Abstract
The extensive Early Jurassic continental strata of southern Africa have yielded an exceptional record of dinosaurs that includes scores of partial to complete skeletons of the sauropodomorph Massospondylus, ranging from embryos to large adults. In 1976 an incomplete egg clutch including in ovo embryos of this dinosaur, the oldest known example in the fossil record, was collected from a road-cut talus, but its exact provenance was uncertain. An excavation program at the site started in 2006 has yielded multiple in situ egg clutches, documenting the oldest known dinosaurian nesting site, predating other similar sites by more than 100 million years. The presence of numerous clutches of eggs, some of which contain embryonic remains, in at least four distinct horizons within a small area, provides the earliest known evidence of complex reproductive behavior including site fidelity and colonial nesting in a terrestrial vertebrate. Thus, fossil and sedimentological evidence from this nesting site provides empirical data on reproductive strategies in early dinosaurs. A temporally calibrated optimization of dinosaurian reproductive biology not only demonstrates the primary significance of the Massospondylus nesting site, but also provides additional insights into the initial stages of the evolutionary history of dinosaurs, including evidence that deposition of eggs in a tightly organized single layer in a nest evolved independently from brooding.
Over the last three decades, numerous discoveries of eggs, embryos, and nesting sites have greatly increased our knowledge of the evolution of reproductive behavior in nonavian dinosaurs (1, 2), including finds of brooding maniraptorans (3, 4), eggs preserved in the body cavity of a mother (5), and vast “rookeries” (6, 7). However, almost all of these fossil localities date from the Late Cretaceous, toward the end of the “Age of Dinosaurs.” Data on the reproductive biology of early dinosaurs have been limited, and, although eggshell and isolated eggs have been reported from Late Jurassic strata (8), to date only two Jurassic examples of dinosaurian egg clutches and in ovo embryonic remains have been documented in detail. One comprises more than 100 eggs and associated embryonic skeletal remains of the allosauroid theropod Lourinhasaurus from the Upper Jurassic of the Paimogo site near Lourinhã, Portugal (9), and eggs containing exquisitely preserved embryos of the sauropodomorph Massospondylus from the Lower Jurassic of Golden Gate Highlands National Park, South Africa (1, 2). The extensive Early Jurassic continental strata of southern Africa have yielded scores of partial to complete, articulated skeletons of the sauropodomorph dinosaur Massospondylus, ranging from embryos to large adults (10). In 1976 a block of reddish-brown siltstone containing partial egg clutch with in ovo embryos of Massospondylus was collected from talus at a roadside exposure, known as Rooidraai (“Red Bend”), in Golden Gate Highlands National Park, South Africa (Fig. 1). The source horizon for the block at the locality was uncertain. An excavation program at the site started in 2006 has led to the discovery of several in situ egg clutches and recognition of the earliest known dinosaurian nesting complex at the Rooidraai locality.
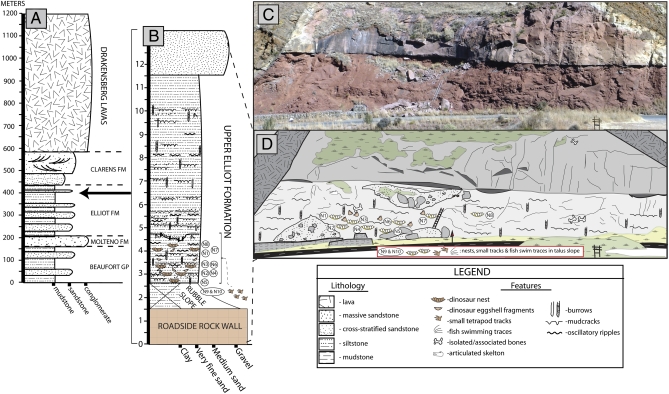
Fig. 1.
Geological section of Rooidraai, Golden Gate Highlands National Park, South Africa. Stratigraphic position of the nesting site within the uppermost (Early Jurassic) part of the Karoo Supergroup (A) and the upper Elliot Formation (B), respectively. Photograph (C) and illustration (D) of the cliff face showing the distribution of the clutches of eggs within the site, as well as additional depositional features. In total, 10 egg clutches (N1–N10) have been identified at Rooidraai, distributed across a 23-m-wide by 2-m-thick interval through the lower portion of the muddy siltstone unit. The egg clutches are found within at least four distinct levels in the 2-m-thick egg-bearing interval. The lowest clutch, N5, is found at the 2.6-m level, whereas the two clusters of eggs occur at the 3.05-m (N2 and N4) and the 3.35-m (N3 and N6) levels, respectively. Egg clutches N1, N7, and N8 are all distributed at approximately the same stratigraphic level, between 4.2 and 4.25 m. Two other clutches (N9 and N10) were recovered from the talus slope at the base of the nest-bearing cliff face; their source layer cannot be located precisely, but the clutches were clearly derived from the clutch-bearing interval.
Results
Our work at the Rooidraai locality has yielded multiple in situ clutches of eggs as well as fragmentary eggshell and bones, all from a 2-m-thick interval of muddy siltstone 25 m from the top of the Lower Jurassic Upper Elliot Formation (“Stormberg Group,” Karoo Supergroup) (11). We refer these finds to the same taxon as the partial clutch containing in ovo embryos of Massospondylus collected in 1976 because the eggs are closely similar in size and structure, and the exposed embryonic bones are indistinguishable from the previously described remains (1, 12).
In total, 10 clutches of eggs (N1–N10), 8 of which occurred in situ, were identified across a 23-m-wide by 2-m-thick outcrop area in the lower portion of the muddy siltstone unit at Rooidraai. The egg clutches occur in at least four discrete levels, with two clutches of uncertain provenance recovered from the talus slope. All eggs were found in clusters at Rooidraai, and there is not a single occurrence of individual eggs. The most completely prepared clutch (Fig. 2B) comprises at least 34 eggs. It is complete on three sides, but it is unclear how many eggs were lost along the eroded edge. As in birds, the eggs form a single layer in each clutch. The eggs are tightly packed together and deposited into recognizable rows (Fig. 2 A and B). Given the large size of adult Massospondylus, it is likely that the mother organized the eggs after laying them in the nest. However, the egg clutches at Rooidraai lack definitive sedimentological evidence of nest construction (13).
Fig. 2.
Egg clutches recovered from Rooidrai. (A) Massospondylus (BP/1/5347a) egg clutch, showing the presence of two exposed skeletons; parts of 7 eggs (numbered) are preserved in this block, and fragments of 4 additional eggs are preserved in the counterpart block (BP/1/5347b, not shown). However, only 6 eggs are sufficiently complete to contain embryos. This clutch was collected as an isolated block in talus in 1976. Of the preserved near complete eggs, 5 contain embryonic remains, but only the remains in 2 eggs (N2 and N6) have been exposed through preparation and removal of most of the eggshell. (B) Part of the most completely prepared egg clutch that contains a total of 34 eggs (BP/1/6229). This clutch was preserved in situ in the cliff face. The matrix in the immediate area around the nest showed extensive bioturbation and lacked the fine laminations that normally characterize much of the nesting site, but there is no definite evidence of a nest beyond the organized nature of the egg clutches. (Scale bar, 5 cm.)
A few meters below the nest-bearing unit is a 17-m-thick fluvial sandstone with large-scale lateral accretion macroforms, which we interpret as a high-sinuosity meandering channel complex, the content of which rapidly fines upward. The egg clutches occur near the base of a 10-m-thick succession of muddy siltstone, which is composed of numerous fine laminations and 1- to 5-cm-thick beds of laminated siltstone that bear current-ripple marks and typically fine upwards into pure claystone. Many of these laminae and beds have small desiccation cracks on their upper surfaces. Other beds within the interval also preserve ripple and wrinkle marks.
Small calcium carbonate nodules of presumably pedogenic origin are distributed fairly randomly within the silty mudstone unit and are mostly allochthonous. The ubiquitous deep red color of the silty mudstone unit indicates rubification, which is typical of oxidation of iron-bearing minerals above the water table. These combined features indicate that the depositional setting was fairly arid during this time period. The fine-grained nature of the sediment and small-scale sedimentary structures that developed at the site attests to the relatively low intensity of repeated flooding events that led to vertical accretion and filling of the floodbasin depression.
Bioturbation is exceptionally common throughout the 10-m-thick muddy siltstone unit and is dominated by small, indeterminate burrows and root traces. The trace fossils comprise common unlined vertical and horizontal burrows (Skolithos and Planolites, respectively), lined, meandering horizontal burrows (Paleophycus), and meniscate, back-filled horizontal burrows (Taenidium).
Although no unequivocal tetrapod tracks have been found in situ within the nest-bearing succession, several talus blocks of muddy siltstone have been found with very small tetrapod tracks and fish swimming traces (Undichna sp.). Numerous tiny prints are scattered over the slabs but it is difficult to trace individual trackways. Nevertheless several manus–pes sets are present and a number of partial trackways can be delineated (BP/1/6923a,b) and are referable to Massospondylus. These footprints are preserved mostly as natural casts (convex hyporeliefs) on the undersides of fine-grained sandstone slabs that have parted along mud drapes. The pes prints closely match those referred to the ichnogenus Otozoum, which have also been attributed to sauropodomorph dinosaurs (14). The digitigrade, mesaxonic pes prints are tetradactyl with subparallel digits II–IV. Digit I is shorter than the other three and tends to diverge slightly. In well-preserved prints, digit I bears a distinct claw impression (Fig. 3 A and C). The clearest pes prints (BP/1/6923a; Fig. 3 A and C) show weakly impressed phalangeal–metatarsal pads behind the digits, including a large, semicoalesced pad behind digits III and IV, which is also present in Otozoum. The principal difference between the Rooidraai tracks and Otozoum is the absence of a posterior pad produced by digit V in the former.
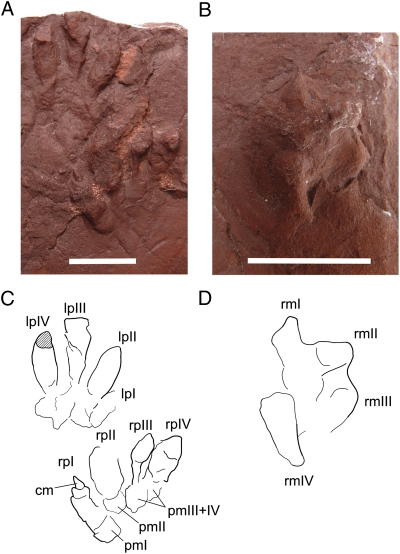
Fig. 3.
Footprints attributed to juvenile Massospondylus (BP/1/6923) from the nesting level at Rooidraai. (A) Pes print pair (BP/1/6923a). (B) Right manus print (BP/1/6923b). (C) Interpretive drawing of pes prints. (D) Interpretive drawing of manus print. (Scale bars, 10 mm.) cm, claw mark; lpI, first left pedal digit; lpII, second left pedal digit; lpIII, third left pedal digit; lpIV, fourth left pedal digit; pmI, phalangeal–metatarsal pad of the first pedal digit; pmII, phalangeal–metatarsal pad of the second pedal digit; pmIII+IV, coalesced phalangeal–metatarsal pad of the third and fourth pedal digits; rmI, first right manual digit; rmII, second right manual digit; rmIII, third right manual digit; rmIV, fourth right manual digit; rpI, first right pedal digit; rpII, second right pedal digit; rpIII, third right pedal digit; rpIV, fourth right pedal digit.
Discussion
Sedimentological data indicate that the climate in this region was at least seasonally arid at the time of deposition (15). Much of the fine-grained silt in the Upper Elliot and Clarens formations has been interpreted as loess (11), which presumably spread across the region as the climate became warmer and drier, in advance of the large dune fields that covered much of southern Africa and characterize the Clarens Formation. Sedimentological evidence also points to a transition from a large, sinuous meandering channel belt to a sequence of relatively homogeneous muddy siltstones, with evidence of ponds and repeated wetting and drying events in the immediate vicinity of the nesting site. This suggests that either an ephemeral lake margin in a flood basin or the vicinity of an oxbow lake was the setting for the dinosaurian nesting site at Rooidraai. The presence of fine laminations, small desiccation cracks, wave ripples, and wrinkle marks at the site indicates a depositional setting characterized by repeated low-energy flooding events and ponding, usually followed by desiccation. Nearly the entire 10-m-thick lake or abandoned channel-belt pond sequence reflects repeated inundation, presumably during seasonal overbank flooding from nearby channels. On the basis of in situ clutches, many eggs and egg clutches are preserved whole, suggesting that they experienced rapid burial and thus were not vulnerable to disintegration, predation, and trampling during subsequent breeding seasons.
Bioturbation in the immediate vicinity of the clutches and the tight arrangement of eggs within clutches in the flood-induced burial suggest that the eggs were at least partially buried in the substrate. The latter interpretation is further supported by the very thin (about 0.1 mm) eggshells, which suggest a low-oxygen, high-carbon dioxide, high-humidity nest environment (16).
Although the precise time interval separating each nest level is difficult to assess, there is sedimentological evidence for up to 20 individual events of low-intensity flooding between the principal nesting levels. The site was presumably a seasonably favorable nesting location because of its proximity to a pond (and associated vegetation) and the availability of soft sediment for excavation of nests. The Rooidraai locality likely preserves many more egg clutches of Massospondylus than identified by fieldwork to date, but the current steep cliff-face exposure of the site limits our ability to map the full spatial extent of the nesting site. We hypothesize that the preserved nests represent exceptional circumstances, where the nesting colony, or part of it, was affected by unusually intense or out-of-season flooding events that led to burial and preservation of egg clutches before hatching.
The Rooidraai nesting site represents by far the oldest known mass accumulation of dinosaurian egg clutches. Similar nesting sites for titanosaurian sauropods and hadrosaurid ornithischians are more than 100 million years younger (6, 17). This abundance of in situ egg clutches within a restricted geographic and stratigraphic interval provides the oldest known evidence of egg-laying behavior in dinosaurs (or for any reptile) and provides further insights into possibly plesiomorphic reproductive behaviors among dinosaurs. The multiple occurrence of egg clutches within at least three stratigraphically distinct layers (Fig. 1B) indicates that at least two or more Massospondylus nested at this site on at least four separate occasions. Similar evidence has been used to infer colonial nesting and/or site fidelity (defined as the repeated use of a preferred nesting site by several members of the same species rather than a single individual) in various Late Cretaceous dinosaurs (18–21).
Additional behavioral evidence is provided by tracks and partial trackways referable to Massospondylus recovered from the site (Fig. 3). They establish that hatchlings were moving around the nesting site in a quadrupedal manner, as was predicted from their skeletal proportions (1, 12). The manus prints are rotated outward relative to the pes prints so that the palm faces the midline of the trackway and manual digit I points anteriorly. This lack of pronation of the manus is in accordance with the skeletal structure of mature Massospondylus. None of the impressions of manual digit I include anything more than the base of the digit, indicating that the large claw of this digit was held clear of the substrate surface during locomotion, as has been inferred for adults (22). On the basis of both the length of the preserved metatarsals of the near-hatching embryonic Massospondylus and the reconstruction of the pes (12), we can estimate that a hatchling would leave a pes print little more than 7 mm in length. At least two class sizes of prints are preserved at the site, the largest pes prints being ∼15 mm long. The presence of these trackways at the site indicates that Massospondylus juveniles remained at the nesting site for some time after hatching, long enough for growth to have at least doubled the linear measurements of the foot, predating previous reports of extended nest site occupancy by more than 100 million years (8).
To unravel the complexities of dinosaurian reproductive biology, we undertook a comprehensive optimization of various behaviors in a time-calibrated phylogenetic framework (Fig. 4). This analysis highlights the fact that well-studied egg occurrences remain unavailable for most nonavian dinosaurian taxa, and their Mesozoic archosaurian relatives, including crocodylians and pterosaurs (23–25). Evidence for avian nesting is also unknown from Mesozoic deposits.
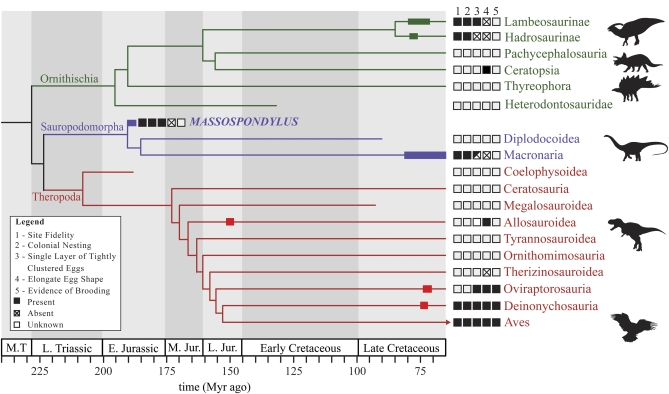
Fig. 4.
Time-calibrated cladogram of Dinosauria showing the known distribution of selected reproductive characters and the known distribution of fossil nests (thick bars). This illustration underscores the rarity of nest information in Dinosauria, and their temporal distribution. Although eggs of pterosaurs and crocodylomorphs have been recently reported, little useful nest information is available for these archosaurian outgroups to dinosaurs (24, 25) and is not included here. Square boxes to the Left of the major clade names indicate presence (solid box), absence (diagonally divided box), or unknown condition (empty box) for the following characters (listed above the boxes): 1, evidence of nesting-site fidelity; 2, evidence of gregarious nesting; 3, nests organization consisting of a single layer of tightly clustered eggs; 4, elongate egg shape (absence denotes spherical eggs); 5, evidence of brooding. Saurischian tree topology is from Zanno and Mackovicky (26) and Martínez et al. (27); ornithischian tree topology is from Butler et al. (28).
Although nesting-site fidelity and gregarious nesting behavior are often associated with extant birds, they are also present among extant crocodylians (23). When data from dinosaurs are interpreted in a phylogenetic context (26–28), gregarious nesting (or at least clustering of nests in a preferred area) and site fidelity appear plesiomorphic for dinosaurs (Fig. 4). Optimization of dinosaurian reproductive behavior within this context also indicates that tight clusters of eggs organized into a single layer and subspherical rather than elongate egg shape also characterize dinosaurs. However, the rarity of well-studied Mesozoic egg occurrences, and the considerable variability of nesting behaviors among extant birds and crocodylians, raises the possibility of multiple, independent evolutionary origins of some of these behaviors. Furthermore, present-day crocodylians and birds have highly specialized modes of life that fundamentally differ from those of nonavian dinosaurs, underscoring the significance of the Massospondylus nesting site for understanding the evolutionary history of reproductive behaviors in dinosaurs.
Another line of evidence (Fig. 5), the relationship of clutch size to adult body mass in Massospondylus, provides additional support for the hypothesis that a relatively primitive form of parental care was plesiomorphic for dinosaurs (29, 30). The egg clutches reported here show that the previous analysis significantly underestimated the clutch size of Massospondylus because its estimate was based on the incomplete original clutch of only 8 eggs. These more complete clutches of eggs indicate that at least one clutch comprises at least 34 eggs. This clutch size for Massospondylus precludes paternal prehatching care of offspring as suggested for some maniraptorans, but is not inconsistent with the plesiomorphic condition of limited parental care, as was previously proposed for this sauropodomorph dinosaur (2).
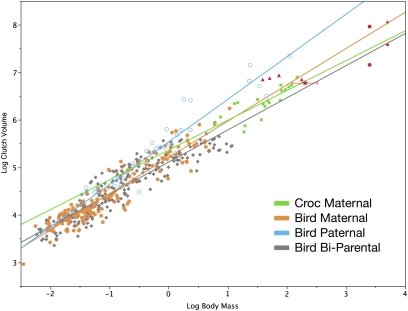
Fig. 5.
Plot of clutch mass versus body mass in archosaurs. Clutch volume versus adult body mass for extant archosaurs is divided into four taxon/care models (29). Color code for all graphs: green, crocodylian-maternal; black, bird-biparental; orange, bird-maternal; blue, bird-paternal. Red symbols represent nonavian dinosaurs: red diamonds, sauropods; red circles, hadrosaurs; red triangles, theropods other than birds; red star, Massospondylus, with range bars indicating minimum and maximum body mass estimates. Dinosaurs were not included in the regression models. The clutch size for Massospondylus precludes paternal care of offspring as suggested for some maniraptorans, but is consistent with the plesiomorphic condition of limited maternal care.
Nest attendance in Massospondylus, and all nonavian dinosaurs, may also be inferred from phylogenetic bracketing, as most extant archosaurs attend their nests (31, 32). However, Massospondylus provides important reproductive data for sauropodomorphs before the evolution of gigantism. The enormous size difference between the tiny hatchlings and often giant adults of titanosaurian sauropods and close proximity between egg clutches in nesting sites of these dinosaurs suggest little if any parental care in this group. The data reported here support the hypothesis that absence of parental care may be a derived condition associated with the evolution of gigantism in this group (6, 7).
The subspherical shape of the eggs of Massospondylus is similar to those of sauropods and hadrosaurs and differs from the oblong shape found in some more derived theropod groups including birds. Eggs are not paired within the clutches of eggs of Massospondylus, which suggests that these dinosaurs resembled extant crocodylians in the shelling and deposition of an entire egg clutch en masse, rather than the derived mode exhibited by maniraptoran theropods, including birds (4, 5, 33). In general, it appears that tight clusters of eggs and organization of eggs into a single layer are the only aspects of reproductive behavior shared by nonavian dinosaurs and birds. Thus, the egg clutches of Massospondylus provide unique insights into reproductive strategies in early dinosaurs, and suggest that certain more derived avian reproductive traits, such as enlarged clutch volumes, bird-like egg laying, and brooding, evolved subsequently only among theropod dinosaurs.
Materials and Methods
Building on preliminary investigations in 2004 and 2005, our team has conducted five additional field trips to Golden Gate Highlands National Park, South Africa, excavating the clutches and collecting detailed sedimentological data. Two clutches of eggs (BP/1/5347a,b and BP/1/6229) were prepared for study at the Royal Ontario Museum and University of Toronto Mississauga. Multiple stratigraphic sections were measured in the park at various outcrop localities to establish the overall depositional context, whereas four highly detailed, centimeter-scale sections were measured across the 20-m-wide clutch-bearing interval at the Rooidraai site. Lithofacies, facies associations, and architectural elements were diagnosed and interpreted following a modified version of Miall's classification system (34). Detailed attention was paid to the identification and interpretation of sedimentary and biogenic structures. Paleocurrent data were measured in the field using the axes of exposed wave and current ripples, trough crossbeds, and on (rare) sole structures. Thin sections were made for rocks from the nest and other horizons to observe microscale fabrics, grain size, and sediment maturity. Photomosaics were produced to assess the lateral relationship between clutches and to establish correlations across the Rooidraai cliff face.
The dataset of Varricchio et al. (29) was used to document the relative clutch size of Massospondylus in the context of other archosaurs and to grossly assess the possible parental care system adopted by Massospondylus and other dinosaurs other than theropods. This was based on regression relationships between body mass (with an updated mass estimate for Massospondylus) and clutch mass relationships and their four parental care types. The clutch presented here of Massospondylus eggs was used to estimate clutch volume for this taxon.
Supplementary Material
Acknowledgments
The authors thank B. Rubidge, M. Raath, and B. Zipfel for ongoing support in South Africa; D. Scott for preparation and illustration of specimens; J. Hancox for advice on depositional environment of the fossil locality; and the staff of Golden Gate Highlands National Park for assistance and support during fieldwork. I. Morrison, H. Maddin, and N. Campione assisted in the field excavations. Fieldwork and research was supported by the National Geographic Society, University of Toronto, Royal Ontario Museum, and Natural Sciences and Engineering Research Council (Canada).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1109385109/-/DCSupplemental.
References
- 1.Reisz RR, Scott D, Sues H-D, Evans DC, Raath MA. Embryos of an early Jurassic prosauropod dinosaur and their evolutionary significance. Science. 2005;309:761–764. doi: 10.1126/science.1114942. [DOI] [PubMed] [Google Scholar]
- 2.Kitching JW. Preliminary report on a clutch of six dinosaurian eggs from the Upper Triassic Elliot Formation, Northern Orange Free State. Palaeontologia Africana. 1979;22:41–45. [Google Scholar]
- 3.Norell MA, Clark JM, Chiappe LM, Dashzeveg D. A nesting dinosaur. Nature. 1995;378:774–776. [Google Scholar]
- 4.Varricchio DJ, Jackson F, Borkowski J, Horner JR. Nest and egg-clutches of the dinosaur Troodon formosus and the evolution of avian reproductive traits. Nature. 1997;385:247–250. [Google Scholar]
- 5.Sato T, Cheng YN, Wu XC, Zelenitsky DK, Hsiao YF. A pair of shelled eggs inside a female dinosaur. Science. 2005;308:375. doi: 10.1126/science.1110578. [DOI] [PubMed] [Google Scholar]
- 6.Chiappe LM, et al. Sauropod embryos from the Late Cretaceous of Patagonia. Nature. 1998;396:258–261. [Google Scholar]
- 7.Sander PM, Peitz C, Jackson F, Chiappe LM. Upper Cretaceous titanosaur nesting sites and their implications for sauropod reproductive biology. Palaeontographica A. 2008;284:69–107. [Google Scholar]
- 8.Carpenter K. In: Dinosaur Eggs and Babies. Carpenter K, Hirsch KF, Horner JR, editors. Cambridge: Cambridge Univ Press; 1994. pp. 288–297. [Google Scholar]
- 9.Mateus O. Upper Jurassic dinosaurs from Lourinha (Portugal) J Vertebr Paleontol. 1999;19:62A. [Google Scholar]
- 10.Sues H-D, Reisz RR, Hinic S, Raath MA. On the skull of Massospondylus carinatus Owen, 1854 (Dinosauria: Sauropodomorpha) from the Elliot and Clarens formations (Lower Jurassic) of South Africa. Ann Carnegie Mus. 2004;73:239–257. [Google Scholar]
- 11.Bordy EM, Hancox PJ, Rubidge BS. Basin development during the deposition of the Elliot Formation (Late Triassic–Early Jurassic), Karoo Supergroup, South Africa. South Afr J Geol. 2004 107:397–412.1. [Google Scholar]
- 12.Reisz RR, Evans DC, Sues H-D, Scott D. Embryonic skeletal anatomy of the sauropodomorph dinosaur Massospondylus from the Lower Jurassic of South Africa. J Vertebr Paleontol. 2010;30:1653–1665. [Google Scholar]
- 13.Chiappe LM, et al. Nest structure for sauropods: Sedimentary criteria for recognition of dinosaur nesting traces. Palaios. 2004;19:89–98. [Google Scholar]
- 14.Rainforth EC. Revision and re-evaluation of the Early Jurassic dinosaurian ichnogenus Otozoum. Palaeontology. 2003;46:803–838. [Google Scholar]
- 15.Eriksson PG. The depositional environment of the Elliot Formation in the Natal Drakensberg and north-east Orange Free State. Trans Geol Soc S Afr. 1985;88:19–26. [Google Scholar]
- 16.Seymour RS. Dinosaur eggs: Gas conductance through shell, water loss during incubation, and clutch size. Paleobiology. 1979;5:1–11. [Google Scholar]
- 17.Horner JR. Evidence of colonial nesting and ‘site fidelity’ among ornithischian dinosaurs. Nature. 1982;297:675–676. [Google Scholar]
- 18.Figueroa C, Powell JE. First International Symposium of Dinosaur Eggs and Embryos. In: Bravo AM, Reyes T, editors. Lleida, Isona, Spain: Imprenta Provincial; 2000. pp. 51–60. [Google Scholar]
- 19.López-Martínez N. First International Symposium of Dinosaur Eggs and Embryos. In: Bravo AM, Reyes T, editors. Lleida, Isona, Spain: Imprenta Provincial; 2000. pp. 95–115. [Google Scholar]
- 20.Mohabey DM. First International Symposium of Dinosaur Eggs and Embryos. In: Bravo AM, Reyes T, editors. Lleida, Isona, Spain: Imprenta Provincial; 2000. pp. 139–154. [Google Scholar]
- 21.Chiappe LM, Jackson F, Coria RA, Dingus L. In: The Sauropods. Wilson JA, Curry-Rogers K, editors. Berkeley: Univ of California Press; 2005. pp. 285–302. [Google Scholar]
- 22.Bonnan MF, Senter P. Were the basal sauropodomorph dinosaurs Plateosaurus and Massospondylus habitual quadrupeds? Spec. Pap. Palaeontol. 2007;77:139–155. [Google Scholar]
- 23.Thorbjarnarson JB, Hernandez G. Reproductive ecology of the Orinoco crocodile (Crocodylus intermedius) in Venezuela. II. Reproductive and social behavior. J Herpetol. 1993;27:371–379. [Google Scholar]
- 24.Lü J, et al. An egg-adult association, gender, and reproduction in pterosaurs. Science. 2011;331:321–324. doi: 10.1126/science.1197323. [DOI] [PubMed] [Google Scholar]
- 25.Oliveira CEM, et al. Crocodylomorph eggs and eggshells from the Adamantina Formation (Bauru Group) Upper Cretaceous of Brasil. Palaeontology. 2011;54:309–321. [Google Scholar]
- 26.Zanno LE, Makovicky PJ. Herbivorous ecomorphology and specialization patterns in theropod dinosaur evolution. Proc Natl Acad Sci USA. 2011;108:232–237. doi: 10.1073/pnas.1011924108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martinez RN, et al. A basal dinosaur from the dawn of the dinosaur era in southwestern Pangaea. Science. 2011;331:206–210. doi: 10.1126/science.1198467. [DOI] [PubMed] [Google Scholar]
- 28.Butler RJ, et al. Lower limits of ornithischian dinosaur body size inferred from a new Upper Jurassic heterodontosaurid from North America. Proc Biol Sci. 2010;277:375–381. doi: 10.1098/rspb.2009.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Varricchio DJ, et al. Avian paternal care had dinosaur origin. Science. 2008;322:1826–1828. doi: 10.1126/science.1163245. [DOI] [PubMed] [Google Scholar]
- 30.Meng Q, Liu J, Varricchio DJ, Huang T, Gao C. Palaeontology: Parental care in an ornithischian dinosaur. Nature. 2004;431:145–146. doi: 10.1038/431145a. [DOI] [PubMed] [Google Scholar]
- 31.Tullberg BS, Ah-King M, Temrin H. Phylogenetic reconstruction of parental-care systems in the ancestors of birds. Philos Trans R Soc Lond B Biol Sci. 2002;357:251–257. doi: 10.1098/rstb.2001.0932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Horner JR. Dinosaur reproduction and parenting. Annu Rev Earth Planet Sci. 2000;28:19–45. [Google Scholar]
- 33.Grellet-Tinner G, Chiappe LM. In: Feathered Dragons. Currie PJ, Koppelhus EB, Shugar MA, Wright JL, editors. Bloomington: Indiana Univ Press; 2004. pp. 185–214. [Google Scholar]
- 34.Miall AD. In: Facies Models: Response to Sea Level Change. Walker RG, James NP, editors. Ottawa: Geological Association of Canada; 1992. pp. 119–142. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.