Abstract
Vitamin D deficiency is a worldwide epidemic, with well known impacts on calcium metabolism and bone health, but increasingly recognized associations with chronic health problems such as bowel and colonic cancer, arthritis, diabetes and cardiovascular disease. In recent years in the Sports Medicine literature, there has been an increased focus on the potential impact that inadequate Vitamin D levels may have on athletic performance.
In the early 20th Century, athletes and coaches felt that ultraviolet rays had a positive impact on athletic performance, and while remaining limited, evidence is accumulating to support this view. Muscle structure and function is recognised to play a key role in athletic performance, and both cross-sectional and longitudinal studies allude to a functional role for Vitamin D in muscle. The identification of the Vitamin D receptor in muscle tissue provides a direct pathway for Vitamin D to impact upon Skeletal Muscle structure and function. This review focuses on the current understanding of the action of Vitamin D within skeletal muscle tissue, and the potential impact on performance.
Keywords: Vitamin D, Sports, Athletic Performance, Exercise, Muscle
INTRODUCTION
It is well recognized that athletic performance is determined by multiple factors, including both genetic and environmental influences [1]. While genetic pre-determinants of performance are being heavily researched, there remain many unanswered questions. Similarly, environmental factors, while well recognized as having the potential to impact upon athletic ability, have only a limited scientific basis. In recent years, Vitamin D has been proposed as a potentially performance limiting factor when in deficiency, and as performance enhancing when present in abundance [2]. Vitamin D deficiency is increasingly recognized as a worldwide epidemic [3–9] and while remaining controversial, it is generally accepted that levels of 20-30 ng/ml 25 OH-Vitamin D represent insufficiency, while levels below 20 and 10 ng/ml represent deficiency and severe deficiency, respectively [10].
The most well documented cause of Vitamin D deficiency is inadequate sunlight exposure, and as such, high latitude countries are known to have a high incidence of deficiency [7]. Paradoxically, despite its high sunlight hours, Vitamin D deficiency is well recognised in Middle Eastern women [11–13], and more recently in inner city young adults in America [5], athletes and dancers in Israel [14], elite gymnasts in Australia [15], young Hawaiian surfers [3], and adolescent girls in England [16]. Given the incidence of Vitamin D deficiency in athletes and non-athletes alike, from all regions of the world, the suggestion that Vitamin D deficiency may impact upon performance has potentially widespread ramifications for athletes.
Vitamin D deficiency may have significant long term health impacts [17, 18] including recognized associations with bowel and colonic cancer, arthritis, diabetes and cardiovascular disease [10, 17–26]. It is also possible that a deficiency will result in more immediate effects on musculoskeletal health, with increased risk of injuries such as stress fractures [20, 27, 28]; whether Vitamin D deficiency will affect injury risk and function of other tissues such as muscle, ligament and tendon remains unknown. Potentially, Vitamin D deficiency may impact upon training quality, injury and illness frequency and duration [29] and as a result, athletic performance. In the early part of the 20th Century, ultraviolet-B (UVB) rays were supposedly being used as an ergogenic aid [2], 30] and research over that period suggested that both cardiovascular fitness and muscular endurance were enhanced with increased exposure to ultraviolet radiation [31]. However, there remains little direct evidence for this [32] and these findings are yet to be reproduced. El-Hajj et al [33] reported a one year prospective double blind, placebo controlled trial of low and high dose Vitamin D3 in 179 adolescent Lebanese girls. In Vitamin D supplemented individuals they found increased lean mass, bone area and bone mass, particularly in pre-menarchal girls, but found no increase in grip strength. Furthermore, there were no significant findings regarding Vitamin D and muscle mass or grip strength in a similar cohort of male adolescents [33]. By contrast, a recent study of 99 post-menarchal adolescent girls in England, found a positive relationship between serum Vitamin D level and jump height, jump velocity and power [16].
While Vitamin D deficiency has long been associated with muscle weakness [34–37], until recently no specific aetiological mechanism had been described. Over the last 30 years a mechanism by which Vitamin D may affect muscle function has slowly been unraveled. Subsequently, while limited, there is evidence from a range of sources relating Vitamin D deficiency to suboptimal muscle function. This review will focus on the current available evidence for the manner in which Vitamin D may affect skeletal muscle, and thereby potentially impair athletic performance.
Vitamin D: Muscle Structure and Function
Myopathy associated with Vitamin D deficient osteomalacia has been recognised for many years, typically presenting as a proximal muscle weakness [34–37]. Until recently, this myopathic presentation was felt to be secondary to disuse, rather than a direct effect of Vitamin D on muscle. However, increased understanding of the Vitamin D metabolic pathways suggests that this presumption may be incorrect [38]. By 1974 electromyographic changes had been observed in patients with muscle weakness associated with osteomalacia [37], which improved with Vitamin D supplementation [36]). The reversibility of the osteomalacic myopathy with Vitamin D correction is now well recognized [35, 39–42]. In a preliminary study Glerup et al [38] examined a small group (n=8) of elderly men and women (mean age 63.1±5.3 years) with known osteomalacia, who had muscle strength assessed using an isokinetic dynamometer before and after 3 months of treatment with alfacalcidol, ergocalciferol and calcium. They found that over three months, muscle power increased significantly in all muscle groups assessed, with a mean improvement of 24.8±8.0%. They then compared a group of Vitamin D deficient Arab women with a control group of Danish women who had normal levels of Vitamin D. At baseline, quadriceps maximum voluntary contraction (MVC), as well as electrically stimulated twitch [Single twitch, Maximum Production Rate (MPR) and Maximal Relaxation Rate (MRR] were all significantly lower in the group of Arab women. Three months of Vitamin D supplementation, without strength training, increased Vitamin D levels and normalized parathyroid hormone (PTH) levels in the Arab women, with a corresponding trend towards normalization of the MVC, MPR and MRR. Further analysis revealed that only 25-OH Vitamin D was significantly associated with Maximum Voluntary Contraction and as a result, the authors concluded that normal levels of 25-OH Vitamin D are necessary for maintaining adequate muscle function.
Both biopsy studies [36,37], and case reports of muscle weakness associated with osteomalacia [35,39], have revealed either non-specific changes or a type II skeletal muscle fibre atrophy. Sato et al [43] assessed the impact of Vitamin D supplementation on muscle histopathology when they biopsied the non-hemiplegic vastus lateralis of 85 Vitamin D deficient elderly stroke patients, before and after a two year supplementation period with either placebo or Vitamin D2. At baseline they found a normal range of type I fibres, but a reduced proportion and diameter of type II muscle fibres. At the two year follow-up, the placebo group had a further reduction in Type II muscle fibre diameter, while in the Vitamin D2 supplemented group the relative content and mean diameter of type II fibres increased. Muscle fibre size was found to correlate with 25-OH Vitamin D levels [43].
Vitamin D, Aging and Muscle Function
While it is well recognized that muscle strength declines with age, this is believed to be due to a number of contributing factors [44] and the role of Vitamin D continues to be debated [45,46]. Cross-sectional studies [47–51] appear to show a relationship between Vitamin D levels and various measures of changes in muscle strength and function with ageing [47–51]. By contrast, a review of randomized controlled trials investigating Vitamin D and/or calcium supplementation, concluded that there was no evidence that Vitamin D alone improved the strength or physical function of elderly people [45]. Paradoxically, recent well controlled and designed studies suggest Vitamin D may have a role in moderating the age related decline in muscle function [52–54]. Visser et al [52] prospectively investigated the impact of low 25-OH Vitamin D and high serum PTH in 1008 men and women aged over 65 years (mean 74 years) and found that individuals with a lower 25-OH Vitamin D and/or higher PTH levels were significantly more likely to lose grip strength and muscle mass and that 30 ng/ml may be a threshold for optimal muscle function. Similarly, Bischoff et al [53] performed a 12 week double blind, randomized controlled trial utilising Vitamin D and calcium versus calcium supplementation alone. Knee flexor and extensor strength, grip strength and functional (timed up and go) testing, all improved in their elderly group following supplementation with Vitamin D and calcium, versus calcium supplementation alone. Gerdhem et al [54], in a three year study of 986 Swedish 75 year old women, found that reduced Vitamin D levels correlated with reduced gait speed, reduced knee flexor and extensor strength and increased risk of falls. Several other prospective studies have reported similar results [43,55–57] but by contrast, in a large prospective randomized controlled trial, Latham et al [58] assessed the relative benefits of home resistance exercise or a single high dose of Vitamin D, on self reported physical health, risk of falls and functional performance. Despite increasing the serum Vitamin D level in those individuals treated, they found no significant impact of Vitamin D on any of the self reported functional outcome parameters [58]. Finally, Vitamin D Receptor (VDR) expression was assessed in female hip and spinal operative patients, and was found to decrease with age, and that expression was unaffected by either 25-OH or 1,25-OH Vitamin D levels. Hence, any age related decline in muscle strength may be related to reduced Vitamin D receptor expression [59] or VDR polymorphisms resulting in variable susceptibility to age related changes [60].
The Vitamin D Receptor
VDR was first recognised within muscle cells in cultured rat myoblast cells in 1985, confirming muscle as a target organ for Vitamin D [61]. The VDR has subsequently been described in tissues such as smooth and heart muscle, liver, lung, colon, gonads and skin [62,63] and was isolated from human skeletal muscle in 2001 [59,64]. It is now recognized that in combination with co-factors “retinoid x receptor” (RXR) and “Steroid Receptor Coactivator 3” (SRC), the VDR: 1,25-OH Vitamin D complex modulates gene expression of a broad range of proteins [65,66]. This includes proteins with roles in calcium metabolism such as calbindin [67], but also proteins not directly related to calcium metabolism such as Insulin-like Growth Factor Binding Protein 3 (IGFBP-3) [65]. The VDR has also been shown to have various genetic polymorphisms, which may affect their function within skeletal muscle [68–70]. To assess the importance of the VDR in the skeletal muscle of mice, a generation of VDR gene deleted mice and myoblast cell lines were examined [71]. VDR null mice had fibre sizes in the quadriceps and other muscle groups 20% smaller than VDR replete mice. In addition, VDR null mice exhibited increased expression of myogenic transcription factors myf5, E2A and myogenin compared to normal mice along with inappropriate expression of embryonic and neonatal type myosin heavy chain (MHC) [71], supporting a direct role for 1,25-OH Vitamin D and the VDR in both the metabolic processes and transcription regulation of skeletal muscle.
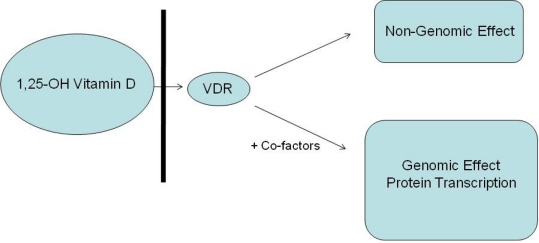
With calcium being a critical modulator of skeletal muscle function, any perturbation to calcium handling may impact on a muscle's contractile properties [72]. Therefore, Vitamin D may affect muscle function through both calcium related protein transcription, and total body calcium levels. However, Vitamin D also has a transcription enhancing role on proteins other than those involved directly with calcium metabolism. One such protein, relevant to the discussion of skeletal muscle, is IGFBP-3. It is recognised that IGFBP-3 expression is regulated by a number of factors, including Vitamin D [65], with a VDRE in the promoter region for human IGFBP-3 recently identified [65]. IGF Binding Protein-3 (IGFBP-3) is a member of the IGFBP family, which bind IGF-1 in the serum, the extracellular matrix and on cell surfaces [73] with high affinity and specificity [65,74]. The binding of IGF-1 to IGFBP's may have both inhibitory and stimulatory effects on IGF-1 function [66,74,75] and as IGF-1 induces proliferation, differentiation and hypertrophy of skeletal muscle [76] and is a key component in muscle regeneration [75], Vitamin D may have a key role to play via IGFBP-3. As a result, IGF-1 is recognized as both a potential means for addressing age related sarcopenia [77] and as an illegal ergogenic aid in sport [78]. In a recent investigation of children with Vitamin D deficient Rickets, the significance of IGF-1 and Vitamin D was recently highlighted [79]. The authors found that growth rates and height of the children increased with Vitamin D supplementation, with a significant correlation between serum concentrations of IGF-1 and the percentage increment in 25-OH Vitamin D concentrations. The authors concluded that the growth spurt observed in children with rickets after Vitamin D supplementation is mediated via through an increase in IGF-1 [79]. Hence, the regulation of IGFBP-3 and subsequently IGF-1 has the potential to impact upon muscle structure and function directly (Fig. 1).
Fig. 1.
Potential action of Vitamin D on cells VDR: Vitamin D Receptor
In addition to the above-mentioned action, a genome independent pathway of Vitamin D action has recently been characterized [80,81] whereby 1,25-OH Vitamin D is involved in the rapid regulation of membrane calcium channels in skeletal muscle cells [82]. A membrane receptor with a higher molecular weight than the intra-nuclear Vitamin D receptor (known as the membrane-associated rapid response steroid binding protein (MARRS) [67]), specific for 1,25-OH Vitamin D has been identified in animal cells [83]. While the exact mechanism of the non-genomic action of Vitamin D remains controversial, it is widely accepted that Vitamin D levels have a rapid effect on the membrane calcium channels of muscle cells in numerous species [67, 81, 84]. As calcium is a critical modulator of skeletal muscle function [72], it follows that Vitamin D levels may have a significant impact on muscle function, performance and potentially injury risk.
VDR Polymporphisms and Performance
Located on Chromosome 12 (12q13.11) [85], the VDR is known to have various genetic polymorphisms including Bsm1, Fok1, Apal, Taq1, which have been associated with various functional outcomes [60,69,70,86]. The Fok1 polymorphism involves a T to C transition in exon 2 of the VDR gene, resulting in a shorter (424) amino acid VDR than the T allele (427) [87], and has been associated with variations in both bone mineral density [88], differential responses of bone density to strength training [89,90], fat-free mass and risk of age related sarcopenia [60]. In patients suffering from chronic obstructive pulmonary disease (COPD), Fok1 C homozygotes (also known as FF) were found to have significantly weaker quadriceps than either CT heterozygotes or T (ff) homozygotes [70]. In a cross sectional study, 501 healthy women over the age of 70, were assessed for quadriceps and grip strength [69], and the VDR genotype Bsm1 (a single nucleotide polymorphism found in intron 8 [87]). The bb genotype was found to be significantly stronger than the BB or heterozygotes genotype. This finding was reproduced in a study involving patients with COPD, whereby the bb polymorphism was again associated with stronger quadriceps muscles [70]. By contrast, Grundberg [68] evaluated the relationship between Bsm1 polymorphisms and muscle strength utilizing 170 pre-menopausal Swedish women and found women homozygous for Bsm1 BB or poly-A repeats to have higher hamstring strength than the bb or LL genotypes. Furthermore, no significant associations were found between VDR polymorphisms and either grip strength or quadriceps strength. Bahat et al [86] found the same trend in elderly men.
CONCLUSION
Vitamin D deficiency is increasingly recognised in modern youth and is now endemic in many communities, with athletes not being spared. Three independent lines of evidence, namely Vitamin D and muscle morphology, age related changes in muscle function, and the presence of the VDR in muscle cells, support the proposition that Vitamin D may play a significant role in muscle structure and function. However, athletic performance at all levels is multi-factorial, and to date there is limited evidence to support the proposition that Vitamin D deficiency is performance limiting, or that maintenance of Vitamin D at supra-physiological levels will result in enhanced muscle development and performance.
CLINICAL RECOMMENDATIONS
Athletes should have their (25-Hydroxy) Vitamin D levels measured regularly throughout the year.
Vitamin D deficient or depleted Athletes should be advised on appropriate UVB exposure or supplemented as required.
Optimal levels of Vitamin D remain controversial, but levels of 25-Hydroxy Vitamin D of 30ng/ml may be considered safe.
ACKNOWLEDGMENTS
The author would like to thank Aspetar (Qatar Orthopaedic and Sports Medicine Hospital) and in particular Dr Hakim Chalabi for the support of this project.
Conflict of interests: The author has no financial or other conflicts of interest in the preparation of this manuscript.
REFERENCES
- 1.MacArthur D, North K. Genes and human elite athletic performance. Hum Genetics. 2005;116:331–9. doi: 10.1007/s00439-005-1261-8. [DOI] [PubMed] [Google Scholar]
- 2.Cannell J, Hollis B, Sorenson M, et al. Athletic performance and vitamin D. Med Sci Sports Exerc. 2009;41:1102–10. doi: 10.1249/MSS.0b013e3181930c2b. [DOI] [PubMed] [Google Scholar]
- 3.Binkley N, Novotny R, Krueger D, et al. Low vitamin D Status despite abundant sun exposure. J Clin Endocrinol Metab. 2007;92:2130–5. doi: 10.1210/jc.2006-2250. [DOI] [PubMed] [Google Scholar]
- 4.Hashemipour S, Larijani B, Adibi H, et al. Vitamin D deficiency and causative factors in the population of Tehran. BMC Public Health. 2004;4:38. doi: 10.1186/1471-2458-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gordon C, DePeter K, Feldman H, et al. Prevalence of vitamin D deficiency among healthy adolescents. Arch Pediatr Adolesc Med. 2004;158:531–7. doi: 10.1001/archpedi.158.6.531. [DOI] [PubMed] [Google Scholar]
- 6.Hannan MT, Litman HJ, Araujo AB, et al. Serum 25-Hydroxyvitamin D and bone mineral density in a racially and ethnically diverse group of men. J Clin Endocrinol Metab. 2008;93:40–6. doi: 10.1210/jc.2007-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andersen R, Molgaard C, Skovgaard L, et al. Teenage girls and elderly women living in northern Europe have low winter vitamin D status. Euro J Clin Nutr. 2005:1–9. doi: 10.1038/sj.ejcn.1602108. [DOI] [PubMed] [Google Scholar]
- 8.Nowson CA, Margerison C. Vitamin D intake and vitamin D status of Australians. Med J Aust. 2002;177:149–52. doi: 10.5694/j.1326-5377.2002.tb04702.x. [DOI] [PubMed] [Google Scholar]
- 9.Rockell J, Green T, Skeaff C, et al. Season and ethnicity are determinants of serum 25-hydroxyvitamin D concentrations in New Zealand children aged 5-15 y. J Nutr. 2005;135:2602–8. doi: 10.1093/jn/135.11.2602. [DOI] [PubMed] [Google Scholar]
- 10.Holick M. Vitamin D deficiency. New Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 11.Allali F, El Aichaoui S, Saoud B, et al. The impact of clothing style on bone mineral density among post menopausal women in Morocco: a case-control study. BMC Public Health. 2006;6:1–6. doi: 10.1186/1471-2458-6-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fonseca V, Tongia R, El-Hazmi M, Abu-Aisha H. Exposure to sunlight and vitamin D deficiency in Saudi Arabian women. Postgrad Med J. 1984;60:589–91. doi: 10.1136/pgmj.60.707.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hatun S, Islam O, Cizmecioglu F, et al. Subclinical vitamin D deficiency is increased in adolescent girls who wear concealing clothing. J Nutr. 2005;135:218–2. doi: 10.1093/jn/135.2.218. [DOI] [PubMed] [Google Scholar]
- 14.Constantini N, Arieli R, Chodick G, Dubnov-Raz G. High prevalence of vitamin D insufficiency in athletes and dancers. Clin J Sport Med. 2010;20:368–371. doi: 10.1097/JSM.0b013e3181f207f2. [DOI] [PubMed] [Google Scholar]
- 15.Lovell G. Vitamin D status of females in an elite gymnastics program. Clin J Sport Med. 2008;18:159–61. doi: 10.1097/JSM.0b013e3181650eee. [DOI] [PubMed] [Google Scholar]
- 16.Ward K, Das G, Berry J, et al. Vitamin D status and muscle function in post-menarchal adolescent girls. Clin Endocrinol Metab. 2009;94:559–63. doi: 10.1210/jc.2008-1284. [DOI] [PubMed] [Google Scholar]
- 17.Giovannucci E, Liu Y, Rimm E, et al. Prospective study of predictors of vitamin D Status and cancer incidence and mortality in men. J Nationl Cancer Inst. 2006;98:451–9. doi: 10.1093/jnci/djj101. [DOI] [PubMed] [Google Scholar]
- 18.Holick M. Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr. 2004;79:362–71. doi: 10.1093/ajcn/79.3.362. [DOI] [PubMed] [Google Scholar]
- 19.Holick M. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81:353–73. doi: 10.4065/81.3.353. [DOI] [PubMed] [Google Scholar]
- 20.Lappe J, Travers-Gastafson D, Davies K, et al. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr. 2007;85:1586–91. doi: 10.1093/ajcn/85.6.1586. [DOI] [PubMed] [Google Scholar]
- 21.Pittas A, Lau J, Hu F, Dawson-Hughes B. The role of vitamin D and calcium in type 2 diabetes. A systematic Review and Meta-Analysis. J Clin Endocrinol Metab. 2007;92:2017–9. doi: 10.1210/jc.2007-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hypponen E, Laara E, Reunanen A, et al. Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet. 2001;358:1500–3. doi: 10.1016/S0140-6736(01)06580-1. [DOI] [PubMed] [Google Scholar]
- 23.Dietrich T, Joshipura K, Dawson-Hughes B, Bischoff-Ferrari H. Association between serum concentrations of 25-hydroxyvitamin D3 and periodontal disease in the US population. Am J Clin Nutr. 2004;80:108–13. doi: 10.1093/ajcn/80.1.108. [DOI] [PubMed] [Google Scholar]
- 24.Hypponen E, Boucher B, Berry D, Power C. 25-Hydroxyvitamin D, IGF-1, and metabolic syndrome at 45 years of Age. Diabetes. 2008;57:298–305. doi: 10.2337/db07-1122. [DOI] [PubMed] [Google Scholar]
- 25.Pani M, Knapp M, Donner H, et al. Vitamin D receptor allele combinations influence genetic susceptibility to type 1 diabetes in germans. Diabetes. 2000;49:504–7. doi: 10.2337/diabetes.49.3.504. [DOI] [PubMed] [Google Scholar]
- 26.Garland C, Garland F, Gorham E, et al. The Role of Vitamin D in Cancer Prevention. Am J Public Health. 2006;96:252–61. doi: 10.2105/AJPH.2004.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruohola J, Laaksi I, Ylikomi T, et al. Association between serum 25(OH)D Concentrations and bone stress fractures in finnish young men. J Bone Min Res. 2006;21:1483–8. doi: 10.1359/jbmr.060607. [DOI] [PubMed] [Google Scholar]
- 28.Lappe J, Cullen D, Haynatzki G, et al. Calcium and vitamin D supplementation decreases incidence of stress fractures in female navy recruits. J Bone Min Res. 2008;23:741–9. doi: 10.1359/jbmr.080102. [DOI] [PubMed] [Google Scholar]
- 29.Halliday T, Peterson N, Thomas J, et al. Vitamin D status rrelative to diet, lifestyle, injury, and illness in college athletes. Med Sci Sports Exerc. 2011;43:335–43. doi: 10.1249/MSS.0b013e3181eb9d4d. [DOI] [PubMed] [Google Scholar]
- 30.Hoberman J. Mortal Engines: The Science of Performance and the Dehumanization of Sport. New York: The Free Press; 1992. Faster, higher, stronger. A History of Doping in Sport; pp. 100–53. [Google Scholar]
- 31.Allen R, Cureton T. Effects of ultraviolet radiation on physical fitness. Arch Phys Med. 1945;26:641–4. [PubMed] [Google Scholar]
- 32.Racinais S, Hamilton B, Li C, Grantham J. Vitamin D and physical fitness in Qatari girls. Arch Dis Child. 2010;95:854–5. doi: 10.1136/adc.2009.163238. [DOI] [PubMed] [Google Scholar]
- 33.El-Hajj G, Nabulsi M, Tamim H, et al. Effect of vitamin D replacement on musculoskeletal parameters in school children: a randomized controlled trial. J Clin Endocrinol Metab. 2006;91:405–12. doi: 10.1210/jc.2005-1436. [DOI] [PubMed] [Google Scholar]
- 34.Ceglia L. Vitamin D and skeletal muscle tissue and function. Mol Aspects Med. 2008;29:407–14. doi: 10.1016/j.mam.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 35.Russell J. Osteomalacic myopathy. Muscle Nerve. 1994;17:578–80. doi: 10.1002/mus.880170603. [DOI] [PubMed] [Google Scholar]
- 36.Irani P. Electromyography in nutritional osteomalaic myopathy. J Neurol Neurosurg Psychiatry. 1976;39:686–93. doi: 10.1136/jnnp.39.7.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Floyd F, Ayyar D, Barwick D, et al. Myopathy in chronic renal failure. Q J Med. 1974;43(172):509–24. [PubMed] [Google Scholar]
- 38.Glerup H, Mikkelsen K, Poulsen L, et al. Hypovitaminosis D myopathy without biochemical signs of osteomalacic bone involvement. Calcif Tissue Int. 2000;66:419–24. doi: 10.1007/s002230010085. [DOI] [PubMed] [Google Scholar]
- 39.Ziambaras K, Dagogo-Jack S. Reversible muscle weakness in patients with vitamin D deficiency. West J Med. 1997;167:435–9. [PMC free article] [PubMed] [Google Scholar]
- 40.Prabhala A, Garg R, Dandona P. Severe myopathy associated with vitamin D deficiency in western New York. Arch Int Med. 2000;160:1199–203. doi: 10.1001/archinte.160.8.1199. [DOI] [PubMed] [Google Scholar]
- 41.Rimaniol J, Authier F, Chariot P. Muscle weakness in intensive care patients: initial manifestation of vitamin D deficiency. Intensive Care Med. 1994;20:591–2. doi: 10.1007/BF01705729. [DOI] [PubMed] [Google Scholar]
- 42.Mingrone G, Greco A, Castagneto M, Gasbarrini G. A woman who left her wheelchair. Lancet. 1999;353:806. doi: 10.1016/s0140-6736(98)10206-4. [DOI] [PubMed] [Google Scholar]
- 43.Sato Y, Iwamoto J, kanoko R, Satoh K. Low-dose vitamin D prevents muscular atrophy and reduces falls and hip fractures in women after stroke: a randomized controlled trial. Cerebrovasc Dis. 2005;20:187–92. doi: 10.1159/000087203. [DOI] [PubMed] [Google Scholar]
- 44.Iannuzzi-Sucich M, Prestwook K, Kenny A. Prevalence of saracopenia and predictors of skeletal muscle mass in healthy, older men and women. J Gerontol. 2002;57A:M772–7. doi: 10.1093/gerona/57.12.m772. [DOI] [PubMed] [Google Scholar]
- 45.Latham N, Anderseon C, Reid I. Effects of vitamin D supplementation on strength, physical performance, and falls in older persons: a systematic review. J Am Geriatr Soc. 2003;51:1219–26. doi: 10.1046/j.1532-5415.2003.51405.x. [DOI] [PubMed] [Google Scholar]
- 46.Janssen H, Samson M, Verhaar H. Vitamin D deficiency, muscle function, and falls in elderly people. Am J Clin Nutr. 2002;75:611–5. doi: 10.1093/ajcn/75.4.611. [DOI] [PubMed] [Google Scholar]
- 47.Wicherts I, van Schoor N, Boeke J, et al. Vitamin D status predicts physical performance and its decline in older persons. J Clin Endocrinol Metabol. 2007;92:2058–65. doi: 10.1210/jc.2006-1525. [DOI] [PubMed] [Google Scholar]
- 48.Mowe M, Haug E, Bohmer T. Low serum calcidiol concentration in older adults with reduced muscular function. J Am Geriatr Soc. 1999;47:220–6. doi: 10.1111/j.1532-5415.1999.tb04581.x. [DOI] [PubMed] [Google Scholar]
- 49.Houston D, Cesari M, Ferrucci L, et al. Association between vitamin D status and physical performance: The InCHIANTI Study. J Gerontol. 2007;62A:440–6. doi: 10.1093/gerona/62.4.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bischoff-Ferrari H, Dietrich T, Orav E, et al. Higher 25-hydroxyvitamin D concentration are associated with better lower-extremity function in both active and inactive persons aged >60y. Am J Clin Nutr. 2004;80:752–8. doi: 10.1093/ajcn/80.3.752. [DOI] [PubMed] [Google Scholar]
- 51.Bischoff H, Stahelin H, Urscheler N, et al. Muscle strength in the elderly: its relation to vitamin D Metabolites. Arch Phys Med Rehab. 1999;80:54–8. doi: 10.1016/s0003-9993(99)90307-6. [DOI] [PubMed] [Google Scholar]
- 52.Visser M, Deeg D, Lips P. Low Vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (Sarcopenia): The Longitudinal Aging Study Amsterdam. J Clin Endocrinol Metabol. 2003;88:5766–72. doi: 10.1210/jc.2003-030604. [DOI] [PubMed] [Google Scholar]
- 53.Bischoff H, Stahelin H, Dick W, et al. Effects of vitamin D and calcium supplementation on falls: a randomized controlled Trial. J Bone Min Res. 2003;18:343–51. doi: 10.1359/jbmr.2003.18.2.343. [DOI] [PubMed] [Google Scholar]
- 54.Gerdhem P, Ringsberg K, Obrant K, Akesson K. Association between 25-hydroxy vitamin D levels, physical activity, muscle strength and fractures in the prospective population-based OPRA study of elderly women. Osteoporosis Int. 2005;16:1425–31. doi: 10.1007/s00198-005-1860-1. [DOI] [PubMed] [Google Scholar]
- 55.Bunout D, Barrera G, Leiva L, et al. Effects of vitamin D supplementation and exercise training on physical performance in Chilean vitamin D deficient elderly subjects. Exp Gerontol. 2006;41:746–52. doi: 10.1016/j.exger.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 56.Dhesi J, Jackson S, Bearne L, et al. Vitamin D supplementation improves neuromuscular function in older people who fall. Age Ageing. 2004;33:589–95. doi: 10.1093/ageing/afh209. [DOI] [PubMed] [Google Scholar]
- 57.Verhaar H, Samson M, Jansen P, et al. Muscle strength, functional mobility and vitamin D in older women. Ageing Clin Exp Res. 2000;12:455–60. doi: 10.1007/BF03339877. [DOI] [PubMed] [Google Scholar]
- 58.Latham N, Anderson C, Lee A, et al. A randomized, controlled trial of quadriceps resistance exercise and vitamin D in Frail Older People: The Frailty Interventions Trial in Elderly Subjects (FITNESS) J Am Geriatr Soc. 2003;51:291–9. doi: 10.1046/j.1532-5415.2003.51101.x. [DOI] [PubMed] [Google Scholar]
- 59.Bischoff-Ferrari H, Borchers M, Durmuller U, et al. Vitamin D receptor expression in human muscle tissue decreases with age. J Bone Miner Res. 2004;19:265–9. doi: 10.1359/jbmr.2004.19.2.265. [DOI] [PubMed] [Google Scholar]
- 60.Roth S, Zmuda J, Cauley J, et al. Vitamin D receptor genotype is associated with fat-free mass aand sarcopenia in elderly men. J Gerontol. 2004;59:10–5. doi: 10.1093/gerona/59.1.b10. [DOI] [PubMed] [Google Scholar]
- 61.Simpson R, Thomas G, Arnold A. Identification of 1,25-dihydroxyvitamin D3 receptors and activities in muscle. J Bio Chem. 1985;260:8882–91. [PubMed] [Google Scholar]
- 62.Nibbelink K, Tishkoff D, Hershey S, et al. 1,25(OH)2vitamin D3 actions on cell proliferation, size, gene expression, and receptor localisation, in the HL-1 cardiac myocyte. J Steroid Biochem Mol Biol. 2007;103:533–7. doi: 10.1016/j.jsbmb.2006.12.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pfeifer M, Begerow B, Minne H. Vitamin D and muscle function. Osteoporosis Int. 2002;13:187–94. doi: 10.1007/s001980200012. [DOI] [PubMed] [Google Scholar]
- 64.Bischoff H, Borchers M, Gudat F, et al. In situ detection of 1,25-dihydroxyvitamin D3 receptor in human skeletal muscle tissue. Histochem J. 2001;33:19–24. doi: 10.1023/a:1017535728844. [DOI] [PubMed] [Google Scholar]
- 65.Peng L, Malloy P, Feldman D. Identification of a functional vitamin D response element in the human insulin-like growth factor binding protein-3 promoter. Mol Endocrinol. 2004;18:1109–19. doi: 10.1210/me.2003-0344. [DOI] [PubMed] [Google Scholar]
- 66.Liao L, Chen X, Wang S, et al. Steroid receptor coactivator 3 maintains circulating insulin-like growth factor 1 (IGF-1) by controlling IGF-binding protein 3 expression. Mol Cell Biol. 2008;28:2460–9. doi: 10.1128/MCB.01163-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fleet J. Rapid, Membrane-initiated actions of 1,25 dihydroxyvitamin D: What are they and what do they mean? J Nutr. 2004;134:3215–8. doi: 10.1093/jn/134.12.3215. [DOI] [PubMed] [Google Scholar]
- 68.Grundberg E, Brandstrom H, Ribom E, et al. Genetic variation in the human vitamin D receptor is associated with muscle strength, fat mass and body weight in Swedish women. Euro J Endocrinol. 2004;150:323–8. doi: 10.1530/eje.0.1500323. [DOI] [PubMed] [Google Scholar]
- 69.Geusens P, Vandevyver C, Vanhoof J, et al. Quadriceps and grip strength are related to vitamin D receptor genotype in elderly nonobese women. J Bone Miner Res. 1997;12:2082–8. doi: 10.1359/jbmr.1997.12.12.2082. [DOI] [PubMed] [Google Scholar]
- 70.Hopkinson N, Li K, Kehoe A, et al. Vitamin D receptor genotypes influence quadriceps strength in obstructive pulmonary disease. Am J Clin Nutr. 2008;87:385–90. doi: 10.1093/ajcn/87.2.385. [DOI] [PubMed] [Google Scholar]
- 71.Endo I, Inoue D, Mitsui T, et al. Deletion of vitamin D receptor gene in mice results in abnormal skeletal muscle development with deregulated expression of myoregulatory transcription factors. Endocrinology. 2003;144:5138–44. doi: 10.1210/en.2003-0502. [DOI] [PubMed] [Google Scholar]
- 72.Berchtold M, Brinkmeirer H, Muntener M. Calcium ion in skeletal muscle: Its crucial role for muscle function, plasticity, and disease. Physiol Rev. 2000;80:1215–65. doi: 10.1152/physrev.2000.80.3.1215. [DOI] [PubMed] [Google Scholar]
- 73.Berg U, Gustafsson T, Sundberg C, et al. Interstitial IGF-1 in exercising skeletal muscle in women. Euro J Endocrinol. 2007;157:427–35. doi: 10.1530/EJE-07-0141. [DOI] [PubMed] [Google Scholar]
- 74.Baxter R. Insulin-like growth factor (IGF)-binding proteins: interactions with IGF's and intrinsic bioactivities. Am J Physiol Endocrinol Metab. 2000;278:E967–976. doi: 10.1152/ajpendo.2000.278.6.E967. [DOI] [PubMed] [Google Scholar]
- 75.Schertzer J, Gehrig S, Ryall J, Lynch GS. Modulation of insulin-like growth factor (IGF-1) and IGF-binding protein interactions enhances skeletal muscle regeneration and ameliorates the dystrophic pathology in mdx mice. Am J Pathol. 2007;171:1180–8. doi: 10.2353/ajpath.2007.070292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Barton-Davis E, Shoturma D, Mausaro A, et al. Viral mediated expression of insulin-like growth factor blocks the aging-related loss of skeletal muscle function. Proc Natl Acad Sci. 1998;95:15603–7. doi: 10.1073/pnas.95.26.15603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Grounds M. Reasons for the degeneration of ageing skeletal muscle: a central role for IGF-1 signalling. Biogerontology. 2002;3:19–24. doi: 10.1023/a:1015234709314. [DOI] [PubMed] [Google Scholar]
- 78.Adams G. Insulin-like growth factor in muscle growth and its potential abuse by athletes. Br J Sport Med. 2000;34:412–3. doi: 10.1136/bjsm.34.6.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Soliman A, Al Khalaf F, Alhemaidi N, et al. Linear growth in relation to the circulating concentrations of insulin-like growth factor 1, parathyroid hormone, and 25-hydroxy vitamin D in children with nutritional rickets before and after treatment: endocrine adaptiation to vitamin D deficiency. Metabolism. 2008;57:95–102. doi: 10.1016/j.metabol.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 80.Boland R, de Boland A, Marinissen M, et al. Avian muscle cells as targets for the secosteroid hormone 1,25-dihydroxy-vitamin D3. Mol Cell Endocrinol. 1995;114:1–8. doi: 10.1016/0303-7207(95)03650-v. [DOI] [PubMed] [Google Scholar]
- 81.Nguyen T, Lieberherr M, Fritsch J, et al. The rapid effects of 1,25-dihydroxyvitamin D3 require the vitamin D receptor and influence 24-hydroxylase activity. J Bio Chem. 2004;279:7591–7. doi: 10.1074/jbc.M309517200. [DOI] [PubMed] [Google Scholar]
- 82.Vazquez G, de Boland A, Boland R. Stimulation of Ca2+ release-activated Ca2+ channels as a potential mechanism involved in non-genomic 1,25(oh)2-vitamin D3-induced Ca2+ entry in skeletal muscle cells. Biochem Biophys Res Commun. 1997;239:562–5. doi: 10.1006/bbrc.1997.7501. [DOI] [PubMed] [Google Scholar]
- 83.Nemere I, Schwartz Z, Pedrozo H, et al. Identification of a membrane receptor for 1,25-dihydroxyvitamin D3 which mediates rapid activation of protein kinase C. J Bone Miner Res. 1998;13:1353–9. doi: 10.1359/jbmr.1998.13.9.1353. [DOI] [PubMed] [Google Scholar]
- 84.Schmidt B, Gerdes D, Feuring M, et al. Rapid, nongenomic steroid actions: A new age? Front Neuroendocrinol. 2000;21:57–94. doi: 10.1006/frne.1999.0189. [DOI] [PubMed] [Google Scholar]
- 85.Bray M, Hagberg JM, Perusse L, et al. The human gene map for performance and health-related fitness phenotypes: The 2006-2007 update. Med Sci Sports Exerc. 2009;41:34–72. doi: 10.1249/mss.0b013e3181844179. [DOI] [PubMed] [Google Scholar]
- 86.Bahat G, Saka B, Erten N, et al. Bsm1 Polymorphism in the vitamin D receptor gene is associated with leg extensor muscle strength in elderly men. Aging Clin Exp Res. 2010;22:198–205. doi: 10.1007/BF03324797. [DOI] [PubMed] [Google Scholar]
- 87.Guo S, Magnuson V, Schiller J, et al. Meta-analysis of vitamin D receptor polymorphisms and type 1 diabetes: A HuGE review of genetic association studies. Am J Epidemiol. 2006;164:711–24. doi: 10.1093/aje/kwj278. [DOI] [PubMed] [Google Scholar]
- 88.Zhang C, Wang C, Liang J, et al. The vitamin D receptor Fok1 polymorphism and bone mineral density in Chinese children. Clin Chim Acta. 2008;395:111–4. doi: 10.1016/j.cca.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 89.Tajima O, Ashizawa N, Ishii T, et al. Interaction of the effects between vitamin D receptor polymorphism and exercise training on bone metabolism. J Applied Physiol. 2000;88:1271–6. doi: 10.1152/jappl.2000.88.4.1271. [DOI] [PubMed] [Google Scholar]
- 90.Rabon-Stith K, Hagberg J, Phares D, et al. Vitamin D receptor FokI genotype influences bone mineral density response to strength training, but not aerobic training. Exp Physiol. 2005;90:653–61. doi: 10.1113/expphysiol.2005.030197. [DOI] [PubMed] [Google Scholar]