Abstract
During pregnancy women can develop B- and T-cell immunity against the inherited paternal antigens (IPAs) of the fetus, such as HLA, peptides of minor histocompatibilty antigens, and possibly onco-fetal antigens. The biological and pathological role of these pregnancy-induced immunological events is only understood in part. However, anti-IPA immunity in the mother persists for many decades after delivery and may reduce relapse in offspring with leukemia after HLA-haploidentical transplantation of maternal hematopoietic stem cells (HSC). We hypothesized that maternal anti-IPA immune elements cross the placenta and might confer a potent graft-versus-leukemia effect when cord blood (CB) is used in unrelated HSC transplantation. In a retrospective study of single-unit CB recipients with all grafts provided by the New York Blood Center, we show that patients with acute myeloid or lymphoblastic leukemia (n = 845) who shared one or more HLA-A, -B, or -DRB1 antigens with their CB donor's IPAs had a significant decrease in leukemic relapse posttransplantation [hazard ratio (HR) = 0.38, P < 0.001] compared with those that did not. Remarkably, relapse reduction in patients receiving CB with one HLA mismatch (HR = 0.15, P < 0.001) was not associated with an increased risk of severe acute graft-versus-host disease (HR = 1.43, P = 0.730). Our findings may explain the unexpected low relapse rate after CB transplantation, open new avenues in the study of leukemic relapse after HSC transplantation (possibly of malignancies in general), and have practical implications for CB unit selection.
Keywords: cord blood stem cell transplantation, birth order
During pregnancy, transplacental trafficking of cells may result in sensitization of maternal cells against HLA that the fetus has inherited from the father [inherited paternal antigens (IPA)]. Not only B-cell but also T-cell immunity is induced against paternal HLA and minor histocompatibility antigens (mHA) (1–6). Importantly, anti-IPA memory T cells persist in the mother for decades. This finding probably explains the recent findings of Stern et al. (7) that HLA-haploidentical hematopoietic stem cell (HSC) transplants from mother to child have lower relapse rates and improved survival compared with paternal grafts, confirming and extending earlier studies (8).
When cord blood (CB) HSC transplantation from unrelated donors became a clinical procedure, there was concern that maternal cells in CB might cause graft-versus-host disease (GVHD) in the recipient. Furthermore, the potential for effective antileukemia activity of CB grafts was in doubt because of proven immunological naivety of fetal cells (9–12). Clinical outcomes of CB transplantation, however, did not support these concerns. Low relapse rates, however, remained an enigma until preliminary evidence suggested that this might be, in part, because of immunity of transplanted CB cells against noninherited maternal antigens (NIMA), at least in patients with myeloid leukemia (13). Why the relapse rate in acute lymphoblastic leukemia (ALL) was not similarly affected remained unclear.
Another mechanism might involve maternal cells that, if they cross the placenta and enter the fetal circulation, could be infused with the CB graft. Based on Stern's observation (7), we hypothesized that microchimeric maternal cells present in CB grafts, sensitized to fetal IPAs during pregnancy, might reduce the risk of relapse, and possibly increase the risk of GVHD. We examined this possibility by comparing relapse and GVHD rates in recipients who shared HLA antigens with their respective CB IPAs, and with those who did not share any IPAs or whose graft had no IPA target for maternal cells. We refer to these, respectively, as shared-IPA transplants and no-shared-IPA transplants (see Materials and Methods and examples in Table 1).
Table 1.
Examples of transplants in patients that had no shared antigen with CB IPAs and of transplants with shared antigens
| HLA mismatch (MM) | A | B | DRB1 |
| No-shared-IPA transplants | |||
| 1 HLA MM | |||
| Mother | 2, 33 | 53, 72 | 08:04, 11:01 |
| CB unit | 2, 33 | 53, 64 | 08:04, 08:04 |
| Patient | 2, 33 | 27, 53 | 08:04, 08:04 |
| IPA assignment | No IPA target (mother and CB match) | IPA = B64 | No IPA target (CB homozygous) |
| Shared IPA | No | No | No |
| Shared IPA ambiguity at allele level | Ambiguous | No ambiguity | No ambiguity |
| Shared-IPA transplants | |||
| 1 HLA MM | |||
| Mother | 3, 66 | 44, 58 | 07:01, 15:03 |
| CB unit | 2, 3 | 45, 58 | 12:01, 15:03 |
| Patient | 2, 3 | 45, 58 | 13:02, 15:03 |
| IPA assignment | IPA = A2 | IPA = B45 | IPA = DRB1*12:01 |
| Shared IPA: | Yes | Yes | No |
| Shared IPA ambiguity at allele level | Ambiguous | Ambiguous | No ambiguity |
In the no-shared-IPA transplants, at a given locus, there is no match between the CB IPA and/or no IPA target for the mother's cells (i.e., the mother and CB donor match for their HLA antigens or the donor is homozygous). Ambiguity at each locus is indicated when allele-level typing could identify an alternate IPA-match assignment.
The New York Blood Center (NYBC) National Cord Blood Program data on patients transplanted for hematological malignancies with single CB units was made available to evaluate this possibility. This same dataset was used in our previous study on the effect of possible CB immunity to NIMA, adding patients with three HLA-A, -B, -DRB1 mismatches to their graft and excluding the few on whom sharing the donor's IPA or identifying an IPA target could not be definitively assigned (13). This analysis used the available HLA-A and -B intermediate-resolution and -DRB1 high-resolution typings, as is currently used in CB unit selection.
Primary study endpoints were acute grade III-IV GVHD and relapse; secondary endpoints were neutrophil and platelet engraftment, chronic GVHD, transplant-related mortality (TRM), overall mortality, and treatment failure (relapse or death, the inverse of disease-free survival). Although this is a retrospective, observational study, the results provide unique evidence that anti-IPA immunity plays a role in the control of relapse in acute myeloid leukemia (AML) and ALL after unrelated CB HSC transplantation.
Results
Largely the same patient dataset used in our previous publication on the impact of NIMA matching was studied (13). Seventy-three percent of patients had ALL or AML (Table S1). Most patients received myeloablative conditioning, including antithymocyte globulins (ATG), and nearly all received a calcineurin inhibitor for GVHD prophylaxis. Among the 1,094 patients given HLA mismatched CB grafts, 64 (6%) were classified as no-shared-IPA transplants, having no shared antigens with their CB's IPAs at all three loci (n = 3), no IPA target for maternal cells at all three loci (n = 19), or a combination of no shared-IPA or no-IPA target (n = 42). The remaining 1,030 patients shared one or more antigens with CB IPAs. In 56 of the 61 patients given a CB unit that was HLA-matched, IPAs could be assigned and all shared at least one CB IPA.
No-shared-IPA transplants did not differ from HLA-mismatched transplants with shared IPAs in patient or treatment characteristics, graft total nucleated cell (TNC) dose, or NIMA match (Table S1). These transplants did, however, differ in the number of HLA mismatches and in the proportion having unidirectional mismatches in the GVH-only direction (17% vs. 3%, respectively), both factors associated with GVHD and relapse risk. The proportion with unidirectional mismatches only in the rejection direction did not differ (3% vs. 2%, respectively).
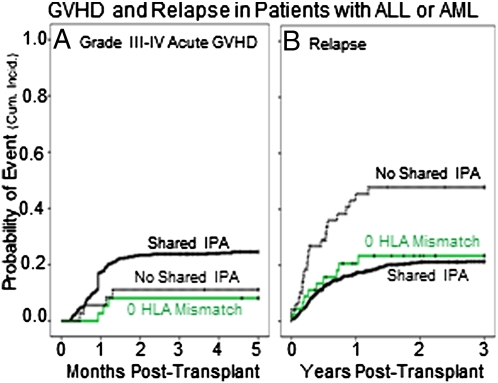
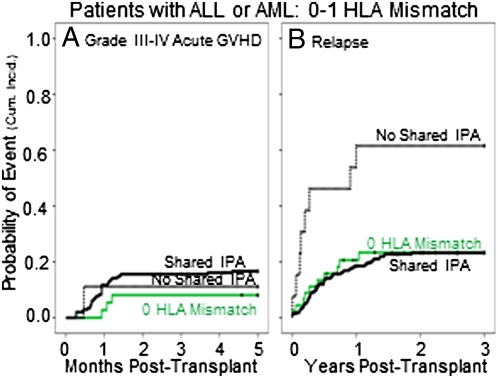
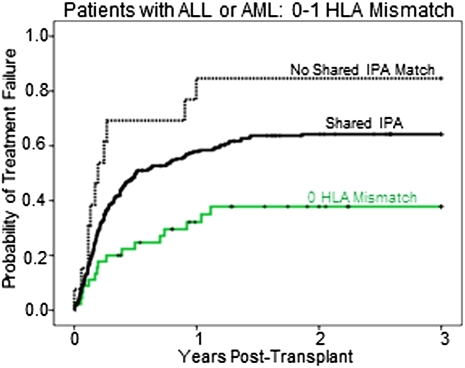
Patients with shared-IPA grafts had a higher rate of acute grade III-IV GVHD (albeit of borderline significance) and a lower relapse rate than did those that had no-shared-IPA (Table S2). The relationship between shared IPAs and relapse was apparent only in patients with ALL and AML (Table S2). We therefore focused subsequent analyses on this subset. Among ALL/AML patients, shared-IPA transplants tended to have a higher cumulative incidence of GVHD than the no-shared-IPA transplants (Fig. 1A and Table 2), a relationship that was strongest in patients with two HLA mismatches (Table S3). Shared-IPA transplants also had a significantly lower cumulative incidence of relapse (Fig. 1B), regardless of disease stage (Table S3). The reduction of relapse was most significant in those with one HLA mismatch, the group in which acute GVHD was not increased significantly (Fig. 2 and Table 2). Among transplants that had one HLA mismatch, the lower incidence of relapse in the shared-IPA group was reflected in a lower rate of treatment failure (Fig. 3), although not in overall survival (Table 2). Within the shared-IPA group, there was no relationship between number of IPAs that were shared and risk of relapse (Table S3). Ambiguity in shared IPA assignments related to low/intermediate HLA-A and -B typing did not diminish the relationship with relapse (Table S3). Of note, relapse risk was lower when the CB unit came from a second or later pregnancy than from a first [hazard ratio (HR) = 0.67, P = 0.014] (Table S3).
Fig. 1.
Probability (cumulative incidence) of acute grade III-IV GVHD and of relapse posttransplant among patients with ALL or AML. (A) GVHD in patients who engrafted. Shared IPA (n = 530), solid black line; 0 HLA mismatch (n = 37), solid green line; no shared IPA (n = 36), dashed black line. (B) Relapse in all patients. Shared IPA (n = 751), solid black line; 0 HLA mismatch (n = 45), solid green line; no shared IPA (n = 49), dashed black line.
Table 2.
Patients with ALL or AML (N = 845): Multivariate analyses of grade III-IV acute GVHD, relapse, transplant-related mortality, overall mortality, and treatment failure (death or relapse) by patient-donor HLA match and shared antigens with donor IPA
| Endpoint/variable | No. | Cumulative incidence | HR (95% CI) | P value |
| GVHD in patients who engrafted (GVHD data were not reported on 44)* | ||||
| 1–3 HLA MM, no shared IPA | 36 | 11% | 1.00 (reference) | |
| 1–3 HLA MM, 1–3 shared IPAs | 528 | 25% | 2.42 (0.89–6.64) | 0.085 |
| 0 HLA MM | 37 | 8% | 1.03 (0.22–4.83) | 0.973 |
| 1 HLA MM | ||||
| No shared IPA | 9 | 11% | 1.00 (reference) | |
| 1–3 shared IPAs | 204 | 17% | 1.43 (0.19–10.96) | 0.730 |
| 2–3 HLA MM | ||||
| No shared IPA | 27 | 11% | 1.00 (reference) | |
| 1–3 shared IPAs | 324 | 30% | 3.92 (0.96–15.97) | 0.057 |
| Relapse during first 3 y posttransplant† | ||||
| 1–3 HLA MM, no shared IPA | 49 | 49% | 1.00 (reference) | |
| 1–3 HLA MM, 1–3 shared IPAs | 751 | 21% | 0.38 (0.24–0.62) | <0.001 |
| 0 HLA MM | 45 | 24% | 0.34 (0.15–0.79) | 0.012 |
| ALL | ||||
| 1–3 HLA MM, no shared IPA | 28 | 38% | 1.00 (reference) | |
| 1–3 HLA MM, 1–3 shared IPAs | 399 | 18% | 0.32 (0.15–0.68) | 0.003 |
| 0 HLA mismatch | 26 | 17% | 0.24 (0.06–0.86) | 0.029 |
| AML | ||||
| 1–3 HLA MM, no shared IPA | 21 | 62% | 1.00 (reference) | |
| 1–3 HLA MM, 1–3 shared IPAs | 352 | 26% | 0.31 (0.16–0.61) | 0.001 |
| 0 HLA mismatch | 19 | 34% | 0.39 (0.13–1.20) | 0.101 |
| 1 HLA MM | ||||
| No shared IPA | 13 | 62% | 1.00 (reference) | |
| 1–3 shared IPAs | 278 | 24% | 0.15 (0.06–0.37) | <0.001 |
| 2–3 HLA MM | ||||
| No shared IPA | 36 | 44% | 1.00 (reference) | |
| 1–3 shared IPAs | 473 | 20% | 0.50 (0.27–0.91) | 0.023 |
| Transplant-related mortality in first 3 y posttransplant‡ | ||||
| 1–3 HLA MM, no shared IPA | 49 | 27% | 1.00 (reference) | |
| 1–3 HLA MM, 1–3 shared IPAs | 751 | 48% | 1.68 (0.96–2.94) | 0.071 |
| 0 HLA MM | 45 | 15% | 0.49 (0.18–1.33) | 0.164 |
| 1 HLA MM | ||||
| No shared IPA | 13 | 23% | 1.00 (reference) | |
| 1–3 shared IPAs | 278 | 40% | 1.27 (0.39–4.12) | 0.694 |
| 2–3 HLA MM | ||||
| No shared IPA | 36 | 28% | 1.00 (reference) | |
| 1–3 shared IPAs | 473 | 53% | 1.69 (0.89–3.20) | 0.107 |
| Overall mortality during first 3 y posttransplant§ | ||||
| 1–3 HLA MM, no shared IPA | 49 | 74% | 1.00 (reference) | |
| 1–3 HLA MM, 1–3 shared IPAs | 751 | 69% | 1.01 (0.70–1.45) | 0.968 |
| 0 HLA MM | 45 | 38% | 0.51 (0.27–0.97) | 0.041 |
| 1 HLA MM | ||||
| No shared IPA | 13 | 74% | 1.00 (reference) | |
| 1–3 shared IPAs | 278 | 64% | 0.58 (0.28–1.20) | 0.143 |
| 2–3 HLA MM | ||||
| No shared IPA | 36 | 73% | 1.00 (reference) | |
| 1–3 shared IPAs | 473 | 72% | 1.12 (0.73–1.73) | 0.601 |
| Treatment failure (relapse or death) during first 3 y posttransplant§ | ||||
| 1–3 HLA MM, no shared IPA | 49 | 78% | 1.00 (reference) | |
| 1–3 HLA MM, 1–3 shared IPAs | 751 | 72% | 0.91 (0.64–1.29) | 0.591 |
| 0 HLA MM | 45 | 40% | 0.46 (0.24–0.85) | 0.014 |
| 1 HLA MM | ||||
| No shared IPA | 13 | 85% | 1.00 (reference) | |
| 1–3 shared IPAs | 278 | 65% | 0.44 (0.22–0.85) | 0.015 |
| 2–3 HLA MM | ||||
| No shared IPA | 36 | 76% | 1.00 (reference) | |
| 1–3 shared IPAs | 473 | 75% | 1.06 (0.69–1.63) | 0.778 |
CI, confidence interval; HR, hazard ratio; MM, mismatch.
*Other significant predictors included in the models for acute GVHD were HLA mismatch and direction (1 vs. 2 vs. 3 bidirectional mismatches vs. GVH only vs. Rejection only vs. mixed-direction mismatches), GVHD prophylaxis (cyclosporine+steroids vs. methotrexate vs. tacrolimus vs. other vs. unknown) and transplant center experience with NYBC CB units (non-United States vs. United States <50 patients vs. United States ≥50 patients transplanted) (Table S3). NIMA match was forced into the model even though not significant in this dataset.
†The other significant predictors in the models for relapse were HLA mismatch and direction (1 vs. 2 vs. 3 bidirectional mismatches vs. GVH only vs. rejection only vs. mixed-direction mismatches) IBMTR relapse risk category (early or intermediate stage vs. late stage vs. unknown stage) and diagnosis (ALL vs. AML), GVHD prophylaxis (cyclosporine+steroids vs. methotrexate vs. tacrolimus vs. other vs. unknown) and birth order of the CB donor (first pregnancy vs. second or higher vs. unknown) (Table S3). NIMA match was forced into the model even though not significant in this dataset.
‡The other significant predictors in the models for transplant related mortality were HLA mismatch and direction (1 vs. 2 vs. 3 bidirectional mismatches vs. GVH only vs. Rejection only vs. mixed direction mismatches), age of the patient (0–9 y vs. ≥ 10 y), TNC dose (continuous log transformed), IBMTR relapse risk category (early-intermediate vs. late vs. unknown stage), pretransplant CMV antibody status (negative vs. positive vs. unknown), transplant center experience with NYBC CB units (non-United States vs. United States <50 patients vs. United States ≥50 patients transplanted) and year of transplant (1993–2002 vs. 2003–2006) (Table S3). NIMA match was forced into models even though not significant in this dataset.
§The other significant predictors in the models for overall mortality and treatment failure were HLA mismatch and direction (1 vs. 2 vs. 3 bidirectional mismatches vs. GVH only vs. Rejection only vs. mixed direction mismatches), birth order of the CB donor (first pregnancy vs. second or higher vs. unknown), TNC dose (continuous log transformed), IBMTR relapse risk category (early-intermediate vs. late vs. unknown stage), pretransplant CMV antibody status (negative vs. positive vs. unknown), transplant center experience with NYBC CB units (non-United States vs. United States <50 patients vs. United States ≥50 patients transplanted) and year of transplant (1993–2002 vs. 2003–2006) (Table S3). NIMA match was forced into models even though not significant in this dataset.
Fig. 2.
Probability (cumulative incidence) of GVHD and relapse in the first 3 y posttransplantation among patients with ALL or AML among patients who received a CB unit with one HLA mismatch. (A) GVHD in patients who engrafted. Shared IPA (n = 205), solid black line; 0 HLA mismatch (n = 37), solid green line; no shared IPA (n = 9), dashed black line. (B) Relapse in all patients. Shared IPA (n = 278), solid black line; 0 HLA mismatch (n = 45), solid green line; no shared IPA (n = 13), dashed black line.
Fig. 3.
Probability of treatment failure (inverse of disease-free survival) in the first 3 y posttransplantation among patients with ALL and AML who received CB units with 0 or 1 HLA mismatch. Shared IPA (n = 278), solid black line; 0 HLA mismatch (n = 45), solid green line; no shared IPA (n = 13), dashed black line.
Because no-shared-IPA graft recipients had a greater chance to have received a unit with a higher number of HLA mismatches or unidirectional GVH-only mismatches, we also performed a case-control study in which shared-IPA controls were chosen to match (1:1) the number of HLA mismatches and mismatch direction as closely as possible to the no-shared-IPA cases (Materials and Methods and Table S4). Although this approach diminished the number of patients analyzed, the relationship between presence of shared IPAs and relapse persisted and remained significant (Table S3).
It is also possible that unprimed maternal cells could explain our findings, because transplants with shared IPAs were more likely to have a higher number of mismatches between the CB donor's mother and recipient than did the no-shared-IPA transplants (Table S1). We therefore examined the relationship between GVHD and relapse and the mother's HLA match to the recipient. No significant differences were detected (Table S5). Moreover, when maternal mismatch was forced as a covariate into the multivariate analysis, the association between shared-IPA transplants and reduced relapse risk remained significant [HR = 0.40, 95% confidence interval (CI) = 0.22–0.73, P = 0. 003]. This finding provides additional evidence that the relapse-reducing effect was related to maternal immunity against IPA and not to the level of HLA-mismatch per se of maternal cells.
Finally, to evaluate whether soluble factors such as antibody to IPA could be responsible for the lower relapse rates, we analyzed associations with CB graft-processing methods. CB units used in the no-shared-IPA transplants were more likely than shared-IPA units to have been volume-reduced (88% vs. 76%, P = 0.057). However, volume reduction processing was not associated with an increased risk of relapse (HR = 1.06, 95% CI = 0.74–1.53, P = 0.741).
Discussion
Our study provides compelling evidence that patients who share one or more HLA antigens with their graft's IPAs had a lower risk of relapse after CB transplantation than those without shared antigens. In all probability, microchimeric maternal effector cells with immunity to the CB donor's IPA were responsible for controlling leukemic relapse. Soluble noncellular elements, such as TNF or specific antibodies, seem unlikely explanations because there was no association with volume reduction prefreeze and most CB units transplanted in this study were washed postthaw. The IPA effect was strongest in shared-IPA graft recipients with a single HLA mismatch, even though their rate of acute GVHD was not increased. This result, seemingly at odds with previous observations that increased graft-versus-leukemia effect after HSC transplantation from unrelated adult donors comes with the price of increased GVHD, may be because of the proven effectiveness of fetal T-regulatory cells (14–17).
We found the IPA relapse reducing effect in both ALL and AML but not in other leukemias or other hematological malignancies, perhaps because of their smaller sample sizes, although biological explanations cannot be excluded. The IPA effect on relapse was independent of other HLA associations, including donor-recipient HLA mismatch level, mismatch direction (vector), and matching for NIMA (13, 18, 19). One might expect that maternal exposure to IPAs in previous pregnancies might also contribute to controlling relapse through sensitization to other IPAs or a booster effect with repeated pregnancies. Indeed, our observation that higher birth order of the CB donor was independently associated with a reduced relapse risk fits with this concept and is in agreement with previous studies of HLA identical sibling bone marrow transplantation (20, 21). Most importantly, we demonstrated that the IPA effect was independent of the HLA mismatch between the CB mother and recipient, further supporting our hypothesis that the antileukemia effect was because of microchimeric maternal cells sensitized to fetal IPAs rather than simply to HLA mismatch between the CB donor's mother and the recipient.
CB IPAs could be identified in this study because maternal HLA typing was routine in the NYBC Program. Hence, inherited maternal antigens were known in most cases and IPAs could be deduced (Table 1). Sharing IPAs was not considered in selecting CB units, however. Thus, the prevalence of shared- and no-shared-IPA transplants reported herein (94% and 6%, respectively) should be representative of what might be expected in CB transplantation by chance. Although the number of no-shared-IPA transplants was relatively small, their relapse risk is substantial and, among one HLA mismatched grafts, is reflected in disease-free survival. Hence, avoidance of CB units without shared IPAs is advisable, at least for patients with ALL or AML.
The IPA match effect on relapse was strongest in the group of patients who received a CB with a single HLA mismatch, although also detected in those with two to three HLA mismatches. The greater number of HLA mismatches in these later cases and the ensuing increase in GVHD may also have contributed to the control of relapse, as previously reported, and may, thereby, have diminished differences related to IPA (14, 15). Although CB grafts with three HLA mismatches are now rarely used, the finding that one-quarter of such donor-recipient pairs had no shared IPA (Table S1) is an additional reason to avoid such units. Lack of high-resolution HLA-A and -B typing and of HLA-C, -DQ, and -DP typing leaves identification of shared IPAs and IPA targets ambiguous for many cases. Nevertheless, the evidence we found of an IPA-related relapse-controlling effect was significant and valid at the level of resolution currently used CB unit selection.
To prove that our observations are a result of microchimerism with IPA-primed maternal cells, we would need to identify the cells involved and their mechanisms of action. We have only very incomplete information, for example, about how frequently cellular anti-IPA immunity is induced. Are the responsible cells CD4+ and/or CD8+ T cells directed against HLA class I and II antigens or MHC-restricted mHA, and to what extent are NK cells involved (1–6, 22, 23)? In this regard, it is of interest that almost all patients received calcineurin inhibitors and the majority also received ATG, in contrast to the patients described by Stern et al. (7). If, indeed, primed CD8+ anti-mHA cytotoxic T-lymphocytes (CTLs) are responsible for the relapse-reducing effect, they appear not to be inhibited by calcineurin and are ATG-resistant. A similar finding has been described in solid organ transplantation where the lytic effect of primed CTLs could not be blocked by anti-CD8 and cyclosporine A but that of unprimed CTLs was (24).
It will also be important that we identify the targets of relapse-reducing immunity. It is helpful that our findings have already identified an immunizing agent (CB IPA) and at least one target (HLA antigens that the recipient shares with the CB). HLA molecules may act either by themselves or as restricting elements for mHA, including onco-fetal antigens (4–6, 25, 26). Such studies would remain incomplete, however, if we do not also take into account quantitative aspects of microchimerism (27–29).
The effect of sensitized maternal cells may also be relevant to double CB unit transplants, possibly contributing to unit predominance and to a lower relapse risk and higher incidence of severe GVHD compared with single-unit grafts (30). Further implications may apply to solid-tumor immune therapy (31). No publications thus far have evaluated whether the T cells responsible for clinical success in tumor therapy were autologous T cells primed by immune stimulators, such as anti–CTL-4 antibody, or were chimeric maternal cells, and hence allogeneic, already primed in utero. We suggest future investigations take this question into account. Similarly, both anti-IPA and anti-NIMA immunity might play a role in known associations between pregnancy and autoimmune disease (32, 33).
Scientifically, an intriguing implication of our observation is the possibility that maternal immunity to IPA might not only help control leukemic relapse after CB HSC transplantation but play a role in cancer surveillance in general. This concept is not new. We have known for decades that maternal antibodies protect the child against bacterial infections during the first months of life, and recent influenza vaccination during pregnancy provided evidence that maternal T-cells also protect the newborn against influenza (34). Thus, it is not too farfetched to suggest that maternal anti-IPA immunity might also have a protective effect against cancer in the offspring, as has been suggested by others (35, 36).
Based on evidence presented herein, we conclude that, in addition to TNC dose and currently used HLA match between donor and recipient, shared IPAs and NIMA matching (both of which require maternal HLA typing) should be taken into account in CB unit selection. Hence, we recommend that search algorithms be modified so that CB match reports can include these characteristics to help transplant centers apply our findings.
Materials and Methods
Patients.
Consecutive patients with a hematological malignancy who were transplanted with a single CB unit from the NYBC National Cord Blood Program between 1993 and 2006 were eligible for this study (n = 1,403). Recipients were excluded if they had four HLA mismatches with their grafts (n = 5), the HLA typing was at a lower level than used in this study (n = 14), maternal HLA typing was incomplete (n = 60), or the IPA could not be defined (n = 24). Patients signed informed consent for CB transplantation at the respective transplant centers. The NYBC program operates under an investigational new drug exemption from the US Food and Drug Administration and patients gave consent for CB as their HSC source.
Transplant centers provided data on patient demographics, diagnosis, treatment, and posttransplant events. Transplant and follow-up reports were reviewed for completeness and consistency (by A.S.). Of the 1,300 eligible patients, 1,155 (89%) have had outcome data reported. Patients with ALL, AML, or chronic myeloid leukemia were classified as low, intermediate, or high risk using International Bone Marrow Transplant Registry (IBMTR) criteria (37).
CB Units.
Methods for collecting, testing, processing, freezing, storage, and postthaw processing of CB units have been previously described in detail (11–13, 18, 19, 38). Among the units used by patients in this study, 76% were volume reduced by removing excess plasma and red blood cells. Postthaw processes were reported by transplant centers on 76% of units and 92% of these were washed as recommended (diluted with dextran-albumin followed by centrifugation and removal of supernatant) before infusion.
Assignment of HLA Mismatch and IPA Match.
CB units and donor mothers were typed for HLA-A, -B, and -DRB1 using serological and DNA methods. CB units were selected based on TNC dose and HLA match to the recipient. Match grades were assigned at low-intermediate resolution level (including splits of broad antigens) for HLA-A and -B, and at high resolution (allele level) for DRB1 and are expressed as 0, 1, 2, or 3 antigen mismatched. HLA mismatch direction and NIMA match was also determined for each donor-recipient pair, as previously reported (11–13, 18, 19, 38). HLA-A, -B, and -DRB1 typing was adequate on all except 59 mothers to assign the number of mismatches between the CB donor's mother and the unit recipient. Because of small numbers, we analyzed the maternal mismatches in four categories: zero to two, three, four, and five to six.
Each donor-recipient pair was evaluated retrospectively to identify CB HLA-A, -B, or -DRB1 IPAs that might sensitize maternal cells (i.e., be a target for maternal cells) and whether any antigens identified as IPAs were also present in the recipient. At a given locus, if the recipient had the same antigen as the CB IPA, the locus was considered to have a shared IPA. When the recipient did not have the same antigen as the donor's IPA (had either a different antigen or was homozygous for the CB IMA), the locus was considered to have no shared IPA. In addition, if the CB unit was homozygous or was HLA matched to both of the mother's antigens at a given locus there would be no IPA target to induce sensitization in maternal cells. These loci with no IPA target also were classified as having no shared IPA. Note that high-resolution typing at the HLA-A and -B loci (not available in this study) might identify alleles that differ between the CB unit and patient, changing a shared IPA to no shared IPA and vice versa.
A total of 64 donor-recipient pairs with one to three HLA mismatches had no shared IPA or no IPA target for all three loci and were classified as no-shared-IPA transplants. All remaining 1,030 recipients of HLA mismatched CB units had one or more shared antigens with the donor's IPAs and were classified as shared-IPA transplants. The number of loci with a shared IPA could be defined in 974 pairs; 56 pairs had at least one shared IPA but the total number shared was uncertain, mainly because of lack of high-resolution HLA-DRB1 typing in the mother. All pairs with zero HLA mismatches that could be evaluated for IPAs (56 of 61) had one or more shared IPA, most (61%) at all three loci.
Case-Control Study.
Because no-shared-IPA transplants tended to have more HLA mismatches and a higher proportion in the GVH-only direction, we performed a case-control study of patients with ALL or AML, selecting shared-IPA transplant controls (selected 1:1) to match as closely as possible for three HLA match characteristics: the number of HLA mismatches, direction of HLA mismatch and match between the donor and CB NIMAs. To balance this small data subset for relapse risk, when there was more than one potential control, we further selected controls to match as closely as possible for the four risk factors associated with relapse: diagnosis, stage of disease, GVHD prophylaxis, and CB donor birth order (Table S4). If there was still more than one possible control, we chose the one with the closest transplant date to the case. Among shared-IPA transplants, we could identify 45 as controls that matched a case for all three HLA characteristics: 9 of these also matched for all four risk factors, 17 for three, and 19 for two. Controls for the other four cases could be matched for only two HLA characteristics: two for all four risk factors, one for three risk factors, and one for two.
Transplant Outcome Endpoints.
Definitions of study endpoints followed standard conventions (12–15, 18, 19, 38). Analyses of acute GVHD among patients who engrafted were based on the grade assigned by the transplant center. Analysis of chronic GVHD was confined to patients who engrafted and survived to at least day 100 posttransplantation. TRM was defined as any death when the patient was in remission and overall mortality was death from any cause. Treatment failure was defined as death or relapse, whichever came first.
Data Analysis and Statistical Methods.
Differences between categorical variables were estimated by χ2 or Fisher's exact test and between means by the Student t test (all two-tailed). The probabilities of death and treatment failure were calculated by the Kaplan–Meier method (39). For endpoints that had competing outcomes, event-specific hazard functions and free-from-event survival rates were calculated to obtain event-specific cumulative incidence rates (40, 41). For myeloid and platelet engraftment, death was the competing event. For GVHD, the competing outcomes were graft failure (documented autologous reconstitution or subsequent transplant following CB graft failure) and death. Relapse was the competing outcome for TRM and death was the competing outcome for relapse. The Cox proportional hazard model was used to estimate hazard ratios for outcome endpoints in uni- and multivariate analyses (42). A stepwise backward regression model was applied, with HLA match variables that were the focus of this analysis (CB unit-recipient HLA mismatch and mismatch direction, NIMA match, shared- vs. no-shared-IPA and, for some analyses, CB mother-recipient HLA mismatch) forced to remain in the model. Analyses were conducted using SPSS, version 19.0.
Supplementary Material
Acknowledgments
We thank the Transplant Centers who reported on patients and their outcome posttransplantation and the New York Blood Center National Cord Blood Program staff who performed all of the tasks needed to ensure the quality of the CB units; the obstetricians in collaborating hospitals who supported the program and the mothers who generously donated their infant's cord blood to any patient who might need it; Carmelita Carrier and Carol Carpenter for supervising all maternal HLA typings used for the study; Fritz Bach (deceased), Machteld Oudshoorn, Anneke Brand, Monique Joris, and Frans Claas for their constructive criticisms; and Pablo Rubinstein for granting access to the data. The Program was supported by grants from the US Public Health Service, National Heart, Lung, and Blood Institute, and the Starr Foundation. This work was also supported by the Macropa Foundation.
Footnotes
The authors declare no conflict of interest.
2Retired.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1119541109/-/DCSupplemental.
See Commentary on page 2190.
References
- 1.van Rood JJ, Eernisse JG, van Leeuwen A. Leucocyte antibodies in sera from pregnant women. Nature. 1958;181:1735–1736. doi: 10.1038/1811735a0. [DOI] [PubMed] [Google Scholar]
- 2.Payne R, Rolfs MR. Fetomaternal leukocyte incompatibility. J Clin Invest. 1958;37:1756–1763. doi: 10.1172/JCI103768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouma GJ, et al. Pregnancy can induce priming of cytotoxic T lymphocytes specific for paternal HLA antigens that is associated with antibody formation. Transplantation. 1996;62:672–678. doi: 10.1097/00007890-199609150-00023. [DOI] [PubMed] [Google Scholar]
- 4.van Kampen CA, Versteeg-vd Voort Maarschalk MF, Langerak-Langerak J, Roelen DL, Claas FH. Kinetics of the pregnancy-induced humoral and cellular immune response against the paternal HLA class I antigens of the child. Hum Immunol. 2002;63:452–458. doi: 10.1016/s0198-8859(02)00396-8. [DOI] [PubMed] [Google Scholar]
- 5.Verdijk RM, et al. Pregnancy induces minor histocompatibility antigen-specific cytotoxic T cells: Implications for stem cell transplantation and immunotherapy. Blood. 2004;103:1961–1964. doi: 10.1182/blood-2003-05-1625. [DOI] [PubMed] [Google Scholar]
- 6.van Halteren AG, et al. Naturally acquired tolerance and sensitization to minor histocompatibility antigens in healthy family members. Blood. 2009;114:2263–2272. doi: 10.1182/blood-2009-01-200410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stern M, et al. Survival after T cell-depleted haploidentical stem cell transplantation is improved using the mother as donor. Blood. 2008;112:2990–2995. doi: 10.1182/blood-2008-01-135285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tamaki S, et al. Japan Society of Hematopoietic Cell Transplantation. Superior survival of blood and marrow stem cell recipients given maternal grafts over recipients given paternal grafts. Bone Marrow Transplant. 2001;28:375–380. doi: 10.1038/sj.bmt.1703146. [DOI] [PubMed] [Google Scholar]
- 9.Gluckman E, et al. Hematopoietic reconstitution in a patient with Fanconi's anemia by means of umbilical-cord blood from an HLA-identical sibling. N Engl J Med. 1989;321:1174–1178. doi: 10.1056/NEJM198910263211707. [DOI] [PubMed] [Google Scholar]
- 10.Broxmeyer HE, Cooper S, Yoder M, Hangoc G. Human umbilical cord blood as a source of transplantable hematopoietic stem and progenitor cells. Curr Top Microbiol Immunol. 1992;177:195–204. doi: 10.1007/978-3-642-76912-2_15. [DOI] [PubMed] [Google Scholar]
- 11.Rubinstein P. Placental blood-derived hematopoietic stem cell for unrelated bone marrow reconstitution. J Hemother. 1993;2:207–210. doi: 10.1089/scd.1.1993.2.207. [DOI] [PubMed] [Google Scholar]
- 12.Rubinstein P, et al. Processing and cryopreservation of placental/umbilical cord blood for unrelated bone marrow reconstitution. Proc Natl Acad Sci USA. 1995;92:10119–10122. doi: 10.1073/pnas.92.22.10119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Rood JJ, et al. Reexposure of cord blood to noninherited maternal HLA antigens improves transplant outcome in hematological malignancies. Proc Natl Acad Sci USA. 2009;106:19952–19957. doi: 10.1073/pnas.0910310106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horowitz MM, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75:555–562. [PubMed] [Google Scholar]
- 15.Eapen M, et al. Outcomes of transplantation of unrelated donor umbilical cord blood and bone marrow in children with acute leukaemia: A comparison study. Lancet. 2007;369:1947–1954. doi: 10.1016/S0140-6736(07)60915-5. [DOI] [PubMed] [Google Scholar]
- 16.Mold JE, et al. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science. 2008;322:1562–1565. doi: 10.1126/science.1164511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mold JE, et al. Fetal and adult hematopoietic stem cells give rise to distinct T cell lineages in humans. Science. 2010;330:1695–1699. doi: 10.1126/science.1196509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barker JN, Scaradavou A, Stevens CE. Combined effect of total nucleated cell dose and HLA match on transplantation outcome in 1061 cord blood recipients with hematologic malignancies. Blood. 2010;115:1843–1849. doi: 10.1182/blood-2009-07-231068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stevens CE, Carrier C, Carpenter C, Sung D, Scaradavou A. HLA mismatch direction in cord blood transplantation: Impact on outcome and implications for cord blood unit selection. Blood. 2011;118:3969–3978. doi: 10.1182/blood-2010-11-317271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bucher C, et al. Role of primacy of birth in HLA-identical sibling transplantation. Blood. 2007;110:468–469. doi: 10.1182/blood-2007-02-076257. [DOI] [PubMed] [Google Scholar]
- 21.Gratwohl A, et al. Birth order and outcome after HLA-identical sibling donor transplantation. Blood. 2009;114:5569–5570. doi: 10.1182/blood-2009-10-249060. [DOI] [PubMed] [Google Scholar]
- 22.Marijt WAE, et al. Hematopoiesis-restricted minor histocompatibility antigens HA-1- or HA-2-specific T cells can induce complete remissions of relapsed leukemia. Proc Natl Acad Sci USA. 2003;100:2742–2747. doi: 10.1073/pnas.0530192100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Willemze R, et al. Eurocord and of the European Group of Blood and Marrow Transplantation. Is there an impact of killer cell immunoglobulin-like receptors and KIR-ligand incompatibilities on outcomes after unrelated cord blood stem cell transplantation? Best Pract Res Clin Haematol. 2010;23:283–290. doi: 10.1016/j.beha.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 24.Roelen DL, et al. Cytotoxic T lymphocytes against HLA-B antigens are less naive than cytotoxic T lymphocytes against HLA-A antigens. Transplantation. 1994;57:446–450. doi: 10.1097/00007890-199402150-00023. [DOI] [PubMed] [Google Scholar]
- 25.Mommaas B, et al. Cord blood comprises antigen-experienced T cells specific for maternal minor histocompatibility antigen HA-1. Blood. 2005;105:1823–1827. doi: 10.1182/blood-2004-07-2832. [DOI] [PubMed] [Google Scholar]
- 26.Zelle-Rieser C, et al. Expression and immunogenicity of oncofetal antigen-immature laminin receptor in human renal cell carcinoma. J Urol. 2001;165:1705–1709. [PubMed] [Google Scholar]
- 27.Scaradavou A, Carrier C, Mollen N, Stevens C, Rubinstein P. Detection of maternal DNA in placental/umbilical cord blood by locus-specific amplification of the noninherited maternal HLA gene. Blood. 1996;88:1494–1500. [PubMed] [Google Scholar]
- 28.Nelson JL. Maternal-fetal immunology and autoimmune disease: Is some autoimmune disease auto-alloimmune or allo-autoimmune? Arthritis Rheum. 1996;39:191–194. doi: 10.1002/art.1780390203. [DOI] [PubMed] [Google Scholar]
- 29.Bianchi DW, et al. Significant fetal-maternal hemorrhage after termination of pregnancy: Implications for development of fetal cell microchimerism. Am J Obstet Gynecol. 2001;184:703–706. doi: 10.1067/mob.2001.111072. [DOI] [PubMed] [Google Scholar]
- 30.Sideri A, et al. An overview of the progress on double umbilical cord blood transplantation. Haematologica. 2011;96:1213–1220. doi: 10.3324/haematol.2010.038836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Callahan MK, Wolchok JD, Allison JP. Anti-CTLA-4 antibody therapy: Immune monitoring during clinical development of a novel immunotherapy. Semin Oncol. 2010;37:473–484. doi: 10.1053/j.seminoncol.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feitsma AL, et al. Protective effect of noninherited maternal HLA-DR antigens on rheumatoid arthritis development. Proc Natl Acad Sci USA. 2007;104:19966–19970. doi: 10.1073/pnas.0710260104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nelson JL, et al. Maternal microchimerism in peripheral blood in type 1 diabetes and pancreatic islet beta cell microchimerism. Proc Natl Acad Sci USA. 2007;104:1637–1642. doi: 10.1073/pnas.0606169104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eick AA, et al. Maternal influenza vaccination and effect on influenza virus infection in young infants. Arch Pediatr Adolesc Med. 2011;165:104–111. doi: 10.1001/archpediatrics.2010.192. [DOI] [PubMed] [Google Scholar]
- 35.Klonisch T, Drouin R. Fetal-maternal exchange of multipotent stem/progenitor cells: Microchimerism in diagnosis and disease. Trends Mol Med. 2009;15:510–518. doi: 10.1016/j.molmed.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 36.Ichinohe T. Long-term feto-maternal microchimerism revisited: Microchimerism and tolerance in hematopoietic stem cell transplantation. Chimerism. 2010;1:39–43. doi: 10.4161/chim.1.1.12743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Szydlo R, et al. Results of allogeneic bone marrow transplants for leukemia using donors other than HLA-identical siblings. J Clin Oncol. 1997;15:1767–1777. doi: 10.1200/JCO.1997.15.5.1767. [DOI] [PubMed] [Google Scholar]
- 38.Rubinstein P, et al. Outcomes among 562 recipients of placental-blood transplants from unrelated donors. N Engl J Med. 1998;339:1565–1577. doi: 10.1056/NEJM199811263392201. [DOI] [PubMed] [Google Scholar]
- 39.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 40.Klein JP, Moeschberger ML. Survival Analysis: Techniques of Censored and Truncated Data. New York, NY: Springer-Verlag; 2003. [Google Scholar]
- 41.Fine J, Gray R. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 42.Cox DR. Regression models and life tables. J R Stat Soc [Ser A] 1972;34:187–220. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.