Abstract
The boronic acid dipeptide bortezomib inhibits the chymotrypsin-like activity of the 26S proteasome and shows significant therapeutic efficacy in multiple myeloma. However, recent studies suggest that bortezomib may have more complex mechanisms of action in treating cancer. We report here that the endocytosis and lysosomal degradation of the receptor tyrosine kinase C-KIT are required for bortezomib- but not tyrosine kinase inhibitor imatinib-caused apoptosis of t(8;21) leukemia and gastrointestinal stromal tumor cells, suggesting that C-KIT may recruit an apoptosis initiator. We show that C-KIT binds and phosphorylates heat shock protein 90β (Hsp90β), which sequestrates apoptotic protease activating factor 1 (Apaf-1). Bortezomib dephosphorylates pHsp90β and releases Apaf-1. Although the activated caspase-3 is not sufficient to cause marked apoptosis, it cleaves the t(8;21) generated acute myeloid leukemia 1-eight twenty one (AML1-ETO) and AML1-ETO9a fusion proteins, with production of cleavage fragments that perturb the functions of the parental oncoproteins and further contribute to apoptosis. Notably, bortezomib exerts potent therapeutic efficacy in mice bearing AML1-ETO9a–driven leukemia. These data show that C-KIT-pHsp90β-Apaf-1 cascade is critical for some malignant cells to evade apoptosis, and the clinical therapeutic potentials of bortezomib in C-KIT–driven neoplasms should be further explored.
Keywords: stem cell factor receptor, internalization, proteolysis, apoptosome
The protooncogene C-KIT (or v-kit Hardy-Zuckerman 4 feline sarcoma viral oncogene homolog) (1) encodes a class III receptor tyrosine kinase composed of five extracellular Ig-like domains, a transmembrane segment, a juxtamembrane domain, and a split cytoplasmic kinase domain. On binding to its ligand, the stem cell factor (SCF), C-KIT rapidly undergoes dimerization, autophosphorylation (2), and clathrin-mediated internalization (3, 4). Through its downstream signal molecules, including PI3K, Rac-serine/threonine-protein kinase (AKT), ERK, v-src sarcoma (Schmidt-Ruppin A-2) viral oncogene homolog (SRC), JAK/STAT, and Rat sarcoma (Ras)/Rapidly Accelerated Fibrosarcoma (Raf)/MAPK cascade (5), C-KIT confers survival/proliferative signals to hematopoietic stem cells, mast cells, germ cells, melanocytes, and interstitial cells of Cajal (6). However, how C-KIT is involved in apoptosis remains obscure.
Aberrant expression and gain of function mutations of C-KIT have been reported in human gastrointestinal stromal tumor (GIST) (7) and hematologic malignancies including acute myeloid leukemia (AML) with inversion 16 [inv(16)] or t(8;21)(q22;q22) (8). The t(8;21), which represents the most common chromosomal anomaly in AML, targets eight twenty one [ETO, also known as myeloid transforming gene on chromosome 8 (MTG8) or RUNX1T1] on chromosome 8 and acute myeloid leukemia 1 [AML1, also known as Runt-related transcription factor 1 (RUNX1), core binding factor (CBF) α2, and Phosphatidylethanolamine-binding protein (PEBP) 2αB] on chromosome 21, yielding two fusion transcripts, the AML1-ETO (AE) (9) and AML1-ETO9a (AE9a), lacking the neuralized homology repeat (NHR)3–4 domains at the C terminus of ETO moiety (10). It has been established that AE9a bears a much stronger leukemogenic activity than AE in murine system (10), and a similar situation might exist in human setting (11). Studies showed that t(8;21) AML follows a stepwise leukemogenesis (i.e., AE/AE9a represent the first, fundamental genetic hit to initiate the disease), whereas activation of the C-KIT pathway may be a second but also crucial hit for the development of a full-blown leukemia (8, 12). Although AE impairs hematopoietic differentiation, aberrant C-KIT increases the stem cell capacity of normal hematopoietic stem cells and enhances the leukemogenic potential of and confers proliferative/survival advantages to AE-positive stem cells (13, 14). However, the mechanism of C-KIT in rendering apoptosis-evading potential to leukemic cells remains elusive. Moreover, novel therapeutic strategy remains a practical need for t(8;21) AML, because the clinical outcome of this subtype of AML remains unsatisfied (in particular, the non-Caucasian patients) (8, 15); additionally, patients receiving chemotherapy (16) or hematopoietic stem cell transplantation (17) had shorter overall survival than those patients with inv(16).
The ubiquitin-proteasome pathway plays a central role in the targeted destruction of endogenous proteins in eukaryotic cells, and its inhibition may result in apoptosis through the accumulation of proapoptotic molecules (18). However, recent studies showed that some proteasome inhibitors may exert effects on cancer through much more complex mechanisms than initially expected. For example, it was reported that (19) bortezomib (BOR), a clinically proven proteasome inhibitor, induces canonical NF-κB activation in multiple myeloma cells; others showed that apoptosis induced by proteasome inhibitor carbobenzoxy-L-leucyl-L-leucyl-L-leucinal (MG-132) can be blocked by caspase inhibitors, whereas caspase activation during apoptosis inhibits proteasome function by cleavage of some key subunits of the 19S regulatory complex (20). Therefore, scrutinized investigation of the complicated relationship between the protein degradation by proteasome-dependent and -independent pathways and cancer cell apoptosis may allow mechanisms of action of classic proteasome inhibitors to be discovered.
Using molecules, including medicinal compounds, as probes, chemical biology can not only reveal key factors/pathways involved in physiology and human diseases such as cancer but also provide drug leads or use of existing drugs. Recently, when conducting chemical biology study in a number of leukemia and solid tumor cell models, we were attracted by unexpected discoveries that, in t(8;21) leukemia and GIST cells with constitutively activated C-KIT, BOR triggered a clathrin-mediated endocytosis and lysosomal degradation of C-KIT, and the dynamin inhibitor dynasore (DY) (21) suppressed BOR- but not tyrosine kinase inhibitor imatinib (IM)-induced apoptosis of these cells. These results suggested that C-KIT may interact with an apoptosis initiator, whereas BOR-triggered degradation but not IM-caused kinase inhibition releases this factor and activates caspases as well as other key downstream molecular cascade. We addressed the hypothesis in this work.
Results
BOR-Induced a Caspase-Dependent Apoptosis of C-KIT–Driven Cells.
We found that BOR significantly inhibited proliferation of t(8;21) AML lines Kasumi-1 and SKNO-1 and GIST line GIST882, with IC50 values of 12.3, 21.9, and 80.5 nM, respectively. BOR inhibited cell growth (Fig. S1A) and induced apoptosis (Fig. S1 B and C) of t(8;21)-positive lines and CD34+ primary leukemia cells isolated from bone marrow from three patients in 24–48 h of treatment time course. BOR inhibited chymotrypsin-like activity (Fig. S1D), down-regulated β5/β5i-component (Fig. S1E), and caused cleavage of the Rpt5 subunit (Fig. S1 F and G) of the proteasome. Interestingly, pan-caspase inhibitor benzyloxycarbonyl-Val-Ala-Asp fluoromethylketone (z-VAD.fmk) suppressed apoptosis of Kasumi-1, chronic myeloid leukemia K562, and myeloma U266 cells induced by treatment with BOR or another proteasome inhibitor Z-Ile-Glu(OtBu)-Ala-Leucinal or PSI (P, 1 μM) for 24 h (Fig. S1C) and reversed BOR-caused Rpt5 cleavage (Fig. S1G). However, z-VAD could not repress BOR-induced inhibition of chymotrypsin-like activity (Fig. S1D) and down-regulation of β5/β5i-component (Fig. S1E) of the proteasome. These results indicate that BOR is a caspase activator with detailed mechanisms in inducing apoptosis that warrant careful dissection.
BOR Induces Internalization and Lysosomal Degradation of C-KIT.
As a cell surface molecule, C-KIT plays a crucial role in leukemogenesis of t(8;21) AML (8, 12), suggesting that it could be targeted by effective therapeutics. We, therefore, tested the effects of BOR on C-KIT and found that treatment with BOR at 10 nM in Kasumi-1 cells resulted in down-regulation of C-KIT expression at the mRNA level (Fig. S2A). Importantly, C-KIT protein was down-regulated at 6 h and became very low at 12 h in cells on BOR (Fig. 1A). Other proteasome inhibitors PSI and MG-132 also caused C-KIT catabolism (Fig. S2B). In CD34+ primary leukemia cells with wild-type (WT) C-KIT, treatment with BOR for 12 h decreased the expression of C-KIT, which was revealed by Western blotting (Fig. S2C) and immunofluorescence assay, which also suggested a C-KIT internalization (Fig. S2D). In GIST882 cells with an activating C-KIT mutation (K642E) (22), treatment with BOR at 100 nM for 12 h markedly down-regulated C-KIT (Fig. 1B). We showed that ectopic expression of a degradable C-KIT with D816V mutation reduced BOR-induced inhibition rate of Kasumi-1 cells, but this reduction is not statistically significant (Fig. S2E). BOR could drastically potentiate the effect of the protein synthesis inhibitor cycloheximide (CHX) in suppressing C-KIT in Kasumi-1 cells (Fig. 1C). We found that, although z-VAD could not prevent BOR-triggered C-KIT turnover (Fig. S2F), lysosome inhibitor chloroquine (Chl; 40 μM) substantially reduced C-KIT catabolism (Fig. 1D).
Fig. 1.
BOR triggers a lysosomal degradation of C-KIT. (A) Kasumi-1 cells were treated with BOR at 10 nM for indicated time points and lysed, and Western blotting was performed using the indicated antibodies. (B) Western blot analysis using lysates of GIST882 cells treated with BOR at 100 nM for 12 h. (C) Kasumi-1 cells were treated with CHX and/or BOR and lysed, and Western blotting was performed. (D) Western blot analyses using lysates of Kasumi-1 cells treated with BOR and/or Chl. (E) Kasumi-1 cells were treated with BOR at 10 nM for indicated time points, and immunofluorescence analyses were performed using the antibodies indicated. (F) Immunofluorescence analyses of Kasumi-1 cells treated with BOR and/or Chl using the antibodies indicated.
Immunofluorescence analyses showed that BOR caused a dynamic change of C-KIT in that, in an early stage (2 h), C-KIT molecules were slightly up-regulated, possibly because of inhibition of proteasomal degradation; however, in a middle stage (6–8 h), they colocalized with lysosomes in cytoplasm and down-regulated. In a relatively late stage (12 h), they became markedly reduced, and the cells underwent apoptosis reflected by nuclear fragmentation with intact cell membrane (Fig. 1E). In the presence of Chl (40 μM), C-KIT was colocalized with lysosomes but not down-regulated (Fig. 1F and Fig. S2G). Nevertheless, z-VAD was unable to perturb C-KIT expression or cellular localization in Kasumi-1 cells on BOR (Fig. S2G).
C-KIT Internalization/Degradation Is Required for BOR-Caused Cell Apoptosis.
C-KIT internalization is mediated by clathrin (3, 4, 23). We evaluated whether clathrin plays a role in BOR-induced C-KIT internalization using DY, a potent inhibitor of dynamin GTPase that is essential for clathrin-dependent coated vesicle formation (21). We found that, although treatment with BOR for 6 h caused C-KIT internalization in Kasumi-1 cells, coincubation with BOR and DY (80 μM) or pretreatment with BOR for 1 h followed by treatment with DY for 5 h rendered C-KIT localization mainly on the cell surface (Fig. 2A), a sign of blockage of internalization. Interestingly, DY not only drastically attenuated BOR-caused inhibition of Kasumi-1 cell growth (Fig. 2B) but also significantly inhibited apoptosis of Kasumi-1, SKNO-1, and GIST882 cells induced by BOR (Fig. 2C). However, DY could not inhibit BOR-caused apoptosis of U266 cells (Fig. 2D) or apoptosis of Kasumi-1 or SKNO-1 cells triggered by IM (Fig. 2E).
Fig. 2.
DY inhibits C-KIT internalization and apoptosis induced by BOR. (A) Kasumi-1 cells were treated with BOR and/or DY (80 μM) for 6 h, or they were pretreated with BOR for 1 h and then incubated with DY for 5 h followed by immunofluorescence assays using indicated antibodies. (B) Cells were treated with BOR and/or DY for indicated time points, and they were assessed by trypan blue exclusion analysis. (C and D) Cells driven by C-KIT (C) or not (D) were treated with BOR (10 nM) in the absence or presence of DY for 12 h, and they were assayed by Annexin V/7-AAD and flow cytometry. (E) Cells were treated with IM (1 μM) and/or DY for 12 h, and apoptotic cells were determined. The mean + SD of three independent experiments is shown.
At the molecular level, DY attenuated BOR-induced C-KIT degradation and reversed BOR-caused suppression of phosphorylated AKT (pAKT), pSTAT3, and pERK, which are C-KIT targets (Fig. S3A). Although BOR up-regulated phospho-Stress-Activated Protein Kinase (pSAPK)/JNK, which is not a C-KIT target, DY could not reverse this effect (Fig. S3A). Furthermore, DY inhibited BOR-caused activation of caspase (Casp)-9 and -3 (reflected by down-regulation of pro–Casp-9 and -3) as well as cleavage of Casp-3 substrate Poly(ADP)-Ribose Polymerase (PARP) (Fig. S3A). However, DY could not reverse IM-induced inhibition of C-KIT signal pathway or cleavage of PARP (Fig. S3B), consistent with the observation fact that DY could not inhibit IM-induced apoptosis (Fig. 2E). Although capable of triggering degradation of C-KIT, SCF did not decrease pAKT or pERK (Fig. S3C) and could not induce apoptosis of Kasumi-1 cells (Fig. S3D). In this context, DY could not inhibit SCF-caused C-KIT catabolism (Fig. S3E). These results indicate that C-KIT internalization and subsequent degradation are required for BOR-induced apoptosis of t(8;21) leukemia and GIST cells, and suggest that C-KIT might directly or indirectly sequestrate a factor that could activate Casp-9/-3, whereas BOR, but not IM, could release this factor and induce programmed cell death.
C-KIT Binds and Phosphorylates Heat Shock Protein 90β.
To identify the putative C-KIT binding factor, Kasumi-1 cells (with N822K mutant C-KIT) were treated with or without BOR and lysed, and the supernatant was immunoprecipitated with a monoclonal anti–C-KIT antibody. The bands of silver-stained gel of eluates were analyzed by tandem mass spectrometric peptide sequencing. Interestingly, heat shock protein 90 (Hsp90) was identified (Fig. S4A). We further confirmed that Hsp90β, but not Hsp90α (Fig. S4B), was the C-KIT binding protein.
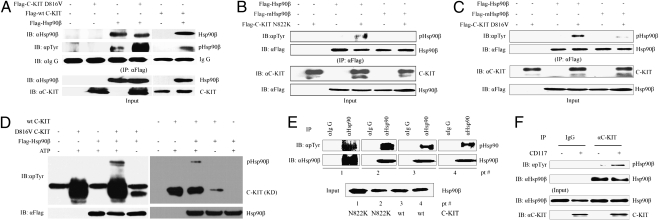
Studies showed that phosphorylation modification modulates the function of Hsp90β (24). We, therefore, tested whether C-KIT could phosphorylate Hsp90β or not. To do this testing, 293T cells were transfected with Flag-Hsp90β and/or Flag-C-KIT with or without D816V mutation and lysed 48 h later, and coimmunoprecipitation assays were performed. We found that, in the presence of mutant or WT C-KIT, the phosphorylated Hsp90β (pHsp90β) was up-regulated (Fig. 3A). C-KIT with N822K mutation was also able to induce phosphorylation of Hsp90β (Fig. 3B). The residue Y301 was shown to be the phosphorylation site of Hsp90β in Src-mediated phosphorylation of Hsp90β in response to VEGF (25). Plasmids containing Flag-Hsp90β, Flag-Hsp90β with Y301F mutation (Flag-mHsp90β), or Flag-C-KIT were transfected into 293T cells. Although C-KIT increased the expression of pHsp90β, Y301F substitution could attenuate this effect (Fig. 3 B and C), suggesting that Y301 is a phosphorylation site. In an in vitro phosphorylation assay, both WT and D816V C-KIT induced phosphorylation of Hsp90β (Fig. 3D). We investigated the expression of pHsp90β in CD34+ cells from t(8;21) AML patients with N822K or WT C-KIT, and we found that pHsp90β was the main type of Hsp90β in these cells (Fig. 3E). Moreover, the expression of pHsp90β was much higher in CD117/C-KIT+ than CD117− cells from bone marrow mononuclear cells of a t(8;21) AML patient with WT C-KIT (Fig. 3F).
Fig. 3.
C-KIT binds and phosphorylates Hsp90β. (A) 293T cells were transfected with the plasmids indicated and lysed, and coimmunoprecipitation and Western blot assays were performed. (B and C) 293T cells were transfected with the plasmids indicated and lysed, and the proteins were purified. The extracts were supplemented with ATP followed by coimmunoprecipitation and Western blot analyses. (D) In vitro phosphorylation assay using kinase domain (KD) of C-KIT, purified Hsp90β, and ATP. Western blot analysis was performed to evaluate pHsp90β. (E) Hsp90β protein was purified from lysates of CD34+ cells isolated from four patients with t(8;21) AML, and pHsp90β was detected using an anti-pTyr antibody. (F) CD117+ and CD117− cells were isolated by fluorescence activated cell sorting from bone marrow mononuclear cells of a t(8;21) AML patient with WT C-KIT and lysed, and coimmunoprecipitation and Western blotting were performed.
C-KIT Indirectly Sequestrates Apoptotic Protease Activating Factor 1, Whereas BOR Releases It.
Studies (24, 26) showed that Hsp90β can bind strongly to apoptotic protease activating factor 1 (Apaf-1), a caspase recruitment domain-containing protein that forms an oligomeric apoptosome on binding cytochrome c and dATP (27). To investigate the possible interaction between C-KIT, Hsp90β, and Apaf-1, plasmids containing Flag-Hsp90β and His-Apaf-1 were transfected into 293T cells, and the proteins were purified and incubated with C-KIT isolated from Kasumi-1 cells. By reciprocal coimmunoprecipitation and Western blot analyses, we found that C-KIT not only induced phosphorylation of Hsp90β but also markedly enhanced the binding affinity between Hsp90β and Apaf-1 (Fig. 4A), suggesting that C-KIT could sequestrate Apaf-1 by phosphorylation of Hsp90β. Y301F substitution in Hsp90β reduced Apaf-1 binding activity (Fig. 4A). In Kasumi-1 cells, pHsp90β bound Apaf-1, whereas BOR decreased phosphorylation of Hsp90β and released Apaf-1 (Fig. 4B). In CD34+ leukemia cells from patients with t(8;21) AML and GIST882 cells, BOR drastically down-regulated pHsp90β and released Apaf-1 from Hsp90β (Fig. 4C).
Fig. 4.
Protein–protein interactions among C-KIT, Hsp90β, and Apaf-1. (A) 293T cells were transfected with the plasmids indicated and lysed, and the proteins were purified by immunoprecipitation followed by incubation with C-KIT isolated from Kasumi-1 cells. Reciprocal coimmunoprecipitation and Western blot analyses were performed. (B and C) The cells were treated without or with BOR and lysed, and reciprocal coimmunoprecipitation and Western blotting were performed. (D) Diagram of constructs of C-KIT. CY, cytoplasmic domain. (E) 293T cells were transfected with pEGFP-C1 plasmids containing different domains of C-KIT and lysed, and coimmunoprecipitation and Western blot assays were conducted.
To define the interaction between C-KIT and Hsp90β, different intracellular domains of C-KIT (Fig. 4D) were subcloned into pEGFP-C1 plasmids and transfected into 293T cells. We found that the tyrosine kinase domains 1 and 2 and the kinase insertion domain could bind Hsp90β, whereas the juxtamembrane domain and the C-terminal region could not (Fig. 4E).
Released Apaf-1 Activates Casp-3, Which Is Not Sufficient to Cause Marked Apoptosis.
We found that, in Kasumi-1 cells treated with BOR for 6 h, the binding affinity between Hsp90β and Apaf-1 was markedly decreased, and cytochrome c was recruited to Apaf-1 (Fig. S4C), which was confirmed by a reciprocal coimmunoprecipitation assay (Fig. S4D). Chronologically, this event was followed by the activation of Casp-9 and -3 with BOR treatment for 6–8 h (Fig. S4E). However, at these time points, BOR failed to induce obvious apoptosis in Kasumi-1 cells (Fig. S4F). Compared with vehicle control, after treatment with BOR for 12 h, only 8% of the cells were committed to apoptosis (Fig. S4F), whereas no significant cell growth inhibition was visible (Fig. S1A). Nevertheless, marked apoptotic effect was seen 24–48 h after coincubation with BOR (Figs. S1B and S4F). In agreement with these observations, apoptosis was seen in CD34+ primary leukemia cells treated with BOR for 24–48 h (Fig. S1B). While Casp-3 is shown to be a major growth-stimulating signal to stimulate the repopulation of tumors undergoing radiotherapy (28), our results indicate that an early activation of Casp-3 is unable to initiate suicide program in t(8;21) cells, and other signals are required to amplify the apoptotic cascade.
We found that DY was able to inhibit BOR-caused down-regulation of pHsp90β and release of Apaf-1 (Fig. S4D), consistent with the fact that DY could attenuate BOR-induced degradation of C-KIT (Fig. S3A). Moreover, we showed that Hsp90β inhibitor 17-allylamino-demethoxy geldanamycin (17-AAG) reduced Kasumi-1 cell proliferation (Fig. S4G), and combined use of BOR (2.5 nM) and 17-AAG (0.1 μM) caused a potentiated suppression of cell proliferation, whereas no such enhanced effect was seen in BOR/IM and 17-AAG/IM combinations (Fig. S4H).
BOR-Triggered Degradation of AE/AE9a and Generation of Cleavage Fragments.
Interestingly, treatment of Kasumi-1 cells with BOR for 12 h resulted in degradation of the AE oncoprotein with generation of a 70-kDa cleavage fragment (CF), ΔAE (Fig. S5A), reminiscent of t(8;21) AML cells treated with oridonin (29) and triptolide (30). These phenomena were also seen in BOR-treated CD34+ cells derived from a t(8;21) patient (Fig. S5B). Moreover, when murine AML with expression of AE9a (10) was used as a model, in vitro and in vivo treatment with BOR caused AE9a down-regulation (Fig. S5C). Indeed, in HeLa cells transfected with a construct of AE9a coding region fused in frame with a construct of GFP (designated AE9a-GFP), BOR at 50 nM generated a CF of ∼70 kDa, including a 43-kDa CF from AE9a (the ΔAE9a) (Fig. S5 D and E).
In Kasumi-1– or AE9a-GFP–expressing 293T cells, pretreatment with caspase inhibitors for 1 h abrogated BOR-triggered degradation of AE/AE9a as well as production of CFs (Fig. S6 A and B). That this cleavage needs action of Casp-3 was further confirmed by an AE9a mutant with amino acid substitution of D188A at an established Casp-3 cutting site (29), which abrogated AE9a catabolism caused by BOR (Fig. S6C). Moreover, when DY was used to pretreat the cells, the CF generation was also substantially abrogated (Fig. S6D), suggesting a causal relationship between C-KIT internalization/lysosomal degradation and caspase-mediated AE cleavage.
AE/AE9a CFs Play an Important Role in BOR-Induced Apoptosis of t(8;21) Leukemia Cells.
The fact that AE-D188A mutant conferred resistance to BOR-induced suppression of U937 cells (Fig. S7A) suggests that AE turnover and production of CFs may have critical roles in the effects of BOR on t(8;21) cells. Indeed, transfection of AE CF (Fig. S5E) into Kasumi-1 cells induced cell death and inhibited cell growth (Fig. S7B) as well as the cells’ colony forming activity (Fig. S7C). Several lines of evidence suggested that AE CF could antagonize the effects of AE. For example, this CF was capable of interfering with the transcriptional regulatory potential of AE by using the luciferase reporter system containing the AML-1 responsive sites of target genes such as MDR1 (Fig. S7D), Bcl-2 (Fig. S7E), and C-KIT (Fig. S7F) or by EMSA with consensus AML1 DNA recognition sequences (Fig. S7G). Notably, treatment with BOR drastically decreased AE-DNA binding activity in Kasumi-1 cells (Fig. S7H). By observation of embryo development of the amphibian model, Xenopus laevis, we showed that microinjection of AE CF mRNA overcame AE-caused defects in embryo development (Fig. S7I). It is worth pointing out that this CF corresponds to almost the entire WT ETO, which is suppressed in t(8;21) AML by unknown epigenetic mechanisms (31); this finding suggests that the WT ETO may bear tumor-suppressing function, which warrants additional investigation.
The AE9a CF (Fig. S5E) suppressed the colony forming activity of AE9a in 32D hematopoietic (Fig. S8 A and B) and Cos-7 cells (Fig. S8C), whereas deletion of NHR2 abrogated this function (Fig. S8 A and C). In X. laevis, microinjection of 500 pg AE9a mRNA into one blastomere of two cell-stage Xenopus embryos from animal pole resulted in slowdown of cell division in the injected side at the late blastula stage of development. After gastrulation, the cells that received exogenous AE9a mRNAs were gradually dying, whereas cells obtaining AE9a CF mRNAs were not affected. Embryos coinjected with AE9a and its CF mRNA developed normally (Fig. S8D). A physical interaction between AE9a and its CF was shown by their reciprocal coimmunoprecipitation (Fig. S8E) and immunofluoresence (Fig. S8F) assays in 293T cells. The results also showed that the NHR2 domain was required for AE9a-CF binding affinity. Size exclusion chromatography and staining Western blots of the fractions showed that, although AE9a and the CF could form homooligomer, respectively, they formed hetero-oligomer when coexpressed in 293T cells (Fig. S8G).
Therapeutic Potential of BOR on AE9a-Driven AML Model.
C57 mice bearing leukemic cells expressing AE9a (10, 29) were randomized into five groups (n = 10 for each group) and treated with 0.9% sodium chloride or BOR (intraperitoneal injection two times per week for 4 wk). Intriguingly, at 1 and 2 mg/kg, BOR significantly prolonged life span of mice compared with control (P = 0.02 and 0.009, respectively) (Fig. 5A). The median survival time of mice treated with control or BOR at 1 or 2 mg/kg was 18, 25, and 34 d, respectively. BOR at 2 mg/kg significantly reduced white blood cell (WBC) count in peripheral blood (Fig. 5B) (P = 0.003) and reduced spleen weight (Fig. 5C). We found that BOR also triggered degradation of C-KIT and AE9a (Fig. 5D and Fig. S5C), and it caused down-regulation of pHsp90β in vivo (Fig. 5E).
Fig. 5.
In vivo therapeutic efficacy of BOR on the murine model for human t(8;21) AML. (A) Survival of C57 mice transplanted with AE9a-expressing cells and treated with BOR (n = 10 for each group). (B) Peripheral WBC count of C57 mice. (C) Spleen weight of mice treated with BOR for 14 d. (D) Leukemia cells were isolated from the spleens of mice, lysed, and analyzed by Western blot assay. (E) Leukemic cells isolated from the spleens of mice were lysed and analyzed by immunoprecipitation and Western blot assays.
Discussion
By using BOR as a chemical probe, we show here that, in t(8;21) AML and GIST cells, C-KIT can bind and phosphorylate Hsp90β and sequestrate Apaf-1 by pHsp90β, which is the main form in t(8;21) AML, leading to apoptosis evading of the cells. BOR triggers internalization and degradation of the kinase, dephosphorylation of pHsp90β, and release of Apaf-1, resulting in formation of apoptosome and activation of caspases. These data, thus, indicate that degradation of C-KIT/dephosphorylation of pHsp90β may be a powerful alternative strategy for kinase inhibition different from the common strategy of occupying the ATP binding pocket.
DY, an inhibitor of the GTPase activity of dynamin that arrests the formation of endocytic clathrin-coated pits and vesicles (21), provides a unique tool to study the role for C-KIT in BOR-induced apoptosis. DY not only retains C-KIT on the cell surface but also inhibits BOR-induced apoptosis of C-KIT–driven neoplastic cells (Fig. 2 A–C). However, DY cannot inhibit BOR-triggered apoptosis of U266 myeloma cells (Fig. 2D). These data indicate that, in different cells, BOR may have different mechanisms, and C-KIT is a crucial target of BOR in the cells that it drives. However, DY cannot suppress IM-induced apoptosis of t(8;21) cells. Although we cannot exclude the possibility that DY might also inhibit endocytosis of other membrane molecules, the above data indicate that functional inhibition of C-KIT tyrosine kinase activity is not responsible to apoptosis induced by BOR, and instead, C-KIT degradation might release an apoptosis initiator. These data also suggest that C-KIT may have an unrecognized role in programmed cell death. Indeed, we identify Hsp90β as both a binding factor and a substrate of C-KIT. We find that, in the presence of C-KIT, Hsp90β–Apaf-1 binding affinity (24, 26) is markedly enhanced; however, on BOR, Apaf-1 is released and then recruits cytochrome c to activate caspases. Therefore, our data not only uncover the critical role in apoptosis for C-KIT by indirect sequestration of Apaf-1 through phosphorylation of Hsp90β, but also unveil mechanisms of action of BOR in cancer.
Ligand-induced down-regulation is an important aspect of the normal physiology of the cell surface receptors. While binding to its receptor, SCF accelerates the turnover of C-KIT by inducing internalization of the receptor ligand complexes followed by polyubiquitination and degradation (32). However, unlike BOR-induced C-KIT degradation, which leads to inactivation of pAKT/pSTAT3/pERK (Fig. S3A), SCF does not inhibit pAKT/pSTAT3 (Fig. S3C) and does not induce cell apoptosis (Fig. S3D). Because AKT is critical for C-KIT–mediated growth and survival of neoplastic cells (33) and AKT inhibitors can induce apoptosis of malignant cells (34), our results may at least partially explain the difference between the effects of BOR and SCF on C-KIT–driven cells. However, why BOR but not SCF inactivates AKT remains elusive, whereas their effects on protein–protein interaction may be critical. This possibility warrants additional exploration.
AE/AE9a-targeting strategies have been emerging in the recent years to further improve clinical outcome of t(8;21) AML (29, 30, 35). We show that AE/AE9a CFs can perturb AE/AE9a oligomerization, leading to inhibition of parental oncoproteins and amplification of the Casp-3 signal to efficiently trigger apoptosis (Fig. S9). In t(8;21) AML, AE and AE9a are associated with C-KIT mutation/overexpression (8, 11), and AE is able to up-regulate C-KIT (8). Therefore, BOR represents a C-KIT, AE/AE9a double targeting agent that triggers a positive feedback signal network to induce apoptosis, and its efficacy on the murine t(8;21) AML model suggests its potential of clinical application in t(8;21) AML as well as other C-KIT–driven cancers.
Methods
Methods and associated references are in SI Methods. The CD34+ primary cells were isolated from patients with t(8;21) AML. The protein extracts of BOR-treated cells were analyzed by immunoprecipitation/Western blot assays. Immunofluorescence microscopy, clonogenic, in vitro phosphorylation, EMSA and luciferase assays were performed. The interested genes were transfected into cells or Xenopus embryos and their biological functions were investigated. AE9a+ cells were injected into irradiated mice, which were then treated with BOR.
Supplementary Material
Acknowledgments
We thank Prof. Dong-Er Zhang at the University of California at San Diego for providing AE9a leukemic cells, Prof. Stephen Swank at Brigham and Women's Hospital for providing GIST882 cells, Prof. Shuo Dong at Baylor College of Medicine for providing the pSG5-AE(ΔNHR2) plasmid, Prof. John D. Schuetz at St. Jude Children's Research Hospital for providing the pGL2-MDR1 promoter luciferase reporter plasmid, Prof. Michael H. Tomasson at Washington University for providing MIG-hKITwt and MIG-hKITD816V plasmids, and Prof. Xiaodong Wang at University of Texas Southwestern Medical Center for providing the pFastBac-His-Apaf-1 plasmid. This work was supported, in part, by National Key Program for Basic Research Grants 2010CB529201 and 2012CB910800, National Natural Science Foundation Grants 30871110 and 81071930, the Special Foundation of President, and Key Project of Knowledge Innovation Program of the Chinese Academy of Sciences Grants KSCX1-YW-R-26 and KSCX2-YW-R-235.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1121341109/-/DCSupplemental.
References
- 1.Yarden Y, et al. Human proto-oncogene c-kit: A new cell surface receptor tyrosine kinase for an unidentified ligand. EMBO J. 1987;6:3341–3351. doi: 10.1002/j.1460-2075.1987.tb02655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Toffalini F, Demoulin JB. New insights into the mechanisms of hematopoietic cell transformation by activated receptor tyrosine kinases. Blood. 2010;116:2429–2437. doi: 10.1182/blood-2010-04-279752. [DOI] [PubMed] [Google Scholar]
- 3.Miyazawa K, et al. Ligand-dependent polyubiquitination of c-kit gene product: A possible mechanism of receptor down modulation in M07e cells. Blood. 1994;83:137–145. [PubMed] [Google Scholar]
- 4.Gommerman JL, Rottapel R, Berger SA. Phosphatidylinositol 3-kinase and Ca2+ influx dependence for ligand-stimulated internalization of the c-Kit receptor. J Biol Chem. 1997;272:30519–30525. doi: 10.1074/jbc.272.48.30519. [DOI] [PubMed] [Google Scholar]
- 5.Masson K, Rönnstrand L. Oncogenic signaling from the hematopoietic growth factor receptors c-Kit and Flt3. Cell Signal. 2009;21:1717–1726. doi: 10.1016/j.cellsig.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Miettinen M, Lasota J. KIT (CD117): A review on expression in normal and neoplastic tissues, and mutations and their clinicopathologic correlation. Appl Immunohistochem Mol Morphol. 2005;13:205–220. doi: 10.1097/01.pai.0000173054.83414.22. [DOI] [PubMed] [Google Scholar]
- 7.Hirota S, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–580. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- 8.Wang YY, et al. AML1-ETO and C-KIT mutation/overexpression in t(8;21) leukemia: Implication in stepwise leukemogenesis and response to Gleevec. Proc Natl Acad Sci USA. 2005;102:1104–1109. doi: 10.1073/pnas.0408831102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erickson P, et al. Identification of breakpoints in t(8;21) acute myelogenous leukemia and isolation of a fusion transcript, AML1/ETO, with similarity to Drosophila segmentation gene, runt. Blood. 1992;80:1825–1831. [PubMed] [Google Scholar]
- 10.Yan M, et al. A previously unidentified alternatively spliced isoform of t(8;21) transcript promotes leukemogenesis. Nat Med. 2006;12:945–949. doi: 10.1038/nm1443. [DOI] [PubMed] [Google Scholar]
- 11.Jiao B, et al. AML1-ETO9a is correlated with C-KIT overexpression/mutations and indicates poor disease outcome in t(8;21) acute myeloid leukemia-M2. Leukemia. 2009;23:1598–1604. doi: 10.1038/leu.2009.104. [DOI] [PubMed] [Google Scholar]
- 12.Wang YY, et al. C-KIT mutation cooperates with full-length AML1-ETO to induce acute myeloid leukemia in mice. Proc Natl Acad Sci USA. 2011;108:2450–2455. doi: 10.1073/pnas.1019625108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng X, Oancea C, Henschler R, Ruthardt M. Cooperation between constitutively activated c-Kit signaling and leukemogenic transcription factors in the determination of the leukemic phenotype in murine hematopoietic stem cells. Int J Oncol. 2009;34:1521–1531. doi: 10.3892/ijo_00000281. [DOI] [PubMed] [Google Scholar]
- 14.Kelly LM, Gilliland DG. Genetics of myeloid leukemias. Annu Rev Genomics Hum Genet. 2002;3:179–198. doi: 10.1146/annurev.genom.3.032802.115046. [DOI] [PubMed] [Google Scholar]
- 15.Lin P, et al. Acute myeloid leukemia harboring t(8;21)(q22;q22): A heterogeneous disease with poor outcome in a subset of patients unrelated to secondary cytogenetic aberrations. Mod Pathol. 2008;21:1029–1036. doi: 10.1038/modpathol.2008.92. [DOI] [PubMed] [Google Scholar]
- 16.Marcucci G, et al. Prognostic factors and outcome of core binding factor acute myeloid leukemia patients with t(8;21) differ from those of patients with inv(16): A Cancer and Leukemia Group B study. J Clin Oncol. 2005;23:5705–5717. doi: 10.1200/JCO.2005.15.610. [DOI] [PubMed] [Google Scholar]
- 17.Kuwatsuka Y, et al. Hematopoietic stem cell transplantation for core binding factor acute myeloid leukemia: t(8;21) and inv(16) represent different clinical outcomes. Blood. 2009;113:2096–2103. doi: 10.1182/blood-2008-03-145862. [DOI] [PubMed] [Google Scholar]
- 18.Ciechanover A. Proteolysis: From the lysosome to ubiquitin and the proteasome. Nat Rev Mol Cell Biol. 2005;6:79–87. doi: 10.1038/nrm1552. [DOI] [PubMed] [Google Scholar]
- 19.Hideshima T, et al. Bortezomib induces canonical nuclear factor-kappaB activation in multiple myeloma cells. Blood. 2009;114:1046–1052. doi: 10.1182/blood-2009-01-199604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun XM, et al. Caspase activation inhibits proteasome function during apoptosis. Mol Cell. 2004;14:81–93. doi: 10.1016/s1097-2765(04)00156-x. [DOI] [PubMed] [Google Scholar]
- 21.Macia E, et al. Dynasore, a cell-permeable inhibitor of dynamin. Dev Cell. 2006;10:839–850. doi: 10.1016/j.devcel.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 22.Tuveson DA, et al. STI571 inactivation of the gastrointestinal stromal tumor c-KIT oncoprotein: Biological and clinical implications. Oncogene. 2001;20:5054–5058. doi: 10.1038/sj.onc.1204704. [DOI] [PubMed] [Google Scholar]
- 23.Broudy VC, et al. Signaling via Src family kinases is required for normal internalization of the receptor c-Kit. Blood. 1999;94:1979–1986. [PubMed] [Google Scholar]
- 24.Kurokawa M, Zhao C, Reya T, Kornbluth S. Inhibition of apoptosome formation by suppression of Hsp90beta phosphorylation in tyrosine kinase-induced leukemias. Mol Cell Biol. 2008;28:5494–5506. doi: 10.1128/MCB.00265-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trepel J, Mollapour M, Giaccone G, Neckers L. Targeting the dynamic HSP90 complex in cancer. Nat Rev Cancer. 2010;10:537–549. doi: 10.1038/nrc2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pandey P, et al. Negative regulation of cytochrome c-mediated oligomerization of Apaf-1 and activation of procaspase-9 by heat shock protein 90. EMBO J. 2000;19:4310–4322. doi: 10.1093/emboj/19.16.4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zou H, Henzel WJ, Liu X, Lutschg A, Wang X. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell. 1997;90:405–413. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]
- 28.Huang Q, et al. Caspase 3-mediated stimulation of tumor cell repopulation during cancer radiotherapy. Nat Med. 2011;17:860–866. doi: 10.1038/nm.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou GB, et al. Oridonin, a diterpenoid extracted from medicinal herbs, targets AML1-ETO fusion protein and shows potent antitumor activity with low adverse effects on t(8;21) leukemia in vitro and in vivo. Blood. 2007;109:3441–3450. doi: 10.1182/blood-2006-06-032250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou GS, et al. Biologic activity of triptolide in t(8;21) acute myeloid leukemia cells. Leuk Res. 2011;35:214–218. doi: 10.1016/j.leukres.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 31.Kozu T, Fukuyama T, Yamami T, Akagi K, Kaneko Y. MYND-less splice variants of AML1-MTG8 (RUNX1-CBFA2T1) are expressed in leukemia with t(8;21) Genes Chromosomes Cancer. 2005;43:45–53. doi: 10.1002/gcc.20165. [DOI] [PubMed] [Google Scholar]
- 32.Zeng S, Xu Z, Lipkowitz S, Longley JB. Regulation of stem cell factor receptor signaling by Cbl family proteins (Cbl-b/c-Cbl) Blood. 2005;105:226–232. doi: 10.1182/blood-2004-05-1768. [DOI] [PubMed] [Google Scholar]
- 33.Harir N, et al. Oncogenic Kit controls neoplastic mast cell growth through a Stat5/PI3-kinase signaling cascade. Blood. 2008;112:2463–2473. doi: 10.1182/blood-2007-09-115477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amaravadi R, Thompson CB. The survival kinases Akt and Pim as potential pharmacological targets. J Clin Invest. 2005;115:2618–2624. doi: 10.1172/JCI26273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wichmann C, et al. Targeting the oligomerization domain of ETO interferes with RUNX1/ETO oncogenic activity in t(8;21)-positive leukemic cells. Cancer Res. 2007;67:2280–2289. doi: 10.1158/0008-5472.CAN-06-3360. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.