Abstract
A supramolecular approach for the specific detection of sarcosine, recently linked to the occurrence of aggressive prostate cancer forms, has been developed. A hybrid active surface was prepared by the covalent anchoring on Si substrates of a tetraphosphonate cavitand as supramolecular receptor and it was proven able to recognize sarcosine from its nonmethylated precursor, glycine, in water and urine. The entire complexation process has been investigated in the solid state, in solution, and at the solid–liquid interface to determine and weight all the factors responsible of the observed specificity. The final outcome is a Si-based active surface capable of binding exclusively sarcosine. The complete selectivity of the cavitand-decorated surface under these stringent conditions represents a critical step forward in the use of these materials for the specific detection of sarcosine and related metabolites in biological fluids.
Keywords: molecular recognition, phosphonate cavitand
Early stage detection of aggressive prostate cancer has been recently linked to the presence of sarcosine in urine (1). Sarcosine forms when the enzyme glycine-N-methyltransferase transfers a methyl group from S-adenosylmethionine to glycine. Given that sarcosine is one of the cancer biomarker candidates (2, 3), effective diagnostic tools for the detection of sarcosine directly in urine are highly desirable. The basic methodology for sarcosine determination is gas chromatography-mass spectroscopy of volatile derivatives (4) which, although highly sensitive and reliable, is hardly applicable for a widespread screening of this pathology. A different approach for sarcosine determination consists in the use of a fluorometric assay (BioVision Research Products). However, this approach requires various reaction steps and it is prone to interference due to unspecific reactions with other (unknown) urinary analytes (2), making it unsuitable for sarcosine measurements directly in the urine. A diffuse screening of prostate cancer requires easy and fast methodologies which minimize sample manipulations, number of reagents, and costs. An important step in this direction could be the development of an interactive surface akin to DNA chips, able to perform the recognition process directly in biological fluids.
In chemical terms, the preparation of a sarcosine detection chip requires (i) the design of a receptor capable of binding exclusively N-methylated amino acids in the presence of overwhelming amounts of amino acids plus many other metabolites in urine, and (ii) the grafting of this receptor on a suitable solid surface, retaining the molecular recognition properties at the solid–liquid interface.
Cavitands, defined as concave organic molecules capable of molecular recognition (5), are particularly versatile synthetic receptors, whose complexation properties depend on size, shape, and functionality of the preorganized cavity (6–8). Among them, tetraphosphonate cavitands are outstanding: Their complexation ability spans from positively charged inorganic and organic species (9) to neutral molecules (10). This diverse complexation ability is the result of three interaction modes, which can be activated either individually or in combination by the host according to the guest requirements: (i) multiple ion–dipole interactions between the inward facing P = O groups and the positively charged guests (11); (ii) single or dual H bonding involving the P = O groups (11, 12); and (iii) CH–π interactions between a methyl group present on the guest and the cavity of the host (13).
The complexation properties of tetraphosphonate cavitands anchored on silicon surface toward N-methyl pyridinium and N-methyl ammonium salts in organic solvents have been recently investigated (14). Tetraphosphonate cavitand ability to distinguish between amino acids and their N-methylated analogues remains to be explored, as well as the possibility to transfer their complexation properties to aqueous and biological environments.
In the present work, we report a comprehensive investigation of the molecular recognition properties of a silicon surface decorated with phosphonate cavitands (Fig. 1) toward glycine and sarcosine in water and urine. The entire complexation process has been investigated in the solid state, in solution, and at the solid–liquid interface to determine and weight all the factors responsible for the observed specificity. The final outcome is a Si-based active surface capable of binding exclusively sarcosine and other N-methylated amino acids in water and urine.
Fig. 1.
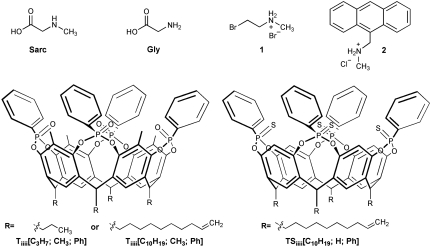
Chemical structure of the guests and cavitands. The short chain footed Tiiii[C3H7,CH3,Ph] cavitand was used for solid state and solution experiments. The double bond-terminated long-chain footed Tiiii[C10H19,CH3,Ph] and TSiiii[C10H19,H,Ph] cavitands were grafted on silicon wafers.
Results and Discussion
Sarcosine Complexation in the Solid State.
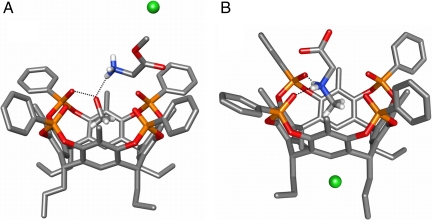
At first, the crystal structures of the complexes formed by tetraphosphonate cavitand Tiiii[C3H7,CH3,Ph] (15), from now onward referred to as Tiiii (16), with glycine methyl ester and sarcosine hydrochlorides were solved to define and compare type, number, and geometry of host–guest interactions present in the solid state in the two cases. Suitable crystals of both complexes were obtained under the same conditions (i.e., via slow evaporation of a methanol/water solution containing the host in the presence of an excess of guest). The complex Tiiii•methanol•glycine methyl ester hydrochloride (the use of glycine hydrochloride led to nondiffracting crystals) features a molecule of methanol into the cavity and the protonated amino acid methyl ester perching on top of the cavity (Fig. 2A). The affinity of this class of cavitand toward methanol has been previously reported (13), and also in this case the alcohol is stabilized within the cavity by a hydrogen bond with one P = O group at the upper rim and by two CH–π interactions between two methyl hydrogens of the guest and two aromatic rings of the host (13). The results indicate that methanol is preferred by the cavitand over glycine methyl ester hydrochloride; the latter could be expected to interact through dipolar interactions between the P = O groups and the positively charged nitrogen atom. But, although the methanol can exploit the synergistic effect of both CH–π interactions and hydrogen bonding, this is not the case for glycine methyl ester hydrochloride, whose interaction with the cavity is mediated by the solvent. The 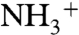 group of the amino acid forms a network of hydrogen bonds both with the methanol hosted inside the cavity and with the three lattice water molecules (SI Appendix, Fig. S1).
group of the amino acid forms a network of hydrogen bonds both with the methanol hosted inside the cavity and with the three lattice water molecules (SI Appendix, Fig. S1).
Fig. 2.
Crystal structures of complexes Tiiii•methanol•glycine methyl ester hydrochloride (A) and Tiiii•sarcosine hydrochloride (B). C, gray; O, red; P, orange; N, blue; Cl, green; H, white; H bonds, black dotted lines. For clarity, the H atoms of the cavitand and those not involved in complexation of the guests have been omitted.
The situation is completely different for the complex Tiiii•sarcosine hydrochloride (Fig. 2B): In this case, all three interaction modes with the guest described in the Introduction are activated. Sarcosine enters the cavity with its methyl group forming two CH–π interactions with two aromatic rings of the host. The complex is further stabilized by two hydrogen bonds involving the positively charged NH2 moiety and two adjacent P = O groups. Methanol does not interact with the cavity even if it is present in the crystal lattice. The chloride ion is located among the four alkyl chains at the lower rim of the cavitand, separated by a distance of 7.136(4) Å from the positive nitrogen atom, forming C-H⋯Cl- interactions with the four α CH2 residues. For a detailed description of all geometrical parameters of the complexes see the SI Appendix.
The different behavior of the two guests toward the cavitand can be attributed to the presence of the methyl residue on the nitrogen in the sarcosine. Its CH3–π interaction with the cavity triggers the formation of the two H bonds and the setting of cation–dipole interactions, which further stabilize the complex. Therefore, sarcosine is preferred over methanol for cavity inclusion.
Density Functional Theory (DFT) calculations (see SI Appendix for computational details) have been performed to estimate the energetic differences between Tiiii•sarcosine and Tiiii•glycine complexes because the theoretical model allows the exclusion of the effects due to the presence of specific solvents. The stabilization introduced by the additional CH3–π interaction has been evaluated in 3.8 kcal mol-1. In both cases, the formation of H bonds between charged NH2 groups and P = O apical fragments is pointed out by the elongations of the P = O bonds with respect to the noninteracting Tiiii (Δ = +0.02 Å) and the parallel elongation of the N–H bond with respect to the noninteracting guest (Δ = +0.02 Å). Regarding the Tiiii•sarcosine adduct, the distance between the C atom of the sarcosine N-methyl group and one benzene centroid (Arcentroid) of the cavitand (3.65 Å) as well as the C-H⋯Arcentroid angle (138.5°) are compatible with a CH–π interaction (17). Because this interaction is absent in the Tiiii•glycine adduct, the difference in energy stabilization between Tiiii•glycine and Tiiii•sarcosine complexes arises mainly from the CH3–π interaction.
Sarcosine Complexation in Solution.
Next, we examined the complexation properties of Tiiii in solution. The Kass of Tiiii and sarcosine methyl ester hydrochloride was determined in methanol at 303 K via isothermal titration calorimetry (ITC) (SI Appendix, Fig. S9). The direct comparison between glycine and sarcosine was not possible because glycine is insoluble in methanol. The determination of the thermodynamic data from the ITC curves requires the knowledge of the binding stoichiometry of the formed complexes. In our case, the Job’s plot provided clear evidence of 1∶1 binding in methanol solution (SI Appendix, Fig. S6). For sarcosine methyl ester hydrochloride, a Kass of 6.8 ± 0.5 × 104 M-1 was obtained. Interestingly, the thermodynamic profile showed that the enthalpic (-14.5 KJ mol-1) and entropic contributions (13.5 KJ mol-1 at 303 K) to the binding are comparable. This large, positive entropic component underlines the importance of desolvation of both host and guest in the binding process. By comparison, the interaction of glycine methyl ester hydrochloride with Tiiii in methanol was too low to be measured by ITC (SI Appendix, Fig. S9). Therefore, the ITC measurements reinforce the crystal structure determinations in supporting the preferential cavity inclusion of sarcosine in polar solvents.
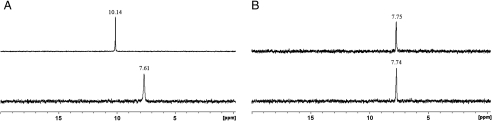
The ability of Tiiii to extract the two amino acids from water was assessed via 31P and 1H NMR in a water/chloroform biphasic system. An aqueous solution of glycine or sarcosine was added to an NMR tube containing the biphasic system, in which the water insoluble Tiiii is confined in the organic phase. In water, both amino acids are in their zwitterionic form. In the case of sarcosine, the 31P resonance of the four P = O groups moved downfield of 2.5 ppm (from 7.6 to 10.1 ppm, Fig. 3A) and the 1H resonance of the N-CH3 moiety moved upfield to -0.5 ppm (SI Appendix, Fig. S10). The downfield shift of P = O signals is a clear indication of the participation of phosphonates in the guest complexation, whereas the upfield shift of the methyl residue is diagnostic of N-CH3 inclusion into the cavity (11). Under the same conditions, glycine produced no detectable variation in the P = O chemical shift (Fig. 3B). This experiment proves that water does not hamper the ability of Tiiii to bind sarcosine, although it completely shuts down glycine uptake. This result can be rationalized by recalling that CH–π interactions, like the ones present between sarcosine and Tiiii, are dispersive in nature, therefore unaffected by the presence of water (18). On the contrary, glycine complexation is suppressed in water, water being a competitive solvent in exohedral Tiiii H bonding (19). The final outcome of these two trends is a boost to sarcosine versus glycine selectivity in water.
Fig. 3.
Sarcosine versus glycine complexation at the chloroform–water interface. (A) 31P NMR spectrum of Tiiii before (Lower) and after (Upper) addition of sarcosine to the water phase. (B) 31P NMR spectrum of Tiiii before (Lower) and after (Upper) addition of glycine to the water phase.
In a separate experiment, addition of solid sarcosine (not zwitterionic in the solid state) to a chloroform solution of Tiiii, did not lead to solubilization through complexation (SI Appendix, Fig. S11). When, instead, solid sarcosine hydrochloride was added, Tiiii was able to complex it efficiently and dissolve it in chloroform (SI Appendix, Figs. S12 and S13). Therefore, Tiiii is capable of binding sarcosine in water both in the protonated and zwitterionic forms, through interaction with the 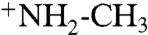 moiety. The acid component does not interfere with complexation either in the zwitterionic or protonated form. This set of experiments qualifies Tiiii as promising receptor for the diagnostics of sarcosine in biological fluids.
moiety. The acid component does not interfere with complexation either in the zwitterionic or protonated form. This set of experiments qualifies Tiiii as promising receptor for the diagnostics of sarcosine in biological fluids.
Sarcosine Complexation at the Solid–Water Interface.
Silicon wafers grafted respectively with Tiiii[C10H19,CH3,Ph] and its complexation-inactive but structurally related thiophosphonate analogue TSiiii[C10H19,H,Ph] (Fig. 1) were prepared via photochemical hydrosilylation of the double bonds on H-terminated Si(100) surfaces (14). The reaction leads to the formation of strong, hydrolytically stable Si–C bonds, capable to withstand the exposure to water and biological fluids in a wide range of pH. To maximize surface passivation, mixed monolayers constituted by Tiiii/1-octene and TSiiii/1-octene were prepared. The use of cavitand/1-octene mixture allows the anchoring of a denser layer in which the octyl chains cover the voids left under the cavitand heads and between cavitands, thus preventing silicon oxidation (20). The four methyl groups in the apical position of Tiiii[C10H19,CH3,Ph] were introduced to enhance CH3–π interactions with the guests with respect to its protio analogue Tiiii[C10H19,H,Ph] (14, 21), as indicated by theoretical modeling (0.5 Kcal/mol energy gain by DFT calculations; see SI Appendix).
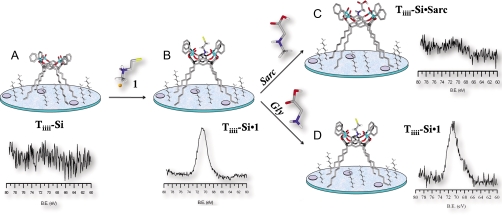
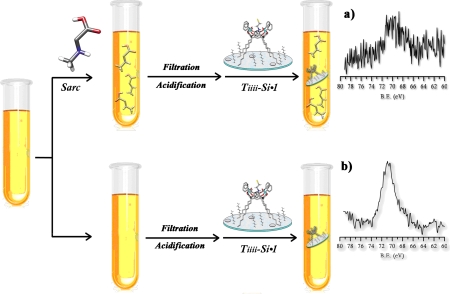
Initially, the complexation properties of the Tiiii-Si surface were tested in water adopting the bromine marked guest 1 (Fig. 4A) as probe. As a control experiment to rule out physisorption phenomena, the complexation-inactive TSiiii-Si surface was similarly treated with guest 1 in water. X-ray photoelectron spectroscopy (XPS) analysis shows that the Br atoms were detected only on the active Tiiii-Si surface, whereas the XPS spectra of the inactive TSiiii-Si surface did not show any Br signal. Because the atomic ratio between Br and P for a 1∶1 complex is one-fourth the yield, the complexation can be calculated as follows:
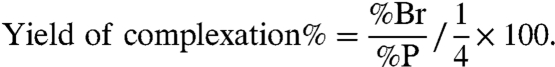 |
[1] |
The complexation yield was estimated in the range of 50–60%.
Fig. 4.
XPS analysis of Br 3D region along all steps of the sarcosine recognition protocol in water. (A) pristine Tiiii-Si wafer and its XPS spectrum. (B) Tiiii-Si•1 and its XPS spectrum after exposure of the wafer to a water solution of 1. (C) Tiiii-Si•Sarc and its XPS spectrum after exposure of the wafer to a water solution of sarcosine. (D) Tiiii-Si•1 and its XPS spectrum after exposure of the wafer to a water solution of glycine.
Because sarcosine/glycine detection on the surface is based on the exchange reaction in water between these amino acids and the Br-marked Tiiii-Si•1 complex (Fig. 4B), the intrinsic stability of Tiiii-Si•1 in pure water was studied as function of pH (Table 1). No Br signal was evident in the Br 3D XPS region of Tiiii-Si•1 surface after dipping it for 10 min in pure water in the pH range 1–7, thus indicating the removal of 1 upon deprotonation. Below pH 1, the XPS Br 3D signal retains a comparable intensity before and after water dipping, proving the Tiiii-Si•1 stability at that pH. The need to acidify the solution at very low pH to avoid guest decomplexation can be rationalized by recalling two surface effects: (i) the apparent pKa of surface groups (22), and in particular of surface bound amines (pKa ∼ 4) (23) are much lower than their intrinsic value in solution (pKa ∼ 10); (ii) guest deprotonation, decomplexation, and diffusion into the bulk solution are not balanced by the reverse reaction because the guest concentration in the solution resulting from surface decomplexation is negligible.
Table 1.
Br/P atomic concentration ratio from XPS data and complexation yield calculated from Eq. 1 as a function of pH
| As prepared* | pH 7–1† | pH 0.7† | pH 0† | |
| Br/P ratio | 0.13 | < 0.02 | 0.14 | 0.13 |
| Complexation yield, % | 54 | / | 56 | 54 |
*Before water dipping.
†After water dipping.
Then, the exchange reaction between sarcosine/glycine and Tiiii-Si•1 was monitored by XPS to test the selectivity of Tiiii-Si surface toward N-methylated amino acids. Two Tiiii-Si•1 wafers were exposed to water solutions of sarcosine and glycine, respectively. Both solutions were at the same concentration (1 mM) and pH (0.7). XPS analyses performed on the two surfaces after the treatment showed that the Br signal disappeared only in the wafer dipped in the sarcosine solution (Fig. 4C). Sarcosine completely replaced guest 1 on the Tiiii-Si surface, whereas glycine was totally ineffective (Fig. 4D). Therefore, the behavior of Tiiii at the silicon–water interface reflects exactly its conduct at the organic–water interface.
Sarcosine Detection in Urine.
The procedure based on the surface exchange reaction was adopted to identify sarcosine directly in urine. A human urine sample was filtered to remove traces of proteins which can clog the surface, then acidified to pH 0.7 and divided into two portions. Sarcosine was added to one of the two portions to simulate its biological occurrence due to prostate cancer (1 mM). The same procedure was repeated adding sarcosine before filtration to mimic the real sampling conditions. Sarcosine concentration before and after filtration remained unchanged as proven by GC-MS analyses (4) (SI Appendix, Fig. S15). Tiiii-Si•1 wafers were exposed to the two urine samples and analyzed via XPS to verify the presence of complexed guest 1 on the surface through its diagnostic Br 3D signal (Fig. 5).
Fig. 5.
Scheme of the detection procedure of sarcosine in human urine: The sample was divided into two portions, one them was added with sarcosine, and after filtration, centrifugation, and acidification, was tested with Tiiii-Si•1 (upper scheme). The other portion, after filtration, centrifugation, and acidification, was tested (control sample) with Tiiii-Si•1 (lower scheme). The XPS Br 3D spectra of Tiiii-Si•1 slides dipped (A) in the sarcosine-added sample and (B) in the control sample are reported sideways.
XPS measurements showed no differences if the sarcosine was added before or after the work-up. The sarcosine-laced urine displaced 1 from the surface, whereas the untreated one did not. None of the potential ionic interferents present in urine (protonated amino acids, 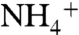 , Na+, K+, Mg2+, and Ca2+, just to mention the major ones) has sufficient affinity for the cavity to replace N-methyl ammonium salts. The resulting exquisite selectivity demonstrate by Tiiii-Si is unprecedented for a synthetic receptor operating at interfaces.
, Na+, K+, Mg2+, and Ca2+, just to mention the major ones) has sufficient affinity for the cavity to replace N-methyl ammonium salts. The resulting exquisite selectivity demonstrate by Tiiii-Si is unprecedented for a synthetic receptor operating at interfaces.
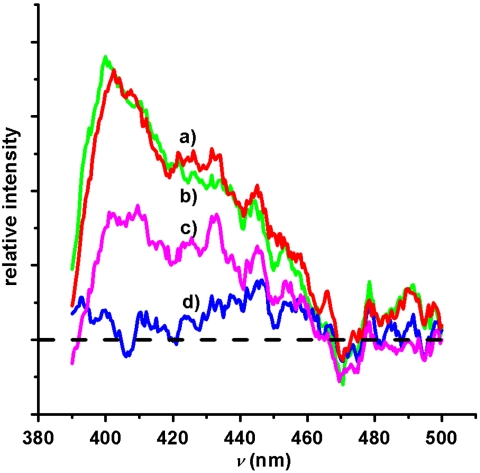
A fluorescence-based detection mode was then developed to prove the potential of Tiiii-Si as active surface for sarcosine detection with optical devices. Sarcosine presence in urine was monitored via fluorescence dye displacement (24), using (9-anthrylmethyl)methyl ammonium chloride 2 as indicator dye (25). Guest 2 binds efficiently to Tiiii-Si retaining its fluorescence, and it can be replaced only by molecules with comparable affinity for the cavity (sarcosine in our case). Tiiii-Si wafers complexed with guest 2 (Tiiii-Si•2) were exposed to three portions of human urine sample, two of them added with sarcosine at different concentrations before filtration (1 and 0.1 mM, respectively). The wafers were then analyzed via fluorescence spectroscopy to detect the presence of residual complexed guest 2 on the surface through its diagnostic fluorescence signal (Fig. 6). The fluorescence results confirmed the XPS experiments because both sarcosine added samples displaced fluorescence guest (traces C and D), whereas the untreated one did not (trace B). Results also indicated that guest 2 displacement depends on sarcosine concentration because a residual fluorescence signal was observed for the sample exposed to the urine with lower sarcosine content (0.1 mM).
Fig. 6.
Luminescence spectra of Tiiii-Si•2: (A) before urine exposure (red line); (B) after dipping for 5 min in urine (green line); (C) after dipping in 0.1 mM sarcosine-added urine (magenta line); (D) after dipping in 1 mM sarcosine-added urine (blue line). Tiiii-Si spectrum was subtracted from all signals. Excitation wavelength λex = 340 nm.
Conclusions
In the present work, we report the use of a silicon active surface for the specific recognition of sarcosine in water and urine using tetraphosphonate cavitand Tiiii as receptor. At the molecular level, the recognition process has been dissected in its three interaction modes and investigated in the solid state, in solution, and at the solid–liquid interface. In aqueous environment, the enhanced role played by the CH3–π interactions leads to complete selectivity toward sarcosine versus glycine. The energy stabilization due to the CH3–π interactions of sarcosine with the cavity has been estimated in 3.8 kcal/mol from DFT calculations.
The complexation properties of Tiiii have been transferred to Si wafers with high fidelity and the exquisite ability of Tiiii-Si to specifically detect sarcosine has been extended to urine, where several potential interferents are present. Complementary XPS and fluorescence guest displacement tests have demonstrated the selectivity of Tiiii-Si under these stringent conditions. In particular, the fluorescence detection mode represents a fundamental requirement for the prospective application of these materials in devices for biomedical diagnostics. These results allow us to envision the use of the Tiiii-Si surface for the specific detection of sarcosine as a marker of the aggressive forms of prostate tumor. Although the present fluorescence dye displacement approach is not yet suitable for sensing sarcosine at the low biological concentrations (1–4 × 10-5 M) (2, 26), we think that this work represents an important step in this direction.
Moreover, given the wide variety of biologically relevant compounds presenting N-CH3 groups (drugs, neurotransmitters, painkillers, antidepressants, etc.), we believe that the use of Tiiii cavitands can be extended to the detection of such compounds (27, 28).
Materials and Methods
Synthesis of Tiiii[C10H19,CH3,Ph].
To a solution of resorcinarene (29) (1 g, 0.91 mmol) in freshly distilled pyridine (10 mL) dichlorophenylphosphine (0.504 mL, 3.72 mmol) was added slowly, at room temperature. After 3 h of stirring at 80 °C, the solution was allowed to cool at room temperature and 4 mL of 35% H2O2 was added. The resulting mixture was stirred for 30 min at room temperature, then the solvent was removed under reduced pressure, and water added. The precipitate obtained in this way was collected by vacuum filtration and profusely rinsed with diethyl ether to give the product in a quantitative yield. The detailed physical data of the product are shown in the SI Appendix.
Tiiii/TSiiii Grafting on Si.
For grafting monolayers, cavitand/1-octene mixtures (χcav = 0.05) were dissolved in mesitylene (solution concentration = 50mM). Cavitand solutions (2.0 mL) were placed in a quartz cell and deoxygenated by stirring in a dry box for at least 1 h. A Si(100) substrate was dipped in H2SO4/H2O2 (3∶1) solution for 12 min to remove organic contaminants, then it was etched in a hydrofluoric acid solution (1% vol/vol) for 90 s, and quickly rinsed with water. The resulting hydrogenated silicon substrate was immediately placed in the mesitylene solution. The cell remained under UV irradiation (254 nm) for 2 h. The sample was then removed from the solution and sonicated in dichloromethane for 10 min to remove residual physisorbed material.
Tiiii-Si Complexation Studies in Water.
Complexation of Tiiii-Si surface with guest 1 was performed in a 1 mM aqueous solution of guests 1 for 30 min and then the wafer was sonicated in CH3CN for 10 min to remove any physisorbed material. Sarcosine recognition was carried out dipping Tiiii-Si•1 wafers in a 1 mM sarcosine solution in water at pH 0.7 for 10 min. As a blank experiment, Tiiii-Si•1 wafers were dipped in a 1 mM glycine solution in water at pH 0.7 for 10 min. All experiments have been repeated three times for consistency, without significant differences.
Urine Treatment.
For XPS detection, individual human urine samples (15 mL) were divided in two portions and one of them was added with solid sarcosine up to 1 mM. Samples were loaded onto 15-mL Vivaspin filters with a molecular weight cutoff of 3,000 Da and centrifuged at 8,000 × g at 15 °C, and then the urine portions were acidified at pH 0.7. Urine filtration does not alter sarcosine concentration in the laced samples, as proven by GC selected ion monitoring MS analyses (4) (SI Appendix, Figs. S15 and S16). Note that the addition of sarcosine to urine before or after the filtration step led to the same results. Tiiii-Si•1 wafers were then dipped in both urine samples for 10 min, washed in water at pH 0.7 for 1 min, and analyzed by XPS.
For fluorescence dye displacement detection, Tiiii-Si•2 wafers were prepared by dipping of Tiiii-Si surface in a 5 mM ethanol solution of guest 2 for 1 h. Then the wafer was sonicated in water at pH 0.7 for 5 min. Urine samples were divided into three portions, two of them were added with sarcosine up to 1 and 0.1 mM, respectively, and then were filtered, centrifuged, and acidified as above described. Tiiii-Si•2 wafers were dipped in all urine samples for 5 min, washed in water at pH 0.7 for 1 min, and then analyzed by fluorescence spectroscopy. All experiments have been repeated three times for consistency, without significant differences.
Supplementary Material
Acknowledgments.
We thank F. Schmidtchen and D. Menozzi for the ITC experiments, and F. Bianchi for GC-MS analyses. Centro Interfacoltà di Misure “G. Casnati” of the University of Parma is acknowledged for the use of NMR and high-resolution MS facilities. Authors also thank Prof. S. Sortino for fluorescence facilities and stimulating discussion. This work was supported by the European Union through the project BION (ICT-2007-213219) and by Fondazione CARIPARMA (project SpA). E.B. thanks Interuniversitario Nazionale per la Scienza e Tecnologia dei Materiali for partial support of her scholarship. Authors also thank Ministero dell’Istruzione, dell’Università e della Ricerca for partial support through COFIN 2008 (project 2008FZK5AC) and CINECA (Grant HP10B0R1E4) for the availability of high-performance computing resources and support.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates and structure factors have been deposited in the Cambridge Structural Database, Cambridge Crystallographic Data Centre, Cambridge CB2 1EZ, United Kingdom, http://www.ccdc.cam.ac.uk (CSD reference nos. CCDC-828311 and CCDC-828312).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1112264109/-/DCSupplemental.
References
- 1.Sreekumar A, et al. Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009;457:910–914. doi: 10.1038/nature07762. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 2.Schalken JA. Is urinary sarcosine useful to identify patients with significant prostate cancer? The trials and tribulations of biomarker development. Eur Urol. 2010;58:19–21. doi: 10.1016/j.eururo.2010.02.025. [DOI] [PubMed] [Google Scholar]
- 3.Cao DL, et al. Efforts to resolve the contradictions in early diagnosis of prostate cancer: A comparison of different algorithms of sarcosine in urine. Prostate Cancer Prostatic Dis. 2011;14:166–172. doi: 10.1038/pcan.2011.2. [DOI] [PubMed] [Google Scholar]
- 4.Bianchi F, et al. Fully automated solid-phase microextraction-fast gas chromatography-mass spectrometry method using a new ionic liquid column for high-throughput analysis of sarcosine and N-ethylglycine in human urine and urinary sediments. Anal Chim Acta. 2011;707:197–203. doi: 10.1016/j.aca.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 5.Cram DJ, Cram JM. Container molecules and their guests. In: Stoddart JF, editor. Monographs in Supramolecular Chemistry. Vol 4. Cambridge, UK: Royal Soc Chem; 1994. pp. 85–106. [Google Scholar]
- 6.Trembleau L, Rebek J., Jr Helical conformation of alkanes in hydrophobic environments. Science. 2003;301:1219–1220. doi: 10.1126/science.1086644. [DOI] [PubMed] [Google Scholar]
- 7.Hooley RJ, Restorp P, Iwasawa T, Rebek J., Jr Cavitands with introverted functionality stabilize tetrahedral intermediates. J Am Chem Soc. 2007;129:15639–15643. doi: 10.1021/ja0756366. [DOI] [PubMed] [Google Scholar]
- 8.Hooley RJ, Rebek J., Jr Chemistry and catalysis in functional cavitands. Chem Biol. 2009;16:255–264. doi: 10.1016/j.chembiol.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dutasta J-P. New phosphorylated hosts for the design of new supramolecular assemblies. Top Curr Chem. 2004;232:55–91. [Google Scholar]
- 10.Pirondini L, Dalcanale E. Molecular recognition at the gas-solid interface: A powerful tool for chemical sensing. Chem Soc Rev. 2007;36:695–706. doi: 10.1039/b516256b. [DOI] [PubMed] [Google Scholar]
- 11.Delangle P, Mulatier J-C, Tinant B, Declercq J-P, Dutasta J-P. Synthesis and binding properties of iiii (4i) stereoisomers of phosphonato cavitands—cooperative effects in cation complexation in organic solvents. Eur J Org Chem. 2001;2001:3695–3704. [Google Scholar]
- 12.Kalenius E, Moiani D, Dalcanale E, Vainiotalo P. Measuring H-bonding in supramolecular complexes by gas phase ion-molecule reactions. Chem Commun. 2007;43:3865–3867. doi: 10.1039/b707842k. [DOI] [PubMed] [Google Scholar]
- 13.Melegari M, et al. Supramolecular sensing with phosphonate cavitands. Chem Eur J. 2008;14:5772–5779. doi: 10.1002/chem.200800327. [DOI] [PubMed] [Google Scholar]
- 14.Biavardi E, et al. Molecular recognition on a cavitand-functionalized silicon surface. J Am Chem Soc. 2009;131:7447–7455. doi: 10.1021/ja901678b. [DOI] [PubMed] [Google Scholar]
- 15.Biavardi E, et al. Fully reversible guest exchange in tetraphosphonate cavitand complexes probed by fluorescence spectroscopy. Chem Commun. 2008;44:1638–1640. doi: 10.1039/b801729h. [DOI] [PubMed] [Google Scholar]
- 16.Pinalli R, Suman M, Dalcanale E. Cavitands at work: From molecular recognition to supramolecular sensors. Eur J Org Chem. 2004;2004:451–462. [Google Scholar]
- 17.Chandra Dey R, Seal P, Chakrabartl S. CH/π interaction in benzene and substituted derivatives with halomethane: A combined density functional and dispersion-corrected density functional study. J Phys Chem A. 2009;113:10113–10118. doi: 10.1021/jp905078p. [DOI] [PubMed] [Google Scholar]
- 18.Nishio M, Umezawa Y, Hirota M, Takeuchi Y. The CH/π interaction: Significance in molecular recognition. Tetrahedron. 1995;51:8665–8701. [Google Scholar]
- 19.Massera C, Melegari M, Kalenius E, Ugozzoli F, Dalcanale E. Supramolecular control of single-crystal-to-single-crystal transformation through selective guest exchange. Chem Eur J. 2011;17:3064–3068. doi: 10.1002/chem.201003407. [DOI] [PubMed] [Google Scholar]
- 20.Condorelli GG, et al. Grafting cavitands on the Si(100) surface. Langmuir. 2006;22:11126–11133. doi: 10.1021/la060682p. [DOI] [PubMed] [Google Scholar]
- 21.Menozzi D, et al. Thermodynamics of host-guest interactions between methylpyridinium salts and phosphonate cavitands. Supramol Chem. 2010;22:768–775. [Google Scholar]
- 22.Faber EJ, et al. pH sensitivity of Si-C linked organic monolayers on crystalline silicon surfaces. ChemPhysChem. 2007;8:101–112. doi: 10.1002/cphc.200600447. [DOI] [PubMed] [Google Scholar]
- 23.Tong Y, et al. Preferential adsorption of amino-terminated silane in a binary mixed self-assembled monolayer. Langmuir. 2011;27:5420–5426. doi: 10.1021/la200497u. [DOI] [PubMed] [Google Scholar]
- 24.Wiskur SL, Ait-Haddou H, Lavigne JJ, Anslyn EV. Teaching old indicators new tricks. Acc Chem Res. 2001;34:963–972. doi: 10.1021/ar9600796. [DOI] [PubMed] [Google Scholar]
- 25.Montalti M, Prodi L. A supramolecular assembly controlled by anions: Threading and unthreading of a pseudorotaxane. Chem Commun. 1998;34:1461–1462. [Google Scholar]
- 26.Cristaldi DA, et al. Sensing of linear alkylammonium ions by a 5-pyrenoylamido-calix[5]arene solution and monolayer using luminescence measurements. J Mater Chem. 22:675–683. [Google Scholar]
- 27.Vachon J, et al. The absolute configuration of an inherently chiral phosphonatocavitand and its use toward the enantioselective recognition of L-adrenaline. Tetrahedron Asymmetry. 2010;21:1534–1541. [Google Scholar]
- 28.Vachon J, et al. Inherently chiral phosphonatocavitands as artificial chemo- and enentio-selective receptors of natural ammoniums. Org Biol Chem. 2011;9:5086–5091. doi: 10.1039/c1ob05194f. [DOI] [PubMed] [Google Scholar]
- 29.Thoden van Velzen EU, Engbersen JFJ, Reinhoudt DN. Synthesis of self-assembling resorcin[4]arene tetrasulfide adsorbates. Synthesis. 1995;1995:989–997. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.