using DPSCs. Samples were prepared using the same procedures as described in Fig. 6, except that the sample was harvested from the SCID mouse 4 months post-implantation. D, original dentin; rD, regenerated dentin-like tissue; rP, regenerated pulp-like tissue. Green arrows in (A) indicate rD; blue arrows in (A) indicate the entrance of blood supply; blue arrow in (B&C) indicate the thin layer of rD under MTA cement; blue arrows in (F&G) indicate the junction of D and rD; black arrow in (G) indicates well-aligned odontoblast-like cells. Scale bars: (A) 1 mm; (B) 200 μm; (C–E) 100 μm; (F&G) 50 μm. (Adapted from (85) with permission)
6. CHALLENGE AND FUTURE PROSPECT ON REGENERATION OF FUNCTIONAL PULP/DENTIN
The most challenging part of tissue regeneration is perhaps the functional tissue engineering and regeneration. Although pulp tissue is very small, it is highly organized and complex. Regenerated pulp tissue in a tooth should be: i) vascularized, ii) containing similar cell density and architecture of the extracellular matrix to those of natural pulp, iii) capable of giving rise to new odontoblasts lining against the existing dentin surface and produce new dentin, and iv) innervated.
Vascularization may be difficult for teeth that the apical canal opening for blood vessel entrance is small (< 1mm). The size of apical opening would affect the ingrowth of blood vessels into the engineered pulp tissue. The larger the opening, the more likely the angiogenesis can occur. Therefore, immature teeth with open apices are the best candidates for pulp tissue regeneration. It was considered that the use of angiogenic inducing factors such as vascular endothelial growth factor (VEGF) and/or platelet-derived growth factor (PDGF) should enhance and accelerate the pulp angiogenesis. Synthetic scaffolds such as PLG can be fabricated with impregnated these growth factors (54, 86–90). Alternatively, the insertion of engineered pulp tissue may have to be separated into multiple steps (17).
As along as good blood supply can be achieved, optimal cell density and the laid down of good quality extracellular matrix should occur. New odontoblast-like cells will form against the existing dentinal wall that has been chemically disinfected as evidenced by our recent report (4). Further, we have shown, as presented herein, new dentin-like tissue can be deposited onto the canal dentinal wall, although the nature of the new mineral tissue remains to be determined in terms of its mechanical and chemical properties.
With respect to innervation, it is likely that regenerated pulp contains ingrown nerve fibers from and adjacent natural tissues. DPSCs have been shown to either produce neurotrophic factors or possess neural differentiation potential (57, 91). However, the specific innervation at the pulp-dentin complex makes the issue not quite straightforward. The reason why dentin is so sensitive to various irritations is the hydrodynamic activities of the dentinal tubules in association with the sensory A-δ fibers extending into the dentinal tubules in the predentin layer. Since the newly generated dentin does not appear to have well-organized dentinal tubules and is similar to reparative dentin, even if the regenerated A-δ fibers reach the pulp-dentin junction, it may not cause the normal dentin sensitivity as the natural teeth.
Another issue on functional regeneration of pulp/dentin is the regeneration of enamel. Intact enamel overlaying dentin is needed to repair the damaged tooth. As noted before, enamel cannot self-regenerate; therefore, engineering approaches have to take place. Enamel regeneration has been tested by various accelular methods using recombinant enamel proteins amelogenin, surfactants, or using simple chemicals like calcium phosphate-containing solutions/paste to restore the enamel layer (92–94). However, these approaches appear to be difficult to apply clinically or can only produce minimal amount of regenerated enamel on existing natural enamel. Thus, the final step of restoring tooth after pulp and dentin regeneration is likely to still require the use of artificial materials.
As mentioned, the availability of cell source for cell-based tissue regeneration is an issue. Tooth and dental stem cell banking are essential infrastructure that has to be established in order to make cell-based therapy practical either for autologous or allogenic applications. Ultimately, the paucity of postnatal stem cells may be resolved by the generation of induced pluripotent (iPS) cells from somatic cells (95, 96) or from dental stem cells (97). Our lab has recently established human iPS from dental stem cells that are capable of differentiating into cells of all three germ layers (Fig. 8). These cells are potentially immortal and may serve as an unlimited cell source for regenerative medicine as well as for pulp/dentin regeneration.
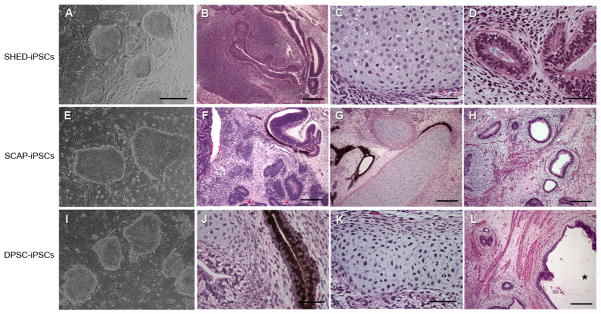
Fig. 8.
Induced pluripotent stem (iPS) cells derived from human dental stem cells. (A, E, I) ES cell-like colonies. SHED, SCA, and DPSCs were reprogrammed into ES-cell like colonies with the 4 factors (Lin28, Nanog, Oct4 and Sox2). These iPS cells form teratomas in SCID mice containing tissues of all three germ layers. (B, F, J) Mainly primitive neural tissues, neural rosettes and retinal epithelium (ectoderm); (C, G, K) mainly cartilage (mesoderm); D, H, L) mainly glandular tissue or respiratory epithelium (endoderm). Scale bars: (A, E, I) 500 μm; (B, F–H, L), 200 μm; (C, J, K), 50 μm. *, in (K) indicates the space of a cavity inside the teratoma. (Adapted from (97) with permission)
7. CONCLUSION
Current clinical protocol of endodontic treatment sacrifices tissues in order to disinfect. After which, no regenerative process can take place as the lost tooth structure is replaced by artificial materials which does not strengthen the tooth. Regeneration of lost pulp and dentin tissues can reverse the deteriorated tooth and avoid more aggressive procedures that can cause tooth structure loss. Dental stem cell-based approaches show capabilities of de novo regenerating pulp and new dentin. Further research is needed to regenerate higher quality of pulp and dentin. With regard to cell source, establishing dental stem cell banking may be a necessary step and further progress on establishing individualized induced pluripotent stem cells for dental tissue regeneration is imminent.
Acknowledgments
This work was supported in part by a grant from the National Institutes of Health R01 DE019156 (G. T.-J. H). The author thanks Dr. Michael Henry, University of Texas Health Science Center at San Antonio, for contributing the images in Figure 2.
Abbreviations
- PDL
periodontal ligament
- MTA
mineral trioxide aggregate
- PLG
D, L-lactide and glycolide
- PGA
polyglycolic acid
- BMSSCs
bone marrow mesenchymal stromal cells
- DPSCs
dental pulp stem cells
- SHED
stem cells from human exfoliated deciduous teeth
- PDLSCs
periodontal ligament stem cells
- SCAP
stem cells for apical papilla
- DFPCs
dental follicle progenitor cells
- ABC
apical bud cell
- MSC
mesenchymal stem cells
- SCID
severe combined immunodeficiency
References
- Mantesso A, Sharpe P. Dental stem cells for tooth regeneration and repair. Expert Opinion on Biological Therapy. 2009;9:1143–1154. doi: 10.1517/14712590903103795. [DOI] [PubMed] [Google Scholar]
- Chen F-M, Jin Y. Periodontal Tissue Engineering and Regeneration: Current Approaches and Expanding Opportunities. Tissue Engineering Part B: Reviews. 2010;16:219–255. doi: 10.1089/ten.TEB.2009.0562. [DOI] [PubMed] [Google Scholar]
- Huang GTJ, Gronthos S, Shi S. Mesenchymal Stem Cells Derived from Dental Tissues vs. Those from Other Sources: Their Biology and Role in Regenerative Medicine. Journal of Dental Research. 2009;88:792–806. doi: 10.1177/0022034509340867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang GTJ, Yamaza T, Shea LD, Djouad F, Kuhn NZ, Tuan RS, Shi S. Stem/Progenitor Cell–Mediated De Novo Regeneration of Dental Pulp with Newly Deposited Continuous Layer of Dentin in an In Vivo Model. Tissue Engineering Part A. 2010;16:605–615. doi: 10.1089/ten.tea.2009.0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panzarini SR, Gulinelli JL, Poi WR, Sonoda CK, Pedrini D, Brandini DA. Treatment of root surface in delayed tooth replantation: a review of literature. Dental Traumatology. 2008;24:277–282. doi: 10.1111/j.1600-9657.2008.00555.x. [DOI] [PubMed] [Google Scholar]
- Young CS, Terada S, Vacanti JP, Honda M, Bartlett JD, Yelick PC. Tissue engineering of complex tooth structures on biodegradable polymer scaffolds. J Dent Res. 2002;81:695–700. doi: 10.1177/154405910208101008. [DOI] [PubMed] [Google Scholar]
- Young CS, Abukawa H, Asrican R, Ravens M, Troulis MJ, Kaban LB, Vacanti JP, Yelick PC. Tissue-engineered hybrid tooth and bone. Tissue Eng. 2005;11:1599–610. doi: 10.1089/ten.2005.11.1599. [DOI] [PubMed] [Google Scholar]
- Yen A, Sharpe P. Stem cells and tooth tissue engineering. Cell and Tissue Research. 2008;331:359–372. doi: 10.1007/s00441-007-0467-6. [DOI] [PubMed] [Google Scholar]
- Ohazama A, Modino SA, Miletich I, Sharpe PT. Stem-cell-based tissue engineering of murine teeth. J Dent Res. 2004;83:518–22. doi: 10.1177/154405910408300702. [DOI] [PubMed] [Google Scholar]
- Sonoyama W, Liu Y, Fang D, Yamaza T, Seo BM, Zhang C, Liu H, Gronthos S, Wang CY, Shi S, Wang S. Mesenchymal stem cell-mediated functional tooth regeneration in Swine. PLoS ONE. 2006;1:e79. doi: 10.1371/journal.pone.0000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney DJ, Powell C, Piana J, Rutherford B. Engineering dental pulp-like tissue in vitro. Biotechnol Prog. 1996;12:865–8. doi: 10.1021/bp960073f. [DOI] [PubMed] [Google Scholar]
- Bohl KS, Shon J, Rutherford B, Mooney DJ. Role of synthetic extracellular matrix in development of engineered dental pulp. Journal of Biomaterials Science Polymer Edition. 1998;9:749–64. doi: 10.1163/156856298x00127. [DOI] [PubMed] [Google Scholar]
- Buurma B, Gu K, Rutherford RB. Transplantation of human pulpal and gingival fibroblasts attached to synthetic scaffolds. European Journal of Oral Sciences. 1999;107:282–9. doi: 10.1046/j.0909-8836.1999.eos107408.x. [DOI] [PubMed] [Google Scholar]
- Tsukamoto Y, Fukutani S, Shin-Ike T, Kubota T, Sato S, Suzuki Y, Mori M. Mineralized nodule formation by cultures of human dental pulp-derived fibroblasts. Arch Oral Biol. 1992;37:1045–55. doi: 10.1016/0003-9969(92)90037-9. [DOI] [PubMed] [Google Scholar]
- About I, Bottero MJ, de Denato P, Camps J, Franquin JC, Mitsiadis TA. Human dentin production in vitro. Exp Cell Res. 2000;258:33–41. doi: 10.1006/excr.2000.4909. [DOI] [PubMed] [Google Scholar]
- Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proceedings of the National Academy of Sciences. 2000;97:13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang GTJ, Sonoyama W, Liu Y, Liu H, Wang S, Shi S. The Hidden Treasure in Apical Papilla: The Potential Role in Pulp/Dentin Regeneration and BioRoot Engineering. Journal of Endodontics. 2008;34:645–651. doi: 10.1016/j.joen.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouhyi J, Dohan Ehrenfest DM, Albrektsson T. The Peri-Implantitis: Implant Surfaces, Microstructure, and Physicochemical Aspects. Clin Implant Dent Relat Res. 2009 doi: 10.1111/j.1708-8208.2009.00244.x. [DOI] [PubMed] [Google Scholar]
- Duailibi MT, Duailibi SE, Young CS, Bartlett JD, Vacanti JP, Yelick PC. Bioengineered teeth from cultured rat tooth bud cells. J Dent Res. 2004;83:523–8. doi: 10.1177/154405910408300703. [DOI] [PubMed] [Google Scholar]
- Komine A, Suenaga M, Nakao K, Tsuji T, Tomooka Y. Tooth regeneration from newly established cell lines from a molar tooth germ epithelium. Biochem Biophys Res Commun. 2007;355:758–63. doi: 10.1016/j.bbrc.2007.02.039. [DOI] [PubMed] [Google Scholar]
- Nakao K, Morita R, Saji Y, Ishida K, Tomita Y, Ogawa M, Saitoh M, Tomooka Y, Tsuji T. The development of a bioengineered organ germ method. Nat Methods. 2007;4:227–30. doi: 10.1038/nmeth1012. [DOI] [PubMed] [Google Scholar]
- Honda MJ, Ohara T, Sumita Y, Ogaeri T, Kagami H, Ueda M. Preliminary Study of Tissue-Engineered Odontogenesis in the Canine Jaw. Journal of Oral and Maxillofacial Surgery. 2006;64:283–289. doi: 10.1016/j.joms.2005.10.020. [DOI] [PubMed] [Google Scholar]
- Kuo T-F, Huang A-T, Chang H-H, Lin F-H, Chen S-T, Chen R-S, Chou C-H, Lin H-C, Chiang H, Chen M-H. Regeneration of dentin-pulp complex with cementum and periodontal ligament formation using dental bud cells in gelatin-chondroitin-hyaluronan tri-copolymer scaffold in swine. Journal of Biomedical Materials Research Part A. 2007 doi: 10.1002/jbm.a.31746. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Zhang W, Abukawa H, Troulis MJ, Kaban LB, Vacanti JP, Yelick PC. Tissue engineered hybrid tooth-bone constructs. Methods. 2009;47:122–128. doi: 10.1016/j.ymeth.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Bogen G, Kim JS, Bakland LK. Direct pulp capping with mineral trioxide aggregate: an observational study. Journal of the American Dental Association. 2008;139:305–15. doi: 10.14219/jada.archive.2008.0160. [DOI] [PubMed] [Google Scholar]
- Olsson H, Rohlin KPM. Formation of a hard tissue barrier after pulp cappings in humans. A systematic review. International Endodontic Journal. 2006;39:429–442. doi: 10.1111/j.1365-2591.2006.01116.x. [DOI] [PubMed] [Google Scholar]
- Alley BS, Kitchens GG, Alley LW, Eleazer PD. A comparison of survival of teeth following endodontic treatment performed by general dentists or by specialists. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;98:115–8. doi: 10.1016/j.tripleo.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Marending M, Peters OA, Zehnder M. Factors affecting the outcome of orthograde root canal therapy in a general dentistry hospital practice. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99:119–24. doi: 10.1016/j.tripleo.2004.06.065. [DOI] [PubMed] [Google Scholar]
- Kojima K, Inamoto K, Nagamatsu K, Hara A, Nakata K, Morita I, Nakagaki H, Nakamura H. Success rate of endodontic treatment of teeth with vital and nonvital pulps. A meta-analysis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:95–9. doi: 10.1016/j.tripleo.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Dammaschke T, Steven D, Kaup M, Ott KH. Long-term survival of root-canal-treated teeth: a retrospective study over 10 years. J Endod. 2003;29:638–43. doi: 10.1097/00004770-200310000-00006. [DOI] [PubMed] [Google Scholar]
- Ruddle CJ. Broken instrument removal. The endodontic challenge. Dent Today. 2002;21:70–2. 74, 76. passim. [PubMed] [Google Scholar]
- Ward JR. The use of an ultrasonic technique to remove a fractured rotary nickel-titanium instrument from the apical third of a curved root canal. Aust Endod J. 2003;29:25–30. doi: 10.1111/j.1747-4477.2003.tb00492.x. [DOI] [PubMed] [Google Scholar]
- Suter B, Lussi A, Sequeira P. Probability of removing fractured instruments from root canals. Int Endod J. 2005;38:112–23. doi: 10.1111/j.1365-2591.2004.00916.x. [DOI] [PubMed] [Google Scholar]
- Bargholz C. Perforation repair with mineral trioxide aggregate: a modified matrix concept. Int Endod J. 2005;38:59–69. doi: 10.1111/j.1365-2591.2004.00901.x. [DOI] [PubMed] [Google Scholar]
- Zenobio EG, Shibli JA. Treatment of endodontic perforations using guided tissue regeneration and demineralized freeze-dried bone allograft: two case reports with 2–4 year post-surgical evaluations. J Contemp Dent Pract. 2004;5:131–41. [PubMed] [Google Scholar]
- Yoldas O, Oztunc H, Tinaz C, Alparslan N. Perforation risks associated with the use of Masserann endodontic kit drills in mandibular molars. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:513–7. doi: 10.1016/j.tripleo.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Andreasen JO, Farik B, Munksgaard EC. Long-term calcium hydroxide as a root canal dressing may increase risk of root fracture. Dental Traumatology. 2002;18:134–7. doi: 10.1034/j.1600-9657.2002.00097.x. [DOI] [PubMed] [Google Scholar]
- Feely L, I, Mackie C, Macfarlane T. An investigation of root-fractured permanent incisor teeth in children. Dent Traumatol. 2003;19:52–4. doi: 10.1034/j.1600-9657.2003.00119.x. [DOI] [PubMed] [Google Scholar]
- Andreasen JO, Andreasen FM, Mejare I, Cvek M. Healing of 400 intra-alveolar root fractures. 1. Effect of pre-injury and injury factors such as sex, age, stage of root development, fracture type, location of fracture and severity of dislocation. Dent Traumatol. 2004;20:192–202. doi: 10.1111/j.1600-9657.2004.00279.x. [DOI] [PubMed] [Google Scholar]
- Bastone EB, Freer TJ, McNamara JR. Epidemiology of dental trauma: a review of the literature. Aust Dent J. 2000;45:2–9. doi: 10.1111/j.1834-7819.2000.tb00234.x. [DOI] [PubMed] [Google Scholar]
- Da Silva AC, Passeri LA, Mazzonetto R, De Moraes M, Moreira RW. Incidence of dental trauma associated with facial trauma in Brazil: a 1-year evaluation. Dent Traumatol. 2004;20:6–11. doi: 10.1111/j.1600-4469.2004.00212.x. [DOI] [PubMed] [Google Scholar]
- Sedgley CM, Messer HH. Are endodontically treated teeth more brittle? J Endod. 1992;18:332–5. doi: 10.1016/S0099-2399(06)80483-8. [DOI] [PubMed] [Google Scholar]
- Huang GT, Schilder H, Nathanson D. Effects of moisture content and endodontic treatment on some mechanical properties of human dentin. J Endodont. 1992;18:209–215. doi: 10.1016/S0099-2399(06)81262-8. [DOI] [PubMed] [Google Scholar]
- Reeh ES, Messer HH, Douglas WH. Reduction in tooth stiffness as a result of endodontic and restorative procedures. J Endod. 1989;15:512–6. doi: 10.1016/S0099-2399(89)80191-8. [DOI] [PubMed] [Google Scholar]
- Piwowarczyk A, Lauer HC, Sorensen JA. Microleakage of various cementing agents for full cast crowns. Dent Mater. 2005;21:445–53. doi: 10.1016/j.dental.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Caplan DJ, Cai J, Yin G, White BA. Root canal filled versus non-root canal filled teeth: a retrospective comparison of survival times. J Public Health Dent. 2005;65:90–6. doi: 10.1111/j.1752-7325.2005.tb02792.x. [DOI] [PubMed] [Google Scholar]
- Pappen AF, Bravo M, Gonzalez-Lopez S, Gonzalez-Rodriguez MP. An in vitro study of coronal leakage after intraradicular preparation of cast-dowel space. J Prosthet Dent. 2005;94:214–8. doi: 10.1016/j.prosdent.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Demirel F, Saygili G, Sahmali S. Microleakage of endodontically treated teeth restored with prefabricated posts and tooth-colored restorative materials. Int J Periodontics Restorative Dent. 2005;25:73–9. [PubMed] [Google Scholar]
- Seltzer S, I, Bender B, Ziontz M. The Dynamics of Pulp Inflammation: Correlations between Diagnostic Data and Actual Histologic Findings in the Pulp. Oral Surg Oral Med Oral Pathol. 1963;16:969–77. doi: 10.1016/0030-4220(63)90201-9. [DOI] [PubMed] [Google Scholar]
- Trowbridge H. Histology of pulpal Inflammation. In: Hargreaves KM, Goodis HE, editors. Seltzer and Bender’s Dental Pulp. Chapter 10. Quintessence Publishing Co., Inc; Carol Stream, IL: 2002. [Google Scholar]
- Ostby BN. The role of the blood clot in endodontic therapy. An experimental histologic study. Acta odontologica Scandinavica. 1961;19:324–53. [PubMed] [Google Scholar]
- Myers WC, Fountain SB. Dental pulp regeneration aided by blood and blood substitutes after experimentally induced periapical infection. Oral Surgery Oral Medicine, Oral Pathology. 1974;37:441–50. doi: 10.1016/0030-4220(74)90119-4. [DOI] [PubMed] [Google Scholar]
- Vacanti CA. The history of tissue engineering. J Cell Mol Med. 2006;10:569–76. doi: 10.1111/j.1582-4934.2006.tb00421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan MH, Shea LD, Peters MC, Mooney DJ. Bioabsorbable polymer scaffolds for tissue engineering capable of sustained growth factor delivery. Journal of Controlled Release. 2000;64:91–102. doi: 10.1016/s0168-3659(99)00138-8. [DOI] [PubMed] [Google Scholar]
- Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–6. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- Nerem RM. Tissue engineering in the USA. Med Biol Eng Comput. 1992;30:CE8–12. doi: 10.1007/BF02446171. [DOI] [PubMed] [Google Scholar]
- Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, DenBesten P, Robey PG, Shi S. Stem cell properties of human dental pulp stem cells. Journal of Dental Research. 2002;81:531–5. doi: 10.1177/154405910208100806. [DOI] [PubMed] [Google Scholar]
- Batouli S, Miura M, Brahim J, Tsutsui TW, Fisher LW, Gronthos S, Robey PG, Shi S. Comparison of stem-cell-mediated osteogenesis and dentinogenesis. Journal of Dental Research. 2003;82:976–81. doi: 10.1177/154405910308201208. [DOI] [PubMed] [Google Scholar]
- Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, Shi S. SHED: Stem cells from human exfoliated deciduous teeth. Proceedings of the National Academy of Sciences. 2003;100:5807–5812. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, Young M, Robey PG, Wang CY, Shi S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–55. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- Sonoyama W, Liu Y, Yamaza T, Tuan RS, Wang S, Shi S, Huang GTJ. Characterization of the Apical Papilla and Its Residing Stem Cells from Human Immature Permanent Teeth: A Pilot Study. Journal of Endodontics. 2008;34:166–171. doi: 10.1016/j.joen.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morsczeck C, Gotz W, Schierholz J, Zeilhofer F, Kuhn U, Mohl C, Sippel C, Hoffmann KH. Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. Matrix Biology. 2005;24:155–165. doi: 10.1016/j.matbio.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Huang GTJ, Sonoyama W, Chen J, Park S. In vitro characterization of human dental pulp cells: various isolation methods and culturing environments. Cell and Tissue Research. 2006;324:225–236. doi: 10.1007/s00441-005-0117-9. [DOI] [PubMed] [Google Scholar]
- Murray PE, Garcia-Godoy F, Hargreaves KM. Regenerative Endodontics: A Review of Current Status and a Call for Action. Journal of Endodontics. 2007;33:377–390. doi: 10.1016/j.joen.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Rutherford RB, Gu K. Treatment of inflamed ferret dental pulps with recombinant bone morphogenetic protein-7. Eur J Oral Sci. 2000;108:202–6. doi: 10.1034/j.1600-0722.2000.108003202.x. [DOI] [PubMed] [Google Scholar]
- Wang X, Thibodeau B, Trope M, Lin LM, Huang GT-J. Histologic Characterization of Regenerated Tissues in Canal Space After the Revitalization/Revascularization Procedure of Immature Dog Teeth With Apical Periodontitis. J Endodont. doi: 10.1016/j.joen.2009.09.039. (In press) [DOI] [PubMed] [Google Scholar]
- Kim JY, Xin X, Moioli EK, Chung J, Lee CH, Chen M, Fu SY, Koch PD, Mao JJ. Regeneration of Dental-Pulp-like Tissue by Chemotaxis-Induced Cell Homing. Tissue Eng Part A. 2010;16:3023–3031. doi: 10.1089/ten.tea.2010.0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, Unda F, Bopp-Kuchler S, Jimenez L, Wang XJ, Haikel Y, Wang SL, Lesot H. Bone Marrow Cells Can Give Rise to Ameloblast-like Cells. J Dent Res. 2006;85:416–421. doi: 10.1177/154405910608500504. [DOI] [PubMed] [Google Scholar]
- Yu J, Wang Y, Deng Z, Tang L, Li Y, Shi J, Jin Y. Odontogenic capability: bone marrow stromal stem cells versus dental pulp stem cells. Biology of the Cell. 2007;099:465–474. doi: 10.1042/BC20070013. [DOI] [PubMed] [Google Scholar]
- Huo N, Tang L, Yang Z, Qian H, Wang Y, Han C, Gu Z, Duan Y, Jin Y. Differentiation of dermal multipotent cells into odontogenic lineage induced by embryonic and neonatal tooth germ cell conditioned medium. Stem Cells and Development. 2009 doi: 10.1089/scd.2009.0048. Epub. [DOI] [PubMed] [Google Scholar]
- Greco SJ, Liu K, Rameshwar P. Functional Similarities Among Genes Regulated by Oct4 in Human Mesenchymal and Embryonic Stem Cells. Stem Cells. 2007;25:3143–3154. doi: 10.1634/stemcells.2007-0351. [DOI] [PubMed] [Google Scholar]
- Gronthos S, Zannettino ACW. A Method to Isolate and Purify Human Bone Marrow Stromal Stem Cells. In: Prockop DJ, Bunnell BA, Phinney DG, editors. Mesenchymal Stem Cells Methods and Protocols. Humana Press; 2008. [DOI] [PubMed] [Google Scholar]
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–7. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- Gronthos S, Zannettino AC, Hay SJ, Shi S, Graves SE, Kortesidis A, Simmons PJ. Molecular and cellular characterisation of highly purified stromal stem cells derived from human bone marrow. J Cell Sci. 2003;116:1827– 1835. doi: 10.1242/jcs.00369. [DOI] [PubMed] [Google Scholar]
- Battula VL, Bareiss PM, Treml S, Conrad S, Albert I, Hojak S, Abele H, Schewe B, Just L, Skutella T, Buhring HJ. Human placenta and bone marrow derived MSC cultured in serum-free, b-FGF-containing medium express cell surface frizzled-9 and SSEA-4 and give rise to multilineage differentiation. Differentiation. 2007;75:279–91. doi: 10.1111/j.1432-0436.2006.00139.x. [DOI] [PubMed] [Google Scholar]
- Miura Y, Miura M, Gronthos S, Allen M, Cao RC, Uveges TE, Bi Y, Ehirchiou D, Kortesidis A, Shi S, Zhang L. Defective osteogenesis of the stromal stem cells predisposes CD18-null mice to osteoporosis. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:14022–14027. doi: 10.1073/pnas.0409397102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S, Gronthos S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res. 2003;18:696–704. doi: 10.1359/jbmr.2003.18.4.696. [DOI] [PubMed] [Google Scholar]
- Laino G, d’Aquino R, Graziano A, Lanza V, Carinci F, Naro F, Pirozzi G, Papaccio G. A new population of human adult dental pulp stem cells: a useful source of living autologous fibrous bone tissue (LAB) Journal of Bone and Mineral Research. 2005;20:1394–402. doi: 10.1359/JBMR.050325. [DOI] [PubMed] [Google Scholar]
- Laino Gregorio, Graziano Antonio, d’Aquino Riccardo, Pirozzi Giuseppe, Lanza Vladimiro, Valiante Salvatore, De Rosa Alfredo, Naro Fabio, Vivarelli Elisabetta, Papaccio Gianpaolo. An approachable human adult stem cell source for hard-tissue engineering. Journal of Cellular Physiology. 2006;206:693–701. doi: 10.1002/jcp.20526. [DOI] [PubMed] [Google Scholar]
- Iohara K, Zheng L, Ito M, Tomokiyo A, Matsushita K, Nakashima M. Side Population Cells Isolated from Porcine Dental Pulp Tissue with Self-Renewal and Multipotency for Dentinogenesis, Chondrogenesis, Adipogenesis, and Neurogenesis. Stem Cells. 2006;24:2493–2503. doi: 10.1634/stemcells.2006-0161. [DOI] [PubMed] [Google Scholar]
- Iohara K, Zheng L, Ito M, Ishizaka R, Nakamura H, Into T, Matsushita K, Nakashima M. Regeneration of dental pulp after pulpotomy by transplantation of CD31-/CD146- side population cells from a canine tooth. Regenerative Medicine. 2009;4:377–385. doi: 10.2217/rme.09.5. [DOI] [PubMed] [Google Scholar]
- Honda MJ, Nakashima F, Satomura K, Shinohara Y, Tsuchiya S, Watanabe N, Ueda M. Side population cells expressing ABCG2 in human adult dental pulp tissue. International Endodontic Journal. 2007;40:949–958. doi: 10.1111/j.1365-2591.2007.01301.x. [DOI] [PubMed] [Google Scholar]
- Nakashima M, Akamine A. The application of tissue engineering to regeneration of pulp and dentin in endodontics. Journal of Endodontics. 2005;31:711–8. doi: 10.1097/01.don.0000164138.49923.e5. [DOI] [PubMed] [Google Scholar]
- Iohara K, Nakashima M, Ito M, Ishikawa M, Nakasima A, Akamine A. Dentin Regeneration by Dental Pulp Stem Cell Therapy with Recombinant Human Bone Morphogenetic Protein 2. J Dent Res. 2004;83:590–595. doi: 10.1177/154405910408300802. [DOI] [PubMed] [Google Scholar]
- Huang G, Yamaza T, Shea LD, Djouad F, Kuhn NZ, Tuan R, Shi S. Stem/progenitor Cell-Mediated De Novo Regeneration of Dental Pulp with Newly Deposited Continuous Layer of Dentin in an In Vivo Model. Tissue Engineering Part A. 2009;0 doi: 10.1089/ten.tea.2009.0518. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanematsu A, Yamamoto S, Ozeki M, Noguchi T, Kanatani I, Ogawa O, Tabata Y. Collagenous matrices as release carriers of exogenous growth factors. Biomaterials. 2004;25:4513–20. doi: 10.1016/j.biomaterials.2003.11.035. [DOI] [PubMed] [Google Scholar]
- Peters MC, Polverini PJ, Mooney DJ. Engineering vascular networks in porous polymer matrices. J Biomed Mater Res. 2002;60:668–78. doi: 10.1002/jbm.10134. [DOI] [PubMed] [Google Scholar]
- Sun Q, Chen RR, Shen Y, Mooney DJ, Rajagopalan S, Grossman PM. Sustained vascular endothelial growth factor delivery enhances angiogenesis and perfusion in ischemic hind limb. Pharm Res. 2005;22:1110–6. doi: 10.1007/s11095-005-5644-2. [DOI] [PubMed] [Google Scholar]
- Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19:1029–34. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- Stiver SI, Tan X, Brown LF, Hedley-Whyte ET, Dvorak HF. VEGF-A angiogenesis induces a stable neovasculature in adult murine brain. J Neuropathol Exp Neurol. 2004;63:841–55. doi: 10.1093/jnen/63.8.841. [DOI] [PubMed] [Google Scholar]
- Nosrat IV, Smith CA, Mullally P, Olson L, Nosrat CA. Dental pulp cells provide neurotrophic support for dopaminergic neurons and differentiate into neurons in vitro; implications for tissue engineering and repair in the nervous system. Eur J Neurosci. 2004;19:2388–98. doi: 10.1111/j.0953-816X.2004.03314.x. [DOI] [PubMed] [Google Scholar]
- Fan Y, Sun Z, Wang R, Abbott C, Moradian-Oldak J. Enamel inspired nanocomposite fabrication through amelogenin supramolecular assembly. Biomaterials. 2007;28:3034–3042. doi: 10.1016/j.biomaterials.2007.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi K, Onuma K, Suzuki T, Okada F, Tagami J, Otsuki M, Senawangse P. Materials chemistry: A synthetic enamel for rapid tooth repair. Nature. 2005;433:819–819. doi: 10.1038/433819a. [DOI] [PubMed] [Google Scholar]
- Yin Y, Yun S, Fang J, Chen H. Chemical regeneration of human tooth enamel under near-physiological conditions. Chem Commun (Camb) 2009:5892–4. doi: 10.1039/b911407f. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced Pluripotent Stem Cell Lines Derived from Human Somatic Cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Yan X, Qin H, Qu C, Tuan RS, Shi S, Huang GTJ. iPS cells reprogrammed from mesenchymal-like stem/progenitor cells of dental tissue origin. Stem Cell Develop. 2009 Oct 1; doi: 10.1089/scd.2009.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]