Abstract
Platelets are important mediators of blood coagulation that lack nuclei, but contain mitochondria. Although the presence of mitochondria in platelets has long been recognized, platelet mitochondrial function remains largely unaddressed. On the basis of a small amount of literature that suggests platelet mitochondria are functional, we hypothesized that the inhibition of platelet mitochondria disrupts platelet function and platelet-activated blood coagulation. To test this hypothesis, members of the tetrazole, thiazole, and 1,2,3-triazole families of small molecule heterocycles were screened for the ability to inhibit isolated mitochondrial respiration and coagulation of whole blood. The families of heterocycles screened were chosen on the basis of the ability of the heterocycle family to inhibit a biomimetic model of cytochrome c oxidase (CcO). The strength of mitochondrial inhibition correlates with each compound's ability to deter platelet stimulation and platelet-activated blood clotting. These results suggest that for this class of molecules, inhibition of blood coagulation may be occurring through a mechanism involving mitochondrial inhibition.
Keywords: synthetic functional model, electron transport system, oxidative phosphorylation
Platelets constitute a key component of blood coagulation. Platelets are directly involved in a number of functions necessary for clotting, including recognition of vascular lesions, triggering activation of the coagulation cascade, and activation of other platelets. The platelet membrane serves as a scaffold for clot formation, and platelets are involved in the activation and co-catalysis of reactions involving many of the soluble clotting factors (1). Like red blood cells, platelets lack nuclei and consequently are unable to replace damaged proteins encoded in the nuclear genome. However, unlike red blood cells, platelets contain actively metabolizing mitochondria (2). Some hints as to the role these mitochondria play in platelet function have been elucidated (3). Along with glycogen granules, platelet mitochondria provide energy that is needed at least indirectly for platelet aggregation and secretion of procoagulant molecules (4). More direct evidence of a role for mitochondria in coagulation rests on observations that changes in the permeability of mitochondrial membranes are linked to changes in coagulation activity (5, 6). These facts imply that inhibition of platelet mitochondrial function should have an inhibitory effect upon platelet-activated blood coagulation.
Experimental investigation led to the discovery of three families of small molecule heterocycles that reversibly inhibit mitochondrial respiration and attenuate platelet-activated blood coagulation. These three families of compounds comprise unique examples of a class of anticoagulants proposed to inhibit blood clotting through a mitochondrial mechanism (Fig. 1).
Fig. 1.
(A) Schematic of a biomimetic model of CcO tethered to a gold electrode via a self-assembled monolayer (SAM). (B) Linear sweep voltammograms showing the model's electrocatalytic O2 reduction (solid black line), its inhibition by a 1-mM solution of tetrazole (red line), and the recovery of its catalysis after removing the solution of tetrazole (dotted black line). (C) Percent inhibition of peak catalytic current by 1-mM solutions of representative compounds from different families of heterocycles.
The discovery of these particular families of platelet inhibitory molecules occurred after initial work from the Collman laboratory related to biomimetic modeling of cytochrome c oxidase (CcO). CcO is the terminal enzyme in the electron transport chain that catalyzes the four-electron reduction of O2 to H2O, and as such, lies at the heart of mitochondrial oxidative metabolism (7). A few years ago, the Collman group developed a synthetic model that faithfully mimics the reaction catalyzed by CcO with high selectivity at physiological pH and potential (8). In the present study, electrochemical measurements of the CcO model attached to a self-assembled monolayer (SAM) on a gold electrode were used to screen various families of heterocycles for their ability to inhibit O2 reduction (Fig. 1). Once representative examples of tetrazoles, thiazoles, and 1,2,3-triazoles were found to inhibit the CcO model, members of the respective molecular families were evaluated for inhibition of mitochondria isolated from fish liver (Fig. 2). A number of members of the tetrazole, thiazole, and 1,2,3-triazole molecular families were found to be active inhibitors of mitochondrial respiratory function, and the addition of these compounds to solutions containing whole blood markedly inhibited platelet-activated coagulation.
Fig. 2.
Heterocycles screened for inhibition of mitochondrial function and blood coagulation.
Results and Discussion
Cytochrome c Oxidase Model Inhibition.
In the absence of an inhibitor, electrocatalytic O2 reduction by the CcO model yields increasing current until it is limited by bulk diffusion of O2 to the catalytic site (Fig. 1B, solid black line). When the catalyst is immersed in a solution of an inhibitor, the O2 reduction current decreases and peaks at a greater overpotential, indicating that the catalysis is inhibited (Fig. 1B, red line). Subsequently removing the inhibitor from the catalyst surface restores the catalytic current to almost its original value (Fig. 1B, dotted black line). These findings demonstrate that the compound is a reversible inhibitor of the CcO model. Members of the tetrazole, thiazole, and 1,2,3-triazole families were found to be inhibitors of the CcO model (Fig. 1C) and identified as possible inhibitors of mitochondrial function.
Mitochondrial Inhibition Studies.
After the identification of tetrazoles, thiazoles, and 1,2,3-triazoles as potential inhibitors of mitochondrial function, a number of examples from each family (Fig. 2, compounds 1a–3c) were evaluated for mitochondrial inhibition by measuring their ability to decrease the rate of oxygen consumed by respiring mitochondria isolated from fish liver. Fish liver mitochondria were chosen due to the relative ease of procurement and their robust properties after isolation (9–12). From a titration curve of each potentially inhibiting compound, the concentration of compound resulting in a 50% inhibition of respiration (IC50) was determined (example shown in Fig. 3). The reversibility of the inhibition was determined by centrifuging the mitochondrial suspension that had been incubated in an inhibiting solution of the compound to produce a mitochondrial pellet. After washing and resuspending the pellet in buffer, the mitochondrial respiration rate was restored to near its original value (Fig. 3, red triangle), indicating the compound's reversibility as an inhibitor of mitochondria. Several of the tetrazoles, thiazoles, and 1,2,3-triazoles tested were found to be reversible inhibitors, although the most potent inhibitor, 2d, proved to be irreversible.
Fig. 3.
Percent inhibition of mitochondrial respiration versus concentration of tetrazole (black circles). Separating tetrazole from the mitochondria by centrifugation after incubating the mitochondria in a 450-mM solution restores respiration to very near its original value (89 ± 7% of initial value, denoted by red triangle).
Platelet Activity and Blood Clotting Inhibition Studies.
The anticoagulant properties of 1a–3c were assessed by adding solutions of the compounds to human whole blood and testing for platelet-activated clotting function using an assay previously developed in the Bull laboratory (13). In this assay, citrated whole blood containing native platelets is recalcified, diluted with a disclosure reagent consisting of calcined diatomaceous earth (no platelet agonists are used), and placed in a temperature-controlled rotating cuvette. Native platelets in this environment will clump (the clumps are easily visualized due to the disclosure reagent); the clumps then aggregate into larger masses that rapidly increase in size and shortly stick to the walls of the cuvette. The times to “clumping” and to “sticking” are two of the three end points recorded for this assay. The final end point (“clotting”) is the time taken for these clumped/aggregated platelets to bring about clotting of the whole blood sample. All three end points are shortened in samples with very active platelets and correspondingly lengthened in samples with inhibited platelets. The times required for platelet clumping, sticking, and clotting are variably affected by platelet activity. Of the three, the time required for platelet clumping is most dependent on platelet function (13).
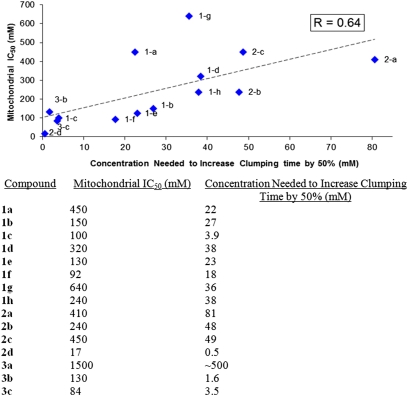
The relationship between inhibitor concentrations required for mitochondrial inhibition and inhibitor concentrations required for inhibition of platelet clumping is shown in Fig. 4. The results indicate that most of the mitochondrial inhibitors increase the time required for platelet clumping, sticking, and blood clotting to occur and that the compounds alter platelet clumping to the greatest extent. In most cases, more than 50% inhibition is observed with inhibitor concentrations of 1–100 mM (Fig. 4 and Fig. S1). Tetrazoles 1b and 1e are among the most effective anticoagulants, increasing clumping and sticking time by ∼10-fold and clotting time by ∼2-fold at higher dosage levels. There is also a positive correlation between the strength of a mitochondrial inhibitor and its ability to deter platelet clumping (R = 0.64, Fig. 4) as well as sticking and clotting (Fig. S1). The correlation between mitochondrial inhibition and platelet inhibition suggests that the effect of the compounds on platelet function is mediated through the mitochondria. The low coefficient of correlation is not especially surprising because isolated mitochondria are unaffected by a compound's membrane transport properties and interactions with other proteins in whole blood.
Fig. 4.
Correlation between strength of mitochondrial inhibition and decrease in platelet clumping activity. IC50 values for mitochondrial respiration were determined using a curve fit to a plot of percent mitochondrial inhibition versus heterocycle concentration as shown in Fig. 3 (remainder of data shown in Table S1). The concentrations required to increase clumping time by 50% were determined using a curve fit to plots of time required for platelet clumping versus heterocycle concentration (data shown in Table S2). Although consistent with the trend, 3a is not shown on the plot due to the difficulty of encompassing all of the data on the same graphical scale.
The concentrations of inhibitors required to induce significant mitochondrial inhibition are very high from a drug-targeting perspective. Whereas this may imply a nonspecific effect, it is noteworthy that oxidative metabolism in platelet mitochondria does not diminish until oxygen concentrations fall below 2.5 μM (14), suggesting CcO and by extension the electron transport system must be markedly inhibited before the rate of oxidative metabolism decreases. It is possible that the high inhibitor concentrations are required to effectively shut down oxidative metabolism, particularly as these compounds are small and relatively simple molecules. As such, they should exhibit generally weak interactions with proteins and enzymes. Although studies of a biomimetic CcO model allowed for the discovery of families of heterocycles as viable candidates for reversible mitochondrial inhibitors, the correlation between the CcO model and mitochondrial inhibition is weak. This suggests that other complexes in the electron transport chain may be implicated in the mitochondrial inhibition; indeed, 2a shares the same core structure as myxothiazol, a well-known inhibitor of complex III (15). Further work is required to fully elucidate the mechanism of inhibition including the precise point(s) of inhibition within the electron transport system.
The connection between platelet functionality and clotting implies possible usefulness of platelet mitochondrial inhibitors as whole blood anticoagulants. These findings may have particular clinical significance as most of the tetrazoles, thiazoles, and 1,2,3-triazoles identified in this paper are reversible inhibitors of mitochondrial respiration. The reversibility of mitochondrial inhibition by these compounds is very important because irreversible inhibitors, such as carbon monoxide, azide, and rotenone, are well-known metabolic poisons (16–18). In addition, many of the heterocycles described herein have low reported toxicities (19–22). Further studies are needed to learn more about the toxicology of these inhibitors and about their tissue distribution, metabolism, and binding affinities to other heme-based proteins such as cyclooxygenase and thromboxane A.
In conclusion, we have discovered a unique class of anticoagulants that inhibit platelet function, presumably by inhibiting mitochondrial respiration. These findings strongly imply that platelet activation and platelet-activated blood clotting are dependent upon mitochondrial function.
Materials and Methods
Chemicals.
Compounds 1a, 3b, and 3c, the CcO model, (4-((4-azidophenyl)ethynyl)phenyl)methanethiol, and Tris-(benzyltriazolylmethyl)amine (TBTA) were synthesized according to literature procedures (23–28). The compound 1d was obtained from the Developmental Therapeutics Program Open Chemical Repository at the National Cancer Institute. The compound 2d was synthesized in two steps from 2-(4-methylthiazol-5-yl)ethanol (Fig. S2). All other chemicals were purchased commercially and used without further purification.
Gold Electrodes.
Silicon wafers (111) were cleaned in piranha solution containing H2O2 (50 mL) and H2SO4 (150 mL) for 1 min (warning: piranha solutions are extremely corrosive and can cause explosions in presence of organic molecules; proper precautions and safety procedures are advised), rinsed thoroughly with water, and dried in an isopropanol bath before metal deposition. A titanium adhesion layer (50 Å) followed by a gold layer (500 Å) were deposited on the cleaned silicon wafers via a home-built electron beam vacuum deposition apparatus.
SAM Formation.
SAM formation was accomplished using a method similar to a published procedure (29). The gold surfaces were first cleaned electrochemically in 0.5 M H2SO4 by repeatedly scanning at 250 mV/s from 0 mV to 1,650 mV and back to 0 mV until a stable gold reduction peak at ∼850 mV was observed. After cleaning, the surfaces were washed with water and ethanol. A solution containing 0.04 mM (4-((4-azidophenyl)ethynyl)phenyl)methanethiol (aromatic azide-terminated thiol) and 0.36 mM 1-octanethiol in ethanol was sonicated for 1 min. The electrode was then immersed in this mixed thiol solution for 1 h before it was washed with ethanol.
Click Reaction.
Azide-alkyne click chemistry was used to attach the CcO model to the gold surface by exposing an azide-modified gold surface to a solution (click solution) containing the CcO model along with all of the reagents necessary to irreversibly attach the model to the surface. Click solutions were prepared in an inert atmosphere box. The solutions were comprised of a mixture of Cu(NO3)2 (800 μM), TBTA (800 μM), sodium ascorbate (800 μM), and the CcO model (80 μM) in 3:2 DMSO:H2O. A mixed azide-modified gold electrode was then transferred to the inert atmosphere box and exposed to the click solution for 2 h. The surface was then removed from the box and rinsed with ethanol, dichloromethane, ethanol, and water and immediately used for electrochemical measurements.
Electrochemical Studies.
Electrochemical studies were carried out using a Pine E-chem station RDE 5 with three electrodes set up with platinum as the counter electrode. An Ag/AgCl/saturated NaCl reference electrode was used and the values reported herein are corrected to normal hydrogen electrode (NHE). Linear sweep voltammetry (LSV) was performed in air-saturated pH 7 phosphate buffer with KPF6 (100 mM) as a supporting electrolyte.
CcO Model Inhibition Studies.
LSV was performed at 50 mV/s from 700 mV to −300 mV. The effect of the inhibitor was then investigated with an additional voltammogram using the same electrochemical parameters. This second voltammogram was performed in a 1-mM solution of the inhibitor in the same pH 7 KPF6 buffer solution.
The strengths of the inhibitors were determined by comparing the peak current of the voltammogram in buffer with the current of the voltammogram with inhibitor at the same potential. For each surface, the percent decay of the catalyst was evaluated, and the data were corrected for the catalyst decay to obtain the actual inhibition percentage.
Isolation of Mitochondria.
Mitochondria were isolated from the liver of tilapia (Sarotheridon mossambica) using a modified literature procedure (10). The fish were purchased from a commercial source, killed by concussion, and stored on ice for less than 1 h. The livers were dissected and then minced on an ice-cooled glass plate in approximately six volumes of pH 7.4 buffer containing KH2PO4 (10 mM), sucrose (250 mM), EDTA (0.5 mM), and fatty acid-free BSA (1 mg/mL). The liver was pulverized using a Dounce homogenizer before it was centrifuged at 500 × g for 10 min at 0 °C. The supernatant was then filtered through glass wool to remove any loose lipid material. The obtained filtrate was centrifuged at 10,000 × g for 10 min at 0 °C to sediment a crude mitochondrial pellet. The gold-colored mitochondrial material of the pellet was then resuspended in fresh pH 7.4 buffer (5 mL), homogenized, and centrifuged at 10,000 × g for 10 min at 0 °C. This resuspension procedure was repeated two additional times to remove any nonmitochondrial material. The final mitochondrial pellet was resuspended in ∼2 mL of pH 7.5 buffer containing Tris- HCl (50 mM), NaCl (100 mM), EDTA (0.1 mM), and DTT (1 mM). Using the Bradford assay (30) with BSA as a standard, the protein content of the suspension was measured to vary between 0.4 and 1.1 mg/mL, depending upon sample preparation.
Inhibition of Mitochondrial Respiration.
All inhibition studies were performed within 12 h of the isolation of the mitochondria. Mitochondrial preparations were stored on ice throughout this time period and remained active. Mitochondrial function began to diminish significantly only after more than 24 h of storage. Mitochondrial respiration rates were measured with a Clark oxygen electrode in a closed glass chamber. The respiration chamber was filled with an air-saturated pH 7.0 respiration buffer (1.2 mL) containing KH2PO4 (100 mM), MgCl2 (500 mM), glycine (500 mM), Hepes (50 mM), ADP (0.17 mM), malic acid (6.7 mM), and succinic acid (6.7 mM). The temperature of the chamber was kept constant at 20 °C using a circulating water bath, and its contents were magnetically stirred.
A suspension of mitochondria (100 μL) was incubated in the appropriate concentration of inhibitor in a pH 7.0 solution of KH2PO4 (100 mM) for 2 min at 0 °C. DMSO was added as a co-solvent if the inhibitor did not fully dissolve. The suspension of mitochondria and the inhibitor were then injected into the respiration chamber via syringe, and the respirometer was allowed to stabilize for 1 min before data collection commenced.
The oxygen concentration of the cell was recorded five times per second for 3 min with data acquisition software (WinDAQ). Respiration rates were averaged over this time period and subtracted by the background rate of oxygen consumption by the electrode. For each inhibitor, the mitochondrial respiration rate was measured at different inhibition incubation concentrations. The percentage of mitochondrial inhibition was then determined by comparing these rates to the respiration rate in the absence of inhibitor in the appropriate solvent system. The half maximal inhibitory incubation concentration (IC50) for a compound was calculated by fitting a linear or sigmoidal equation to the percent inhibition versus incubation concentration of inhibitor. For a mitochondrial preparation containing 0.50 mg/mL protein, tetrazole had an IC50 value of 450 mM. Inhibition by tetrazole was measured on each fresh batch of mitochondria. The IC50 values of other inhibitors were then multiplied by a normalization factor of measured tetrazole inhibition to correct for differences in mitochondrial activity among different mitochondrial preparations.
Reversibility Measurements.
A suspension of mitochondria (100 μL) was incubated in the appropriate volume of inhibitor in a pH 7.0 solution of KH2PO4 (100 mM) for 2 min at 0 °C to give the IC50 concentration. The mitochondria were then pelleted out of solution by centrifugation at 10,000 × g for 4 min at 0 °C, resuspended in the respiration buffer described above (1.2 mL), and centrifuged and resuspended once more, before they were monitored with the oxygen electrode. An inhibitor was considered “reversible” if the mitochondrial respiration rate after removing the inhibitor returned to greater than 75% of its value in the absence of inhibitor. Reversibility measurements were performed ∼15 min after the initial inhibitor incubation period.
Evaluation of Blood Clotting.
Platelet function testing was performed using a method previously described in the literature (13). The method allows for simultaneous evaluation of platelet function and platelet-activated blood clotting. Testing is performed by adding 150 μL of citrated blood to a test tube containing a calcium/saline suspension of Celite and glass beads. The test tube containing the mixture is then placed nearly horizontally into a device that rotates the tube at a steady rate while holding temperature constant at 37 °C and illuminating the tube for easy visualization. Normal platelets undergo visible clumping, followed by the clumps sticking to the walls of the revolving tube. Ultimately, a blood clot forms. End points are determined visually and inhibition is calculated as the percent change from baseline time for each of the end points.
In preparation for the studies, an additive solution was made by suspending 160 mg of Celite in 8 mL of water. A total of 10–15 glass beads and 50 μL of the additive solution were added to a 12 × 75 mm test tube. The tube and its contents were frozen and lyophilized until dry (a minimum of 4 h). The final contents of the tube included 10–15 glass beads and 1 mg of Celite. The tube was capped with Parafilm and stored indefinitely at room temperature until needed.
A stock solution of each inhibitor to be tested was made by dissolving a known quantity of each inhibitor in 0.9% NaCl solution. The stock solution was then diluted with additional 0.9% NaCl solution and used as diluent to make a 25 mM CaCl2 working solution containing the required inhibitor dosage. A control solution was made in the same manner except the inhibitor was omitted.
Blood specimens were obtained from healthy volunteer donors who were not taking any platelet-inhibiting medications. All blood collections and studies of human subjects were cleared through the institutional review board at Loma Linda University. The donors were drawn by trauma-free venipuncture into standard citrate-containing Vacutainer tubes. After phlebotomy, the blood-containing tubes were mixed gently by inversion four to five times. Other than this gentle inversion, agitation was avoided. After mixing the collection tube by inversion, the blood was immediately transferred to a 5-mL plastic syringe. If immediate transfer could not be made, the blood was mixed by two to three inversions immediately before transferring.
A total of 450 μL of the inhibitor or control solution was added to a prewarmed lyophilized Celite 289 tube. A total of 150 μL of blood was added and a timer was started. End points were determined visually by observing platelet clumping, sticking, and clotting as previously described. Clumping is defined as occurring when the platelets begin to adhere to each other and with the Celite, forming visible clumps, but the clumps do not stick to the walls of the tube. The clumping end point is recorded when the clumps are approximately the same size as the glass beads. Sticking is defined when the platelet clumps adhere to the walls and are carried around with the rotating tube. Clotting is recorded when fibrin strands form within the sample tube.
Recorded times for clumping, sticking, and clotting in the presence of inhibitor were divided by the corresponding times in the absence of inhibitor and multiplied by 100 to yield a percent baseline. A separate control was performed for each experiment. Repeat testing of control material demonstrated variability of less than 20%. Consequently, 120% was subtracted from each result, yielding a minimum percent inhibition. The absolute inhibitor quantity present in the tube during testing was divided by the volume of whole blood (150 μL) present in the tube to yield an inhibitor dosage relative to whole blood.
Supplementary Material
Acknowledgments
We thank Dr. Ali Hosseini for help with the electrochemical work and Sheryl Aka for technical assistance with the platelet function assays. This work was supported by National Institutes of Health Grant GM-069658 and a grant-in-aid from the Department of Pathology, Loma Linda University School of Medicine.
Footnotes
Conflict of interest statement: A patent application has been filed by the authors on the compounds described in this paper.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1120645109/-/DCSupplemental.
References
- 1.Kaushansky K, et al. Williams Hematology. 8th Ed. New York: McGraw-Hill; 2010. pp. 1735–1814. [Google Scholar]
- 2.Harmening DM. Clinical Hematology and Fundamentals of Hemostasis. 5th Ed. Philadelphia: FA Davis; 2009. pp. 550–551. [Google Scholar]
- 3.Hillman R, Ault K, Leporrier M, Rinder H. Hematology in Clinical Practice. 4th Ed. Columbus, OH: McGraw-Hill; 2005. [Google Scholar]
- 4.Salganicoff L, Fukami MH. Energy metabolism of blood platelets. I. Isolation and properties of platelet mitochondria. Arch Biochem Biophys. 1972;153:726–735. doi: 10.1016/0003-9861(72)90391-8. [DOI] [PubMed] [Google Scholar]
- 5.Remenyi G, Szasz R, Friese P, Dale GL. Role of mitochondrial permeability transition pore in coated-platelet formation. Arterioscler Thromb Vasc Biol. 2005;25:467–471. doi: 10.1161/01.ATV.0000152726.49229.bf. [DOI] [PubMed] [Google Scholar]
- 6.Jobe SM, et al. Critical role for the mitochondrial permeability transition pore and cyclophilin D in platelet activation and thrombosis. Blood. 2008;111:1257–1265. doi: 10.1182/blood-2007-05-092684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferguson-Miller S, Babcock GT. Heme/copper terminal oxidases. Chem Rev. 1996;96:2889–2908. doi: 10.1021/cr950051s. [DOI] [PubMed] [Google Scholar]
- 8.Collman JP, et al. A cytochrome C oxidase model catalyzes oxygen to water reduction under rate-limiting electron flux. Science. 2007;315:1565–1568. doi: 10.1126/science.1135844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toninello A, Salvi M, Colombo L. The membrane permeability transition in liver mitochondria of the great green goby Zosterisessor ophiocephalus (Pallas) J Exp Biol. 2000;203:3425–3434. doi: 10.1242/jeb.203.22.3425. [DOI] [PubMed] [Google Scholar]
- 10.Tan AM, Xie CL, Qu SS, Ping K, Yu G. Microcalorimetric study of mitochondria isolated from fish liver tissue. J Biochem Biophys Methods. 1996;31:189–193. doi: 10.1016/0165-022x(95)00034-o. [DOI] [PubMed] [Google Scholar]
- 11.Weinstein RB, Somero GN. Effects of temperature on mitochondrial function in the Antarctic fish Trematomus bernacchii. J Comp Physiol B. 1998;168:190–196. [Google Scholar]
- 12.Johnson D, Lardy H. Isolation of liver or kidney mitochondria. Methods Enzymol. 1967;10:94–96. [Google Scholar]
- 13.Pappas JM, Westengard JC, Bull BS. Population variability in the effect of aspirin on platelet function. Implications for clinical trials and therapy. Arch Pathol Lab Med. 1994;118:801–804. [PubMed] [Google Scholar]
- 14.Arthur PG, Ngo CT, Moretta P, Guppy M. Lack of oxygen sensing by mitochondria in platelets. Eur J Biochem. 1999;266:215–219. doi: 10.1046/j.1432-1327.1999.00846.x. [DOI] [PubMed] [Google Scholar]
- 15.Thierbach G, Reichenbach H. Myxothiazol, a new inhibitor of the cytochrome b-c1 segment of th respiratory chain. Biochim Biophys Acta. 1981;638:282–289. doi: 10.1016/0005-2728(81)90238-3. [DOI] [PubMed] [Google Scholar]
- 16.Alonso JR, Cardellach F, López S, Casademont J, Miró O. Carbon monoxide specifically inhibits cytochrome c oxidase of human mitochondrial respiratory chain. Pharmacol Toxicol. 2003;93:142–146. doi: 10.1034/j.1600-0773.2003.930306.x. [DOI] [PubMed] [Google Scholar]
- 17.Palmieri F, Klingenberg M. Inhibition of respiration under the control of azide uptake by mitochondria. Eur J Biochem. 1967;1:439–446. doi: 10.1007/978-3-662-25813-2_60. [DOI] [PubMed] [Google Scholar]
- 18.Degli Esposti M. Inhibitors of NADH-ubiquinone reductase: An overview. Biochim Biophys Acta. 1998;1364:222–235. doi: 10.1016/s0005-2728(98)00029-2. [DOI] [PubMed] [Google Scholar]
- 19.O'Neal C, Sargent E, Bagdon W. Acute toxicologic evaluation of 4-methylthiazole. J Am Coll Toxicol. 1978;1:182–183. [Google Scholar]
- 20.Gross EG, Featherstone RM. Studies with tetrazole derivatives; some pharmacologic properties of 1,5-disubstituted tetrazoles. J Pharmacol Exp Ther. 1946;87:299–305. [PubMed] [Google Scholar]
- 21.Aguilar F, et al. Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food. Eur Food Safe Auth. 2007;455:1–76. [Google Scholar]
- 22.Mizojiri K, et al. Disposition of moxalactam and N-methyltetrazolethiol in rats and monkeys. Antimicrob Agents Chemother. 1987;31:1169–1176. doi: 10.1128/aac.31.8.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mihina JS, Herbst RM. The reaction of nitriles with hydrazoic acid: Synthesis of monosubstituted tetrazoles. J Org Chem. 1950;15:1082–1092. [Google Scholar]
- 24.Wang XJ, Zhang L, Krishnamurthy D, Senanayake CH, Wipf P. General solution to the synthesis of N-2-substituted 1,2,3-triazoles. Org Lett. 2010;12:4632–4635. doi: 10.1021/ol101965a. [DOI] [PubMed] [Google Scholar]
- 25.Mikhat'ev BI, Shatalov GV, Galkin VD, Kimsanov BK, Khuseinov K. Alkyl esters of mono- and dicarboxylic acids with 1,2,3-triazole rings. Dokl Akad Nauk Tadzh SSR. 1974;17:38–42. [Google Scholar]
- 26.Decréau RA, Collman JP, Yang Y, Yan Y, Devaraj NK. Syntheses of hemoprotein models that can be covalently attached onto electrode surfaces by click chemistry. J Org Chem. 2007;72:2794–2802. doi: 10.1021/jo062349w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Devaraj NK, Decreau RA, Ebina W, Collman JP, Chidsey CED. Rate of interfacial electron transfer through the 1,2,3-triazole linkage. J Phys Chem B. 2006;110:15955–15962. doi: 10.1021/jp057416p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan TR, Hilgraf R, Sharpless KB, Fokin VV. Polytriazoles as copper(I)-stabilizing ligands in catalysis. Org Lett. 2004;6:2853–2855. doi: 10.1021/ol0493094. [DOI] [PubMed] [Google Scholar]
- 29.Collman JP, Hosseini A, Eberspacher TA, Chidsey CED. Selective anodic desorption for assembly of different thiol monolayers on the individual electrodes of an array. Langmuir. 2009;25:6517–6521. doi: 10.1021/la8043363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.