Abstract
Members of the order Psittaciformes (parrots and cockatoos) are among the most long-lived and endangered avian species. Comprehensive data on lifespan and breeding are critical to setting conservation priorities, parameterizing population viability models, and managing captive and wild populations. To meet these needs, we analyzed 83, 212 life history records of captive birds from the International Species Information System and calculated lifespan and breeding parameters for 260 species of parrots (71% of extant species). Species varied widely in lifespan, with larger species generally living longer than smaller ones. The highest maximum lifespan recorded was 92 years in Cacatua moluccensis, but only 11 other species had a maximum lifespan over 50 years. Our data indicate that while some captive individuals are capable of reaching extraordinary ages, median lifespans are generally shorter than widely assumed, albeit with some increase seen in birds presently held in zoos. Species that lived longer and bred later in life tended to be more threatened according to IUCN classifications. We documented several individuals of multiple species that were able to breed for more than two decades, but the majority of clades examined had much shorter active reproduction periods. Post-breeding periods were surprisingly long and in many cases surpassed the duration of active breeding. Our results demonstrate the value of the ISIS database to estimate life history data for an at-risk taxon that is difficult to study in the wild, and provide life history data that is crucial for predictive modeling of future species endangerment and proactively managing captive populations of parrots.
Keywords: captive breeding, ISIS, life history, lifespan, parrot, Psittaciformes
INTRODUCTION
Earth is facing a biodiversity crisis of enormous proportions, with extinction rates estimated to be 1,000 – 10,000 times greater than normal background rates (Brooks et al.; 2006, Wilson, 1991). Zoos and aquariums play a critical role in conserving biodiversity (Miller et al., 2004) through research, education, conservation of habitat and genomic materials, and captive breeding (Fischer & Lindenmayer, 2000; Foose & Wiese, 2006; Mace et al., 2007; Price & Soorae, 2003; Walters et al., 2010). Captive breeding maintains viable populations and in some notable cases provides the only source of individual for reintroductions (Beck et al., 1994; Seddon, Armstrong & Maloney, 2007), as with the black-footed ferret, Mustela nigripes (Biggins et al., 1999), California condor, Gymnogyps californianus (Snyder & Snyder, 1989), Przewalski’s horse, Equus caballus przewalskii (Bouman, 2000), and Arabian oryx, Oryx leucoryx (Rahbek, 1993). Another important, albeit less widely recognized, role for captive populations is to provide behavioral, physiological and life history data that are difficult, costly or time-consuming to obtain in field studies (see Ricklefs & Cadena, 2007). These data are useful for predictive modeling and management of wild populations and for setting management priorities for captive populations (Conde et al., 2011).
One important step in managing captive populations is to assess conservation priorities at the larger taxonomic scales of family or order. Zoos and aquariums have organized Taxon Advisory Groups (TAGs) to set priorities for maintaining and managing captive populations across higher-level taxa. TAGs determine which species to propagate based primarily on captive population numbers and conservation status (AZA, 2007; Hutchins, 2003; Wilkinson, 2000). The TAGs further divide species into Regional Collection Plans (RCP) which are represented worldwide. All of these programs were initiated in the 1980s to track and manage the genetics and demographics of captive animal populations in studbooks so as to meet overall management goals for that species (Hutchins & Wiese, 1991). A critical role for TAGs is to prioritize efforts across different species because both space and funding for captive animals are limited (Baker, 2007; Hutchins, 2003; Hutchins & Wiese, 1991; Smith et al., 2002).
TAGs face the issue of surplus animals, animals that have already made a genetic contribution to the program either directly or via kin (Hutchins & Wiese, 1991; Lindburg & Lindburg, 1995), and are now consuming resources that could otherwise be invested in breeding animals that would further enhance genetic diversity. To allocate limited zoo resources optimally, TAGs should work with RCPs to predict and control numbers of surplus animals (Graham, 1996; Lacy, 1995; Lindburg & Lindburg, 1995), a task which requires comprehensive data on a species’ lifespan, breeding parameters, IUCN status, current numbers, and demographics. Demographic and reproductive data are especially important for captive breeding programs (Hutchins, 2003; Hutchins & Wiese, 1991), and authors of captive-management manuals have been advised to incorporate data on lifespan and duration of active reproduction to improve breeding and reintroduction efforts (Jackson, 2003; Seddon et al., 2007). These recommendations have been followed in a few cases, most notably for elephants (Hutchins & Thompson, 2008; Wiese & Willis, 2004; Wiese & Willis, 2006). However, the comprehensive life history data needed for optimal management of captive populations are not readily available for most taxa (Baker, 2007; Hutchins & Thompson, 2008).
Here we provide comprehensive lifespan and reproductive data for the order Psittaciformes (parrots and cockatoos, hereafter ‘parrots’). The parrots are an important group in which to investigate general patterns of captive longevity and breeding. The order contains a high proportion of endangered species, with 36% of the 365 extant species of parrots (Forshaw & Knight, 2006) listed as being at risk (IUCN, 2009) and at least 18 confirmed extinctions by the end of the 20th century, making parrots the most threatened speciose order of birds (Forshaw & Knight, 2006). They are also the longest-lived order of birds for their size (Prinzinger, 1993) with some reported lifespans exceeding 50 years (Brouwer et al., 2000). Furthermore, they are commonly held in captivity, with upwards of 20,000 parrots housed in zoos and other animal holding facilities (ISIS, 2009) and millions more held in private hands (World Parrot Trust, 2009). Successful reintroductions with captive bred parrots are challenging (Snyder et al., 1996), but feasible (Brightsmith et al., 2005; Collazo et al., 2003; Sanz & Grajal, 1998; White Jr, Collazo & Vilella, 2005). The majority of bird supplementation in the wild has come from captive breeding programs (Fischer & Lindenmayer, 2000), but these efforts are stymied by a lack of captive breeding populations for many species of high conservation concern. Instead, the current zoo population of parrots is biased toward large species that are more attractive to humans (Frynta et al., 2010). While the conventional role of zoos in the past has been entertainment (Hatchwell et al., 2007), the World Association of Zoos and Aquariums recently has asserted that the “major goal of zoos and aquariums will be to integrate all aspects of their work with conservation activities” (WAZA, 2005). Overall, the large numbers, long lifespans, and high level of endangerment of parrots results in a high burden on space in zoos and a critical need to set breeding and husbandry goals on the basis of conservation priorities.
Efforts to set conservation priorities for parrots have been hampered by a lack of life history data. While there are a few exemplary studies of life history and reproduction in wild populations (Beissinger, Wunderle Jr & Meyers, 2008; Buckland, Rowley & Williams, 1983; Heinsohn & Legge, 2003; Holdsworth and Dettmann, 2010; Koenig, 2008; Murphy, Legge & Heinsohn, 2003; Powlesland et al., 1992; Renton & Salinas-Melgoza, 2004; Rowley, 1983; Sandercock et al., 2000; Saunders, 1982), it is difficult to age adults and field studies are generally short in duration relative to lifespans. While data from captive individuals may not precisely predict lifespans in wild animals given the different stresses faced by each, a significant positive relationship between captive and wild maximum lifespans has been demonstrated generally in birds (Wasser & Sherman, 2010) as have similar rates of actuarial senescence (Ricklefs, 2000). Previous studies in parrots have provided some data on captive lifespans: Brouwer and colleagues (2000) reported maximum recorded ages for 176 species and subspecies of parrots, while Vanstreels and colleagues (Vanstreels et al., 2010) examined lifespans of confiscated wild-caught parrots in a Brazilian zoo. Neither study reported reproductive parameters. Parrot studbooks are maintained regionally and internationally, but less than 10% of all parrot species and subspecies housed in zoos are currently represented by studbooks worldwide (Bingaman Lackey, personal observation). In sum, these sources provide valuable information for some species, but there remains a pressing need for comprehensive life history data for the order as a whole.
The International Species Information System (ISIS) database contains thousands of records of parrot births, deaths, and reproduction contributed by zoos and other animal holding collections from approximately 845 member institutions in 80 countries (ISIS, 2009). This database represents a wealth of valuable information on parrots, and many other taxa, that has been largely untapped by the zoological and scientific community.
We provide a species-level analysis of lSIS records to present comprehensive life history data for parrots. We collated data from over 87,000 individuals representing over 260 species of parrots from the ISIS database to characterize lifespan and breeding parameters for each species, examine general patterns across major clades of parrot, and test the effects of mass and sex on lifespan.
METHODS
Data coding
We compiled individual lifespan records representing all available parrot species from ISIS. We used Forshaw and Knight (2006) as the taxonomic authority for common and scientific names. Our only departure from the classification of Forshaw and Knight (2006) was to elevate the three subspecies of rosellas under Platycercus elegans to full species based on (Joseph et al., 2008): the crimson rosella (P. elegans), the yellow rosella (P. flaveolus), and the orange-red and yellow rosella (P. adelaidae); otherwise we did not distinguish between subspecies. Individual birds that hatched in an ISIS facility received a HATCH date, while those that were transferred into an ISIS facility from a non-ISIS institution received an IN date. Birds transferred out of an ISIS facility received an OUT data, whereas birds that died in an ISIS facility received a DEATH date. We eliminated individuals with records that had an IN or HATCH date before the 1800s, or which were missing these dates entirely. We excluded individuals recorded as surviving less than one day from further analysis. Sorting and formatting of the data was conducted with Access 2003 (Microsoft Inc., Redmond, WA), and statistical analyses were run using JMP 8.0 (SAS Institute, Cary, NC).
Lifespan across species
To reveal trends in basic lifespan data across species, we first calculated the median lifespan and maximum lifespan for each species. Preliminary analysis indicated that many species followed a Type III survivorship curve (Ricklefs, 2008), with high initial mortality that reached an asymptote at four years of age. Thus we calculated lifespan statistics on two different datasets: a) all individuals who lived past their first day, b) individuals who survived to age four years or older. Four years exceeds the age of first reproduction for many species included in the analysis, but preliminary analyses found this age to be the best single threshold for avoiding juvenile mortality across all 260 species analyzed. We also calculated the median living adult age for individuals that were still alive as of March 24th, 2008 as a measure of lifespan for the currently-living captive population.
We calculated the median instead of the mean as an indicator of central tendencies because the lifespan data was non-normally distributed and exhibited a positively skewed unimodal distribution (Zar, 1999). While we report these summary statistics for all species, for the purpose of statistical tests of life history relationships we excluded species with fewer than 20 individual records to increase reliability of the data and ensure that general trends would not be distorted by a few aberrant individuals. We tested the effect of sex on the maximum and median lifespan by performing the nonparametric Wilcoxon signed-rank test, which treated the two sexes of each species as a paired comparison (Zar, 1999). We then examined the relationship between body size and lifespan with least-squares regressions of log of mass versus log of maximum lifespan, median adult lifespan, and median adult age. Least-squares regressions of maximum lifespan versus median adult lifespan and median adult age were performed on log transformed data. Positive residuals from these regressions indicated species with a single individual, represented by the maximum lifespan, that lived substantially longer than their conspecifics, represented by median adult lifespan or age; negative residuals indicated species with a median adult lifespan or age that was closer to the maximum lifespan within that species.
Lifespan trends for clades
In addition to the summary statistics described across species, we examined data for species within selected clades of particular interest to zoos and captive population managers. These clades were i) Cacatua and allies (Cacatua, Callocephalon, Eolophus), ii) Trichoglossus and allies (Chalcopsitta, Eos, Trichoglossus), iii) Platycercus and allies (Barnardius, Platycercus, Psephotus, Purpureicephalus), iv) Ara and allies (Ara, Orthopsittaca, Propyrrhura), v) Aratinga, and vi) Amazona. We again excluded species that had fewer than 20 individual records from these analyses. A generalized linear model (GLM) was performed to test for the joint effects of mass and clade on the means of maximum lifespan, median adult lifespan, and median adult age and Tukey-Kramer HSD was used for post-hoc comparison between pairs of clades.
Breeding parameters
To describe breeding parameters for each species, we analyzed ISIS breeding information for female parrots. Males were not included in this analysis because paternity could not be unambiguously determined. For these analyses we excluded species with fewer than five individuals to maintain an adequate sample size while minimizing the effect of aberrant individuals. Several other types of exclusions were performed on the breeding data to balance maximizing the number of records available for analysis with maintaining accurate and conservative estimates of reproductive parameters (summarized in Table 1). We calculated medians of the age of first breeding, age of last breeding, duration of active breeding, and duration of post-breeding. Values for age of last breeding are conservative estimates as birds with an IN date were treated as newly hatched birds. Values of the post-breeding period are also conservative because we treated individuals transferred out of the system as deaths. A one-way ANOVA was performed to test for differences in the means of these four breeding parameters among the six major clades.
Table 1.
Criteria for calculation of breeding parameters
| Parameters used | Types of individual records included in analysis | |
|---|---|---|
| Age of first breeding | Known HATCH date, age at first breeding > 0 | Birds that reproduced within an ISIS facility |
| Age at last breeding | Both HATCH and IN date used if age at last breeding > 0 | Birds whose last reproduction was after their transfer into an ISIS facility |
| Duration of active reproduction | Both HATCH and IN date used if age of first reproduction > 0 | Birds that reproduced within an ISIS facility, included durations = 0, where an individual reproduced only once |
| Duration of post-active reproduction | Both HATCH and IN date used if age at last breeding > 0, both DEATH and OUT date used | Birds whose last reproduction was after their transfer into an ISIS facility, included durations = 0, where an individual died on the day of last reproduction |
Conservation status
To determine whether conservation status is associated with particular demographic parameters, one-way ANOVAs were conducted to test for an effect of IUCN status on lifespan (maximum and median adult) and median breeding variables (age of first and last breeding, duration of active breeding and post-breeding).
RESULTS
Lifespan across species
We compiled 87,777 individual parrot records representing 262 species (72% of all parrot species) from ISIS. After excluding those individuals hatched prior to the 1800s or who failed to survive their first day, and species in which no individuals lived past a year, 83,212 individuals representing 260 species remained for analysis. Parrot species in captivity ranged dramatically in their maximum and median lifespans. The highest maximum lifespan recorded was 92 years for the salmon-crested cockatoo (Cacatua moluccensis). Only 12 species (< 5% of the 260 species) had an individual live past 50 years of age. Of all the species held in ISIS institutions, 50% never had an individual live beyond 22 years of age, and only 30% of these species had a median adult lifespan ≥ 10 years even after limiting data to individuals who survived juvenile mortality (≥ 4 years). In contrast, when only living animals were considered, 58% of species had a median age ≥ 10 years (Table 2, see Supplementary Table 1 for medians with quartiles).
Table 2.
Lifespan summary across species (in years)
| Species common name | Scientific name | Lifespan: Max | Lifespan: Alla | Lifespan: Adults ≥ 4 | Age: Adults ≥ 4b | IUCN statusc | |||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| N | Median | N | Median | N | Median | ||||
| Palm cockatoo | Probosciger aterrimus | 39.97 | 522 | 5.29 | 299 | 11.60 | 144 | 13.30 | LC |
| Yellow-tailed black cockatoo | Calyptorhynchus funereus | 47.41 | 324 | 2.84 | 136 | 9.78 | 63 | 10.95 | LC |
| Red-tailed black cockatoo | Calyptorhynchus banksii | 55.26 | 354 | 4.20 | 180 | 11.84 | 82 | 13.02 | LC |
| Glossy black cockatoo | Calyptorhynchus lathami | 37.72 | 41 | 5.32 | 22 | 8.15 | 13 | 9.07 | LC |
| Gang gang cockatoo | Callocephalon fimbriatum | 27.57 | 291 | 2.07 | 96 | 9.73 | 25 | 13.77 | LC |
| Galah | Eolophus roseicapilla | 72.82 | 1889 | 2.00 | 687 | 9.33 | 249 | 10.08 | LC |
| Sulphur-crested cockatoo | Cacatua galerita | 72.95 | 1668 | 3.71 | 804 | 10.92 | 309 | 12.38 | LC |
| Yellow-crested cockatoo | Cacatua sulphurea | 39.97 | 977 | 4.15 | 497 | 8.90 | 221 | 11.23 | CR |
| Blue-eyed cockatoo | Cacatua ophthalmica | 37.95 | 84 | 7.78 | 50 | 11.66 | 21 | 11.60 | VU |
| White-crested cockatoo | Cacatua alba | 32.24 | 967 | 3.49 | 460 | 9.76 | 228 | 11.15 | VU |
| Salmon-crested cockatoo | Cacatua moluccensis | 92.55 | 1675 | 4.56 | 896 | 9.51 | 314 | 11.07 | VU |
| Major Mitchell’s cockatoo | Cacatua leadbeateri | 74.86 | 949 | 2.95 | 419 | 9.64 | 135 | 12.00 | LC |
| Slender-billed corella | Cacatua tenuirostris | 43.45 | 333 | 1.93 | 126 | 11.70 | 52 | 12.09 | LC |
| Little corella | Cacatua sanguinea | 44.01 | 455 | 2.45 | 189 | 11.52 | 56 | 12.49 | LC |
| Ducorps’s corella | Cacatua ducorpsii | 57.81 | 73 | 3.75 | 36 | 9.58 | 23 | 9.58 | LC |
| Goffin’s corella | Cacatua goffini | 30.39 | 581 | 3.90 | 289 | 10.65 | 131 | 13.46 | NT |
| Red-vented corella | Cacatua haematuropygia | 33.59 | 125 | 5.30 | 67 | 11.76 | 28 | 12.02 | CR |
| Cockatiel | Nymphicus hollandicus | 35.92 | 2850 | 2.53 | 949 | 7.06 | 241 | 8.98 | LC |
| Black lory | Chalcopsitta atra | 17.58 | 140 | 2.78 | 56 | 7.66 | 15 | 9.64 | LC |
| Yellow-streaked lory | Chalcopsitta scintillata | 25.01 | 176 | 2.13 | 60 | 9.48 | 20 | 9.03 | LC |
| Brown lory | Chalcopsitta duivenbodei | 26.54 | 248 | 1.66 | 69 | 8.90 | 30 | 10.07 | LC |
| Cardinal lory | Chalcopsitta cardinalis | 18.23 | 64 | 4.72 | 37 | 9.70 | 23 | 10.23 | LC |
| Dusky lory | Pseudeos fuscata | 19.59 | 568 | 2.76 | 239 | 7.34 | 100 | 7.46 | LC |
| Black-winged lory | Eos cyanogenia | 22.53 | 142 | 1.67 | 43 | 7.06 | 5 | 5.98 | VU |
| Violet-necked lory | Eos squamata | 18.23 | 166 | 2.28 | 58 | 6.57 | 20 | 6.82 | LC |
| Blue-streaked lory | Eos reticulata | 27.83 | 353 | 2.16 | 121 | 7.91 | 33 | 11.41 | NT |
| Red and blue lory | Eos histrio | 16.08 | 56 | 6.14 | 35 | 9.58 | 18 | 10.48 | EN |
| Red lory | Eos bornea | 29.48 | 881 | 2.78 | 380 | 6.92 | 145 | 7.86 | LC |
| Blue-eared lory | Eos semilarvata | 12.23 | 14 | 4.06 | 7 | 10.02 | 6 | 10.82 | LC |
| Ornate lorikeet | Trichoglossus ornatus | 18.49 | 143 | 3.27 | 62 | 6.45 | 30 | 7.91 | LC |
| Rainbow lorikeet | Trichoglossus haematodus | 37.94 | 6805 | 2.39 | 2424 | 6.47 | 1325 | 6.70 | LC |
| Scaly-breasted lorikeet | Trichoglossus chlorolepidotus | 24.17 | 187 | 2.60 | 72 | 6.62 | 25 | 6.62 | LC |
| Olive-headed lorikeet | Trichoglossus euteles | 18.74 | 167 | 2.59 | 63 | 7.40 | 23 | 8.15 | LC |
| Yellow and green lorikeet | Trichoglossus flavoviridis | 12.28 | 33 | 2.27 | 11 | 5.36 | LC | ||
| Mindanao lorikeet | Trichoglossus johnstoniae | 17.77 | 81 | 1.85 | 28 | 7.27 | 12 | 8.79 | NT |
| Varied lorikeet | Psitteuteles versicolor | 12.47 | 173 | 1.42 | 48 | 6.64 | 2 | 8.47 | LC |
| Iris lorikeet | Psitteuteles iris | 27.04 | 147 | 1.58 | 39 | 7.71 | 22 | 10.01 | NT |
| Goldie’s lorikeet | Psitteuteles goldiei | 24.13 | 605 | 1.61 | 161 | 7.01 | 31 | 8.90 | LC |
| Musk lorikeet | Glossopsitta concinna | 19.3 | 467 | 2.22 | 141 | 5.88 | 22 | 5.69 | LC |
| Little lorikeet | Glossopsitta pusilla | 20.11 | 153 | 1.41 | 34 | 5.27 | 6 | 9.75 | LC |
| Purple-crowned lorikeet | Glossopsitta porphyrocephala | 12.67 | 476 | 1.28 | 81 | 5.47 | 6 | 5.35 | LC |
| Blue-crowned lorikeet | Vini australis | 37.36 | 241 | 2.05 | 74 | 6.62 | 31 | 9.51 | LC |
| Kuhl’s lorikeet | Vini kuhlii | 2.21 | 1 | 2.21 | EN | ||||
| Blue lorikeet | Vini peruviana | 21.12 | 91 | 1.21 | 30 | 11.66 | 1 | 17.95 | VU |
| Collared lorikeet | Phigys solitarius | 18.23 | 106 | 3.66 | 50 | 9.22 | 36 | 9.99 | LC |
| Purple-bellied lory | Lorius hypoinochrous | 18.13 | 12 | 10.66 | 11 | 11.09 | 6 | 11.19 | LC |
| Black-capped lory | Lorius lory | 30.83 | 482 | 3.13 | 219 | 7.56 | 107 | 7.50 | LC |
| Purple-naped lory | Lorius domicella | 19.23 | 181 | 2.79 | 77 | 7.16 | 29 | 9.00 | VU |
| Yellow-bibbed lory | Lorius chlorocercus | 13.81 | 134 | 5.27 | 87 | 8.88 | 47 | 13.81 | LC |
| Chattering lory | Lorius garrulus | 27.24 | 586 | 2.43 | 220 | 7.59 | 65 | 7.89 | EN |
| Red-fronted lorikeet | Charmosyna rubronotata | 2.34 | 3 | 2.30 | LC | ||||
| Red-flanked lorikeeet | Charmosyna placentis | 19.59 | 198 | 1.47 | 56 | 5.91 | 28 | 6.91 | LC |
| Fairy lorikeet | Charmosyna pulchella | 20.78 | 71 | 1.43 | 21 | 6.65 | 3 | 15.58 | LC |
| Duchess lorikeet | Charmosyna margarethae | 4 | 4 | 4.00 | 4 | 4.00 | NT | ||
| Josephine’s lorikeet | Charmosyna josefinae | 10.2 | 22 | 3.57 | 10 | 5.26 | LC | ||
| Papuan lorikeet | Charmosyna papou | 22.43 | 552 | 1.38 | 140 | 6.80 | 33 | 8.23 | LC |
| Whiskered lorikeet | Oreopsittacus arfaki | 1.56 | 2 | 1.56 | LC | ||||
| Musschenbroek’s lorikeet | Neopsittacus musschenbroekii | 26.54 | 73 | 2.34 | 26 | 7.23 | 4 | 16.89 | LC |
| Kea | Nestor notabilis | 50.53 | 773 | 2.88 | 339 | 10.49 | 114 | 10.80 | VU |
| Kaka | Nestor meridionalis | 35.46 | 191 | 2.13 | 64 | 12.55 | 17 | 12.25 | EN |
| Pesquet’s parrot | Psittrichas fulgidus | 27.63 | 159 | 4.84 | 87 | 8.29 | 39 | 9.73 | VU |
| Orange-breasted fig parrot | Cyclopsitta gulielmitertii | 12.23 | 1 | 12.23 | 1 | 12.23 | 1 | 12.23 | LC |
| Double-eyed fig parrot | Cyclopsitta diophthalma | 14.35 | 287 | 1.81 | 83 | 6.54 | 16 | 6.47 | LC |
| Desmarest’s fig parrot | Psittaculirostris desmarestii | 17.46 | 136 | 1.06 | 25 | 5.29 | 2 | 13.24 | LC |
| Edwards’s fig parrot | Psittaculirostris edwardsii | 20.4 | 122 | 1.66 | 36 | 7.34 | 8 | 9.81 | LC |
| Salvadori’s fig parrot | Psittaculirostris salvadorii | 14.89 | 14 | 3.69 | 7 | 9.73 | 3 | 9.73 | VU |
| Guaiabero | Bolbopsittacus lunulatus | 1.99 | 3 | 1.86 | LC | ||||
| Blue-rumped parrot | Psittinus cyanurus | 15.23 | 28 | 2.60 | 9 | 6.85 | 2 | 11.04 | NT |
| Red-cheeked parrot | Geoffroyus geoffroyi | 5.54 | 2 | 3.07 | 1 | 5.54 | LC | ||
| Blue-crowned racquet-tailed parrot | Prioniturus discurus | 1.64 | 4 | 0.06 | LC | ||||
| Golden-mantled racquet-tailed parrot | Prioniturus platurus | 24.65 | 17 | 4.79 | 9 | 15.78 | 4 | 24.65 | LC |
| Eclectus parrot | Eclectus roratus | 40.76 | 2521 | 1.92 | 949 | 9.00 | 416 | 10.08 | LC |
| Great-billed parrot | Tanygnathus megalorhynchos | 14.56 | 24 | 1.97 | 8 | 6.86 | 1 | 14.56 | LC |
| Blue-naped parrot | Tanygnathus lucionensis | 13.19 | 36 | 2.52 | 10 | 5.90 | 1 | 13.19 | NT |
| Blue-backed parrot | Tanygnathus sumatranus | 26.3 | 10 | 6.81 | 6 | 7.18 | 3 | 7.18 | LC |
| Alexandrine parakeet | Psittacula eupatria | 29.06 | 562 | 5.06 | 308 | 6.23 | 181 | 5.23 | LC |
| Rose-ringed parakeet | Psittacula krameri | 33.63 | 1719 | 3.58 | 797 | 7.69 | 274 | 9.58 | LC |
| Mauritius parakeet | Psittacula echo | 12.35 | 6 | 6.72 | 4 | 7.63 | 2 | 10.43 | EN |
| Malabar parakeet | Psittacula columboides | 14.08 | 15 | 5.18 | 8 | 7.24 | LC | ||
| Emerald-collared parakeet | Psittacula calthorpae | 10.59 | 2 | 10.41 | 2 | 10.41 | LC | ||
| Plum-headed parakeet | Psittacula cyanocephala | 19.86 | 410 | 4.22 | 209 | 5.62 | 117 | 5.23 | LC |
| Blossom-headed parakeet | Psittacula roseata | 17.15 | 21 | 6.07 | 13 | 9.07 | 6 | 10.12 | LC |
| Slaty-headed parakeet | Psittacula himalayana | 18.46 | 19 | 6.82 | 13 | 7.77 | 11 | 7.77 | LC |
| Derbyan parakeet | Psittacula derbiana | 28.22 | 408 | 2.24 | 167 | 9.07 | 62 | 9.81 | LC |
| Nicobar parakeet | Psittacula caniceps | 6.54 | 14 | 0.46 | 4 | 5.79 | NT | ||
| Red-breasted parakeet | Psittacula alexandri | 20.24 | 282 | 3.03 | 119 | 8.13 | 47 | 10.23 | LC |
| Long-tailed parakeet | Psittacula longicauda | 13.05 | 55 | 1.68 | 12 | 8.83 | 7 | 10.23 | NT |
| Gray-headed lovebird | Agapornis canus | 16.01 | 120 | 2.79 | 45 | 7.12 | 20 | 7.12 | LC |
| Red-faced lovebird | Agapornis pullarius | 19.23 | 123 | 4.22 | 62 | 7.55 | 13 | 7.73 | LC |
| Black-winged lovebird | Agapornis taranta | 15.82 | 164 | 2.38 | 59 | 7.01 | 2 | 6.16 | LC |
| Peach-faced lovebird | Agapornis roseicollis | 34.1 | 1943 | 2.33 | 663 | 6.74 | 120 | 8.23 | LC |
| Masked lovebird | Agapornis personatus | 24.24 | 997 | 1.46 | 282 | 5.73 | 106 | 5.56 | LC |
| Fischer’s lovebird | Agapornis fischeri | 32.24 | 1402 | 1.75 | 397 | 5.94 | 203 | 7.80 | NT |
| Nyasa lovebird | Agapornis lilianae | 19.2 | 227 | 3.63 | 108 | 7.44 | 4 | 6.38 | NT |
| Black-cheeked lovebird | Agapornis nigrigenis | 13.75 | 675 | 1.71 | 224 | 6.83 | 152 | 6.88 | VU |
| Vernal hanging parrot | Loriculus vernalis | 13 | 50 | 6.93 | 39 | 8.10 | 11 | 9.62 | LC |
| Ceylon hanging parrot | Loriculus beryllinus | 3.56 | 2 | 2.71 | LC | ||||
| Philippine hanging parrot | Loriculus philippensis | 13.33 | 21 | 4.14 | 12 | 5.05 | 1 | 10.23 | LC |
| Blue-crowned hanging parrot | Loriculus galgulus | 21.25 | 837 | 2.18 | 253 | 6.33 | 65 | 9.00 | LC |
| Maroon-rumped hanging parrot | Loriculus stigmatus | 13.03 | 24 | 4.37 | 12 | 7.93 | LC | ||
| Green hanging parrot | Loriculus exilis | 3.45 | 3 | 3.10 | NT | ||||
| Yellow-throated hanging parrot | Loriculus pusillus | 2.23 | 1 | 2.23 | NT | ||||
| Moluccan king parrot | Alisterus amboinensis | 29.2 | 112 | 1.94 | 41 | 7.56 | 17 | 8.16 | LC |
| Papuan king parrot | Alisterus chloropterus | 22.35 | 77 | 6.06 | 45 | 10.26 | 10 | 11.66 | LC |
| Australian king parrot | Alisterus scapularis | 31.32 | 699 | 1.53 | 171 | 7.63 | 59 | 7.75 | LC |
| Olive-shouldered parrot | Aprosmictus jonquillaceus | 14.84 | 7 | 11.12 | 6 | 11.18 | NT | ||
| Red-winged parrot | Aprosmictus erythropterus | 27.3 | 390 | 1.83 | 137 | 9.03 | 41 | 10.69 | LC |
| Superb parrot | Polytelis swainsonii | 24.21 | 421 | 2.86 | 166 | 7.18 | 47 | 6.81 | VU |
| Regent parrot | Polytelis anthopeplus | 27.49 | 365 | 2.51 | 125 | 7.23 | 36 | 7.21 | LC |
| Princess parrot | Polytelis alexandrae | 23.98 | 614 | 2.55 | 210 | 7.36 | 76 | 7.45 | NT |
| Red-capped parrot | Purpureicephalus spurius | 17.9 | 138 | 1.06 | 29 | 8.00 | 4 | 12.91 | LC |
| Mallee ringneck parrot | Barnardius barnardi | 31.62 | 181 | 1.05 | 52 | 6.75 | 8 | 4.84 | not available |
| Port Lincoln parrot | Barnardius zonarius | 17.96 | 140 | 3.66 | 68 | 7.81 | 13 | 8.06 | LC |
| Green rosella | Platycercus caledonicus | 7.98 | 37 | 3.37 | 15 | 6.35 | 4 | 6.61 | LC |
| Crimson rosella | Platycercus elegans | 20.14 | 1010 | 1.03 | 139 | 6.50 | 36 | 7.53 | LC |
| Yellow rosella | Platycercus flaveolus | 13.31 | 59 | 2.50 | 21 | 6.18 | 1 | 4.23 | LC |
| Adelaide rosella | Platycercus adelaidae | 17.35 | 58 | 0.26 | 11 | 8.44 | 5 | 7.23 | LC |
| Eastern rosella | Platycercus eximius | 37.44 | 1302 | 1.61 | 334 | 6.58 | 93 | 6.81 | LC |
| Pale-headed rosella | Platycercus adscitus | 25.03 | 267 | 1.76 | 75 | 6.35 | 23 | 7.18 | LC |
| Northern rosella | Platycercus venustus | 21.57 | 191 | 2.57 | 69 | 6.81 | 4 | 7.81 | LC |
| Western rosella | Platycercus icterotis | 31.64 | 274 | 1.03 | 64 | 6.58 | 7 | 5.87 | LC |
| Bluebonnet | Northiella haematogaster | 15.4 | 173 | 1.44 | 40 | 6.06 | 2 | 10.42 | LC |
| Red-rumped parrot | Psephotus haematonotus | 17.74 | 792 | 1.93 | 238 | 5.27 | 107 | 5.23 | LC |
| Mulga parrot | Psephotus varius | 11.79 | 116 | 1.93 | 25 | 5.61 | 3 | 4.81 | LC |
| Golden-shouldered parrot | Psephotus chrysopterygius | 20.96 | 812 | 1.38 | 221 | 7.91 | 32 | 9.49 | EN |
| Swift parrot | Lathamus discolor | 14.18 | 184 | 1.26 | 28 | 6.28 | 11 | 6.24 | EN |
| Antipodes green parakeet | Cyanoramphus unicolor | 12.66 | 147 | 2.77 | 60 | 6.61 | 7 | 5.37 | VU |
| Red-fronted parakeet | Cyanoramphus novaezelandiae | 36.45 | 510 | 1.73 | 129 | 7.23 | 51 | 10.05 | VU |
| Yellow-fronted parakeet | Cyanoramphus auriceps | 16.48 | 171 | 1.30 | 51 | 6.54 | 20 | 6.96 | NT |
| Orange-fronted parakeet | Cyanoramphus malherbi | 12.28 | 17 | 1.87 | 4 | 7.56 | 1 | 12.28 | CR |
| Horned parakeet | Eunymphicus cornutus | 12.9 | 30 | 2.75 | 11 | 6.93 | 6 | 11.98 | EN |
| Masked shining parrot | Prosopeia personata | 17.73 | 4 | 11.46 | 4 | 11.46 | NT | ||
| Red shining parrot | Prosopeia tabuensis | 23.67 | 35 | 5.42 | 21 | 9.89 | LC | ||
| Blue-winged parrot | Neophema chrysostoma | 15.85 | 165 | 0.60 | 30 | 5.47 | LC | ||
| Elegant parrot | Neophema elegans | 14.91 | 207 | 1.72 | 54 | 6.22 | LC | ||
| Rock parrot | Neophema petrophila | 16.92 | 68 | 2.17 | 25 | 6.36 | LC | ||
| Orange-bellied parrot | Neophema chrysogaster | 13.27 | 426 | 0.81 | 81 | 6.29 | 47 | 7.23 | CR |
| Turquoise parrot | Neophema pulchella | 26.54 | 461 | 2.15 | 142 | 6.65 | 15 | 7.52 | LC |
| Scarlet-chested parrot | Neophema splendida | 25.41 | 1284 | 1.05 | 202 | 5.91 | 32 | 13.05 | LC |
| Bourke’s parrot | Neopsephotus bourkii | 19.4 | 511 | 2.11 | 155 | 6.06 | 43 | 6.56 | LC |
| Budgerigar | Melopsittacus undulatus | 18.01 | 4840 | 0.97 | 819 | 5.64 | 324 | 5.20 | LC |
| Ground parrot | Pezoporus wallicus | 1.17 | 1 | 1.17 | LC | ||||
| Brehm’s tiger parrot | Psittacella brehmii | 1.92 | 1 | 1.92 | LC | ||||
| Vasa parrot | Coracopsis vasa | 29.06 | 190 | 5.20 | 109 | 9.76 | 49 | 11.57 | LC |
| Black parrot | Coracopsis nigra | 37.69 | 132 | 4.89 | 73 | 8.95 | 22 | 9.90 | LC |
| Gray parrot | Psittacus erithacus | 48.26 | 4742 | 2.66 | 1979 | 8.23 | 882 | 8.75 | NT |
| Brown-necked parrot | Poicephalus robustus | 36 | 377 | 3.08 | 172 | 8.15 | 89 | 8.36 | LC |
| Jardine’s parrot | Poicephalus gulielmi | 20.2 | 227 | 4.30 | 118 | 6.81 | 44 | 9.20 | LC |
| Brown-headed parrot | Poicephalus cryptoxanthus | 16.2 | 169 | 2.27 | 63 | 6.61 | 30 | 8.58 | LC |
| Niam-niam parrot | Poicephalus crassus | 10.06 | 2 | 10.06 | 2 | 10.06 | 2 | 10.06 | LC |
| Meyer’s parrot | Poicephalus meyeri | 31.02 | 275 | 4.53 | 150 | 8.05 | 60 | 9.63 | LC |
| Rüppell’s parrot | Poicephalus rueppellii | 20.54 | 49 | 5.23 | 35 | 5.23 | 21 | 5.23 | LC |
| Red-bellied parrot | Poicephalus rufiventris | 22.23 | 192 | 1.92 | 65 | 7.49 | 34 | 7.72 | LC |
| Senegal parrot | Poicephalus senegalus | 27.16 | 736 | 3.88 | 361 | 6.60 | 186 | 5.47 | LC |
| Hyacinth macaw | Anodorhynchus hyacinthinus | 54.26 | 568 | 12.52 | 422 | 18.23 | 141 | 21.77 | EN |
| Lear’s macaw | Anodorhynchus leari | 43.57 | 13 | 14.56 | 11 | 17.86 | CR | ||
| Spix’s macaw | Cyanopsitta spixii | 32.22 | 9 | 6.18 | 7 | 15.18 | 3 | 20.21 | CR |
| Blue and yellow macaw | Ara ararauna | 48.52 | 2124 | 6.60 | 1297 | 12.55 | 273 | 20.78 | LC |
| Blue-throated macaw | Ara glaucogularis | 32.79 | 23 | 12.55 | 17 | 18.20 | 12 | 18.66 | CR |
| Scarlet macaw | Ara macao | 48.26 | 1360 | 8.36 | 896 | 14.59 | 188 | 21.24 | LC |
| Green-winged macaw | Ara chloroptera | 63.04 | 981 | 9.51 | 670 | 14.44 | 180 | 19.19 | LC |
| Military macaw | Ara militaris | 54.43 | 581 | 8.57 | 385 | 14.16 | 110 | 20.12 | VU |
| Great green macaw | Ara ambigua | 34.75 | 78 | 10.73 | 56 | 19.88 | 16 | 24.83 | EN |
| Red-fronted macaw | Ara rubrogenys | 36.21 | 241 | 6.48 | 143 | 13.84 | 42 | 19.77 | EN |
| Chestnut-fronted macaw | Ara severus | 39.67 | 135 | 5.73 | 80 | 10.57 | 13 | 19.80 | LC |
| Yellow-collared macaw | Primolius auricollis | 25.21 | 153 | 3.03 | 68 | 9.58 | 9 | 19.60 | LC |
| Blue-headed macaw | Primolius couloni | 13.3 | 2 | 13.30 | 2 | 13.30 | 2 | 13.30 | EN |
| Blue-winged macaw | Primolius maracana | 24.95 | 93 | 6.87 | 61 | 14.48 | 23 | 18.23 | NT |
| Red-bellied macaw | Orthopsittaca manilata | 9.72 | 43 | 2.01 | 16 | 6.89 | LC | ||
| Red-shouldered macaw | Diopsittaca nobilis | 22.91 | 157 | 2.51 | 59 | 11.64 | 15 | 18.98 | LC |
| Thick-billed parrot | Rhynchopsitta pachyrhyncha | 35.24 | 359 | 6.21 | 202 | 15.60 | 40 | 20.74 | EN |
| Golden conure | Guaruba guarouba | 60.9 | 373 | 4.59 | 190 | 14.10 | 34 | 21.68 | EN |
| Blue-crowned conure | Aratinga acuticaudata | 22.49 | 99 | 2.89 | 42 | 8.81 | 4 | 19.23 | LC |
| White-eyed conure | Aratinga leucophthalmus | 28.45 | 93 | 2.28 | 28 | 10.15 | 6 | 23.35 | LC |
| Green conure | Aratinga holochlora | 21.06 | 44 | 0.89 | 8 | 7.96 | LC | ||
| Hispaniolan conure | Aratinga chloroptera | 30.84 | 14 | 18.23 | 14 | 18.23 | 13 | 18.23 | VU |
| Cuban conure | Aratinga euops | 14.71 | 15 | 1.79 | 6 | 7.63 | VU | ||
| Finsch’s conure | Aratinga finschi | 14.16 | 1 | 14.16 | 1 | 14.16 | LC | ||
| Red-fronted conure | Aratinga wagleri | 24.86 | 35 | 8.32 | 30 | 8.32 | 2 | 15.58 | LC |
| Mitred conure | Aratinga mitrata | 27.83 | 93 | 3.45 | 41 | 8.44 | 11 | 27.83 | LC |
| Red-masked conure | Aratinga erythrogenys | 26.64 | 108 | 4.40 | 56 | 8.93 | 2 | 21.02 | NT |
| Golden-capped conure | Aratinga auricapillus | 18.85 | 25 | 6.73 | 19 | 8.43 | 1 | 18.85 | NT |
| Jandaya conure | Aratinga jandaya | 22.24 | 165 | 1.92 | 69 | 9.53 | 7 | 19.98 | LC |
| Sun conure | Aratinga solstitialis | 29.7 | 529 | 2.07 | 200 | 10.98 | 23 | 18.98 | EN |
| Dusky-headed conure | Aratinga weddellii | 24.57 | 12 | 5.39 | 8 | 9.27 | 1 | 24.57 | LC |
| Brown-throated conure | Aratinga pertinax | 19.73 | 31 | 1.42 | 10 | 8.58 | 1 | 13.73 | LC |
| Olive-throated conure | Aratinga nana | 7.12 | 5 | 2.09 | 1 | 7.12 | LC | ||
| Orange-fronted conure | Aratinga canicularis | 28.62 | 131 | 0.87 | 23 | 8.80 | 2 | 23.42 | LC |
| Peach-fronted conure | Aratinga aurea | 15.34 | 124 | 2.19 | 25 | 6.58 | LC | ||
| Cactus conure | Aratinga cactorum | 9.08 | 15 | 6.26 | 10 | 6.47 | LC | ||
| Nanday conure | Nandayus nenday | 30.24 | 411 | 3.39 | 185 | 9.00 | 19 | 20.79 | LC |
| Patagonian conure | Cyanoliseus patagonus | 34.12 | 439 | 7.11 | 294 | 11.19 | 62 | 18.56 | LC |
| Monk parakeet | Myiopsitta monachus | 24.78 | 455 | 3.76 | 217 | 6.65 | 5 | 22.24 | LC |
| Slender-billed conure | Enicognathus leptorhynchus | 20.17 | 23 | 3.87 | 11 | 9.28 | LC | ||
| Maroon-bellied conure | Pyrrhura frontalis | 17.42 | 40 | 1.52 | 13 | 11.05 | LC | ||
| Green-cheeked conure | Pyrrhura molinae | 8.34 | 3 | 8.22 | 3 | 8.22 | LC | ||
| Maroon-tailed conure | Pyrrhura melanura | 12.05 | 3 | 1.24 | 1 | 12.05 | LC | ||
| Crimson-bellied conure | Pyrrhura perlata | 22.34 | 21 | 7.41 | 15 | 8.44 | LC | ||
| Fiery-shouldered conure | Pyrrhura egregia | 12.16 | 2 | 12.13 | 2 | 12.13 | LC | ||
| White-eared conure | Pyrrhura leucotis | 10.61 | 2 | 6.57 | 1 | 10.61 | NT | ||
| Painted conure | Pyrrhura picta | 17.75 | 77 | 0.57 | 12 | 11.26 | LC | ||
| Black-capped conure | Pyrrhura rupicola | 6.09 | 6 | 0.01 | 2 | 6.09 | LC | ||
| Blue-throated conure | Pyrrhura cruentata | 20.38 | 29 | 10.57 | 17 | 16.85 | 4 | 18.53 | VU |
| Barred parakeet | Bolborhynchus lineola | 6.03 | 3 | 3.73 | 1 | 6.03 | LC | ||
| Andean parakeet | Bolborhynchus orbygnesius | 9.65 | 28 | 2.24 | 9 | 7.53 | LC | ||
| Mexican parrotlet | Forpus cyanopygius | 12.02 | 35 | 6.37 | 22 | 8.10 | LC | ||
| Green-rumped parrotlet | Forpus passerinus | 11.58 | 22 | 2.42 | 7 | 4.72 | LC | ||
| Blue-winged parrotlet | Forpus xanthopterygius | 27.9 | 15 | 3.03 | 7 | 9.16 | 2 | 27.90 | LC |
| Spectacled parrotlet | Forpus cospicillatus | 7.12 | 2 | 4.18 | 1 | 7.12 | 1 | 7.12 | LC |
| Pacific parrotlet | Forpus coelestis | 30.38 | 145 | 1.27 | 28 | 6.07 | 2 | 24.26 | LC |
| Yellow-faced parrotlet | Forpus xanthops | 10.5 | 22 | 4.47 | 12 | 6.18 | VU | ||
| Plain parakeet | Brotogeris tirica | 4.21 | 24 | 1.74 | 3 | 4.21 | LC | ||
| White-winged parakeet | Brotogeris versicolurus | 17.84 | 89 | 1.88 | 29 | 6.82 | LC | ||
| Gray-cheeked parakeet | Brotogeris pyrrhopterus | 13.78 | 20 | 3.48 | 9 | 8.03 | EN | ||
| Orange-chinned parakeet | Brotogeris jugularis | 22.79 | 43 | 0.04 | 5 | 7.05 | LC | ||
| Tui parakeet | Brotogeris sanctithomae | 15.15 | 6 | 1.86 | 2 | 10.01 | LC | ||
| Black-capped parrot | Pionites melanocephala | 27.07 | 96 | 3.40 | 45 | 13.21 | 8 | 20.71 | LC |
| White-bellied parrot | Pionites leucogaster | 23.14 | 34 | 4.97 | 26 | 5.79 | 3 | 20.28 | LC |
| Pileated parrot | Pionopsitta pileata | 19.51 | 29 | 8.27 | 22 | 9.20 | 1 | 19.51 | LC |
| Caica parrot | Pionopsitta caica | 28.24 | 1 | 28.24 | 1 | 28.24 | 1 | 28.24 | LC |
| Blue-headed parrot | Pionus menstruus | 23.03 | 79 | 3.07 | 37 | 7.36 | 1 | 18.90 | LC |
| Red-billed parrot | Pionus sordidus | 5.7 | 3 | 2.83 | 1 | 5.70 | LC | ||
| Scaly-headed parrot | Pionus maximiliani | 23.86 | 31 | 8.47 | 21 | 10.25 | 2 | 16.63 | LC |
| Plum-crowned parrot | Pionus tumultuosus | 13.76 | 8 | 4.37 | 4 | 11.29 | LC | ||
| White-capped parrot | Pionus seniloides | 7.6 | 1 | 7.60 | 1 | 7.60 | LC | ||
| White-crowned parrot | Pionus senilis | 13.45 | 7 | 12.63 | 4 | 13.02 | LC | ||
| Bronze-winged parrot | Pionus chalcopterus | 18.98 | 36 | 2.15 | 13 | 15.23 | 5 | 18.23 | LC |
| Dusky parrot | Pionus fuscus | 9.1 | 16 | 1.50 | 7 | 7.26 | LC | ||
| Yellow-billed amazon | Amazona collaria | 11.61 | 7 | 7.91 | 6 | 8.10 | VU | ||
| Cuban amazon | Amazona leucocephala | 30.83 | 292 | 4.09 | 165 | 7.77 | 24 | 21.24 | NT |
| Hispaniolan amazon | Amazona ventralis | 29.06 | 117 | 3.61 | 53 | 6.30 | 1 | 14.15 | VU |
| Black-billed amazon | Amazona agilis | 8 | 2 | 8.00 | 2 | 8.00 | VU | ||
| Puerto Rican amazon | Amazona vittata | 27.23 | 63 | 1.50 | 28 | 9.03 | CR | ||
| Tucuman amazon | Amazona tucumana | 26.19 | 109 | 5.08 | 66 | 5.41 | 10 | 18.96 | NT |
| Red-specacled amazon | Amazona pretrei | 24.23 | 18 | 1.26 | 5 | 23.54 | 4 | 23.69 | VU |
| White-fronted amazon | Amazona albifrons | 35.24 | 133 | 3.76 | 64 | 8.76 | 9 | 20.05 | LC |
| Yellow-lored amazon | Amazona xantholora | 22.05 | 11 | 12.33 | 10 | 12.33 | 1 | 22.05 | LC |
| Green-cheeked amazon | Amazona viridigenalis | 39.45 | 311 | 5.24 | 180 | 11.59 | 33 | 20.68 | EN |
| Lilac-crowned amazon | Amazona finschi | 30.73 | 133 | 4.47 | 74 | 11.13 | 15 | 25.29 | VU |
| Red-lored amazon | Amazona autumnalis | 37.84 | 192 | 7.28 | 127 | 11.75 | 26 | 18.44 | LC |
| Festive amazon | Amazona festiva | 30.31 | 35 | 9.66 | 28 | 18.11 | 11 | 21.10 | LC |
| Red-tailed amazon | Amazona brasiliensis | 23.2 | 16 | 18.89 | 15 | 21.93 | 8 | 22.34 | VU |
| Red-crowned amazon | Amazona rhodocorytha | 26.94 | 39 | 14.75 | 32 | 15.37 | 11 | 24.25 | EN |
| Blue-cheeked-amazon | Amazona dufresniana | 22.42 | 15 | 8.43 | 15 | 8.43 | 6 | 19.48 | NT |
| Yellow-shouldered amazon | Amazona barbadensis | 34.62 | 46 | 8.77 | 38 | 10.45 | 8 | 9.46 | VU |
| Yellow-faced amazon | Amazona xanthops | 24.73 | 28 | 6.92 | 23 | 11.90 | 4 | 24.73 | NT |
| Blue-fronted amazon | Amazona aestiva | 37.44 | 646 | 5.37 | 395 | 10.22 | 73 | 19.32 | LC |
| Yellow-crowned amazon | Amazona ochrocephala | 34.45 | 401 | 5.18 | 240 | 10.46 | 50 | 17.75 | LC |
| Yellow-naped amazon | Amazona auropalliata | 66.81 | 185 | 7.91 | 124 | 11.45 | 38 | 19.48 | LC |
| Yellow-headed amazon | Amazona oratrix | 41.5 | 620 | 3.37 | 285 | 10.48 | 54 | 20.78 | EN |
| Orange-winged amazon | Amazona amazonica | 39.02 | 289 | 5.43 | 174 | 8.44 | 30 | 18.15 | LC |
| Scaly-naped amazon | Amazona mercenaria | 6.57 | 2 | 4.52 | 1 | 6.57 | LC | ||
| Mealy amazon | Amazona farinosa | 42.9 | 100 | 6.11 | 62 | 10.40 | 11 | 16.93 | LC |
| Vinaceous amazon | Amazona vinacea | 26.94 | 43 | 4.28 | 22 | 12.15 | 6 | 21.65 | VU |
| St. Vincent amazon | Amazona guildingii | 36.58 | 32 | 17.30 | 29 | 19.87 | 3 | 34.70 | VU |
| St. Lucia amazon | Amazona versicolor | 38.75 | 27 | 13.57 | 17 | 19.92 | 7 | 32.04 | VU |
| Red-necked amazon | Amazona arausiaca | 29.65 | 3 | 29.61 | 2 | 29.63 | VU | ||
| Hawk-headed parrot | Deroptyus accipitrinus | 38.25 | 163 | 6.50 | 101 | 13.57 | 15 | 21.30 | LC |
| Purple-bellied parrot | Triclaria malachitacea | 15.14 | 12 | 5.80 | 9 | 7.99 | NT | ||
| Carolina parakeet | Conuropsis carolinensis | 15.55 | 4 | 10.02 | 3 | 15.03 | EX | ||
Does not include chicks that died day of hatch.
Individuals that were still alive as of 3/24/2008.
2009 Red List status: LC = Least Concern, NT = Near Threatened, VU = Vulnerable, EN = Endangered, CE = Critically Endangered, EX = Extinct
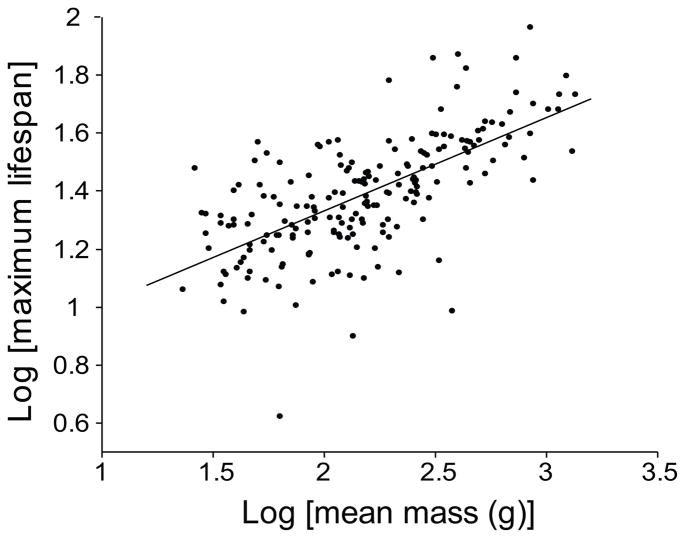
When further excluding species that do not have at least 20 individual records, 82,777 individuals from 199 species remained in the dataset used for the following lifespan analyses. Matched pairs analysis indicated that living adult median age is significantly different than median adult lifespan (age = 11.12 ± 4.79, lifespan = 8.81 ± 2.62; z97 = 2036.00, P < 0.0001) with living adults today surviving longer on average than adults collectively over the last 200 years. Least-squares regressions of log maximum lifespan and log median adult lifespan on log body mass revealed that mass was a significant predictor of maximum lifespan (F1, 195 = 148.79, P < 0.0001, R2 = 0.43; Fig. 1) median adult lifespan (F1, 167 = 182.65, P < 0.0001, R2 = 0.52 and median adult age (F1, 96 = 71.11, P < 0.0001, R2 = 0.43; Supplementary Fig. 1) such that larger species were generally longer lived than smaller species.
Figure 1.
The regression of log of maximum lifespan on log of adult mass by parrot species with n ≥ 20 individuals. The slope of the fit line is 0.3215 ± 0.0213.
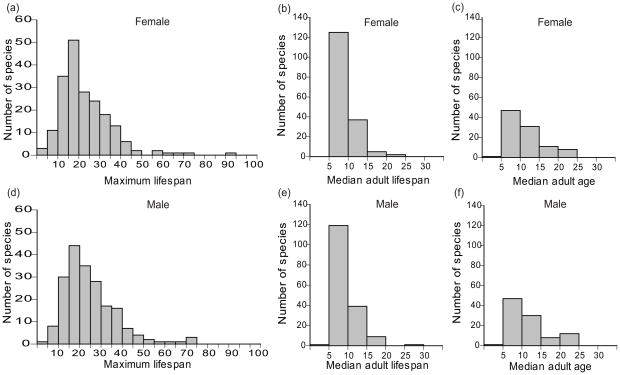
Matched pairs analysis revealed that sex affected maximum lifespan (males = 24.79 ± 12.58, females = 23.05 ± 12.52; z196 = 3027.00, P < 0.0001), median adult lifespan (males = 9.12 ± 3.19, females = 8.93 ± 3.09; z168 = 1671.50, P = 0.0049), and median living adult age (males = 11.37 ± 5.00, females = 11.15 ± 4.91.; z97 = 488.00, P = 0.0490) with males living longer on average (Fig. 2).
Figure 2.
Histograms of lifespan by parrot species with n ≥ 20 individuals of female (a) maximum and (b) median adult lifespan and (c) median adult age, and of male (d) maximum and (e) median adult lifespan and (f) median adult age.
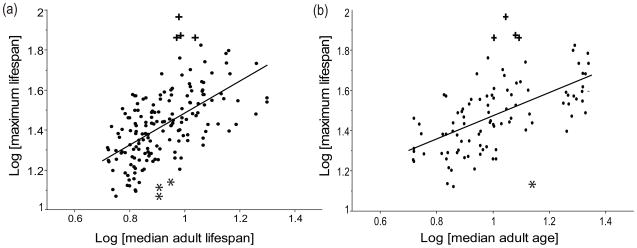
Least-squares regressions of log maximum lifespan on log median adult lifespan and log median adult age revealed that both were significant predictors of maximum lifespan (lifespan: F1, 167 = 78.56, P < 0.0001, R2 = 0.32; age: F1, 96 = 42.75, P < 0.0001, R2 = 0.31; Fig. 3). For both regressions the cockatoos were the most notable positive outliers.
Figure 3.
Regression of log of maximum lifespan on (a) log of median adult lifespan (slope = 0.7975 ± 0.0223) and (b) log of median adult age (slope = 0.5759 ± 0.0303). Points represent individual parrot species, plus signs indicate the species with a residual value greater than 0.3, and asterisks indicate the species with a residual value less than −0.3.
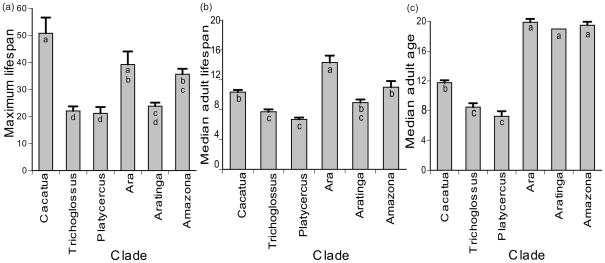
Lifespan trends for clades
Our selected clades of parrots differed in most lifespan parameters. A one-way ANOVA testing for differences among clades in the means of maximum lifespan (F5, 77 = 13.27, P < 0.0001), median adult lifespan (F5, 69 = 15.65, P < 0.0001), and median adult age (F5, 37 = 112.14, P < 0.0001) indicated that clades differed significantly in these parameters (Fig. 4). To assess whether these differences could be attributed to the size differences among clades, both clade and weight were run as factors in a GLM. There was no significant interaction between clade and weight for maximum lifespan (χ5, 71 = 5.35, P = 0.3741); when this interaction term was removed and the GLM rerun, both clade (χ5, 68 = 32.07, P < 0.0001), and weight (χ1, 68 = 21.66, P < 0.0001), had a significant effect on maximum lifespan. In contrast, there was a significant interaction for median adult lifespan (χ5, 63 = 16.86, P = 0.0048), but neither weight (χ1, 63 = 3.69, P = 0.0547) nor clade (χ5, 63 = 5.71, P = 0.3357) had significant effects with this interaction term in the model. There were insufficient degrees of freedom to run a GLM with an interaction for median adult age, but the effect of clade was significant (χ5, 36 = 101.88, P < 0.0001) while that of weight was not (χ1, 36 = 0.56, P = 0.4544). The Cacatua clade (cockatoos) showed the greatest mean of maximum lifespan at 50.78 years. In contrast, the Ara clade had the highest mean of median adult lifespan at 14.31 years. Overall, the Cacatua clade included some of the longest-lived individuals in the entire database, but out of the species held in captivity, 65% of them had never had an individual live past 50 years old. Mean median adult lifespan for this clade was notably low in captivity (10.36 years), significantly less than the Ara clade, and did not differ from the Aratinga or Amazona clades whose mean maximum lifespans were 15–25 years less than that of the cockatoos. The median age of living birds is higher than the median lifespan of all birds for all six clades, but this increase is much less dramatic in the cockatoos than in Ara, Aratinga, and Amazona (Fig. 4).
Figure 4.
Mean and SE of (a) maximum lifespan, (b) median adult lifespan and (c) median adult age for major clades of parrots. Clades that do not share the same letter within the bar are significantly different based on a Tukey-Kramer HSD post hoc test.
Breeding parameters
Breeding parameters in captivity varied greatly across the 193 species for which breeding data was available (Supplementary Table 2). When restricted to species with data for ≥ five individuals, the lowest median age at first breeding was 1.10 years for the orange-bellied parrot (Neophema chrysogaster). The highest median age of last breeding was 19.75 years for the St. Vincent amazon (Amazona guildingii). The blue-eyed cockatoo (Cacatua ophthalmica) had the longest median breeding duration at 5.92 years. The longest median post-breeding duration was recorded at 5.16 years for Pesquet’s parrot (Psittrichas fulgidus); Supplementary Table 2).
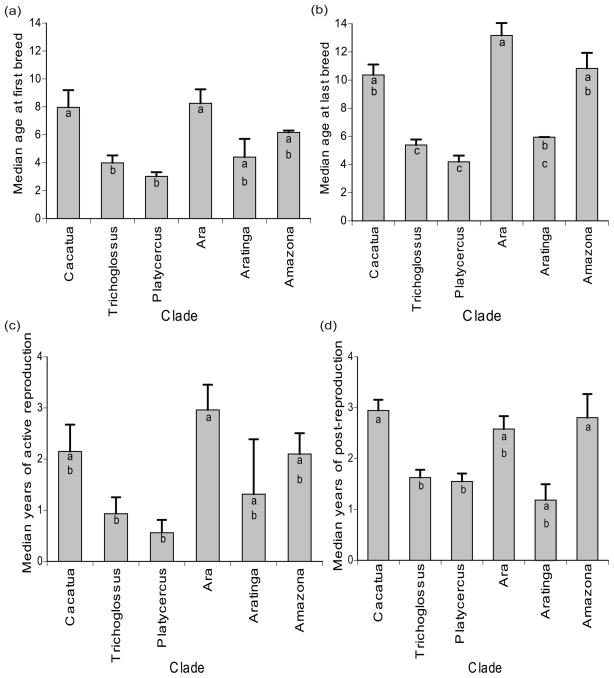
A one-way ANOVA testing for differences in the means of reproduction data among the six selected clades indicated that they differed in the median age of first breeding (F5,27 = 5.39, P = 0.0015), median age of last breeding (F5, 49 = 17.77, P < 0.0001), median duration of active reproduction (F5, 49 = 4.18, P = 0.0031), and median duration of post-reproduction (F5, 45 = 5.66, P = 0.0004). Notably, the mean median duration of post-reproduction was longer than the mean median duration of active reproduction for Trichoglossus, Cacatua, Amazona, and Platycercus (Fig. 5).
Figure 5.
Mean and SE of (a) median age at first breeding, (b) median age at last breeding, (c) median duration of active breeding, (d) median duration of post-breeding for major clades of parrots. Clade bars that do not share the same letter are significantly different based on a Tukey-Kramer HSD post hoc test.
Life history and IUCN status
After classifying species using the 2009 IUCN Red List, we found 68% of species were of Least Concern (LC), 10% were Near Threatened (NT), 11% were Vulnerable (VU), 7% were Endangered (EN), and 3% were Critically Endangered (CR; Table 1). One-way ANOVAs of lifespan and breeding parameters by IUCN status revealed that there was a detectable difference in adult median lifespan (F4, 163 = 9.00, P < 0.0001), median adult age (F4, 93 = 6.44, P = 0.0001), median age of last breeding (F4,125 = 3.55, P = 0.0088), and median duration of active breeding (F4,125 = 4.65, P = 0.0016) among the IUCN status groups. The species with VU, EN or CR (the classifications of highest threat) had greater average values for maximum lifespan, median adult lifespan, median age of last breeding, and median duration of active breeding than did species classified as LC or NT.
DISCUSSION
Parrots have a reputation for being one of the longest-lived avian taxa (Prinzinger, 1993). This analysis of 260 species of captive parrots spanning the order Psittaciformes demonstrates that even closely related clades of parrots can differ dramatically in lifespan and duration of reproduction. While a few individual parrots have lived for nearly a century, the majority of parrots in captivity did not live much beyond two decades. Even when accounting for juvenile mortality, only 30% of the 260 species had median adult lifespans ≥ 10 years. Clearly, most captive parrots are not living as long as generally thought. However, we found that lifespan in captivity appears to be increasing, as the median age of living adult birds is significantly greater than the median lifespan of all birds in the database, despite the truncating effect on lifespan of considering only living birds. This increase is likely due to advances in animal husbandry and indicates that modern zoos have improved their care and maintenance of parrots. Below we discuss these general trends and their implications for the conservation of parrots.
Life history trends in parrots
As found in a smaller analysis of parrot lifespan (Munshi-South & Wilkinson, 2006), we found that larger parrots had longer lifespans than smaller parrots. Even though parrots with a larger body mass generally lived longer than smaller bodied parrots, on average the difference in mean median adult lifespan and age was only about a decade within the six clades examined. When body mass was included as a covariate in the analysis mass and clade had a significant interaction for median adult lifespan, suggesting the effect of body mass on median adult lifespan varied with different clades. In contrast, body mass and clade independently affect maximum lifespan.
Male parrots had statistically longer maximum and median lifespans than females. However, this difference was small (1.74 yrs longer max lifespan, 0.22 yrs longer median lifespan) and may not be biologically important. In general, there is no consistent pattern of sex differences in avian lifespans; some sources report that in many species of birds, males live longer than females (Holmes et al., 2003), but other sources cite females as the sex with the typically longer lifespan (Christe, Keller & Roulin, 2006).
Breeding parameter patterns for captive female parrots vary greatly across species. Some smaller species were able to breed before they were a year old, while many larger species still bred when they were past 40 years old (Supplementary Table 2). Notably, several species also had very long post-breeding periods, and clade means of the median duration of active reproduction was similar to the median duration of the post-reproductive period. This similarity suggests that either a) parrots are not being housed in situations where they can realize their breeding potential fully, b) parrots have an unusually early reproductive senescence compared to other birds (Holmes et al., 2003), or c) female parrots have an extended lifespan in captivity relative to wild parrots and can live past the constraints on egg production (as seen in domestic quail; vom Saal, Finch & Nelson, 1994). Housing is likely influencing the breeding data as not all zoo parrots have access to a sexually mature, opposite-sex conspecific in ideal breeding conditions, but this effect cannot be teased apart from the other factors until data on opportunity to breed is also recorded. While this is not always feasible, especially in monomorphic species, it would be beneficial for zoos to enter as much of these data as possible into ISIS, so the impact of biological factors could potentially be assessed.
Caveats
While our analysis provides an important demonstration of the utility of the ISIS database for providing lifespan data on long-lived species, there are important caveats concerning the reliability of the data. The ISIS database is composed of data contributed by many different institutions that do not necessarily adhere to the same standards of accuracy, reliability, diligence, and comprehensiveness in record keeping. While we tried to eliminate clearly erroneous records during our initial compilation of the data, some questionable values remain (e.g. the age at first breeding of 0.29 years from Cyanoramphus novaezelandiae, Supplementary Table 2). Data accuracy can depend on the species, as parentage is harder to ascertain in group-living species than for species housed in pairs. In many cases, individual records may also represent an incomplete account of the entire lifespan due to transfers of animals in and out of ISIS member institutions. Overall, we suggest that the greatest care be exercised in generalizing from breeding data, as captive breeding is dependent on opportunities provided by housing arrangements and thus most subject to biases introduced by captivity. Renewed commitment of all ISIS members to record keeping protocols would improve the value of this large database for species maintenance, reproduction and conservation. A more fundamental issue is that ISIS data are from captive animals. While captive animals rarely suffer levels of predation and starvation seen in wild populations, they may experience higher rates of inbreeding, unusual social group composition and captive conditions that produce physical and psychological stress (Meehan & Mench, 2006). It is difficult to assess the relative importance of these factors, but there are some indications that lifespan data from captive animals are a generally reliable predictor of lifespan in the wild (Ricklefs, 2000; Wasser & Sherman, 2010).
Conservation Implications
This taxon-wide analysis of parrot lifespan and breeding parameters has several implications for conservation. First, survival in captivity should be taken into account when deciding which species to propagate. For example, the swift parrot (Lathamus discolor) had low residuals in the maximum lifespan on medium adult lifespan regression (Supplementary Table 3), meaning that many individuals of that species live nearly as long as the oldest surviving members. In contrast, the cockatoos had the highest residuals, and patterns in lifespan data that suggest while cockatoos have the biological potential to live for a very long time, few individuals are realizing that potential in captivity. We suggest that in the short term, zoos focus resources on propagating endangered species that fare well in captivity in order to create populations for potential reintroductions. International or regional studbooks should be created for the species that fit these requirements, which includes the swift parrot (L. discolor), golden-shouldered parrot (Psephotus chrysopterygius), and sun conure (Aratinga solstitialis). Long-term goals should include research aimed at improving husbandry and welfare so that endangered species that currently do not survive well in captivity, such as the cockatoos, can become better candidates for captive propagation programs.
Second, our data are the most comprehensive to date regarding lifespan and breeding in parrots. Such data are critical for parameterizing population viability models for wild populations. It is difficult to compare our captive data to data from wild populations, as the life history traits of interest have been studied for relatively few species over a limited scope of time in comparison to parrot lifespan and reproduction. The majority of these studies estimate survival rates or fecundity (Beissinger, Wunderle Jr & Meyers, 2008; Buckland, Rowley & Williams, 1983; Heinsohn & Legge, 2003; Koenig, 2008; Murphy, Legge & Heinsohn, 2003; Powlesland et al., 1992; Renton & Salinas-Melgoza, 2004; Rowley, 1983; Sandercock et al., 2000; Saunders, 1982;). A study on wild orange-bellied parrots reported life history measures comparable to our captive data (Holdsworth, Dettmann & Baker, in press). Furthermore, two of the general trends we detected have particular importance for the viability of wild parrot populations, namely the shorter median lifespans than generally considered, and the long periods of post-reproductive lifespans. Taken together these trends suggest that wild populations may be more vulnerable to rapid declines than previously thought.
Third, our results suggest the suitability of older individuals for captive breeding should be carefully assessed. Many species in our dataset exhibited long post-breeding durations (see Supplementary materials: Fig. 2 and Table 2); it is not clear whether this phenomenon also occurs in wild parrots or is an artifact of captive conditions. Efforts to house pairs together could potentially increase the duration of active breeding and thereby maximize their value for conservation. On the other hand, if these long post-breeding durations are generally characteristic of parrot life history, then many individuals will be surplus animals for as long a period as they were contributing active breeders. If true, this trend would put additional pressure on TAGs to refine their prioritization efforts.
Fourth, these data on average lifespan and breeding parameters may be used by TAGs as a rough guide for predicting future endangerment of species and proactively planning captive management priorities. We found that larger-bodied species that lived longer and bred later in life tended to be more threatened according to IUCN classifications. These trends suggest that TAGs should add lifespan and breeding measures to their existing criteria of number of individuals in captivity and IUCN status (AZA, 2007) in prioritizing the management of captive parrot populations for conservation.
Finally, this study demonstrates the general value and utility of the ISIS database and provides a baseline for demographic comparisons with wild populations. Even though caution must be exercised, ISIS provides a tremendous source of unrivaled information which can be used to parameterize population viability models for wild populations and adaptively manage captive populations according to conservation priorities.
Supplementary Material
Acknowledgments
We thank Christine Dahlin for statistical advice, Breanne Cordier and Aaron Hobson for assistance with data organization, and Nadine Lamberski of the San Diego Zoo and Wild Animal Park for sponsoring our research request to ISIS. Funding for this research was provided by National Institutes of Health grant S06 GM008136 and National Science Foundation grant IOS-0725032 to T.F.W. This study was made possible by the dedicated record keeping of the staff members of ISIS and its member institutions.
References
- Association of Zoos and Aquariums (AZA) Regional Collection Plan (RCP) Handbook. 2007. [Google Scholar]
- Baker A. Animal ambassadors: An analysis of the effectiveness and conservation impact of ex situ breeding efforts. In: Zimmermann A, Hatchwell M, Dickie L, West C, editors. Zoos in the 21st century: Catalysts for conservation? New York: Cambridge University Press; 2007. pp. 139–154. [Google Scholar]
- Beck BB, Rapaport LG, Price MS, Wilson AC. Reintroduction of captive-born animals. In: Olney PJS, Mace GM, Feistner ATC, editors. Creative conservation: Interactive management of wild and captive animals. London: Chapman & Hall; 1994. pp. 265–284. [Google Scholar]
- Beissinger SR, Wunderle JM, Jr, Meyers JM, Saether BE, Engen S. Anatomy of a bottleneck: Diagnosing factors limiting population growth in the Puerto Rican Parrot. Ecological Monographs. 2008;78:185–203. [Google Scholar]
- Biggins DE, Vargas A, Godbey JL, Anderson SH. Influence of prerelease experience on reintroduced black-footed ferrets (Mustela nigripes) Biological Conservation. 1999;89:121–129. [Google Scholar]
- Bouman I. The reintroduction of Przewalski horses in the Hustain Nuruu mountain forest steppe reserve in Mongolia; an integrated conservation development project. Gazella. 2000;27:27–51. [Google Scholar]
- Brightsmith D, Hilburn J, Del Campo A, Boyd J, Frisius M, Frisius R, Janik D, Guillen F. The use of hand-raised psittacines for reintroduction: A case study of scarlet macaws (Ara macao) in Peru and Costa Rica. Biological Conservation. 2005;121:465–472. [Google Scholar]
- Brooks TM, Mittermeier RA, Da Fonseca GAB, Gerlach J, Hoffmann M, Lamoreux JF, Mittermeier CG, Pilgrim JD, Rodrigues ASL. Global biodiversity conservation priorities. Science. 2006;313:58. doi: 10.1126/science.1127609. [DOI] [PubMed] [Google Scholar]
- Brouwer K, Jones ML, King CE, Schifter H. Longevity records for Psittaciformes in captivity. International Zoo Yearbook. 2000;37:299–316. [Google Scholar]
- Buckland ST, Rowley I, Williams DA. Estimation of survival from repeated sightings of tagged galahs. Journal of Animal Ecology. 1983;52:563–573. [Google Scholar]
- Christe P, Keller L, Roulin A. The predation cost of being a male: Implications for sex-specific rates of ageing. Oikos. 2006;114:381. [Google Scholar]
- Collazo JA, White TH, Jr, Vilella FJ, Guerrero SA. Survival of captive-reared Hispaniolan parrots released in Parque Nacional del Este, Dominican Republic. Condor. 2003;105:198–207. [Google Scholar]
- Conde DA, Flesness N, Colchero F, Jones OR, Scheuerlein A. An emerging role of zoos to conserve biodiversity. Science. 2011;331:1390–1391. doi: 10.1126/science.1200674. [DOI] [PubMed] [Google Scholar]
- Fischer J, Lindenmayer DB. An assessment of the published results of animal relocations. Biological Conservation. 2000;96:1–11. [Google Scholar]
- Foose TJ, Wiese RJ. Population management of rhinoceros in captivity. International Zoo Yearbook. 2006;40:174–196. [Google Scholar]
- Forshaw JM, Knight F. Parrots of the world: an identification guide. Princeton, NJ: Princeton University Press; 2006. [Google Scholar]
- Frynta D, Lišková S, Bültmann S, Burda H, Mappes T. Being attractive brings advantages: the case of parrot species in captivity. PloS one. 2010;5:e12568. doi: 10.1371/journal.pone.0012568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham S. Issues of surplus animals. In: Kleiman DG, Allen ME, Thompson KV, Lumpkin S, editors. Wild mammals in captivity: Principles and techniques. Chicago: University of Chicago Press; 1996. [Google Scholar]
- Hatchwell M, Rubel A, Dickie LA, West C, Zimmerman A. The future of zoos. In: Zimmerman A, Hatchwell M, Dickie LA, West C, editors. Zoos in the 21st century: Catalysts for conservation? New York: Cambridge University Press; 2007. pp. 343–360. [Google Scholar]
- Heinsohn R, Legge S. Breeding biology of the reverse-dichromatic, cooperative parrot Eclectus roratus. Journal of Zoology. 2003;259:197–208. [Google Scholar]
- Holdsworth M, Dettmann B, Baker B. Survival in the orange-bellied parrot. Neophema chrysogaster. Emu. In press. Accessed online http://www.publish.csiro.au/nid/97/aid/13034.htm.
- Holmes DJ, Thomson SL, Wu J, Ottinger MA. Reproductive aging in female birds. Experimental gerontology. 2003;38:751–756. doi: 10.1016/s0531-5565(03)00103-7. [DOI] [PubMed] [Google Scholar]
- Hutchins M. Zoo and aquarium animal management and conservation: Current trends and future challenges. International Zoo Yearbook. 2003;38:14–28. [Google Scholar]
- Hutchins M, Thompson SD. Zoo and aquarium research: Priority setting for the coming decades. Zoo Biology. 2008;27:488–497. doi: 10.1002/zoo.20167. [DOI] [PubMed] [Google Scholar]
- Hutchins M, Wiese RJ. Beyond genetic and demographic management: The future of the species survival plan and related AAZPA conservation efforts. Zoo Biology. 1991;10:285–292. [Google Scholar]
- International Species Information System (ISIS) ISIS species holdings. 2009 Downloaded at < http://app.isis.org/abstracts/abs.asp> on 28 July 2009.
- International Union for the Conservation of Nature (IUCN) The IUCN red list of threatened species. 2009 Downloaded at < http://www.iucnredlist.org> on 19 June 2009.
- Jackson SM. Standardizing captive-management manuals: Guidelines for terrestrial vertebrates. International Zoo Yearbook. 2003;38:229–243. [Google Scholar]
- Joseph L, Dolman G, Donnellan S, Saint KM, Berg ML, Bennett ATD. Where and when does a ring start and end? Testing the ring-species hypothesis in a species complex of Australian parrots. Proceedings of the Royal Society B. 2008;275:2431. doi: 10.1098/rspb.2008.0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig SE. Black-billed parrot (Amazona agilis) population viability assessment (PVA): A science-based prediction for policy makers. Ornitologia Neotropical. 2008;19 (Suppl):135–149. [Google Scholar]
- Lacy R. Culling surplus animals for population management. In: Norton B, Hutchins M, Stevens EF, Maple TF, editors. Ethics on the ark: Zoos, animal welfare, and wildlife conservation. Washington DC: Smithsonian Institution Press; 1995. pp. 187–194. [Google Scholar]
- Lindburg DG, Lindburg L. Success breeds a quandary: To cull or not to cull. In: Norton B, Hutchins M, Stevens EF, Maple TF, editors. Ethics on the ark: Zoos, animal welfare, and wildlife conservation. Washington DC: Smithsonian Institution Press; 1995. pp. 195–208. [Google Scholar]
- Mace GM, Balmford A, Leader-Williams N, Manica A, Walter O, West C, Zimmermann A. Measuring conservation success: Assessing zoos’ contribution. In: Zimmermann A, Hatchwell M, Dickie L, West C, editors. Zoos in the 21st century: Catalysts for conservation? New York: Cambridge University Press; 2007. pp. 322–342. [Google Scholar]
- Meehan C, Mench J. Captive parrot welfare. In: Luescher AU, editor. Manual of parrot behavior. Ames: Blackwell Publishing; 2006. pp. 301–318. [Google Scholar]
- Miller B, Conway W, Reading RP, Wemmer C, Wildt D, Kleiman D, Monfort S, Rabinowitz A, Armstrong B, Hutchins M. Evaluating the conservation mission of zoos, aquariums, botanical gardens, and natural history museums. Conservation Biology. 2004;18:86–93. [Google Scholar]
- Munshi-South J, Wilkinson GS. Diet influences life span in parrots (Psittaciformes) The Auk. 2006;123:108–118. [Google Scholar]
- Murphy S, Legge S, Heinsohn R. The breeding biology of palm cockatoos (Probosciger aterrimus): a case of a slow life history. Journal of Zoology. 2003;261:327–339. [Google Scholar]
- Powlesland RG, Lloyd BD, Best HA, Merton DV. Breeding biology of the kakapo Strigops habroptilus on Stewart Island, New Zealand. Ibis. 1992;134:361–373. [Google Scholar]
- Price MRS, Soorae PS. Reintroductions: Whence and whither? International Zoo Yearbook. 2003;38:61–75. [Google Scholar]
- Prinzinger R. Life span in birds and the ageing theory of absolute metabolic scope. Comparative Biochemistry and Physiology. A. Comparative physiology. 1993;105:609–615. [Google Scholar]
- Rahbek C. Captive breeding—a useful tool in the preservation of biodiversity? Biodiversity and Conservation. 1993;2:426–437. [Google Scholar]
- Renton K, Salinas-Melgoza A. Climatic variability, nest predation, and reproductive output of lilac-crowned parrots (Amazona finschi) in tropical dry forest of western Mexico. The Auk. 2004;121:1214–1225. [Google Scholar]
- Ricklefs RE. Intrinsic aging-related mortality in birds. Journal of Avian Biology. 2000;31:103–111. [Google Scholar]
- Ricklefs RE. The Economy of Nature. 6. New York: WH Freeman & Co; 2008. [Google Scholar]
- Ricklefs RE, Cadena CD. Lifespan is unrelated to investment in reproduction in populations of mammals and birds in captivity. Ecology Letters. 2007;10:867–872. doi: 10.1111/j.1461-0248.2007.01085.x. [DOI] [PubMed] [Google Scholar]
- Rowley I. Mortality and dispersal of juvenile galahs, Cacatua roseicapilla, in the western Australian wheatbelt. Australian Wildlife Research. 1983;10:329–342. [Google Scholar]
- Sandercock BK, Beissinger SR, Stoleson SH, Melland RR, Hughes CR. Survival rates of a Neotropical parrot: Implications for latitudinal comparisons of avian demography. Ecology. 2000;81:1351–1370. [Google Scholar]
- Sanz V, Grajal A. Successful reintroduction of captive-raised yellow-shouldered amazon parrots on Margarita Island, Venezuela. Conservation Biology. 1998;12:430–441. [Google Scholar]
- Saunders DA. The breeding-behavior and biology of the short-billed form of the white-tailed black cockatoo Calyptorhynchus-funereus. Ibis. 1982;124:422–455. [Google Scholar]
- Seddon PJ, Armstrong DP, Maloney RF. Developing the science of reintroduction biology. Conservation Biology. 2007;21:303. doi: 10.1111/j.1523-1739.2006.00627.x. [DOI] [PubMed] [Google Scholar]
- Smith B, Hutchins M, Allard R, Warmolts D. Regional collection planning for speciose taxonomic groups. Zoo Biology. 2002;21:313–320. [Google Scholar]
- Snyder NFR, Derrickson SR, Beissinger SR, Wiley JW, Smith TB, Toone WD, Miller B. Limitations of captive breeding in endangered species recovery. Conservation Biology. 1996;10:338–348. [Google Scholar]
- Snyder NFR, Snyder HA. Biology and conservation of the California condor. Current Ornithology. 1989;6:175–263. [Google Scholar]
- Vanstreels RET, Teixeira RHF, Camargo LC, Nunes ALV, Matushima ER. Zoo Biology. 2010. Impacts of animal traffic on the Brazilian amazon parrots (Amazona species) collection of the Quinzinho de Barros Municipal Zoological Park, Brazil, 1986–2007. [DOI] [PubMed] [Google Scholar]
- Vom Saal FS, Finch CE, Nelson JF. Natural history and mechanisms of reproductive aging in humans, laboratory rodents, and other selected vertebrates. In: Knobil E, Neill J, editors. The Physiology of Reproduction. New York: Raven Press; 1994. pp. 1213–1314. [Google Scholar]
- Walters JR, Derrickson SR, Michael Fry D, Haig SM, Marzluff JM, Wunderle JM., Jr Status of the California condor (Gymnogyps californianus) and efforts to achieve its recovery. The Auk. 2010;127:969–1001. [Google Scholar]
- Wasser DE, Sherman PW. Avian longevities and their interpretation under evolutionary theories of senescence. Journal of Zoology. 2010;280:103–155. [Google Scholar]
- WAZA. The World Zoo and Aquarium conservation strategy: Building a future for wildlife. Liebefeld-Bern: WAZA; 2005. [Google Scholar]
- White TH, Jr , Collazo JA, Vilella FJ. Survival of captive-reared Puerto rican parrots released in the Caribbean national forest. Condor. 2005:424–432. [Google Scholar]
- Wiese RJ, Willis K. Calculation of longevity and life expectancy in captive elephants. Zoo Biology. 2004;23:365–373. [Google Scholar]
- Wiese RJ, Willis K. Population management of zoo elephants. International Zoo Yearbook. 2006;40:80–87. [Google Scholar]
- Wilkinson R. An overview of captive-management programmes and regional collection planning for parrots. International Zoo Yearbook. 2000;37:36–58. [Google Scholar]
- Wilson EO. The current state of biological diversity. In: Willers WB, editor. Learning to listen to the land. Washington, DC: Island Press; 1991. pp. 17–37. [Google Scholar]
- World Parrot Trust. 2009 Downloaded at < http://www.parrots.org> on 20 November 2009.
- Zar JH. Biostatistical analysis. 4. New Jersey: Prentice Hall; 1999. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.