Abstract
Peripheral serotonin, synthesized by tryptophan hydroxylase-1 (TPH1), has been shown to play a key role in several physiological functions. Recently, controversy has emerged about whether peripheral serotonin has any effect on bone density and remodeling.We therefore decided to investigate in detail bone remodeling in growing and mature TPH1 knockout mice (TPH1−/−). Bone resorption in TPH1−/− mice, as assessed by biochemical markers and bone histomorphometry, was markedly decreased at both ages. Using bone marrow transplantation, we present evidence that the decrease in bone resorption in TPH1−/− mice is cell-autonomous. Cultures from TPH1−/− in the presence of macrophage colony-stimulating factor and receptor activator for NF-KB ligand (RANKL) displayed fewer osteoclasts, and the decreased differentiation could be rescued by adding serotonin. Our data also provide evidence that in the presence of RANKL, osteoclast precursors express TPH1 and synthesize serotonin. Furthermore, pharmacological inhibition of serotonin receptor 1B with SB224289, and of receptor 2A with ketanserin, also reduced the number of osteoclasts. Our findings reveal that serotonin has an important local action in bone, as it can amplify the effect of RANKL on osteoclastogenesis.
Keywords: neuromediator, osteopetrosis
Bone remodeling is a highly integrated process that continuously renews mineralized tissue throughout the skeleton to assure harmonious growth, maintenance, and repair throughout the lifespan of the individual. It couples the resorption of mineralized bone by osteoclasts and bone formation by osteoblasts. Osteoblasts originate from mesenchymal stem cells (1), and osteoclasts are multinucleated cells derived from a hematopoietic precursor of the monocyte macrophage lineage (2). Dysregulation of osteoclast function or differentiation results in an osteopetrotic phenotype, with a marked increase in bone density. In contrast, increased bone resorption is associated with bone loss in diseases such as osteoporosis, arthritis, and metastatic bone lesions. Molecular communication between osteoblasts and osteoclasts is required to regulate the commitment, proliferation, and differentiation of bone cell precursors. The main osteoclastogenic signals are the receptor activator for NF-KB ligand (RANKL) and macrophage colony-stimulating factor (M-CSF), both of which are cytokines secreted by osteoblasts (3).
Serotonin, or 5-hydroxytryptamine (5-HT), mediates a wide range of central functions, such as mood, behavior, sleep, blood pressure, and thermoregulation (4). Peripherally, serotonin is involved mainly in the regulation of vascular and heart functions (5, 6) and in gastrointestinal mobility (7). The diverse actions of 5-HT result from the presence of multiple 5-HT receptors (5-HTRs). These various different receptors have been divided into seven classes (5-HT1R to 5-HT7R) (8). Tryptophan hydroxylase (TPH) is the rate-limiting enzyme in 5-HT biosynthesis. There are two isoforms of this enzyme: TPH2 is mainly expressed in brain, and TPH1 is expressed elsewhere in the body (9, 10). 5-HT is produced by TPH1 in the enterochromaffin cells of the gastrointestinal tract and accumulates in platelet-dense granules, which constitute the body's main 5-HT reservoir (11). The action of serotonin is mainly paracrine and involves a complex action through its transporter and different receptors (4). In the periphery, although the main site of serotonin synthesis is the enterochromaffin cells of the gut, TPH1 expression and local serotonin synthesis have been shown to have a functional role in the cardiovascular system (12), mammary gland (13), and pancreas (14).
The action of serotonin in bone has recently been the subject of controversy. Using specific invalidation of TPH1 in different murine tissues, Yadav et al. reported that serotonin produced in the gut decreased bone formation via the 5-HT1B receptor that they observed in osteoblasts (15). This group also published data showing that the pharmacological inhibition of TPH1 was able to prevent bone loss in ovariectomized rodents (16). In contrast, Cui et al. observed no change in bone density measured by dual-energy X-ray absorptiometry in TPH1−/− mice 4 and 6 mo of age (17). However, in these studies, the main point of interest was whether changes in serotonin could be responsible for the bone phenotype of LRP5−/− mice (18). In brief, data from the Karsenty group suggest that the bone formation defect in LRP5−/− mice is not cell-autonomous but is induced by an increase in the serotonin level in serum (15), whereas Cui et al., using osteoblast- and osteocyte-specific knockout and knockins of the LRP5 mutation that induces a high and low bone mass phenotype in humans, claimed that LRP5 has a direct effect in the osteoblast without any change in whole-blood serotonin (17).
In these two conflicting studies, only a putative endocrine action of serotonin on bone was explored. However, various data about the action of serotonin on bone had previously been reported in vivo and in vitro. Osteoblasts were found to express the 5-HT transporter (SERT) and various serotonin receptors (19, 20), and we have shown that invalidation of the 5-HT2B receptor induced decreased bone formation in aging mice (21). The lack of SERT also induces a decrease in bone accrual during growth in mice (22).
To reevaluate these controversial findings, we decided to study bone remodeling in TPH1−/− mice while they were growing and at maturity. Our findings show that serotonin deficiency is responsible for a decrease in osteoclastic differentiation both during growth and at maturity. During growth, this decrease is responsible for an increase in bone density that resolves in adult animals due to the subsequent decrease in bone formation. We identified 5-HT as a regulator of osteoclast differentiation and function both in vivo and ex vivo. Furthermore, we show that osteoclast precursors are able to synthesize serotonin, and that the decrease in osteoclastic resorption is cell-autonomous in TPH1−/− mice.
Results
Bone Density and Formation Decrease from Growth to Maturity in TPH1−/− Mice.
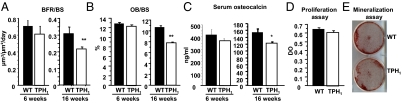
To determine bone phenotype, the bone mineral density (BMD) of male TPH1−/− and WT mice was assessed at 6 and 16 wk. At 6 wk, while they were still growing, TPH1−/− mice displayed higher BMD than WT mice in total body (+22%), at the femur (+17%), and at the vertebrae (+15%). In both genotypes, BMD increased significantly from 6 to 16 wk. However, the increase was lower in TPH1−/− mice than in WT mice, and BMD was not significantly different in mature WT and TPH1−/− mice at 16 wk (Table 1). To analyze the bone phenotype further, we carried out bone histomorphometry in 6- and 16-wk-old WT and TPH1−/− mice. A higher trabecular bone volume to total bone volume ratio (BV/TV) (+67%) was observed in TPH1−/− mice than in WT mice at 6 wk. The BV/TV measured in mature TPH1−/− mice at 16 wk was no longer significantly different from WT values, and was dramatically lower than that of BV/TV at 6 wk of age (Table 1). To find out whether bone formation was affected by TPH1 invalidation, we measured osteoblast surface per bone surface and the dynamic parameter of bone formation [bone formation rate (BFR); per bone surface (BS)] (Fig. 1 A and B) and quantified serum osteocalcin, a biochemical marker of bone formation (Fig. 1C). All of these parameters decreased in WT and TPH1−/− mice between 6 and 16 wk of age. At 16 wk, the bone formation parameters had decreased to a greater extent in TPH1−/− mice than in WT mice. This observation may in part explain the considerable decrease in trabecular bone volume and trabecular thickness (Table 1) that occurred in TPH1−/− mice between 6 and 16 wk of age. To find out whether TPH1−/− mice had an intrinsic osteoblast defect, we cultured primary osteoblasts from WT and TPH1−/− mice and did not observe any change in their proliferation or mineralization (Fig. 1 D and E). Furthermore, no serotonin could be detected in the osteoblast lysate. We therefore hypothesized that the high bone mass phenotype observed in young TPH1−/− mice could be attributed to a bone resorption defect, as suggested by the lower trabecular separation observed in 6- and 16-wk-old TPH1−/− mice than in their WT littermates (Table 1).
Table 1.
Bone density and trabecular microarchitecture in 6- and 16-wk-old TPH1−/− and WT mice
| 6-wk-old |
16-wk-old |
|||
| WT | TPH1−/− | WT | TPH1−/− | |
| Bone mineral density (mg/cm2) | ||||
| BMD whole-body | 0.037 ± 0.003 | 0.042 ± 0.002** | 0.055 ± 0.002††† | 0.055 ± 0.003††† |
| BMD femur | 0.046 ± 0.006 | 0.054 ± 0.004* | 0.077 ± 0.005††† | 0.073 ± 0.005††† |
| BMD vertebrae | 0.040 ± 0.005 | 0.046 ± 0.002* | 0.059 ± 0.001†† | 0.060 ± 0.003††† |
| Bone structure | ||||
| Bone volume/tissue volume (%) | 12.76 ± 1.29 | 21.1 ± 2.63*** | 13.4 ± 0.6 | 12.7 ± 1.2 |
| Trabecular separation (μm) | 182.15 ± 15.94 | 117.89 ± 12.69*** | 221.7 ± 15.9 | 193.5 ± 25.8** |
| Trabecular thickness (μm) | 26.21 ± 2.19 | 31.08 ± 2.52* | 34.3 ± 2.8 | 28.3 ± 3.3*** |
| Trabecular number (Tb.N,1/mm)** | 5.25 ± 0.4 | 7.36 ± 0.1*** | 4.12 ± 0.2 | 5.56 ± 0.1** |
Values are shown as mean ± SD (n = 8 per genotype). *P < 0.01 versus WT, **P < 0.001 versus WT, ***P < 0.0001 versus WT, ††P < 0.001 versus 16-wk-old mice in the same genotype, †††P < 0.0001 versus 16-wk-old mice in the same genotype.
Fig. 1.
Bone formation in WT and TPH1−/− mice during growth and maturity. Static and dynamic histomorphometric parameters were measured in 6- and 16-wk-old animals. (A) Bone formation rate/bone surface (BFR/BS) and (B) osteoblast surface / bone surface (OB/BS) were unchanged in both genotypes (WT, black bars; TPH1−/−, white bars) at 6 wk and were reduced at 16 wk. (C) Biochemical markers of bone formation. Serum osteocalcin levels were determined when the mice were 6 and 16 wk old. No significant difference was observed when the mice were 6 wk old, whereas a significant decrease in the osteocalcin level was observed when they were 16 wk old. (D) Proliferation was assessed after a 3-d culture period by BrdU incorporation in WT and TPH1−/− calvarial osteoblasts, and no difference was observed. (E) The capacity of WT and TPH1−/− primary osteoblasts to produce mineralized nodules was determined by alizarin staining after 18 d in culture, and no difference was observed. Data are shown as mean ± SEM; n = 8 mice per genotype. *P < 0.01 versus WT, **P < 0.001 versus WT, ***P < 0.0001 versus WT.
Both Growing and Mature TPH1−/− Mice Display Reduced Bone Resorption Due to an Osteoclastic Differentiation Defect.
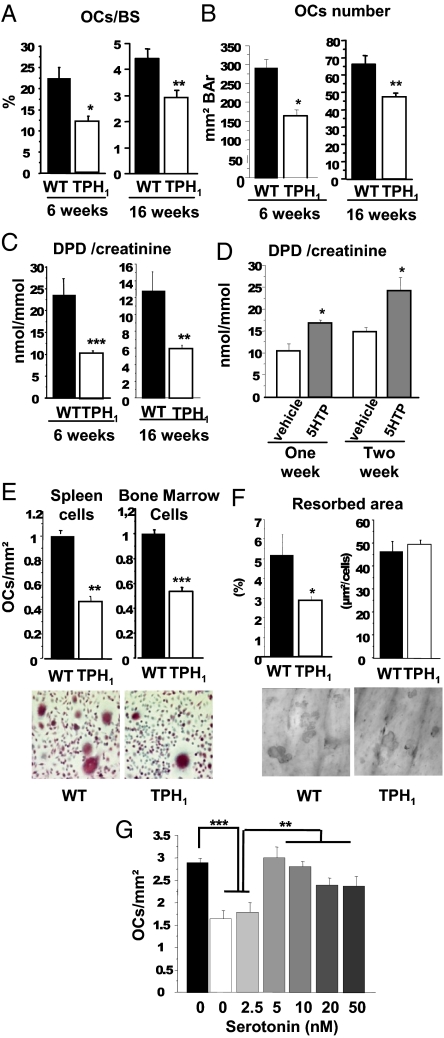
In light of these results, we first used bone histomorphometry to measure the osteoclast number as tartrate-resistant acid phosphatase (TRAP)-positive cells in 6- and 16-wk-old mice. As shown in Fig. 2A, the osteoclastic surfaces at the femoral metaphysis were lower in TPH1−/− mice than in WT at 6 wk (−44.4%), and this phenotype persisted at 16 wk (−34.8%). Furthermore, the osteoclast number was also reduced at both ages (Fig. 2B). We next measured urinary d-pyridinoline (DPD), a marker of osteoclast activity, in 6- and 16-wk-old WT and TPH1−/− mice (Fig. 2C). DPD/creatinine ratios were dramatically reduced at both times in TPH1−/− mice (TPH1−/− vs. WT, −60% at 6 wk; −55% at 16 wk). Finally, to confirm in vivo that the decreased bone resorption observed in TPH1−/− mice was associated with a lack of 5-HT, the mutant animals were treated for 2 wk with 5-hydroxytryptophan (5-HTP) to override the TPH1 invalidation. Consistent with the previous in vivo results, we found that the DPD ratio was increased in mice treated with 5-HTP from the first week of treatment, and that the DPD ratio measured after 2 wk of treatment was in the same range as that measured in the 6-wk-old WT mice (Fig. 2D).
Fig. 2.
Lack of serotonin induces in vivo and in vitro decrease in bone resorption. Histomorphometric analysis was performed at 6 and 16 wk. TRAP staining of a section of the trabecular region of the distal femur was performed to identify osteoclasts. Bone resorption parameters were determined. (A) The number of osteoclasts per bone surface (OCs/BS) showed a marked decrease, and TPH1−/− mice had a lower osteoclast count than WT mice. (B) Osteoclast number/bone area (OCs number/BAr). (C) Biochemical markers of bone resorption. Urinary deoxypyridinoline cross-links normalized by the amount of creatinine present (DPD/creat) were measured in 6- and 16-wk-old WT and TPH1−/− mice. DPD levels were lower in 6- and 16-wk-old male TPH1−/− mice. WT, black bars; TPH1−/−, white bars. Results are reported as mean ± SEM; n = 8 mice per genotype. (D) TPH1−/− mice were treated twice a day with vehicle (white bars) or with 50 mg/kg body weight of 5-HTP (gray bars). DPD levels were significantly increased by 5-HTP administration to 4-wk-old mice after 1 or 2 wk of treatment; vehicle, n = 6; 5-HTP treatment, n = 9. (E) Osteoclastogenesis was assessed in WT and TPH1−/− spleen cells and bone marrow cells cultured with dialyzed serum without serotonin in the presence of M-CSF (25 ng/mL) for 4 d and of M-CSF and RANKL (30 ng/mL) for a further 5 d. Counts of OCLs in WT (black bars) and TPH1−/− (white bars) culture; osteoclast numbers were lower in TPH1−/− cultures than in WT cultures at the end of the differentiation of spleen cells and bone marrow cells. Representative pictures of WT and TPH1−/− spleen cell cultures (20× magnification). (F) OCL activity was assessed by pit assays on dentin slices. The resorbed area was stained with toluidine blue (10× magnification). The resorbed area was strongly decreased in TPH1−/− cultures, whereas the ratio pit area:OC number was unchanged. (G) WT spleen cells were cultured without any serotonin treatment, and TPH1−/− cells were treated with serotonin (2.5, 5, 10, 20, and 50 nM) throughout the culture in the presence of RANKL. From 5 nM 5-HT the number of OCLs increased to equal WT levels, without any dose effect. *P < 0.01 versus WT, **P < 0.001 versus WT, ***P < 0.0001 versus WT.
To investigate the cell defects that lead to low bone resorption, we assessed osteoclastic differentiation from spleen cells and bone marrow macrophages in medium supplemented with M-CSF, RANKL, and dialyzed serum without 5-HT (Fig. 2E). We showed that in TPH1−/−cultures, there were markedly fewer multinucleated TRAP-positive cells (−55%), assimilated to osteoclasts (OCLs), than in WT cultures. The osteoclast activity was determined by a pit assay on dentin slices. The pit area was lower in TPH1−/− than in WT cultures (Fig. 2F). Furthermore, the pit area per osteoclast number was the same in both genotypes (Fig. 2F). These findings suggest that 5-HT is involved in osteoclast differentiation, and that it does not affect osteoclast activity. In line with these functional findings, the expression of osteoclast markers (TRAP, Cathepsin K, and NFATc1) was significantly lower at the end of the culture in TPH1−/− than in WT, whereas RANK expression remained unchanged. These findings indicate that the number of precursors was the same in TPH1−/− and WT cultures (Fig. S1A). Finally, to confirm that 5-HT plays a role during osteoclast differentiation, we treated TPH1−/− cell cultures with physiological concentrations of 5-HT (2.5, 5, 10, 20, and 50 nM). At concentrations from 5 nM 5-HT, and without any dose effect, the number of OCLs increased to reach WT levels (Fig. 2G). Taken together, these findings suggest that an osteoclast defect was responsible for the decreased bone resorption in TPH1−/− mice.
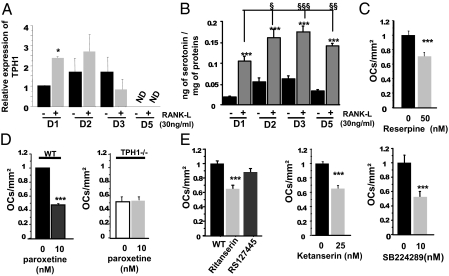
Synthesis, Storage, and Reuptake of 5-HT in WT Osteoclast Precursors.
From the above data, and as TPH1−/− spleen and marrow macrophage cultures in the presence of RANKL have been shown to have reduced osteoclast differentiation capacity, we hypothesized that 5-HT was produced by the osteoclast precursors themselves. Indeed, we detected TPH1 mRNA in WT cells, and its expression was blunted as the cells differentiated in the presence of RANKL (Fig. 3A). RANKL significantly stimulated the expression of TPH1 mRNA after exposure for 1 d (Fig. 3A). We next investigated 5-HT production in spleen cell cultures after different culture times. We showed that 5-HT was produced in the cell lysate, was increased by RANKL, and then diminished toward the end of the culture period (Fig. 3B). We went on to demonstrate that the 5-HT synthesized in WT osteoclast precursors is subsequently stored in intracellular granules by vesicular monoamine transporters (VMATs). We first showed that the expression of VMAT1 and VMAT2 mRNA at the end of the culture did not differ significantly in the two genotypes (Fig. S1B). We treated the WT culture with reserpine, an inhibitor of both VMATs, and as in TPH1−/− cultures this induced a decrease in osteoclast differentiation, suggesting that intracellular storage of 5-HT plays a positive role in osteoclast differentiation (Fig. 3C).
Fig. 3.
Synthesis, storage, and reuptake of serotonin in WT osteoclast precursors. (A) After 4 d of treatment with M-CSF (25 ng/mL), total RNAs were isolated at different times [day (D)1, 2, 3, and 5] in the presence (gray bar) and absence (black bar) of RANKL (30 ng/mL), and RT-PCR was performed. TPH1 mRNA was expressed from day 1 to day 3. The presence of RANKL increased TPH1 mRNA expression, but only at day 1. (B) Serotonin levels were measured by HPLC in WT cell lysates. Serotonin was detected from day 1 to day 5 in the presence or absence of RANKL. An increase in serotonin level in the presence of RANKL was observed during the culture. (C) WT cells were treated with several different doses of reserpine, an inhibitor of vesicular monoamine transporter (50,100, and 200 nM), which induced a dose-dependent decrease in OCL number in WT cultures. (D) WT and TPH1−/− cultures were treated with paroxetine (10 nM), an inhibitor of SERT. Paroxetine also induced a significant decrease in OCL number in WT culture but not in TPH1−/− culture. (E) Various different substances were added to the WT culture in the presence of M-CSF and RANKL. Ritanserin (100 nM), an inverse agonist of the 5-HT2 family, induced a decrease in osteoclast number, whereas RS127445 (20 nM), a specific antagonist of 5-HT2BR, did not induce any change. Several different doses of ketanserin (25 nM), an antagonist of 5-HT2AR, were added to the WT culture. A significant dose-related decrease in osteoclast number was observed. SB224289 (10 nM) 5-HT1BR antagonist treatments of WT cells decreased the OCL number, but without any dose effect. Data are reported as mean ± SEM of four independent experiments. *P < 0.01 versus WT, **P < 0.001 versus WT, ***P < 0.0001 versus WT.
We next evaluated the possible reuptake of 5-HT by SERT, the plasma membrane serotonin transporter. Osteoclasts from WT mice expressed SERT (Fig. S1B). To find out whether the inhibition of the cellular 5-HT influx played any role in the lower osteoclastogenesis, we treated the cells with paroxetine, a specific inhibitor of SERT. We observed that paroxetine markedly reduced osteoclast differentiation in WT osteoclast cultures, whereas it had no effect on osteoclast differentiation in TPH1−/− cultures (Fig. 3D). These findings demonstrate that 5-HT was produced and stored in osteoclast precursor cells, and that 5-HT plays a significant role in osteoclast differentiation.
Serotonin Modulates Osteoclastic Differentiation via a Paracrine Pathway.
As 5-HT is produced locally, we thought it was worth investigating the expression of the different 5-HT receptors able to mediate a paracrine effect in osteoclast precursors. We evaluated the expression of the different 5-HT receptors by quantitative PCR at the end of WT cultures in the presence or absence of RANKL (Fig. S1C). 5-HT1AR and 5-HT4R mRNAs were not detected in WT spleen cell cultures. 5-HT1BR was increased by the presence of RANKL after 5 d of culture. The level of 5-HT2AR was the same in the presence or absence of RANKL. And, finally, 5-HT2BR was significantly decreased in the presence of RANKL. To assess the functional role of these receptors in osteoclastogenesis, we next evaluated the effect of selective 5-HT receptor antagonists on these cultures. When WT spleen cells were treated with ritanserin, an inverse agonist of the 5-HT2 receptor, we observed a significant decrease in osteoclast formation (Fig. 3E). In contrast, RS127445, a specific 5-HT2BR antagonist, did not produce any significant effect (Fig. 3E), whereas at 25 nM, ketanserin, a specific 5-HT2AR antagonist, induced effects similar to those of ritanserin (Fig. 3E). Thus, 5-HT2AR is the member of the 5-HT2 family involved in osteoclast differentiation. In parallel, we treated WT cultures with SB224289, a 5-HT1B receptor antagonist, and found that at 10 nM, this compound also had a significant effect on the number of osteoclasts (Fig. 3E). These findings show that the 5-HT1B and 5-HT2A receptors were both implicated in osteoclast differentiation.
In Vivo Decrease in Bone Resorption in TPH1−/− Mice Is Due to an Intrinsic Osteoclast Defect.
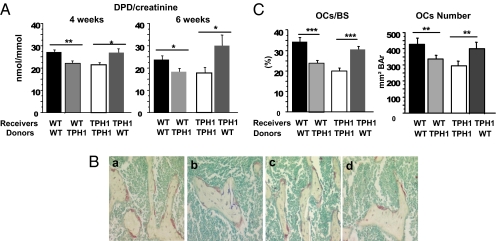
Our in vitro data support the existence of an intrinsic osteoclast defect in TPH1−/− mice. The low bone resorption observed in vivo in these mice could be directly related to a low level of serotonin in the bone microenvironment. To investigate this hypothesis in vivo, we carried out a series of bone marrow transplantations. Newborn WT and TPH1−/− mice were treated with busulfan and then transplanted with lineage-negative cells isolated from WT or TPH1−/− donors. As expected, 4 wk after transplantation, the DPD:creatinine ratio of TPH1−/− mice transplanted with TPH1−/− cells was lower than that of WT mice transplanted with WT cells. We then observed that the DPD:creatinine ratio in WT mice transplanted with TPH1−/− cells was lower than that of WT mice transplanted with WT cells and, finally, that the transplantation of WT cells into TPH1−/− mice rescued the DPD:creatinine ratio to the same level as that of WT mice transplanted with WT cells (Fig. 4A). These DPD results were sustained 6 wk after transplantation. Furthermore, the histomorphometric study showed that both osteoclast surface area and osteoclast numbers decreased when mice were transplanted with TPH1−/− cells, and increased when transplantation was performed with WT cells (Fig. 4 B and C). Taken together, these data support the existence of an osteoclast intrinsic defect that is responsible for the low bone resorption of TPH1−/− mice in vivo.
Fig. 4.
In vivo decrease in bone resorption in TPH1−/− mice is due to an intrinsic osteoclast defect. Newborn WT and TPH1−/− mice were treated with busulfan (10 mg/kg) and transplanted with WT and TPH1−/− bone marrow cells. (A) At age 4 and 6 wk, urine samples were taken, and DPD was measured. We found that TPH1−/− mice transplanted with TPH1−/− cells had a lower DPD:creatinine ratio than WT mice transplanted with WT cells. Moreover, when WT mice were transplanted with TPH1−/− cells, the DPD ratio decreased, and the transplantation of WT cells into TPH1−/− mice led to a rescue of the DPD ratio. (B) Histomorphometric analysis was performed at 6 wk on the transplanted mice. TRAP staining of a section of the trabecular region of the distal femur was performed to identify osteoclast multinucleated and red cells (20× magnification): (a) WT mice transplanted with WT cells, (b) WT mice transplanted with TPH1−/− cells, (c) TPH1−/− mice transplanted with WT cells, and (d) TPH1−/− mice transplanted with TPH1−/− cells. (C) Bone resorption parameters were also determined: osteoclast number per bone surface (OCs/BS) and the osteoclast number. These parameters showed a similar pattern to the DPD ratio: When WT cells were transplanted into TPH1−/− mice, we observed an increase in the bone resorption parameter, and when TPH1−/− cells were transplanted into WT mice, we found that rescue of bone resorption occurred. Data are shown as mean ± SEM; n = 5–8 mice per genotype. *P < 0.05 versus WT, **P < 0.005 versus WT, ***P < 0.0005 versus WT.
Discussion
The findings presented in this paper establish a function for local serotonin in bone remodeling. We were not able to show cell-autonomous change in osteoblast function in the absence of serotonin, but we did find both in vivo and in vitro evidence that serotonin acts on the differentiation of monocytes/macrophages into osteoclasts via an autocrine/paracrine loop. We also show here that serotonin is synthesized by osteoclast precursors, and that bone resorption decreases in the absence of serotonin synthesis by osteoclast precursors. We were also able to demonstrate by in vivo and in vitro rescues that serotonin is indeed responsible for the low bone resorption in mutant mice and, using marrow transplantation, that this low bone resorption is cell-autonomous in TPH1−/− mice. We therefore conclude that serotonin has complex physiological actions in bone, as in other tissues (4).
Our findings complete and can reconcile those of previous studies of serotonin in bone. In contrast to a present study, Yadav and colleagues (15) analyzed a mouse line with a specific inactivation of TPH1 affecting either the gut or the osteoblasts, and could not, therefore, detect any specific function of 5-HT produced by osteoclasts. Cui and colleagues (17) have shown that TPH1−/− mice had no change in BMD at 4 and 6 mo, but did not investigate bone remodeling. Here, in accordance with the Cui et al. data, we show an unchanged BMD at 16 wk. However, when deep phenotyping was performed, we observed that this unchanged BMD at 16 wk in TPH1−/− mice was associated with a decrease in both bone resorption and formation at that time (Fig. 1). Interestingly, although low bone resorption was observed in both growing and mature mice, high trabecular bone volume was only observed in growing TPH1−/− mice.
Although gut is the main organ responsible for peripheral 5-HT synthesis, several other peripheral tissues have recently been demonstrated to be 5-HT producers with important physiological roles, even though the amounts of serotonin measured in these tissues were far lower than those in the gut (12–14, 23). Serotonin was synthesized by osteoclast precursors, as we could detect TPH1 mRNA expression and serotonin at different times during the cultures with RANKL. The level of serotonin present in the osteoclast precursors was in the same range as that necessary to rescue osteoclast differentiation in TPH1−/− cultures. The synthesis of serotonin was increased by RANKL during the first day, and then decreased as the cells differentiated. In these experiments, we used primary spleen cell cultures, composed mainly of monocytes/macrophages, cultured with M-CSF and RANKL. These cells were engaged in osteoclast differentiation, and we show here that local serotonin synthesis was increased by RANKL in osteoclast precursors. Interestingly, it has recently been shown that the bone marrow of TPH1−/− mice contains fewer nucleated cells (24). Although only erythropoiesis was studied, it is not impossible that in TPH1−/− mice the myeloid lineage could also have been altered, and that the reduced osteoclast differentiation we observed from spleen and bone marrow monocytes/macrophages was already present in earlier precursors.
It had previously been shown that serotonin can act on the cells of the immune system (7, 25). Battaglino and colleagues demonstrated the presence of serotonin receptors 1B, 2B, and 4 in the RAW264.7 cell line, induced by M-CSF and RANKL in the osteoclast lineage (26). This is why we focused on the 1, 2, and 4 families of 5-HT receptors. Using RT-PCR, we demonstrated that the 5-HT1B, 5-HT2A, and 5-HT2B receptors are expressed in osteoclast precursors of the monocyte/macrophage lineage, whereas 5-HT1A and 5-HT4 receptors were not detected. We also demonstrated increased 5-HT1BR expression after induction with RANKL, which suggests that this receptor is associated with the differentiation of the osteoclast precursors. In contrast, the expression of 5-HT2AR did not change in the presence of RANKL, which suggests that it acts before the osteoclast lineage begins. Finally, as the monocytes differentiated into osteoclasts, the expression of 5-HT2BR decreased. We tested the functional role of these receptors by specific pharmacological inhibitors, and were able to show that two of them (5-HT1BR and 5-HT2AR) could mediate the action of serotonin on osteoclast differentiation. These serotonin receptors are known to be implicated in the regulation of intracellular calcium concentration via several pathways (27, 28). Furthermore, during osteoclast differentiation, intracellular calcium signaling activates NFATc1, the master regulator of osteoclastogenesis (29, 30). We can, therefore, hypothesize that these two serotonin receptors could be implicated in osteoclast differentiation.
To maintain healthy bone tissue, osteoclastic bone resorption and osteoblast-mediated bone formation have to be tightly coupled. As low osteoclastic bone resorption was observed in mice lacking peripheral serotonin, we expected to find reduced bone formation. However, surprisingly, bone formation was maintained in 6-wk-old TPH1−/− mice, resulting in an increase in trabecular bone volume at this age. However, in mature 16-wk-old mice, we observed that low bone resorption was accompanied by the expected low bone formation that may have resulted from cellular coupling, as no intrinsic osteoblast defect could be demonstrated in TPH1−/− mice. Osteoclasts are now recognized as having a direct key role in bone remodeling. More recently, it has been revealed that osteoclasts can directly release soluble factors, and it is suspected that they may be involved in the coupling between resorption and formation (31, 32). We can therefore speculate that serotonin secreted by osteoclast precursors could not only play a role in osteoclast differentiation but also act on neighboring osteoblasts via a paracrine mechanism. Furthermore, circulating serotonin could also act on osteoblasts. However, the role of the physiological concentration of serotonin in osteoblast proliferation remains to be elucidated, as so far only pharmacological concentrations have been investigated (15).
In summary, our results show that physiological levels of serotonin enhance osteoclastic resorption. We also demonstrate that RANKL enhances the expression of TPH1 and the synthesis of serotonin by the osteoclasts themselves. When RANKL is produced by osteoblasts, serotonin synthesized by osteoclast precursors could act synergistically with RANKL signaling and further increase osteoclast differentiation (Fig. S2). In conclusion, we propose that serotonin can increase osteoclast differentiation via the autocrine/paracrine pathway. These findings are of importance with regard to the role of serotonin in bone remodeling.
Materials and Methods
Skeletal Phenotyping.
The generation of mice lacking TPH1 on an Sv129 background has been reported previously (10). All of the experiments were approved by the Ethics Committee of Paris Diderot University, and complied fully with French government animal welfare policy. Histomorphometry was carried out as described previously (21) in accordance to American Society for Bone and Mineral Research nomenclature (33, 34).
5-HTP Treatment.
5-Hydroxytryptophan (5-HTP) (Sigma-Aldrich) was administered s.c. to TPH1−/− mice twice a day for 2 wk from 4 to 6 wk of age. Control mice received saline solution, the vehicle, during the same period. d-pyridinolines were measured in the urine at baseline, and then after 1 and 2 wk of treatment with either 5-HTP or vehicle.
Measurement of 5-HT Levels by HPLC.
The cells were washed and suspended in 200 μL of NaCl 0.9% and sonicated in 10 volumes (v/w) of 0.1 N perchloric acid/0.05% disodium EDTA/0.05% sodium metabisulfite. 5-HT was extracted, and 10-μL samples were injected onto a Beckman Ultrasphere 5-μm IP column (Beckman). Eluted 5-HT was quantified electrochemically (at 0.65 V), and the results are reported in nanograms of serotonin per milligrams of proteins. Detectable concentration was 17.6 ng/L.
Bone Marrow Transplantation.
Bone marrow was harvested from WT and TPH1−/− donor mice (4- to 6-wk-old mice). Hematopoietic stem cells and progenitor cells (lineage-negative cells) from single-cell suspensions of bone marrow were obtained using a Mouse Hematopoietic Progenitor Enrichment Kit (STEMCELL Technologies). For bone marrow transplantation into newborn mice, 1- to 2-d-old WT and TPH1−/− pups were given single i.p. injections of busulfan (10 mg/kg) (Busilvex; Pierre Fabre). The newborn mice then underwent transplantation 24 h after the busulfan injection by a temporal vein injection of 1 × 106 lineage-negative cells negatively selected from WT and TPH1−/− donor mice.
Statistics.
Results are expressed as mean ± SEM. Statistical analyses were carried out by the StatView analysis program (SAS Institute, Cary, NC), using a two-way ANOVA to compare differences between genotypes. P values less than 0.01 were considered to be significant. *P < 0.01 versus WT, **P < 0.001 versus WT, ***P < 0.0001 versus WT.
For additional details, see SI Materials and Methods.
Supplementary Material
Acknowledgments
We are grateful to Pascale Chanterenne and Hugo Becque for maintaining the mouse colony. We thank Agnès Ostertag and Caroline Marty for their excellent technical assistance, and finally our gratitude goes to Robert Olaso for designing the primers. We also thank François Xavier Dieudonné for help in carrying out the bone marrow transplantations. The work was funded by Agence Nationale de la Recherche (07-e-RARE-010-01 OSTEOPETR). Y.C.-A. was the recipient of a fellowship from the Ministère Supérieur de l'Enseignement et de la Recherche.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1117792109/-/DCSupplemental.
References
- 1.Bianco P, Riminucci M, Gronthos S, Robey PG. Bone marrow stromal stem cells: Nature, biology, and potential applications. Stem Cells. 2001;19(3):180–192. doi: 10.1634/stemcells.19-3-180. [DOI] [PubMed] [Google Scholar]
- 2.Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289:1504–1508. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- 3.Khosla S. Minireview: The OPG/RANKL/RANK system. Endocrinology. 2001;142:5050–5055. doi: 10.1210/endo.142.12.8536. [DOI] [PubMed] [Google Scholar]
- 4.Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Annu Rev Med. 2009;60:355–366. doi: 10.1146/annurev.med.60.042307.110802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaumann AJ, Levy FO. 5-Hydroxytryptamine receptors in the human cardiovascular system. Pharmacol Ther. 2006;111:674–706. doi: 10.1016/j.pharmthera.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Hamel E. Serotonin and migraine: Biology and clinical implications. Cephalalgia. 2007;27:1293–1300. doi: 10.1111/j.1468-2982.2007.01476.x. [DOI] [PubMed] [Google Scholar]
- 7.Spiller R. Serotonin and GI clinical disorders. Neuropharmacology. 2008;55:1072–1080. doi: 10.1016/j.neuropharm.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 8.Hannon J, Hoyer D. Molecular biology of 5-HT receptors. Behav Brain Res. 2008;195:198–213. doi: 10.1016/j.bbr.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 9.Walther DJ, et al. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science. 2003;299:76. doi: 10.1126/science.1078197. [DOI] [PubMed] [Google Scholar]
- 10.Côté F, et al. Disruption of the nonneuronal tph1 gene demonstrates the importance of peripheral serotonin in cardiac function. Proc Natl Acad Sci USA. 2003;100:13525–13530. doi: 10.1073/pnas.2233056100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Da Prada M, Pletscher A. Mechanisms of 5-hydroxytryptamine storage in subcellular organelles of blood platelets. Adv Biochem Psychopharmacol. 1974;10:311–320. [PubMed] [Google Scholar]
- 12.Ni W, et al. The existence of a local 5-hydroxytryptaminergic system in peripheral arteries. Br J Pharmacol. 2008;154:663–674. doi: 10.1038/bjp.2008.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsuda M, et al. Serotonin regulates mammary gland development via an autocrine-paracrine loop. Dev Cell. 2004;6:193–203. doi: 10.1016/s1534-5807(04)00022-x. [DOI] [PubMed] [Google Scholar]
- 14.Paulmann N, et al. Intracellular serotonin modulates insulin secretion from pancreatic β-cells by protein serotonylation. PLoS Biol. 2009;7:e1000229. doi: 10.1371/journal.pbio.1000229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yadav VK, et al. Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cell. 2008;135:825–837. doi: 10.1016/j.cell.2008.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yadav VK, et al. Pharmacological inhibition of gut-derived serotonin synthesis is a potential bone anabolic treatment for osteoporosis. Nat Med. 2010;16:308–312. doi: 10.1038/nm.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cui Y, et al. Lrp5 functions in bone to regulate bone mass. Nat Med. 2011;17:684–691. doi: 10.1038/nm.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goltzman D. LRP5, serotonin, and bone: Complexity, contradictions, and conundrums. J Bone Miner Res. 2011;26:1997–2001. doi: 10.1002/jbmr.462. [DOI] [PubMed] [Google Scholar]
- 19.Bliziotes MM, Eshleman AJ, Zhang XW, Wiren KM. Neurotransmitter action in osteoblasts: Expression of a functional system for serotonin receptor activation and reuptake. Bone. 2001;29:477–486. doi: 10.1016/s8756-3282(01)00593-2. [DOI] [PubMed] [Google Scholar]
- 20.Westbroek I, van der Plas A, de Rooij KE, Klein-Nulend J, Nijweide PJ. Expression of serotonin receptors in bone. J Biol Chem. 2001;276:28961–28968. doi: 10.1074/jbc.M101824200. [DOI] [PubMed] [Google Scholar]
- 21.Collet C, et al. The serotonin 5-HT2B receptor controls bone mass via osteoblast recruitment and proliferation. FASEB J. 2008;22:418–427. doi: 10.1096/fj.07-9209com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Warden SJ, Robling AG, Sanders MS, Bliziotes MM, Turner CH. Inhibition of the serotonin (5-hydroxytryptamine) transporter reduces bone accrual during growth. Endocrinology. 2005;146:685–693. doi: 10.1210/en.2004-1259. [DOI] [PubMed] [Google Scholar]
- 23.Slominski A, et al. Serotoninergic and melatoninergic systems are fully expressed in human skin. FASEB J. 2002;16:896–898. doi: 10.1096/fj.01-0952fje. [DOI] [PubMed] [Google Scholar]
- 24.Amireault P, et al. Ineffective erythropoiesis with reduced red blood cell survival in serotonin-deficient mice. Proc Natl Acad Sci USA. 2011;108:13141–13146. doi: 10.1073/pnas.1103964108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dürk T, et al. 5-Hydroxytryptamine modulates cytokine and chemokine production in LPS-primed human monocytes via stimulation of different 5-HTR subtypes. Int Immunol. 2005;17:599–606. doi: 10.1093/intimm/dxh242. [DOI] [PubMed] [Google Scholar]
- 26.Battaglino R, et al. Serotonin regulates osteoclast differentiation through its transporter. J Bone Miner Res. 2004;19:1420–1431. doi: 10.1359/JBMR.040606. [DOI] [PubMed] [Google Scholar]
- 27.Roth BL. Multiple serotonin receptors: Clinical and experimental aspects. Ann Clin Psychiatry. 1994;6(2):67–78. doi: 10.3109/10401239409148985. [DOI] [PubMed] [Google Scholar]
- 28.Bohn LM, Schmid CL. Serotonin receptor signaling and regulation via β-arrestins. Crit Rev Biochem Mol Biol. 2010;45:555–566. doi: 10.3109/10409238.2010.516741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mao D, Epple H, Uthgenannt B, Novack DV, Faccio R. PLCγ2 regulates osteoclastogenesis via its interaction with ITAM proteins and GAB2. J Clin Invest. 2006;116:2869–2879. doi: 10.1172/JCI28775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Negishi-Koga T, Takayanagi H. Ca2+-NFATc1 signaling is an essential axis of osteoclast differentiation. Immunol Rev. 2009;231:241–256. doi: 10.1111/j.1600-065X.2009.00821.x. [DOI] [PubMed] [Google Scholar]
- 31.Martin TJ, Sims NA. Osteoclast-derived activity in the coupling of bone formation to resorption. Trends Mol Med. 2005;11(2):76–81. doi: 10.1016/j.molmed.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 32.Karsdal MA, Martin TJ, Bollerslev J, Christiansen C, Henriksen K. Are nonresorbing osteoclasts sources of bone anabolic activity? J Bone Miner Res. 2007;22:487–494. doi: 10.1359/jbmr.070109. [DOI] [PubMed] [Google Scholar]
- 33.Parfitt AM. Bone histomorphometry: Proposed system for standardization of nomenclature, symbols, and units. Calcif Tissue Int. 1988;42:284–286. doi: 10.1007/BF02556360. [DOI] [PubMed] [Google Scholar]
- 34.Parfitt AM, et al. Bone histomorphometry: Standardization of nomenclature, symbols, and units: Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.