Abstract
Purpose
Our study aimed to profile the effect of fasting during the Ramadan month on cognitive function in a group of healthy Muslim athletes.
Methods
Eighteen male athletes underwent computerized neuropsychological testing during (fasting) and after (non-fasting) Ramadan. Diet was standardized, and tests were performed at 0900h and 1600h to characterize potential time-of-day (TOD) interactions. Psychomotor function (processing speed), vigilance (visual attention), visual learning and memory, working memory (executive function), verbal learning and memory were examined. Capillary glucose, body temperature, urine specific gravity, and sleep volume were also recorded.
Results
Fasting effects were observed for psychomotor function (Cohen's d=1.3, P=0.01) and vigilance (d=0.6, P=0.004), with improved performance at 0900h during fasting; verbal learning and memory was poorer at 1600h (d=-0.8, P=0.03). A TOD effect was present for psychomotor function (d=-0.4, P<0.001), visual learning (d=-0.5, P=0.04), verbal learning and memory (d=-1.3, P=0.001), with poorer performances at 1600h. There was no significant fasting effect on visual learning and working memory.
Conclusions
Our results show that the effect of fasting on cognition is heterogeneous and domain-specific. Performance in functions requiring sustained rapid responses was better in the morning, declining in the late afternoon, whereas performance in non-speed dependent accuracy measures was more resilient.
Keywords: Psychomotor Function, Cognition, Ramadan Fasting, Memory
INTRODUCTION
Fasting is a practice that is observed by many cultures and religions. In Islam, during the month of Ramadan, able-bodied Muslims abstain from food and fluid for 12-14 hours daily. They rise early for prayers, eat before sunrise, retire later and consume large meals after sunset to replenish energy and fluid levels. This leads to alterations in feeding habits, sleep duration, pattern and architecture [1,2]. As major sporting calendars do not take religious observances into account, many athletes continue to train or compete while fasting.
Well-documented effects of Ramadan fasting include changes in circadian rhythms [3,4], physiological [5], metabolic and endocrine function [6–8], as well as reductions in daytime hydration, blood glucose and body temperature[6,9,10]. Physical and cognitive performances are also affecte. [11].
Cognition allows the athlete to pay attention, learn, remember new skills and solve problems. It enables the athlete to react appropriately to stimuli, and is crucial in disciplines such as shooting, martial arts, road cycling and motorsports, where impairment can change the outcome of a contest or result in injury. Several studies have demonstrated that psychomotor performance, subjective alertness and memory are adversely affected during Ramadan [10,12–14]. However, evidence also exists regarding the relative robustness of memory, attention, executive function, information processing and verbal function during fasting [15–19]. The conflicting findings may be due to methodological differences and the unavailability of sensitive computerised neuropsychological instruments in some studies.
Cognitive function is also subject to the influence of circadian rhythms, with performance fluctuating over the day [4,9,20,21]. Thus far, to the best of our knowledge, only two published studies on cognitive performance during fasting have considered this [10,22].
Meal timing and macronutrient composition can also affect cognitive function [23,24]. They may either impair reaction time and attention or improve memory and learning ability [23,25]. High protein meals can slow memory scanning and increase susceptibility to distraction [23]. The effects are attributed to diet-induced changes in neurotransmitter activity, sympathetic activation, hypothalamic-pituitary-adrenal axis function and serum glucocorticoid levels [24].
Hence, our study was designed to examine the effect of fasting during Ramadan on cognitive function, taking into account the influence of circadian rhythms, meal composition and timing. We posit that the alterations in feeding and sleep schedules may affect cognitive function via changes in blood glucose levels, body temperature and circadian rhythms.
METHODS AND SUBJECTS
Subjects
Eighteen healthy male volunteers were recruited from the national pencak silat squad. Pencak silat is a traditional Southeast Asian martial art form that is currently practised worldwide with regular sanctioned competitions. The participants’ weekly training volume during Ramadan [8.9 (±2.8) hours; range, 3-14 hours] was not significantly different from the non-Ramadan period (P=0.8). To enhance external validity, only Muslim athletes with previous fasting experience during Ramadan were selected. Written informed consent was obtained and the study conducted in 2009 after approval by the Ethics Committee of the Singapore Sports Council.
Experimental procedure
To characterize potential interactions between the time-of-day (TOD) and fasting, testing was carried out at two diurnal time points (0900h and 1600h), with each participant attending two sessions during (fasting) and two sessions after Ramadan (non-fasting) (Fig. 1). To minimize any potential carryover effect of Ramadan fasting, the non-fasting sessions were conducted two weeks after the end of Ramadan. Participants served as their own controls. Familiarization with all test procedures was performed before Ramadan, and the test sequence was identical throughout all sessions. To minimize fatigue, each session for the same participant was separated by an interval of at least 24 hours.
Fig. 1.
Study design
Meal schedule and composition
A standardized meal with 2g of carbohydrate/kg body mass, 0.5g of protein/kg body mass and 200ml of fluid was consumed between 0530h–0630h for the 0900h test sessions and between 1230h–1330h for the non-Ramadan 1600h session. The menu was identical throughout the experiment. Outside of the test occasions, the athletes were allowed to consume a free diet. However, they had to inform the investigators if they were ill or consuming medication, and they were not allowed to consume caffeinated products, sedatives, stimulants, alcohol or smoke in the 24 hours prior to testing.
Protocol and physiological measurements
Upon arrival at the laboratory, participants rested quietly for 20 minutes, during which they answered questionnaires regarding their past 24 hours diet and sleep duration. A urine sample was collected and specific gravity measured using a digital refractometer (UG-1, Atago, Japan). Oral temperature was recorded using a digital thermometer (accuracy, 0.50C; Terumo Corporation, Japan) and blood glucose level measured via a finger-prick sample (Accu-check, Roche Diagnostics, Germany).
Cognitive function
Neuropsychological testing was performed using CogState software (version 1.0, Cogstate Ltd., Melbourne, Australia). Computerised testing was used as it allows standardised and accurate recording of reaction times, electronic data capture and processing, thus minimising human error and response bias. The entire battery required 25-30 minutes to complete and consisted of 5 different tasks (Table 1), detailed descriptions of which have previously been published [26]. The tests were selected because of their parametric properties and demonstrated utility for within-subject experimental designs [27]. These could also be administered at short intervals without significant practice effects[28,29].
Table 1.
Performance measures for CogState tests and change scores (negative values indicate performance decline)
| CogState test | Cognition domain | Performance measure (unit) | Change score (fasting vs non-fasting) | Change score (0900h vs 1600h) | ||
|---|---|---|---|---|---|---|
| 0900h | 1600h | Fasting | Non-fasting | |||
| Detection task | Psychomotor function (processing speed) | Speed (Log10 milliseconds)1 | 0.124* (0.033) | − 0.028 (0.014) | 0.138# (0.02) | − 0.014 (0.02) |
| Identification Task | Vigilance (visual attention) | Speed (Log10 milliseconds)1 | 0.044* (0.017) | 0.028 (0.017) | − 0.006 (0.015) | − 0.022 (0.02) |
| One Card Learning | Visual learning & memory | Accuracy (arcsine proportion)2 | − 0.004 (0.024) | − 0.071 (0.038) | 0.085# (0.035) | 0.018 (0.033) |
| One Back Memory | Executive function (working memory) | Accuracy (arcsine proportion)2 | − 0.062 (0.039) | − 0.026 (0.043) | 0.006 (0.003) | 0.093 (0.04) |
| International Shopping List Recall | Verbal learning & memory | Number of correct responses3 | 0.444 (0.544) | −1.557* (0.682) | 2.28# (0.51) | 0.28 (0.522) |
Mean of the log10 transformed reaction times for correct responses;
Arcsine transformation of the square root of the proportion of correct responses;
Total number of correct responses made in remembering the list after a delay;
Significant change in performance between fasting and non-fasting
Significant change in performance between 0900h and 1600h
Each task was in the form of a card game presented in succession against a green background. Prior to each task, written instructions were presented and participants were given an interactive demonstration. Once they had successfully given a sufficient number of correct responses to demonstrate their awareness of the rules, the task began. Participants used the same computer for each session, and the battery was always administered in the same order.
Statistical analysis
This was performed using SPSS software (version 15.0; SPSS Inc., Chicago, Illinois). Comparison of quantitative data between Ramadan (fasting) and non-Ramadan (non-fasting) was performed using paired t-tests if normality assumptions were satisfied; otherwise non-parametric tests were used. The fasting and TOD effects were assessed using 2 x 2 repeated measures ANOVA, with (0900h, 1600h) and (fasting, non-fasting) as factors. If a significant interaction was present, single factor pair-wise comparison was performed to determine its direction. Effect size was quantified using Cohen's d, which was computed as the difference of the two group means expressed as a function of the pooled variance associated with each individual mean. Change scores for performance differences were computed for both fasting and non-fasting states as well as between the two diurnal time-points. Two-tailed statistics were used and statistical significance set at P<0.05.
RESULTS
Eighteen healthy male volunteers aged between 17 and 29 years [20.9 (±3.3) years] with a mean education level of 11.8 (±1.4) years (range, 10-15 years) were enrolled in this study.
Cognitive function
Performance differences were observed in the detection (P=0.01), identification (P=0.02), and international shopping list recall (P=0.03) tasks during Ramadan (Table 1). There were also significant performance differences between the 0900h and 1600h sessions for the detection (P<0.001), one-card learning (P=0.02), and international shopping list recall (P<0.001) tasks (Table 1). The TOD effect was significant only during the fasting sessions.
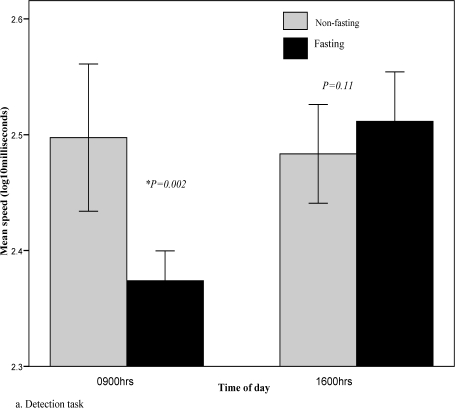
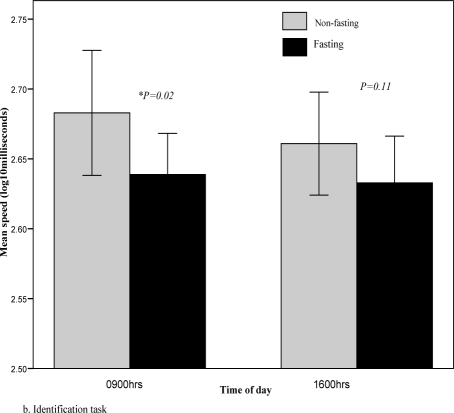
Repeated-measures ANOVA analysis revealed a significant fasting effect on the speed-dependent detection and identification tasks (Table 2). These assess psychomotor function/processing speed and visual attention/vigilance. Performance was significantly better at 0900h during fasting when compared to the non-fasting period (Fig. 2a, b). The magnitude of the fasting effect was relatively large (Cohen's d=1.3) for the detection task and moderate (d=0.6) for the identification task. Performance in the international shopping list recall task, which assesses verbal learning and short-term memory, was also adversely affected by fasting (d=-0.8). There was no significant fasting effect on the one-card learning and one-back memory tasks, both of which are non-speed dependent accuracy measures.
Table 2.
Effect of Time-of-Day (TOD) and Fasting on CogState task performance
| Cogstate task | Time-of-day effect | Fasting effect | Fasting & time-of-day interaction effect | |||
|---|---|---|---|---|---|---|
| F(1,34) | P-value | F(1,34) | P-value | F(1,34) | P-value | |
| Detection task | 15.19 | 0.0011 | 6.95 | 0.012 | 17.51 | <0.0013 |
| Identification task | 1.26 | 0.27 | 9.40 | 0.0042 | 0.46 | 0.5 |
| One Card Learning | 4.59 | 0.041 | 2.79 | 0.10 | 2.19 | 0.15 |
| One Back Memory | 4.03 | 0.05 | 0.40 | 0.53 | 2.27 | 0.14 |
| International Shopping List Recall | 12.24 | 0.0011 | 5.23 | 0.032 | 5.26 | 0.034 |
Significant time-of-day effect
Significant fasting effect
Better performance at 0900h during fasting
Poorer performance at 1600h during fasting
Fig. 2a.
Detection task during fasting and non-fasting, 0900hrs vs 1600hrs. Lower scores indicate better performance.
Fig. 2b.
Identification task during fasting and non-fasting, 0900hrs vs 1600hrs. Lower scores indicate better performance.
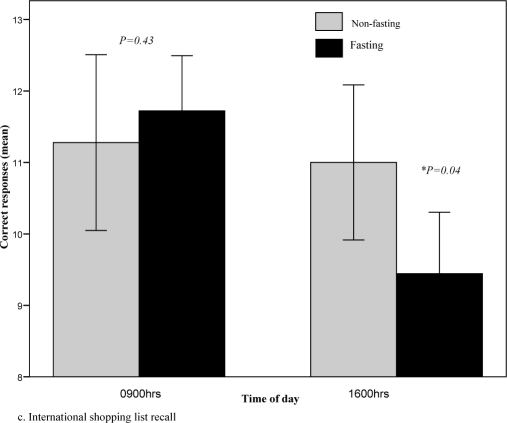
A significant TOD effect was present for the psychomotor function/processing speed, visual learning, verbal learning and memory tasks (Table 2). Performance was significantly poorer at 1600h compared to 0900h and the magnitude of the effect was greater during fasting. The largest effect size was observed for verbal learning and memory (d=-1.3, fasting; d=-0.1, non-fasting) (Fig. 2c). Smaller effect sizes were present for psychomotor function (d=-0.4, fasting; d=-0.1, non-fasting) and visual learning (d=-0.5, fasting; d=-0.2, non-fasting).
Fig. 2c.
International shopping list recall task during fasting and non-fasting, 0900hrs vs 1600hrs. Higher scores indicate better performance.
There was also a significant interaction between TOD and fasting for both the detection and international shopping list recall tasks.
Physiological parameters
During Ramadan, both mean body mass and capillary glucose levels were significantly lower at 1600h (P=0.001, Table 3). There was a 0.8mmol/l reduction in mean capillary glucose level and a 0.9kg decrease in mean body mass at 1600h. A significant TOD (F=11.93, P<0.001) and fasting effect (F=8.06, P=0.008) was observed for blood glucose levels. A significant TOD effect was also present for oral temperature (F=6.79, P=0.01), but the fasting effect was not significant (F =3.88, P=0.06)
Table 3.
Physiological parameters and sleep volume
| Parameters | Time | Non – fasting | Fasting | P-value (Fasting vs non-fasting) |
|---|---|---|---|---|
| Body mass(kg) | 0900h | 69.8 (16.8) | 69.3 (16.3) | 0.6 |
| 1600h | 69.8 (17.0) | 68.4 (16.1) | <0.001 | |
| Capillary glucose level(mmol/l) | 0900h | 5.8 (1.2) | 5.4 (0.6) | 0.3 |
| 1600h | 5.4 (0.9) | 4.6 (0.3) | 0.001 | |
| Temperature(°C) | 0900h | 36.6 (0.2) | 36.5 (0.3) | 0.07 |
| 1600h | 36.7 (0.3) | 36.6 (0.2) | 0.05 | |
| Urine specific gravity | 0900h | 1.020 (0.009) | 1.021 (0.009) | 0.8 |
| 1600h | 1.021 (0.007) | 1.023 (0.004) | 0.3 | |
| Total nocturnal sleep(h) | 7.0 (1.7) | 5.3 (1.8) | 0.005 | |
| Total daytime nap(h) | 0.3 (0.4) | 3.0 (2.1) | 0.001 |
Sleep
The mean duration of nocturnal sleep during Ramadan was significantly shorter (P=0.005, Table 3). The duration of daytime naps was also increased (P=0.001).
DISCUSSION
The physiological measurements we obtained are in line with published data showing lower blood glucose levels and body temperature in the afternoon during Ramadan [3,7,8,10]. The main findings from our neuropsychological test panel suggest that the influence of Ramadan fasting on cognitive function is not homogeneous. During Ramadan, performance in the domains of psychomotor function/processing speed and visual attention/vigilance was better at 0900h. By late afternoon, there was a significant decrease in psychomotor function/processing speed, verbal learning and memory performance. This may be attributed to the combination of food, fluid and sleep restriction.
As glucose is an essential substrate for the central nervous system, with an increase in its metabolism documented in specific brain areas during cognitive activity [30,31], the performance impairment we observed at 1600h may be due to lowered blood glucose levels. The brain cannot manufacture its own glucose and is dependent on a continual peripheral supply, which will be limited in the late afternoon in fasted subjects.
The effect was not uniform across all the domains tested. This may be due to the different susceptibility of the various cognition domains. Doniger's study, performed during a 12-16 hour Jewish religious fast [22], showed that both time-dependent (attention and information processing) and spatial relation perception (non-verbal recognition memory, problem solving, verbal naming, visual-spatial skills) tasks were more likely to be adversely affected during fasting [23].
Electroencephalogram (EEG) studies provide further support for the domain specific effect. These show that although performance decrements and slower reaction times were present in fasted participants carrying out frontal-parietal dominant tasks requiring decision determination and response implementation, there was no significant effect on simple attention tasks involving stimulus encoding, memory retrieval and decision making [32].
Alpha frequency activity in the occipital cortex, which is the primary area associated with visual sensory stimuli processing and integration, was also not significantly altered during fasting [32]. This is consistent with our findings of a lack of any significant performance decline in the non-speed dependent, predominantly occipital cortex centred one-card learning and one-back memory tasks, which supports the previously demonstrated robustness of these domains [15–17,22].
The improved performance in the visual attention/ vigilance and psychomotor function/processing speed tasks at 0900h in our participants may be the result of post feeding neural activity enhancement as well as reduced sleep inertia due to earlier awakening for prayers. In Pivik's study, frontal lobe activity was enhanced in fed participants performing frontal dominant processes related to stimulus encoding, memory retrieval, decision making and motor response execution [32].
In line with Dinges's study [33], sleep deprivation may be associated with the late afternoon decline in working memory and sustained attention observed in our participants. In sleep restricted subjects, Blood Oxygenation Level Dependent functional MRI (BOLD-fMRI) studies show a decrease in both global and task-specific brain activation during cognitive activity [34]. EEG data also demonstrate reductions in intraparietal sulci and superior parietal lobe activation during short-term memory related tasks [34–36]. BOLD-fMRI studies suggest that compensatory mechanisms exist to aid attention focus and suppress extraneous mental processes in sleep restricted individuals [37,38]. This may ameliorate some of the adverse effects of sleep deprivation and explain the resilience of tasks involved in vigilance and simple reaction.
The reduction in mean body mass and increase in urine specific gravity indicates that the participants in our study were mildly dehydrated (1.3%) at 1600h. Dehydration has a dose-dependent effect on cognitive function. Speed and accuracy dependent tasks are adversely affected within 3 hours of fluid deprivation [39], and mathematical ability, short-term working memory, perceptive discrimination and eye-hand coordination are affected by levels of dehydration between 1%-4% [40,41]. The effects may be mediated via altered cerebral neurotransmission due to hyperosmolality and electrolyte shifts and/or changes in blood brain barrier permeability. Current theories include alterations in the activity of nitric oxide synthase, which is a key enzyme facilitating learning and memory, and changes in central dopaminergic and noradrenergic pathways [42,43]. The reticular activating system subserving attention and wakefulness; the autonomic structures regulating psychomotor functions; and the cortical and mid-brain structures responsible for thought, memory and perception appear most vulnerable, thus contributing to the late afternoon performance decline.
Our study was limited to a small group of athletes from a single martial art discipline. Furthermore, the participants’ lifestyle and training in between test sessions were not controlled. Other potential confounders include the duration of the previous night's sleep and daytime naps, as well as the time of awakening, which were not regulated in this study.
CONCLUSION
Our results suggest that during Ramadan fasting, performance in cognitive functions requiring sustained rapid responses was better in the morning, declining in the late afternoon, whereas performance in non-speed dependent accuracy measures was more resilient. Although the tests were laboratory based and may not reflect cognitive ability during training or competition, our findings suggest that during Ramadan, skill-based or training requiring high levels of psychomotor, learning or memory ability should be performed in the morning. This will help Muslim athletes and their coaches avoid the late afternoon performance nidus and circumvent the potentially detrimental effects of fasting.
ACKNOWLEDGMENTS
The authors would like to acknowledge Ms Lee Hong Choo from the Sports Physiology Unit, Singapore Sports Institute and Professor Paul Maruff from the School of Psychological Science, La Trobe University, Melbourne, Australia for their invaluable assistance.
Conflict of interests: None
REFERENCES
- 1.Roky R, Chapotot F, Benchekroun M, et al. Daytime sleepiness during Ramadan intermittent fasting: polysomnographic and quantitative waking EEG study. J Sleep Res. 2003;12:95–101. doi: 10.1046/j.1365-2869.2003.00341.x. [DOI] [PubMed] [Google Scholar]
- 2.Waterhouse J, Alabed H, Edwards B, et al. Changes in sleep, mood and subjective and objective responses to physical performance during the daytime in Ramadan. Biol Rhythm Res. 2009;40:367–83. [Google Scholar]
- 3.Iraki L, Bogdan A, Hakkou F, et al. Ramadan diet restrictions modify the circadian time structure in humans. Study on plasma gastrin, insulin, glucose and calcium and on gastric Ph. J Clin Endocrinol Metab. 1997;82:1261–73. doi: 10.1210/jcem.82.4.3860. [DOI] [PubMed] [Google Scholar]
- 4.Reilly T, Waterhouse J. Altered sleep-wake cycles and food intake: the Ramadan model. Physiol Behav. 2007;90:219–28. doi: 10.1016/j.physbeh.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Roky R, Houti I, Moussamih S, et al. Physiological and chronobiological changes during Ramadan intermittent fasting. Ann Nutr Metab. 2004;48:296–303. doi: 10.1159/000081076. [DOI] [PubMed] [Google Scholar]
- 6.Leiper JB, Molla AM, Molla AM. Effects on health of fluid restriction during fasting in Ramadan. Eur J Clin Nutr. 2003;57:S30–8. doi: 10.1038/sj.ejcn.1601899. [DOI] [PubMed] [Google Scholar]
- 7.Nomani MZ, Hallak MH, Nomani S, et al. Changes in blood urea and glucose and their association with energy containing nutrients in men on hypocaloric diets during Ramadan fasting. Am J Clin Nutr. 1989;49:1141–5. doi: 10.1093/ajcn/49.6.1141. [DOI] [PubMed] [Google Scholar]
- 8.Larijani B, Zahedi F, Sanjari M, et al. The effect of Ramadan fasting on fasting serum glucose in healthy adults. Med J Malaysia. 2003;58:678–80. [PubMed] [Google Scholar]
- 9.Monk TH, Folkard S, Leng VC. Circadian rhythms in subjective alertness and core body temperature. Chronobiologia. 1983;10:49–55. [PubMed] [Google Scholar]
- 10.Roky R, Iraki L, HajKhlifa R, et al. Daytime alertness, mood, psychomotor performances, and oral temperature during Ramadan intermittent fasting. Ann Nutr Metab. 2000;44:101–7. doi: 10.1159/000012830. [DOI] [PubMed] [Google Scholar]
- 11.Aziz AR, Png W. Practical tips to exercise training during the Ramadan fasting month. Institut Sukan Negara Bulletin. 2008;1:13–9. [Google Scholar]
- 12.Hakkou F, Wast D, Jaouen C. Does Ramadan impair vigilance and memory. Psychopharmacology. 1988;96:213. [Google Scholar]
- 13.Ali MR, Amir T. Effects of fasting on visual flicker fusion. Percept Mot Skills. 1989;69:627–631. doi: 10.2466/pms.1989.69.2.627. [DOI] [PubMed] [Google Scholar]
- 14.Hakkou F, Iraki L, Tazi A. Ramadan, chronobiology and health. Chronobiol Int. 1994;11:340–2. doi: 10.3109/07420529409057250. [DOI] [PubMed] [Google Scholar]
- 15.Liebermeister H, Schroter K. Absence of detrimental changes of cognitive parameters during fasting. Int J Obes. 1983;7:45–51. [PubMed] [Google Scholar]
- 16.Green MW, Elliman NA, Rogers PJ. Lack of effect of short-term fasting on cognitive function. J Psychiatr Res. 1995;29:245–53. doi: 10.1016/0022-3956(95)00009-t. [DOI] [PubMed] [Google Scholar]
- 17.Green MW, Elliman N, Rogers PJ. The effects of food deprivation and incentive motivation on blood glucose levels and cognitive function. Psychopharmacology (Berlin) 1997;134:88–94. doi: 10.1007/s002130050429. [DOI] [PubMed] [Google Scholar]
- 18.Gutierrez A, Gonzalez-Gross M, Delgado M, et al. Three days fast in sportsmen decreases physical work capacity but not strength or perception-reaction time. Int J Sport Nutr Exerc Metab. 2001;11:420–9. doi: 10.1123/ijsnem.11.4.420. [DOI] [PubMed] [Google Scholar]
- 19.Szinnai G, Schachinger H, Arnaud MJ, et al. Effect of water deprivation on cognitive-motor performance in healthy men and women. Am J Physiol Regul Integr Comp Physiol. 2005;289:275–80. doi: 10.1152/ajpregu.00501.2004. [DOI] [PubMed] [Google Scholar]
- 20.Reilly T, Atkinson G, Edwards B, et al. Diurnal variation in temperature, mental and physical performance, and tasks specifically related to football (soccer) Chronobiol Int. 2007;24:507–10. doi: 10.1080/07420520701420709. [DOI] [PubMed] [Google Scholar]
- 21.Monk TH, Buysse DJ, Reynolds CF, et al. Circadian rhythms in human performance and mood under constant conditions. J Sleep Res. 1997;6:9–18. doi: 10.1046/j.1365-2869.1997.00023.x. [DOI] [PubMed] [Google Scholar]
- 22.Doniger GM, Simon ES, Zivotofsky AZ. Comprehensive computerized assessment of cognitive sequelae of a complete 12-16 hour fast. Behav Neurosci. 2006;120:804–16. doi: 10.1037/0735-7044.120.4.804. [DOI] [PubMed] [Google Scholar]
- 23.Dye L, Lluch A, Blundell JE. Macronutrients and mental performance. Nutrition. 2000;16:1021–34. doi: 10.1016/s0899-9007(00)00450-0. [DOI] [PubMed] [Google Scholar]
- 24.Gibson EL, Green MW. Nutritional influences on cognitive function: Mechanisms of susceptibility. Nutr Res Rev. 2002;15:169–206. doi: 10.1079/NRR200131. [DOI] [PubMed] [Google Scholar]
- 25.Benton D, Parker PY. Breakfast, blood glucose, and cognition. Am J Clin Nutr. 1998;67:772S–78S. doi: 10.1093/ajcn/67.4.772S. [DOI] [PubMed] [Google Scholar]
- 26.Falleti MG, Maruff P, Collie A, et al. Qualitative similarities in cognitive impairment associated with 24h of sustained wakefulness and a blood alcohol concentration of 0.05% J Sleep Res. 2003;12:265–74. doi: 10.1111/j.1365-2869.2003.00363.x. [DOI] [PubMed] [Google Scholar]
- 27.Maruff P, Thomas E, Cysique L, et al. Validity of the CogState brief battery: relationship to standardized tests and sensitivity to cognitive impairment in mild traumatic brain injury, schizophrenia, and AIDS dementia complex. Arch Clin Neuropsychol. 2009;24:165–78. doi: 10.1093/arclin/acp010. [DOI] [PubMed] [Google Scholar]
- 28.Collie A, Maruff P, Darby DG, et al. The effects of practice on the cognitive test performance of neurologically normal individuals assessed at brief test-retest intervals. J Int Neuropsychol Soc. 2003;9:419–28. doi: 10.1017/S1355617703930074. [DOI] [PubMed] [Google Scholar]
- 29.Falleti MG, Maruff P, Collie A, et al. Practice effects associated with the repeated measurement of cognitive function using the CogState battery at 10-minute, one week and one month test-retest intervals. J Clin Exp Neuropsychol. 2006;28:1095–112. doi: 10.1080/13803390500205718. [DOI] [PubMed] [Google Scholar]
- 30.Donohoe RT, Benton D. Cognitive functioning is susceptible to the level of blood glucose. Psychopharmacology (Berl) 1999;145:378–85. doi: 10.1007/s002130051071. [DOI] [PubMed] [Google Scholar]
- 31.Hertz L, Dienel GA. Energy metabolism in the brain. Int Rev Neurobiol. 2002;51:1–102. doi: 10.1016/s0074-7742(02)51003-5. [DOI] [PubMed] [Google Scholar]
- 32.Pivik RT, Dykman RA. Event-related variations in alpha band activity during an attentional task in preadolescents: Effect of morning nutrition. Clin Neurophysiol. 2007;118:615–32. doi: 10.1016/j.clinph.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 33.Dinges DF, Pack F, Williams K, et al. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4-5 hours per night. Sleep. 1997;20:267–77. [PubMed] [Google Scholar]
- 34.Mu Q, Nahas Z, Johnson KA, et al. Decreased cortical response to verbal working memory following sleep deprivation. Sleep. 2005;28:55–67. doi: 10.1093/sleep/28.1.55. [DOI] [PubMed] [Google Scholar]
- 35.Chee MW, Choo WC. Functional imaging of working memory after 24 hr of total sleep deprivation. J Neurosci. 2004;24:4560–7. doi: 10.1523/JNEUROSCI.0007-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chee MW, Chuah LY, Venkatraman V, et al. Functional imaging of working memory following normal sleep and after 24 and 35 h of sleep deprivation: Correlations of frontoparietal activation with performance. Neuroimage. 2006;31:419–28. doi: 10.1016/j.neuroimage.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 37.Drummond SP, Meloy MJ, Yanagi MA, et al. Compensatory recruitment after sleep deprivation and the relationship with performance. Psychiatry Res. 2005;140:211–23. doi: 10.1016/j.pscychresns.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 38.Beebe DW, Difrancesco MW, Tlustos SJ, et al. Preliminary fMRI findings in experimentally sleep-restricted adolescents engaged in a working memory task. Behav Brain Funct. 2009;5:9–15. doi: 10.1186/1744-9081-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petri NM, Dropulic N, Kardum G. Effects of voluntary fluid intake deprivation on mental and psychomotor performance. Croat Med J. 2006;47:855–61. [PMC free article] [PubMed] [Google Scholar]
- 40.Sharma VM, Sridharan K, Pichan G, et al. Influence of heat-stress induced dehydration on mental functions. Ergonomics. 1986;29:791–9. doi: 10.1080/00140138608968315. [DOI] [PubMed] [Google Scholar]
- 41.Gopinathan PM, Pichan G, Sharma VM. Role of dehydration in heat stress-induced variations in mental performance. Arch Environ Health. 1988;43:15–17. doi: 10.1080/00039896.1988.9934367. [DOI] [PubMed] [Google Scholar]
- 42.Wilson MM, Morley JE. Impaired cognitive function and mental performance in mild dehydration. Eur J Clin Nutr. 2003;57:S24–29. doi: 10.1038/sj.ejcn.1601898. [DOI] [PubMed] [Google Scholar]
- 43.Maughan RJ. Exercise, heat, hydration and the brain. J Am Coll Nutr. 2007;26:604S–12S. doi: 10.1080/07315724.2007.10719666. [DOI] [PubMed] [Google Scholar]