Abstract
Previously, we observed that hypoxia increases the expression of the β1-subunit (KCNMB1) of the calcium-sensitive potassium channel (BKCa). Herein, we elucidate the mechanism whereby hypoxia increases KCNMB1 expression in human pulmonary artery smooth muscle cells (hPASMC). In response to hypoxia, the expression of both the transcription factor hypoxia-inducible factor 1-α (HIF-1α) and KCNMB1 are increased. Knockdown of HIF-1α using a shRNA plasmid blocked the hypoxic induction of KCNMB1 expression. Chromatin immunoprecipitation (ChIP) demonstrated HIF-1α binding to three discrete regions of the human KCNMB1 promoter known to contain hypoxia response elements (HREs). A KCNMB1 promoter reporter assay combined with site-directed mutagenesis identified two adjacent HREs located between −3,540 bp and −3,311 bp that are essential for the hypoxic induction of KCNMB1 promoter activity. Furthermore, additional ChIP assays demonstrated recruitment of the HIF-1α transcriptional coactivator, p300, to this same promoter region. Treatment of hPASMC with the histone deacetylase inhibitor, trichostatin, prolonged the increase in KCNMB1 observed with hypoxia, suggesting that alterations in chromatin remodeling function to limit the hypoxic induction of KCNMB1. Finally, KCNMB1 knockdown potentiated the hypoxia-induced increase in cytosolic calcium in hPASMC, highlighting the contribution of the β1-subunit in modulating vascular SMC tone in response to acute hypoxia. In conclusion, HIF-1α increases KCNMB1 expression in response to hypoxia in hPASMC by binding to two HREs located at −3,540 to −3,311 of the KCNMB1 promoter. We speculate that selective modulation of KCNMB1 expression may serve as a novel therapeutic approach to address diseases characterized by an increase in vascular tone.
Keywords: calcium-sensitive K+ channel, oxygen sensing
regulation of vascular tone is of critical importance. Evidence underscores the significance of the calcium-sensitive potassium channel (BKCa) in ensuring that blood flow to vital organs is preserved across the spectrum of development, as well during physiological and pathophysiological stress (17). In vascular smooth muscle cells (SMC), the BKCa channel is formed by four ion-conducting α-subunits (8, 14) and regulatory β-subunits. The β-subunits serve to optimally well adapt the BKCa α-pore (KCNMA1) channel to the functional requirements of the cell (3, 16). In vascular SMC, activation of the BKCa channel results in K+ efflux, membrane hyperpolarization, closure of voltage-dependent calcium channels, a decrease in cytosolic calcium, and a subsequent decrease in tone (2).
The β1-subunit (KCNMB1) is highly expressed in SMC but not in other tissues and serves to increase the voltage and calcium sensitivity of the pore-forming α-subunit of the channel (6). Evidence supporting the importance of the β1-subunit includes the observation that, in mice lacking KCNMB1, calcium sparks are uncoupled from the BKCa channels, resulting in vasoconstriction, systemic hypertension, and left ventricular hypertrophy (19). In several rat models of hypertension, elevated blood pressure is associated with downregulation of the β- but not the α-subunit (1). In animal models, α- and β1-subunit expression of the BKCa channel may decrease with maturation (24). The well-characterized protective effects of the female sex on the risk of essential hypertension may be the result of the activation of the BKCa channel by estradiol (26). Moreover, a large epidemiological study indicates that a single nucleotide substitution in the third exon of the KCNMB1 gene (E65K) confers protection against diastolic hypertension. These data support the notion that, in humans, augmented β1 expression serves a protective function by increasing BKCa activity, further illustrating the importance of the BKCa channel and the β1-subunit (7).
Despite data that underscore the importance of the β1-subunit of the BKCa channel, the factors that regulate its expression remain incompletely understood. Data from our laboratory have established a central role for the BKCa channel in mediating the transition of the pulmonary circulation. Nitric oxide (25), shear stress (27), rhythmic distention of the lung (28), and oxygen (4) are each critically important physiological stimuli that cause perinatal pulmonary vasodilation through activation of the BKCa channel. With maturation and ongoing exposure to the relatively oxygen-rich environment of air-breathing life, pulmonary vascular BKCa expression decreases while voltage-gated K+ channels expression increases (24).
Two observations from our laboratory have at least partially informed our understanding of the factors that account for the developmental regulation of the pulmonary vascular K+ channels. First, the normally low oxygen tension in the fetal environment increases BKCa channel subunit expression (22). Second, hypoxia-inducible factor-1α (HIF-1α), a transcription factor that represents a universal response element to hypoxia, is relatively more stable and less prone to destruction in fetal and neonatal pulmonary artery (PA) SMC, compared with adult PA SMC (23). Taken together, these two observations suggest that the biologically imperative vasodilatory response of the perinatal pulmonary circulation to oxygen is guaranteed by robust HIF-1α expression that functions to increase BKCa channel β1-subunit expression.
Thus the present experimental series was undertaken to test the hypothesis that hypoxia increases β1-subunit expression through HIF-1α-mediated transcriptional regulation. Here we demonstrate that HIF-1α increases KCNMB1 expression in response to hypoxia and identify the two discrete, adjacent hypoxia response elements (HREs) located between −3,540 bp and −3,311 bp of the KCNMB1 promoter that are essential for this HIF-1α-mediated induction. Furthermore, we go on to demonstrate that this regulation possesses functional implications, as the response of hPASMC to acute hypoxia is accentuated in the absence of KCNMB1.
MATERIALS AND METHODS
Cell culture.
Human PASMC (hPASMC) derived from a 2.5-mo-old human were purchased from Lonza (Walkersville, MD) and grown according to the manufacturer's protocol. Cells were maintained in either normoxic (20% O2-5% CO2-balance nitrogen) or hypoxic conditions (1% O2-5% CO2-balance nitrogen). Oxygen tension in the hypoxic medium ranged between 9 and 14 Torr.
Western immunoblot analysis.
Total cellular extracts of PASMC were prepared according to the manufacturer's protocol (TransFactor extraction kit; Clontech, Mountain View, CA). Protein lysates were quantified using the Bradford method and then subjected to SDS-PAGE. Membranes were incubated with primary antibodies to detect KCNMA1 (Santa Cruz Biotechnology, Santa Cruz, CA), KCNMB1 (Santa Cruz Biotechnology), HIF-1α (Cayman Chemical, Ann Arbor, MI), or α-tubulin (GE Life Sciences, Piscataway, NJ) followed by detection with enhanced chemiluminescence reagents (GE Life Sciences).
Real-time quantitative PCR.
Total RNA was extracted using the RNeasy kit (Qiagen, Valencia, CA) and reverse transcribed using Superscript II (Invitrogen, Carlsbad, CA) per the manufacturer's protocol. Primers for real-time PCR are outlined in the Table 1. PCR was performed using SYBR Green (Applied Biosystems, Carlsbad, CA) with a GeneAmp 7900 sequence detection system (Applied Biosystems) for 40 cycles of PCR under the following conditions: 2 min at 50°C and 10 min at 95°C for 1 cycle, followed by 40 cycles of 15 s at 95°C and 1 min at 60°C. The expression of the target genes was represented as the fold increase (2−ΔΔCt), where ΔΔCt = [ΔCt(normoxia)] − [ΔCt(hypoxia)] and ΔCt = [Ct(sample)] − [Ct(18S)].
Table 1.
Primers for qPCR
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| KCNMA1 | 5′-CGGTTAGTGGAAGAAACACA-3′ | 5′-GAGGACGGAACCCTGATAAAA-3′ |
| KCNMB1 | 5′-CTGTACCACACGGAGGACACT-3′ | 5′-GTAGAGGCGCTGGAATAGGAC-3′ |
| HIF-1α | 5′-TTTGCTGAAGACACAGAAGC AAAGA-3′ | 5′-TTGAGGACTTGCGCTTTCAGG-3′ |
| Human 18S | 5′-AACGGCTACCACATCCAAGG-3′ | 5′-GGGAGTGGGTAATTTGCGC-3′ |
KCNMA1, α1-subunit of the calcium-sensitive potassium channel; KCNMB1, β1-subunit of the calcium-sensitive potassium channel; HIF, hypoxia-inducible factor.
Depletion of HIF-1α with shRNA plasmid.
HIF-1α shRNA plasmid and related negative control shRNA plasmid were purchased from Santa Cruz Biotechnology. A total of 2 × 105 hPASMCs were transfected with either 2.5 μg of HIF-1α or nonsilencing shRNA plasmids using the Lipofectamine LTX reagent (Invitrogen) according to the manufacturer's protocol. Cells were plated in six-well plates in SMC-specific media with 5% FBS and incubated under normoxic conditions at 37°C, 5% CO2. After 6 h of incubation, the media was exchanged, and hPASMC were incubated for an additional 72 h and subsequently exposed to acute hypoxia for up to 12 h. Cells were then harvested for use in Western blotting assays and real-time quantitative PCR. For the studies wherein cytosolic calcium was measured in hPASMC, HIF-1α siRNA and related negative control siRNA were purchased from Dharmacon (ON-TARGETplus SMARTpool HIF1A; Dharmacon, Chicago, IL). hPASMC were transfected with 50 nM siRNA using Lipofectamine RNAiMAX (Invitrogen) according to the manufacturer's protocol. After 6 h of incubation, the media was exchanged, and hPASMC were incubated for an additional 24 h and subsequently rendered acutely hypoxic.
ChIP assay.
Chromatin immunoprecipitation (ChIP) assays were performed using the ChIP-IT kit (Active Motif, Carlsbad, CA), per the manufacturer's instructions, and 5 × 106 hPASMCs were used per condition. The sonicated chromatin complexes were immunoprecipitated with specific antibodies for HIF-1α (Cayman) or p300 (Millipore, Billerica, MA). Input DNA and DNA immunoprecipitated with either specific antibodies or immunoglobulin G (IgG) were amplified by PCR using primers flanking the proximal KCNMB1 promoter (+81 to −3,540) (Table 2). Reaction mixtures were cycled with an initial melt step at 94°C for 5 min and then 24–30 cycles of 94°C for 45 s, 55°C for 30 s, and 72°C for 30 s, followed by 72°C for 10 min. Products were analyzed by electrophoresis on a 2% agarose gel. As a negative control, primers corresponding to a genomic region distal to the KCNMB1 promoter from −20,969 to −20,668 were used (Table 2).
Table 2.
Primers for chromatin immunoprecipitation
| KCNMB1 Promoter Region | Forward Primer | Reverse Primer |
|---|---|---|
| A | 5′-TAACACAAGTGAGAAAACTGAG-3′ | 5′-TCAGCCTGGTATATTTGGAA-3′ |
| B | 5′-AGTGAAGGGTGGAGATAAGC-3′ | 5′-AAATCAGCAACAAAGGGCAG-3′ |
| C | 5′-TGAGATCTGAGCTGAGGCT-3′ | 5′-GCTTCGTAAGGGCCTCAATAG-3′ |
| D | 5′-AGCAGACATCATGAAGAAAC-3′ | 5′-CATACACATGGACATTCCTC-3′ |
| Neg | 5′-CCTTATTAATCCTGCCCTGACC-3′ | 5′-AGGAGATCGCAGACACAG-3′ |
Neg, negative.
Transient transfection and luciferase assay.
We constructed pGL3-A, pGL3-BA, and pGL3-DCBA plasmids by inserting a series of PCR-amplified KCNMB1 promoter fragments into pGL3-Basic Luciferase Reporter Vector (Promega, Madison, WI). To identify which of the HRE(s) within promoter fragment D possessed transcriptional activity, three different mutants (two single mutants and a double mutant, RCGTG→GTACG) were also constructed and used for luciferase assays to determine the functional HRE(s) within the KCNMB1 promoter. hPASMC were transfected with the various luciferase reporter constructs in combination with a HIF-1α overexpression plasmid (15), using Lipofectamine LTX. Forty-eight hours after transfection, groups of cells were either maintained in normoxia, or exposed to 6 h of hypoxia, and luciferase was measured with the Dual Luciferase Assay Kit (Promega) per the manufacturer's instructions. Luciferase activity was measured over a 30-s time interval in an EG&G Lumat LB 9507 luminometer (Berthold Technologies, Bad Wideband, Germany). As a negative control, cells were transfected with the pGL3 basic vector (pGL3-basic), which lacks the KCNMB1 or any other promoter. Data were reported as fold increase in luciferase activity over normoxic control samples.
Site-directed mutagenesis.
Mutation in the HIF-1α binding site in the upstream promoter region of KCNMB1 gene was introduced via PCR-based, site-directed mutagenesis (QuickChange XL-mutagenesis kit; Stratagene, Santa Clara, CA). The HRE sites, RCGTG, in the upstream promoter region of KCNMB1 were changed to XhoI site (CTCGAG) using mutant primer sets (Table 3). Double mutants for HRE were made with a single mutant as a template and corresponding mutant primer set. All mutants were verified by DNA sequencing (Elimpharma, Hayward, CA).
Table 3.
Primers for site-directed mutagenesis
| HRE Mutants | Forward Primer | Reverse Primer |
|---|---|---|
| mHRE6.D | 5′-CACATCACACGCTAGGGCTCGAACCTTGGGAAAATCACTTTG-3′ | 5′-CAAAGTGATTTTCCCAAGGTTC GAGCCCTAGCGTGTGATGTG-3′ |
| mHRE7.D | 5′-CAGGCTCCATCACATCATCGAGTAGGGGCGTGACCTTGG-3′ | 5′-CCAAGGTCACGCCCCTACTCGATGATGTGATGGAGCCTG-3′ |
Underlined segments are mutant sequences. HRE, hypoxia response element.
Ca2+ imaging.
To assess dynamic changes in cytosolic calcium ([Ca2+]i) in individual hPASMC, the Ca2+-sensitive fluorophore fura-2 AM (Molecular Probes, Carlsbad, CA) was used. Subconfluent monolayers of hPASMC on 25-mm2 glass coverslips were placed on the stage of an inverted microscope (Nikon Diaphot). Cells were loaded with 10 nM fura-2 AM and 2.5 μg/ml pluronic acid (Molecular Probes) for 20 min, followed by 20 min in Ca2+-containing solution to allow for deesterification before the experiment. After a stable baseline was measured, oxygen tension was decreased to ∼20 Torr and [Ca2+]i measurements were continued. Ratiometric imaging was performed with the excitation wavelengths of 340 nm and 380 nm and an emission wavelength of 510 nm. Imaging was performed with an ICCD camera (Photonic Science, Robertsbridge, UK) using Axon Instruments (Foster City, CA) or Metafluor (Fryer, Bloomington, MN) image capture and analysis software. Ca2+ calibration was achieved by measuring a maximum (with 1 mM ionomycin) and a minimum (with 10 mM EGTA). For each experiment ∼5–10 cells per coverslip were visualized, and ratiometric data were acquired from individual cells.
Statistical analysis.
Statistical significance between two groups was determined using Student's t-test and differences between multiple groups using an ANOVA with Bonferroni posttest analysis. P < 0.05 was taken as the threshold level for statistical significance. All results are expressed as means ± SE.
RESULTS
Hypoxic induction of KCNMB1 expression is associated with an increase in HIF-1α protein.
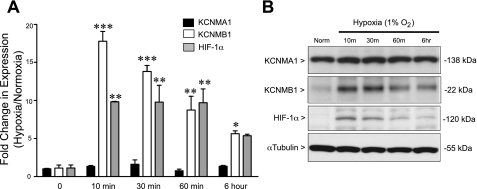
We demonstrated previously that hypoxia increases KCNMB1 expression both in vivo and in vitro in the rat (22). To determine whether the hypoxic induction of KCNMB1 is conserved across species, hPASMC were maintained in culture under either normoxic or hypoxic conditions, and total mRNA (Fig. 1A) and protein (Fig. 1B) of KCNMA1, KCNMB1, and HIF-1α were measured. Whereas the expression of KCNMA1 did not change with acute hypoxia, both KCNMB1 gene and protein rapidly increased upon exposure to hypoxia. By 10 min of hypoxic exposure, KCNMB1 mRNA and protein expression increased markedly, followed by a gradual decrease over time, but remained elevated compared with normoxic control at 6 h. As expected, hypoxia also caused an increase in HIF-1α gene and protein, which temporally mirrored the hypoxic induction of KCNMB1. These data suggested that KCNMB1 might be under the transcriptional control of HIF-1α.
Fig. 1.
Hypoxia increases the β1-subunit (KCNMB1) of the calcium-sensitive potassium channel (BKCa) and hypoxia-inducible factor-1α (HIF-1α) in human pulmonary artery smooth muscle cells (hPASMC). A: qRT-PCR in hPASMC demonstrates a significant increase in the α1-subunit of BKCa (KCNMB1) and HIF-1α mRNA expression after 10 min to 6 h of hypoxia compared with normoxia. No difference was found in KCNMA1 gene expression. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. normoxia by one-way ANOVA followed by Bonferroni posttest analysis, with n = 3. B: representative Western immunoblot to detect KCNMA1, KCNMB1, and HIF-1α protein expression in hPASMC demonstrates that hypoxia increases KCNMB1 protein within 10 min of hypoxic exposure and persists through 6 h, which mirrors the increase in HIF-1α protein expression.
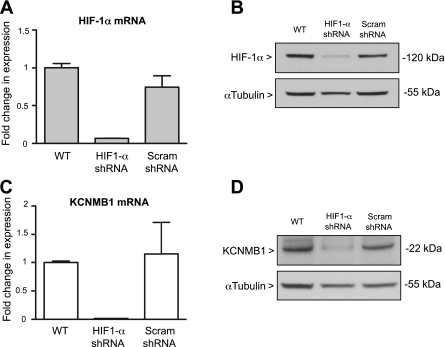
HIF-1α depletion decreases KCNMB1 expression in hPASMC.
To identify a role for HIF-1α in regulating KCNMB1 expression in hPASMC, the cells were transfected with a HIF-1α-specific shRNA plasmid. As depicted in Fig. 2, shRNA against HIF-1α effectively decreased HIF-1α mRNA (Fig. 2A) and protein (Fig. 2B) expression in hPASMC incubated in hypoxia for 6 h. Furthermore, depletion of HIF-1α prevented the hypoxic increase in KCNMB1 mRNA and protein expression (Fig. 2, C and D). Transfection with the scrambled shRNA had no effect on either HIF-1α or KCNMB1 expression, confirming the specificity of the HIF-1α shRNA plasmid. The observation that silencing of HIF-1α blocks the upregulation of KCNMB1 in response to hypoxia provides further evidence for the notion that HIF-1α is a direct transcriptional regulator of KCNMB1.
Fig. 2.
Silencing HIF-1α prevents the hypoxic induction of KCNMB1. A and B: qRT-PCR and Western immunoblot demonstrate that stable transfection of hPASMC with shRNA to target HIF-1α effectively decreases HIF-1α gene and protein expression. C and D: qRT-PCR and Western immunoblot to detect KCNMB1 mRNA and protein show that knockdown of HIF-1α prevents the hypoxic induction of KCNMB1 expression. In Western immunoblotting experiments, α-tubulin was used as a protein loading control. WT, wild-type; Scram; scrambled.
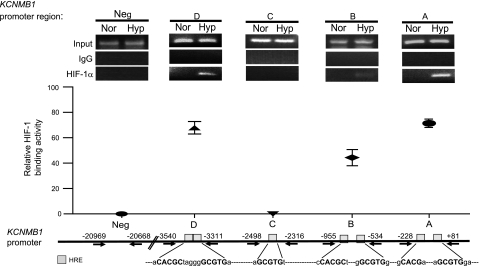
Hypoxia increases the binding of HIF-1α to select HRE(s) within the KCNMB1 promoter.
The human KCNMB1 promoter contains ten putative HREs located in seven separate regions (12). To demonstrate that hypoxia increases HIF-1α binding to the KCNMB1 promoter, a ChIP assay was performed. On the basis of the distribution of the HRE regions within the KCNMB1 promoter and location of restriction enzyme sites (Figs. 3 and 4A), the promoter was dissected and fragments were arbitrarily named (A, B, C, and D). With the use of anti-HIF-1α-specific antibody and primer sets specific to each fragment (Table 2), data from four distinct ChIP assays (Fig. 3, top) were obtained. DNA was amplified with primers that included the HRE(s) located within each specific region of the KCNMB1 promoter. Although no PCR product was detectable after immunoprecipitation of HIF-1α under normoxic conditions, PCR products corresponding to the A, B, and D regions of KCNMB1 promoter were readily apparent under hypoxic conditions (1% O2, 6 h). The levels of binding differed significantly between promoter regions, with promoter regions A, B, and D demonstrating the greatest level of HIF-1α binding (Fig. 3, top and middle). Evidence of the specificity of the assay included the observation that no PCR product was amplified after immunoprecipitation with IgG or after immunoprecipitation with HIF-1α antibody, followed by PCR using primers corresponding to an upstream region of the KCNMB1 promoter that does not contain an HRE (Neg). Taken together, this data provides evidence that HIF-1α binds directly to HRE(s) located within the A, B, and D regions of the KCNMB1 promoter.
Fig. 3.
Hypoxia increases binding of HIF-1α to discrete regions of the human KCNMB1 promoter. Chromatin immunoprecipitation (ChIP) demonstrated that hypoxia induced binding of HIF-1α-containing complexes and the KCNMB1 promoter regions A, B, and D. Data are presented as relative binding activity determined as the intensity of the PCR product obtained after HIF-1α IP relative to the input signal and expressed as a percent, with n = 3. HRE, hypoxia response element.
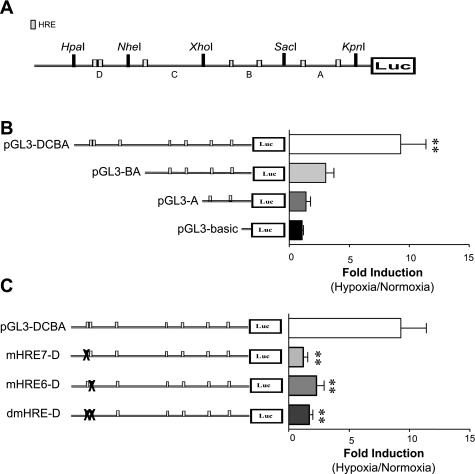
Fig. 4.
HIF-1α-mediated transcription of KCNMB1 requires binding to the HREs located between −3,540 and −3,311 bp of the promoter. A: schematic of the KCNMB1 promoter divided into fragments arbitrarily named (A, B, C, and D) based on the distribution of HREs (gray boxes) and location of restriction enzyme sites. B: hPASMC were transfected with 1 of 3 luciferase reporter plasmids containing different fragments of the KCNMB1 promoter, or the negative control plasmid, pGL3-basic. Transfected hPASMCs were then exposed to 1% O2 for 6 h, and luciferase reporter activity was measured and reported as the fold induction of reporter activity after hypoxia vs. normoxia. **P < 0.01 vs. pGL3-basic, with data presented as the means ± SE of 3 independent experiments. C: hPASMC transfected with luciferase reporter constructs containing mutations within each of the 2 HRE located within the D region (mHRE7-D and mHRE6-D), or a double mutant with both HRE mutated (dmHRE-D). Transfected cells were exposed to 1% O2 for 6 h, and luciferase reporter activity was measured and reported as the fold induction of reporter activity after hypoxia vs. normoxia. **P < 0.01 vs. pGL3-DCBA, with data presented as the means ± SE of 3 independent experiments.
HIF-1α-mediated transcription of KCNMB1 requires binding to the two HREs located between −3,540 and −3,311 bp of the promoter.
To identify which specific HRE(s) account for the HIF-1α-mediated induction of KCNMB1, we next employed a luciferase reporter assay. We cloned components of the KCNMB1 promoter fragments (A, B, C, and D) (Fig. 4A) and subcloned them into the luciferase reporter vector. hPASMC were transiently transfected with the reporter vectors, in addition to a HIF-1α overexpression vector. In the transfected hPASMC exposed to hypoxia (1% O2, 6 h), only transfection with the construct containing promoter region D (pGL3-DCBA) resulted in a significant increase in luciferase activity over that observed with the pGL3-basic control plasmid (Fig. 4B). In addition, mutating either of the two adjacent HREs located in this portion of the KCNMB1 promoter (mutants mHRE7-D and mHRE6-D) completely prevented the hypoxia-mediated increase in luciferase activity, and mutating both regions (dmHRE-D) had no additional effect (Fig. 4C). These studies indicate that binding of HIF-1α to both of the HREs located within the D regions (−3,540 to −3,311) is essential for the hypoxic induction of KCNMB1, and that binding to a single HRE within that region is insufficient to increase KCNMB1 expression.
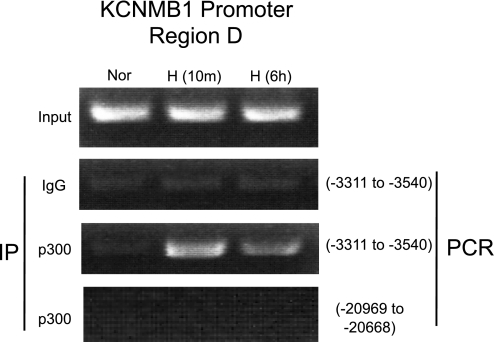
Normally, HIF-1α activates transcription through recruitment of the coactivator, p300/CBP (9, 31). Thus additional ChIP assays were conducted to determine whether hypoxia enhances p300 recruitment to the D region of the KCNMB1 promoter. Using the primers spanning D (−3,540 to −3,311) regions, we demonstrated that, after only 10 min of hypoxia, p300 is recruited to the KCNMB1 promoter. The recruitment of p300 to the KCNMB1 promoter was time limited, as after 6 h of hypoxia p300 recruitment decreased (Fig. 5), paralleling the decrease in KCNMB1 expression we observed at 6 h. Under normoxic conditions, no PCR products were detected following immunoprecipitation with p300 antibody, confirming that the interaction between the KCNMB1 promoter and p300 is oxygen sensitive (Fig. 5). The specificity of the approach was demonstrated by the absence of PCR products when IP was performed using control IgG, or after IP with HIF-1α antibody and PCR using primers corresponding to an upstream region of the KCNMB1 promoter that does not contain an HRE (−20,969 to −20,668). These results suggest that HIF-1α regulates KCNMB1 expression by binding and recruiting p300 to the D region of KCNMB1 promoter.
Fig. 5.
Hypoxia increases binding of the transcriptional coactivator, p300, to the KCNMB1 promoter at 3,540 to −3,311 bp. Representative ChIP after IP with an antibody against p300 followed by and PCR using primers designed to detect the D region (−3,540 to −3,311 bp) of the KCNMB1 promoter in hPASMC under normoxia, and after either 10 min or 6 h of hypoxia. Control experiments were performed by immunoprecipitating with an isotype control antibody (Ig), and after IP using the p300 antibody followed by PCR using primers corresponding to an upstream region of the KCNMB1 promoter that does not contain an HRE.
Histone deacetylase activity limits the hypoxic induction of KCNMB1.
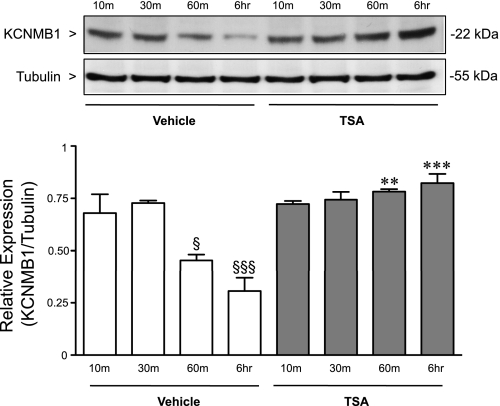
The hypoxic induction of KCNMB1 peaked at 10 to 30 min, and decreased with persistent exposure to hypoxia. Sustained hypoxia can silence gene expression through epigenetic mechanisms (11, 18). Thus we investigated whether changes in histone acetylation contributed to the decrease in KCNMB1 expression after prolonged hypoxia. hPASMC were maintained under either hypoxic or normoxic conditions, and KCNMB1 protein expression was measured after treatment with the histone deacetylase (HDAC) inhibitor, trichostatin A. Interestingly, in contrast with the time-limited increase in KCNMB1 expression in vehicle-treated cells, the hypoxia-induced increase in KCNMB1 expression was sustained over an extended interval in the presence of HDAC inhibitor, TSA (Fig. 6).
Fig. 6.
Histone deacetylase (HDAC) inhibition prolongs the hypoxic induction of KCNMB1. Western immunoblot to detect KCNMB1 protein after treatment of the hPASMC with either vehicle, or the HDAC inhibitor, trichostatin A (TSA), and exposure to hypoxia for 10 min to 6 h. α-Tubulin was used as a loading control. §P < 0.05 vs. vehicle 30 min, and §§§P < 0.001 vs. vehicle 10 min and 30 min. **P < 0.01 vs. vehicle 60 min, and ***P < 0.001 vs. vehicle 6 h.
KCNMB1 expression modulates the level of cytosolic calcium in hPASMC in response to acute hypoxia.
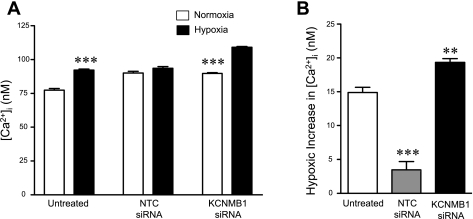
We hypothesized that depletion of KCNMB1 might possess physiological implications for the response of hPASMC to acute hypoxia, a stimulus that increases cytosolic calcium ([Ca2+]i). Therefore, hPASMC were transfected with either siRNA to target KCNMB1 or nontargeted control siRNA. Transfection with the KCNMB1 siRNA effectively silenced both KCNMB1 mRNA and protein expression (data not shown). Acute hypoxia for 20 min induced a significant increase in [Ca2+]i from 78 ± 1 to 92 ± 1 nM (P < 0.001, vs. normoxia) in untransfected control cells (Fig. 7). Interestingly, transfection itself appears to blunt the hypoxic-mediated increase in [Ca2+]i, as hypoxia did not induce a statistically significant increase in [Ca2+]i in control siRNA-transfected cells. In contrast, silencing of KCNMB1 with siRNA accentuated the response to hypoxia, resulting in an increase in [Ca2+]i increasing from 90 ± 1 (normoxia) to 109 ± 1 nM (hypoxia; P < 0.001, vs. normoxia). Thus KCNMB1 knockdown potentiated and accentuated the response to acute hypoxia, demonstrating a role for KCNMB1 in modulating the response of PASMC to hypoxia.
Fig. 7.
Depletion of KCNMB1 potentiates the response of hPASMC to hypoxia. hPASMC were transfected with either nontargeting (control) or KCNMB1 siRNA. Untreated and siRNA-transfected cells were loaded with fura-2 AM and exposed to acute hypoxia for 20 min. A: hypoxia caused a significant increase in cytosolic calcium (P < 0.05 vs. normoxia; n = 14). Transfection of hPASMC with nontargeted control siRNA blunted hypoxia-induced increase in of cytosolic calcium (n = 15). However, KCNMB1 knockdown with siRNA accentuated the response of hPASMC to hypoxia, resulting in significant increase in cytosolic calcium (***P < 0.001, vs. normoxic control for that treatment group, with n = 14–15, per group). B: after 20 min of hypoxia, untreated hPASMC demonstrated a significant increase in cytosolic calcium. Compared with untreated cells, the response to hypoxia in hPASMC treated with nontargeting (control) siRNA was attenuated (***P < 0.001). In hPASMC treated with KCNMB1 siRNA, the response to hypoxia was accentuated, compared with untreated cells (**P < 0.01). NTC, non targeted control.
DISCUSSION
Previously, we reported that chronic hypoxia induces significant increase in BKCa α- and β1-subunits in ovine PASMC and the lung of Sprague-Dawley rats (22). In the present report, we found that acute hypoxia increases BKCa β1-subunit (KCNMB1) expression in hPASMC through promoter activation and that this induction is mediated by HIF-1α. We demonstrate that hypoxia causes a rapid, significant, but time-limited increase in HIF-1α protein in hPASMC that is paralleled, qualitatively and temporally, by the increase in KCNMB1 expression. Further, we provide definitive information that the hypoxic induction of KCNMB1 results from binding of HIF-1α to two specific HREs of the KCNMB1 promoter. We add further detail to the molecular regulation of the hypoxic induction of the KCNMB1 gene by demonstrating that epigenetic regulation contributes to the time-limited increase in the expression of the β1-subunit. Finally, we demonstrate the functional significance of the KCNMB1 gene, as the response of hPASMC to acute hypoxia is meaningfully potentiated in the absence of the β1-subunit.
Data from the ChIP studies in Fig. 3 indicate that HIF-1α binds most avidly to regions A, B, and D of the KCNMB1 promoter. However, promoter reporter assays on HIF-1α overexpressing hPASMC demonstrated that only region D (−3,540 to −3,311 bp) of the KCNMB1 promoter is crucial for the HIF-1α-mediated transcriptional activation of KCNMB1. We conclude that HIF-1α is primarily responsible for the hypoxic induction of KCNMB1 expression in hPASMC. Furthermore, by individually mutating the two HREs located within the D region of the KCNMB1 promoter, we concluded that both are essential for the hypoxic induction of KCNMB1 promoter activity. As mutations in either of the HREs in region D attenuate expression, we conclude that the juxtaposition of two HREs immediately adjacent to one another is required for the high degree of hypoxic sensitivity that characterizes the KCNMB1 promoter. There is precedent for the notion that HREs positioned immediately adjacent to one another might have implications for the hypoxic induction of a specific gene (10).
As seen in Fig. 5, our data are consistent with the notion that, with hypoxia, HIF-1α recruits p300 to the HRE of the KCNMB1 promoter, specifically to the D region of the promoter. As HIF-1α normally activates transcription through recruitment of the coactivator, p300/CBP (9, 31), the observation provides further evidence that HIF-1α mediates the hypoxic induction of KCNMB1 expression. Moreover, the observation that hypoxia-induced recruitment of p300 to the D region of the KCNMB1 was time-limited is entirely consistent with the data presented in Fig. 1, thereby lending still further strength to the notion that HIF-1α mediates the increase in KCNMB1 expression.
In these experiments, KCNMB1 expression increased rapidly in response to hypoxia. However, by 6 h of hypoxic exposure, the level of KCNMB1 was decreasing. Epigenetic mechanisms of gene silencing have been implicated in the repression of genes in response to chronic hypoxia (11, 18). This observation prompted us to interrogate the possibility that epigenetic mechanisms may contribute to the regulation of the KCNMB1 gene during hypoxia (20). To address the issue, we used the relatively nonspecific inhibitor of histone deacetylase activity, tricostatin (30), to demonstrate a role for epigenetic regulation in modulating the hypoxic induction of the KCNMB1 gene product. Interestingly, in the presence of the HDAC inhibition, the increase in KCNMB1 expression was sustained over the entire 6-h study period, suggesting that epigenetic mechanisms likely play a role in preventing the sustained increase in hPASMC expression of the β1-subunit (Fig. 6). This observation may have implications for conditions wherein pulmonary vascular tone is increased. If the capacity to augment KCNMB1 expression is developmentally regulated, then a tool to compensate for an increase in pulmonary vascular tone may not be available in more mature organisms.
Finally, we demonstrated that loss of KCNMB1 has substantial functional significance in hPASMC. Previous work from our laboratory led us to believe that the β1-subunit plays a role in modulating the pulmonary vascular response of PASMC to changes in oxygen tension (22). Given evidence that HIF-1α regulates KCNMB1 expression, we postulated that depletion of KCNMB1 expression would potentiate the physiological response to acute hypoxia in PASMC. As expected, hypoxic induction of cytosolic calcium was further exacerbated in KCNMB1-depleted hPASMC compared with that in control hPASMCs. Therefore, upregulation of KCNMB1 in response to acute hypoxia likely provides a compensatory mechanism to modulate the hypoxia-induced cytosolic calcium and vasoconstriction. These results provide further support for the notion that KCNMB1 expression has physiological significance in the pulmonary circulation. This result is logical from a mechanistic perspective, given previous data from our laboratory that underscore the importance of the BKCa channel in mediating perinatal pulmonary dilation (4, 25). The presence of the β1-subunit might serve to mitigate hypoxic pulmonary vasoconstriction, a physiological response that in the perinatal period might lead to marked untoward sequelae, such as extrapulmonary shunting and severe arterial hypoxemia (13).
These studies were performed in hPASMC derived from 2.5-mo-old human infant. Given previous data from our laboratory demonstrating the importance of the BKCa in the perinatal pulmonary circulation (4, 27, 28) superimposed on the observation that expression of the channel decreases with maturation (5), it may be the case that the robust increase in KCNMB1 expression in response to hypoxia is developmentally regulated. It may be important to undertake a similar line of investigation at several different developmental stages. Indeed, if the response to hypoxia is developmentally regulated, it is possible that the relatively greater level of resiliency in the pulmonary circulation of neonates with persistent pulmonary hypertension of the newborn (13) compared with older people results, in part, from the capacity of neonatal PASMC to increase KCNMB1 in response to injurious stimuli such as hypoxia.
There are limitations in the present study. First, all the experiments were performed in vitro. The strength of the findings would be amplified by in vivo experiments. Moreover, as the experiments were performed in a cell line, there is potential for phenotypic drift that may have influenced the findings. However, in studies performed in rats (22), hypoxia caused a similar increase in β1-subunit expression in the pulmonary arteries, suggesting that the present findings have fidelity with the biology seen in whole animals.
In conclusion, we have identified two HREs located between bp −3,540 and −3,311 of the KCNMB1 promoter that function as cis-acting elements to regulate the KCNMB1 gene induction by hypoxia. Evidence that HIF-1α interacts with these specific HREs provides mechanistic information regarding how HIF-1α regulates KCNMB1 gene transcription in hPASMC. Modulating expression of the β1-subunit of BKCa channel in hPASMC might represent a novel and potentially powerful strategy to address pulmonary vascular disease.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants RO1-HL-60784 and RO1-HL-70628 (D. Cornfield), 1P30HL101315-01 (D. Cornfield and C. Alvira), and the American Heart Association Fellow to Faculty Award #0875001N (C. Alvira).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
The findings were presented, in part, at the Society for Pediatric Research Meeting on May 5, 2011. D. N. Cornfield is an Established Investigator of the American Heart Association.
REFERENCES
- 1. Amberg GC, Santana LF. Downregulation of the BK channel beta1 subunit in genetic hypertension. Circ Res 93: 965–971, 2003 [DOI] [PubMed] [Google Scholar]
- 2. Brayden JE, Nelson MT. Regulation of arterial tone by activation of calcium-dependent potassium channels. Science 256: 534–535, 1992 [DOI] [PubMed] [Google Scholar]
- 3. Brenner R, Peréz GJ, Bonev AD, Eckman DM, Kosek JC, Wiler SW, Patterson AJ, Nelson MT, Aldrich RW. Vasoregulation by the beta1 subunit of the calcium-activated potassium channel. Nature 407: 870–876, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Cornfield DN, Reeve HL, Tolarova S, Weir EK, Archer SL. Oxygen causes fetal pulmonary vasodilation through activation of a calcium-dependent potassium channel. Proc Natl Acad Sci USA 93: 8089–8094, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cornfield DN, Saqueton CB, Porter VA, Herron JM, Resnik ER, Haddad IY, Reeve HL. Voltage-gated K+ channel activity in the ovine pulmonary vasculature is developmentally regulated. Am J Physiol Lung Cell Mol Physiol 278: L1297–L1304, 2000 [DOI] [PubMed] [Google Scholar]
- 6. Cox DH, Aldrich RW. Role of the β1 subunit in large-conductance Ca2+-activated K+ channel gating energetics: mechanisms of enhanced Ca2+ sensitivity. J Gen Physiol 116: 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fernandez-Fernandez JM, Tomas M, Vazquez E, Orio P, Latorre R, Senti M, Marrugat J, Valverde MA. Gain-of-function mutation in the KCNMB1 potassium channel subunit is associated with low prevalence of diastolic hypertension. J Clin Invest 113: 1032–1039, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Garcia-Calvo M, Knaus HG, McManus OB, Giangiacomo KM, Kaczorowski GJ, Garcia ML. Purification and Reconstitution of the high conductance, calcium-activated potassium channel from tracheal smooth muscle. J Biol Chem 269: 676–682, 1994 [PubMed] [Google Scholar]
- 9. Goodman RH, Smolik S. CBP/p300 in cell growth, transformation, and development. Genes Dev 14: 1553–1577, 2000 [PubMed] [Google Scholar]
- 10. Guadall A, Orriols M, Rodríguez-Calvo R, Calvayrac O, Crespo J, Aledo R, Martínez-González J, Rodríguez C. Fibulin-5 is up-regulated by hypoxia in endothelial cells through a hypoxia-inducible factor-1 (HIF-1α)-dependent mechanism. J Biol Chem 286: 7093–7103, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jeon HW, Lee YM. Inhibition of histone deacetylase attenuates hypoxia-induced migration and invasion of cancer cells via the restoration of RECK expression. Mol Cancer Ther 9: 1361–1370, 2010 [DOI] [PubMed] [Google Scholar]
- 12. Jiang Z, Wallner M, Meera P, Toro L. Human and rodent MaxiK channel β-subunit genes: Cloning and characterization. Genomics 55: 57–67, 1999 [DOI] [PubMed] [Google Scholar]
- 13. Kinsella JP, Abman SH. Recent developments in the pathophysiology and treatment of persistent pulmonary hypertension of the newborn. J Ped Surg 126: 853–864, 1995 [DOI] [PubMed] [Google Scholar]
- 14. Knaus HG, Eberhart A, Koch ROA, Munujos P, Kaczoroxski GJ, Garcia ML. Characterization of tissue-expressed alpha subunits of the high conductance calcium-activated K+ channel. J Biol Chem 270: 22434–22439, 1995 [DOI] [PubMed] [Google Scholar]
- 15. Mazure NM, Chen EY, Laderoute KR, Giaccia AJ. Induction of vascular endothelial growth factor by hypoxia is modulated by a phosphatidylinositol 3-kinase/Akt signaling pathway in Ha-ras-transformed cells through a hypoxia inducible factor-1 transcriptional element. Blood 90: 332–331, 1997 [PubMed] [Google Scholar]
- 16. McManus OB, Helms LM, Pallanck L, Ganetzky B, Swanson R, Leonard RJ. Functional role of the beta subunit of high conductance calcium-activated potassium channels. Neuron 14: 645–650, 1995 [DOI] [PubMed] [Google Scholar]
- 17. Nelson MT, Cheng H, Rubart M, Santana LF, Bonev AD, Knot HJ, Lederer WJ. Relaxation of arterial smooth muscle by arterial sparks. Science 270: 633–637, 1995 [DOI] [PubMed] [Google Scholar]
- 18. Patterson AJ, Chen M, Xue Q, Xiao D, Zhang L. Chronic prenatal hypoxia induces epigenetic programming of PKCepsilon gene repression in rat hearts. Circ Res 107: 365–373, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Plüger S, Faulhabe J, Fürstenau M, Löhn M, Waldschütz R, Gollasch M, Haller H, Luft FC, Ehmke H, Pongs O. Mice with disrupted BK channel beta1 subunit gene feature abnormal Ca(2+) spark/STOC coupling and elevated blood pressure. Circ Res 87: E53–E60, 2000 [DOI] [PubMed] [Google Scholar]
- 20. Ptashne M, Gann A. Transcriptional activation by recruitment. Nature 386: 569–577, 1997 [DOI] [PubMed] [Google Scholar]
- 21. Reeve HL, Archer SL, Weir EK, Cornfield DN. Maturational changes in K+ channel activity and oxygen sensing in the ovine pulmonary vasculature. Am J Physiol Lung Cell Mol Physiol 275: L2019–L2025, 1998 [Google Scholar]
- 22. Resnik E, Herron J, Fu R, Ivy DD, Cornfield DN. Oxygen tension modulates the expression of pulmonary vascular BKCa channel alpha- and beta-subunits. Am J Physiol Lung Cell Mol Physiol 290: L761–L768, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Resnik ER, Herron JM, Lyu SC, Cornfield DN. Developmental regulation of hypoxia-inducible factor 1 and prolyl-hydroxylases in pulmonary vascular smooth muscle cells. Proc Natl Acad Sci USA 104: 18789–18794, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rhodes MT, Porter VA, Saqueton CB, Herron JM, Resnik ER, Cornfield DN. Pulmonary vascular response to normoxia and KCa channel activity is developmentally regulated. Am J Physiol Lung Cell Mol Physiol 280: L1250–L1257, 2001 [DOI] [PubMed] [Google Scholar]
- 25. Saqueton CB, Miller RM, Porter VA, Millla CM, Cornfield DN. Nitric oxide causes perinatal pulmonary vasodilation through K+ channel activation and requires intracellular calcium release. Am J Physiol Lung Cell Mol Physiol 276: L925–L932, 1999 [DOI] [PubMed] [Google Scholar]
- 26. Senti M, Fernandez-Fernandez JM, Tomas M, Vazquez E, Elosua R, Marrugat J, Valverde MA. Protective effect of the KCNMB1 E65K genetic polymorphism against diastolic hypertension in aging women and its relevance to cardiovascular risk. Circ Res 97: 1360–1365, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Storme L, Rairigh RL, Parker TA, Cornfield DN, Kinsella JP, Abman SH. K+ channel blockade inhibits shear stress-induced pulmonary vasodilation in the ovine fetus. Am J Physiol Lung Cell Mol Physiol 276: L220–L228, 1999 [DOI] [PubMed] [Google Scholar]
- 28. Tristani-Firouzi M, Martin EB, Tolarova S, Weir EK, Archer SL, Cornfield DN. Ventilation-induced pulmonary vasodilation at birth is modulated by potassium channel activity. Am J Physiol Heart Circ Physiol 271: H2353–H2359, 1996 [DOI] [PubMed] [Google Scholar]
- 29. Wang GL, Salisbury E, Shi X, Timchenko L, Medrano EE, Timchenko NA. HDAC1 cooperates with C/EBPalpha in the inhibition of liver proliferation in old mice. J Biol Chem 2883: 26169–26178, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang GG, Allis CD, Chi P. Chromatin remodeling and cancer, part I: covalent histone modifications.. Trends Mol Med 13: 363–372, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Yamashita K, Discher DJ, Hu J, Bishopric NH, Webster KA. Molecular Regulation of the Endothelin-1 Gene by Hypoxia Contributions of Hypoxia-Inducible factor-1, Activator protein-1, GATA-2 and p300/CBP. J Biol Chem 276: 12645–12653, 2001 [DOI] [PubMed] [Google Scholar]