Abstract
Dopamine receptors are G protein-coupled receptors that are divided into two subgroups, “D1-like” receptors (D1 and D5) that couple to the Gs protein and “D2-like” receptors (D2, D3, and D4) that couple to Gi. Although inhaled dopamine has been reported to induce bronchodilation in patients with asthma, functional expression of dopamine receptor subtypes has never been described on airway smooth muscle (ASM) cells. Acute activation of Gi-coupled receptors inhibits adenylyl cyclase activity and cAMP synthesis, which classically impairs ASM relaxation. In contrast, chronic activation of Gi-coupled receptors produces a paradoxical enhancement of adenylyl cyclase activity referred to as heterologous sensitization. We questioned whether the dopamine D2-like receptor is expressed on ASM, whether it exhibits classical Gi-coupling, and whether it modulates ASM function. We detected the mRNA encoding the dopamine D2 receptor in total RNA isolated from native human ASM and from cultured human airway smooth muscle (HASM) cells. Immunoblots identified the dopamine D2 receptor protein in both native human and guinea pig ASM and cultured HASM cells. The dopamine D2 receptor protein was immunohistochemically localized to both human and guinea pig ASM. Acute activation of the dopamine D2 receptor by quinpirole inhibited forskolin-stimulated adenylyl cyclase activity in HASM cells, which was blocked by the dopamine D2 receptor antagonist L-741626. In contrast, the chronic pretreatment (1 h) with quinpirole potentiated forskolin-stimulated adenylyl cyclase activity, which was inhibited by L-741626, the phospholipase C inhibitor U73122, or the protein kinase C inhibitor GF109203X. Quinpirole also stimulated inositol phosphate synthesis, which was inhibited by L-741626 or U73122. Chronic pretreatment (1 h) of the guinea pig tracheal rings with quinpirole significantly potentiated forskolin-induced airway relaxation, which was inhibited by L-741626. These results demonstrate that functional dopamine D2 receptors are expressed on ASM and could be a novel therapeutic target for the relaxation of ASM.
Keywords: reverse transcriptase polymerase chain reaction, immunoblot, guinea pig, organ bath
dopamine is an endogenous catecholamine that influences many cellular activities, including behavior, hormone synthesis and release, blood pressure, and intracellular ion transport. The dopamine receptor is a member of the superfamily of heptahelical G-protein-coupled receptors that have been divided into two subgroups, “D1-like” receptors (D1 and D5) that couple to the Gs protein and “D2-like” receptors (D2, D3, and D4) that couple to Gi (27, 29).
In patients with asthma, inhalation of dopamine has a bronchodilatory effect during bronchial obstruction (10, 12, 26). Although the dopamine D2-like receptor has been identified in the dorsal root ganglia and airway projecting sensory neurons (33, 34), the functional expression of this receptor has never been described on airway smooth muscle cells.
The stimulation of Gi-coupled receptors leads to inhibition of adenylyl cyclase activity and a consequent reduction in cellular cAMP levels (9), which classically impairs airway smooth muscle relaxation (38). However, when the Gi-coupled receptors are chronically activated, there is a paradoxical and perhaps compensatory enhancement of adenylyl cyclase activity, which is called heterologous sensitization (48). This sensitization has been demonstrated following the persistent activation of a number of Gi-coupled receptors, including dopamine D2 and D4, A3 adenosine, α2-adrenergic, and μ-opioid receptors (3, 22, 31, 46, 48).
In the present study, we provide evidence that the dopamine D2-like receptor is expressed in native human and guinea pig airway smooth muscle and cultured human airway smooth muscle (HASM) cells, exhibits classical Gi-coupling, and following chronic stimulation induces airway smooth muscle relaxation.
METHODS
Materials.
HASM cells were cultured in M-199 smooth muscle medium containing human fibroblast growth factor, human epidermal growth factor, FBS, and antibiotic-antimycotic which were purchased from Invitrogen (Grand Island, NY). Lysates of human brain cerebral cortex were used as a positive protein control on immunoblots and were obtained from BD Biosciences (Palo Alto, CA). Total RNA from whole human brain was purchased from Clontech (Mountain View, CA). Pertussis toxin (PTX) and protease inhibitor cocktail III was purchased from EMD Biosciences (San Diego, CA). α-[32P]ATP (800 Ci/mmol), [3H]cAMP (32 Ci/mmol), and [3H]myo-inositol (20 Ci/mmol) were obtained from MP Biomedicals (Irvine, CA). Quinpirole, L-741626, and U73122 were purchased from Tocris Bioscience (Ellisville, MO). All other chemicals were obtained from Sigma (St. Louis, MO) unless otherwise stated.
Cell culture.
Primary cultures of HASM cells came from two sources. Cells were obtained from Lonza (Walkersville, MD) or at the University of Pennsylvania, which were obtained from lung transplant donors in accordance with procedures approved by the University of Pennsylvania Committee on Studies Involving Human Beings as previously described (32). Cells were grown in culture medium [M-199, supplemented with 10% FBS, 1 ng/ml human fibroblast growth factor, 250 pg/ml human epidermal growth factor, and an antibiotic-antimycotic mix (100 U/ml penicillin G sodium, 100 μg/ml streptomycin sulfate, and 0.25 μg/ml amphotericin B)] at 37°C in a humidified atmosphere of 5% CO2-95% air. For immunoblotting, cells were grown to confluency in T75 flasks. Cells were rinsed with cold PBS and scraped in cold PBS. Cells were pelleted (500 g, 10 min, 4°C) and lysed in cold lysis buffer (50 mM Tris·HCl pH 8.0, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, 150 mM NaCl, 1 mM EDTA, 1:200 dilution of protease inhibitor cocktail III, 1 mM Na3VO4, and 1 mM NaF). Lysed cells were centrifuged (15,000 g, 15 min, 4°C), and an aliquot of the supernatant was subjected to protein analysis before solubilizing the samples by heating at 95°C for 5 min in sample buffer (final concentrations: 50 mM Tris·HCl pH 6.8, 2.5% SDS, 6% glycerol, 2.5% 2-mercaptoethanol, and bromophenol blue) and storing at −20°C.
Isolation of smooth muscle from human trachea and guinea pig for RNA and protein analysis.
All studies were approved by the Columbia University's Institutional Review Board and deemed not human subjects research under 45 CFR 46. All animal studies were approved by the Columbia University Institutional Animal Care and Use Committee. These studies were also reviewed by the Committee on the Ethics of Animal Experiments in Tohoku University School of Medicine, and they were carried out in accordance with both the Guidelines for Animal Experiments issued by the Tohoku University and The Law (No. 105) and Notification (No. 6) issued by the Japanese Government.
Human trachea came from two sources. Snap frozen tracheas obtained at autopsy from nonasthmatic adults within 8 h of death were obtained from the National Disease Research Exchange (NDRI, Philadelphia, PA). Additional trachea were obtained from discarded regions of healthy donor lungs harvested for lung transplantation at Columbia University. Lung transplant excess tissue was transported to the laboratory on ice and submerged in cold (4°C) Krebs-Henseleit (KH) buffer (in mM: 118 NaCl, 5.6 KCl, 0.5 CaCl2, 0.24 MgSO4, 1.3 NaH2PO4, 25 NaHCO3, and 5.6 glucose pH 7.4).
Adult male guinea pigs were deeply anesthetized by intraperitoneal pentobarbital (50 mg/kg), the chest cavity was opened, and the animal exsanguinated before dissection of the trachea. The trachea or whole brain were surgically removed and placed in cold (4°C) KH buffer.
The exteriors of either the human or guinea pig trachea were meticulously dissected free of adherent connective tissue under a dissecting microscope. Tracheas were opened longitudinally along the anterior border, the epithelium was removed, and the airway smooth muscle between the noncontiguous ends of the cartilaginous tracheal rings was dissected free. Airway smooth muscle or whole brain was homogenized (Tekmar Ultra Turrax T25 high-speed homogenizer set at top speed for 30 s) in Trizol reagent (Ambion, Austin, TX) for total RNA extraction according to the manufacturer's recommendations or in cold (4°C) buffer (50 mM Tris, 10 mM HEPES pH 7.4, and 1 mM EDTA with a 1:200 dilution of protease inhibitor cocktail III) for total protein isolation. The homogenate was filtered through 125-μm Nitex mesh and centrifuged twice at 500 g for 15 min. The supernatant was transferred into new tubes and centrifuged at 50,000 g for 30 min at 4°C. The final membrane pellet was resuspended in the same buffer for protein concentration determinations and stored at −80°C.
RNA isolation and RT-PCR.
Total RNA was extracted from freshly dissected native human or guinea pig airway smooth muscle, cultured HASM cells, and guinea pig whole brain using Trizol Reagent (Ambion, Austin, TX) according to the manufacturer's recommendations. Total RNA from whole human brain (Clontech, Mountain View, CA) was used as a positive control. With the use of the Advantage RT-for-PCR kit (Clontech), 1 μg of total RNA was reverse transcribed at 42°C for 1 h in 20 μl including 200 U of Moloney murine leukemia virus reverse transcriptase, 20 U of RNase inhibitor, 20 pmol oligo (dT) primer, and 0.5 mM each of dNTP mix in reaction buffer (50 mM Tris·HCl pH 8.3, 75 mM KCl, and 3 mM MgCl2).
PCR was performed by addition of 5 μl of newly synthesized cDNA to a 45 μl reaction mixture yielding final concentrations of 0.2 mM of each dNTP, 1× Advantage 2 polymerase mix, PCR buffer (Clontech), and 0.4 μM of both sense and antisense primers for the D2 subgroup (D2, D3, and D4) of dopamine receptors (Table 1). Two-step PCR (annealing and extension at same temperature) was performed for 1 min with a PTC-200 Peltier thermal cycler (MJ Research) for all PCR reactions, and all reactions included an initial denaturation step at 94°C for 1 min followed by 40 cycles of denaturation (94°C for 10 s) and annealing/extension at 72°C for 1 min. PCR products were electrophoresed on 5% nondenaturing polyacrylamide gels in 1× Tris, acetate, EDTA buffer. The gel was stained with ethidium bromide (Molecular Probes, Eugene, OR), visualized using ultraviolet illumination, and analyzed using Quantity One software (Bio-Rad, Hercules, CA).
Table 1.
Primer sequences for dopamine D2-like receptor subtypes
| Target | Sequence of Primer | Amplicon Size, bp |
|---|---|---|
| Human dopamine D2 | FP: 5′-GGA CCT CCC TCA AGA CCA TGA GCC GTA-3′ | 287 |
| RP: 5′-GCA GCA GAG TCA GCA GTG GAG GAT CTT CAG-3′ | ||
| Human dopamine D3 | FP: 5′-GAG TCC CAC CAT AGC GCC CAA GCT CAG-3′ | 241 |
| RP: 5′-ACT GTA AAG CTC TGG GGA CAC GTG GCA-3′ | ||
| Human dopamine D4 | FP: 5′-GCT CTC CTG GTG CTG CCG CTC TTC GTC TAC T-3′ | 168 |
| RP :5′-CAC GGC CAC GGC CAC GAA CCT GT-3′ |
FR, forward primer; RP, reverse primer.
Western blot analysis.
Membrane lysates were electrophoresed (8% SDS-PAGE) and transferred to PVDF membranes. The PVDF membranes were blocked for 1 h at room temperature with 5% membrane blocking agent (RPN2125V; GE Healthcare, Arlington Heights, IL) in TBS with 0.1% Tween 20 and were then probed with antibodies directed against the dopamine D2 receptor protein (rabbit polyclonal 1:1,000; AB5084P; Millipore, Seattle, WA) overnight at 4°C. After being washed three times, membranes were incubated for 1 h at room temperature with horseradish peroxidase-conjugated secondary anti-rabbit antibodies (1:5,000; NA931V; GE Healthcare). The signals from the immunoreactive bands were detected by ECL Plus (GE Healthcare) according to the manufacturer's recommendations and the signal was captured by luminescent image analyzer (LAS-1,000; Fujifilm, Tokyo, Japan).
Immunohistochemistry.
Human tracheal rings were fixed with 4% paraformaldehyde/1% glutaraldehyde in 0.1 M phosphate buffer for 4 h at 4°C. Guinea pig tracheal rings were fixed with 4% paraformaldehyde in 0.1 M phosphate buffer for 24 h at 4°C. Tracheal rings were paraffin embedded, sectioned (10 μm), dewaxed in xylene, and rehydrated in a graded alcohol series to water. Heat-mediated antigen retrieval was performed with Tris-EDTA buffer (10 mM Tris base and 1 mM EDTA pH 9.0) for 2 min using a pressure cooker. Endogenous peroxidase was blocked using 3% hydrogen peroxide for 15 min at room temperature. Sections were blocked with 10% normal serum for 30 min. After being washed with PBS with 0.1% Triton X-100 (PBST), an avidin biotin blocking kit (Vector Laboratories, Peterborough, UK) was used (in 10% serum in PBS) to block endogenous biotin. Slides were rinsed with PBST and incubated overnight at 4°C in primary antibody against dopamine D2 receptor protein (for human tracheal ring sections: rabbit polyclonal 1:2,000; HPA015691; Sigma; for guinea pig tracheal ring sections: rabbit polyclonal 1:2,000; AB5084P; Millipore) in 2% normal goat serum in PBST or against α-smooth muscle actin (mouse monoclonal 1:10,000; MAB1522; Millipore) in 2% normal horse serum in PBST. A tracheal ring section was incubated with the appropriate isotype IgG (rabbit IgG or mouse IgG2a) antibody as a negative control. Following overnight incubation at 4°C, slides were washed three times with PBST and primary antibodies were detected using biotinylated anti-rabbit or anti-mouse antibodies (Vector Laboratories, Peterborough, UK) at a concentration of 1:100. After incubation with the ABC- horseradish peroxidase complex (Vector Laboratories, Peterborough, UK) for 30 min, the antigen antibody complex was then visualized with the peroxidase substrate kit (DAB; SK-4100; Vector Laboratories). Sections were counterstained with hematoxylin (Vector Laboratories), dried, dehydrated in a graded alcohol series and xylene, and coverslipped using Poly-mount (Polysciences, Warrington, PA).
Adenylyl cyclase assays.
Adenylyl cyclase activity was measured as previously described (19). Briefly, HASM cells in 24-well plates were washed once with warm PBS (37°C). In some experiments, the cells were pretreated for 4 h with PTX (100 ng/ml) or for 30 min with L-741626 (1 μM) in cell culture medium before being washed with PBS. In acute experiments, 100 μl of warm PBS were added to each well. Subsequently, 50 μl of 3× adenylyl cyclase buffer (19) were added directly to the wells [to achieve final concentrations of 50 mM HEPES pH 8.0, 50 mM NaCl, 0.4 mM EGTA, 1 mM cAMP, 7 mM MgCl2, 0.1 mM ATP (20 μCi/ml 32P-α-ATP), 0.1 mg/ml BSA, 50 U/ml creatine phosphokinase, and 7 mM phosphocreatine] in the absence (basal activity) or presence of 10 μM forskolin ± 1 μM quinpirole (dopamine D2-like receptor agonist) ± 1 μM L-741626 (selective dopamine D2 receptor antagonist) and the plates were incubated at 37°C for 15 min. In chronic experiments examining the sensitization of adenylyl cyclase activity, HASM cells were initially pretreated with either vehicle, 1 μM L-741626 (30 min), 5 μM U73122 [phospholipase C (PLC) inhibitor; 30 min], 100 nM GF109203X [protein kinase C (PKC) inhibitor; 1 h], or 100 ng/ml PTX (Gi protein blocker; 4 h) and then treated for 1 h with quinpirole (1 μM) in the M-199 culture medium at 37°C in a humidified incubator with 5% CO2. Cells were then washed twice with PBS before stimulation with 10 μM forskolin for 15 min at 37°C in the presence of L-741626 (1 μM) in those wells pretreated with L-741626 to prevent activation of dopamine D2 receptor by residual quinpirole (48).
For all experiments, the reactions were terminated by the addition of 100 μl stop buffer [50 mM HEPES pH 7.5, 2 mM ATP, 0.5 mM cAMP (0.5 μCi/ml 3H-cAMP), and 2% SDS] and synthesized 32P-cAMP was separated and quantitated by sequentially column chromatography over dowex and alumina as described previously (37).
Inositol phosphate assays.
Synthesis of total [3H]inositol phosphates was measured in confluent HASM cells in 24-well tissue culture plates as described previously (19, 23). Briefly, after overnight loading with 3H-myo-inositol (10 μCi/ml, 20 Ci/mmol) in inositol-free and serum-free DMEM (Chemicon, Temecula, CA), plates were washed three times (37°C, 500 μl HBSS with 10 mM LiCl). Incubation of cells with quinpirole (1 μM) in a final volume of 300 μl (HBSS with 10mM LiCl) at 37°C for 30 min was performed in the absence or presence of PTX (100 ng/ml; 4 h before the addition of quinpirole), U73122 (5 μM; 30 min before the addition of quinpirole), or L-741626 (1 μM; 30 min before the addition of quinpirole). Incubation of the cells with L-741626 (1 μM) alone in a final volume of 300 μl at 37°C for 30 min was also performed to determine whether L-741626 by itself exerts any effect on inositol phosphate synthesis. Reactions were terminated, and total [3H]inositol phosphates were recovered by column chromatography (19).
In vitro effects of chronic treatment with dopamine D2 receptor agonist on guinea pig airway smooth muscle relaxation.
All studies were approved by the Columbia University's Institutional Animal Care and Use Committee. Force measurements were performed on closed guinea pig tracheal rings suspended in organ baths as previously described (23). Briefly, Hartley male guinea pigs (∼400 g body wt) were anesthetized with 50 mg/kg ip pentobarbital, the tracheas were removed promptly and dissected into closed rings comprised of two cartilagenous rings from which mucosa, connective tissue, and epithelium were removed. Silk threads were tied to the rings such that the threads were at each end of the posterior aspect of the ring (lacking in cartilage), ∼180° from one another. One thread was attached to a fixed point at the bottom of 4-ml organ baths (Radnoti Glass Technology, Monrovia, CA), and the opposing thread was attached to a Grass FT03 force transducer (Grass-Telefactor, West Warwick, RI) coupled to a computer via Biopac hardware and Acknowledge 7.3.3 software (Biopac Systems, Goleta, CA) for continuous digital recording of muscle tension. The rings were suspended in 4 ml of KH buffer solution (composition in mM: 118 NaCl, 5.6 KCl, 0.5 CaCl2, 0.2 MgSO4, 25 NaHCO3, 1.3 NaH2PO4, and 5.6 d-glucose) with 10 μM indomethacin (DMSO vehicle final concentration of 0.01%), which was continuously bubbled with 95% O2-5% CO2 at pH 7.4, 37°C. The rings were equilibrated at 1 g of isotonic tension for 1 h with new KH buffer added every 15 min. All rings were precontracted with two cycles of cumulatively increasing concentration of acetylcholine (0.1 μM-1 mM) with extensive buffer washes between and after these two cycles with resetting of the resting tension to 1.0 g. Rings were chronically pretreated with or without the dopamine D2-like receptor agonist quinpirole (10 μM) for 1 h to chronically activate the dopamine D2-like receptor to test for adenylyl cyclase sensitization. Rings were then contracted with acetylcholine (an individual EC50 calculated for each ring). Following the achievement of a stable contraction (typically 15 min), forskolin (5 μM) was added to the buffer in the baths. In separate experiments, rings were pretreated with the dopamine D2 receptor selective antagonist L-741626 (10 μM) 30 min before the quinpirole (10 μM) treatment. Control-contracted rings received vehicle (KH buffer) to serve as time controls.
Statistical analysis.
Statistical analysis was performed using repeated-measures ANOVA, followed by Bonferroni posttest comparison using GraphPad Instat 3.0.6 software (GraphPad Software, San Diego, CA). Data are presented as means ± SE. P < 0.05 was considered significant.
RESULTS
RT-PCR analysis of dopamine D2-like receptors in airway smooth muscle.
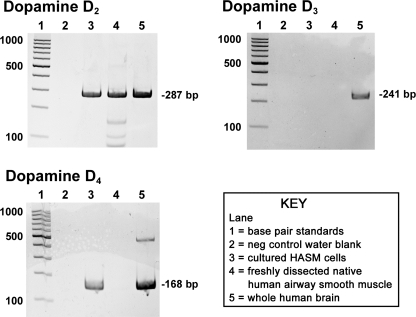
Initially, we assessed the expression of mRNA encoding dopamine D2-like receptors (D2, D3, and D4) in freshly isolated HASM and in primary cultures of HASM cells. Total RNA from whole brain was used as a positive control. Freshly dissected native HASM expressed mRNA encoding the dopamine D2 receptor (Fig. 1). Messenger RNA encoding the dopamine D3 and D4 receptors was not detected in freshly dissected airway smooth muscle from human upper airways despite their detection in control human brain RNA. In primary cultures of HASM cells, mRNA encoding the dopamine D2 and D4 receptors was detected whereas D3 was not detected (Fig. 1).
Fig. 1.
Representative gel images of RT-PCR analysis of total RNA using primers specific for each of the known human dopamine D2-like receptor subtypes (D2, D3, and D4). Total RNA extracted from primary cultures of human airway smooth muscle (HASM) cells or freshly dissected human tracheal airway smooth muscle was analyzed. Lane 1: base pair standards; lane 2: negative control water blanks; lane 3: total RNA from primary cultured HASM cells; lane 4: total RNA from freshly dissected native human airway smooth muscle; lane 5: total RNA from whole human brain.
Immunoblot analysis of dopamine D2-like receptors in airway smooth muscle.
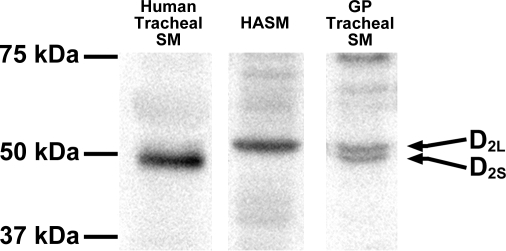
Lysates prepared from freshly dissected native HASM, freshly dissected native guinea pig airway smooth muscle and cultured HASM cells were subjected to immunoblot analysis using a specific antidopamine D2 receptor antibody (Fig. 2). Immunoreactive bands of appropriate molecular mass of ∼50 kDa for dopamine D2 receptor were identified airway smooth muscle from all three sources. The antibody used to detect the dopamine D2 receptor protein is capable of identifying two isoforms of the dopamine D2 receptor (D2L and D2S). The higher band of the appropriate molecular mass for dopamine D2L (50.6 kDa) was identified in primary cultures of HASM cells and in freshly isolated airway smooth muscle from guinea pig, while the D2S (47.4 kDa) was identified in freshly isolated airway smooth muscle from human and guinea pig (Fig. 2).
Fig. 2.
Representative immunoblot analyses using antibodies against the dopamine D2 receptor using protein prepared from cultured HASM cells (100 μg), freshly dissected native human tracheal airway smooth muscle (SM; 100 μg), and freshly dissected native guinea pig (GP) tracheal SM (100 μg) identifying two known isoforms of the dopamine D2 receptor (D2L and D2S). White spaces between the 3 lanes indicate that these lanes were located on the same immunoblot but were not located in neighbouring lanes on the original gel and immunoblot image.
Immunohistochemical detection of dopamine D2 receptor expression in guinea pig airway smooth muscle.
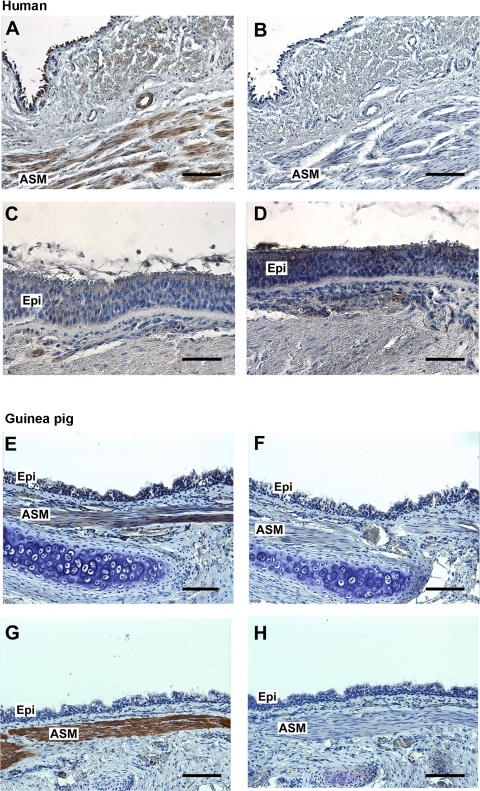
To confirm the localization of dopamine D2 receptor protein to airway smooth muscle, immunohistochemistry was performed using a rabbit polyclonal antibody that recognizes the dopamine D2 receptor proteins in paraffin sections of both human and guinea pig tracheal rings. Specific immunoreactivity was detected in the airway smooth muscle layer of both human and guinea pig trachea (indicated by brown color) with no staining in the negative control sections using an isotype-specific rabbit IgG or mouse IgG2a control primary antibodies. In addition, no specific immunoreactivity was detected in the airway epithelial layer of both human and guinea pig trachea (Fig. 3).
Fig. 3.
A and C: representative immunohistochemical staining of dopamine D2 receptor in paraformaldehyde/glutaraldehyde-fixed human trachea (A) and airway epithelium (C). E and G: representative immunohistochemical staining of dopamine D2 receptor (E) and α-smooth muscle actin (G) in paraformaldehyde-fixed guinea pig trachea. B and D: anti-rabbit IgG isotype negative control in serial sections of human trachea (B) and airway epithelium (D). F and H: anti-rabbit IgG (F) or anti-mouse IgG2a (H) isotype negative control in sections of guinea pig trachea. All sections were counterstained with hematoxylin. Calibration bar: A, B, E–H = 100 μm; C and D = 50 μm. Epi, airway epithelium. Images are representative of ≥3 independent immunohistochemical analyses from both human and guinea pig trachea.
Agonist-induced adenylyl cyclase activity in HASM cells.
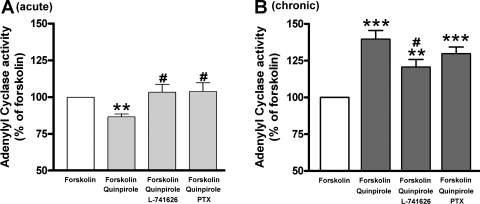
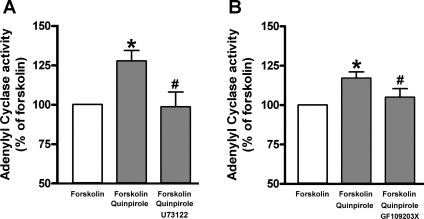
Dopamine D2 receptor-mediated acute inhibition and chronic activation of adenylyl cyclase activity via the Gi protein are well known in neurons (48). To examine whether a dopamine D2 receptor agonist has similar effects on adenylyl cyclase activities in cultured HASM cells, we measured adenylyl cyclase activity in the presence or absence of the dopamine D2 receptor agonist quinpirole. Acute activation of the dopamine D2 receptor with quinpirole (1 μM) significantly inhibited the forskolin-stimulated adenylyl cyclase activity (P < 0.01; n = 13), which was significantly blocked by pretreatment with the Gi inhibitor PTX (100 ng/ml; 4 h; P < 0.05, n = 13) or the dopamine D2 receptor antagonist L-741626 (1 μM; P < 0.05; n = 13; Fig. 4A). In contrast, chronic pretreatment with the same drugs resulted in a heterologous sensitization of adenylyl cyclase activity. The chronic (1 h) pretreatment with quinpirole (1 μM) significantly enhanced the forskolin-stimulated adenylyl cyclase activity (P < 0.001; n = 14), which was significantly blocked by the selective dopamine D2 receptor antagonist L-741626 (1 μM; P < 0.05; n = 14) in a PTX (100 ng/ml; 4 h)-insensitive manner (NS; n = 14; Fig. 4B). In addition, as shown in Fig. 5, this sensitization of adenylyl cyclase activity by chronic activation of the dopamine D2 receptor with quinpirole was significantly blocked by either the PLC inhibitor U73122 (5 μM; P < 0.05; n = 8; Fig. 5A) or the PKC inhibitor GF109203X (100 nM; P < 0.05; n = 9; Fig. 5B).
Fig. 4.
A: forskolin (10 μM)-stimulated adenylyl cyclase activity in cultured HASM cells induced by acute (15-min pretreatment) activation of dopamine D2 receptor by quinpirole (1 μM) (n = 13). Cells were pretreated with L-741626 (1 μM for 30-min pretreatment before quinpirole treatment) or pertussis toxin (PTX; 100 ng/ml for 4-h pretreatment before quinpirole treatment). Data represent means ± SE. **P < 0.01, compared with forskolin alone. #P < 0.05, compared with forskolin + quinpirole. B: forskolin (10 μM)-stimulated adenylyl cyclase activity in cultured HASM cells induced by chronic (1-h pretreatment) activation of dopamine D2 receptor by quinpirole (1 μM; n = 14). Cells were pretreated with L-741626 (1 μM for 30-min pretreatment before quinpirole treatment) or PTX (100 ng/ml for 4-h pretreatment before quinpirole treatment). Data represent means ± SE. **P < 0.01 and ***P < 0.001, compared with forskolin alone. #P < 0.05, compared with forskolin + quinpirole. In each experiment, values were determined in triplicate.
Fig. 5.
A: effect of phospholipase C (PLC) inhibitor (U73122; 5 μM) on forskolin (10 μM)-stimulated adenylyl cyclase activity in cultured HASM cells induced by chronic activation of dopamine D2 receptor by quinpirole (1 μM; n = 8). *P < 0.05, compared with forskolin alone. #P < 0.05, compared with forskolin + quinpirole. B: effect of protein kinase C (PKC) inhibitor (GF109203X; 100 nM) on forskolin (10 μM)-stimulated adenylyl cyclase activity in cultured HASM cells induced by chronic activation of dopamine D2 receptor by quinpirole (1 μM; n = 9). Data represent means ± SE. *P < 0.05, compared with forskolin alone. #P < 0.05, compared with forskolin + quinpirole. In each experiment, values were determined in triplicate.
Agonist-induced stimulation of inositol phosphate synthesis in HASM cells.
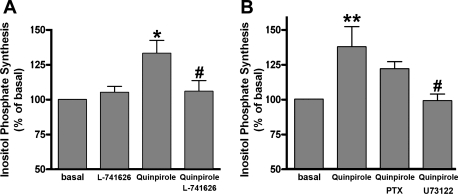
Classically receptors that couple to the Gq protein activate PLC increasing inositol phosphates and intracellular calcium. Similarly, in Gi-coupled receptors, cross-talk to PLCβ1 from liberated Giα or Giβγ subunits has been described in other cell types (1, 14, 16, 42). To examine whether the dopamine D2 receptor agonist stimulate inositol phosphate synthesis in cultured HASM cells, we measured the quinpirole-induced inositol phosphate synthesis in the presence or absence of L-741626, U73122, or PTX. Quinpirole (1 μM) significantly increased inositol phosphate synthesis (P < 0.05; n = 8), which was significantly blocked by L-741626 (P < 0.05; n = 8; 1 μM; Fig. 6A). Pretreatment the cells with U73122 (5 μM; 30 min) also significantly blocked the quinpirole-induced inositol phosphate synthesis, whereas the pretreatment with PTX (100 ng/ml; 4 h) did not significantly block quinpirole-induced synthesis of inositol phosphates (NS; n = 5; Fig. 6B).
Fig. 6.
A: effect of the selective dopamine D2 receptor antagonist L-741626 (1 μM) on quinpirole (1 μM)-stimulated inositol phosphate synthesis in HASM cells (n = 8). In separate experiments, the effect of L-741626 by itself on inositol phosphate synthesis was measured (n = 6). Data represent means ± SE, presented as percentages of basal values. *P < 0.05, compared with basal. #P < 0.05, compared with quinpirole. In each experiment, values were determined in triplicate. B: effects of PTX (100 ng/ml) or U73122 (5 μM) on quinpirole (1 μM)-stimulated inositol phosphate synthesis in HASM cells (n = 5). Data represent means ± SE, presented as percentages of basal values. **P < 0.01, compared with basal. #P < 0.05, compared with quinpirole. In each experiment, values were determined in triplicate.
Functional studies of dopamine D2 receptor function in intact guinea pig airway rings.
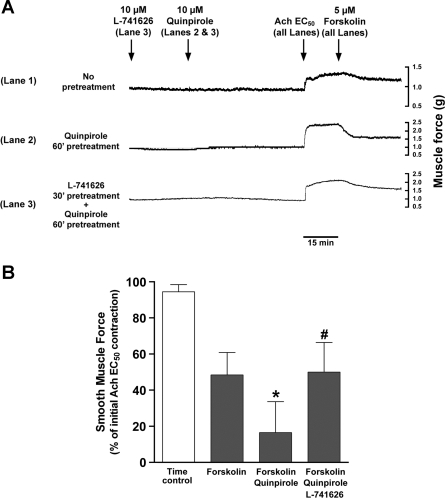
Molecular identification of multiple subunits of dopamine D2 receptors led us to question whether chronic activation of the functional dopamine D2 receptor could modulate airway smooth muscle tone. Guinea pig tracheal rings suspended in organ baths subjected to chronic activation of the dopamine D2 receptor with quinpirole (10 μM) demonstrated significantly increased forskolin (5 μM)-induced relaxation of an acetylcholine (EC50)-induced contraction. Under identical conditions the pretreatment of tracheal rings with the selective dopamine D2 receptor antagonist L-741626 (10 μM) significantly attenuated the potentiation induced by quinpirole (10 μM; Fig. 7).
Fig. 7.
A: representative tension tracings in guinea pig tracheal ring illustrating the potentiation of forskolin (5 μM)-induced relaxation of acetylcholine (EC50) contraction by the chronic activation (1 h) of dopamine D2 receptor with quinpirole (10 μM) and attenuation of relaxation by pretreatment (30 min) with the dopamine D2 receptor antagonist L-741626 (10 μM). Lane 1: no pretreatment control; lane 2: quinpirole (10 μM) 1-h pretreatment; lane 3: L-741626 (10 μM) 30-min pretreatment followed by quinpirole 1-h pretreatment. B: chronic activation (1 h) of dopamine D2 receptor with quinpirole (10 μM) significantly potentiates the relaxation induced by forskolin (5 μM), which is inhibited by L-741626 (10 μM) pretreatment (30 min). Data represent means ± SE, presented as percentages of initial force after an acetylcholine (EC50) contraction. *P < 0.05, compared with forskolin alone group. #P < 0.05, compared with forskolin + quinpirole group; n = 6.
DISCUSSION
The primary findings of the present study include the first demonstration of dopamine D2 receptor mRNA and protein expression in human and guinea pig airway smooth muscle cells and the ability of a dopamine D2 receptor-selective agonist to facilitate the relaxation against acetylcholine-induced contraction in airway smooth muscle through adenylyl cyclase sensitization.
The dopamine D2-like receptor is ubiquitously expressed in the central nervous system, and the modulation of neuronal activity by dopamine has been extensively studied. Numerous studies (17, 18, 20, 25, 28, 40) have also indicated the expression of functional dopamine D2 receptors in peripheral and nonneuronal cells such as pulmonary artery (36), renal tubules, glomeruli (postganglionic sympathetic nerve terminals), and zona glomerulosa cells of the adrenal cortex.
Identification of mRNA in freshly isolated HASM ensures that native expression of the dopamine D2-like receptor (D2, D3, and D4) is being identified. However, even with careful dissection freshly isolated tissue is contaminated by other cells types that could give rise to cDNA products during sensitive RT-PCR amplification. Therefore, we also evaluated the expression of the dopamine D2-like receptor in established primary cultures of HASM cells, which are a homogenous population of airway smooth muscle cells. Despite their cellular purity, cultured cell systems can result in an altered phenotype of protein expression during culture. Despite these limitations, there was remarkable agreement but some differences between the expression of dopamine D2-like receptor in freshly isolated vs. cultured HASM. We detected mRNA encoding the dopamine D2 receptor in dual sources of HASM studied including human freshly isolated airway smooth muscle and cultured HASM cells. In contrast, the dopamine D4 receptor was not identified in freshly isolated HASM but was readily detected in cultured HASM cells. This raises the possibility that there is a phenotypic switch from the dopamine D2 receptor to the D4 receptor in cultured HASM cells, the functional consequence of which is unknown.
Identification of mRNA encoding dopamine D2 receptor in airway smooth muscle led us to investigate its protein expression by immunoblotting and immunohistochemistry. In agreement with our mRNA analysis, the dopamine D2 receptor protein was identified in airway smooth muscle from human and guinea pig airway smooth muscle and cultured HASM cells. The antibody used to detect dopamine D2 receptor identified the two isoforms of the dopamine D2 receptor, named long (D2L) and short (D2S) isoforms (13), which differ by an insertion of 29 amino acids in the third intracellular loop of the D2L receptor. We resolved two immunoreactive bands of 47.4 kDa (D2S) and 50.6 kDa (D2L) in freshly dissected guinea pig airway smooth muscle. In contrast, only a single band corresponding to 47.4 kDa was identified in freshly dissected HASM, whereas only the larger 50.6-kDa band was identified in cultured HASM cells. The smooth muscle-specific expression of dopamine D2 receptor was further confirmed immunohistochemically in both human and guinea pig airway smooth muscle. Although RNA of all dopamine D2-like receptors have been reported to be expressed in HASM cells by using expression microarrays (15), the present study is the first to demonstrate the protein expression of dopamine D2 receptor localized to airway smooth muscle.
After demonstrating the mRNA and protein expression of dopamine D2 receptor in airway smooth muscle, we sought to assess its bronchodilatatory effect. Numerous studies have demonstrated that the acute activation of Gi-coupled receptors inhibits adenylyl cyclase activity, whereas chronic activation of these inhibitory receptors results in a compensatory increase in cAMP accumulation (47). Consistent with these previous findings (48), our findings also suggest that acute activation of dopamine D2 receptor on HASM cells inhibited the adenylyl cyclase activity by a PTX-sensitive pathway, while chronic activation of dopamine D2 receptor stimulated adenylyl cyclase activity but not via a Gi pathway.
This heterologous sensitization of adenylyl cyclase represents an adaptive response to persistent activation of several Gi-coupled receptors including dopamine D2 and D4, μ-opioid, δ-opioid, α2-adrenergic, M2 and M4 muscarinic, CB1 cannabinoid, and 5HT1A serotonin receptors (2–5, 7, 22, 30, 31, 39, 41, 43, 44, 48). Although long-term (18 h) agonist pretreatment has been reported to sensitize the adenylyl cyclase activity, short-term agonist pretreatment (2 h) still caused sensitization (48) and was apparent after 20–30 min of agonist treatments (24). The sensitization of adenylyl cyclase has also been described in HASM cells (7, 8). Previous studies (7) have showed that the chronic activation of the Gi-coupled receptors with their agonist induced adenylyl cyclase sensitization in HASM cells in PKC-insensitive, PTX-sensitive manner, suggesting that this sensitization is Gi-protein mediated. However, in the present study, adenylyl cyclase sensitization induced by chronic activation of the dopamine D2 receptor on HASM cells was not attenuated by PTX, while both a PLC inhibitor and a PKC inhibitor significantly blocked this sensitization. In addition, activation of the dopamine D2 receptor stimulated inositol phosphate synthesis in a PLC-sensitive manner. Classically Gq-coupled receptors are known to stimulate inositol phosphate synthesis through PLC. However, evidence exists in multiple cell types that activated Giα or Giβγ subunits released from Gi-coupled receptor can also cross-activate PLC, which is located downstream of the Gq-coupled receptor pathway (1, 14, 16, 42). PLC promotes the hydrolysis of phosphoinositol 4,5-bisphosphate into inositol phosphates and 1,2-diacylglycerol, which promotes the translocation of PKC from cytoplasm to the membrane and its subsequent activation. Rashid et al. (35) stated that dopamine D2 receptor stimulates PLC via a cross-talk mechanism through Giα or Giβγ subunits released from the dopamine D2 receptor. However, this cross-talk mechanism is inconsistent with our findings in that both the adenylyl cyclase sensitization induced by chronic activation of the dopamine D2 receptor and inositol phosphate synthesis induced by activation of this receptor were not attenuated by PTX, indicating that dopamine D2 receptor agonist has promiscuous effects on G protein coupling. There is increasing evidence that the dopamine D2 receptor is capable of forming a heterodimer with the dopamine D1 receptor and acts as a Gq-coupled receptor (35). Yan et al. (49) also suggested that the dopamine D2 receptor that classically couples to Gi can couple to the Gq/PLCβ pathway, causing an elevation of intracellular Ca2+ and activation of PKC. In our studies, the dopamine D1 receptor as well as the D2 receptor protein is expressed in airway smooth muscle (data not shown), suggesting that sensitization of the adenylyl cyclase through Gq pathway may be triggered through dopamine D1-D2 receptor heterodimerization. However, detailed mechanisms of this in airway smooth muscle cells have not been investigated.
PKC is involved in the mechanism of heterologous sensitization of adenylyl cyclase isoform VI after activation of Gi-coupled receptors (21, 45, 47). For example, in Chinese hamster ovary cells, prolonged δ-opioid receptor activation sensitizes subsequent stimulation of adenylyl cyclase isoform VI phosphorylation, and PKC attenuated the sensitization of cAMP response (43, 44). In contrast, in HEK293 cells expressing both recombinant adenylyl cyclase VI and the dopamine D2L receptor, a PKC inhibitor did not attenuate adenylyl cyclase VI sensitization after persistent D2L receptor activation (6). These findings indicate that the mechanism of adenylyl cyclase sensitization differ between cell types. The present study indicated that, in HASM cells, adenylyl cyclase sensitization are mediated via PKC. The purpose of such adenylyl cyclase sensitization in HASM cells is unclear but may involve the need to maintain cAMP synthesis in the face of persistent Gi-coupled receptor activation.
In cases of bronchoconstriction, the administration of dopamine, systemically or topically, has a bronchodilatory effect (11, 12, 26). In the present study, chronic pretreatment of the guinea pig tracheal rings with a dopamine D2 receptor agonist potentiated forskolin-induced airway relaxation. These findings suggest that the continuous use of a dopamine D2 receptor agonist with a stimulator of adenylyl cyclase such as a Gs-coupled receptor agonist (i.e., β2 adrenergic receptor agonist) would cooperatively relax the airway smooth muscle through sensitization of adenylyl cyclase activity.
In summary, we demonstrate for the first time the molecular expression of the dopamine D2 receptor in human and guinea pig airway smooth muscle. We demonstrate that a selective dopamine D2 receptor agonist can potentiate the relaxation of intact airway smooth muscle contracted with acetylcholine. These studies suggest that dopamine D2 receptor on airway smooth muscle would be a novel pharmacologic target for the relaxation of airway smooth muscle.
GRANTS
This work was supported by National Institutes of Health Grant (to C. W. Emala) (GM065281), Grant-in-Aid for Young Scientists (A) (20689036) from Japan Society for the Promotion of Science (to K. Mizuta), a research grant from the Uehara Memorial Foundation (to K. Mizuta), and a research grant from Takeda Science foundation (to K. Mizuta).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
K.M. and C.W.E. conceived and designed these studies; K.M., Y.Z., D.X., and C.W.E. performed experiments; K.M., Y.Z., D.X., and C.W.E. analyzed data; K.M., Y.Z., D.X., and C.W.E. interpreted results of experiments; K.M. and C.W.E. prepared figures; K.M., E.M., R.A.P., and C.W.E. drafted manuscript; K.M., E.M., R.A.P., and C.W.E. edited and revised manuscript; K.M., E.M., R.A.P., and C.W.E. approved final version of manuscript.
Supplementary Material
REFERENCES
- 1. Abebe W, Mustafa SJ. A1 adenosine receptor-mediated Ins(1,4,5)P3 generation in allergic rabbit airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 275: L990–L997, 1998 [DOI] [PubMed] [Google Scholar]
- 2. Avidor-Reiss T, Bayewitch M, Levy R, Matus-Leibovitch N, Nevo I, Vogel Z. Adenylylcyclase supersensitization in μ-opioid receptor-transfected Chinese hamster ovary cells following chronic opioid treatment. J Biol Chem 270: 29732–29738, 1995 [DOI] [PubMed] [Google Scholar]
- 3. Avidor-Reiss T, Nevo I, Levy R, Pfeuffer T, Vogel Z. Chronic opioid treatment induces adenylyl cyclase V superactivation. Involvement of GβY. J Biol Chem 271: 21309–21315, 1996 [DOI] [PubMed] [Google Scholar]
- 4. Avidor-Reiss T, Nevo I, Saya D, Bayewitch M, Vogel Z. Opiate-induced adenylyl cyclase superactivation is isozyme-specific. J Biol Chem 272: 5040–5047, 1997 [DOI] [PubMed] [Google Scholar]
- 5. Avidor-Reiss T, Zippel R, Levy R, Saya D, Ezra V, Barg J, Matus-Leibovitch N, Vogel Z. κ-Opioid receptor-transfected cell lines: modulation of adenylyl cyclase activity following acute and chronic opioid treatments. FEBS Lett 361: 70–74, 1995 [DOI] [PubMed] [Google Scholar]
- 6. Beazely MA, Watts VJ. Activation of a novel PKC isoform synergistically enhances D2L dopamine receptor-mediated sensitization of adenylate cyclase type 6. Cell Signal 17: 647–653, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Billington CK, Hall IP, Mundell SJ, Parent JL, Panettieri RA, Jr, Benovic JL, Penn RB. Inflammatory and contractile agents sensitize specific adenylyl cyclase isoforms in human airway smooth muscle. Am J Respir Cell Mol Biol 21: 597–606, 1999 [DOI] [PubMed] [Google Scholar]
- 8. Billington CK, Pascual RM, Hawkins ML, Penn RB, Hall IP. Interleukin-1β and rhinovirus sensitize adenylyl cyclase in human airway smooth-muscle cells. Am J Respir Cell Mol Biol 24: 633–639, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Birnbaumer L, Abramowitz J, Brown AM. Receptor-effector coupling by G proteins. Biochim Biophys Acta 1031: 163–224, 1990 [DOI] [PubMed] [Google Scholar]
- 10. Cabezas GA, Israili ZH, Velasco M. The actions of dopamine on the airways. Am J Ther 10: 477–486, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Cabezas GA, Lezama Y, Velasco M. Dopaminergic modulation of human bronchial tone. Arch Med Res 32: 143–147, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Cabezas GA, Lezama Y, Vidal A, Velasco M. Inhaled dopamine induces bronchodilatation in patients with bronchial asthma. Int J Clin Pharmacol Ther 37: 510–513, 1999 [PubMed] [Google Scholar]
- 13. Dal Toso R, Sommer B, Ewert M, Herb A, Pritchett DB, Bach A, Shivers BD, Seeburg PH. The dopamine D2 receptor: two molecular forms generated by alternative splicing. EMBO J 8: 4025–4034, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dickenson JM, Hill SJ. Involvement of G-protein βγ subunits in coupling the adenosine A1 receptor to phospholipase C in transfected CHO cells. Eur J Pharmacol 355: 85–93, 1998 [DOI] [PubMed] [Google Scholar]
- 15. Einstein R, Jordan H, Zhou W, Brenner M, Moses EG, Liggett SB. Alternative splicing of the G protein-coupled receptor superfamily in human airway smooth muscle diversifies the complement of receptors. Proc Natl Acad Sci USA 105: 5230–5235, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ethier MF, Madison JM. Adenosine A1 receptors mediate mobilization of calcium in human bronchial smooth muscle cells. Am J Respir Cell Mol Biol 35: 496–502, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Felder CC, McKelvey AM, Gitler MS, Eisner GM, Jose PA. Dopamine receptor subtypes in renal brush border and basolateral membranes. Kidney Int 36: 183–193, 1989 [DOI] [PubMed] [Google Scholar]
- 18. Felder RA, Blecher M, Eisner GM, Jose PA. Cortical tubular and glomerular dopamine receptors in the rat kidney. Am J Physiol Renal Fluid Electrolyte Physiol 246: F557–F568, 1984 [DOI] [PubMed] [Google Scholar]
- 19. Hotta K, Emala CW, Hirshman CA. TNF-α upregulates Giα and Gqα protein expression and function in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 276: L405–L411, 1999 [DOI] [PubMed] [Google Scholar]
- 20. Hussain T, Lokhandwala MF. Renal dopamine receptors and hypertension. Exp Biol Med (Maywood) 228: 134–142, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Johnston CA, Watts VJ. Sensitization of adenylate cyclase: a general mechanism of neuroadaptation to persistent activation of Gαi/o-coupled receptors? Life Sci 73: 2913–2925, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Jones SB, Bylund DB. Characterization and possible mechanisms of α2-adrenergic receptor-mediated sensitization of forskolin-stimulated cyclic AMP production in HT29 cells. J Biol Chem 263: 14236–14244, 1988 [PubMed] [Google Scholar]
- 23. Jooste E, Zhang Y, Emala CW. Rapacuronium preferentially antagonizes the function of M2 versus M3 muscarinic receptors in guinea pig airway smooth muscle. Anesthesiology 102: 117–124, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Lisinicchia JG, Watts VJ. Sensitization of adenylate cyclase by short-term activation of 5-HT1A receptors. Cell Signal 15: 1111–1117, 2003 [DOI] [PubMed] [Google Scholar]
- 25. Lokhandwala MF, Amenta F. Anatomical distribution and function of dopamine receptors in the kidney. FASEB J 5: 3023–3030, 1991 [DOI] [PubMed] [Google Scholar]
- 26. Michoud MC, Amyot R, Jeanneret-Grosjean A. Dopamine effect on bronchomotor tone in vivo. Am Rev Respir Dis 130: 755–758, 1984 [DOI] [PubMed] [Google Scholar]
- 27. Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev 78: 189–225, 1998 [DOI] [PubMed] [Google Scholar]
- 28. Missale C, Pizzi M, Memo M, Picotti GB, Carruba MO, Spano PF. Postsynaptic D1 and D2 dopamine receptors are present in rabbit renal and mesenteric arteries. Neurosci Lett 61: 207–211, 1985 [DOI] [PubMed] [Google Scholar]
- 29. Neve KA, Seamans JK, Trantham-Davidson H. Dopamine receptor signaling. J Recept Signal Transduct Res 24: 165–205, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Nevo I, Avidor-Reiss T, Levy R, Bayewitch M, Heldman E, Vogel Z. Regulation of adenylyl cyclase isozymes on acute and chronic activation of inhibitory receptors. Mol Pharmacol 54: 419–426, 1998 [DOI] [PubMed] [Google Scholar]
- 31. Palmer TM, Harris CA, Coote J, Stiles GL. Induction of multiple effects on adenylyl cyclase regulation by chronic activation of the human A3 adenosine receptor. Mol Pharmacol 52: 632–640, 1997 [DOI] [PubMed] [Google Scholar]
- 32. Panettieri RA, Murray RK, DePalo LR, Yadvish PA, Kotlikoff MI. A human airway smooth muscle cell line that retains physiological responsiveness. Am J Physiol Cell Physiol 256: C329–C335, 1989 [DOI] [PubMed] [Google Scholar]
- 33. Peiser C, Andreae DA, Groneberg DA, Dinh QT, Muller B, Wahn U, Fischer A. Dopamine D2 receptor mRNA expression is increased in the jugular-nodose ganglia of rats with nitrogen dioxide-induced chronic bronchitis. Neurosci Lett 465: 143–146, 2009 [DOI] [PubMed] [Google Scholar]
- 34. Peiser C, Trevisani M, Groneberg DA, Dinh QT, Lencer D, Amadesi S, Maggiore B, Harrison S, Geppetti P, Fischer A. Dopamine type 2 receptor expression and function in rodent sensory neurons projecting to the airways. Am J Physiol Lung Cell Mol Physiol 289: L153–L158, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Rashid AJ, O'Dowd BF, Verma V, George SR. Neuronal Gq/11-coupled dopamine receptors: an uncharted role for dopamine. Trends Pharmacol Sci 28: 551–555, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Ricci A, Mignini F, Tomassoni D, Amenta F. Dopamine receptor subtypes in the human pulmonary arterial tree. Auton Autacoid Pharmacol 26: 361–369, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Salomon Y, Londos C, Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem 58: 541–548, 1974 [DOI] [PubMed] [Google Scholar]
- 38. Sankary RM, Jones CA, Madison JM, Brown JK. Muscarinic cholinergic inhibition of cyclic AMP accumulation in airway smooth muscle. Role of a pertussis toxin-sensitive protein. Am Rev Respir Dis 138: 145–150, 1988 [DOI] [PubMed] [Google Scholar]
- 39. Sharma SK, Klee WA, Nirenberg M. Dual regulation of adenylate cyclase accounts for narcotic dependence and tolerance. Proc Natl Acad Sci USA 72: 3092–3096, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sokoloff P, Giros B, Martres MP, Bouthenet ML, Schwartz JC. Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature 347: 146–151, 1990 [DOI] [PubMed] [Google Scholar]
- 41. Thomas JM, Hoffman BB. Isoform-specific sensitization of adenylyl cyclase activity by prior activation of inhibitory receptors: role of βγ subunits in transducing enhanced activity of the type VI isoform. Mol Pharmacol 49: 907–914, 1996 [PubMed] [Google Scholar]
- 42. Tomura H, Itoh H, Sho K, Sato K, Nagao M, Ui M, Kondo Y, Okajima F. βγ subunits of pertussis toxin-sensitive G proteins mediate A1 adenosine receptor agonist-induced activation of phospholipase C in collaboration with thyrotropin. A novel stimulatory mechanism through the cross-talk of two types of receptors. J Biol Chem 272: 23130–23137, 1997 [DOI] [PubMed] [Google Scholar]
- 43. Varga EV, Rubenzik MK, Stropova D, Sugiyama M, Grife V, Hruby VJ, Rice KC, Roeske WR, Yamamura HI. Converging protein kinase pathways mediate adenylyl cyclase superactivation upon chronic δ-opioid agonist treatment. J Pharmacol Exp Ther 306: 109–115, 2003 [DOI] [PubMed] [Google Scholar]
- 44. Varga EV, Stropova D, Rubenzik M, Waite S, Roeske WR, Yamamura HI. Phosphorylation of adenylyl cyclase VI upon chronic δ-opioid receptor stimulation. Eur J Pharmacol 364: R1–R3, 1999 [DOI] [PubMed] [Google Scholar]
- 45. Watts VJ. Molecular mechanisms for heterologous sensitization of adenylate cyclase. J Pharmacol Exp Ther 302: 1–7, 2002 [DOI] [PubMed] [Google Scholar]
- 46. Watts VJ, Neve KA. Activation of type II adenylate cyclase by D2 and D4 but not D3 dopamine receptors. Mol Pharmacol 52: 181–186, 1997 [DOI] [PubMed] [Google Scholar]
- 47. Watts VJ, Neve KA. Sensitization of adenylate cyclase by Gαi/o-coupled receptors. Pharmacol Ther 106: 405–421, 2005 [DOI] [PubMed] [Google Scholar]
- 48. Watts VJ, Neve KA. Sensitization of endogenous and recombinant adenylate cyclase by activation of D2 dopamine receptors. Mol Pharmacol 50: 966–976, 1996 [PubMed] [Google Scholar]
- 49. Yan Z, Feng J, Fienberg AA, Greengard P. D2 dopamine receptors induce mitogen-activated protein kinase and cAMP response element-binding protein phosphorylation in neurons. Proc Natl Acad Sci USA 96: 11607–11612, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.