Abstract
Objective
Cortical gray matter (GM) abnormalities in patients with childhood-onset schizophrenia (COS) progress during adolescence ultimately localizing to prefrontal and temporal cortices by early adult age. A previous study of 52 nonpsychotic siblings of COS probands had significant prefrontal and temporal GM deficits that appeared to “normalize” by age 17 years. Here we present a replication with nonoverlapping groups of healthy full siblings and healthy controls.
Method
Using an automated measure and prospectively acquired anatomical brain magnetic resonance images, we mapped cortical GM thickness in nonpsychotic full siblings (n = 43, 68 scans; ages 5 through 26 years) of patients with COS, contrasting them with age-, gender-, and scan interval–matched healthy controls (n = 86, 136 scans). The false-discovery rate procedure was used to control for type I errors due to multiple comparisons.
Results
As in our previous study, young nonpsychotic siblings (<17 years) showed significant GM deficits in bilateral prefrontal and left temporal cortices and, in addition, smaller deficits in the parietal and right inferior temporal cortices. These deficits in nonpsychotic siblings normalized with age with minimal abnormalities remaining by age 17.
Conclusions
Our results support previous findings showing nonpsychotic siblings of COS probands to have early GM deficits that ameliorate with time. At early ages, prefrontal and/or temporal loss may serve as a familial/trait marker for COS. Late adolescence appears to be a critical period for greatest localization of deficits in probands or normalization in nonpsychotic siblings.
Keywords: childhood-onset schizophrenia, cortical thickness, endophenotype, gray matter
Progressive cortical gray matter (GM) loss in prefrontal and temporal cortices is an established feature in schizophrenia.1–5 The cortical GM loss in childhood-onset schizophrenia (COS) seems more profound and spreads in a dynamic parieto-frontal pattern during adolescence, ultimately localizing to prefrontal and temporal cortices by early adulthood.6–9 However, cross-sectional studies of nonpsychotic first-degree relatives of schizophrenia patients have been inconsistent.10–12 Although nonpsychotic relatives have shared GM abnormalities, these have been mostly in deeper structures such as the hippocampus.13 The majority of the studies show lack of cortical GM abnormalities in non-psychotic relatives, suggesting that they are primarily illness related.14 Similarly, the only small longitudinal study in nonpsychotic siblings of adult-onset patients also failed to show cortical deficits at baseline or 5-year follow up.15
Our prior longitudinal study on a large sample of 52 nonpsychotic COS siblings showed a unique pattern of shared cortical development. In comparison with matched controls, nonpsychotic siblings less than 15 years of age had significant GM deficits in the prefrontal and temporal cortices, which appeared to “normalize” by age 17 years.16 That is, although both groups showed early cortical thinning, healthy controls had a higher rate (steeper slope) of GM loss during adolescence in which cortical thickness differences disappeared with time. This pattern of normalizing GM deficits indicates age-specific familial/genetic influence on cortical GM abnormalities. These results may also help to explain the observed lack of cortical GM deficits in the adult studies in which the average age of nonpsychotic relatives is beyond the age when the deficits are normalized in nonpsychotic COS siblings. The normalization of GM deficits by late adolescence also suggests the role of protective factors that probably develop by late adolescence. Given the unexpected nature of the cortical developmental patterns in nonpsychotic siblings in our first study, we studied additional siblings and controls for replication. We hypothesized that a deficit pattern of early prefrontal and temporal deficits would normalize by late adolescence confirming a trait nature of GM deficits in early ages.
METHOD
COS Probands
Since 1991, through national recruitment and pre-screening of more than 2000 submitted case records and in-person screening of more than 300 subjects, 112 patients to date have met unmodified DSM-III-R/DSM-IV criteria for schizophrenia with onset of psychosis before their 13th birthday. Patients with a history of significant medical problems, substance abuse, or an IQ of less than 70 before the onset of psychotic symptoms were excluded. Further details of patient selection, including those for the COS sample used for comparison in this study, are described elsewhere.17,18
Siblings of Patients With COS
Available biological full siblings of patients with COS participated in the study and were scanned prospectively every 2 years along with their probands. Siblings were interviewed using structured psychiatric interviews for Axis I (using either the Schedule for Affective Disorders and Schizophrenia [SADS]19 or the Schedule for Affective Disorders and Schizophrenia for School-Age Children [K-SADS].20 Axis II diagnoses were estimated using the Structured Interview for the DSM-III Personality Disorders [SIDP].21 All diagnoses (Axis I and Axis II) were made by a child psychiatrist. Siblings were considered to be nonpsychotic if they were free of any psychotic or schizophrenia spectrum disorder (which included schizophrenia, schizoaffective disorder on Axis I, or paranoid, schizotypal, schizoid, or avoidant personality disorders on Axis II).22 In addition, symptom counts for all Axis II disorders were calculated both for individual disorders and also cumulatively for schizophrenia spectrum diagnoses. Since our 2007 study, a total of 53 siblings were screened, of whom 49 full siblings were considered nonpsychotic. Of these individuals, 43 had at least one magnetic resonance image available for cortical thickness estimation.
Control Subjects
A second group of 86 unrelated healthy controls was selected from a sample of community volunteers recruited as part of a prospective study of normal brain development and matched for gender, age, number of repeated scans, and scan interval. Controls were free of lifetime medical or psychiatric disorders as determined by clinical examination and standardized interview. Psychiatric illness in a first-degree relative was also exclusionary.23 Each control used in our study belonged to a unique family. It should be noted that the sample of controls, COS patients and siblings were entirely new and unrelated to the sample used in our original study.
The research protocol was approved by the National Institute of Mental Health (NIMH) institutional review board. Written informed consent was obtained from parents and controls and patients older than 18 years, and written informed assent was obtained from minors.
Magnetic Resonance Image Acquisition and Analysis
Briefly, T1-weighted images with contiguous 1.5-mm slices in the axial plane were obtained using a three-dimensional spoiled gradient recalled echo sequence in the steady state. Imaging parameters were echo time of 5 milliseconds, repetition time of 24 milliseconds, flip angle of 45°, acquisition matrix of 256 × 192, number of excitations equaled 1, and a 24-cm field of view. Head placement was standardized as previously described.24 Magnetic resonance images were registered into standardized space using a linear transformation and corrected for nonuniformity artifacts.25 Registered and corrected volumes were segmented with an advanced neural net classifier26 and GM and white matter surfaces were fitted with a surface deformation algorithm, which first determines the white matter surface, then expands outward to find the GM–cerebrospinal fluid intersection.27,28 Cortical thickness measurements, defined as the distance between linked vertices of the GM and white matter boundaries using a 30-mm surface-based blurring kernel (which has been shown to maximize statistical power), have been previously validated and were calculated in native space at 40,962 cortical points.29
Statistical Analysis
Differences in sibling/control group demographics were tested using t tests or Mann–Whitney U tests (if assumptions were violated) for continuous variables, c2 tests of independence for categorical variables, and the Fisher exact test for categorical variables if there was an expected cell count of less than 5.
We performed a linear mixed effects regression model at each of the 40,962 GM points per hemisphere. Eight of 25 families contributed more than one sibling. Controls were unrelated. Because multiple siblings per family and multiple scans per person over time were included in the analyses, two random intercepts were included for all analyses: one intercept to model within-family dependence, and one intercept to model person within-family dependence. At each point, the dependent variable was GM thickness. Fixed effects included cortical thickness against group, age (centered at the sample average age), and group × age. A straight-line fit was deemed adequate to model cortical thickness development because the combined contribution of quadratic age terms for both groups was not a significant contributor to the explanatory power of the model across the cortical surface after using a false-discovery rate correction. Cortical thickness was measured in native space, and the siblings and controls did not differ significantly in either total cerebral volume or mean cortical thickness. Type 1 error was controlled per hemisphere using the False Discovery Rate (FDR) procedure30 with q set at 0.05 (i.e., no more than 5% are false positive). The model was run with gender and socioeconomic status covariates to account for variance in cortical thickness due to gender.
Age Recentering
These analyses entailed the same model as in the Statistical Analysis section but recentered age at approximate 3-year intervals within the middle 80% of the age range. If stratified by age, the individual groups become too small and lose power, and the cross- sectional analyses become vulnerable to cohort effects. This approach of age recentering permits interpretation of the intercept and group differences at the centered age (instead of at age zero). It also permits maximal use of the entire data set without separating participants into age groups and thereby diminishing statistical power. We have successfully applied this strategy in prior studies on cortical thickness.9,16,31 The differences that are visually represented at different ages are essentially snapshots of the differences between the two groups’ regression lines at the specified age, where all scans are informing all pictures in Figure 1. A video (Videos S1, available online) using this methodology was created to further illustrate how cortical differences between the two groups change dynamically over time. This video used a combined group of COS siblings from both the original16 and current study so as to include the largest sample of COS siblings to demonstrate effect.
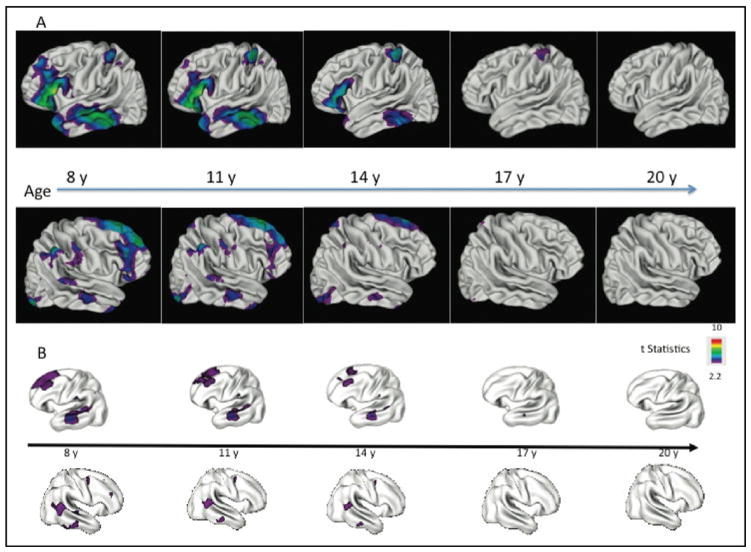
FIGURE 1.
(A) Comparison of cortical gray matter (GM) thickness (right and left hemisphere) of nonpsychotic siblings of patients with childhood-onset schizophrenia (COS) (n = 43, 68 scans) versus age-, gender-, and scan interval–matched healthy controls (n = 83, 136 scans) between ages 5 and 26 years. Note: Nonpsychotic siblings show significant GM deficits in bilateral prefrontal and left temporal cortices with smaller deficits in the parietal cortices and right inferior temporal cortices. These deficits in nonpsychotic siblings normalize with age, with no abnormalities remaining by age 20 years. Color bar shows the t statistic with the threshold to control for multiple comparisons using the false-discovery rate procedure with q = 0.05. (B) GM thickness of COS nonpsychotic siblings (n = 52, 113 scans) compared with healthy controls (n = 52, 108 scans) between ages 7 and 26 years. Note: Cortical GM thickness is adjusted for mean cortical thickness. These data are published elsewhere16 and are used here only for visual comparison with the GM developmental pattern of the current study (A).
RESULTS
Demographics for both the original and current study are shown in Table 1. The groups were well matched on all the variables with the exception of socioeconomic status32 (p = .001) that were lower for siblings of patients with COS than for the unrelated healthy controls.
TABLE 1.
Sample Demographics for Siblings of Patients With Childhood-Onset Schizophrenia and Healthy Controls
| Demographic Characteristic | Healthy Controls (n = 86; 136 Scans) | Siblings (n = 43; 68 Scans) | Test Statistic | P Value |
|---|---|---|---|---|
| Men/Women | 34/52 | 17/26 | χ21 = 0 | .99 |
| No. of scans at | χ22 = 0 | .99 | ||
| Time 1 | 86 | 43 | ||
| Time 2 | 40 | 20 | ||
| Time 3 | 10 | 5 | ||
| Age at scan, y, mean (SD) [range] | ||||
| All scans | 13.68 (5.66) [5.47–25.57] | 13.66 (5.64) [5.25–24.95] | t202 = −0.027 | .98 |
| Time 1 | 13.36 (5.942) | 13.36 (5.942) | t127 = 0.005 | .97 |
| Time 2 | 13.78 (4.882) | 13.71 (4.983) | t58 = −0.052 | .96 |
| Time 3 | 16.08 (6.171) | 16.00 (6.098) | t13 = −0.025 | .98 |
| Socioeconomic statusa | 42.03 (22.32) | 53.19 (19.59) | t199 = 3.49 | .001 |
| Ethnicity | ||||
| White | 63 | 30 | ||
| African American | 16 | 6 | ||
| Hispanic | 4 | 5 | ||
| Other | 3 | 2 | ||
| Comorbid Axis I disorders (lifetime) in nonpsychotic siblings | ||||
| Anxiety disorder | 16 | |||
| Alcohol/substance abuse | 3 | |||
| Behavioral disorder | 8 | |||
| MR or LD | 4 | |||
| Bipolar disorder | 1 | |||
| Depressive disorder (major/minor depression, dysthymia) | 23 |
Note: Statistically significant p value is in boldface. LD = learning disability; MR = mental retardation.
Socioeconomic status was measured using the Hollingshead31 scale. Higher scores reflect lower socioeconomic status.
There were no significant mean cortical thickness differences between the nonpsychotic siblings and their matched controls based on the average of all scans. A comparison of cortical GM development of the two groups between ages 5 and 26 years revealed nonpsychotic siblings had an early restricted pattern of GM loss (Figure 1). Furthermore, nonpsychotic siblings showed significant GM deficits in bilateral prefrontal and left temporal cortices with smaller deficits in the parietal and right inferior temporal cortices. These deficits in nonpsychotic siblings normalized with age, with no abnormalities remaining by age 17 year (Figure 1). There were smaller areas of significant parietal GM loss on the right side of the brain (middle parietal cortices) and the left side of the brain (superior parietal cortices) that also normalized. A reanalysis of the data excluding 14 siblings (19 scans) and 28 healthy volunteers (38 scans) to make the sample independent (one subject per family) did not significantly change the results. Similarly, to address whether the presence of depression affected our findings, we comparatively analyzed the subgroup of COS siblings (n = 23, time 1; n = 12, time 2; n = 2, time 3) with depressive disorders to a group of matched healthy controls. The subgroup also demonstrated a normalization of gray matter findings over time with no apparent gray matter deficits by age 17 (p = .01). However, the findings in this subgroup did not survive adjustments for multiple comparisons.
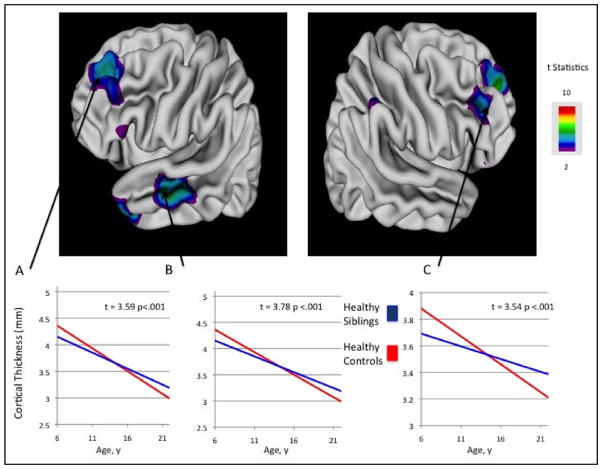
The developmental trajectories of selected regional cortical gray matter thickness are compared between the two groups in Figure 2. Healthy controls had a significantly steeper slope (group × age interaction effects) of GM loss than in the siblings in these areas (Figure 2). This eventually leads to an equilibration of GM between the two groups during adolescence. A separate analysis looking at average GM loss rates for nonpsychotic siblings compared to controls and found a positive slope for siblings at the rate of 3% to 5% per year depending on region.
FIGURE 2.
Longitudinal trajectories (slopes) of selected regional cortical gray matter (GM) thickness in nonpsychotic siblings of patients with childhood-onset schizophrenia (COS) compared with those for the same regions for healthy controls, showing group × age interaction effects. Note: Siblings of patients with COS ”normalize” by age 17 years (by slower thinning) in the left prefrontal cortices (A) (df = 71, t = 3.59, p < .001) by age 16 years in the left middle temporal cortices (B) (df = 71, t = 3.78, p < .001), and right prefrontal cortices (C) (df = 71, t = 3.54, p < .001). Graphs represent region of interest analyses at selected regions. Color bar shows the t statistic with the threshold to control for multiple comparisons using the false-discovery rate procedure with q = 0.05.
DISCUSSION
The main goal of this study was to replicate and extend our prior unexpected finding of early frontal and temporal GM deficits in COS siblings that “normalized” by late adolescence. A nonoverlapping sample of nonpsychotic sibling scans and of matched controls again showed early cortical GM deficits that normalizeby age 17 years. When the two samples were combined, the consistency was striking, as shown in the supplementary movie (Video S1, available online). Both groups showed cortical thinning, with healthy controls having a higher rate (steeper slope) of gray matter loss during adolescence. Compared with controls, nonpsychotic siblings appeared to normalize GM at an annual rate of 3% to 5%, depending on region. This suggests that prefrontal and temporal cortical deficits in schizophrenia are age-specific familal/genetic trait markers.
Whether the cortical GM deficits in schizophrenia represent familial/genetic trait markers has remained an unsettled issue. Recent studies of the first-degree relative studies appear to point against such evidence and fail to show any focal cortical GM deficits in siblings/relatives/cotwins, thus arguing against the trait status of the deficits.14,33,34 In addition, studies of ultra-high risk subjects support the idea that cortical volume loss in schizophrenia is illness related,35,36 as brain deficits were not found in subjects who did not convert to psychosis. Furthermore, reductions in GM volume have been related to duration of untreated psychosis37 and duration of psychotic illness,38 bolstering support for GM loss being reflective of disease state.
Interestingly, the GM deficits appear to normalize around the same age at which GM deficits in probands become circumscribed to prefrontal and temporal regions. The normalization of GM deficits in nonpsychotic siblings could then be viewed as a parallel to the overall developmental process in probands, as they display early prefrontal and temporal GM deficits with earlier normalization. Adolescence appears to be a critical time of plasticity, as reflected by the relative restoration of prefrontal/temporal cortical thinning in siblings.
The mechanism by which cortical normalization occurs and its implications are unclear, although they clearly reflect plasticity of the brain both in schizophrenia as well as in nonpsychotic siblings and during adolescence. Prior in vitro and in vivo studies have shown significant alterations in prefrontal cortex synaptic plasticity induction with schizophrenia,39,40 and neurodevelopmental animal models suggest that the illness is associated with abnormal synaptic plasticity.39,41,42 By changing synaptic plasticity and reorganizing neuronal inputs/outputs,43 one could, in theory, alter the balance of information processing and avoid onset of psychotic illness, a process that is probably more efficient in healthy siblings.
The normalization could have functional advantage. Higher intelligence is associated with a delayed trajectory of frontal cortical development,44,45 and a positive correlation exists between cortical thickness and intelligence from late childhood onward.44 Recently, Hartberg et al. investigated the relationship between cortical thickness and cognitive performance in 67 patients with schizophrenia and 69 healthy controls, and found a similar relationships between prefrontal cortical thickness and verbal abilities and executive functioning.46 It is expected that the process of GM normalization in nonpsychotic siblings could theoretically modulate the preservation and enhancement of cognitive and language abilities.
Overall, there is a dearth in the literature on cognitive deficits in siblings of early-onset probands. Our comparison of neurocognitive measures (Trail Making Tests A and B and the Weschler Intelligence Scale–Revised Digit Span and Vocabulary) in 24 siblings and 67 parents of COS probands matched with 114 community controls revealed that COS siblings had significantly poorer performance and subtle deficits involving oculomotor/psychomotor speed than did community controls.47 However, the average age of the siblings examined was 19.3 years (standard deviation [SD] 7.0 years), which is past the age in which structural GM deficits would occur. Previous work has established that subtle cognitive deficits including abnormalities of attention, verbal memory, and executive functioning are seen in healthy relatives of patients with AOS.48,49 Our structural findings would support the notion that not only do cognitive deficits exist in nonpsychotic siblings but these deficits are potentially more severe at earlier ages. We are currently expanding our neuropsychological battery on the sibling sample as well as use multimodal imaging to gain insights into specific neurocircuitries.
In the same light, although the structural brain abnormalities normalize in nonpsychotic siblings, it is possible that they have long-term functional abnormalities. A recent systematic review of functional magnetic resonance imaging (fMRI) studies in nonpsychotic relatives of schizophrenic patients found consistent increases in activation in the right ventral prefrontal cortex and right parietal cortex.50 Similarly, a handful of studies examining white matter deficiencies in nonpsychotic siblings of schizophrenia patients found decreased fractional anisotropy in a variety of areas including left inferior frontal gyrus as well as in multiple regions of the cingulate WM bilaterally and in angular gyral WM bilaterally.51,52 Further longitudinal studies are needed to address whether more subtle disruptions of neural connectivity are indeed present in both childhood and adolescence in nonpsychotic siblings.
This study had a number of limitations. A total of 35 nonpsychotic siblings were less than 20 years of age and not past the age risk for schizophrenia and/or spectrum disorders, and in theory could develop these conditions as adults, although the use of stringent inclusionary criteria makes this unlikely. Second, a small percentage of siblings had three or more scans, making our study only partially longitudinal and potentially susceptible to cohort effects. In addition, older siblings were less likely to have follow-up scans compared with younger siblings. Ideally, it would be nice to see the same subjects from early ages through late adolescence, thus requiring more than 10 years of follow-up. Consequently, restrictions in study power and missing data could have affected the distribution and magnitude of cortical thickness differences between the two groups. For example, our study finding of linear rather than quadratic neuroanatomic GM changes over the specified age range might be due to limited power. Further attention and reanalysis using larger samples is clearly warranted to further characterize cortical development over time. Third, we were not able to match healthy controls to nonpsychotic siblings in a 2:1 fashion for the Hispanic/Other ethnic groups. As a result, these subgroups may have been overrepresented in our sibling sample. Finally, some siblings had a lifetime history of comorbid Axis I diagnoses, which theoretically could affect their cortical brain development. This was reflected in our analysis of the subgroup of nonpsychotic siblings with depressive disorders in which adjustments for multiple comparisons failed to localize deficits at the prefrontal and temporal cortices. Collectively, these results should be interpreted with caution, given the limitations of power and sample size.
In conclusion, we replicate findings from our previous studies showing nonpsychotic siblings of COS probands to have early GM structural deficits that ameliorate with time. Although the intimate mechanisms of this normalization are yet to be defined, continued exploration of this process could lead to identification of a potential intermediate phenotype and a future target for genetic studies and for treatment.
Footnotes
Disclosure: Drs. Mattai, Greenstein, Clasen, Miller, Tossell, Rapoport, and Gogtay, and Mr. Weisinger and Ms Stidd report no biomedical financial interests or potential conflicts of interest.
Supplemental material cited in this article is available online.
References
- 1.Wright IC, Rabe-Hesketh S, Woodruff PW, David AS, Murray RM, Bullmore ET. Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatry. 2000;157:16–25. doi: 10.1176/ajp.157.1.16. [DOI] [PubMed] [Google Scholar]
- 2.Lawrie SM, Abukmeil SS. Brain abnormality in schizophrenia. A systematic and quantitative review of volumetric magnetic resonance imaging studies. Br J Psychiatry. 1998;172:110–120. doi: 10.1192/bjp.172.2.110. [DOI] [PubMed] [Google Scholar]
- 3.Honea R, Crow TJ, Passingham D, Mackay CE. Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. Am J Psychiatry. 2005;162:2233–2245. doi: 10.1176/appi.ajp.162.12.2233. [DOI] [PubMed] [Google Scholar]
- 4.Kuperberg GR, Broome MR, McGuire PK, et al. Regionally localized thinning of the cerebral cortex in schizophrenia. Arch Gen Psychiatry. 2003;60:878–888. doi: 10.1001/archpsyc.60.9.878. [DOI] [PubMed] [Google Scholar]
- 5.Narr KL, Bilder RM, Toga AW, et al. Mapping cortical thickness and gray matter concentration in first episode schizophrenia. Cereb Cortex. 2005;15:708–719. doi: 10.1093/cercor/bhh172. [DOI] [PubMed] [Google Scholar]
- 6.Rapoport JL, Giedd JN, Blumenthal J, et al. Progressive cortical change during adolescence in childhood-onset schizophrenia. A longitudinal magnetic resonance imaging study. Arch Gen Psychiatry. 1999;56:649–654. doi: 10.1001/archpsyc.56.7.649. [DOI] [PubMed] [Google Scholar]
- 7.Giedd JN, Jeffries NO, Blumenthal J, et al. Childhood-onset schizophrenia: progressive brain changes during adolescence. Biol Psychiatry. 1999;46:892–898. doi: 10.1016/s0006-3223(99)00072-4. [DOI] [PubMed] [Google Scholar]
- 8.Thompson PM, Vidal C, Giedd JN, et al. Mapping adolescent brain change reveals dynamic wave of accelerated gray matter loss in very early-onset schizophrenia. Proc Natl Acad Sci U S A. 2001;98:11650–11655. doi: 10.1073/pnas.201243998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenstein D, Lerch J, Shaw P, et al. Childhood onset schizophrenia: cortical brain abnormalities as young adults. J Child Psychol Psychiatry. 2006;47:1003–1012. doi: 10.1111/j.1469-7610.2006.01658.x. [DOI] [PubMed] [Google Scholar]
- 10.Cannon TD, van Erp TG, Huttunen M, et al. Regional gray matter, white matter, and cerebrospinal fluid distributions in schizophrenic patients, their siblings, and controls. Arch Gen Psychiatry. 1998;55:1084–1091. doi: 10.1001/archpsyc.55.12.1084. [DOI] [PubMed] [Google Scholar]
- 11.McDonald C, Marshall N, Sham PC, et al. Regional brain morphometry in patients with schizophrenia or bipolar disorder and their unaffected relatives. Am J Psychiatry. 2006;163:478–487. doi: 10.1176/appi.ajp.163.3.478. [DOI] [PubMed] [Google Scholar]
- 12.Staal WG, Hulshoff Pol HE, Schnack HG, Hoogendoorn ML, Jellema K, Kahn RS. Structural brain abnormalities in patients with schizophrenia and their healthy siblings. Am J Psychiatry. 2000;157:416–421. doi: 10.1176/appi.ajp.157.3.416. [DOI] [PubMed] [Google Scholar]
- 13.Boos HB, Aleman A, Cahn W, Hulshoff Pol H, Kahn RS. Brain volumes in relatives of patients with schizophrenia: a meta-analysis. Arch Gen Psychiatry. 2007;64:297–304. doi: 10.1001/archpsyc.64.3.297. [DOI] [PubMed] [Google Scholar]
- 14.Goldman AL, Pezawas L, Mattay VS, et al. Widespread reductions of cortical thickness in schizophrenia and spectrum disorders and evidence of heritability. Arch Gen Psychiatry. 2009;66:467–477. doi: 10.1001/archgenpsychiatry.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brans RG, van Haren NE, van Baal GC, et al. Longitudinal MRI study in schizophrenia patients and their healthy siblings. Br J Psychiatry. 2008;193:422–423. doi: 10.1192/bjp.bp.107.041467. [DOI] [PubMed] [Google Scholar]
- 16.Gogtay N, Greenstein D, Lenane M, et al. Cortical brain development in nonpsychotic siblings of patients with childhood-onset schizophrenia. Arch Gen Psychiatry. 2007;64:772–780. doi: 10.1001/archpsyc.64.7.772. [DOI] [PubMed] [Google Scholar]
- 17.McKenna K, Gordon CT, Lenane M, Kaysen D, Fahey K, Rapoport JL. Looking for childhood-onset schizophrenia: the first 71 cases screened. J Am Acad Child Adolesc Psychiatry. 1994;33:636–644. doi: 10.1097/00004583-199406000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Kumra S, Frazier JA, Jacobsen LK, et al. Childhood-onset schizophrenia. A double-blind clozapine-haloperidol comparison. Arch Gen Psychiatry. 1996;53:1090–1097. doi: 10.1001/archpsyc.1996.01830120020005. [DOI] [PubMed] [Google Scholar]
- 19.Endicott J, Spitzer RL. Schedule for Affective Disorders and Schizophrenia (SADS) Acta Psychiatr Belg. 1987;87:361–516. [PubMed] [Google Scholar]
- 20.Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 21.Stangl D, Pfohl B, Zimmerman M, Bowers W, Corenthal C. A structured interview for the DSM-III personality disorders. A preliminary report. Arch Gen Psychiatry. 1985;42:591–596. doi: 10.1001/archpsyc.1985.01790290073008. [DOI] [PubMed] [Google Scholar]
- 22.Asarnow RF, Nuechterlein KH, Fogelson D, et al. Schizophrenia and schizophrenia-spectrum personality disorders in the first-degree relatives of children with schizophrenia: the UCLA family study. Arch Gen Psychiatry. 2001;58:581–588. doi: 10.1001/archpsyc.58.6.581. [DOI] [PubMed] [Google Scholar]
- 23.Giedd JN, Blumenthal J, Jeffries NO, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 24.Castellanos FX, Giedd JN, Berquin PC, et al. Quantitative brain magnetic resonance imaging in girls with attention-deficit/hyper-activity disorder. Arch Gen Psychiatry. 2001;58:289–295. doi: 10.1001/archpsyc.58.3.289. [DOI] [PubMed] [Google Scholar]
- 25.Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- 26.Zijdenbos AP, Forghani R, Evans AC. Automatic ”pipeline” analysis of 3-D MRI data for clinical trials: application to multiple sclerosis. IEEE Trans Med Imaging. 2002;21:1280–1291. doi: 10.1109/TMI.2002.806283. [DOI] [PubMed] [Google Scholar]
- 27.Kim JS, Singh V, Lee JK, et al. Automated 3-D extraction and evaluation of the inner and outer cortical surfaces using a Laplacian map and partial volume effect classification. Neuroimage. 2005;27:210–221. doi: 10.1016/j.neuroimage.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 28.MacDonald D, Kabani N, Avis D, Evans AC. Automated 3-D extraction of inner and outer surfaces of cerebral cortex from MRI. Neuroimage. 2000;12:340–356. doi: 10.1006/nimg.1999.0534. [DOI] [PubMed] [Google Scholar]
- 29.Lerch JP, Evans AC. Cortical thickness analysis examined through power analysis and a population simulation. Neuroimage. 2005;24:163–173. doi: 10.1016/j.neuroimage.2004.07.045. [DOI] [PubMed] [Google Scholar]
- 30.Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- 31.Mattai A, Chavez A, Greenstein D, et al. Effects of clozapine and olanzapine on cortical thickness in childhood-onset schizophrenia. Schizophr Res. 2010;116:44–48. doi: 10.1016/j.schres.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hollingshead A. Four Factor Index for Social Status. New Haven, CT: Yale University; 1975. [Google Scholar]
- 33.Goldman AL, Pezawas L, Mattay VS, et al. Heritability of brain morphology related to schizophrenia: a large-scale automated magnetic resonance imaging segmentation study. Biol Psychiatry. 2008;63:475–483. doi: 10.1016/j.biopsych.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 34.Honea RA, Meyer-Lindenberg A, Hobbs KB, et al. Is gray matter volume an intermediate phenotype for schizophrenia? A voxel-based morphometry study of patients with schizophrenia and their healthy siblings. Biol Psychiatry. 2008;63:465–474. doi: 10.1016/j.biopsych.2007.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lawrie SM, Whalley HC, Abukmeil SS, et al. Temporal lobe volume changes in people at high risk of schizophrenia with psychotic symptoms. Br J Psychiatry. 2002;181:138–143. doi: 10.1017/s0007125000161860. [DOI] [PubMed] [Google Scholar]
- 36.Sun D, Phillips L, Velakoulis D, et al. Progressive brain structural changes mapped as psychosis develops in ‘at risk’ individuals. Schizophr Res. 2009;108:85–92. doi: 10.1016/j.schres.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lappin JM, Morgan K, Morgan C, et al. Gray matter abnormalities associated with duration of untreated psychosis. Schizophr Res. 2006;83:145–153. doi: 10.1016/j.schres.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 38.Cahn W, Rais M, Stigter FP, et al. Psychosis and brain volume changes during the first five years of schizophrenia. Eur Neuropsychopharmacol. 2009;19:147–151. doi: 10.1016/j.euroneuro.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 39.Goto Y, Yang CR, Otani S. Functional and dysfunctional synaptic plasticity in prefrontal cortex: roles in psychiatric disorders. Biol Psychiatry. 2010;67:199–207. doi: 10.1016/j.biopsych.2009.08.026. [DOI] [PubMed] [Google Scholar]
- 40.Stephan KE, Baldeweg T, Friston KJ. Synaptic plasticity and dysconnection in schizophrenia. Biol Psychiatry. 2006;59:929–939. doi: 10.1016/j.biopsych.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 41.Yang CR, Chen L. Targeting prefrontal cortical dopamine D1 and N-methyl-D-aspartate receptor interactions in schizophrenia treatment. Neuroscientist. 2005;11:452–470. doi: 10.1177/1073858405279692. [DOI] [PubMed] [Google Scholar]
- 42.Jensen J, Willeit M, Zipursky RB, et al. The formation of abnormal associations in schizophrenia: neural and behavioral evidence. Neuropsychopharmacology. 2008;33:473–479. doi: 10.1038/sj.npp.1301437. [DOI] [PubMed] [Google Scholar]
- 43.Navarro X. Chapter 27: Neural plasticity after nerve injury and regeneration. Int Rev Neurobiol. 2009;87:483–505. doi: 10.1016/S0074-7742(09)87027-X. [DOI] [PubMed] [Google Scholar]
- 44.Shaw P, Greenstein D, Lerch J, et al. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440:676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- 45.Narr KL, Woods RP, Thompson PM, et al. Relationships between IQ and regional cortical gray matter thickness in healthy adults. Cereb Cortex. 2007;17:2163–2171. doi: 10.1093/cercor/bhl125. [DOI] [PubMed] [Google Scholar]
- 46.Hartberg CB, Lawyer G, Nyman H, et al. Investigating relationships between cortical thickness and cognitive performance in patients with schizophrenia and healthy adults. Psychiatry Res. 2010;182:123–133. doi: 10.1016/j.pscychresns.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 47.Gochman PA, Greenstein D, Sporn A, et al. Childhood onset schizophrenia: familial neurocognitive measures. Schizophr Res. 2004;71:43–47. doi: 10.1016/j.schres.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 48.Faraone SV, Seidman LJ, Kremen WS, Toomey R, Pepple JR, Tsuang MT. Neuropsychological functioning among the nonpsychotic relatives of schizophrenic patients: a 4-year follow-up study. J Abnorm Psychol. 1999;108:176–181. doi: 10.1037//0021-843x.108.1.176. [DOI] [PubMed] [Google Scholar]
- 49.Chen WJ, Faraone SV. Sustained attention deficits as markers of genetic susceptibility to schizophrenia. Am J Med Genet. 2000;97:52–57. doi: 10.1002/(sici)1096-8628(200021)97:1<52::aid-ajmg7>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 50.MacDonald AW, 3rd, Thermenos HW, Barch DM, Seidman LJ. Imaging genetic liability to schizophrenia: systematic review of FMRI studies of patients’ nonpsychotic relatives. Schizophr Bull. 2009;35:1142–1162. doi: 10.1093/schbul/sbn053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hao Y, Yan Q, Liu H, et al. Schizophrenia patients and their healthy siblings share disruption of white matter integrity in the left prefrontal cortex and the hippocampus but not the anterior cingulate cortex. Schizophr Res. 2009;114:128–135. doi: 10.1016/j.schres.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 52.Hoptman MJ, Nierenberg J, Bertisch HC, et al. A DTI study of white matter microstructure in individuals at high genetic risk for schizophrenia. Schizophr Res. 2008;106:115–124. doi: 10.1016/j.schres.2008.07.023. [DOI] [PubMed] [Google Scholar]