Abstract
Retinal pigment epithelial (RPE) cells play an important role in normal functioning of retina and photoreceptors, and some retinal degenerations arise due to malfunctioning RPE. Retinal pigment epithelium transplantation is being explored as a strategy to rescue degenerating photoreceptors in diseases such as age-related macular degeneration (AMD) and retinitis pigmentosa (RP). Additionally, RPE-secreted factors could rescue degenerating photoreceptors by prolonging survival or by their ability to differentiate and give rise to photoreceptors by transdifferentiation. In this study, we have explored what role cell density could play in differentiation induced in a human retinal progenitor cell line, in response to RPE-secreted growth factors. Retinal progenitors plated at low (1 × 104 cells/cm2), medium (2–4 × 104 cells/cm2), and high (1 × 105 cells/cm2) cell density were exposed to various dilutions of RPE-conditioned medium (secreted factors) under conditions of defined medium culture. Progenitor cell differentiation was monitored phenotypically (morphological, biochemical analysis, and immunophenotyping, and western blot analysis were performed). Our data show that differentiation in response to RPE-secreted factors is modulated by cell density and dilutions of conditioned medium. We conclude that before embarking on RPE transplantation as a modality for treatment of RP and AMD, one will have to determine the role that cell density and inhibitory and stimulatory neurotrophins secreted by RPE could play in the efficacy of survival of transplants. We report that RPE-conditioned medium enhances neuronal phenotype (photoreceptors, bipolars) at the lowest cell density in the absence of cell–cell contact. Eighty percent to 90% of progenitor cells differentiate into photoreceptors and bipolars at 50% concentration of conditioned medium, while exposure to 100% conditioned medium might increase multipolar neurons (ganglionic and amacrine phenotypes) to a small degree. However, no clear-cut pattern of differentiation in response to RPE-secreted factors is noted at higher cell densities.
Keywords: Retinal progenitors, Retinal pigment epithelium, Secreted factors, Differentiation, Density
Introduction
Various ocular diseases, including retinitis pigmentosa, cone dystrophy, and age-related macular degeneration, are characterized by a loss of photoreceptor cells, leading to blindness [1]. Some of the strategies currently being explored for treatment of retinal diseases (RP, AMD) are tissue replacement and the rescue of degenerating photoreceptors by exogenously provided neurotrophins.
Cells/tissues being tested for replacement include retinal progenitors, sheets of photoreceptors from cadavers, and retinal/retinal pigment epithelial (RPE) sheets, including some thoughts to transplantation of RPE which could rescue degenerating retina by secretion of neurotrophins or by transdifferentiation into photoreceptors after transplantation [2–9]. Other suggestions are transplantation of partially differentiated progenitors to transplantation of 3-D constructs of retina generated from progenitors in a 3-D bioreactor culture [7, 10–12]. Others are proposing transplantation of RPE induced from pluripotent stem cells, which could differentiate into photoreceptors in vivo [13].
Photoreceptors have a highly evolved phenotype, and cell–cell interaction is critical to maintenance of this phenotype. A potential source of interaction is cells within the retina (Muller, horizontal, bipolar, etc.), as well as adjacent tissue such as the RPE [14–17]. Retinal/RPE interactions are critical not only during development but also vital for normal functioning of adult retina. Soluble secreted signals could induce or modify interaction between various retinal cell types, specifically photoreceptors [18]. In vivo retina develops from multipotential progenitors in which cell–cell interaction seems to play a critical role.
Although cell–cell interactions have been reported, there are also reports to confirm that in chick, primate, and human retinal cell lines, photoreceptors could develop independent of cell–cell interaction, which could be a specific trait of cone-rich retina. However, for rodent photoreceptor development, cell–cell interaction seems vital [19, 20]. Others have reported photoreceptor differentiation in chick retinal cultures and human retinal cell lines in the absence of cell–cell contact [21–23].
Short-range signals, such as growth factors and hormones, appear to be necessary to induce specific retinal cell types [16, 24–27]. In particular, fibroblast growth factor (FGF) signaling has been implicated in retinal cell fate choice. Members of the FGF family [28–31] and their receptors [32, 33] are expressed in the developing retina of many species.
Of these, only the effects of basic fibroblast growth factor (bFGF) on retinal cell genesis in vivo and in culture have been investigated in detail. Basic fibroblast growth factor influences the specification of several different retinal cell types including retinal ganglion cells [34, 35] and Muller glia [36]. In Xenopus, pigment epithelium treated with bFGF transdifferentiates, generating all retinal cell types including both neural retina and glia [37]. We have reported that bFGF and transforming growth factor alpha (TGFα) induce photoreceptor differentiation in this retinal progenitor cell line in a density-dependent manner [23, 38].
It has been suggested by some that chick photoreceptor differentiation seen in low-density cultures reported by Adler and Hatlee [21] could be due to signaling molecules provided from culture medium in the form of growth factors. It has been suggested that growth medium itself contains both stimulatory and inhibitory molecules and some factors could have opposing effects across species [27]. Some of the factors implicated in photoreceptor differentiation and maintenance are bFGF, ciliary neurotrophic factor (CNTF), TGFα, etc. [23, 39, 40]. Ezeonu et al. [23] have shown density-dependent photoreceptor differentiations in response to bFGF and TGFα in their in vitro studies with a human progenitor cell line.
Multipotential retinal progenitors have been induced toward photoreceptor phenotype by transfection with receptors and exposure to growth factors (bFGF, TGFα, CNTF, etc.) In vivo signaling molecules, necessary for differentiation, could be provided by retina or RPE.
The RPE is developmentally and anatomically close to the neural retina. Unlike retinal neurons, RPE cells are nonneural and can reenter the cell cycle on stimulation. Furthermore, their progenies may differentiate into cell types other than RPE. Classical experiments show that embryonic chick RPE at early developmental stages can be triggered to transdifferentiate into a neural retina. This RPE-to-retina transdifferentiation occurs in vivo and in vitro under the induction of fibroblast growth factor. We have shown that adult RPE can be induced into neuronal phenotype by transfection of H-ras Val [12] gene [41].
Cultured RPE cells secrete numerous growth factors including pigment epithelium-derived factor (PEDF), bFGF, epithelial growth factor, nerve growth factor (NGF), vascular endothelial growth factor, brain-derived neurotrophic factor (BDNF), etc. We have shown that a higher degree of differentiation in retinal progenitor cells cultured in a bioreactor is mediated by upregulation of bFGF, TGFα, CNTF, and BDNF [42].
We have a well-characterized system of human multipotential retinal progenitor cell lines in which retinal cell differentiation can be monitored in response to neurotrophins [10, 11, 23, 42–44] or 3-D culture models of growth [10]. Our results show that in monolayer culture, photoreceptor differentiation occurs in response to bFGF and TGFα in a density-dependent manner confirming the studies of the default model of cone photoreceptor differentiation established by Belecky-Adams et al. [22].
Also, in a 3-D model of retinal progenitor differentiation, we have shown that retinal progenitors and RPE cells express bFGF, TGFα, CNTF, and BDNF levels that are much higher in 3-D culture when compared to monolayers [42]. In the present study, we have explored the role of RPE-secreted factors in monolayer cultures (conditioned media on a non-transformed human retinal cell line) [43, 44] characterized in monolayer culture [23, 38] and 3-D culture [10, 11, 42].
In this study, we report:
Conditioned medium from RPE enhances neuronal differentiation in this progenitor cell line in a dose- and density-dependent manner, inducing neurons with long, extended neurites. The majority of the neurons generated have photoreceptor or bipolar phenotype. The degree of differentiation is inversely proportional to cell density. The highest degree of differentiation is seen at the lowest cell density.
Cells with neuronal phenotype express photoreceptor and bipolar cell markers AaNAT, D4 receptor, rhodopsin, and PKCα.
Also, the RPE-secreted factor effect on photoreceptor differentiation is maximum at 50% dilution of conditioned medium, suggesting that RPE-conditioned medium contains both stimulatory and inhibitory signals for photoreceptor differentiation.
We suggest that one of the factors responsible for photoreceptor differentiation could be bFGF.
Materials and methods
The spontaneously immortalized human progenitor cell line in passages 50 and 52 was used in this study. The well-characterized cell line [23, 43, 44] is from a clone, which became spontaneously immortalized. The cell line has been tested and is negative for SV-40T antigens. D407 [45], a retinal pigment epithelial cell line, was a generous gift from Dr. Richard Hunt (Department of Microbiology University of South Carolina at Columbia). The cells used in this study were at passage 72 [45].
Cell culture
Both of the cell lines were maintained routinely in Dulbecco modified Eagle medium (DMEM): nutrient mixture F-12 1:1(Ham) (Bethesda, MD, USA) supplemented with 10% fetal bovine serum (Hyclone Laboratories, Logan, UT, USA), 10% serum plus supplement (JRH Biosciences, Lenexa, KS, USA), 2 mM l-glutamine, 0.075% sodium bicarbonate, penicillin 100 U/ml, and streptomycin 100 μg/ml (GIBCO) [43, 44].
Mitogenic and differentiation potential of RPE-secreted factors
To determine the role of RPE-secreted growth factors on cell proliferation and differentiation, cells were plated at three densities. To determine the potential role of growth factors in cell differentiation and proliferation, cells were plated at a density of 1 × 104 cells/cm2 (low), 2–4 × 104 cells/cm2 (medium), or 1 × 105 cells/cm2 (high) on plastic culture dishes or glass coverslips placed in tissue culture wells. The cells were allowed to attach in serum-containing medium for 6 h and subsequently switched to serum-free defined medium comprising DMEM: F-12 (Ham) nutrient mixture (1:1) supplemented with 2 mM HEPES buffer, putrescine 8.8 ng/ml, transferrin 10 μg/ml (Sigma Chemical, St. Louis, MO, USA), 2 mM l-glutamine, insulin 5 μg/ml, and penicillin (100 U/ml)–streptomycin (100 μg/ml).
Collection of RPE-conditioned medium
Retinal pigment epithelial cells in passages 70–71 were plated in 75-cm2 flasks in complete medium (serum and serum supplement) described above and allowed to grow to confluency. At this time, serum-containing medium was discarded, and excess 40-ml serum-free DMEM/F12 (1:1) with antibiotics was added for 24 h. After this point, medium was discarded. Additional serum-free medium (40 ml) was added to the flask for an additional 24–36 h and discarded. To collect RPE-secreted factors from these confluent RPE serum-depleted cultures, 7–8 ml of serum-free medium was added and conditioned medium collected twice. The twice collected conditioned medium was pooled and filtered through 22-μm Millipore filter and frozen at −20°C. Conditioned medium was added, either straight (100%) or diluted (50%, 25%, 12.5%), for various analyses to be performed.
Mitogenic potential of RPE-secreted factor
To determine the mitogenic potential, the cells were plated at a density of 1 × 104 or 4 × 104 cells/cm2 and allowed to attach in 5% serum-containing medium for 4–6 h and subsequently switched to serum-free medium for an additional 18–24 h. The medium was discarded and cells were cultured in conditioned medium at 100%, 50%, 25%, and 12.5% concentration. Controls included serum-containing and serum-free cultures.
After overnight equilibration in defined medium, the cells were exposed at 100%, 50%, 25%, and 12.5% in RPE-conditioned medium. Tritiated thymidine (NEN, Boston, MA, USA) was added to the cultures (4 μCi/ml) for 24 h. Trichloroacetic acid (TCA) precipitation was performed by lysing the cells with 0.3 M sodium hydroxide and trapping the DNA on GS filters (0.22 μm) (Millipore Corporation, Bedford, MA, USA). The filters were washed twice with cold 10% TCA before counting in a Beckman scintillation counter (Beckman Instruments, Inc., Fullerton, CA, USA). Total protein content was measured by Lowry’s method. The mean counts per minute and standard deviations were calculated, and values were expressed as counts per minute per milligram of protein. Increased counts per minute values were correlated with increased cell proliferation and mitogenic activity of growth factors. The experiment was repeated at least three times, and each experiment had three to five replicates. The data represent a single experiment with five samples.
Morphological analysis of the retinal progenitors exposed to RPE-conditioned medium
To determine the effects of growth factors on cell differentiation, retinal cells in passages 50–52 were plated at different densities on plastic culture dishes or on dishes coated with polylysine (Sigma). The cells were allowed to attach in serum-containing medium before being switched to serum-free defined medium. After overnight equilibration in defined medium, the cells were treated with different dilutions of conditioned medium.
Cells plated at low, medium, and high cell density were exposed to varying concentrations of conditioned medium and scored by counting various neuronal cell types. The scoring was done by two separate individuals in a blind study. Simultaneously, phase contrast morphology and scanning electron micrographs were generated. Cultures were monitored and scored at days 1, 2, and 3. The experiment was repeated three times. Current data are from a single experiment average of three to five flasks/culture/dose condition.
Progenitors have epithelial morphology; ganglions have large cell body and multiple processes. Bipolar cells have small, rounded cell bodies and two very thin-cell processes on both sides. Cones have short neurites on one side and rods elongated with a slender shape.
Immunophenotyping of neurons generated in response to RPE-conditioned medium
Immunophenotyping was performed on cells plated at a density 1 × 104 cells/cm2. This density revealed the highest degree of neuronal differentiation, and the RPE-conditioned medium was tested at 50%. Cells were plated on polylysine-coated coverslips and exposed to conditioned medium for 36–48 h and processed for immunolabeling by our previously published protocols [23, 38]. Cells plated at low density in serum-free culture were exposed to 50% conditioned medium and assessed for the expression of various antigens by western blot analysis to confirm immunophenotyping data at the same time.
Immunophenotyping of cells
Cells were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) for 20 min at room temperature and permeabilized with 0.2% Triton X-100 at 37°C for 10 min. Nonspecific binding was blocked by treatment with 10% goat serum overnight at 4°C or 37°C for 1 h. Controls were included in each determination by the omission of primary and secondary antibodies. Cells were reacted with the primary antibody at room temperature for 1 h and the secondary antibody for 1 h at room temperature. Secondary antibodies used were goat anti-rabbit Alexa Fluor 488 dilution (1:500) color green and goat anti-rabbit Alexa Fluor 594 dilution (1:500) color red (Source, Molecular Probes, Eugene, OR, USA). Nuclear staining is DAP1 blue. All proteins were green except neurofilament protein, which is identified by the color red. Superimposed blue (DAP1) over red neurofilament proteins looks purplish in color. Primary antibodies PKCα, D4 receptor, calbindin, tyrosine hydroxylase, and thy 1.1 were procured from Santa Cruz, (Santa Cruz, CA, USA) and were used at a dilution of 1:50 and 1:200 for immunohistochemistry and 1:200 and 1:2,000 for western blot analysis. Antibodies AaNat, rhodopsin 1D4, Nr2e3, and GNB3 were procured from Chemicon (Temecula, CA, USA) and used at a dilution of 1:200 for immunohistochemistry and at 1:1,000 for western blot analysis. Actin antibody from Sigma-Aldrich Corp., St. Louis, MO, USA was used at a 1:500 dilution [10, 11, 42].
Western blot analysis
For western blot analysis, our previously published protocols were used [11]. Whole-cell extracts were prepared from cells treated with various dilutions of conditioned medium. The cells were washed thrice with PBS and were lysed with lysis buffer, which consisted of 20 mM Tris, pH 7.5, 150 mM sodium chloride (NaCl), 1 mM ethylenediaminetetraacetic acid, 1 mM ethylene glycol tetraacetic acid, 1% Triton X-100, 0.2 mM 4(2 aminoethyl) benzene sulfonyl fluoride hydrochloride, 0.5 mM benzamidine, 2 mg/ml aprotinin, 0.5 mM leupeptin, and 5 mg/ml pepstatin A. Cell debris and detergent insoluble material were removed by centrifugation at 15,000 rpm for 15 min at 48°C. The protein content of the whole-cell extract was determined using the BCA protein assay reagent kit (Pierce, Rockford, IL, USA) according to manufacturer’s instructions. Whole-cell extract containing 50 mg proteins was separated by electrophoresis on 10% sodium dodecyl sulfate–polyacrylamide gels. After the separation, the proteins were electrophoretically transferred to a polyvinylidene difluoride membrane (Immobilon R; Millipore, Bedford, MA, USA). The nonspecific binding sites were blocked by immersing the membrane in 5% (W/V) nonfat dry milk powder in Tris-buffered saline Tween-20 (25 mM Tris–HC1, pH 6.5, 150 mM NaCl, and 0.05% Tween-20) for 2 h at room temperature. The antibody for neuron-specific enolase was obtained from Zymed, and all other primary and secondary antibodies were purchased from Chemicon. Expression of specific proteins was determined by binding to specific primary antibodies (dilution is as indicated), PKCα (1:500), TH (1:100), calbindin (1:500), and dopamine receptor D4 antibodies (1:300). Detection was done with enhanced chemiluminescence (Amersham, Arlington Heights, IL, USA) using secondary antibodies conjugated with peroxidase and exposed to X-ray film. The level of expression was determined using densitometer and Scion Image analysis software (Frederick, MD, USA).
Results
Description of human progenitor cell line
We have previously reported the characterization of a human progenitor cell line, which became spontaneously immortalized (clone 208). The cell line is from first trimester spontaneously aborted fetus [23, 38, 43, 44]. The cell line grows in a contact inhibited manner and is SV40T antigen negative (data not included). The cell line was used in 52 passages. The precursor cells form a flat layer (arrowheads) overlay of occasional neurons with neuritic processes (arrows) (Fig. 5a (a)). The cell line is very similar to another cell line established by us and is multipotential [44].
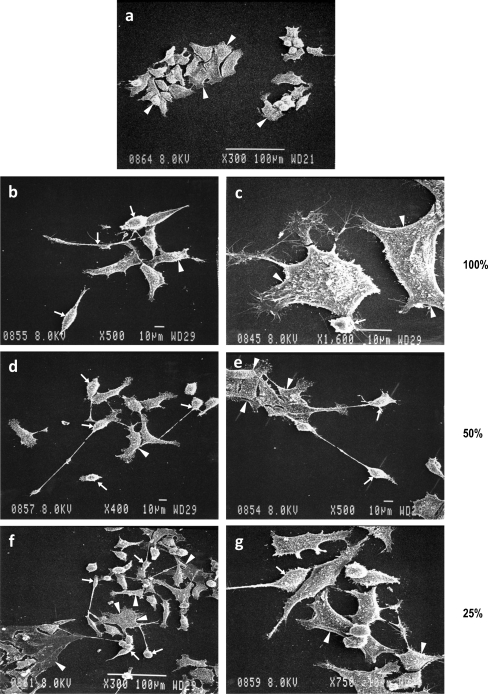
Fig. 5.
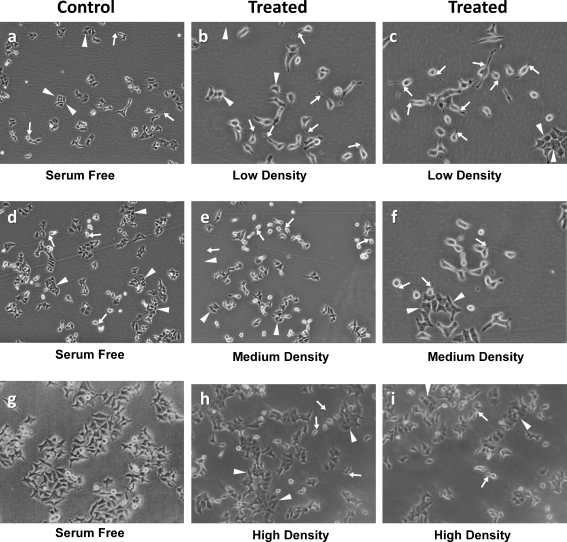
The scanning electron micrographs of low-density cultures exposed to various dilutions of conditioned medium. Note in a the a progenitors with epithelial cell phenotype in serum-free culture day 2 (arrowhead) progenitors. ab, c Cells treated with 100% conditioned medium. Arrows point to cells with photoreceptor phenotype (arrowheads, possible multipolar neurons and some progenitors). Note in a the d, e cells treated with 50% conditioned medium with an increase in the number of cells with photoreceptor phenotype (arrows). af, g Cells exposed to 12.5% conditioned medium showing a mixture of neurons. Although the majority of the cells have progenitor phenotype, some multipolar neuronal cell phenotype is also noted (arrowheads). b Morphological analysis and phase micrography of differentiated cells. Cells processed in a manner similar to Figs. 2, 3, and 4 and a. Cells plated at low (1 × 104 cells/cm2), medium (4 × 104 cells/cm2), and high density (1 × 105 cells/cm2) were treated with 50% conditioned medium (700–800 cells scored in blind study). Note that the cell differentiation is indirectly proportional to the cell density. The highest number of cells with neuronal phenotype are seen at the lowest cell density (arrows; b (b, c)) and progenitors (arrowheads; b), followed by medium-density cultures (e, f) and there is hardly any differentiation at higher cell density (g, h)
Mitogenic potential of RPE-conditioned medium on retinal progenitor cell line
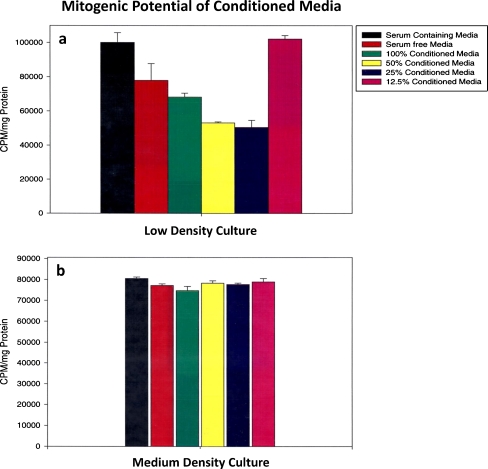
Retinal progenitors were plated at low (1 × 104 cells/cm2), medium, (4 × 104 cells/cm2), and high density (1 × 105 cells/cm2) and cultured in serum-containing medium, serum-free medium, and RPE-conditioned medium straight at 100%, 50%, 25%, and 12.5%. Culture medium contained 4 μCi/ml of tritiated thymidine. Twenty-four hours later, TCA-precipitated counts were expressed per milligram protein. The experiment was repeated three times. Representative data from a single experiment average of five samples is represented in Fig. 1a, b. At low cell density, conditioned medium reduced cell proliferation only slightly at 100% concentration; however, significant reductions were noted at 50% and 25% concentrations. At 12.5% concentration of conditioned medium, the cell proliferation was very similar to serum-containing medium (Fig. 1a).
Fig. 1.
Mitogenic potential of RPE-conditioned medium on retinal progenitors plated at low and medium cell density. Retinal progenitors plated at 1 × 104 cells/cm2 (low) and 4 × 104 cells/cm2 (medium) were treated with various dilutions of conditioned medium (100%, 50%, 25%, 12.5%). Also, 4 μCi/ml of tritiated thymidine was included in the cultures. Serum-treated and serum-free control cultures were also included. Trichloroacetic acid precipitated samples were evaluated for 3Htdr incorporation at 24 h. Data are from five separate samples for each condition. a, b3HTdr incorporation in low- and medium-density cultures. Data represent an average of five samples. Note in a that 3Htdr incorporation, reflecting cell proliferation, is the highest in serum-treated cultures followed by serum-free cultures, followed by cells treated with 100% conditioned medium. Mitogenic potential was less in cells treated with 50% and 25% conditioned medium, suggesting that these dilutions inhibited cell proliferation, whereas exposure to 12.5% conditioned medium had no inhibitory effect. b Medium-density cultures treated in similar fashion. Note that 3Htdr incorporation at this cell density is similar to serum-containing cultures and various dilutions of conditioned medium had no effect
In cells plated at medium density (Fig. 1b), there were no differences in cell proliferation/differentiation in serum-containing serum-free or various dilutions of conditioned medium. This suggests that the cells plated in medium density are making their growth factors thus overriding the effects of RPE-conditioned growth factors. At high cell density, cells were contact inhibited and very little 3Htdr incorporation was noted under all culture conditions. Similar results were seen in high-density cultures (data not included).
Conditioned medium induced differentiation in progenitors in low-density culture
To determine the role of cell density and dose of conditioned medium on progenitor cell differentiation, cells plated at a density of 1 × 104 cells/cm2 in 25-cm flasks were exposed to serum-containing medium, serum-free medium, and RPE-conditioned medium (100%, 50%, 25%, and 12.5%).
Emerging phenotypes were scored by two independent investigators in a blind study. Data presented are from three to five flasks (700–800 cells scored/flask). The experiment was repeated three times. Cells were scored by the morphological criterion described in the “Materials and methods” sections. Additionally, 100–200 cells exposed to RPE-conditioned medium were immunophenotyped and scored for the presence of various retinal antigens. Photoreceptors (Fig. 5a, b, arrows) were identified by phase contrast microscopy on the basis of elongated shape, the presence of single short neurite and elongated axon. Also, apparent was the polarized appearance and characteristic structure at the distal position of the inner segment showing some microvilli.
It was easy to distinguish photoreceptors (arrows) from multipolar neurons (ganglion and other neurons). These neurons were identified by a large cell body and multiple processes. Bipolars mostly had a small rounded cell body, two very thin processes on both sides, as opposed to short neurites seen in cones and elongated slender shape of rod photoreceptors. The progenitor cells had very epithelial phenotype. The composite represents photoreceptors (rods and cones), multipolar neurons with multiple processes (ganglion and amacrine cells), and progenitors with flat epithelial cell morphology and lack of processes.
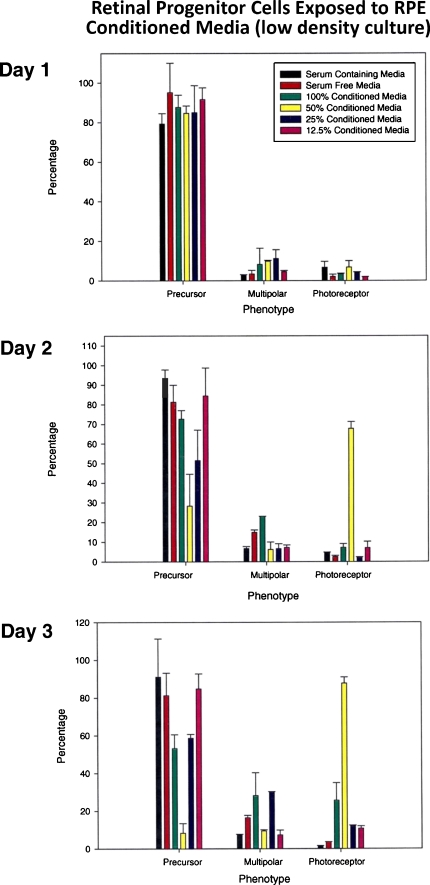
In the low-density culture exposed to conditioned medium, no significant differences in emerging phenotypes were noted on day 1 (progenitors vs. photoreceptors or multipolars). On day 2, between 24 and 48 h, there was a significant drop in the number of progenitors exposed to 50% conditioned medium, accompanied by a simultaneous increase in the number of cells with photoreceptor phenotype reaching as high as 68%. There was also a slight increase in the number of cells with multipolar neuronal phenotype in cultures exposed to 100% conditioned medium. Between days 2 and 3, the number of cells with photoreceptor phenotype had reached close to 90% in flasks exposed to 50% conditioned medium, with a corresponding drop in the number of cells with progenitor cell phenotype (Fig. 2).
Fig. 2.
Conditioned medium induced differentiation in retinal progenitors in low-density cultures. Retinal progenitors plated at a density of 1 × 104 cells/cm2 were treated with RPE-conditioned medium (100%, 50%, 25%, 12.5%) and analyzed on days 1–3 for differentiation. Seven hundred to 800 cells were scored for phenotypic analysis by phase microscopy/photomicrographs and analysis in a blind study by two investigators. Emerging phenotype grouped as progenitors, multipolars (ganglion and amacrine cells), and photoreceptors (rods and cones) as described in “Materials and methods” section and results. This figure is the composite of data collected. Note that no significant differences were noted on day 1. Note on day 2 that a drop in the number of progenitors is seen in cells treated with 50% conditioned medium accompanied by a simultaneous increase in the number of photoreceptors reaching as high as 70%. There was a slight increase in the number of cells with multipolar neuronal phenotype in cells exposed to 100% conditioned medium. On day 3, the number of cells with photoreceptor phenotype in cells treated with 50% conditioned medium was close to 90% with a simultaneous drop in the number of progenitors
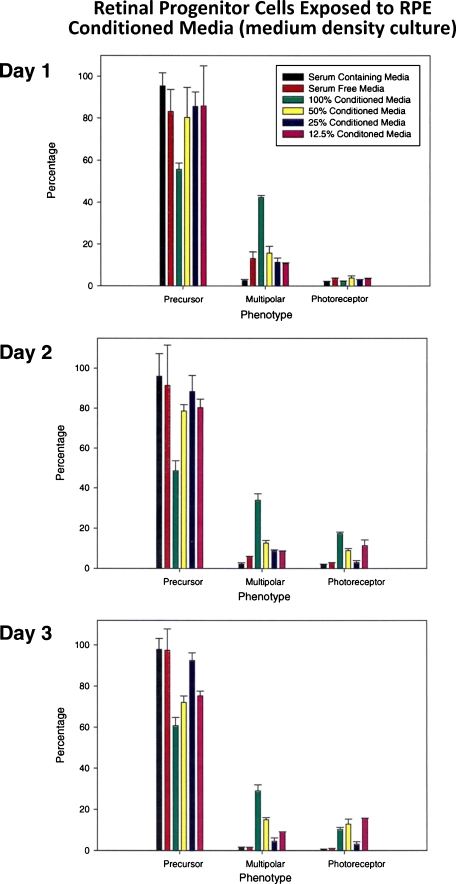
Medium-density cultures
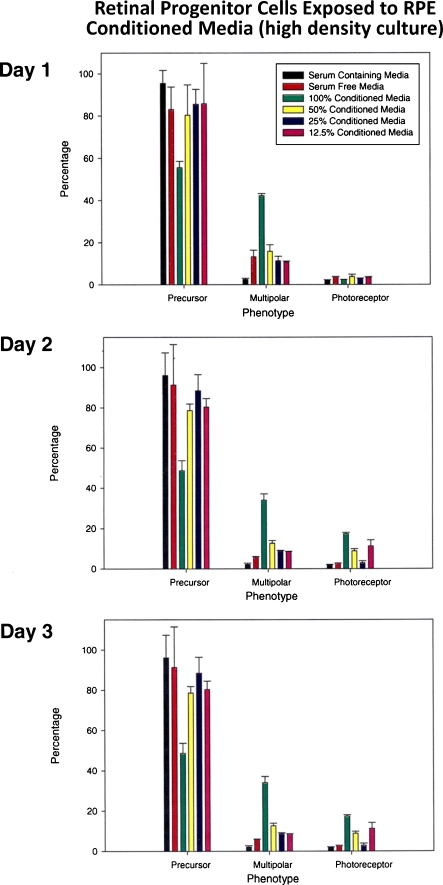
Medium density cells (4 × 104 cells/cm2) in cultures were exposed to various dilutions of RPE-conditioned medium and were scored for differentiation as in Fig. 2. There were no dramatic changes noted under various culture conditions between days 1 and 3 other than a slight increase in the number of cells with multipolar neuronal phenotype in cells exposed to 100% condition medium. The number of multipolar neurons was close to 40% on day 1, followed by a slight drop on days 2 and 3, suggesting that 100% conditioned medium along with some unknown factor made by progenitors themselves is inducing multipolar neuronal phenotype (possibly ganglionic or amacrine phenotype, Fig. 3). This increase in the number of cells with neuronal phenotype was accompanied by a drop in the number of progenitor cells in these flasks. Very similar results were noted in high-density cultures (1 × 105 cells/cm2) treated similarly (Fig. 4).
Fig. 3.
Conditioned medium induced differentiation in medium-density retinal cultures. Retinal cells plated at 4 × 104 cells/cm2 were treated with various dilutions of conditioned medium and assessed similar to Fig. 2. Note that at this cell density, no significant increase in the number of photoreceptors was seen in response to exposure to various dilutions of conditioned medium. However, cell exposure to 100% conditioned medium reflected an increase in the number of multipolar neurons reaching as high as 40% on day 1, with a simultaneous drop in the number of progenitors. This pattern was maintained on days 2 and 3 with a slight drop in the number of multipolars possibly reflecting cell death
Fig. 4.
Differentiation in response to conditioned medium in high-density cultures (1 × 105 cells/cm2). Note in cells exposed to 100% conditioned medium an increase in the number of multipolar neurons reaching as high as 40% on day 1 followed by a slight drop in numbers on days 2 and 3
Morphological phenotype of progenitors exposed to RPE-conditioned medium: scanning electron micrography
Figure 5a represents data on low-density cultures (1 × 104 cells/cm2) exposed to various dilutions of conditioned medium. Cells plated at 1 × 104 cells/cm2 were exposed to conditioned medium at various dilutions. The composite depicts scanning electron micrograph at 36–48 h post-treatment.
In Fig. 5a (a), note the epithelial cell-like morphology of the cells cultured in serum-free medium (arrowheads). Note in Fig. 5a (b, c) the multipolar neuronal phenotype of cells exposed to 100% conditioned medium (arrowheads). Note the photoreceptor-like phenotype with elongated axonal processes seen in cells plated at low density exposed to 50% and 25% conditioned medium (arrows). Progenitors are identified by arrowheads (Fig. 5a (a–g)).
In Fig. 5b, note the morphological analysis by phase micrography of cells plated at different cell densities. Cells were processed in a manner similar to Figs. 2, 3, 4, and 5a. Cells plated at low (1 × 104 cells/cm2), medium (4 × 104 cells/cm2), and high (1 × 105 cells/cm2) density were treated with 50% conditioned medium and 700–800 cells scored in the blind study. Note that the cell differentiation is indirectly proportional to cell density with the highest number of cells with neuronal phenotype seen at the lowest cell density (arrows; Fig. 5b (b, c)) and progenitors (arrowheads). This level of differentiation is followed by medium-density cultures (e, f), and there is hardly any differentiation at the higher cell density (h, i). Most cells have progenitor phenotype.
Immunophenotypic analysis of differentiated progenitors
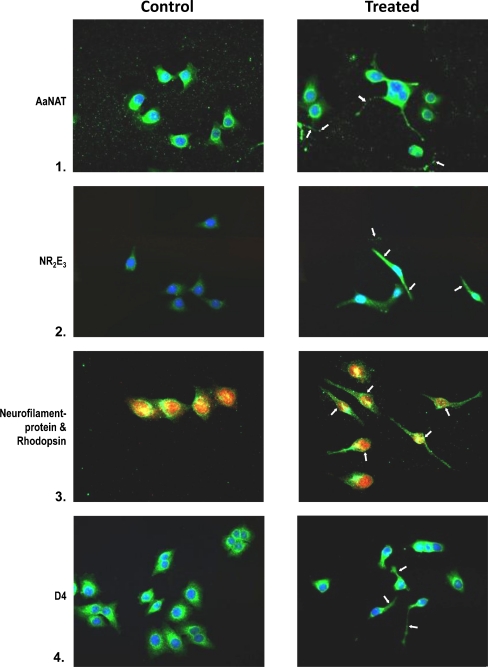
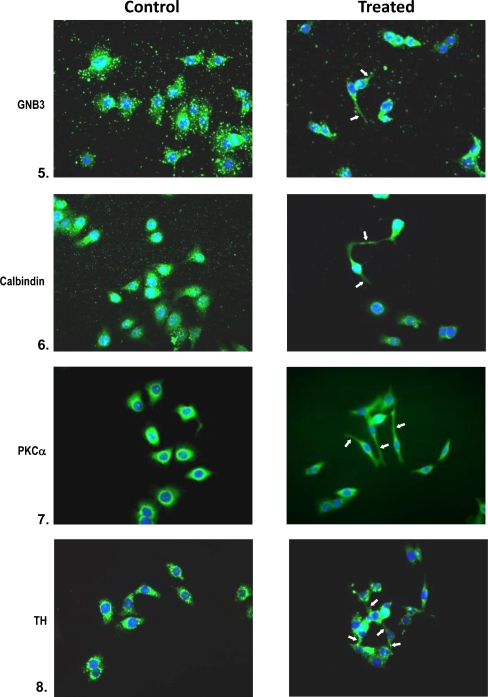
Immunophenotypic analysis of differentiated progenitors confirms differentiation seen in cells plated at low cell density (1 × 104 cells/cm2) in response to 50% conditioned medium. Cells grown in serum-free medium served as controls. Cells were immunophenotyped for the expression of several retinal specific proteins. Controls with the exclusion of primary and secondary antibody were also processed simultaneously; 50–100 cells were scored for each antibody. The composite reflects the overall pattern. Note the expression of AaNat in conditioned medium cultures. The arrows point to neuritic processes. Similarly, cells treated with conditioned medium are positive for calbindin, D4 receptor, rhodopsin, and Nr2e3. All are cell markers for photoreceptor cells. Also note the double-labeled cells which are positive for neurofilament protein (red) and rhodopsin (green) rod photoreceptors (Fig. 6). Note the bipolar phenotype of cells expressing PKCα (rod, cone bipolar) and the large cell body phenotype of cells expressing tyrosine hydroxylase, a marker for amacrine cells. Also, note the neuronal phenotype of cells expressing GNB3, a cone transducin. Most of the antigens are also expressed in serum-free cultures; however, differentiated neuronal phenotype is lacking, and the levels of antigens are also lower in these cultures.
Fig. 6.
Immunophenotyping of retinal progenitors exposed to 50% conditioned medium in low-density cultures on day 2. Controls are untreated cultures. Treated cells were analyzed for the expression of AaNat, Nr2e3, rhodopsin, GNB3, calbindin (rods, cones), PKCα (rod and cone bipolars), and TH (amacrine cells). Note in the composite the neuronal phenotype with extended neurites and axons in treated cultures (arrows). Note the photoreceptor phenotype (1, 2, 3, 4, 5). Note in 3 that photoreceptors are positive for neurofilament protein (red) and rhodopsin (green). Note the bipolars expressing PKCα in 7. In untreated cells, antigens were present but neuronal phenotype was missing
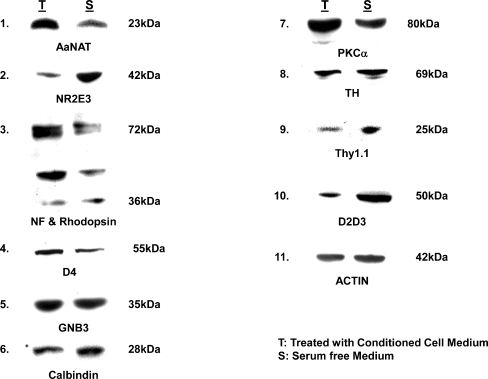
Western blot analysis
Western blot analysis performed on low-density cell cultures exposed to 50% RPE-conditioned medium confirms the results obtained by phenotypic analysis (morphological and immunophenotyping). Photoreceptor differentiation in response to conditioned medium is confirmed in western blot (Fig. 7) by upregulation of rhodopsin. Both monomeric and dimeric forms of rhodopsin are expressed. Slight upregulation of GNB3 (cone transducin) is also noted, and significant upregulation of PKCα expressed in both rod and cone bipolar is also seen. D4 receptors (photoreceptors) and AaNat expressed in rod photoreceptors, Nr2e3, a transcription factor for rod photoreceptor differentiation, were, however, expressed at higher levels in serum-free cultures. Similarly, D2D3 and thy 1.1 (ganglion cell markers) were expressed at a higher level in serum-free cultures. Actin levels were similar in RPE-conditioned medium-treated vs serum-free cultures.
Fig. 7.
Western blot analysis of retinal cells plated at low cell density (1 × 104 cells/cm2) were treated with 50% conditioned medium and assessed by western blot analysis for expression of retinal antigens, day 2. Note the significant upregulation of AaNat and rhodopsin in treated cultures confirming rod photoreceptor differentiation. Both monomeric and dimeric forms of rhodopsin are expressed. However, Nr2e3, a transcription factor for rod photoreceptors, is expressed at higher levels in the controls. A similar increase in treated cultures is seen in the expression of PKCα (expressed in rod and cone bipolars). Also, a slight increase in GNB3, a cone transducin, is noted. No dramatic differences were seen in the expression of other antigens. Actin levels in treated and untreated cultures were similar
Discussion
Interactions between photoreceptors and RPE cells are essential for the development, differentiation, and maintenance of visual function [46, 47]. There is an abundance of data that points to the fact that RPE or factors supplied by RPE are critical to the development and survival of photoreceptors; however, the nature of these interactions is poorly understood [48]. Several studies have reported mutations in RPE genes in several forms of retinopathies [48–50].
Several lines of evidence indicate that transplantation of both neural retina and RPE tissue improves vision in animals and humans, suggesting that combined transplantation of retinal progenitors and RPE cells could be more effective [51]. Akrami et al. [52] reported the plasticity of RPE cells to generate stem cells, which could be harvested for therapeutic purposes.
In addition to replacing photoreceptors, it should be possible to regenerate retina from retinal progenitors and from labile tissue such as RPE, which is being explored as a tissue for replacement by transdifferentiation into photoreceptors in vivo. Cultured RPE cells retain their remarkable regenerative capabilities. The potential of RPE toward transdifferentiation has been well documented. Cells guided to transdifferentiate along the photoreceptor pathway by bFGF and neuroD developed a highly ordered cellular structure and could integrate into the outer nuclear layer [53]. These data suggest that through genetic reprogramming, RPE cells could be a potential source of photoreceptor cells. Also, we had previously reported [41] that adult RPE cultures transdifferentiate into neural phenotype by H-ras (Val12) transfection.
Carr et al. [13] have shown that RPE induced from pluripotent stem cells upon transplantation perform the function of normal RPE in the Royal College of Surgeons rat model of retinal degeneration, at least for a short time. Additionally, Jablonski et al. [54] reported that the removal of RPE resulted in the aberrant assembly of photoreceptor out segments and subcellular structures which could be restored by the addition of PEDF.
Since RPE is being considered as one of the replacement tissues and since there are reports of varied RPE-secreted factors playing a role [55–57] in retinal differentiation and survival, we have explored a role that cell density might play in such rescue and differentiation. We report in this study that RPE-secreted factors enhance neuronal/photoreceptor differentiation and that (a) this differentiation is density dependent and (b) RPE might be secreting both photoreceptor differentiation and inhibition factors. We saw the highest degree of (photoreceptor) differentiation at 50% dilution of conditional medium.
Previously, Sheedlo et al. [58] reported that the mouse photoreceptor cell line GG1W [59] has the characteristics of retinal progenitor cells in which mature photoreceptors could be generated in response to bFGF, but the same was inhibited by NGF and RPE-secreted conditioned medium. Our results in more than one aspect complement their studies. However, in our retinal progenitor cell line, conditioned medium in low-density cultures enhanced neuronal photoreceptor differentiation at 50% dilution, suggesting that conditioned medium contains both stimulatory and inhibitory signals. The differences in our studies could be also due to species differences or developmentally restricted states of progenitor cell lines used in the two studies or culture conditions themselves. For example, the conditioned medium has multiple factors, and these various factors could be operating in opposing directions. The 50% dilution could have diluted the molecule that is inhibitory toward photoreceptor differentiation. Additionally, the cell density itself could play a role by progenitor cells themselves secreting growth factors.
Thus, our studies confirm some aspects of their [58] findings but differ in others. These differences could be related to differences in species. Their cell line is mouse-derived whereas ours is a human progenitor cell line. Secondly, their cell line was generated by transfection of SV-40T antigen whereas ours became spontaneously immortalized. Our studies confirm their previously published findings that bFGF enhances photoreceptor differentiation. We think that the neurotrophin most likely responsible for the increase in the number of photoreceptors in low-density cultures exposed to 50% conditioned medium is bFGF [10, 38, 60].
RPE has been shown to be a natural source of bFGF, and one of its trophic effects on retina may be to help maintain its normal structure. This effect has also been reported in retina–RPE aggregate culture, where the presence of RPE enforced proper retinal orientation [61]. More recently, German et al. [62] report that RPE is critical in defining spatial organization in retina. Data from several labs including ours [23, 34, 38, 63, 64] have shown that bFGF and TFGα could influence cell fate in uncommitted retinal precursors. Basic fibroblast growth factor has been identified in normal developing retina [65, 66].
We are cognizant of other reports where bFGF has been reported to have no effect on the appearance of the rod photoreceptors in an in vitro model. This discrepancy has been attributed to age of the tissue used or some other yet unidentified variable [67].
To summarize, our studies confirm and are closest to that of Sheedlo et al. [58]. However, in some aspects, our results on RPE-conditioned medium on photoreceptor differentiation differ from theirs. They did not find RPE-conditioned medium conducive to neuronal/photoreceptor differentiation. We have shown that RPE-conditioned medium enhances neuronal/photoreceptor differentiation in low-density culture at 50% dilution. Finally, we would like to point out that in cell transplantation studies where progenitors/RPE cells are being tested for their efficacy to give rise to photoreceptors, it might be worthwhile to explore how cell density could alter the outcome.
Acknowledgments
The authors thank Renarder Pressley for the excellent secretarial assistance, Mr. Patrick Abramson for the graphics, and Ms. Suzanne Alexander for the critical reading of the manuscript (Morehouse School of Medicine). We thank Mr. L. Brako for scanning electron micrographs (Morehouse School of Medicine). We thank Ms. T. Douglas-Williams, information specialist (Morehouse School of Medicine). We also thank Dr. Myrtle Thierry-Palmer, Dr. Sandra Harris-Hooker, and Dr. Marjorie Smith for their friendship and support. The work was supported by RCMI Grant 5 G12RR03034 (K.D.) and NASA NCC-11-112 (K.D.).
References
- 1.Rattner A, Nathans J. Macular degeneration: recent advances and therapeutic opportunities. Nat Rev Neurosci. 2006;7:860–872. doi: 10.1038/nrn2007. [DOI] [PubMed] [Google Scholar]
- 2.Kaplan HJ, Tezel TH, Berger AS, Wolf ML, Priore LV. Human photoreceptor transplantation in retinitis pigmentosa. A safety study. Arch Ophthalmol. 1997;115:1168–1172. doi: 10.1001/archopht.1997.01100160338012. [DOI] [PubMed] [Google Scholar]
- 3.Chacko DM, Rogers JA, Tuner JE, Ahmad I. Survival and differentiation of cultured retinal progenitors transplanted in the subretinal space of the rat. Biochem Biophys Res Commun. 2000;268:842–846. doi: 10.1006/bbrc.2000.2153. [DOI] [PubMed] [Google Scholar]
- 4.Radtke ND, Aramant RB, Seiler MJ, Petry HM, Pidwell D. Vision change after sheet transplant of fetal retina with retinal pigment epithelium to a patient with retinitis pigmentosa. Arch Ophthalmol. 2004;122:1159–1165. doi: 10.1001/archopht.122.8.1159. [DOI] [PubMed] [Google Scholar]
- 5.Binder S, Stanzel B, Krebs I, Glittenberg C. Transplantation of the RPE in AMD. Prog Retin Eye Res. 2007;26:516–554. doi: 10.1016/j.preteyeres.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 6.Joussen AM, Heussen FM, Joeres S, Llacer H, Prinz B, Rohrschneider K, et al. Autologous translocation of the choroid and retinal pigment epithelium in age-related macular degeneration. Am J Ophthalmol. 2006;142(1):17–30. doi: 10.1016/j.ajo.2006.01.090. [DOI] [PubMed] [Google Scholar]
- 7.MacLaren RE, et al. Retinal repair by transplantation of photoreceptor precursors. Nature. 2006;444:203–207. doi: 10.1038/nature05161. [DOI] [PubMed] [Google Scholar]
- 8.Osakada F, Ikeda H, Mandai M, Wataya T, Watanabe K, Yoshimura N, et al. Toward the generation of rod and cone photoreceptors from mouse, monkey and human embryonic stem cells. Nat Biotechnol. 2008;26:215–224. doi: 10.1038/nbt1384. [DOI] [PubMed] [Google Scholar]
- 9.West EL, Pearson RA, MacLaren RE, Sowden JC, Ali RR. Cell transplantation strategies for retinal repair. Prog Brain Res. 2009;175:3–21. doi: 10.1016/S0079-6123(09)17501-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dutt K, Harris-Hooker S, Ellerson D, Layne D, Kumar R, Hunt R. Generation of 3D retina-like structures from a human retinal cell line in a NASA bioreactor. Cell Transplant. 2003;12:717–731. doi: 10.3727/000000003108747334. [DOI] [PubMed] [Google Scholar]
- 11.Dutt K, Cao Y. Engineering retina from human retinal progenitors. Tissue Eng. 2009;15:1–13. doi: 10.1089/ten.tea.2007.0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lamba DA, Gust J, Reh TA. Transplantation of human embryonic stem cell derived photoreceptors restores some visual function in Crx-deficient mice. Cell Stem Cell. 2009;4:73–79. doi: 10.1016/j.stem.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carr AJ, Vugler AA, Hikita ST, Lawrence JM, Gias C, Chen LL, et al. Protective effects of human iPS-derived retinal pigment epithelium cell transplantation in the retinal dystrophic rat. PLoS One. 2009;4(12):e8152. doi: 10.1371/journal.pone.0008152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hinds JW, Hinds PL. Differentiation of photoreceptors and horizontal cells in the embryonic mouse retina: an electron microscopic, serial section analysis. J Comp Neurol. 1979;187:495–511. doi: 10.1002/cne.901870303. [DOI] [PubMed] [Google Scholar]
- 15.Watanabe T, Raff MC. Rod photoreceptor development in vitro intrinsic properties of proliferating neuroepithelial cells change as development proceeds in the rat retina. Neuron. 1990;4:461–467. doi: 10.1016/0896-6273(90)90058-n. [DOI] [PubMed] [Google Scholar]
- 16.Harris WA, Messersmith SL. Two cellular inductions involved in photoreceptor determination in the Xenopus retina. Neuron. 1992;9:357–372. doi: 10.1016/0896-6273(92)90174-c. [DOI] [PubMed] [Google Scholar]
- 17.Strauss O. The retinal pigment epithelium in visual function. Physiol Rev. 2005;85:845–881. doi: 10.1152/physrev.00021.2004. [DOI] [PubMed] [Google Scholar]
- 18.Wässle H, Boycott BB. Functional architecture of the mammalian retina. Physiol Rev. 1991;71:447–480. doi: 10.1152/physrev.1991.71.2.447. [DOI] [PubMed] [Google Scholar]
- 19.Raymond SM, Jackson IJ. The retinal pigmented epithelium is required for development and maintenance of the mouse neural retina. Curr Biol. 1995;5:1286–1295. doi: 10.1016/s0960-9822(95)00255-7. [DOI] [PubMed] [Google Scholar]
- 20.Levine EM, Fuhrmann S, Reh TA. Soluble factors and the development of rod photoreceptors. CMLS Cell Mol Life Sci. 2000;57:224–234. doi: 10.1007/PL00000686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adler R, Hatlee M. Plasticity and differentiation of embryonic retinal cells after terminal mitosis. Science. 1989;243:391–393. doi: 10.1126/science.2911751. [DOI] [PubMed] [Google Scholar]
- 22.Belecky-Adams T, Cook B, Adler R. Correlations between terminal mitosis and differentiated fate of retinal precursor cells in vivo and in vitro: analysis with the “window-labeling” technique. Dev Biol. 1996;178:304–315. doi: 10.1006/dbio.1996.0220. [DOI] [PubMed] [Google Scholar]
- 23.Ezeonu I, Wang M, Kumar R, Dutt K. Density-dependent differentiation in nontransformed human retinal progenitor cells in response to basic fibroblast growth factor- and transforming growth factor-alpha. DNA Cell Biol. 2003;22:607–620. doi: 10.1089/104454903770238085. [DOI] [PubMed] [Google Scholar]
- 24.Altshuler D, Cepko C. A temporally regulated, diffusible activity is required for rod photoreceptor development in vitro. Development. 1992;114:947–957. doi: 10.1242/dev.114.4.947. [DOI] [PubMed] [Google Scholar]
- 25.Lillien L, Cepko C. Control of proliferation in the retina: temporal changes in responsiveness to FGF and TGFα. Development. 1991;115:253–266. doi: 10.1242/dev.115.1.253. [DOI] [PubMed] [Google Scholar]
- 26.Reh TA. Cellular interactions determine neuronal phenotypes in rodent retinal cultures. J Neurobiol. 1992;23:1067–1083. doi: 10.1002/neu.480230811. [DOI] [PubMed] [Google Scholar]
- 27.Fuhrmann S, Kirsch M, Hofmann HD. Ciliary neurotrophic factor promotes chick photoreceptor development in vitro. Development. 1995;121:2695–2706. doi: 10.1242/dev.121.8.2695. [DOI] [PubMed] [Google Scholar]
- 28.Bugra K, Olivier L, Jacquemin E, Laurent M, Courtois Y, Hicks D. Acidic fibroblast growth factor is expressed abundantly by photoreceptors within the developing and mature rat retina. Eur J Neurosci. 1993;6:1586–1595. doi: 10.1111/j.1460-9568.1993.tb00228.x. [DOI] [PubMed] [Google Scholar]
- 29.Consigli SA, Lyser KM, Joseph-Silverstein J. The temporal and spatial expression of basic fibroblast growth factor during ocular development in the chicken. Invest Ophthalmol Vis Sci. 1993;34:559–566. [PubMed] [Google Scholar]
- 30.Iongh R, McAvoy JW. Spatio-temporal distribution of acidic and basic FGF indicates a role for FGF in rat lens morphogenesis. Dev Dynamics. 1993;198:190–202. doi: 10.1002/aja.1001980305. [DOI] [PubMed] [Google Scholar]
- 31.Gao H, Hollyfield JG. Basic fibroblast growth factor in retinal development: differential levels of bFGF expression and content in normal and retinal degeneration (rd) mutant mice. Dev Biol. 1995;169:168–184. doi: 10.1006/dbio.1995.1135. [DOI] [PubMed] [Google Scholar]
- 32.Wanaka A, Milbrandt J, Johnson EM. Expression of FGF receptor gene in rat development. Development. 1991;11:455–468. doi: 10.1242/dev.111.2.455. [DOI] [PubMed] [Google Scholar]
- 33.Patstone G, Pasquale EB, Maher PA. Different members of the fibroblast growth factor receptor family are specific to distinct cell types in the developing chicken embryo. Dev Biol. 1993;155:107–123. doi: 10.1006/dbio.1993.1011. [DOI] [PubMed] [Google Scholar]
- 34.Guillemot F, Cepko CL. Retinal fate and ganglion cell differentiation are potentiated by acidic FGF in an in vitro assay of early retinal development. Development. 1992;114:743–754. doi: 10.1242/dev.114.3.743. [DOI] [PubMed] [Google Scholar]
- 35.Pittack C, Grunwald BG, Reh AT. Fibroblast growth factors are necessary for neural retina but not pigmented epithelium differentiation in chick embryos. Development. 1997;124:805–816. doi: 10.1242/dev.124.4.805. [DOI] [PubMed] [Google Scholar]
- 36.Tcheng M, Oliver L, Courtois Y, Jeanny JC. Effects of exogenous FGFs on growth, differentiation, and survival of chick neural retina cells. Exp Cell Res. 1994;212:30–35. doi: 10.1006/excr.1994.1114. [DOI] [PubMed] [Google Scholar]
- 37.Sakaguchi DS, Janick LM, Reh TA. Basic fibroblast growth factor (FGF-2) induced transdifferentiation of retinal pigment epithelium: generation of retinal neurons and glia. Dev Dyn. 1997;209:387–398. doi: 10.1002/(SICI)1097-0177(199708)209:4<387::AID-AJA6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 38.Ezeonu I, Derrickson B, Dutt K. Cell fate decisions in a human retinal precursor cell line; basic fibroblast growth factor, and transforming growth factor-alpha-mediated differentiation. DNA Cell Biol. 2000;19:527–537. doi: 10.1089/104454900439764. [DOI] [PubMed] [Google Scholar]
- 39.Carwile ME, Culbert RB, Sturdivant RL, Kraft TW. Rod outer segment maintenance is enhanced in the presence of bFGF, CNTF and GDNF. Exp Eye Res. 1998;66:791–805. doi: 10.1006/exer.1998.0488. [DOI] [PubMed] [Google Scholar]
- 40.Lavail MM, Yasumura D, Matthes MT, Lau-Villacorta C, Unoki K, Sung CH, et al. Protection of mouse photoreceptors by survival factors in retinal degenerations. Invest Ophthalmol Vis Sci. 1998;39:592–602. [PubMed] [Google Scholar]
- 41.Dutt K, Scott M, Sternberg P, Linser P, Srinivasan A. Transdifferentiation of adult human pigment epithelium into retinal cells by transfection with an activated H-ras proto-oncogene. DNA Cell Biol. 1993;12:667–673. doi: 10.1089/dna.1993.12.667. [DOI] [PubMed] [Google Scholar]
- 42.Kumar R, Dutt K. Enhanced neurotrophin synthesis and molecular differentiation in non-transformed human retinal progenitor cells cultured in a rotating bioreactor. Tissue Eng. 2006;12:141–158. doi: 10.1089/ten.2006.12.141. [DOI] [PubMed] [Google Scholar]
- 43.Dutt K, Scott M, Wang M, Semple E, Sharma GP, Srinivasan A. Establishment of a human retinal cell line by transfection of SV40 T antigen gene with potential to undergo neuronal differentiation. DNA Cell Biol. 1994;13:909–921. doi: 10.1089/dna.1994.13.909. [DOI] [PubMed] [Google Scholar]
- 44.Dutt K, Ezeonu I, Scott M, Semple E, Srinivasan A. Proto-oncogene expression in cAMP and TPA-mediated neuronal differentiation in a human retinal cell line KGLDMSM. Curr Eye Res. 1996;15:477–485. doi: 10.3109/02713689609000759. [DOI] [PubMed] [Google Scholar]
- 45.Davis AA, Bernstein S, Bok D, Turner J, Nachtigal M, Hunt RC. A human retinal pigment epithelial cell line that retains epithelial characteristics after prolonged culture. Invest Ophthalmol Vis Sci. 1995;36:955–964. [PubMed] [Google Scholar]
- 46.Hollyfield J, Witkovsky P. Pigmented retinal epithelium involvement in photoreceptor development and function. J Exp Zool. 1974;189:357–378. doi: 10.1002/jez.1401890309. [DOI] [PubMed] [Google Scholar]
- 47.Rizzolo L. Polarity and the development of the outer blood retinal barrier. Histol Histopathol. 1997;12:1057–1067. [PubMed] [Google Scholar]
- 48.Cao W, Tombran-Tink J, Elias R, Sezate S, Mrazek D, et al. In vivo protection of photoreceptors from light damage by pigment epithelium-derived factor. Invest Ophthalmol Vis Sci. 2001;42:1646–1652. [PubMed] [Google Scholar]
- 49.Goliath R, Tombran-Tink J, Rodriques R, Chader GJ, Rajkumar R, Greenberg J. The gene for PEDF, a retinal growth factor, is a prime candidate for retinitis pigmentosa and is tightly linked to the RP13 locus on chromosome 17p13.3. Mol Vis. 1996;2:5. [PubMed] [Google Scholar]
- 50.Gaur VP, Liu Y, Turner JE. Retinal fate and ganglion cell differentiation are potentiated by acidic FGF in an in vitro assay of early retinal development. Development. 1997;114:743–754. doi: 10.1242/dev.114.3.743. [DOI] [PubMed] [Google Scholar]
- 51.Aramant RB, Seiler MJ. Transplanted sheets of human retina and retinal pigment epithelium develop normally in nude rats. Exp Eye Res. 2002;75:115–125. doi: 10.1006/exer.2002.2001. [DOI] [PubMed] [Google Scholar]
- 52.Akrami H, Soheili ZS, Khalooghi K, Ahmadieh H, Rezaie-Kanavi M, Samiei S, et al. Retinal pigment epithelium culture; a potential source of retinal stem cells. J Ophthalmic Vis Res. 2009;4(3):134–141. [PMC free article] [PubMed] [Google Scholar]
- 53.Yan RT, Wang SZ. Differential induction of gene expression by basic fibroblast growth factor and neuroD in cultured retinal pigment epithelial cells. Vis Neurosci. 2000;17:157–164. doi: 10.1017/s0952523800171172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jablonski MM, Tombran-Tink J, Mrazek AD, Lannaccone A. Pigment epithelium-derived factor supports normal development of photoreceptor neurons and opsin expression after retinal pigment epithelium removal. J Neurosci. 2000;20(19):7149–7157. doi: 10.1523/JNEUROSCI.20-19-07149.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liang L, Yan RT, Ma W, Zhang H, Wang SZ. Exploring RPE as a source of photoreceptors: differentiation and integration of transdifferentiating cells grafted into embryonic chick eyes. Invest Ophthalmol Vis Sci. 2006;47(11):5066–5074. doi: 10.1167/iovs.06-0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tezel TH, Priore LV, Berger AS, Kaplan HJ. Adult retinal pigment epithelial transplantation in exudative age-related macular degeneration. Am J Ophthalmol. 2007;143:584–595. doi: 10.1016/j.ajo.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 57.Ma Z, Han L, Wang C, Dou H, Hu Y, et al. Autologous transplantation of retinal pigment epithelium–Bruch’s membrane complex for hemorrhagic age-related macular degeneration. Invest Ophthalmol Vis Sci. 2009;50:2975–2981. doi: 10.1167/iovs.08-2573. [DOI] [PubMed] [Google Scholar]
- 58.Sheedlo HJ, Bartosh TJ, Wang Z, Srinivasan B, Brun-Zinkernagel AM, Roque RS. RPE-derived factors modulate photoreceptor differentiation: a possible role in the retinal stem cell niche. In Vitro Cell Dev Biol Animal. 2007;43:361–370. doi: 10.1007/s11626-007-9051-3. [DOI] [PubMed] [Google Scholar]
- 59.Al-Ubaidi M, Font RL, Quiambao AB, Keener MJ, Loiu GI, Overbeek PA, et al. Bilateral retinal and brain tumors in transgenic mice expressing simian virus 40 large T-antigen under control of the human interphotoreceptor retinoid-binding protein promoter. J Cell Biol. 1992;119:1681–1687. doi: 10.1083/jcb.119.6.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hicks D, Courtois Y. Fibroblast growth factor stimulates photoreceptor differentiation in vitro. J Neurosci. 1992;12:2022–2033. doi: 10.1523/JNEUROSCI.12-06-02022.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Layer PG, Willbold E. Regeneration of the avian retina by retinospheroid technology. Prog Ret Eye Res. 1994;13:197–229. [Google Scholar]
- 62.German OL, Buzzi E, Potstein NP, Rodriguez-Boulan E, Politi LE. Retinal pigment epithelial cells promote spatial reorganization and differentiation of retina photoreceptors. J Neurosci Res. 2008;86:3503–3514. doi: 10.1002/jnr.21813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Blanks JC, Barron E, Schmidt SY. bFGF enhanced photoreceptor differentiation in normal and rd mice in vitro: effects of cell density. Invest Ophthalmol Vis Sci. 1996;37:S436. [Google Scholar]
- 64.Plouet J. Molecular interaction of fibroblast growth factors light-activated rhodopsin and s-antigen. In: Piatigorsky J, Toshimichi S, Zelenka PS, editors. Molecular biology of the eye: genes, vision and ocular disease. New York: Liss; 1988. pp. 83–92. [Google Scholar]
- 65.Hageman GS, Kirchoff-Rempe MA, Lewis GP, Fisher SK, Anderson DH. Sequestration of basic fibroblast growth factor in the primate retinal interphotoreceptor matrix. Proc Natl Acad Sci USA. 1991;88:6706–6710. doi: 10.1073/pnas.88.15.6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Raymond PA, Bartheil LK, Rounsifer ME. Immunolocalization of basic fibroblast growth factor in its receptor in adult goldfish retina. Exp Neurol. 1992;115:73–78. doi: 10.1016/0014-4886(92)90225-f. [DOI] [PubMed] [Google Scholar]
- 67.Zhao S, Barnstable CJ. Differential effects of bFGF on development of the rat retina. Brain Res. 1996;723:169–176. doi: 10.1016/0006-8993(96)00237-5. [DOI] [PubMed] [Google Scholar]