Abstract
Tryptophan catabolism mediated by indoleamine 2,3-dioxygenase (IDO1) is an important mechanism of peripheral immune tolerance contributing to tumoral immune resistance, and IDO1 inhibition is an active area of drug development. Tryptophan 2,3-dioxygenase (TDO) is an unrelated hepatic enzyme that also degrades tryptophan along the kynurenine pathway. Here, we show that enzymatically active TDO is expressed in a significant proportion of human tumors. In a preclinical model, TDO expression by tumors prevented their rejection by immunized mice. We developed a TDO inhibitor, which, upon systemic treatment, restored the ability of mice to reject TDO-expressing tumors. Our results describe a mechanism of tumoral immune resistance based on TDO expression and establish proof-of-concept for the use of TDO inhibitors in cancer therapy.
Keywords: cancer immunotherapy, small molecule inhibitor, immunomodulation, immune suppression
Tryptophan 2,3-dioxygenase (TDO) is a homotetrameric heme-containing cytosolic enzyme encoded by gene TDO2 and expressed at high levels in the liver. It catalyses the first and rate-limiting step of tryptophan degradation along the kynurenine pathway and thereby regulates systemic tryptophan levels. The same reaction can be catalyzed by another heme-containing cytosolic enzyme, named indoleamine 2,3-dioxygenase (IDO1), which has no sequence similarity with TDO, is not expressed in the liver, and is monomeric. IDO1 has been the focus of attention in recent years because of its immunosuppressive effects on T lymphocytes, resulting partly from tryptophan depletion and partly from direct effects of tryptophan catabolites (1–3). IDO1 is expressed constitutively in the placenta, where it plays a key role in feto-maternal tolerance (2), and in many tumors, where it contributes to tumoral resistance to immune rejection (4–7). IDO1 expression is also inducible in many cells, including dendritic cells, and appears to play a role in peripheral immune tolerance and the retro-control of immune responses (8). In contrast, little is known about the effect of TDO expression on the immune response. A recent report indicated that human cells transfected with TDO depleted tryptophan and thereby prevented both the growth of pathogens and the proliferation of allogeneic T lymphocytes (9). These results suggested that TDO might mediate immunosuppressive effects similar to those of IDO1. We set out to examine whether tumor cells express TDO and thereby inhibit T-cell–mediated immune responses.
Results
We first observed that many human tumor samples expressed gene TDO2 as measured by real-time RT-PCR (Table 1). This was the case for 41% of bladder carcinomas, 50% of melanomas and 100% of hepatocarcinomas. To confirm the activity of TDO in tumor cells, we selected a series of human tumor cell lines that also expressed the TDO2 mRNA. We incubated cells for 24 h in medium containing a known concentration of tryptophan, and measured by HPLC in the supernatant the concentration of tryptophan and kynurenine, which is the main tryptophan catabolite (Table 2). HEK-293 cells transfected or not with human TDO2 were used as positive and negative controls. We observed a clear activity in cell lines expressing more than one copy of TDO2 mRNA per cell. Because some of these tumor lines also expressed IDO1, we used TDO inhibitor 680C91 (10) and IDO1 inhibitor 1-methyl-l-tryptophan (1MT) (4) to distinguish the activity of both enzymes in the cellular assay. In cell lines expressing TDO and not IDO1, tryptophan degradation was completely blocked by 680C91 and not by 1MT, as expected (Table 2). In cell lines expressing both enzymes, an additive effect of both inhibitors was observed (Table 2 and Fig. S1). The TDO activity of those lines and its direct inhibition by 680C91 were confirmed in an enzymatic assay performed on crude cell extracts, which is not dependent on the transport of the substrate and the inhibitor across the cell membrane (Table 2 and Fig. S2).
Table 1.
TDO2 expression in human samples
|
TDO2 mRNA/cell in positive samples |
|||
| Sample type | TDO2-positive samples (no. positive/no. tested) | Mean | Min–max |
| Tumors | |||
| Bladder carcinoma | 9/22 | 23.7 | (2.6–125) |
| Hepatocarcinoma | 7/7 | 791.7 | (47.4–4,000) |
| Melanoma | 10/20 | 5.6 | (2.0–16.7) |
| Mesothelioma | 2/4 | 8.5 | (2.4–14.6) |
| Neuroblastoma | 2/3 | 5.2 | (2.1–8.4) |
| Sarcoma | 1/5 | 5.9 | (5.9) |
| Breast carcinoma | 2/17 | 12.2 | (7.8–16.7) |
| Leukemia | 1/25 | 16.7 | (16.7) |
| Renal cell carcinoma | 1/7 | 88.4 | (88.4) |
| Colorectal carcinoma | 2/7 | 9.2 | (4.8–13.6) |
| Head & neck carcinoma | 1/9 | 9.0 | (9.0) |
| Lung carcinoma | 2/8 | 11.4 | (3.6–19.2) |
| Brain tumor | 2/10 | 4.7 | (4.2–5.3) |
| Normal tissues | |||
| Liver | 3/3 | 876.3 | (406.1–1515.7) |
| Skin | 0/2 | 0.0 | (0.0) |
| Bladder | 0/2 | 0.1 | (0.05–0.2) |
| Breast | 0/3 | 0.1 | (0.0–0.1) |
| Blood | 0/2 | 0.0 | (0.0) |
Expression of TDO2 mRNA was measured by quantitative RT-PCR. Each cDNA was performed at least in duplicate. TDO2 expression was normalized to actin and the amount of mRNA molecules per cell was calculated. Tumor samples containing at least two molecules of TDO2 mRNA per cell were considered positive. For normal tissues, means and min-max are provided for all samples.
Table 2.
TDO expression and activity in tumor cell lines
| Kynurenine production (pmol kynurenine/hr/106 cells) |
||||||||
| Cellular assay† |
Assay on crude extract‡ |
|||||||
| Inhibitor | TDO2 mRNA/cell* | IDO1 mRNA/cell* | - | 680C91 | 1MT | 680C91+1MT | - | 680C91 |
| Human cell lines | ||||||||
| 293-E | 0.0 | 0.0 | 0 | 0 | 0 | |||
| 293-E hTDO cl105 | 993.1 | 0.0 | 2,462 | 0 | 2,051 | 0 | 1,111 (±13) | 61 (±9) |
| 293-E hTDO cl119 | 1,231.1 | 0.0 | 3,743 | 94 | 3,333 | 105 | 544 (±14) | 15 (±3) |
| HepG2 (hepatocarcinoma) | 0.0 | 0.0 | 0 | 0 | 0 | 0 | ||
| Huh7 (hepatocarcinoma) | 1.9 | 0.0 | 41 | 0 | 0 | 0 | ||
| LB159-CRC (colorectal carcinoma) | 67.0 | 0.0 | 2,007 | 0 | 1,869 | 0 | ||
| SK-CO-11 (colorectal carcinoma) | 0.5 | 0.0 | 0 | 0 | 0 | 0 | ||
| LB1317-SCCHN (head & neck carcinoma) | 58.3 | 19.2 | 2,973 | 715 | 2,310 | 303 | 3,252 (±14) | 277 (±19) |
| A172 (glioblastoma) | 31.2 | 1.6 | 3,753 | 119 | 3,775 | 0 | 708 (±7) | 18 (±5) |
| HTZ-349 (glioblastoma) | 0.1 | 0.0 | 0 | 0 | 0 | 0 | ||
| U-87-MG (astrocytoma) | 8.7 | 0.0 | 413 | 0 | 559 | 0 | ||
| SK-Mes-1 (lung carcinoma) | 6.3 | 0.4 | 1,117 | 926 | 515 | 354 | ||
| LB2259-MEL (melanoma) | 0.1 | 0.0 | 0 | 0 | 0 | 0 | ||
| MZ2-MEL (melanoma) | 0.3 | 0.0 | 0 | 0 | 0 | 0 | ||
| MZ-CHA-3 (gall-bladder carcinoma) | 4.5 | 35.9 | 534 | 366 | 217 | 144 | ||
| Murine cell lines | ||||||||
| Mastocytoma P815, subline P815B | 0.0 | 0.0 | ND | ND | ||||
| Transfected P815B cells: | ||||||||
| P815B cl1 (control) | 0.0 | 0.0 | 0 | 0 | 0 | |||
| P815B mTDO cl8 | 129.6 | 0.0 | 4,857 | 103 | 2,330 (±32) | 55 (±5) | ||
| P815B mTDO cl12 | 166.8 | 0.0 | 5,483 | 136 | 4,090 (±24) | 128 (±8) | ||
*Expression of human or mouse TDO2 and IDO1 mRNA was measured by quantitative RT-PCR, normalized to actin and expressed as the amount of mRNA molecules per cell. Each cDNA was tested at least in duplicate.
†Enzymatic activity was estimated by measuring tryptophan degradation and kynurenine production in the supernatant of 14-h (murine lines) or 24-h (human lines) cultures performed in the absence or presence of TDO inhibitor 680C91 (20 μM), IDO1 inhibitor 1-methyl-l-tryptophan (1MT, 400 μM) or both. Tryptophan and kynurenine were measured by HPLC. ND, not determined.
‡Enzymatic activity (± SEM) was estimated by measuring kynurenine production in the clarified cell extracts with l-tryptophan at 1 mM in the absence or presence of TDO inhibitor 680C91 (25 μM). The rate of catalysis was calculated within the linear phase of N-formylkynurenine production. Results are the mean value of three independent experiments performed in duplicate and with two measures for each duplicate.
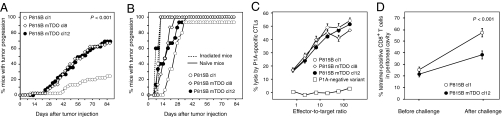
To determine whether TDO expression allows tumor cells to resist immune rejection by T cells, we used the P815 mouse tumor model (4). P815 tumor cells regularly produce tumors when injected intraperitoneally into naïve syngeneic DBA/2 mice, even though they are clearly immunogenic and express several tumor antigens recognized by cytolytic T lymphocytes (CTL). One of these antigens is encoded by cancer germ-line gene P1A and is the major target of the rejection response of mice immunized against P815 (11). Mice immunized against this antigen reject a challenge of P815 tumor cells injected in the peritoneal cavity (12). We transfected subline P815B, which does not express TDO2, with an expression plasmid containing the mouse TDO2 cDNA. We selected transfected P815B mTDO clones 8 and 12, which degraded tryptophan at levels similar to human tumor lines (Table 2). We then immunized mice against the P1A antigen and injected them 4 wk later with an i.p. challenge of transfected P815 cells. As expected, most mice rejected the control P815B clone 1, which was transfected with an empty vector and did not express TDO (Fig. 1A). In contrast, most mice injected with TDO-expressing tumor cells developed progressive tumors and died. This was true for both TDO-transfected clones. The three clones produced progressive tumors in all naïve mice, but the TDO-expressing tumors grew somewhat faster (Fig. 1B). This might result from the occurrence of a primary immune response that retards the growth of the tumors and is abolished with the TDO-expressing tumors. The intrinsic tumorigenicity of the three clones was similar, as indicated by their identical progression in irradiated mice (Fig. 1B). They also expressed identical levels of P1A mRNA, as measured by semiquantitative RT-PCR, and were equally lysed by P1A-specific CTL (Fig. 1C). These results were very similar to those we reported previously with IDO1-expressing tumor cells, and suggested that the progression of TDO-expressing tumors in P1A-immunized mice resulted from the ability of those tumors to prevent their rejection by T lymphocytes. To visualize this effect on T lymphocytes, we used H-2Ld/P1A tetramers to measure the number of P1A-specific CTL in the peritoneal cavity of immunized mice before and after the challenge with P815 tumor cells. Immunized mice contained detectable amounts of P1A-specific CTL, which further increased four days after challenge with P815B control cells (Fig. 1D). This increase was limited in mice challenged with TDO-expressing P815B mTDO cl12, suggesting that TDO expression reduces T lymphocyte proliferation locally.
Fig. 1.
Immune resistance of TDO-expressing tumors. (A) Mice (n = 45 per group) were immunized by i.p. injection of living L1210 cells expressing P1A and B7-1, and challenged 4 wk later by i.p. injection of 4 × 105 cells either P815B cl1 (○), or P815B-mTDO cl8 (◇), or P815B-mTDO cl12 (●). Tumor progression was monitored. Shown are the compiled results of three experiments, each involving groups of 15 mice. P < 0.001 for cl1 versus cl8 and for cl1 versus cl12 (Logrank test). (B) Naïve (solid line) (n = 15 per group) or irradiated mice (dashed line) (n = 5 per group, 650 cGy) were injected as in A. One representative experiment out of five is shown. (C) Lysis of TDO-transfected P815B cells by P1A-specific CTLs. P1A-specific CTLs were incubated with 51Cr-loaded TDO-transfected P815B cells at varying effector/target ratios. P815 variant P1.istA-B-, which has lost gene P1A, was used as a control target (□) P1A-negative variant). One representative experiment out of three is shown. (D) Proportion of P1A-specific T cells among CD8+ T cells in the peritoneal cavity of immunized mice, estimated using H-2Ld/P1A tetramers 1 d before or 4 d after i.p. challenge with 106 cells of P815B cl1 (○, n = 30 mice) or P815B-mTDO cl12 (●, n = 30 mice). Error bars represent SEM. P < 0.001 for cl1 versus cl12 after challenge (two-tailed Student t test). One representative experiment out of two is shown.
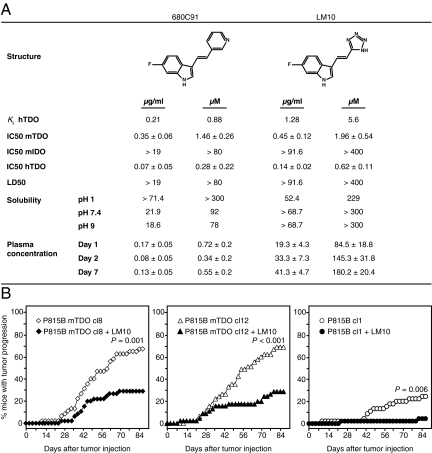
The results outlined above indicated that pharmacological inhibition of TDO could combat tumoral immune resistance and promote tumor rejection. So far, several compounds were reported as TDO inhibitors (10, 13–15). One series, initially developed by Madge and Salter as combined TDO/serotonin reuptake inhibitors for depression therapy, is characterized by a (fluoro)indole scaffold substituted in the 3-position by a pyridinyl-vinyl side chain. Among these compounds, the analog 680C91 represents a very attracting compound, as it is endowed with a good TDO inhibition potency and is deprived of activity on serotonin reuptake (10). As a first step, we assessed bioavailability of 680C91 in mice by the oral route. After administration of 160 mg/kg/day in the drinking water up to 7 d, we measured plasma concentrations of 680C91 below 0.2 μg/mL (0.8 μM), indicating the poor bioavailability of the compound, presumably related to its poor solubility (Fig. 2A). We thus started a medicinal chemistry program aimed at improving the aqueous solubility and bioavailability in this series (16). This eventually led to the discovery of compound LM10, which is characterized by a 6-fluoro-indole substituted in the 3-position by a tetrazolyl-vinyl side chain and displays a good TDO inhibition (Ki = 5.6 μM) with a competitive inhibition profile (Fig. 2A; ref. 16). LM10 does not inhibit IDO and has a high solubility and bioavailability (Fig. 2A). The plasma concentration of LM10 after oral administration of 160 mg/kg/day was between 20 and 40 μg/mL (87-175 μM), a concentration about 40 times above the IC50 measured in the cellular assay performed with the physiological concentration of plasma tryptophan (80 μM; Fig. 2A; ref. 17).
Fig. 2.
Reversal of immune resistance by systemic inhibition of TDO. (A) Structure, activity, physicochemical features, and bioavailability of TDO inhibitors 680C91 and LM10. Ki were measured on recombinant human TDO (hTDO) (16). A cellular assay based on mouse P815B-mTDO, mouse P815B-mIDO, or human 293E-hTDO cells was used to measure IC50, defined as the concentration giving 50% inhibition of TDO activity at a l-tryptophan concentration of 80 μM. Values ± SEM are shown corresponding to the mean of three independent experiments. LD50 was estimated by an MTT assay evaluating cell viability in the same cultures. Solubility was evaluated at room temperature as described in ref. 16. Bioavailability was estimated by measuring plasma concentration of the compounds after 1, 2, or 7 d of oral administration of 160 mg/kg/day. Mice (n = 10) received either normal drinking water or a solution of 1 mg/mL 680C91 at pH 2.5 or 1 mg/mL LM10 at pH 9, of which they drank an average of 4 mL/day. (B) Tumor progression in immunized mice (n = 45 per group) challenged by i.p. injection of 4 × 105 cells from either P815B-mTDO cl8 (diamonds, Left), P815B-mTDO cl12 (triangles, Center), or P815B cl1 (circles, Right) and treated (black symbol) or not (white symbol) with 160 mg/kg/day LM10 in the drinking water, starting one day before the injection of tumor cells. Shown are the compiled results of three experiments, each involving groups of 15 mice. The untreated groups are identical to those shown on Fig. 1A. Mice received either normal drinking water or a solution of LM10 at 1 mg/mL P = 0.001, P < 0.001, and P = 0.006 for treated versus untreated mice after challenge with cl8, cl12 and cl1, respectively (Logrank test).
To determine whether systemic treatment with LM10 was able to promote rejection of TDO-expressing tumors, we immunized and challenged mice as above and administered LM10 in the drinking water (Fig. 2B). We observed that systemic treatment of immunized mice with LM10 (160 mg/kg/day) prevented the growth of TDO-expressing P815 tumor cells. This was true for both TDO-transfected clones. Surprisingly, LM10 treatment also promoted better rejection of control clone P815B cl1, which does not express TDO. One explanation for this might be the expression of TDO by macrophages at the tumor site, as activation of macrophages by phorbol esters or intracellular infection was shown to induce TDO expression (18, 19). We confirmed expression of TDO2 mRNA in F4/80+ macrophages isolated from the peritoneal cavity of both control mice and immunized mice carrying ascitic P815B cl1 tumor cells (Fig. S3).
Mice treated with LM10 did not show obvious signs of toxicity. Because TDO is mostly expressed in the liver, we evaluated liver damage at the end of the experiment as a first attempt to evaluate the safety of LM10 treatment. After more than 100 d of systemic administration of LM10, we found no significant difference between treated and untreated mice for their plasma level of hepatic enzymes alkaline phosphatase (ALAP), γ-glutamyltransferase (γ-GT), alanine aminotransferase (ALAT), and aspartate aminotransferase (ASAT) (Table 3). Those levels remained within the normal range, suggesting the lack of liver toxicity of prolonged TDO inhibition with LM10.
Table 3.
Evaluation of liver toxicity of LM10 in mice
| Liver enzyme* | ALAT (IU/mL ± SEM) | ALAP (IU/mL ± SEM) | ASAT (IU/mL ± SEM) | γ-GT (IU/mL ± SEM) |
| Control† | 34.8 ± 2.4 | 74.4 ± 2.3 | 106.7 ± 8.8 | 3.0 ± 0.2 |
| LM10-treated‡ | 47.5 ± 3.3 | 92.7 ± 3.0 | 156.9 ± 8.1 | 2.8 ± 0.2 |
| Normal range§ | 21–83 | 68–89 | 70–215 | 1–4 |
*Enzyme activities were measured in the plasma. ALAP: alkaline phosphatase; γ-GT: γ-glutamyl transferase; ALAT: alanine aminotransferase, ASAT: aspartate aminotransferase.
†Group of 43 mice from the experiment reported in Fig. 2B, fed with normal drinking water and tested 107 d after tumor challenge. Shown are the compiled results of three independent assays.
‡Group of 82 mice from the experiment reported in Fig. 2B, fed with a solution of LM10 (1 mg/mL pH 9; 160 mg/kg/day) and tested 107 d after tumor challenge. Shown are the compiled results of three independent assays.
§Group of 7 untreated normal DBA/2 mice.
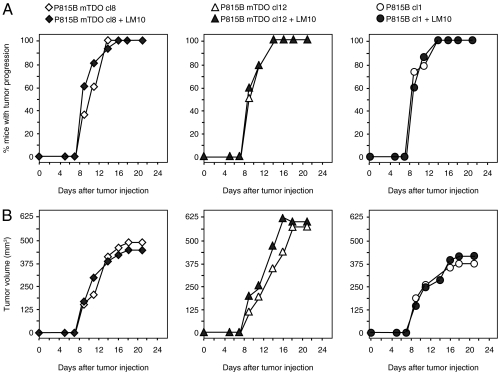
To determine whether the antitumor effects of LM10 resulted only from promoting tumor rejection or whether LM10 also had a direct effect on tumor cells, we injected immunodeficient RAG2 knockout mice with TDO-expressing P815 cells and treated them with LM10 as above. Tumor growth was not affected by LM10 treatment in RAG2 knockout mice, indicating that the effect of LM10 was dependent on the immune system in this model (Fig. 3).
Fig. 3.
Lack of antitumor effect of LM10 in RAG2 knockout mice. RAG2 knockout mice (15 per group) received a s.c. injection of 2 × 105 cells of the indicated P815 tumor clones (either P815B-mTDO cl8 (diamonds, Left), P815B-mTDO cl12 (triangles, Center), or P815B cl1 (circles, Right), and were treated (filled symbols) or not (open symbols) with 160 mg/kg/day LM10 in the drinking water (1 mg/mL) as in Fig. 2. The proportion of mice bearing progressive tumors in A and the mean tumor volume in B are reported. Tumor volume was calculated with formula (π x L x l2)/12. One representative experiment is shown out of two.
Discussion
Cancer immunotherapy is emerging as a promising approach for cancer treatment. Various strategies to modulate antitumor responses have been tested for many years and some of them recently proved clinically useful or even gained FDA approval (20–26). However, it is also becoming clear that an important limitation of cancer immunotherapy results from the ability of some tumors to resist immune rejection (27). A variety of mechanisms can account for such resistance, only some of which are amenable to modulation to improve the efficacy of cancer immunotherapy. One such mechanism is based on tryptophan catabolism by IDO1, which is frequently expressed in tumors (4, 6). We previously observed in a preclinical model that rejection of IDO1-expressing tumors was promoted by systemic treatment with an IDO1 inhibitor (4). The search for IDO1 inhibitors that can be used clinically is ongoing (7, 28–30). We show here that tumors also use another means to degrade tryptophan and resist immune rejection: they express TDO, the tryptophan-degrading enzyme normally expressed almost exclusively in the liver. Interestingly, the immunosuppressive effect of liver-expressed TDO might contribute to the allograft tolerance usually observed after liver transplantation, as recently suggested (9). Similarly, low levels of TDO were observed in early concepti, where they might contribute to tolerance of the embryo by the maternal immune system (31–33).
Using a TDO inhibitor, we proved, in a preclinical model, the concept that TDO inhibition promotes tumoral immune rejection. It will be of interest to evaluate the role of TDO in additional tumor models expressing various levels of TDO. The adverse effects of TDO inhibition appear limited. TDO is normally expressed at a high level in the liver and at low level in the brain (34). We did not observe obvious toxicity in mice treated with LM10 for 3 months. Moreover, mice genetically deficient for TDO2 were recently obtained and found viable and healthy (35). The main observation in those mice is a reduced anxiety, which may result from their highly increased serum levels of tryptophan. Indeed, tryptophan is the precursor of serotonin, and TDO inhibitors were previously considered as potential antidepressants (10).
A recent report published during the reviewing process of this paper corroborates our findings by showing that TDO is expressed constitutively in human glioblastomas and promotes tumor progression through the production of kynurenine acting as endogenous ligand of the aryl hydrocarbon receptor, resulting in increased tumor cell survival and motility, and reduced antitumor immune responses (36). In our tumor model, however, we did not observe a tumor cell autonomous effect of TDO activity, as TDO inhibition in vivo resulted in tumor control in immune competent but not in immunodeficient mice (Fig. 3).
Blocking both TDO and IDO1 to improve the efficacy of cancer immunotherapy would be complementary, not redundant: in a series of 104 human tumor lines of various histological types, we observed 20 tumors expressing only TDO2, 17 expressing only IDO1 and 16 expressing both (Table 4). Therefore, targeting both IDO1 and TDO would allow reaching 51% of tumors instead of 32% with IDO1 or 35% with TDO alone. Moreover, our results suggest that TDO inhibition might be useful also for tumors that do not intrinsically express TDO. If confirmed, this notion would further increase the proportion of tumors eligible for such a therapy.
Table 4.
TDO2 and IDO1 expression in human tumor cell lines
| Tumor type | TDO2 only | IDO1 only | TDO2 and IDO1 | Total |
| Colorectal carcinoma | 5/11 | 1/11 | 0/11 | 6/11 |
| Glioblastoma | 1/8 | 3/8 | 1/8 | 5/8 |
| Leukemia | 1/4 | 0/4 | 0/4 | 1/4 |
| Lymphoma | 0/4 | 0/4 | 0/4 | 0/4 |
| Melanoma | 0/12 | 2/12 | 0/12 | 2/12 |
| Mesothelioma | 1/7 | 2/7 | 3/7 | 6/7 |
| Myeloma | 1/3 | 0/3 | 0/3 | 1/3 |
| Head and neck carcinoma | 1/11 | 2/11 | 5/11 | 8/11 |
| Ovarian carcinoma | 0/1 | 1/1 | 0/1 | 1/1 |
| Pancreatic adenocarcinoma | 0/5 | 3/5 | 1/5 | 4/5 |
| NSCLC | 5/7 | 0/7 | 1/7 | 6/7 |
| SCLC | 0/6 | 0/6 | 0/6 | 0/6 |
| Sarcoma | 0/6 | 0/6 | 2/6 | 2/6 |
| Breast carcinoma | 1/4 | 1/4 | 0/4 | 2/4 |
| Bladder carcinoma | 0/3 | 1/3 | 2/3 | 3/3 |
| Hepatocarcinoma | 0/2 | 0/2 | 0/2 | 0/2 |
| Gall-bladder carcinoma | 0/1 | 0/1 | 1/1 | 1/1 |
| Renal cell carcinoma | 4/9 | 1/9 | 0/9 | 5/9 |
| Total | 20/104 | 17/104 | 16/104 | 53/104 |
Expression of TDO2 an IDO1 was evaluated by RT-PCR on RNA isolated from human tumor cell lines. NSCLC: non small cell lung carcinoma, SCLC: small cell lung carcinoma. In some tumor types, such as melanoma, the proportion of TDO2-expressing tumor lines is lower than the proportion of TDO2-expressing tumor samples (see Table 1). Two factors may explain this difference: (i) during establishment of the cell lines, TDO-expressing tumor cells may have been counterselected because of their tryptophan-depleting effect, as in most cases the cell lines were established in the absence of TDO inhibitor, and (ii) TDO might also be expressed by some stromal cells, such as activated macrophages, which are present in tumor samples and absent in tumor cell lines.
In conclusion, pharmacological inhibition of TDO might represent a safe and efficient approach for cancer therapy acting by promoting tumoral immune rejection, thereby leveraging cancer immunotherapy.
Materials and Methods
Mice.
DBA/2 mice aged 6–8 wk were purchased from Harlan, provided food and water ad libitum, and housed under specific pathogen-free conditions. Animals were immunized by injection of 106 live L1210.P1A.B7-1 cells into the peritoneal cavity and challenged 4 wk later by i.p. injection of 4 × 105 tumor cells, as described (4, 12). As indicated, mice were given LM10 in the drinking water (1 mg/mL, pH 9), of which they drank an average of 4 mL/day. For assessment of bioavailability, naïve mice (n = 10) were given a solution of 680C91 (1 mg/mL, pH 2.5) or LM10 (1 mg/mL, pH 9) instead of drinking water. Blood was collected from the retro-orbital sinus in Lithium-Heparin tubes (Microvette 500 LH, Sarstedt). After centrifugation at 11,000 × g for 10 min, plasma was collected and the concentration of compound was measured by HPLC-UV at 345 nm (680C91) or 326 nm (LM10). For the HPLC analysis, 50 μl of supernatant were mixed with 500 μl of acetonitrile to precipitate the proteins. After centrifugation, the supernatant was collected, concentrated in a speedvac, resuspended in a final volume of 100 μl of water, and injected onto an Onyx Monolithic C18 column (Phenomenex). Hepatic enzymes were assayed on a Synchron CX system according to the manufacturer's instructions (Beckman Coulter). The ethical committee of the Faculty of Medicine, Université Catholique de Louvain, approved all mouse experiments.
Further Details.
See SI Materials and Methods for details about:
- Cell lines and tumor samples
- Cellular assay for TDO and IDO activity
- Enzymatic assay for TDO activity on crude extracts
- Lysis assay
- Tetramer staining and fluorescence-activated cell sorting analysis
- Quantitative RT-PCR analysis of mRNA expression
- Cellular assay for TDO inhibition
- Compounds synthesis and characterization
- Synthesis of trans-3-(6-fluoro-1H-indol-3-yl)acrylonitrile
- Synthesis of trans-6-Fluoro-3-[2-(1H-tetrazol-5-yl)vinyl]-1H-indole (LM10)
- Statistics.
Supplementary Material
Acknowledgments
We are grateful to Dr. Lionel Pochet and Laurence Moineaux for their assistance in solubility evaluation and chemical synthesis, respectively. We also thank Marc Dieu for his assistance in HRMS determination, Dr. Francis Brasseur for providing tumor cDNA samples, Dr. Antonia Busse for statistical analysis, Dr. Pierre Coulie for critical reading of the manuscript, Dr. Ivan Théate for help with some mouse experiments, Sahida El Yassini for help with expression studies, Thérèse Aerts and Debora Piccolo for technical assistance, and Julie Klein for editorial assistance. This work was supported in part by grants from the Fonds National de la Recherche Scientifique (FNRS-Télévie, Grant 7.4.543.07F, Belgium), the European Union under the Sixth Programme (CancerImmunotherapy; LSHC-CT-2006-518234), the Fondation Contre le Cancer (Belgium), the Walloon Region (Programme d'Excellence CIBLES), and the Fonds Maisin (Belgium). R.F. is greatly indebted to the FNRS for the award of a postdoctoral research grant.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. V.C. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1113873109/-/DCSupplemental.
References
- 1.Munn DH, et al. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med. 1999;189:1363–1372. doi: 10.1084/jem.189.9.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Munn DH, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–1193. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 3.Terness P, et al. Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase-expressing dendritic cells: mediation of suppression by tryptophan metabolites. J Exp Med. 2002;196:447–457. doi: 10.1084/jem.20020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uyttenhove C, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. 2003;9:1269–1274. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 5.Muller AJ, DuHadaway JB, Donover PS, Sutanto-Ward E, Prendergast GC. Inhibition of indoleamine 2,3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Nat Med. 2005;11:312–319. doi: 10.1038/nm1196. [DOI] [PubMed] [Google Scholar]
- 6.Munn DH, Mellor AL. Indoleamine 2,3-dioxygenase and tumor-induced tolerance. J Clin Invest. 2007;117:1147–1154. doi: 10.1172/JCI31178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Löb S, Königsrainer A, Rammensee HG, Opelz G, Terness P. Inhibitors of indoleamine-2,3-dioxygenase for cancer therapy: can we see the wood for the trees? Nat Rev Cancer. 2009;9:445–452. doi: 10.1038/nrc2639. [DOI] [PubMed] [Google Scholar]
- 8.Grohmann U, Bronte V. Control of immune response by amino acid metabolism. Immunol Rev. 2010;236:243–264. doi: 10.1111/j.1600-065X.2010.00915.x. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt SK, et al. Antimicrobial and immunoregulatory properties of human tryptophan 2,3-dioxygenase. Eur J Immunol. 2009;39:2755–2764. doi: 10.1002/eji.200939535. [DOI] [PubMed] [Google Scholar]
- 10.Salter M, Hazelwood R, Pogson CI, Iyer R, Madge DJ. The effects of a novel and selective inhibitor of tryptophan 2,3-dioxygenase on tryptophan and serotonin metabolism in the rat. Biochem Pharmacol. 1995;49:1435–1442. doi: 10.1016/0006-2952(95)00006-l. [DOI] [PubMed] [Google Scholar]
- 11.Van den Eynde B, Lethé B, Van Pel A, De Plaen E, Boon T. The gene coding for a major tumor rejection antigen of tumor P815 is identical to the normal gene of syngeneic DBA/2 mice. J Exp Med. 1991;173:1373–1384. doi: 10.1084/jem.173.6.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brändle D, et al. The shared tumor-specific antigen encoded by mouse gene P1A is a target not only for cytolytic T lymphocytes but also for tumor rejection. Eur J Immunol. 1998;28:4010–4019. doi: 10.1002/(SICI)1521-4141(199812)28:12<4010::AID-IMMU4010>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 13.Civen M, Knox WE. The specificity of tryptophan analogues as inducers, substrates, inhibitors, and stabilizers of liver tryptophan pyrrolase. J Biol Chem. 1960;235:1716–1718. [PubMed] [Google Scholar]
- 14.Eguchi N, Watanabe Y, Kawanishi K, Hashimoto Y, Hayaishi O. Inhibition of indoleamine 2,3-dioxygenase and tryptophan 2,3-dioxygenase by beta-carboline and indole derivatives. Arch Biochem Biophys. 1984;232:602–609. doi: 10.1016/0003-9861(84)90579-4. [DOI] [PubMed] [Google Scholar]
- 15.Frieden E, Westmark GW, Schor JM. Inhibition of tryptophan pyrrolase by serotonin, epinephrine and tryptophan analogs. Arch Biochem Biophys. 1961;92:176–182. doi: 10.1016/0003-9861(61)90233-8. [DOI] [PubMed] [Google Scholar]
- 16.Dolusić E, et al. Tryptophan 2,3-dioxygenase (TDO) inhibitors. 3-(2-(pyridyl)ethenyl)indoles as potential anticancer immunomodulators. J Med Chem. 2011;54:5320–5334. doi: 10.1021/jm2006782. [DOI] [PubMed] [Google Scholar]
- 17.Widner B, Werner ER, Schennach H, Wachter H, Fuchs D. Simultaneous measurement of serum tryptophan and kynurenine by HPLC. Clin Chem. 1997;43:2424–2426. [PubMed] [Google Scholar]
- 18.Ren Q, Robertson SJ, Howe D, Barrows LF, Heinzen RA. Comparative DNA microarray analysis of host cell transcriptional responses to infection by Coxiella burnetii or Chlamydia trachomatis. Ann N Y Acad Sci. 2003;990:701–713. doi: 10.1111/j.1749-6632.2003.tb07447.x. [DOI] [PubMed] [Google Scholar]
- 19.Kohro T, et al. A comparison of differences in the gene expression profiles of phorbol 12-myristate 13-acetate differentiated THP-1 cells and human monocyte-derived macrophage. J Atheroscler Thromb. 2004;11:88–97. doi: 10.5551/jat.11.88. [DOI] [PubMed] [Google Scholar]
- 20.Hodi FS, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hwu P. Treating cancer by targeting the immune system. N Engl J Med. 2010;363:779–781. doi: 10.1056/NEJMe1006416. [DOI] [PubMed] [Google Scholar]
- 22.Longo DL. New therapies for castration-resistant prostate cancer. N Engl J Med. 2010;363:479–481. doi: 10.1056/NEJMe1006300. [DOI] [PubMed] [Google Scholar]
- 23.Kantoff PW, et al. IMPACT Study Investigators Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 24.Higano CS, et al. Sipuleucel-T. Nat Rev Drug Discov. 2010;9:513–514. doi: 10.1038/nrd3220. [DOI] [PubMed] [Google Scholar]
- 25.Robert C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 26.Ledford H. Melanoma drug wins US approval. Nature. 2011;471:561. doi: 10.1038/471561a. [DOI] [PubMed] [Google Scholar]
- 27.Boon T, Coulie PG, Van den Eynde BJ, van der Bruggen P. Human T cell responses against melanoma. Annu Rev Immunol. 2006;24:175–208. doi: 10.1146/annurev.immunol.24.021605.090733. [DOI] [PubMed] [Google Scholar]
- 28.Röhrig UF, et al. Rational design of indoleamine 2,3-dioxygenase inhibitors. J Med Chem. 2010;53:1172–1189. doi: 10.1021/jm9014718. [DOI] [PubMed] [Google Scholar]
- 29.Yue EW, et al. Discovery of potent competitive inhibitors of indoleamine 2,3-dioxygenase with in vivo pharmacodynamic activity and efficacy in a mouse melanoma model. J Med Chem. 2009;52:7364–7367. doi: 10.1021/jm900518f. [DOI] [PubMed] [Google Scholar]
- 30.Katz JB, Muller AJ, Prendergast GC. Indoleamine 2,3-dioxygenase in T-cell tolerance and tumoral immune escape. Immunol Rev. 2008;222:206–221. doi: 10.1111/j.1600-065X.2008.00610.x. [DOI] [PubMed] [Google Scholar]
- 31.Minatogawa Y, Suzuki S, Ando Y, Tone S, Takikawa O. Tryptophan pyrrole ring cleavage enzymes in placenta. Adv Exp Med Biol. 2003;527:425–434. doi: 10.1007/978-1-4615-0135-0_50. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki S, et al. Expression of indoleamine 2,3-dioxygenase and tryptophan 2,3-dioxygenase in early concepti. Biochem J. 2001;355:425–429. doi: 10.1042/0264-6021:3550425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tatsumi K, et al. Induction of tryptophan 2,3-dioxygenase in the mouse endometrium during implantation. Biochem Biophys Res Commun. 2000;274:166–170. doi: 10.1006/bbrc.2000.3115. [DOI] [PubMed] [Google Scholar]
- 34.Miller CL, et al. Expression of the kynurenine pathway enzyme tryptophan 2,3-dioxygenase is increased in the frontal cortex of individuals with schizophrenia. Neurobiol Dis. 2004;15:618–629. doi: 10.1016/j.nbd.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 35.Kanai M, et al. Tryptophan 2,3-dioxygenase is a key modulator of physiological neurogenesis and anxiety-related behavior in mice. Mol Brain. 2009;2:8. doi: 10.1186/1756-6606-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Opitz CA, et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011;478:197–203. doi: 10.1038/nature10491. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.