Abstract
Globins constitute a superfamily of proteins widespread in all kingdoms of life, where they fulfill multiple functions, such as efficient O2 transport and modulation of nitric oxide bioactivity. In plants, the most abundant Hbs are the symbiotic leghemoglobins (Lbs) that scavenge O2 and facilitate its diffusion to the N2-fixing bacteroids in nodules. The biosynthesis of Lbs during nodule formation has been studied in detail, whereas little is known about the green derivatives of Lbs generated during nodule senescence. Here we characterize modified forms of Lbs, termed Lbam, Lbcm, and Lbdm, of soybean nodules. These green Lbs have identical globins to the parent red Lbs but their hemes are nitrated. By combining UV-visible, MS, NMR, and resonance Raman spectroscopies with reconstitution experiments of the apoprotein with protoheme or mesoheme, we show that the nitro group is on the 4-vinyl. In vitro nitration of Lba with excess nitrite produced several isomers of nitrated heme, one of which is identical to those found in vivo. The use of antioxidants, metal chelators, and heme ligands reveals that nitration is contingent upon the binding of nitrite to heme Fe, and that the reactive nitrogen species involved derives from nitrous acid and is most probably the nitronium cation. The identification of these green Lbs provides conclusive evidence that highly oxidizing and nitrating species are produced in nodules leading to nitrosative stress. These findings are consistent with a previous report showing that the modified Lbs are more abundant in senescing nodules and have aberrant O2 binding.
Globins constitute a superfamily of proteins widespread in bacteria, protozoa, fungi, plants, and animals (1). Not surprisingly, they are structurally and functionally diverse. Flavohemoglobins of bacteria and yeast are chimeric Hbs with heme and FAD reductase domains, and are involved in nitric oxide (NO•) metabolism because of their high NO• dioxygenase activity (2). In humans and other vertebrates, Hb and Mb play key roles in efficient O2 transport and storage but are also involved in NO• homeostasis, whereas the recently discovered neuroglobin and cytoglobin might assist in O2 transport to the mitochondria and act as NADH oxidases and O2 sensors (2, 3). Plants contain up to three types of Hbs: symbiotic, nonsymbiotic, and truncated. Symbiotic Hbs, which include leghemoglobins (Lbs) of legumes and some Hbs of actinorhizal plants, scavenge O2 and facilitate its diffusion to the N2-fixing microbial symbionts in nodules (4). Nonsymbiotic Hbs are further classified into two groups based on phylogeny and O2-binding properties. Class 1 Hbs are expressed at ∼100 nM in most plant tissues, display extremely high affinity for O2, and participate in NO• metabolism and in the maintenance of cell energetics under hypoxia (5–7), whereas class 2 Hbs have similar O2 affinities to Lbs but unknown function (8). Plant truncated Hbs resemble their bacterial counterparts in having a 2/2 helical sandwich secondary structure instead of the canonical 3/3 structure of other Hbs, and have not yet been assigned any role (1).
Legume nodules are an interesting model to study Hb function and regulation as they express the three types of plant globins (9). Specifically, Lbs are present at concentrations of 2–3 mM and maintain a free O2 concentration of 20–40 nM in the cytosol of host cells (10). This range of O2 concentration permits an adequate supply of ATP for N2 fixation but avoids nitrogenase inactivation (4). In nodules, Lbs are usually found as multiple components, the relative proportions of which vary with age (11). In soybean nodules, there are four major components (a, c1, c2, c3), encoded by different genes, and four minor components (b, d1, d2, d3), originated by posttranslational modification (11, 12). Considerable progress has been made on elucidating the regulatory pathways of Lb biosynthesis (13, 14, and references therein), whereas the mechanisms implicated in its degradation are virtually unknown. In animals and plants, the conversion of heme to biliverdins is catalyzed by heme oxygenase (15, 16), but can be carried out also nonenzymatically (coupled oxidation) at pH 7.5 in the presence of ascorbate and O2 (17, 18). In plants, biliverdin-like pigments perform important functions in photosynthesis and photomorphogenesis (15) and are also associated with a decrease of N2 fixation activity and Lb content in senescent nodules (17, 19). Legume nodule senescence, whether natural or stress-induced, is a complex and poorly studied process, with potential agricultural and ecological relevance as it limits the functional lifespan of nodules, and thereby N2 fixation (19–21).
The green proteins derived from Lb in nodules have not yet been characterized. More than 60 y ago, Virtanen and Laine (17) reported the presence in legume nodules of a green pigment similar to animal choleglobin, and proposed that it was generated from Lb through the breaking of the tetrapyrrole ring without the release of Fe. Much more recently, a different type of green proteins was isolated from soybean nodules (22). The “modified” proteins, termed Lbam and Lbcm, derive from Lba and Lbc (22). Spectroscopic analysis of Lbam, purified by isoelectric focusing (IEF), revealed that this protein has an amino acid sequence identical to Lba but an unknown alteration of the tetrapyrrole ring (22, 23). Identification of the heme modifications in Lbam and Lbcm is important because they are increasingly produced during nodule senescence and exhibit aberrant binding to O2 (24). In this article we show that soybean Lbam and Lbcm have a 4-nitrovinyl in their heme groups, and that these modified hemoproteins can be reproducibly generated in vitro by exposure of functional Lba and Lbc to nitrite (NO2−) via reactive nitrogen species (RNS). This finding reveals that Lbs are a target of nitration in vivo and demonstrates the production of powerful oxidant and nitrating species in nodules, particularly during senescence.
Results
Purification and Identification of Lb Components and Modified Forms.
The major Lb components and their derivatives were purified from soybean nodules by ammonium sulfate fractionation followed by several chromatographic steps (22). Fractions containing Lbs were further purified by IEF using a narrow range of pH, which allowed us to separate Lba, Lbc1, and Lbc2+c3 from the corresponding green derivatives. It was not possible to fully resolve Lbc2 and Lbc3, as their pI values differ by only 0.01 units (11), and the same problem was encountered with the respective modified forms Lbc2m and Lbc3m. To confirm the identification of Lbs and their modified forms and to detect possible chemical modifications in the polypeptides, all bands containing Lbs were carefully excised from the IEF gels and the proteins were eluted and analyzed by MALDI-TOF/MS. The molecular masses of the apoproteins of Lba, Lbc1, Lbc2, and Lbc3, as well as those of their respective modified forms, were found to be 15,241, 15,256, 15,393, and 15,451 Da, respectively, which matched ± 1 Da those predicted from the amino acid sequences excluding the initial Met. The lack of this Met residue is common in Lbs, which usually have Gly or Val at the N terminus (25). We also purified two fractions containing the Lbd and Lbdm components. The molecular masses of the apoproteins of Lbd1, Lbd2, and Lbd3 were found to be 15,299, 15,436, and 15,492 Da, which exceed by 42 ± 1 Da those of Lbc1, Lbc2, and Lbc3, respectively. This mass difference was in agreement with the presence of an N-terminal acetylation as confirmed by MALDI-TOF peptide mass fingerprinting of the tryptic digests. As occurred for the other Lb-modified forms, the apoproteins of the Lbdm derivatives have identical molecular masses to those of the parent proteins. We thus conclude that all four minor Lb components of soybean arise from the major components by N-terminal acetylation, and that all of the green Lb derivatives are affected in the hemes and not in the globins.
Structural Elucidation of Modified Hemes.
Purified Lba and Lbcm from soybean nodules were used for comparative structural analyses of the protoheme and the modified heme (Fig. S1) by using UV-visible, MS, NMR, and resonance Raman (RR) spectroscopies. In some cases, Lbc was also used as a model because the spectral properties of Lba, Lbc1, and Lbc2+c3 are almost identical. The major features of the UV-visible spectra of Lba and Lbcm, as well as those of some representative complexes, are shown in Table S1. In sharp contrast to the ferric form of typical Lbs, ferric Lbcm exhibits a Soret band at 389 nm with a shoulder at 436 nm, and a charge-transfer absorption band at 615 nm. The pyridine hemochrome spectrum of Lbcm was identical to that of Lbam (23), exhibiting prominent absorption bands at 553 nm (α-band) and 522 nm (β-band) and a new peak at 580 nm. However, the spectra of the deoxyferrous forms or of the ferrous complexes with NO• or nicotinate were similar for Lba and Lbcm. These data indicate that the heme of Lbcm is not cleaved and still retains the capacity to bind ligands, but also that it is chemically affected on the tetrapyrrole ring itself or on the vinyl groups.
To determine precisely the nature of the modification, heme structures were exhaustively analyzed by MSn fragmentation with microelectrospray ionization-linear ion trap and with Fourier transform-ion cyclotron mass spectrometers. Initial analyses were performed on the isolated modified hemes but they were relatively unstable. Consequently, the whole proteins were directly subjected to MS analysis, which was optimized for maximal yield of the heme molecular ions. The hemes of Lba, Lbc, and Lbd had a m/z 616, as expected for protoheme, whereas those from Lbam, Lbcm, and Lbdm had a m/z 661. High-resolution MS of these molecular ions proved that the 45-Da difference was a result of the insertion of a NO2 group (Table S2). The molecular ions were extensively fragmented (MS2 to MS4) and the elemental compositions of the most relevant fragments elucidated by high-resolution MS. These analyses revealed that one propionic group, at least the α-carbon and carboxyl of the other propionic group, and at least three Me groups of the tetrapyrrole, were intact in the modified hemes (Table S2). Notably, the fragmentation patterns up to MS4 of the Lbam, Lbcm, and Lbdm hemes were identical, thus confirming, together with the UV-visible spectroscopy data, that all of them bear a NO2 group.
Further structural information on the modified hemes was obtained by 1H NMR spectroscopy using the ferric-cyano forms of the unmodified Lba and the modified Lbcm proteins, rather than the free hemes, to avoid problems encountered with instability and artifactual chemical alteration during heme isolation from Lbcm. As a standard for comparison, the Lba sample was found to have a 1D 1H NMR spectrum with identical proton signals to that already published (26), but with assignments, made via the Water-Eliminated Fourier Transform-Nuclear Overhauser and Exchange SpectroscopY (WEFT-NOESY) spectrum (Fig. S2), which showed the heme to be reversed in vinyl substituent placement within the protein, as reported for the nicotinate complex (27). All resonances of the Lba heme were assigned except for those of the 4-vinyl group and meso-γ-H (Table S3), the resonances of which were buried in the protein resonance region. Because the sample of Lbcm protein was relatively small and composed of a mixture of Lbc1m and Lbc2m+c3m (see above), it was not possible to assign as many of the heme resonances of these Lbcm isoproteins (Fig. S3). The chemical shifts of all of the heme Me resonances of the two major species of the Lbcm sample were changed by the heme modification, in part because the greater size of the modified substituent changed the heme seating by about 7° (28). The chemical shifts of the 2-vinyl group and the 6-propionate α- and β-protons were not significantly modified, nor were those of the 7-propionate α-protons. The 7β protons could not be unambiguously assigned in this dilute sample; however, because the 7α protons do not show large chemical shifts relative to those of Lba, the modification cannot be at the 7β carbon. Thus, the modification of the heme appears to be at the 4-vinyl substituent. Unfortunately, none of the protons of the 4-vinyl group of Lbcm could be identified, and thus our study was complemented with RR spectroscopy and reconstitution experiments.
The RR spectra of ferric Lba and Lbcm are compared in Fig. S4. The mode notations and the band assignments are based on refs. 29–31. The high-frequency regions of RR spectra reveal the binding of a NO2 group to the protoheme of Lbcm, with a signature at 1,320 cm−1, specific of a nitroaromatic group (30). In the mid-frequency and low-frequency RR spectra, the frequencies of modes involving the peripheral vinyl and Me groups are significantly modified, further indicating binding of a NO2 group to a vinyl (29, 31). In-plane and out-of-plane porphyrin modes show changes in frequency in accordance with an increased porphyrin distortion upon nitration (32). This increased protoheme distortion likely originates from a minimization of the steric contacts between the nitrovinyl and its adjacent Me groups.
Reconstitution of Lbs with Mesoheme and in Vitro Nitration.
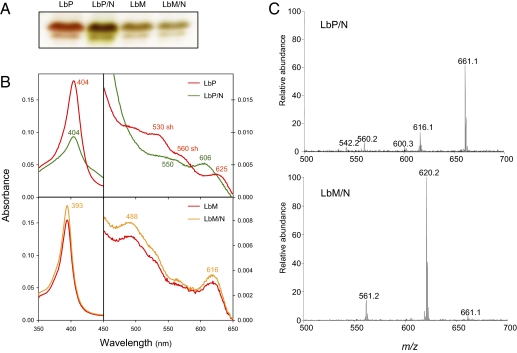
The possibility that the nitrated heme originated by a substitution of a proton by NO2 on a vinyl group was suggested by previous work on nitriMb (33) and nitriHb (34, 35). These green Mb and Hb derivatives contain a 2-nitrovinyl group and are generated in vitro by exposing the proteins to excess NO2−. Thus, we prepared apoLba and apoLbc, reconstituted the holoproteins with protoheme or mesoheme (heme with ethyl groups replacing vinyls), and attempted to nitrate them (Fig. 1). The apoLb reconstituted with protoheme yielded green protein products having modified visible spectra and heme groups with m/z 661, which had identical fragmentation patterns to the hemes of nitriMb, Lbam, Lbcm, and Lbdm. In contrast, the apoLb reconstituted with mesoheme remained unaffected after the NaNO2 treatment, based on the IEF, Soret and visible spectra, RR, and MS analyses of the protein. The MS analysis showed a molecular ion of m/z 620, characteristic of the Fe-mesoporphyrin lacking NO2 (Fig. 1). Taking these results together with the MS, NMR, and RR data, we conclude that the NO2 group of the modified Lb hemes is on the 4-vinyl and that several structural isomers are produced by nitration of the protoheme. To substantiate the presence of several isomers of Lb hemes, Lba purified from soybean nodules was nitrated with NaNO2 at pH 7.0 or 5.5 at room temperature and the resulting proteins were resolved on preparative IEF gels (Fig. 2). Nitration was faster at pH 5.5 than at pH 7.0, being completed within ∼1 and ∼2 d, respectively, when ∼200 μM Lb and ∼200 mM NO2− were used. At pH 5.5, heme nitration required ∼3 d to complete with ∼20 mM NO2− and was far from completion after 5 d with ∼2 mM NO2−.
Fig. 1.
In vitro reconstitution and nitration of Lb. (A) ApoLbc was reconstituted with either protoheme (LbP) or mesoheme (LbM) and treated for 24 h at pH 6.5 with a 1,000-fold excess of NaNO2. The products (LbP/N and LbM/N) were loaded on an analytical IEF gel and let to proceed until separation of Lbc1 (Upper band) and Lbc2+c3 (Lower band). Green nitrated derivatives were formed from the Lb bearing heme with vinyls (LbP/N) and not from the Lb bearing heme with ethyl groups (LbM/N). (B) Soret and visible spectra of aliquot samples of the proteins loaded on the gel. Note that LbM and LbM/N have identical spectra, whereas LbP/N is being converted to green derivatives, with a Soret band of lower intensity and a hypsochromic shift of the 625-nm charge transfer absorption band. (C) Mass spectra of the hemes from Lba reconstituted with protoheme or mesoheme and then nitrated. Note the absence of nitration (m/z 620) in the mesoheme. Experiments shown in A and B were repeated three times and the experiment shown in C was repeated twice, each with a different apoLb preparation, producing identical results.
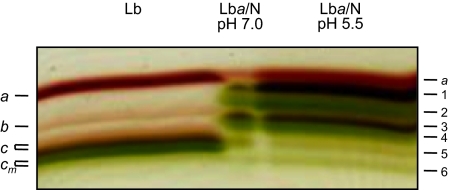
Fig. 2.
Nitration of Lba and separation of the nitrated products on preparative IEF gels. (Left) Mixture of Lba, Lbb, Lbc, and Lbcm standards. The two Lbc protein bands correspond to Lbc1 and Lbc2+c3. (Right) Lba (500 μM) purified from soybean nodules was nitrated with NaNO2 (500 mM) for 48 h in citrate buffer (pH 5.5), yielding six derivatives (LbaN1 to LbaN6). (Center) A similar pattern of LbaN derivatives was obtained when nitration was performed in phosphate buffer (pH 7.0). These experiments were repeated at least twice with identical results.
Typically, six Lba derivatives were produced, four of which (LbaN2, LbaN4, LbaN5, and LbaN6) were green (Fig. 2). LbaN6 was low abundant and could not be studied further. All other derivatives had pyridine hemochromes, with a 580-nm band that is absent in unmodified Lbs (Table S1). The ferric aquo forms had Soret bands at 391–403 nm, with shoulders at 433–436 nm, as well as a charge-transfer band at 615 nm. The Soret bands of LbaN4 and LbaN5 showed the closest match to those of Lbam or Lbcm (Table S1). This similarity was confirmed by RR spectroscopy (Fig. S5). Based on the relative intensity of the bands at ∼1,320 and 1,373 cm−1, the spectrum of the LbaN4 is the closest one to that of Lbcm. Furthermore, in the 1,400–1,700 cm−1 region, the spectra of Lbcm and LbaN4 were most similar in terms of band shape and frequency. This similarity was also found in the mid- and low-frequency regions. Thus, we conclude that LbaN4 has an identical modified heme to Lbam or Lbcm.
All LbaN derivatives had hemes with a m/z 661 and identical high-order fragmentation profiles. Similarly, all of the apoLbaN derivatives were found to have a molecular mass of 15,240 ± 1 Da, as determined by MALDI-TOF/MS, and hence do not bear any modification in their amino acid residues. Consequently, the in vitro nitration of Lbs with excess NO2− can reproducibly generate the modified Lbs found in nodules, as well as several isomers of nitrated hemes.
Involvement of RNS in Heme Nitration.
Both NO2− and NO• are unable to directly nitrate proteins, whereas other RNS derived therefrom can do it in vitro and presumably in vivo. These oxidant and nitrating RNS include peroxynitrite (ONOO−), nitrogen dioxide radical (NO2•), and nitronium (NO2+) salts (36–38). Experiments were carried out with recombinant or purified soybean Lba and with equine Mb for comparative purposes to elucidate the nature of the RNS and the pathways involved in heme nitration. This task is complicated because ONOO−, when present as peroxynitrous acid (ONOOH, pKa= 6.8), can undergo homolytic cleavage to NO2• and hydroxyl radical (•OH), and because nitrous acid (HNO2, pKa= 3.2) can give rise to NO2+ (Fig. 3). We obtained similar results with Mb and Lb. Addition of 10 mM cyanide, a strong ligand of both hemoproteins, completely prevented nitration, indicating that the reaction involves the heme Fe. To examine whether ONOO− was the nitrating agent, we used 3-morpholinosydnonimine hydrochloride (SIN-1) because synthetic ONOO− is a very short-lived molecule in buffered solutions. Spontaneous decomposition of SIN-1 yields NO• and superoxide anion radicals (O2−•), which react with each other to form ONOO−, and thus SIN-1 can mimic a slow exposure of the protein to ONOO− (Fig. 3). Incubation of Mb or Lba with 0.5–1 mM SIN-1 at pH 5.5 or 7.0 for up to 4 h did not nitrate the heme, excluding any contribution of free ONOO− to nitration. Likewise, an exogenous supply of superoxide dismutase (50–100 μg) or catalase (50–100 μg) did not prevent nitration, and therefore production of O2−• radicals or H2O2 outside the protein is not involved in the reaction. Addition of 30–100 μM H2O2 did not promote nitration, confirming that peroxide is apparently not required. In contrast, incubation of Mb or Lba with 1 mM desferrioxamine (DFO) for 2–48 h inhibited nitration substantially (Fig. S6). DFO is a natural Fe chelator commonly used to establish the dependence of biological reactions on free Fe2+/3+ ions, but can also intercept free radicals (39). To gain information on the inhibitory effect of DFO and the role of metals on Lba nitration, we used ferrioxamine (1 mM), prepared by equimolar mixing of DFO and Fe3+ ions, and two powerful metal chelators, diethylenetriamine pentaacetic acid (1 mM) and Chelex resin (5 mg). Neither FO (Fig. S6) nor the other two compounds had any effect on heme nitration when added to the hemoprotein before NO2−. Therefore, free-metal ions are not required for the reaction and DFO needs to have the hydroxamic moities unblocked to inhibit nitration, which is consistent with the high affinity binding of DFO for heme (40).
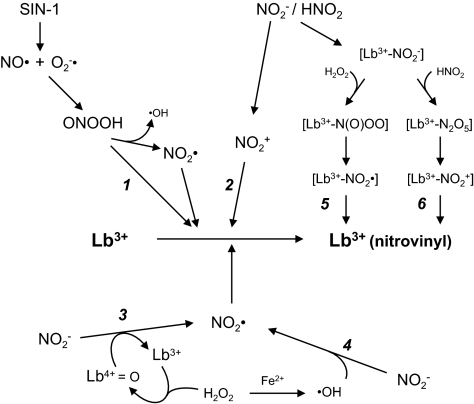
Fig. 3.
Mechanisms that may be operative in the nitration of Tyr residues and heme groups of hemoproteins. The pathways have been exemplified for Lb but are also extensive to Mb and Hb. Some intermediates are indicated in square brackets only to mean that they are formed inside the heme pocket but, except for the nitrite and peroxynitrite complexes, are not necessarily bound to the heme. Experiments designed to test these pathways are described in the text. Additional abbreviations: Lb3+, ferric Lb; Lb4+=O, ferryl Lb; Lb3+(nitrovinyl), ferric Lb bearing a vinyl-bound NO2 group in the heme.
Discussion
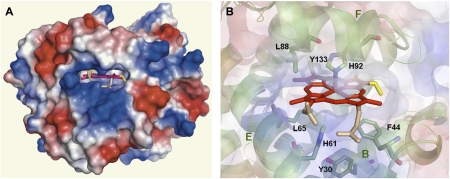
Green pigments and nitrated derivatives have been generated in vitro from animal and plant hemoproteins. Thus, human and equine Mb can be nitrated in the heme and in the Tyr103 and Tyr146 residues, depending on the Mb source and on the relative concentrations of NO2− and H2O2 (33, 41). In the case of plant hemoproteins, HRP was found to be nitrated on the vinyl groups (42) and a green derivative of Lb has been produced by oxidative reaction with H2O2 (43). The latter authors surmised that the green Lb species was formed at least in part by heme-globin cross-linking. We failed to detect similar compounds in vivo but found instead that the green Lbs of soybean originated by nitration of the heme. Furthermore, spectroscopic and reconstitution analyses of the hemoprotein revealed that the NO2 group is on the 4-vinyl (Fig. S1). The modified Lbs were reproducibly synthesized in vitro by exposing the proteins to excess NO2−. These findings are fully consistent with a recent study showing that nitration of HRP heme occurs preferentially on the 4-vinyl rather than on the 2-vinyl (42). Nevertheless, nitriMb and nitriHb are nitrated on the 2-vinyl (33–35), suggesting that multiple isomers can be formed during nitration and that the vinyl group that is preferentially nitrated may be predicted by its relative availability to nitration reagents within the heme pocket. The regiospecificity of Lb heme nitration is indeed consistent with the crystal structure of soybean Lba (44), which shows a greater accessibility of the 4-vinyl relative to the 2-vinyl when considering both surface electrostatic charges and steric restrictions for insertion of a NO2 group (Fig. 4). It also should be noted that the NMR spectra tell us that the Lb protein imposes strict binding of the unsymmetrical heme molecule in only one orientation at equilibrium. Thus, the specific placement of the 2- and 4-vinyl groups, as shown in Fig. 4, defines a strong thermodynamic preference for the observed heme orientation, which, along with the accessibility of the 4-vinyl group seen in the structure, dictates that only the 4-vinyl group is attacked. In an early study, however, three Lbam derivatives were found to exhibit virtually identical Soret-visible spectra (23), and here we found also different Lbam products from in vitro nitration of Lba. In light of the present results, we propose that these products are isomers differing in the site of the NO2 group on the 4-vinyl, such as the α- or β-carbons and/or cis- or trans-configuration (Fig. S1).
Fig. 4.
Heme pocket of soybean Lba [PDB accession 1BIN (44)] showing relevant α-helices and amino acid residues. (A) Electrostatic potential surface of the whole protein, showing heme localization. (B) Detail of the heme pocket. Ribbon diagram showing side-chains B, E, and F, with stick representation of the relevant amino acid residues, including proximal His92 and distal His61. The vinyl groups of the heme are highlighted in yellow and the propionic groups in pink. Electrostatic potential surface is overlapped as transparency. Molecular structures were inspected, analyzed, and plotted with PyMol (56).
How was the nitrated heme produced? We used RNS scavengers and releasing compounds, antioxidants, and metal chelators to gain insights on the nature of nitrating molecules (Fig. 3 and Fig. S6). Nitration of Mb and Lb requires binding of NO2− to the heme because it was inhibited by cyanide. The reaction is strongly pH-dependent, which points out the implication of a nitrating agent derived from HNO2 rather than from NO2− itself. We can exclude a direct involvement of ONOOH formed outside the protein (pathway 1) because SIN-1 did not nitrate the Lb heme and superoxide dismutase and catalase did not prevent nitration. The same conclusion can be drawn for an oxidative attack of NO2+, which may be formed outside the protein from HNO2 decomposition (pathway 2), because addition of 1–10 mM nitronium tetrafluoroborate (NO2BF4) did not elicit heme nitration. Two alternative mechanisms, involving oxidation of NO2− to NO2• by ferryl Lb (pathway 3) or by •OH generated via Fenton reactions (pathway 4), can be also discarded because nitration did not require H2O2 and was not dependent on free-metal ions. A mechanism of protein nitration based on Fenton chemistry with free metals or heme was initially proposed as an alternative to the ONOO− pathway (45). Exogenous H2O2 is not required either for the nitration of HRP heme (42) or for the production of NO2-Tyr on a plant Hb (46). In these two cases, the nitrating agent is proposed to be the NO2• radical based on the peroxidase activity (pathway 3) of the hemoproteins (42, 46). In fact, in these and our own studies, the possibility that H2O2 might be generated inside the heme pocket cannot be entirely ruled out. This possibility is unlikely, however, because addition of up to 100 μM H2O2 did not accelerate nitration. Recently, two additional mechanisms have been proposed for nitration of Mb (38, 41) and Hb (34) with a large excess of NO2−. As in our case, these two pathways require binding of NO2− to the heme. The first one (pathway 5) entails a subsequent reaction of the [heme-NO2−] complex with H2O2 to form a heme-bound peroxynitrite [heme-N(O)OO] species (38, 41). The nitration potential of this pathway for Lb could nevertheless be markedly diminished, as ferric Lb isomerizes ONOO− to NO3− at rates that are 10-fold higher than those for Mb or Hb (47). This pathway would require formation of H2O2 inside the heme crevice and probably decomposition of the protonated species [heme-N(O)OOH] to NO2• radical. The second one (pathway 6) proposes that N2O5 is an intermediate (34). In this case, the [heme-NO2−] complex would react with another molecule of HNO2, giving rise to N2O5, which in turn would decompose to NO2+ and NO3−. Our findings that HNO2 is the precursor of the nitrating agent and that a [heme-NO2−] complex is a prerequisite for nitration are fully consistent with this hypothesis. Specifically, we propose that nitration is mainly a result of an electrophilic attack on the vinyl by the NO2+ generated from HNO2 inside the heme pocket according to pathway 6, although we cannot discard the simultaneous formation of NO2• radical by pathway 5, as mentioned earlier.
The deoxyferrous and oxyferrous forms of Lb are predominant in nodules, but other heme oxidation states and Lb complexes are also present. These include ferric Lb and the ferrous Lb-NO• (nitrosyl) complex that have been detected in intact nodules (48, 49). Ferric Lb can arise from several oxidative reactions, including the autoxidation of oxyferrous Lb or the reaction of NO• with oxyferrous Lb (47). In nodules, NO2− and NO• are mainly produced as a result of the nitrate reductase activities in the cytosol and bacteroids (50–52). Under natural conditions, nitration reactions are likely to occur because Lb may be exposed to NO2− over weeks or months and because the pH decreases to 5.5 during nodule senescence (53). The identification of Lbs bearing a nitrovinyl in their hemes provides conclusive evidence that nitrating and oxidizing RNS are produced in nodules. These reactive molecules are increasingly produced during aging or stressful conditions, in accord with the enhanced concentrations of Lbam and Lbcm observed in senescing nodules (24). Because these green proteins appear not to be competent for O2 transport (23, 24), it will be of interest to determine whether they are unavoidable by-products of Lb-mediated RNS detoxification or perform as yet unknown functions in legume nodules.
Materials and Methods
Biological Material.
Soybean plants (Glycine max cvs Hobbit or Williams × Bradyrhizobium japonicum strains 61A89 or USDA110) were grown under environment controlled conditions until the late vegetative growth stage (22). Nodules were harvested in liquid nitrogen and stored at –80 °C.
Purification of Lbs, Protein Identification, and Molecular Mass Determination.
Soybean Lbs were purified using ammonium sulfate precipitation and chromatographic steps in hydroxylapatite, Sephadex G-75, and DE-52 columns (22, 23). Proteins were subjected to in-gel digestion with trypsin using a Digest MSPro (Intavis). Peptide and protein identification was performed by peptide mass fingerprinting in a MALDI-TOF instrument (Applied Biosystems) as previously described (54). The molecular masses of Lbs were determined by MALDI-TOF/MS. Details of all these procedures are provided in SI Materials and Methods.
Structural Analyses of Hemes and Hemoproteins.
Details of equipment and protocols used for MS, NMR, and RR analyses are given in SI Materials and Methods.
Production of Recombinant Soybean Lba.
Recombinant Lba was used instead of protein purified from soybean nodules to duplicate nitration experiments, producing identical results. The recombinant protein was expressed using conventional protocols described in SI Materials and Methods.
Reconstitution and in Vitro Nitration of Hemoproteins.
The apoproteins of Mb and Lba were obtained by the acid-butanone method (55). After neutralization of the aqueous phase with phosphate buffer (pH 7.0), the apoproteins were incubated overnight with a twofold excess of protoheme or mesoheme, dialyzed, and nitrated. For time-course studies of nitration, hemoproteins (150–200 μM) were treated with NaNO2 (200 mM) in 50 mM phosphate buffer (pH 5.5 or 7.0) for 2–48 h at room temperature. The mixtures were dialyzed, concentrated, and resuspended in water (IEF analysis) or in 10 mM NH4HCO3 (MS analysis).
Supplementary Material
Acknowledgments
We thank Hyung-Kyun Jun, Gautam Sarath, Jose F. Moran, Bob Klucas, and Fred Wagner for stimulating discussions on Lbs and hemes in the early 1990s; Paul Ortiz de Montellano and Rafael Radi for their useful advice on hemoprotein reconstitution and nitration reactions, respectively; and Inmaculada Yruela and Montse Carrascal for invaluable help with Fig. 4 and MS analyses, respectively. This work was funded by Ministerio de Ciencia e Innovación-Fondo Europeo de Desarrollo Regional Grants AGL2008-01298 and AGL2011-24524; Gobierno de Aragón-Fondo Social Europeo Group A53; and Junta para la Ampliación de Estudios-Doc contracts from the Consejo Superior de Investigaciones Científicas (to J.N. and M.G.). The Proteomics Laboratory-Consejo Superior de Investigaciones Científicas/Universidad Autónoma de Barcelona is a member of the ProteoRed-Instituto de Salud Carlos III network.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1116559109/-/DCSupplemental.
References
- 1.Vinogradov SN, et al. Three globin lineages belonging to two structural classes in genomes from the three kingdoms of life. Proc Natl Acad Sci USA. 2005;102:11385–11389. doi: 10.1073/pnas.0502103102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gardner PR. Nitric oxide dioxygenase function and mechanism of flavohemoglobin, hemoglobin, myoglobin and their associated reductases. J Inorg Biochem. 2005;99:247–266. doi: 10.1016/j.jinorgbio.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Pesce A, et al. Neuroglobin and cytoglobin. Fresh blood for the vertebrate globin family. EMBO Rep. 2002;3:1146–1151. doi: 10.1093/embo-reports/kvf248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Appleby CA, Bergersen FJ. In: Methods for Evaluating Biological Nitrogen Fixation. Bergersen FJ, editor. Chichester: John Wiley; 1980. pp. 315–335. [Google Scholar]
- 5.Igamberdiev AU, Hill RD. Nitrate, NO and haemoglobin in plant adaptation to hypoxia: An alternative to classic fermentation pathways. J Exp Bot. 2004;55:2473–2482. doi: 10.1093/jxb/erh272. [DOI] [PubMed] [Google Scholar]
- 6.Perazzolli M, Romero-Puertas MC, Delledonne M. Modulation of nitric oxide bioactivity by plant haemoglobins. J Exp Bot. 2006;57:479–488. doi: 10.1093/jxb/erj051. [DOI] [PubMed] [Google Scholar]
- 7.Sturms R, DiSpirito AA, Hargrove MS. Plant and cyanobacterial hemoglobins reduce nitrite to nitric oxide under anoxic conditions. Biochemistry. 2011;50:3873–3878. doi: 10.1021/bi2004312. [DOI] [PubMed] [Google Scholar]
- 8.Trevaskis B, et al. Two hemoglobin genes in Arabidopsis thaliana: The evolutionary origins of leghemoglobins. Proc Natl Acad Sci USA. 1997;94:12230–12234. doi: 10.1073/pnas.94.22.12230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bustos-Sanmamed P, et al. Regulation of nonsymbiotic and truncated hemoglobin genes of Lotus japonicus in plant organs and in response to nitric oxide and hormones. New Phytol. 2011;189:765–776. doi: 10.1111/j.1469-8137.2010.03527.x. [DOI] [PubMed] [Google Scholar]
- 10.Becana M, Klucas RV. Oxidation and reduction of leghemoglobin in root nodules of leguminous plants. Plant Physiol. 1992;98:1217–1221. doi: 10.1104/pp.98.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fuchsman WH, Appleby CA. Separation and determination of the relative concentrations of the homogeneous components of soybean leghemoglobin by isoelectric focusing. Biochim Biophys Acta. 1979;579:314–324. doi: 10.1016/0005-2795(79)90059-x. [DOI] [PubMed] [Google Scholar]
- 12.Whittaker RG, Moss BA, Appleby CA. Determination of the blocked N-terminal of soybean leghemoglobin b. Biochem Biophys Res Commun. 1979;89:552–558. doi: 10.1016/0006-291x(79)90665-x. [DOI] [PubMed] [Google Scholar]
- 13.Marcker A, Lund M, Jensen EØ, Marcker KA. Transcription of the soybean leghemoglobin genes during nodule development. EMBO J. 1984;3:1691–1695. doi: 10.1002/j.1460-2075.1984.tb02033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Brian MR. Heme synthesis in the rhizobium-legume symbiosis: A palette for bacterial and eukaryotic pigments. J Bacteriol. 1996;178:2471–2478. doi: 10.1128/jb.178.9.2471-2478.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown SB, Houghton JD, Wilks A. Heme degradation and biosynthesis of bilins. In: Dailey HA, editor. Biosynthesis of Heme and Chlorophylls. New York: McGraw-Hill; 1990. pp. 543–575. [Google Scholar]
- 16.Baudouin E, Frendo P, Le Gleuher M, Puppo A. A Medicago sativa haem oxygenase gene is preferentially expressed in root nodules. J Exp Bot. 2004;55:43–47. doi: 10.1093/jxb/erh020. [DOI] [PubMed] [Google Scholar]
- 17.Virtanen AI, Laine T. Red, brown and green pigments in leguminous root nodules. Nature. 1946;157:25–26. doi: 10.1038/157025a0. [DOI] [PubMed] [Google Scholar]
- 18.Lehtovaara P, Perttilä U. Bile-pigment formation from different leghaemoglobins. Methine-bridge specificity of coupled oxidation. Biochem J. 1978;176:359–364. doi: 10.1042/bj1760359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roponen I. The effect of darkness on the leghemoglobin content and amino acid levels in the root nodules of pea plants. Physiol Plant. 1970;23:452–460. [Google Scholar]
- 20.Puppo A, et al. Legume nodule senescence: Roles for redox and hormone signalling in the orchestration of the natural aging process. New Phytol. 2005;165:683–701. doi: 10.1111/j.1469-8137.2004.01285.x. [DOI] [PubMed] [Google Scholar]
- 21.Becana M, Matamoros MA, Udvardi M, Dalton DA. Recent insights into antioxidant defenses of legume root nodules. New Phytol. 2010;188:960–976. doi: 10.1111/j.1469-8137.2010.03512.x. [DOI] [PubMed] [Google Scholar]
- 22.Jun H-K, Sarath G, Wagner FW. Detection and purification of modified leghemoglobins from soybean root nodules. Plant Sci. 1994;100:31–40. [Google Scholar]
- 23.Jun H-K, et al. Characteristics of modified leghemoglobins isolated from soybean (Glycine max Merr.) root nodules. Plant Physiol. 1994;104:1231–1236. doi: 10.1104/pp.104.4.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wagner FW, Sarath G. In: Plant Senescence: Its Biochemistry and Physiology. Thomson WW, Nothnagel EA, Huffaker RC, editors. Rockville: Am Soc Plant Physiologists; 1987. pp. 190–197. [Google Scholar]
- 25.Lehtovaara P, Ellfolk N. Purification and properties of Phaseolus vulgaris leghemoglobin (PhLb) Acta Chem Scand B. 1975;29:56–60. doi: 10.3891/acta.chem.scand.29b-0056. [DOI] [PubMed] [Google Scholar]
- 26.Trewhella J, Wright PE. 1H-NMR studies of ferric soybean leghemoglobin: Assignment of hyperfine shifted resonances of complexes with cyanide, nicotinate, pyridine and azide. Biochim Biophys Acta. 1980;625:202–220. doi: 10.1016/0005-2795(80)90284-6. [DOI] [PubMed] [Google Scholar]
- 27.Mabbutt BC, Wright PE. 1H NMR studies of complexes of ferric leghemoglobin with substituted pyridines and nicotinic acids. J Inorg Biochem. 1983;18:123–132. [Google Scholar]
- 28.Shokhireva TK, Shokhirev NV, Berry RE, Zhang H, Walker FA. Assignment of the ferriheme resonances of high- and low-spin forms of the symmetrical hemin-reconstituted nitrophorins 1-4 by 1H and 13C NMR spectroscopy: The dynamics of heme ruffling deformations. J Biol Inorg Chem. 2008;13:941–959. doi: 10.1007/s00775-008-0381-8. [DOI] [PubMed] [Google Scholar]
- 29.Desbois A, Mazza G, Stetzkowski F, Lutz M. Resonance Raman spectroscopy of protoheme-protein interactions in oxygen-carrying hemoproteins and in peroxidases. Biochim Biophys Acta. 1984;785:161–176. [Google Scholar]
- 30.Lin-Vien D, Colthup NB, Fateley WG, Grasselli JG. The Handbook of Infrared and Raman Characteristic Frequencies of Organic Molecules. San Diego: Academic Press; 1991. pp. 179–190. [Google Scholar]
- 31.Hu S, Smith KM, Spiro TG. Assignment of protoheme resonance Raman spectrum by heme labeling in myoglobin. J Am Chem Soc. 1996;118:12638–12646. [Google Scholar]
- 32.Picaud T, Le Moigne C, Loock B, Momenteau M, Desbois A. Nonplanar distortions of bis-base low-spin iron(II)-porphyrinates: Absorption and resonance Raman investigations of cross-trans-linked iron(II)-basket-handle porphyrin complexes. J Am Chem Soc. 2003;125:11616–11625. doi: 10.1021/ja034710r. [DOI] [PubMed] [Google Scholar]
- 33.Bondoc LL, Timkovich R. Structural characterization of nitrimyoglobin. J Biol Chem. 1989;264:6134–6145. [PubMed] [Google Scholar]
- 34.Otsuka M, et al. Covalent modifications of hemoglobin by nitrite anion: Formation kinetics and properties of nitrihemoglobin. Chem Res Toxicol. 2010;23:1786–1795. doi: 10.1021/tx100242w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yi J, Thomas LM, Musayev FN, Safo MK, Richter-Addo GB. Crystallographic trapping of heme loss intermediates during the nitrite-induced degradation of human hemoglobin. Biochemistry. 2011;50:8323–8332. doi: 10.1021/bi2009322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olah GA, Narang SC, Olah JA, Lammertsma K. Recent aspects of nitration: New preparative methods and mechanistic studies (a review) Proc Natl Acad Sci USA. 1982;79:4487–4494. [Google Scholar]
- 37.Brennan ML, et al. A tale of two controversies: Defining both the role of peroxidases in nitrotyrosine formation in vivo using eosinophil peroxidase and myeloperoxidase-deficient mice, and the nature of peroxidase-generated reactive nitrogen species. J Biol Chem. 2002;277:17415–17427. doi: 10.1074/jbc.M112400200. [DOI] [PubMed] [Google Scholar]
- 38.Nicolis S, Monzani E, Roncone R, Gianelli L, Casella L. Metmyoglobin-catalyzed exogenous and endogenous tyrosine nitration by nitrite and hydrogen peroxide. Chemistry. 2004;10:2281–2290. doi: 10.1002/chem.200304989. [DOI] [PubMed] [Google Scholar]
- 39.Bartesaghi S, et al. Reactions of desferrioxamine with peroxynitrite-derived carbonate and nitrogen dioxide radicals. Free Radic Biol Med. 2004;36:471–483. doi: 10.1016/j.freeradbiomed.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 40.Baysal E, Monteiro HP, Sullivan SG, Stern A. Desferrioxamine protects human red blood cells from hemin-induced hemolysis. Free Radic Biol Med. 1990;9:5–10. doi: 10.1016/0891-5849(90)90043-i. [DOI] [PubMed] [Google Scholar]
- 41.Nicolis S, et al. Easy oxidation and nitration of human myoglobin by nitrite and hydrogen peroxide. Chemistry. 2006;12:749–757. doi: 10.1002/chem.200500361. [DOI] [PubMed] [Google Scholar]
- 42.Wojciechowski G, de Montellano PR. Radical energies and the regiochemistry of addition to heme groups. Methylperoxy and nitrite radical additions to the heme of horseradish peroxidase. J Am Chem Soc. 2007;129:1663–1672. doi: 10.1021/ja067067s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moreau S, Davies MJ, Puppo A. Reaction of ferric leghemoglobin with H2O2: Formation of heme-protein cross-links and dimeric species. Biochim Biophys Acta. 1995;1251:17–22. doi: 10.1016/0167-4838(95)00087-b. [DOI] [PubMed] [Google Scholar]
- 44.Hargrove MS, et al. Characterization of recombinant soybean leghemoglobin a and apolar distal histidine mutants. J Mol Biol. 1997;266:1032–1042. doi: 10.1006/jmbi.1996.0833. [DOI] [PubMed] [Google Scholar]
- 45.Thomas DD, Espey MG, Vitek MP, Miranda KM, Wink DA. Protein nitration is mediated by heme and free metals through Fenton-type chemistry: An alternative to the NO/O2- reaction. Proc Natl Acad Sci USA. 2002;99:12691–12696. doi: 10.1073/pnas.202312699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sakamoto A, et al. Three distinct Arabidopsis hemoglobins exhibit peroxidase-like activity and differentially mediate nitrite-dependent protein nitration. FEBS Lett. 2004;572:27–32. doi: 10.1016/j.febslet.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 47.Herold S, Puppo A. Oxyleghemoglobin scavenges nitrogen monoxide and peroxynitrite: A possible role in functioning nodules? J Biol Inorg Chem. 2005;10:935–945. doi: 10.1007/s00775-005-0046-9. [DOI] [PubMed] [Google Scholar]
- 48.Lee KK, Shearman LL, Erickson BK, Klucas RV. Ferric leghemoglobin in plant-attached leguminous nodules. Plant Physiol. 1995;109:261–267. doi: 10.1104/pp.109.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mathieu C, Moreau S, Frendo P, Puppo A, Davies MJ. Direct detection of radicals in intact soybean nodules: Presence of nitric oxide-leghemoglobin complexes. Free Radic Biol Med. 1998;24:1242–1249. doi: 10.1016/s0891-5849(97)00440-1. [DOI] [PubMed] [Google Scholar]
- 50.Becana M, Minchin FR, Sprent JI. Short-term inhibition of legume N2 fixation by nitrate. I. Nitrate effects on nitrate-reductase activities of bacteroids and nodule cytosol. Planta. 1989;180:40–45. doi: 10.1007/BF02411408. [DOI] [PubMed] [Google Scholar]
- 51.Meakin GE, et al. The contribution of bacteroidal nitrate and nitrite reduction to the formation of nitrosylleghaemoglobin complexes in soybean root nodules. Microbiology. 2007;153:411–419. doi: 10.1099/mic.0.2006/000059-0. [DOI] [PubMed] [Google Scholar]
- 52.Horchani F, et al. Both plant and bacterial nitrate reductases contribute to nitric oxide production in Medicago truncatula nitrogen-fixing nodules. Plant Physiol. 2011;155:1023–1036. doi: 10.1104/pp.110.166140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pladys D, Barthe P, Rigaud J. Changes in intracellular pH in French-bean nodules induced by senescence and nitrate treatment. Plant Sci. 1988;56:99–106. [Google Scholar]
- 54.Casanovas A, Carrascal M, Abián J, López-Tejero MD, Llobera M. Discovery of lipoprotein lipase pI isoforms and contributions to their characterization. J Proteomics. 2009;72:1031–1039. doi: 10.1016/j.jprot.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 55.Ascoli F, Fanelli MR, Antonini E. Preparation and properties of apohemoglobin and reconstituted hemoglobins. Methods Enzymol. 1981;76:72–87. doi: 10.1016/0076-6879(81)76115-9. [DOI] [PubMed] [Google Scholar]
- 56.DeLano WL. The PyMol User's Manual. Palo Alto, CA: DeLano Scientific; 2002. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.