Abstract
The proinflammatory stimulus of chorioamnionitis is commonly associated with preterm delivery. Women at risk of preterm delivery receive antenatal glucocorticoids to functionally mature the fetal lung. However, the effects of the combined exposures of chorioamnionitis and antenatal glucocorticoids on the fetus are poorly understood. Time-mated ewes with singleton fetuses received an intra-amniotic injection of lipopolysaccharide (LPS) either preceding or following maternal intramuscular betamethasone 7 or 14 days before delivery, and the fetuses were delivered at 120 days gestational age (GA) (term = 150 days GA). Gestation matched controls received intra-amniotic and maternal intramuscular saline. Compared with saline controls, intra-amniotic LPS increased inflammatory cells in the bronchoalveolar lavage and myeloperoxidase, Toll-like receptor 2 and 4 mRNA, PU.1, CD3, and Foxp3-positive cells in the fetal lung. LPS-induced lung maturation measured as increased airway surfactant and improved lung gas volumes. Intra-amniotic LPS-induced inflammation persisted until 14 days after exposure. Betamethasone treatment alone induced modest lung maturation but, when administered before intra-amniotic LPS, suppressed lung inflammation. Interestingly, betamethasone treatment after LPS did not counteract inflammation but enhanced lung maturation. We conclude that the order of exposures of intra-amniotic LPS or maternal betamethasone had large effects on fetal lung inflammation and maturation.
Keywords: chorioamnionitis, lung inflammation, surfactant, bronchopulmonary dysplasia, respiratory distress syndrome
chorioamnionitis, defined as inflammation of the fetal membranes, complicates up to 70% of preterm deliveries before 30 wk of gestation (16). The epidemiological associations of preterm infants exposed to chorioamnionitis are fetal systemic inflammation and lung, brain, and gastrointestinal injury (17, 42). Lung inflammation may initiate a progressive injury that results in bronchopulmonary dysplasia (26). However, the exposure of the fetal lung to inflammation associated with chorioamnionitis also increases surfactant proteins and lipids with salutary effects on respiratory distress syndrome (1, 30, 36).
Antenatal corticosteroids are a standard treatment given to mothers at risk of imminent preterm birth to induce lung maturation (41). Lung maturation induced by corticosteroids results from the simultaneous induction and suppression of multiple genes that have the net effects of increasing surfactant lipids, surfactant proteins, and thinning of the lung mesenchyme to increase the potential air spaces (23, 46, 48). Clinical studies and experimental evidence suggest that antenatal corticosteroids are also efficacious in the setting of chorioamnionitis (18, 39, 40). The delivery of many women given corticosteroids is delayed for several days to weeks (5), and chorioamnionitis is clinically silent in a majority of women (1). Therefore, the combined exposures of antenatal corticosteroids and chorioamnionitis are common in the preterm fetus, but the order of exposures can vary.
We reported previously that simultaneous exposure of the preterm sheep fetus to antenatal corticosteroids and lipopolysaccharide (LPS) suppressed the pulmonary inflammation for 1 to 2 days after the exposure but amplified the inflammatory response to chorioamnionitis 5 to 15 days after the exposure (28, 33). However, it is not known whether the order of exposure is important in mediating lung inflammation and maturation. Therefore, we aimed to study the interactive effects of chorioamnionitis and antenatal corticosteroids on the inflammatory and maturational response in the fetal lungs in a clinically relevant preterm sheep model of chorioamnionitis (32). We tested the hypothesis that the order of exposure to maternal betamethasone and intra-amniotic LPS will differentially impact lung surfactants and inflammatory responses in the preterm fetus. Fetal sheep were exposed in utero to intra-amniotic LPS or antenatal maternal intramuscular betamethasone, with an interval of 7 days between the two interventions. Furthermore, we asked whether the order of exposure to antenatal corticosteroids and intra-amniotic LPS altered fetal lung outcomes.
METHODS
Animal model and sampling protocol.
All studies were approved by the Animal Ethics Committees at The University of Western Australia and Cincinnati Children's Hospital Medical Center. Time-mated ewes with singleton fetuses were randomly assigned to one of six treatment groups to receive an intra-amniotic injection of lipopolysaccharide (LPS) (10 mg Escherichia coli 055:B5, Sigma Chemical, St. Louis, MO), intramuscular injection of betamethasone [Celestone Soluspan, Schering-Plough, North Ryde, New South Wales (NSW), Australia, 0.5 mg/kg maternal weight], or an equivalent injection of saline (controls) at 107 days and/or 114 days GA in different permutations and combinations (Fig. 1). All ewes in this study received a single intramuscular injection of 150 mg medroxyprogesterone acetate (Depo-Provera, Kenral, NSW, Australia) at 100 days gestational age to prevent preterm birth induced by betamethasone treatment (24). Lambs were surgically delivered at 120 days GA (term = 150 days GA) and euthanized with 100 mg/kg pentobarbital. The fetus was weighed and fetal cord blood was collected. Following opening of the chest, a deflation air pressure-volume curve was measured from static inflation of the lung to 40 cmH2O airway pressure (25). The lungs were removed, separated, and weighed prior to a bronchoalveolar lavage of the left lung with 0.9% NaCl (25). The bronchoalveolar lavage fluid (BALF) was used for differential cell counts and surfactant measurements. Lung tissue from the right lower lobe (RLL) was snap frozen and the right upper lobe (RUL) was inflation fixed in 10% buffered formalin with 30 cmH2O pressure for 24 h.
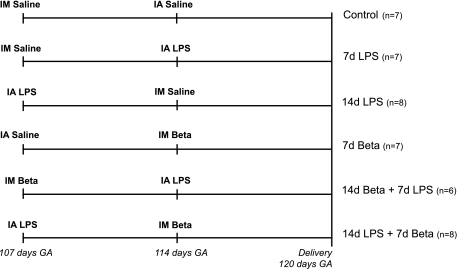
Fig. 1.
Study design. Pregnant ewes received an intra-amniotic (IA) injection of lipopolysaccharide (LPS) and/or an intramuscular (IM) injection of betamethasone (Beta) and/or an equivalent injection of saline for control animals at 107 days and/or 114 days gestation (GA). Lambs were delivered preterm by cesarean section at 120 days GA (term = 150 days GA). 7d, 7-day exposure; 14d, 14-day exposure.
Surfactant proteins and cytokine mRNA quantitation.
Total RNA was isolated from frozen lung tissue of the RLL by a modified Chomzynski method, and mRNA quantitation was performed by real-time PCR (27). Gene expression was measured for surfactant proteins and cytokines: SP-A, SP-B, SP-C, IL-1β, IL-6, IL-8, MCP-1, and Serum amyloid A3. The mRNA was reverse transcribed to yield a single-strand cDNA (verso cDNA kit, Thermo Scientific), which was used as a template with primers and TaqMan probes (Applied Biosystems, Carlsbad, CA) specific to sheep sequences (29). The values for each cytokine were normalized to the internal 18S rRNA. Data were expressed as fold increased over control values.
Toll-like receptor mRNA quantitation.
For the Toll-like receptor mRNA measurements, total RNA was extracted from frozen lung tissue of the RLL by using the SV Total RNA Isolation system (Z3100, Promega, Madison, WI) according to the manufacturer's instructions. Genomic DNA contamination was removed by treatment with RQ1 DNase (M610A, Promega), and the RNA was tested for the presence of genomic GAPDH. Briefly, PCR amplification for the detection of genomic DNA was performed with Taq DNA polymerase (M124B, Promega) at 95°C for 5 min followed by 40 cycles at 95°C for 30 s, 55°C for 45 s, and 72°C for 30 s. Total RNA was used as a template. PCR products were analyzed on 1.5% agarose gels. Total RNA was reverse transcribed with the First Strand cDNA synthesis kit (4379012001, Roche-Applied, Mannheim, Germany) according to manufacturer's instructions by use of anchored oligo primers. Primers for real-time PCR (RT-PCR) were constructed based on published ovine or bovine cDNA sequences (Table 1). Dilution experiments were performed to ensure similar PCR amplification efficiency of the primers. RT-PCR reactions were performed in duplicate with the LightCycler 480 SYBR Green I Master mix (4707516001, Roche-Applied) on a LightCycler 480 Instrument according to the manufacturer's instructions. RT-PCR results were normalized to cyclophilin A, a household gene, and mean fold changes in mRNA expression were calculated by the ΔΔCt method (37).
Table 1.
Primers used for RT-PCR
| Gene | Sequence (5′-3′) | Amplicon Size | Tm | Accession Code (RefSeq) | |
|---|---|---|---|---|---|
| TLR2 | Fw | GGCTGTAATCAGCGTGTTCA | 160 bp | 64°C | NM_001048231.1 |
| Rv | GATCTCGTTGTCGGACAGGT | ||||
| TLR4 | Fw | GAGAAGACTCAGAAAAGCCTTGCT | 200 bp | 65°C | NM_001135930.1 |
| Rv | GCGGGTTGGTTTCTGCAT | ||||
| TLR9 | Fw | CCCTGGAGAAGCTGGACAT | 175 bp | 60°C | NM_001011555.1 |
| Rv | GACAGGTCCACGAAGAGCAG |
Tm, melt temperature; RefSeq, reference sequence; Fw, forward; Rv, reverse.
Surfactant Sat PC and SP-D.
Pulmonary surfactant protein D (SP-D) in the BALF was measured with a sandwich ELISA using rabbit anti-ovine SP-D as coating antibody and guinea pig anti-ovine SP-D a secondary antibody (19, 20). Saturated phosphatidylcholine (Sat PC) was isolated from the BALF by organic solvent extraction followed by oxidation with osmium tetroxide and recovery from neutral alumina, followed by a phosphorus measurement (7).
Immunohistology.
The following antibodies were used to identify different cell types: CD3 for T cells, myeloperoxidase (MPO) for activated neutrophils and monocytes, PU.1 for maturation marker for myeloid cells, precursor form of surfactant protein C (SP-C; pro-SP-C) for maturation marker for alveolar type II cells, thyroid transcription factor-1 (TTF-1) for alveolar type II cells, Foxp3 for regulatory T cells. Paraffin-embedded RUL lung sections (4 μm, transverse) were stained for CD3 (DAKO A0452, Dakocytomation, Glostrup, Denmark), MPO (DAKO A0398, Dakocytomation), PU.1 (sc-352, Santa Cruz Biotechnology, Santa Cruz), Foxp3 (14–7979, eBioscience, San Diego), TTF-1 (WRAB-1231, Seven Hills Bioreagents, Cincinnati, OH) or pro-SP-C (WRAB-9337, Seven Hills Bioregeants). Briefly, the sections were deparaffinized in an ethanol series and endogenous peroxidase-activity was blocked by incubation with 0.3% H2O2 in 1 × phosphate buffered saline (PBS, pH 7.4) or methanol (for TTF-1 and pro-SP-C). Antigen retrieval was performed by incubating the sections in heated citrate buffer (10 mM, pH 6.0) for 30 min. To block nonspecific binding, the slides were incubated with 20% normal goat serum (NGS) in PBS (for MPO and Foxp3), 5% bovine serum albumin in PBS (for CD3 and PU.1) or 2% NGS in PBS (for TTF-1 and pro-SP-C). Sections were incubated overnight at 4°C with the diluted primary antibody (CD3 1:200, MPO 1:500, PU.1 1:400, Foxp3 1:30, TTF-1 1:100, pro-SP-C 1:1,500). After incubation with a goat-anti-mouse biotin labeled (for Foxp3) (DAKO E0433, Dakocytomation) or swine-anti-rabbit biotin-labeled secondary antibody (DAKO E0353, Dakocytomation), immunostaining was enhanced with Vectastain ABC Peroxidase Elite kit (PK-6200, Vector Laboratories, Burlingame, CA) and stained with nickel sulfate-diaminobenzidine. Subsequently, the sections were rinsed in Tris-saline and incubated with Tris-cobalt. After counterstaining with 0.1% Nuclear Fast Red, the sections were washed, dehydrated, and coverslipped.
Since the air space expansion and tissue characteristics were different in the different groups, we measured tissue area in the lung sections. Blinded measurements of tissue fractions (expressed relative to the total lung area) were performed in five random nonoverlapping fields (×20 objective) for each animal and for at least four animals per group using the color threshold function of the program MetaMorph v6.1r0 (Molecular Devices/Universal Imaging, Sunnyvale, CA). The average measurement from each lamb was used to compute a group average. The tissue fractions in different experimental groups were normalized to the control group average to obtain a correction factor. For example, if the tissue fraction in an experimental group was 1.2 times control, then the cells counted per microscopic field were divided by 1.2. Thus the expressed cell counts per microscopic field incorporated this correction factor.
Evaluation was performed by light microscopy (Axioskop 40, Zeiss) with LeicaQWin Pro v.3.4.0 software (Leica Microsystems). MPO-, CD3-, PU.1-, and pro-SP-C-positive cells were counted in three representative high-power fields at ×200 magnification by a blinded observer and averaged per animal. Because FoxP3-positive cells were unevenly distributed throughout the lung tissue, sections were scored for positive signal for Foxp3 with a semiquantitative scoring system by a blinded observer: 1, little staining; 2, light staining; 3, heavy staining. For TTF-1, a computer-aided manual count of five random fields at ×200 magnification per animal was performed, using the computer program MetaMorph (Image-Pro Plus v7.0). The MPO, CD3, and TTF-1 cell counts per microscopic field incorporated the correction factor for tissue fraction. The SP-C-positive cells were expressed relative to TTF-1-positive cells to assess whether changes were due to increased expression in each of the alveolar type II cell vs. increased numbers of alveolar type II cells in different groups.
Data analysis.
Results are given as means ± SE. The groups were compared by one-way ANOVA with Tukey's test for post hoc analysis as appropriate. Statistical analysis was performed by GraphPad Prism v5.0. Significance was accepted at P < 0.05.
RESULTS
Description of animals.
The experiments were prospectively designed to test the interactions of antenatal corticosteroids and intra-amniotic LPS exposure. Despite the medroxyprogesterone acetate treatment, animals exposed to maternal betamethasone experienced fetal losses, such that we reassigned animals from the group that only received betamethasone 14 days before delivery to other treatment groups because our priority was to test the interaction of betamethasone and LPS. The intra-amniotic LPS injections were not associated with fetal losses, but fetal deaths identified by ultrasound and fetal losses were frequent in the betamethasone-treated groups (Table 2). All animals had comparable birth weights except for a lower birth weight for animals with combined exposure to LPS 14 days and betamethasone 7 days before delivery. Cord blood pH values and lung-to-body-weight ratios were comparable across all groups.
Table 2.
Variables at birth
| Fetal Outcomes |
|||||||
|---|---|---|---|---|---|---|---|
| Treatment Group | Fetal Death | Abortion | Alive | Birth Weight, kg | Cord Blood, pH | Sex | Lung Wt/Body Wt, g/kg |
| Control | 1 | 7 | 2.7 ± 0.2 | 7.24 ± 0.03 | 3/4 | 32.7 ± 0.9 | |
| 7d LPS | 7 | 2.5 ± 0.1 | 7.26 ± 0.02 | 3/4 | 33.6 ± 1.2 | ||
| 14d LPS | 8 | 2.5 ± 0.1 | 7.27 ± 0.02 | 5/3 | 37.1 ± 1.8 | ||
| 7d Beta | 2 | 1 | 7 | 2.7 ± 0.2 | 7.20 ± 0.04 | 4/3 | 36.7 ± 0.8 |
| 14d Beta + 7d LPS | 3 | 6 | 2.7 ± 0.1 | 7.25 ± 0.04 | 1/5 | 36.2 ± 2.4 | |
| 14d LPS + 7d Beta | 1 | 1 | 8 | 2.1 ± 0.1* | 7.25 ± 0.03 | 4/4 | 39.9 ± 2.7 |
Data are expressed as means ± SE.
P < 0.05 vs. controls by 1-way ANOVA with Tukey's post hoc test. LPS, lipopolysaccharide; Beta, betamethasone; GA, gestational age; Wt, weight; M, male; F, female; 7d, 7-day exposure; 14d, 14-day exposure.
Lung inflammation.
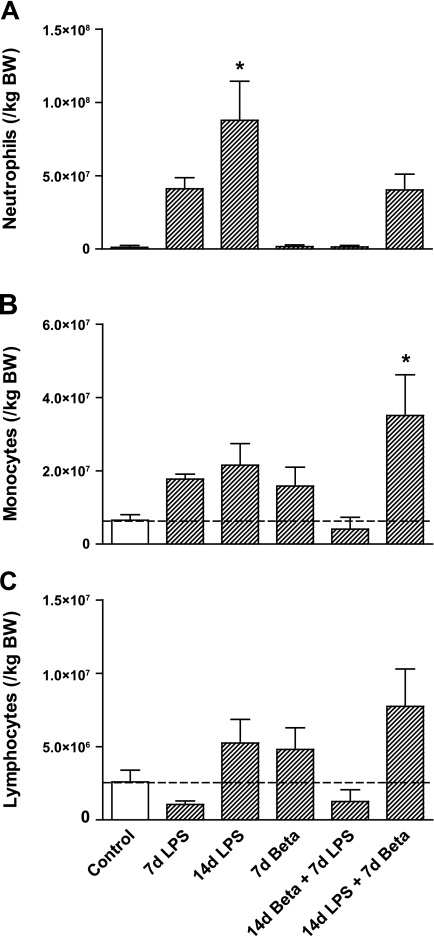
Pulmonary inflammation resulting from LPS and the anti-inflammatory effect of betamethasone exposures were assessed by differential cell counts on the BALF (Fig. 2). Neutrophil levels were modestly increased 7 days after the exposure to LPS and significantly increased 14 days after LPS exposure (Fig. 2A). This increase did not occur in lambs exposed to maternal betamethasone 7 days after intra-amniotic LPS. In this group, however, there was a 10-fold increase in monocytes, which was not seen in the groups exposed to LPS only (Fig. 2B). Lymphocyte numbers did not differ in experimental groups compared with controls (Fig. 2C).
Fig. 2.
Differential cell count of the bronchoalveolar lavage. A: neutrophil levels increased 14 days after the exposure to LPS. B: combined exposure to LPS for 14 days and betamethasone for 7 days increased monocytes in the bronchoalveolar lavage. C: lymphocyte count did not differ in any of the treatment groups compared with controls. *P < 0.05 vs. controls by 1-way ANOVA with Tukey's post hoc test. BW, body weight.
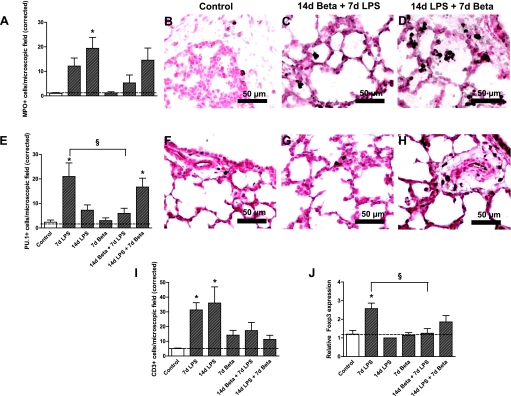
Lung inflammation was further characterized by identifying markers for activation of inflammatory cells (Fig. 3). MPO-positive cells were significantly increased 14 days after LPS exposure (Fig. 3, A–D). Betamethasone pretreatment 7 days before the LPS exposure prevented the increase of MPO-positive cells in the fetal lung. However, betamethasone given 7 days after LPS only partially inhibited the influx of LPS-mediated MPO-positive cells. PU.1 expression, which is a transcription factor that indicates maturation of monocytes (34), increased 7 days after LPS exposure in the fetal lung (Fig. 3, E–H). This increase in PU.1-positive cells could be prevented by pretreatment with betamethasone 7 days before the LPS exposure. However, exposure to 14 days of LPS followed by betamethasone for 7 days increased PU.1-expressing cells in the lung. LPS exposure for either 7 or 14 days increased the number of CD3-positive cells in the fetal lung (Fig. 3I). The groups exposed to both betamethasone and LPS did not show a significant increase in CD3-positive cells compared with controls, indicating that betamethasone pre- and posttreatment both countered the LPS-induced influx of CD3-positive cells. Foxp3-positive cells only increased in the lung tissue 7 days after LPS exposure (Fig. 3J), which was inhibited by betamethasone pretreatment.
Fig. 3.
Characterization of inflammatory cells in the fetal lung tissue. A: quantitation of myeloperoxidase (MPO)-expressing cells in lung sections per microscopic field corrected for the tissue fraction (see methods for details). Representative photomicrographs of immunostaining against myeloperoxidase using lung sections from controls (B), 14d betamethasone + 7d LPS (C), and 14d LPS + 7d betamethasone (D). MPO-positive cells increased in the lung tissue 14 days after LPS exposure compared with controls. E: quantitation of PU.1 expressing cells in lung sections per microscopic field. Representative photomicrographs of immunostaining against PU.1 using lung sections from controls (F), 14d betamethasone + 7d LPS (G), and 14d LPS + 7d betamethasone (H). LPS exposure for 7 days and combined exposure to LPS for 14 days and betamethasone for 7 days increased the number of PU.1-positive cells in the fetal lung. Betamethasone treatment 7 days before the LPS exposure prevented this increase. I: number of CD3-positive cells increased significantly 7 and 14 days after LPS exposure compared with controls. J: Foxp3 expression increased 7 days after LPS exposure compared with controls. Betamethasone treatment before the LPS exposure prevented this increase. *P < 0.05 vs. controls; §P < 0.05 between experimental groups by 1-way ANOVA with Tukey's post hoc test.
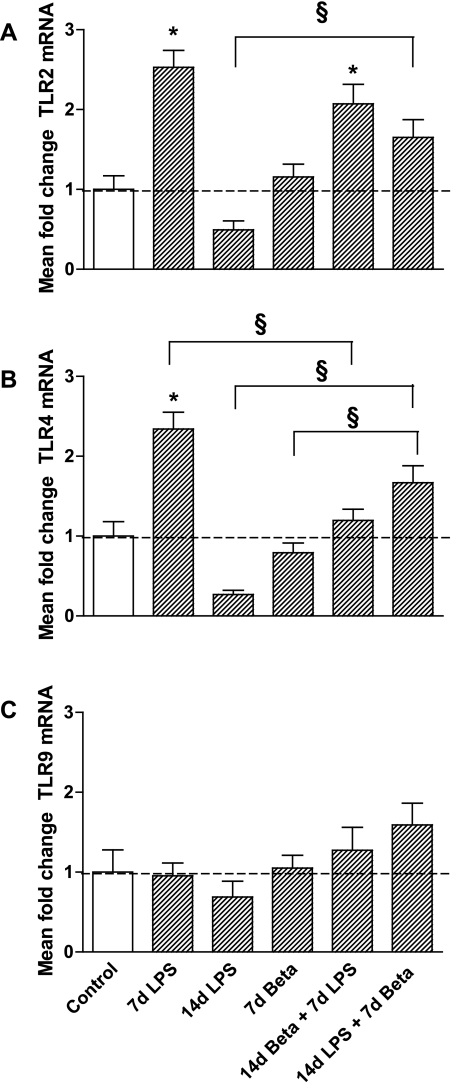
Consistent with our previous observation of maximal induction of cytokines 2 d after exposure (32, 35), the mRNA expression of IL-1β, IL-6, IL-8, and MCP-1 were only modestly increased 7 days after LPS exposure compared with controls (Table 3). Combined exposure to LPS and betamethasone had no significant effect on lung cytokine mRNA expression. The mRNA levels of Serum amyloid A, an acute-phase reaction protein expressed in the lung and liver (49), increased 7 days after the LPS exposure compared with controls. Betamethasone pretreatment prior to the LPS exposure suppressed this increase. The mRNA levels of TLR2 (Fig. 4A) and TLR4 (Fig. 4B) more than doubled after 7 days of LPS exposure but returned to control levels 14 days after exposure. Pretreatment with betamethasone 7 days prior decreased the LPS-induced increase in TLR4 but not TLR2 mRNA. Levels of TLR9 mRNA did not change in experimental groups compared with controls (Fig. 4C).
Table 3.
Cytokine and acute-phase reactant expression in the fetal lung
| Treatment Group | IL-1β mRNA | IL-6 mRNA | IL-8 mRNA | MCP-1 mRNA | Serum Amyloid A3 mRNA |
|---|---|---|---|---|---|
| Control | 1.0 ± 0.2 | 1.0 ± 0.3 | 1.0 ± 0.2 | 1.0 ± 0.2 | 1.0 ± 0.7 |
| 7d LPS | 3.5 ± 0.5* | 0.9 ± 0.1 | 4.7 ± 0.8 | 3.7 ± 0.4 | 29 ± 10* |
| 14d LPS | 3.1 ± 0.6 | 1.1 ± 0.2 | 4.2 ± 1.5 | 3.8 ± 0.9 | 12 ± 8.0 |
| 7d Beta | 1.2 ± 0.3 | 1.0 ± 0.3 | 1.0 ± 0.4 | 1.3 ± 0.3 | 1.2 ± 0.5 |
| 14d Beta + 7d LPS | 1.8 ± 0.4 | 0.8 ± 0.1 | 2.1 ± 0.8 | 1.7 ± 0.5 | 4.3 ± 3.0 |
| 14d LPS + 7d Beta | 3.2 ± 1.5 | 1.6 ± 0.8 | 4.3 ± 2.3 | 5.0 ± 3.2 | 31 ± 26 |
Data are expressed as means ± SE of the fold increase of cytokine relative to controls.
P < 0.05 vs. controls by 1-way ANOVA with Tukey's post hoc test.
Fig. 4.
Expression of Toll-like receptors (TLR) 2, 4, and 9. TLR2 (A) and TLR4 (B) mRNA expression were upregulated 7 days after LPS exposure but returned to baseline 14 days after exposure to LPS. Betamethasone treatment before LPS exposure prevented increased TLR4 expression, but not TLR2 expression. TLR2 and TLR4 mRNA levels increased in the lungs of lambs that received betamethasone after LPS exposure, although these levels were not higher than levels measured in controls. C: TLR9 was not differently expressed in experimental groups compared with controls. *P < 0.05 vs. controls; §P < 0.05 between experimental groups by 1-way ANOVA with Tukey's post hoc test.
Surfactant components.
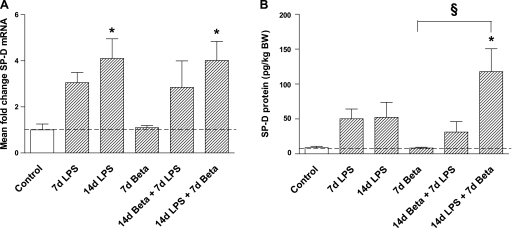
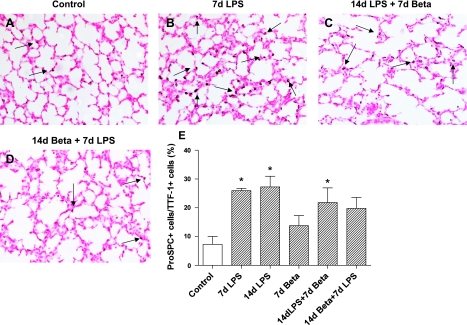
Exposure to LPS alone modestly and variably increased SP-A and SP-B mRNA levels (nonsignificant) (Table 4). However, the combined exposure to LPS followed by betamethasone resulted in a more consistent and significant increase in SP-A and SP-B mRNAs. SP-C mRNA expression did not change with either the betamethasone or LPS exposure. SP-D mRNA expression increased 14 days after the exposure to LPS alone or with betamethasone 7 days after LPS (Fig. 5A). Consistent with the mRNA data, combined exposure at 14 days to LPS followed by a 7-day exposure to betamethasone significantly increased SP-D protein in the BALF (Fig. 5B). To better assess the changes in the alveolar type II cells, we immunostained fetal lung sections using antibodies against TTF-1 and pro-SP-C (38). TTF-1, a transcription factor for alveolar type II cells (38), did not change in any of the treatment groups, indicating no change in the alveolar type II cell numbers after exposure to either LPS or betamethasone (Table 4). However, compared with controls, the number of pro-SP-C-positive cells increased significantly 7 and 14 days after the exposure to LPS, and after 14 days LPS + 7 days betamethasone, indicating maturation of alveolar type-II cells (Fig. 6, A–E).
Table 4.
Markers of lung maturation
| Treatment Group | SP-A mRNA | SP-B mRNA | SP-C mRNA | TTF-1+cells/HPF |
|---|---|---|---|---|
| Control | 1.0 ± 0.4 | 1.0 ± 0.3 | 1.0 ± 0.6 | 514 ± 84 |
| 7d LPS | 4.6 ± 0.6 | 1.9 ± 0.2 | 1.7 ± 0.7 | 453 ± 37 |
| 14d LPS | 7.5 ± 2.6 | 2.4 ± 0.4 | 0.4 ± 0.1 | 362 ± 19 |
| 7d Beta | 1.4 ± 0.2 | 1.1 ± 0.1 | 0.2 ± 0.1* | 395 ± 28 |
| 14d Beta + 7d LPS | 5.6 ± 3.3 | 1.8 ± 0.6 | 1.0 ± 0.3 | 295 ± 51 |
| 14d LPS + 7d Beta | 10.5 ± 2.2* | 2.7 ± 0.3* | 0.6 ± 0.1 | 346 ± 26 |
Thyroid transcription factor-1 (TTF-1)-positive cells represent counts per high power microscopic field (HPF) corrected for the tissue space fraction (see methods for details). pulonary surfactant proteins A, B, and C (SP-A, SP-B, and SP-C) mRNA are expressed as fold increase relative to controls. Data are expressed as means ± SE
P < 0.05 vs. controls by 1-way ANOVA with Tukey's post hoc test.
Fig. 5.
Pulmonary surfactant protein D (SP-D) mRNA and protein expression. A: SP-D mRNA levels increased 14 days after the exposure to LPS irrespectively of the betamethasone posttreatment. B: only combined exposure to LPS for 14 days and betamethasone for 7 days increased SP-D protein expression in the bronchoalveolar lavage fluid. Exposure to LPS for 14 days did not increase SP-D protein expression. *P < 0.05 vs. controls; §P < 0.05 between experimental groups by 1-way ANOVA with Tukey's post hoc test.
Fig. 6.
Pro-pulmonary surfactant protein C (pro-SP-C) immunostaining in the lung. The number of pro-SP-C-positive cells was expressed as a percentage of TTF-1-positive cells (data in Table 4). Representative photomicrographs of immunostaining using lung sections from controls (A), 7d LPS (B), 14d LPS + 7d betamethasone (C), and 14d betamethasone + 7d LPS (D). E: quantitation of pro-SP-C-expressing cells in lung sections per microscopic field via a ×20 objective. LPS exposure increased pro-SP-C expression in the alveolar type II cells in the lung. (*P < 0.05 vs. controls, scale bar is 50 μm).
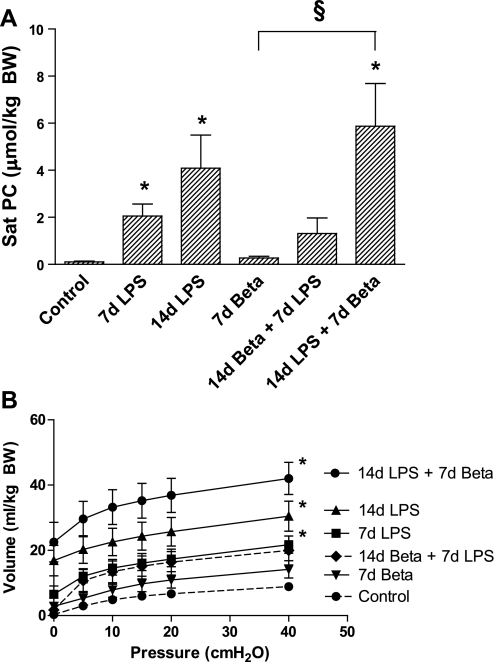
Sat PC is the major surfactant lipid (22). Exposure to intra-amniotic LPS for 7 or 14 days increased Sat PC in the fetal air spaces (Fig. 7A). Betamethasone alone did not significantly increase Sat PC expression. However, the 14-day LPS exposure followed by betamethasone for 7 days had the highest airway Sat PC levels (Fig. 7A). Consistent with increases in surfactant Sat PC, lung volumes, a measure of compliance, increased in all the LPS groups regardless of betamethasone exposure, with the highest lung volumes recorded in the 14-day LPS followed by 7-day betamethasone exposure group (Fig. 7B).
Fig. 7.
Saturated phosphatidylcholine (Sat PC) and pressure-volume curves. A: Sat PC levels in the bronchoalveolar lavage fluid were increased 7 and 14 days after exposure to intra-amniotic LPS. Betamethasone posttreatment 7 days after the exposure to LPS increased Sat PC levels further. B: pressure-volume curve of animals after 14 days of LPS exposure was significantly higher compared with controls irrespectively of treatment with betamethasone. *P < 0.05 vs. controls; §P < 0.05 between experimental groups by 1-way ANOVA with Tukey's post hoc test.
DISCUSSION
Lung inflammation, increased airway surfactants, and increased lung volumes consistent with clinical lung maturation are the major effects of experimental chorioamnionitis (32, 35). In this study we evaluated whether the exposure to the commonly used anti-inflammatory drug betamethasone altered the responses to LPS in the fetal lung. The major conclusion was that betamethasone when administered 7 days after intra-amniotic LPS did not inhibit lung inflammation but resulted in a striking lung maturation. However, when betamethasone was given 7 days before intra-amniotic LPS, lung inflammation was suppressed and lung maturation was more modest.
Intra-amniotic LPS caused lung inflammation at 7 and 14 days after exposure. Both neutrophils and T lymphocytes were recruited to the lung. Furthermore, the neutrophils were activated as demonstrated by the MPO expression (10). The influx of proinflammatory cells was also accompanied by an increase in Foxp3-positive cells, a prototypic marker of the anti-inflammatory T-regulatory cells (13). The number of cells expressing PU.1 also increased, which suggests maturation of lung monocytes, a characteristic for LPS-mediated pulmonary inflammation (34). Both the TLR2 and TLR4 mRNA levels increased in the lung tissue after LPS. Consistent with our previous results demonstrating an early rapid induction profile for cytokine expression (32), the mRNAs for proinflammatory cytokines were only modestly increased 7 days after exposure in the present study. Interestingly, betamethasone treatment 7 days prior to LPS significantly decreased LPS-induced lung inflammation. However, when LPS exposure preceded betamethasone treatment, lung inflammation was not altered. We previously reported that a concomitant administration of maternal betamethasone and LPS resulted in an early suppression but later amplification of lung inflammation (28, 33). Thus the timing of exposure to betamethasone in relation to the proinflammatory stimulus is a major determinant of modulation of lung inflammation.
Maternal betamethasone and intra-amniotic LPS have different pharmacokinetic profiles in the fetus. In a human study, peak fetal serum betamethasone levels were measured 1–2 h after maternal betamethasone treatment with a return to baseline within 2 days of treatment (4). In fetal sheep, maternal betamethasone treatment resulted in peak fetal betamethasone levels 3 h after treatment with a decrease to 50% of the peak levels at 6 h (9). In contrast, the half-life of endotoxin was measured as ∼30 h in the amniotic fluid of preterm lambs after an intra-amniotic administration and exhibited first order kinetics of elimination (39). Neither antenatal betamethasone nor intra-amniotic LPS significantly change the levels of endogenous cortisol in the preterm fetus (21, 25). These results suggest that fetal betamethasone levels 7 days after maternal treatment must be negligible. Therefore, the inhibition of LPS-induced fetal lung inflammation 7 days after maternal betamethasone suggests priming or conditioning of the inflammatory response cells rather than a straight forward drug-drug interaction. This interpretation is also consistent with our previous observation of an early suppression with a late amplification of lung inflammation with concomitant administration of maternal betamethasone and intra-amniotic LPS (28, 33). Our results also demonstrate that modulation of LPS-induced fetal inflammation does not occur when betamethasone is administered after LPS.
Intra-amniotic exposure to LPS followed by betamethasone 7 days before delivery strikingly increased lung maturation as seen by increased Sat PC, SP-D, and the increase in the pressure-volume curve. In contrast, betamethasone pretreatment followed by exposure to LPS 7 days before delivery induced less lung maturation. The large effect of LPS on SP-D expression is consistent with recently reported upregulation of LPS-induced SP-D expression in fetal mice (43). SP-D is an innate host-defense molecule protecting the host against a number of pulmonary pathogens (12). Alveolar type II cell maturation was demonstrated by increased pro-SPC expression. The expression of surfactant protein mRNAs for SP-B increased but SP-C was unchanged after LPS. Betamethasone alone did not increase expression of any of the surfactant protein mRNAs. We previously reported a differential effect of LPS on SP-B and SP-C mRNA levels (2). In vitro, these two mRNAs are differentially regulated by betamethasone (3). The more pronounced effect of LPS compared with betamethasone in increasing mRNA levels of surfactant proteins in this study is consistent with previous reports showing a rapid but reversible effect of betamethasone, but a more lasting effect of intra-amniotic LPS on surfactant protein mRNA expression (2, 44). Previous reports suggest that the effects of simultaneous exposure to explants of fetal rabbits to IL-1α and glucocorticoids could stimulate or inhibit surfactant protein mRNA depending on the gestational age (45). Taken together, the combined effects of a proinflammatory exposure and glucocorticoids on the fetal lung are variable and dependent on context including gestational age and the order of exposure.
We reported previously that intra-amniotic LPS but not maternal betamethasone improved lung compliance, ventilatory efficiency index, and the alveolar wash Sat PC pool sizes 15 days after treatment (2, 22). We did not study the effects of betamethasone alone 14 days after exposure, because of high rate of abortions in this group. Interestingly, the highest expression of surfactant components and the consequent increase in lung volumes was in the group exposed to LPS for 14 days along with betamethasone 7 days before delivery. This group of animals also had the most pronounced lung inflammation. These results are consistent with our previous studies demonstrating that lung inflammation was required for LPS-induced lung maturation (30, 31).
Clinical studies indicate that maternal glucocorticoids can decrease fetal weight and head size (14). We reported previously that simultaneous exposures of fetal sheep to maternal corticosteroid and intra-amniotic LPS protected the fetus from the growth restriction caused by the antenatal corticosteroids (40). In this experiment, the maternal corticosteroid exposure did not decrease fetal weight, a result that probably represents animal variability. The new observation is that the combination of 14-day LPS + 7-day betamethasone caused fetal growth restriction. Since exposure to intrauterine inflammation is the major cause for preterm labor and cervical dilation leading to preterm birth, pretreatment with betamethasone will rarely be an option to diminish or counteract inflammation in the clinical setting. An inference from the study is that prenatal betamethasone in conjunction with inflammation promotes the maturity of the surfactant system resulting in reduced respiratory distress syndrome, but does not prevent bronchopulmonary dysplasia as noted in clinical studies (8, 47). However, the interactions of LPS and betamethasone on fetal growth are complex and not fully understood, and this observation needs verification by replication.
Antenatal corticosteroids are routinely administered to mothers who are at risk of preterm birth to mature the fetal organs, suppress inflammation, and thus reduce perinatal morbidity and mortality (12a). Antenatal steroids also reduce adverse neonatal outcome after preterm birth associated with chorioamnionitis (15). Our experiments do not identify new concerns for the use of antenatal corticosteroids. It remains unknown whether these antenatal corticosteroids also prevent or amplify the developmental and structural complications in the fetal lung, which are induced by infection/inflammation (11, 48). In summary, this study provides further molecular evidence into the protective effects of antenatal corticosteroids on the fetal lung and its interactions with proinflammatory stimuli in the setting of chorioamnionitis.
GRANTS
This research was supported by HD-57869 (S. G. Kallapur) from the National Institute of Health; the National Health and Medical Research Council of Australia; the Women and Infants Research Foundation, Western Australia; Veni BWK-016096141 from the Dutch Scientific Research Organization and the Research School for Oncology and Developmental Biology (GROW), Maastricht University; and 6FY09235 (M. Ikegami) from the March of Dimes Foundation.
DISCLOSURES
None of the authors have a commercial interest in any entity related to subject of the manuscript or have a conflict of interest relative to the manuscript.
ACKNOWLEDGMENTS
We thank Mustapha Tahir, Richard Dalton, Joe Derwort, Masatoshi Saito, Clare Berry, Carryn McLean, Shaofu Li, Andrea Lee, James Robinson, and Jennifer Henderson for excellent technical support.
REFERENCES
- 1. Andrews WW, Goldenberg RL, Faye-Petersen O, Cliver S, Goepfert AR, Hauth JC. The Alabama Preterm Birth study: polymorphonuclear and mononuclear cell placental infiltrations, other markers of inflammation, and outcomes in 23- to 32-week preterm newborn infants. Am J Obstet Gynecol 195: 803–808, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Bachurski CJ, Ross GF, Ikegami M, Kramer BW, Jobe AH. Intra-amniotic endotoxin increases pulmonary surfactant proteins and induces SP-B processing in fetal sheep. Am J Physiol Lung Cell Mol Physiol 280: L279–L285, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Ballard PL, Ertsey R, Gonzales LW, Gonzales J. Transcriptional regulation of human pulmonary surfactant proteins SP-B and SP-C by glucocorticoids. Am J Respir Cell Mol Biol 14: 599–607, 1996 [DOI] [PubMed] [Google Scholar]
- 4. Ballard PL, Granberg P, Ballard RA. Glucocorticoid levels in maternal and cord serum after prenatal betamethasone therapy to prevent respiratory distress syndrome. J Clin Invest 56: 1548–1554, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Banks BA, Cnaan A, Morgan MA, Parer JT, Merrill JD, Ballard PL, Ballard RA. Multiple courses of antenatal corticosteroids and outcome of premature neonates. North American Thyrotropin-Releasing Hormone Study Group. Am J Obstet Gynecol 181: 709–717, 1999 [DOI] [PubMed] [Google Scholar]
- 7. Bartlett GR. Phosphorus assay in column chromatography. J Biol Chem 234: 466–468, 1959 [PubMed] [Google Scholar]
- 8. Been JV, Degraeuwe PL, Kramer BW, Zimmermann LJ. Antenatal steroids and neonatal outcome after chorioamnionitis: a meta-analysis. BJOG 118: 113–122, 2011 [DOI] [PubMed] [Google Scholar]
- 9. Berry LM, Polk DH, Ikegami M, Jobe AH, Padbury JF, Ervin MG. Preterm newborn lamb renal and cardiovascular responses after fetal or maternal antenatal betamethasone. Am J Physiol Regul Integr Comp Physiol 272: R1972–R1979, 1997 [DOI] [PubMed] [Google Scholar]
- 10. Cheah FC, Pillow JJ, Kramer BW, Polglase GR, Nitsos I, Newnham JP, Jobe AH, Kallapur SG. Airway inflammatory cell responses to intra-amniotic lipopolysaccharide in a sheep model of chorioamnionitis. Am J Physiol Lung Cell Mol Physiol 296: L384–L393, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Collins JJ, Kallapur SG, Knox CL, Nitsos I, Polglase GR, Pillow JJ, Kuypers E, Newnham JP, Jobe AH, Kramer BW. Inflammation in fetal sheep from intra-amniotic injection of Ureaplasma parvum. Am J Physiol Lung Cell Mol Physiol 299: L852–L860, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Crouch E, Persson A, Chang D, Heuser J. Molecular structure of pulmonary surfactant protein D (SP-D). J Biol Chem 269: 17311–17319, 1994 [PubMed] [Google Scholar]
- 12a. Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consensus Development Panel on the Effect of Corticosteroids for Fetal Maturation on Perinatal Outcomes JAMA 273: 413–418, 1995 [DOI] [PubMed] [Google Scholar]
- 13. Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol 4: 330–336, 2003 [DOI] [PubMed] [Google Scholar]
- 14. French NP, Hagan R, Evans SF, Godfrey M, Newnham JP. Repeated antenatal corticosteroids: size at birth and subsequent development. Am J Obstet Gynecol 180: 114–121, 1999 [DOI] [PubMed] [Google Scholar]
- 15. Goldenberg RL, Andrews WW, Faye-Petersen OM, Cliver SP, Goepfert AR, Hauth JC. The Alabama preterm birth study: corticosteroids and neonatal outcomes in 23- to 32-week newborns with various markers of intrauterine infection. Am J Obstet Gynecol 195: 1020–1024, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med 342: 1500–1507, 2000 [DOI] [PubMed] [Google Scholar]
- 17. Gomez R, Romero R, Ghezzi F, Yoon B, Mazor M, Berry S. The fetal inflammatory response syndrome. Am J Obstet Gynecol 179: 194–202, 1998 [DOI] [PubMed] [Google Scholar]
- 18. Harding JE, Pang J, Knight DB, Liggins GC. Do antenatal corticosteroids help in the setting of preterm rupture of membranes? Am J Obstet Gynecol 184: 131–139, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Ikegami M, Carter K, Bishop K, Yadav A, Masterjohn E, Brondyk W, Scheule RK, Whitsett JA. Intratracheal recombinant surfactant protein d prevents endotoxin shock in the newborn preterm lamb. Am J Respir Crit Care Med 173: 1342–1347, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ikegami M, Grant S, Korfhagen T, Scheule RK, Whitsett JA. Surfactant protein-D regulates the postnatal maturation of pulmonary surfactant lipid pool sizes. J Appl Physiol 106: 1545–1552, 2009 [DOI] [PubMed] [Google Scholar]
- 21. Ikegami M, Jobe A, Newnham J, Polk D, Willet K, Sly P. Repetitive prenatal glucocorticoids improve lung function and decrease growth in preterm lambs. Am J Respir Crit Care Med 156: 178–184, 1997 [DOI] [PubMed] [Google Scholar]
- 22. Ikegami M, Jobe AH, Yamada T, Seidner S. Relationship between alveolar saturated phosphatidylcholine pool sizes and compliance of preterm rabbit lungs. The effect of maternal corticosteroid treatment. Am Rev Respir Dis 139: 367–369, 1989 [DOI] [PubMed] [Google Scholar]
- 23. Ikegami M, Polk D, Jobe A. Minimum interval from fetal betamethasone treatment to postnatal lung responses in preterm lambs. Am J Obstet Gynecol 174: 1408–1413, 1996 [DOI] [PubMed] [Google Scholar]
- 24. Jobe AH, Newnham JP, Moss TJ, Ikegami M. Differential effects of maternal betamethasone and cortisol on lung maturation and growth in fetal sheep. Am J Obstet Gynecol 188: 22–28, 2003 [DOI] [PubMed] [Google Scholar]
- 25. Jobe AH, Newnham JP, Willet KE, Moss TJ, Ervin MG, Padbury JF, Sly PD, Ikegami M. Endotoxin-induced lung maturation in preterm lambs is not mediated by cortisol. Am J Respir Crit Care Med 162: 1656–1661, 2000 [DOI] [PubMed] [Google Scholar]
- 26. Kallapur SG, Ikegami M. Physiological consequences of intrauterine insults. Paediatr Respir Rev 7: 110–116, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Kallapur SG, Kramer BW, Knox CL, Berry CA, Collins JJ, Kemp MW, Nitsos I, Polglase GR, Robinson J, Hillman NH, Newnham JP, Chougnet C, Jobe AH. Chronic fetal exposure to ureaplasma parvum suppresses innate immune responses in sheep. J Immunol 187: 2688–2695, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kallapur SG, Kramer BW, Moss TJ, Newnham JP, Jobe AH, Ikegami M, Bachurski CJ. Maternal glucocorticoids increase endotoxin-induced lung inflammation in preterm lambs. Am J Physiol Lung Cell Mol Physiol 284: L633–L642, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Kallapur SG, Kramer BW, Nitsos I, Pillow JJ, Collins JJ, Polglase GR, Newnham JP, Jobe AH. Pulmonary and systemic inflammatory responses to intra-amniotic IL-1α in fetal sheep. Am J Physiol Lung Cell Mol Physiol 301: L285–L295, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kallapur SG, Moss TJM, Ikegami M, Jasman RL, Newnham JP, Jobe AH. Recruited inflammatory cells mediate endotoxin induced lung maturation in preterm fetal lambs. Am J Respir Crit Care Med 172: 1315–1321, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kallapur SG, Nitsos I, Moss TJ, Polglase GR, Pillow JJ, Cheah FC, Kramer BW, Newnham JP, Ikegami M, Jobe AH. IL-1 mediates pulmonary and systemic inflammatory responses to chorioamnionitis induced by lipopolysaccharide. Am J Respir Crit Care Med 179: 955–961, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kallapur SG, Willet KE, Jobe AH, Ikegami M, Bachurski CJ. Intra-amniotic endotoxin: chorioamnionitis precedes lung maturation in preterm lambs. Am J Physiol Lung Cell Mol Physiol 280: L527–L536, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Kramer BW, Ikegami M, Moss TJ, Nitsos I, Newnham JP, Jobe AH. Antenatal betamethasone changes cord blood monocyte responses to endotoxin in preterm lambs. Pediatr Res 55: 764–768, 2004 [DOI] [PubMed] [Google Scholar]
- 34. Kramer BW, Joshi SN, Moss TJ, Newnham JP, Sindelar R, Jobe AH, Kallapur SG. Endotoxin-induced maturation of monocytes in preterm fetal sheep lung. Am J Physiol Lung Cell Mol Physiol 293: L345–L353, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Kramer BW, Moss TJ, Willet KE, Newnham JP, Sly PD, Kallapur SG, Ikegami M, Jobe AH. Dose and time response for inflammation and lung maturation after intra-amniotic endotoxin in preterm lambs. Am J Respir Crit Care Med 164: 982–988, 2001 [DOI] [PubMed] [Google Scholar]
- 36. Lahra MM, Jeffery HE. A fetal response to chorioamnionitis is associated with early survival after preterm birth. Am J Obstet Gynecol 190: 147–151, 2004 [DOI] [PubMed] [Google Scholar]
- 37. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 38. Maeda Y, Dave V, Whitsett JA. Transcriptional control of lung morphogenesis. Physiol Rev 87: 219–244, 2007 [DOI] [PubMed] [Google Scholar]
- 39. Newnham JP, Kallapur SG, Kramer BW, Moss TJ, Nitsos I, Ikegami M, Jobe AH. Betamethasone effects on chorioamnionitis induced by intra-amniotic endotoxin in sheep. Am J Obstet Gynecol 189: 1458–1466, 2003 [DOI] [PubMed] [Google Scholar]
- 40. Newnham JP, Moss TJ, Padbury JF, Willet KE, Ikegami M, Ervin MG, Sly P, Jobe A. The interactive effects of endotoxin with prenatal glucocorticoids on short-term lung function in sheep. Am J Obstet Gynecol 185: 190–197, 2001 [DOI] [PubMed] [Google Scholar]
- 41. Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev 3: CD004454, 2006 [DOI] [PubMed] [Google Scholar]
- 42. Romero R, Gomez R, Ghezzi F, Yoon B, Mazor M, Edwin S, Berry S. A fetal systemic inflammatory response is followed by the spontaneous onset of preterm parturition. Am J Obstet Gynecol 179: 186–193, 1998 [DOI] [PubMed] [Google Scholar]
- 43. Schmiedl A, Behrens J, Zscheppang K, Purevdorj E, von Mayersbach D, Liese A, Dammann CE. Lipopolysaccharide-induced injury is more pronounced in fetal transgenic ErbB4-deleted lungs. Am J Physiol Lung Cell Mol Physiol 301: L490–L499, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tan RC, Ikegami M, Jobe AH, Yao LY, Possmayer F, Ballard PL. Developmental and glucocorticoid regulation of surfactant protein mRNAs in preterm lambs. Am J Physiol Lung Cell Mol Physiol 277: L1142–L1148, 1999 [DOI] [PubMed] [Google Scholar]
- 45. Vayrynen O, Glumoff V, Hallman M. Regulation of surfactant proteins by LPS and proinflammatory cytokines in fetal and newborn lung. Am J Physiol Lung Cell Mol Physiol 282: L803–L810, 2002 [DOI] [PubMed] [Google Scholar]
- 46. Wade KC, Guttentag SH, Gonzales LW, Maschhoff KL, Gonzales J, Kolla V, Singhal S, Ballard PL. Gene induction during differentiation of human pulmonary type II cells in vitro. Am J Respir Cell Mol Biol 34: 727–737, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wapner R, Jobe AH. Controversy: antenatal steroids. Clin Perinatol 38: 529–545, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Willet KE, Jobe AH, Ikegami M, Brennan S, Newnham J, Sly PD. Antenatal endotoxin and glucocorticoid effects on lung morphometry in preterm lambs. Pediatr Res 48: 782–788, 2000 [DOI] [PubMed] [Google Scholar]
- 49. Wilson TC, Bachurski CJ, Ikegami M, Jobe AH, Kallapur SG. Pulmonary and systemic induction of SAA3 after ventilation and endotoxin in preterm lambs. Pediatr Res 58: 1204–1209, 2005 [DOI] [PubMed] [Google Scholar]