Abstract
The Müller cell is the only glial cell type generated from the retinal neuroepithelium. This cell type controls normal retina homeostasis and has been suggested to play a neuroprotective role. Recent evidence suggests that mammalian Müller cells can de-differentiate and return to a progenitor or stem cell stage following injury or disease. In vivo exploration of the molecular mechanisms of Müller cell differentiation and proliferation will add essential information to manipulate Müller cell functions. Signal transduction pathways that regulate Müller cell responses and activity are a critical part of their cellular machinery. In this study, we focus on mitogen-activated protein kinase (MAPK) signaling pathway during Müller glial cell differentiation and proliferation. We found that both MAPK and STAT3 signaling pathways are present during Müller glial cell development. Ciliary neurotrophic factor (CNTF)-stimulated Müller glial cell proliferation is associated with early developmental stages. Specific inhibition of MAPK phosphorylation significantly reduced the number of Müller glial cells with or without CNTF stimulation. These results suggested that the MAPK signal transduction pathway is important in the formation of Müller glial cells during retina development.
Keywords: Müller cell, Neuroprotection, STAT3, Ciliary neurotrophic factor (CNTF), MAPK
Introduction
How cell differentiation is specified during development of nervous systems remains a central question of developmental neurobiology [1, 2]. It is believed that extracellular factors, which bind to cell surface receptors and generate signals for regulation of intrinsic factors, have a decisive role in cell specification [3]. For example, in Drosophila eye development, growth factors and their receptors activate the RAS-MAP kinase pathway, which plays an essential role in specification of photoreceptors [4]. However, it remains to be shown whether any signaling pathway initiated by growth factors plays a critical role during vertebrate retina development.
The vertebrate retina has frequently been used as a model for studies of cell fate determination during neural development. Pioneering lineage analysis studies have shown that a common pool of dividing retinal progenitor cells becomes progressively restricted in their potential for differentiation during development [1, 5–7]. For example, one late pool of precursor cells present in perinatal mouse retina can give rise to rod photoreceptors, bipolar cells and Müller glial cells [1, 6]. There is also evidence that extracellular factors, in collaboration with intrinsic factors, determine retinal cell specification [8–13]. Although no factor has yet been isolated that can direct a progenitor cell into a specific retinal cell fate, it has been shown that ciliary neurotrophic factor (CNTF) and leukemia inhibitory factor (LIF) can specifically block the production of rod photoreceptors in both mice and chicks [14–16]. However, it is not understood how these cytokines control cell specification, particularly what pathway is used use to transduce their signal into the cell.
The signal transducer and activator of transcription (STAT) proteins were initially discovered from interferon-stimulated transcriptional complexes [17]. They contain an SH2 domain and are phosphorylated directly by protein tyrosine kinases [18, 19]. After phosphorylation, the activated STAT proteins dimerize in the cytoplasm and then translocate to the nucleus where they can modulate transcription [20]. It has been shown that many cytokines and growth factors can activate the STAT signaling pathway, and STAT proteins have essential functions in cell growth, differentiation, and survival [21–24]. Cytokines of the IL-6 family, including CNTF and LIF, activate both STAT3 and MAPK in their signal transduction through gp130 family common receptors [25–29]. STAT3 has been characterized as a regulator for glial differentiation [25, 27].
We have shown that STAT3 is expressed and activated during mouse retina development [30], and STAT3 but not MAPK have the respective roles on rod photoreceptor fate determination [31]. Although STAT3 is sufficient to control the transition into a rod differentiation pathway, CNTF and LIF also activate MAPK. The function of the MAPK pathway in retinal development has not been well documented.
Here, we report that MAPK plays a significant role in Müller glial cell defferentiation within the retina. The actions of MAPK in Müller glial cell differentiation and proliferation are stage-dependent. The results strongly support a model whereby activation of the MAPK signaling pathway can promote the entry of progenitors into a Müller glial cell differentiation pathway during embryonic stages but not later postnatal stages of retina development.
Materials and methods
Reagents
Recombinant rat CNTF was purchased from R&D Systems (Minneapolis, MN) and MAPK inhibitor, PD98059 [32], from Cell Signaling. Anti-STAT3 (C20) polyclonal antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-p44/p42 MAPK (P-Tyr185/P-Thr183) polyclonal antibodies were purchased from Cell Signaling (Billerica, MA). Anti-2A1 monoclonal antibody recognizes against filamentous components specifically in Müller cells [33].
Animals and retina explant cultures
Timed-pregnant CD-1 mice were purchased from Charles River Laboratories (Boston, MA). All procedures of animal used were approved by Penn State Hershey IACUC. Most of the litters were born on E20, which was considered equivalent to postnatal day 0 (PN0). Embryos were dissected in cooled phosphate-buffered saline (PBS) for retina isolation. Whole retinas were isolated from embryos or postnatal mice, respectively [13, 30, 31, 34]. Retinas were cultured individually in 1 ml basal medium (UltraCulture™, Cambrex Bio Science Rockland, ME) without serum supplement as described previously [13]. Every 2 days, half of the total medium was replaced by fresh medium. CNTF and PD98059 were added at E17.5 or PN1 as indicated in the text. CNTF was dissolved in basal medium and PD98059 was dissolved in 0.01% dimethyl sulfoxide (DMSO).
Histology and immunohistochemistry
Explanted retinas were fixed with 4% paraformaldehyde in PBS for at least 12 h at 4°C. After three washes with PBS, fixed explants were dehydrated through a series of graded ethanols and embedded in paraffin. All samples for one experiment were placed in the same block and sectioned for immunohistochemistry or in situ hybridization. A standard immunohistochemistry protocol [30, 31] was employed for single or double staining using peroxidase-conjugated (Vector Laboratory, Burlingame, CA) or fluorescein-conjugated (Jackson Immuno-Research Laboratory, West Grove, PA) secondary antibodies. Stained sections were imaged by using Olympus microscopes equipped with digital cameras or an Olympus FluoView FV1000 confocal microscope.
Statistical analysis
Student’s t test was used for the statistical analysis between control and experimental groups, p < 0.05 will be considered as significant differences.
Results
STAT3 and MAPK are expressed in retina Müller glial cells
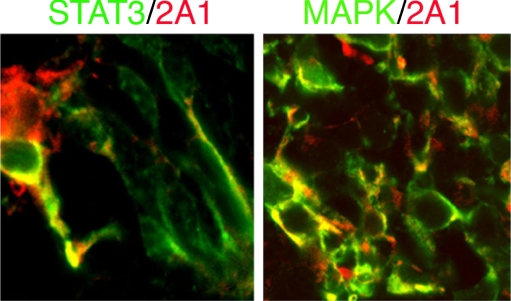
2A1 is a monoclonal antibody that reacts with intermediate filament components in Müller cells and shows no overlap in staining with GFAP positive astrocytes [33]. Double staining of 2A1 with STAT3 or MAPK (pp44/42) on sections from explant cultures from E17.5 retinas for 7 days showed co-localization of these two molecules in the same cells, respectively (Fig. 1). The overlap signaling from both molecules can be identified in the soma and dendrite of Müller glial cells. This result indicated that both STAT3 and MAPK signaling pathways are present during Müller glial cell development.
Fig. 1.
Confocal imaging of Müller glial cells from cultured retina explants. 2A1-positive cells (red) are co-localized with STAT3 (green, left panel) and MAPK (green, right panel) signaling pathways. Co-localization presented as yellow, ×40 in magnification
CNTF stimulated Müller glial proliferation is dependent on developmental stage
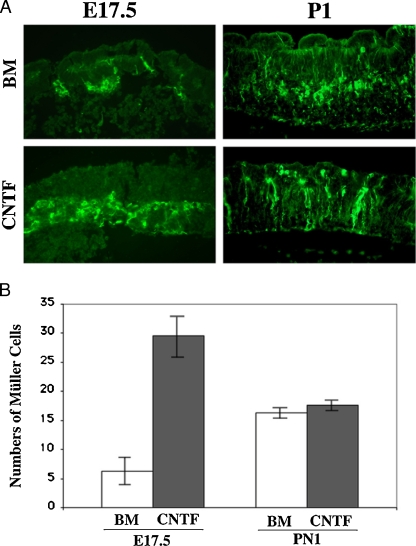
The numbers of Müller glial cells increased after CNTF treatment in E17.5 cultured retina explants. However, it presented a different pattern when we treated the retina explants from postnatal stages. As shown in Fig. 2, we have compared two stages, using retina explants from embryonic day 17.5 (E17.5) and PN1 to examine the numbers of MAPK positive cells treated with or without CNTF, respectively. We found that a significantly elevated the numbers of MAPK positive cells in the explant culture from E17.5 retinas (Fig. 2a, b, p < 0.05, n = 6), but there are no differences in the numbers of MAPK positive cells in the explants cultured from PN1 retinas (Fig. 2a, b). This result indicated that activation of MAPK by CNTF increases Müller glial cell productions, but it is dependent on the developmental stages.
Fig. 2.
Stage-dependent CNTF-induced Müller glial cell proliferation during retina development. a Fluorescent images with pp44/42 staining (green) from E17.5 (left panels) and PN1 (right panels) retinas cultured to postnatal day 7 with (lower panels) or without (upper panels) CNTF application. BM basal medium; ×20 in magnification; b Bar graphs show average numbers of Müller glial cells with or without CNTF stimulation. A significantly increase of Müller glial cell numbers are examined in the E17.5 groups after CNTF treatment (p < 0.05, n = 6), while no differences are found in PN1 groups (p > 0.05, n = 6)
MAPK inhibitor blocks both normal Müller glial development and CNTF-induced Müller glial proliferation
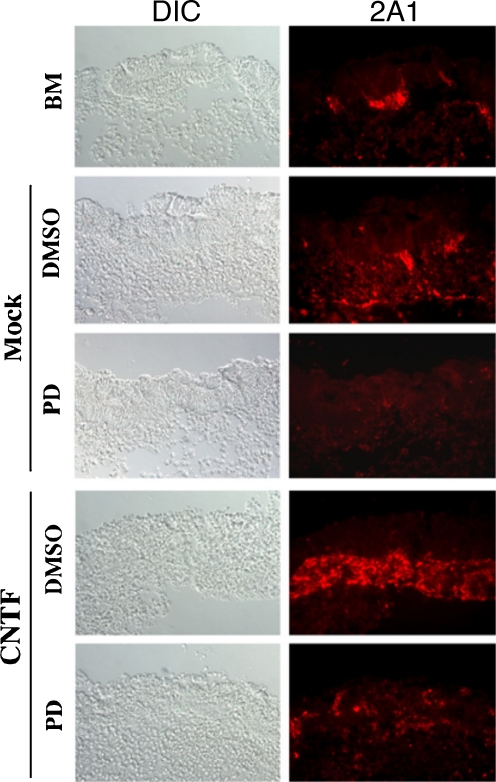
PD98059 is a specific MAPKK inhibitor and pretreatment of the prenatal retina explants with PD98059 specifically diminished MAPK function to monitor the changes of 2A1-positive Müller glial cells with or without CNTF treatment. As shown in Fig. 3, we found that reduction of 2A1-positive cell numbers was consistently with the decreasing of MAPK (pp44/42) expression levels (data not shown) in both CNTF treated or untreated groups compared with their mock DMSO controls. This result indicated that MAPK (pp44) is a critical signaling molecule for 2A1-positive Müller glial cell differentiation.
Fig. 3.
Treatment with MAPK inhibitor PD98059 reduced Müller glial cell numbers in retinal explant cultures with or without CNTF application. Microscopic images show differential interference contrast (DIC, left panels) and fluorescence 2A1 staining (red, right panels). BM basal medium; PD PD98059; DMSO dimethyl sulfoxide, ×20 in magnification
Discussion
In this study, we have shown that MAPK (pp44/42) is an important mediator for Müller glial development in mammalian retina. In previous studies, we showed that CNTF directly stimulates STAT3 and MAPK (pp44/42) cascades in the retina explant cultures [31, 35]. STAT3 but not MAPK (pp44/42) activation is responsible for the inhibitory role of CNTF on rod differentiation [31]. In additional studies, we and other groups have obtained results strongly suggesting that STAT3 signaling is an underlying component of neural responsiveness to stress stimuli, especially in retina ganglion cells [36, 37].
Müller glial cells are among the last neural cells to be born during retina development. CNTF has been shown to cause a small increase of Müller glial cell numbers in a rat model [14]. Later in a mouse model, Goureau et al. showed that CNTF promoted Müller glial differentiation from the postnatal retinal progenitor pool [38]. In our study, we showed that the numbers of Müller glial cells were only slightly increased by CNTF in explant cultures from PN1 retina, in agreement with the results from rat model [14]. However, we found that a significant increase in the number of Müller glial cells induced by CNTF in explant cultures from embryonic day 17.5 (E17.5) retina. Although the differences could be caused by the use of different Müller glial cell markers or different animal models, we suggest that CNTF trigger more significant changes in Müller cell production in early developmental stages.
Previous studies have shown that perturbation of STAT and MAPK (pp44/42) signaling using protein kinase inhibitors and a dominant-negative STAT3 mutant demonstrates that both CNTF-induced STAT and MAPK (pp44/42) (ERK) activation are involved in promoting Müller cell production [38]. In this study, we co-localized MAPK (pp44/42) with a specific marker 2A1 [33] but not a marker of activated Müller cells, GFAP [14]. We found that MAPK (pp44/42) was exactly co-localized with the 2A1 marker by confocal imaging and the changes of MAPK (pp44/42) expression induced by stimulating with CNTF also corresponded to the changes of 2A1. Furthermore, a MAPK (pp44/42) inhibitor PD98059 inhibited both MAPK (pp44/42) activation and 2A1 expression, suggesting that MAPK (pp44/42) is a crucial factor for Müller cell production and itself can be a useful marker for developing Müller glial cells.
Recent evidence suggests that some retina Müller cells can de-differentiate and return to a progenitor or stem cell stage [39]. MAPK signaling has been shown to be important for Müller cell differentiation and proliferation in other systems and following retinal injury. MAPK signaling can stimulate Müller glial to proliferate in acutely damaged chicken retina in response to fibroblast growth factor [40]. Recent studies by the same group showed that chick Notch is downstream of FGF/MAPK signaling to drive the proliferation of Müller glial cells in response to stress [41]. Further defining the activators and targets of MAPK signaling in mammals following injury is crucial to develop a full understanding of Müller glial de-differentiation and subsequent fate choices.
Acknowledgments
We acknowledge support from the NIH (CJB), the Macula Vision Research Foundation (CJB), the Pennsylvania State Sight Conservation & Eye Research Fund (SSZ), and an Eye and Vision Research Award of Penn State Hershey Eye Center (SSZ).
References
- 1.Livesey FJ, Cepko CL. Vertebrate neural cell-fate determination: lessons from the retina. Nat Rev Neurosci. 2001;2:109–118. doi: 10.1038/35053522. [DOI] [PubMed] [Google Scholar]
- 2.McKay R. Stem cells and the cellular organization of the brain. J Neurosci Res. 2000;59:298–300. doi: 10.1002/(SICI)1097-4547(20000201)59:3<298::AID-JNR2>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 3.Segal RA, Greenberg ME. Intracellular signaling pathways activated by neurotrophic factors. Annu Rev Neurosci. 1996;19:463–489. doi: 10.1146/annurev.ne.19.030196.002335. [DOI] [PubMed] [Google Scholar]
- 4.Dominguez M, Wasserman JD, Freeman M. Multiple functions of the EGF receptor in Drosophila eye development. Curr Biol. 1998;8:1039–1048. doi: 10.1016/S0960-9822(98)70441-5. [DOI] [PubMed] [Google Scholar]
- 5.Holt CE, Bertsch TW, Ellis HM, Harris WA. Cellular determination in the Xenopus retina is independent of lineage and birth date. Neuron. 1988;1:15–26. doi: 10.1016/0896-6273(88)90205-X. [DOI] [PubMed] [Google Scholar]
- 6.Turner DL, Cepko CL. A common progenitor for neurons and glial persists in rat retina late in development. Nature. 1987;328:131–136. doi: 10.1038/328131a0. [DOI] [PubMed] [Google Scholar]
- 7.Wetts R, Fraser SE. Multipotent precursors can give rise to all major cell types of the frog retina. Science. 1988;239:1142–1145. doi: 10.1126/science.2449732. [DOI] [PubMed] [Google Scholar]
- 8.Altshuler D, Cepko C. A temporally regulated, diffusible activity is required for rod photoreceptor development in vitro. Development. 1992;114:947–957. doi: 10.1242/dev.114.4.947. [DOI] [PubMed] [Google Scholar]
- 9.Austin CP, Feldman DE, Ida JA, Jr, Cepko CL. Vertebrate retinal ganglion cells are selected from competent progenitors by the action of Notch. Development. 1995;121:3637–3650. doi: 10.1242/dev.121.11.3637. [DOI] [PubMed] [Google Scholar]
- 10.Hicks D, Courtois Y. Fibroblast growth factor stimulates photoreceptor differentiation in vitro. J Neurosci. 1992;12:2022–2033. doi: 10.1523/JNEUROSCI.12-06-02022.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelley MW, Turner JK, Reh TA. Regulation of proliferation and photoreceptor differentiation in fetal human retinal cell cultures. Invest Ophthalmol Vis Sci. 1995;36:1280–1289. [PubMed] [Google Scholar]
- 12.Watanabe T, Raff MC. Diffusible rod-promoting signals in the developing rat retina. Development. 1992;114:899–906. doi: 10.1242/dev.114.4.899. [DOI] [PubMed] [Google Scholar]
- 13.Zhao S, Barnstable CJ. Differential effects of bFGF on development of the rat retina. Brain Res. 1996;723:169–176. doi: 10.1016/0006-8993(96)00237-5. [DOI] [PubMed] [Google Scholar]
- 14.Ezzeddine ZD, Yang X, DeChiara T, Yancopoulos G, Cepko CL. Postmitotic cells fated to become rod photoreceptors can be respecified by CNTF treatment of the retina. Development. 1997;124:1055–1067. doi: 10.1242/dev.124.5.1055. [DOI] [PubMed] [Google Scholar]
- 15.Kirsch M, Lee MY, Meyer V, Wiese A, Hofmann HD. Evidence for multiple, local functions of ciliary neurotrophic factor (CNTF) in retinal development: expression of CNTF and its receptors and in vitro effects on target cells. J Neurochem. 1997;68:979–990. doi: 10.1046/j.1471-4159.1997.68030979.x. [DOI] [PubMed] [Google Scholar]
- 16.Neophytou C, Vernallis AB, Smith A, Raff MC. Muller-cell-derived leukaemia inhibitory factor arrests rod photoreceptor differentiation at a postmitotic pre-rod stage of development. Development. 1997;124:2345–2354. doi: 10.1242/dev.124.12.2345. [DOI] [PubMed] [Google Scholar]
- 17.Fu X-Y, Schindler C, Improta T, Aebersold R, Darnell JE. The proteins of ISGF-3, the interferon a-induced transcriptional activator, define a gene family involved in signal transduction. Proc. Natl. Acad. Sci. USA. 1992;89:7840–7843. doi: 10.1073/pnas.89.16.7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu XY. A transcription factor with SH2 and SH3 domains is directly activated by an interferon alpha-induced cytoplasmic protein tyrosine kinase(s) Cell. 1992;70:323–335. doi: 10.1016/0092-8674(92)90106-M. [DOI] [PubMed] [Google Scholar]
- 19.Schindler C, Fu XY, Improta T, Aebersold R, Darnell JE., Jr Proteins of transcription factor ISGF-3: one gene encodes the 91-and 84-kDa ISGF-3 proteins that are activated by interferon alpha. Proc Natl Acad Sci USA. 1992;89:7836–7839. doi: 10.1073/pnas.89.16.7836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Darnell JE, Jr, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 21.Chin YE, Kitagawa M, Su WC, You ZH, Iwamoto Y, Fu XY. Cell growth arrest and induction of cyclin-dependent kinase inhibitor p21 WAF1/CIP1 mediated by STAT1. Science. 1996;272:719–722. doi: 10.1126/science.272.5262.719. [DOI] [PubMed] [Google Scholar]
- 22.Darnell JE., Jr STATs and gene regulation. Science. 1997;277:1630–1635. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- 23.Fu X-Y. From PTK-STAT signaling to caspase expression and apoptosis induction. Cell Death Differ. 1999;6:1201–1208. doi: 10.1038/sj.cdd.4400613. [DOI] [PubMed] [Google Scholar]
- 24.Leonard WJ, O'Shea JJ. Jaks and STATs: biological implications. Annu Rev Immunol. 1998;16:293–322. doi: 10.1146/annurev.immunol.16.1.293. [DOI] [PubMed] [Google Scholar]
- 25.Bonni A, Sun Y, Nadal-Vicens M, Bhatt A, Frank DA, Rozovsky I, Stahl N, Yancopoulos GD, Greenberg ME. Regulation of gliogenesis in the central nervous system by the JAK-STAT signaling pathway. Science. 1997;278:477–483. doi: 10.1126/science.278.5337.477. [DOI] [PubMed] [Google Scholar]
- 26.Hirano T. Interleukin 6 and its receptor: ten years later. Int Rev Immunol. 1998;16:249–284. doi: 10.3109/08830189809042997. [DOI] [PubMed] [Google Scholar]
- 27.Rajan P, McKay RD. Multiple routes to astrocytic differentiation in the CNS. J Neurosci. 1998;18:3620–3629. doi: 10.1523/JNEUROSCI.18-10-03620.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taga T, Kishimoto T. GP130 and the interleukin-6 family of cytokines. Annu Rev Immunol. 1997;15:797–819. doi: 10.1146/annurev.immunol.15.1.797. [DOI] [PubMed] [Google Scholar]
- 29.Takeda K, Akira S. STAT family of transcription factors in cytokine-mediated biological responses. Cytokine Growth Factor Rev. 2000;11:199–207. doi: 10.1016/S1359-6101(00)00005-8. [DOI] [PubMed] [Google Scholar]
- 30.Zhang SS, Wei J, Li C, Barnstable CJ, Fu XY. Expression and activation of STAT proteins during mouse retina development. Exp Eye Res. 2003;76:421–431. doi: 10.1016/S0014-4835(03)00002-2. [DOI] [PubMed] [Google Scholar]
- 31.Zhang SS, Wei J, Qin H, Zhang L, Xie B, Hui P, Deisseroth A, Barnstable CJ, Fu XY. STAT3-mediated signaling in the determination of rod photoreceptor cell fate in mouse retina. Invest Ophthalmol Vis Sci. 2004;45:2407–2412. doi: 10.1167/iovs.04-0003. [DOI] [PubMed] [Google Scholar]
- 32.Pang L, Sawada T, Decker SJ, Saltiel AR. Inhibition of MAP kinase kinase blocks the differentiation of PC-12 cells induced by nerve growth factor. J Biol Chem. 1995;270:13585–13588. doi: 10.1074/jbc.270.36.21040. [DOI] [PubMed] [Google Scholar]
- 33.Drager UC, Edwards DL, Barnstable CJ. Antibodies against filamentous components in discrete cell types of the mouse retina. J Neurosci. 1984;4:2025–2042. doi: 10.1523/JNEUROSCI.04-08-02025.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sparrow JR, Hicks D, Barnstable CJ. Cell commitment and differentiation in explants of embryonic rat neural retina. Comparison with the developmental potential of dissociated retina. Brain Res Dev Brain Res. 1990;51:69–84. doi: 10.1016/0165-3806(90)90259-2. [DOI] [PubMed] [Google Scholar]
- 35.Zhang SS, Liu MG, Kano A, Zhang C, Fu XY, Barnstable CJ. STAT3 activation in response to growth factors or cytokines participates in retina precursor proliferation. Exp Eye Res. 2005;81:103–115. doi: 10.1016/j.exer.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 36.Peterson WM, Wang Q, Tzekova R, Wiegand SJ. Ciliary neurotrophic factor and stress stimuli activate the Jak-STAT pathway in retinal neurons and glia. J Neurosci. 2000;20:4081–90. [DOI] [PMC free article] [PubMed]
- 37.Zhang C, Li H, Liu MG, Kawasaki A, Fu XY, Barnstable CJ, Zhang SS. STAT3 activation protects retinal ganglion cell layer neurons in response to stress. Exp Eye Res. 2008;86:991–997. doi: 10.1016/j.exer.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 38.Goureau O, Rhee KD, Yang XJ. Ciliary neurotrophic factor promotes muller glial differentiation from the postnatal retinal progenitor pool. Dev Neurosci. 2004;26:359–370. doi: 10.1159/000082278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fischer AJ, Omar G. Transitin, a nestin-related intermediate filament, is expressed by neural progenitors and can be induced in Müller glial in the chicken retina. J Comp Neurol. 2005;484:1–14. doi: 10.1002/cne.20406. [DOI] [PubMed] [Google Scholar]
- 40.Fischer AJ, Scott MA, Ritchey ER, Sherwood P. Mitogen-activated protein kinase-signaling regulates the ability of Müller glial to proliferate and protect retinal neurons against excitotoxicity. Glia. 2009;57:1538–1552. doi: 10.1002/glia.20868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghai K, Zelinka C, Fischer AJ. Notch signaling influences neuroprotective and proliferative properties of mature Müller glia. J Neurosci. 2010;30:3101–3112. doi: 10.1523/JNEUROSCI.4919-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]