Abstract
Invasive species represent a significant threat to global biodiversity and a substantial economic burden. Burmese pythons, giant constricting snakes native to Asia, now are found throughout much of southern Florida, including all of Everglades National Park (ENP). Pythons have increased dramatically in both abundance and geographic range since 2000 and consume a wide variety of mammals and birds. Here we report severe apparent declines in mammal populations that coincide temporally and spatially with the proliferation of pythons in ENP. Before 2000, mammals were encountered frequently during nocturnal road surveys within ENP. In contrast, road surveys totaling 56,971 km from 2003–2011 documented a 99.3% decrease in the frequency of raccoon observations, decreases of 98.9% and 87.5% for opossum and bobcat observations, respectively, and failed to detect rabbits. Road surveys also revealed that these species are more common in areas where pythons have been discovered only recently and are most abundant outside the python's current introduced range. These findings suggest that predation by pythons has resulted in dramatic declines in mammals within ENP and that introduced apex predators, such as giant constrictors, can exert significant top-down pressure on prey populations. Severe declines in easily observed and/or common mammals, such as raccoons and bobcats, bode poorly for species of conservation concern, which often are more difficult to sample and occur at lower densities.
Keywords: invasion biology, population declines, top-down regulation, reptiles
Invasive species represent one of the most significant threats to global biodiversity and ecosystem function (1). In the United States the cost of invasive species management exceeds $120 billion annually (2). Invasive species affect native ecosystems via alteration of habitat structure (3), competition (4), reduction of native predator populations (5), and alteration of trophic structure (6). Invasive predators can reduce or even extirpate native prey populations (7, 8).
Nonnative reptiles are increasingly recognized as problematic invaders (9). Most reptiles are predators that, as ectotherms, can direct large proportions of assimilated energy to growth, storage, and reproduction (9), often allowing them to persist at high densities and pose major risks to native wildlife (10). For example, Brown treesnakes (Boiga irregularis) introduced to Guam before 1950 devastated populations of native vertebrates (11), greatly altering natural ecosystems (12). However, treesnakes were not implicated in the decline of native vertebrates for more than 30 y (13). Unfortunately, the time from the establishment of an invasive reptile species to the recognition of impacts often is decades, and for many invasions, the historical data necessary to evaluate impacts are unavailable (10).
Burmese pythons (Python molurus bivittatus), large (up to 5.5 m) constrictors native to Southeast Asia (14), now are established across thousands of square kilometers in southern Florida, including all of Everglades National Park (ENP) (Fig. 1) (15). Pythons were sighted intermittently in ENP for about 20 y before 2000, when they first were recognized as being established (16); subsequently, the number of pythons removed annually from ENP has increased dramatically (Fig. 2). Pythons in Florida consume a wide range of mammals and birds, including species classified as threatened or endangered under the US Endangered Species Act, such as the Key Largo woodrat (Neotoma floridana smalli) and wood stork (Mycteria americana) (14–15, 17–18). Pythons also occasionally prey on American alligators (Alligator mississippiensis) (14, 18). Although hundreds of prey items and more than 40 prey species for pythons in Florida have been documented, the impacts of python predation on native prey populations are essentially unknown. We used systematic road surveys to sample mammals in ENP before and after the proliferation of pythons. Road surveys also were conducted in areas where pythons have been documented only recently. Here, we present spatial and temporal data supporting the hypothesis that Burmese pythons have severely reduced populations of several species of formerly common mammals in ENP within 11 y of being recognized as an established invasive species.
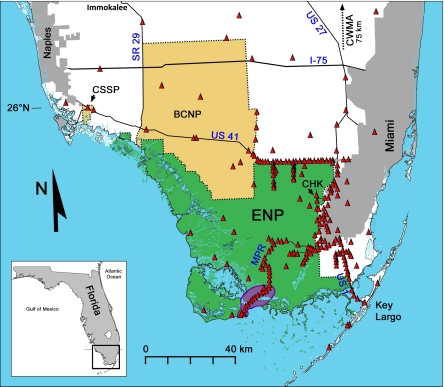
Fig. 1.
Map of South Florida illustrating sampling locations in relation to python distribution. Road surveys for mammals were conducted in the 1990s and 2000s along the Main Park Road (MPR) in Everglades National Park (ENP). Areas recently invaded by pythons and surveyed for mammals in 2009–2011 include Big Cypress National Preserve (BCNP), Collier-Seminole State Park (CSSP), Chekika (CHK), and Key Largo. Immokalee and Corbett Wildlife Management Area (CWMA; north of the map) are two sampled sites where pythons have not yet become established. The purple region represents the area of ENP where pythons were found in the 1990s and where reproduction was first reported (16). Red triangles represent localities of pythons found during 2008–2009.
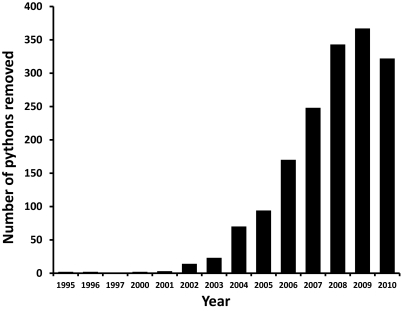
Fig. 2.
Python removals from ENP and its environs from 1995–2010. Note that data include captures resulting from opportunistic encounters of pythons and thus are not corrected for effort. The slight decrease in numbers of pythons captured during 2010 may be the result of a severe freeze in South Florida during January of that year (43).
Results
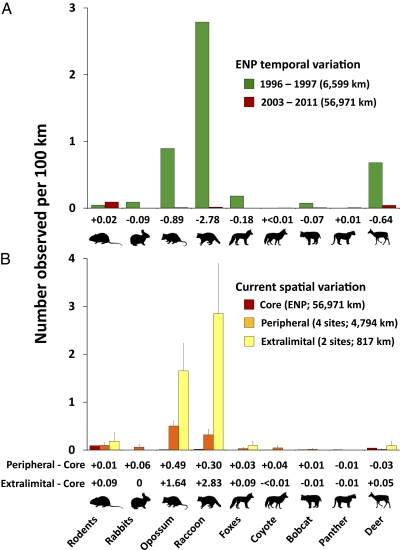
From 1993–1999, raccoons (Procyon lotor), Virginia opossums (Didelphis virginiana), and rabbits (Sylvilagus spp.) were the most common mammals found during roadkill surveys in ENP (Fig. 3). Encounter rates of live and dead mammals during systematic nocturnal road surveys in 1996–1997 corroborated this pattern, with raccoons (2.8 observations/100 km) and opossums (0.9/100 km) being the most frequently encountered species. Substantial decreases in the encounter rates of several species of mammals were apparent from 2003–2011 (Fig. 4A). Despite consistency of survey methods, we observed no rabbits or foxes (Urocyon cinereoargenteus and Vulpes vulpes) between 2003 and 2011, found a 99.3% decrease in raccoon observations and decreases of 98.9%, 94.1%, and 87.5% for opossums, white-tailed deer (Odocoileus virginianus), and bobcats (Lynx rufus), respectively. Observations of other mammals, including rodents, coyotes (Canis latrans), and Florida panthers (Puma concolor coryi) increased slightly (<0.02/100 km), but the overall numbers of observations for these groups were low.
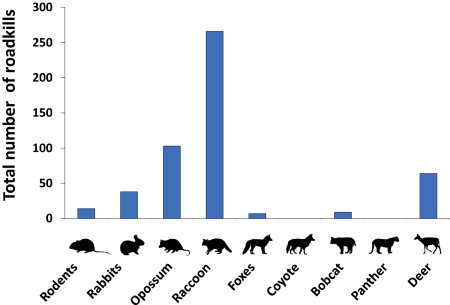
Fig. 3.
Encounter rates of mammal taxa in ENP reflected in roadkills recorded by park staff from 1993–1999, before pythons become common. Note that these data represent only the number of overall observations and are not corrected for distance (i.e., kilometers driven).
Fig. 4.
Temporal and spatial variation in mammal abundances in South Florida. (A) Temporal variation in mammal-encounter rates in ENP, as reflected in distance-corrected road survey counts (live and roadkill) before (1996–1997) and after (2003–2011) pythons became common. Numbers below bars represent the change in number of observations/100 km for each species or group. (B) Current (2008–2011) spatial variation in mammal-encounter rates as reflected in distance-corrected road survey counts in core (southern ENP), peripheral, and extralimital regions of python range; data for one of the two extralimital sites were taken from Holbrook and Chesnes (21). Pythons have been recorded in the core region for at least a decade and in peripheral locations more recently. Numbers below bars represent the change in number of observations/100 km for each species or group for peripheral locations vs. core python habitat (Upper) and extralimital sites vs. core python habitat (Lower). Errors bars represent SEM.
We also found considerable spatial variation in mammal observations. At peripheral locations, where pythons have been documented only recently and python densities presumably are lower, mammal encounter rates generally were intermediate between the 1996–1997 and 2003–2011 values for ENP (Fig. 4B). Specifically, mean encounter rates of opossums, raccoons, and foxes in recent surveys at peripheral sites were 44%, 89%, and 83% lower, respectively, than historical encounter rates in ENP (Fig. 4B). Rabbits were observed at only one peripheral site during recent surveys (Table S1). Observation frequency of raccoons and opossums at two extralimital locales were similar to historical sighting frequencies in ENP and were substantially higher than sighting rates in recent surveys of ENP and peripheral locales (Fig. 4B) (21). However, this pattern did not hold for deer, which were sighted less frequently in recent surveys at all sites than in ENP before python proliferation. Details of data by year, site, and species are provided in Table S1.
Discussion
Numerous lines of evidence implicate introduced Burmese pythons as the primary cause of dramatic declines of several species of once-abundant mammals in ENP. First, the timing of the python proliferation in ENP (19) coincides with reductions in mammal abundances. Second, spatial variation in encounter rates of mammals correlates strongly with the spread of pythons throughout ENP and surrounding areas. In areas where pythons have been established longest (southern ENP), mammal populations appear to have been reduced severely; in peripheral areas where pythons have been documented only recently (20), several species of mammals appear to occur at lower densities than at sites where pythons have not been documented (21). Third, raccoons, opossums, bobcats, deer, and rabbits have been documented in the diet of pythons in ENP (14, 15); these animals represent several diverse taxonomic and trophic groups (i.e., Carnivora, Didelphimorpha, Artiodactyla, Lagomorpha), arguing against a single disease as the agent of decline. Fourth, raccoons and opossums often forage near the water's edge, a microhabitat frequented by ambushing pythons (22). Fifth, in addition to frequenting habitats used by foraging pythons, mammals such as raccoons, opossums, deer, and bobcats may be naive to predation by large snakes. Boid snakes went extinct in the eastern United States during the Miocene, concomitant with other climatic, vegetation, and faunal, [e.g., the rise of colubroid snakes (23)] changes. The most recent large boids in the eastern United States are those from the Hemingfordian (20.6–16.3 Mya) Thomas Farm, Florida site (see ref. 24 for taxonomic discussion of these fossils, which might be synonymous with Boa constrictor). Thus, for at least 16 million years, there have been no snakes in Florida large enough to prey on medium-sized mammals (24). Finally, ENP represents a vast natural area where hunting is prohibited; other than changes in water-management regimes, anthropogenic impacts in ENP that might result in mammal declines have not changed markedly during the last two decades (25).
Severe declines in mammal populations have occurred across the globe and are attributable to various factors. In Asia, declines of mammals are coincident with declines in other animal taxa and have been attributed to deforestation, wildfire, bushmeat hunting, and the wildlife trade (26, 27). Although habitat loss and overexploitation are thought to be the primary threats to mammal populations in the United States (28), ENP is largely protected from these impacts, and the declines we observed were most severe in the remote southern portion of ENP (25). Diseases, such as canine distemper, have resulted in declines of African predators, most notably silver-backed jackals (Canis mesomelas), wild dogs (Lycaon pictus), and bat-eared foxes (Otocyon megalotis (29). Limited evidence of disease has been noted in the varied mammalian taxa that have declined in ENP during the time period we examined, and there is no evidence of a disease that could have resulted in the widespread patterns of declines we have documented across taxa. In Australia, mammal declines since European settlement have been attributed to various factors including persecution of top-predators (dingos; Canis lupus dingo), which has allowed introduced predators, notably cats (Felis catus) and red foxes (Vulpes vulpes) to proliferate (30). Similar to the declines we document in ENP, declines in Australian mammals have occurred in a number of taxa and lend support to the top-down effects apex predators can have on ecosystems (31, 32).
Numerous published accounts and anecdotal observations by ENP personnel and others lend further support that dramatic declines in mammal populations have occurred in ENP since the proliferation of pythons (SI Text). Marsh rabbits and raccoons were once described as the most commonly seen mammals in the Everglades (25, 33). In the 1980s, raccoons were such nuisances in campgrounds and visitor-use areas that a control program was initiated in ENP. The number of human–raccoon incidents documented by ENP has declined precipitously since the 1990s, and although raccoons still are found in some coastal areas around ENP, no nuisance raccoon incidents have been reported from the southern part of ENP since 2005. Interviews with naturalists who have visited ENP regularly for decades reveal that none have seen rabbits in the core of ENP in recent years. Although the spatiotemporal patterns are correlative, the preponderance of evidence supports the hypothesis that pythons have severely reduced mammal populations within ENP.
The mammal species we focus on here are some of the most tractable for population monitoring because their abundance and behaviors make them easily observable from roads (34). These species can serve as proxies for species of conservation concern that often are more difficult to monitor because of low densities, spotty distributions, or secretive behavior. Pythons have been reported to consume leopards in their native range (35), and thus even top predators, such as the Florida panther, may be at risk. Approximately 25% of all pythons found in ENP contain bird remains (17), and although quantifying impacts on birds is difficult, species such as rails, limpkins, grebes, herons, egrets, and the federally endangered wood stork may be particularly vulnerable to python predation.
Most medium-sized mammals showed severe declines after python proliferation. Although deer observations declined by 94%, and deer are known prey of pythons in South Florida (18), the relatively low number of deer observed in recent surveys at peripheral and extralimital sites raises the possibility that factors other than pythons may have contributed to declines in deer populations (36). Additionally, we documented slight increases in sighting rates of rodents, coyotes, and Florida panthers within ENP. However, overall numbers for these groups are low both before and after python proliferation, making firm conclusions regarding the status of their current populations difficult. Although rodents are common prey items for young pythons, the severe declines in other major predators of rodents (e.g., bobcats and foxes) may have reduced overall predation pressure after python proliferation (37). Additionally, the high reproductive potential of many rodents (38) may make them better able to withstand python predation than larger mammal species.
The effects of declining mammal populations on ecosystem function are likely complex and difficult to predict (39). Declines in bobcats and foxes could be the result of direct predation or of exploitation competition for shared prey such as rabbits. Prey declines could negatively affect other predators that are not frequently consumed by pythons, such as large native snakes and raptors. For some species, indirect effects of pythons may be positive. Reductions in raccoons, which frequently prey on eggs of oviparous amniotes (40), may increase nesting success and recruitment of some turtles, crocodilians, and birds.
Attempts to assess responses of organisms to emerging threats (e.g., invasive species, disease, climate change) often are hampered by lack of historical or baseline abundance data (41). We were fortunate to have available effort-corrected data from 1996–1997 comparable to the data on mammal relative abundances we have collected since the proliferation of pythons, allowing us to document declines in numerous mammal species accurately. However, our reliance on indirect estimates of mammal abundance in ENP is the result of a nearly complete absence of actual density or population size estimates based on rigorous and repeatable field methods. Therefore, baseline monitoring efforts of even common species are needed to allow accurate assessment of temporal trends in wildlife populations, whether resulting from invasive species, climate change, disease, hydrological management, or other factors.
Our results also suggest that giant snakes, acting as generalist apex predators, can exert significant top-down pressure on vertebrate populations, even in a complex ecosystem with an exceedingly wide array of available prey species. The significance of top predators for ecosystem function has been demonstrated when such predators are removed from marine, terrestrial, and freshwater ecosystems (32). The addition of such predators is similarly informative. The introduction of predators has resulted in major impacts to insular faunas (13, 42). Here, we suggest that introduction of a novel top predator to a complex continental ecosystem has resulted in the severe decline of several mammal populations. Whether mammal populations will remain suppressed or will rebound remains to be seen. The magnitude of these declines underscores the apparent incredible density of pythons in ENP and justifies intensive investigation into how the addition of novel apex predators affects overall ecosystem processes.
Methods
We compiled records of road-killed mammals from surveys conducted by National Park Service rangers within ENP from 1993–1999, before pythons were common in ENP. These surveys were conducted by park rangers who kept track of all road-killed animals while working in ENP but did not keep track of distance driven, preventing us from estimating survey effort. From February 1996 to January 1997, we conducted weekly systematic mammal surveys within ENP and counted both live and road-killed animals. Surveys were conducted along the Main Park Road (MPR) and Research Road (both paved with asphalt) from the Daniel Beard Research Center near Royal Palm to Flamingo and back. Driving speed typically was between 55 and 70 km/h; traffic volumes were not measured but usually were very low. The number of observers per vehicle varied between one and four but was usually one or two. Surveys (130-km round trip) began at sunset and totaled 6,599 km over 51 nights in 1996–1997. Road-killed animals were removed from the road, and we did not count animals that were obvious resightings of previously observed individuals. Although some individual animals may have been observed on more than one occasion, there is no reason to suspect that the likelihood of resightings changed over time or otherwise biased our conclusions about temporal or spatial shifts in mammal abundances.
From 2003–2011, after pythons became common in ENP, we conducted road surveys on Research Road and MPR between sunset and sunrise (totaling 56,971 km on 313 nights) at varying intervals. From 2009–2011, we conducted a total of 4,794 km of surveys on 26 nights at four locations (peripheral sites) more recently colonized by pythons (Collier-Seminole State Park, Florida City to Key Largo, the Chekika area on the eastern border of ENP, and Big Cypress National Preserve) (Fig. 1). For comparison, we also include data for two locations (extralimital sites) that have habitats similar to ENP but are north of the area where pythons are known to be established. These data include one night (278 km) from the vicinity of Immokalee, Florida and nine nights (539 km) of road surveys conducted by other researchers at Corbett Wildlife Management Area (21). Rodents and rabbits [marsh rabbits (Sylvilagus palustris) and eastern cottontails (Sylvilagus floridanus)] often were not identifiable to species and were grouped together as “rodents” and “rabbits” for analyses. Red foxes and gray foxes (Urocyon cinereoargenteus) also were grouped to simplify analyses. See Table S1 for more detail on mammal observations and survey effort.
Supplementary Material
Acknowledgments
We thank J. Holbrook, C. Gillette, S. Goetz, S. Pfaff, R. Rozar, and D. Smith for providing data and information used in this study. S. Price and G. H. Rodda provided comments that improved the manuscript. Support was provided by Davidson College, Duke Energy, the J. E. and Majorie B. Pittman Foundation, Inc., the Center for Forest Sustainability at Auburn University, US Geological Survey Ecosystems Program, US Geological Survey Priority Ecosystem Science Program, and the National Park Service.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1115226109/-/DCSupplemental.
References
- 1.Clout MN, Williams PA. Invasive Species Management: A Handbook of Principles and Techniques. Oxford, UK: Oxford Univ Press; 2009. p. 336. [Google Scholar]
- 2.Pimentel D, Zuniga R, Morrison D. Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecol Econ. 2005;52:273–288. [Google Scholar]
- 3.Crooks JA. Characterizing ecosystem-level consequences of biological invasions: The role of ecosystem engineers. Oikos. 2002;97:153–166. [Google Scholar]
- 4.Human KG, Gordon DM. Exploitation and interference competition between the invasive Argentine ant, Linepithema humile, and native ant species. Oecologia. 1996;105:405–412. doi: 10.1007/BF00328744. [DOI] [PubMed] [Google Scholar]
- 5.Letnic M, Webb JK, Shine R. Invasive cane toads (Bufo marinus) cause mass mortality of freshwater crocodiles (Crocodylus johnstoni) in tropical Australia. Biol Conserv. 2008;141:1773–1782. [Google Scholar]
- 6.Levin LA, Neira C, Grosholz ED. Invasive cordgrass modifies wetland trophic function. Ecology. 2006;87:419–432. doi: 10.1890/04-1752. [DOI] [PubMed] [Google Scholar]
- 7.Gurevitch J, Padilla DK. Are invasive species a major cause of extinctions? Trends Ecol Evol. 2004;19:470–474. doi: 10.1016/j.tree.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 8.Caut S, Angulo E, Courchamp F. Dietary shift of an invasive predator: Rats, seabirds and sea turtles. J Appl Ecol. 2008;45:428–437. doi: 10.1111/j.1365-2664.2007.01438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pough FH. Advantages of ectothermy for tetrapods. Am Nat. 1980;115:92–112. [Google Scholar]
- 10.Kraus F. Alien Reptiles and Amphibians: A Scientific Compendium and Analysis. New York: Springer; 2009. p. 563. [Google Scholar]
- 11.Rodda GH, Savidge JA. Biology and impacts of Pacific island invasive species. 2. Boiga irregularis, the Brown Tree Snake (Reptilia: Colubridae) Pac Sci. 2007;61:307–324. [Google Scholar]
- 12.Fritts TH, Rodda GH. The role of introduced species in the degradation of island ecosystems: A case history of Guam. Annu Rev Ecol Syst. 1998;29:113–140. [Google Scholar]
- 13.Savidge JA. Extinction of an island forest avifauna by an introduced snake. Ecology. 1987;68:660–668. [Google Scholar]
- 14.Reed RN, Rodda GH. Giant Constrictors: Biological and Management Profiles and an Establishment Risk Assessment for Nine Large Species of Pythons, Anacondas, and the Boa Constrictor. 2009 US Geological Survey Open-File Report 2009–1202. (US Geological Survey, Reston, VA), p 302. [Google Scholar]
- 15.Snow RW, et al. In: Biology of the Boas and Pythons. Henderson RW, Powell R, editors. Eagle Mountain, UT: Eagle Mountain Publ; 2007. pp. 416–438. [Google Scholar]
- 16.Meshaka WE, Jr., Loftus WF, Steiner T. The herpetofauna of Everglades National Park. Fla Sci. 2000;63:84–103. [Google Scholar]
- 17.Dove CJ, Snow RW, Rochford MR, Mazzotti FJ. Birds consumed by the invasive Burmese python (Python molurus bivittatus) in Everglades National Park, Florida, USA. The Wilson Journal of Ornithology. 2011;123:126–131. [Google Scholar]
- 18.Rochford M, et al. Python molurus bivittatus (Burmese Python). Diet. Herpetol Rev. 2010;41:97. [Google Scholar]
- 19.Willson JD, Dorcas ME, Snow RW. Identifying plausible scenarios for the establishment of invasive Burmese pythons (Python molurus) in southern Florida. Biol Invasions. 2011;13:1493–1504. [Google Scholar]
- 20.Pifer EK, Hart KM, Rice KG, Mazzotti FJ. Small and Medium-Sized Mammal Inventory for Everglades National Park and Big Cypress National Preserve. 2011 (US National Park Service, Davie, FL), p 177. [Google Scholar]
- 21.Holbrook J, Chesnes T. An effect of Burmese pythons (Python molurus bivittatus) on mammal populations in southern Florida. Fla Sci. 2011;74:17–24. [Google Scholar]
- 22.Dorcas ME, Willson JD, Gibbons JW. Can invasive Burmese pythons inhabit temperate regions of the southeastern United States? Biol Invasions. 2011;13:793–802. [Google Scholar]
- 23.Holman JA. Fossil Snakes of North America. Bloomington, IN: Indiana Univ Press; 2000. p. 367. [Google Scholar]
- 24.Kluge AG. Relationships of the Cenozic Boine snakes Paraepicrates and Pseudoepicrates. J Vertebr Paleontol. 1988;8:229–230. [Google Scholar]
- 25.Lodge TE. The Everglades Handbook: Understanding the Ecosystem. 3rd Ed. Boca Raton< FL: CRC; 2010. p. 392. [Google Scholar]
- 26.Sodhi NS, Koh LP, Brook BW, Ng PKL. Southeast Asian biodiversity: An impending disaster. Trends Ecol Evol. 2004;19:654–660. doi: 10.1016/j.tree.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 27.Brook BW, Sodhi NS, Ng PKL. Catastrophic extinctions follow deforestation in Singapore. Nature. 2003;424:420–426. doi: 10.1038/nature01795. [DOI] [PubMed] [Google Scholar]
- 28.Wilcove DS, Rothstein D, Dubow J, Phillips A, Losos E. Quantifying threats to imperiled species in the United States. Bioscience. 1998;48:607–615. [Google Scholar]
- 29.Alexander KA, Appel MJG. African wild dogs (Lycaon pictus) endangered by a canine distemper epizootic among domestic dogs near the Masai Mara National Reserve, Kenya. J Wildl Dis. 1994;30:481–485. doi: 10.7589/0090-3558-30.4.481. [DOI] [PubMed] [Google Scholar]
- 30.Woinarski JCZ, et al. The disappearing mammal fauna of northern Australia: Context, cause, and response. Conserv. Lett. 2011;4:192–201. [Google Scholar]
- 31.Bilney RJ, Cooke R, White JG. Underestimated and severe: Small mammal decline from the forests of south-eastern Australia since European settlement, as revealed by a top-order predator. Biol Conserv. 2010;143:52–59. [Google Scholar]
- 32.Estes JA, et al. Trophic downgrading of planet Earth. Science. 2011;333:301–306. doi: 10.1126/science.1205106. [DOI] [PubMed] [Google Scholar]
- 33.Layne JN. In: Environments of South Florida: Present and Past. Gleason PJ, editor. Miami, FL: Miami Geological Society; 1974. pp. 386–412. [Google Scholar]
- 34.Main MB, Allen GM. Landscape and seasonal influences on roadkill of wildlife in southwest Florida. Fla Sci. 2002;65:149–158. [Google Scholar]
- 35.Begbie A. The food of pythons. J. Bombay Nat. Hist. Soc. 1907;17:1021. [Google Scholar]
- 36.Garrison E, et al. Status of White-Tailed Deer in the Stairstep Unit of Big Cypress National Preserve. 2011 (US National Park Service, Gainesville, FL), p 30. [Google Scholar]
- 37.Maehr DS, Brady JR. Food habits of bobcats in Florida. J Mammal. 1986;67:133–138. [Google Scholar]
- 38.Pianka ER. On r and K selection. Am Nat. 1970;104:592–597. [Google Scholar]
- 39.Terborgh J, Estes JA. Trophic Cascades: Predators, Prey, and the Changing Dynamics of Nature. Washington, DC: Island; 2010. p. 464. [Google Scholar]
- 40.Ernst CH, Lovich JE. Turtles of the United States and Canada. Baltimore: Johns Hopkins Univ Press; 2009. p. 827. [Google Scholar]
- 41.Collins C, Kays R. Causes of mortality in North American populations of large and medium-sized mammals. Anim Conserv. 2011;14:474–483. [Google Scholar]
- 42.Hays WST, Conant S. Biology and impacts of Pacific Island invasive species. 1. A worldwide review of effects of the small Indian mongoose, Herpestes javanicus (Carnivora: Herpestidae) Pac Sci. 2007;61:3–16. [Google Scholar]
- 43.Mazzotti FJ, et al. Cold-induced mortality of invasive Burmese pythons in south Florida. Biol Invasions. 2011;13:143–151. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.