Abstract
We recently implicated two recurrent somatic mutations in an adrenal potassium channel, KCNJ5, as a cause of aldosterone-producing adrenal adenomas (APAs) and one inherited KCNJ5 mutation in a Mendelian form of early severe hypertension with massive adrenal hyperplasia. The mutations identified all altered the channel selectivity filter, producing increased Na+ conductance and membrane depolarization, the signal for aldosterone production and proliferation of adrenal glomerulosa cells. We report herein members of four kindreds with early onset primary aldosteronism of unknown cause. Sequencing of KCNJ5 revealed that affected members of two kindreds had KCNJ5G151R mutations, identical to one of the prevalent recurrent mutations in APAs. These individuals had severe progressive aldosteronism and hyperplasia requiring bilateral adrenalectomy in childhood for blood pressure control. Affected members of the other two kindreds had KCNJ5G151E mutations, which are not seen in APAs. These subjects had easily controlled hypertension and no evidence of hyperplasia. Surprisingly, electrophysiology of channels expressed in 293T cells demonstrated that KCNJ5G151E was the more extreme mutation, producing a much larger Na+ conductance than KCNJ5G151R, resulting in rapid Na+-dependent cell lethality. We infer that this increased lethality limits adrenocortical cell mass and the severity of aldosteronism in vivo, accounting for the milder phenotype among these patients. These findings demonstrate striking variations in phenotypes and clinical outcome resulting from different mutations of the same amino acid in KCNJ5 and have implications for the diagnosis and pathogenesis of primary aldosteronism with and without adrenal hyperplasia.
Keywords: adrenal gland, inwardly rectifying potassium channel, Kir3.4
Hypertension affects >1 billion people worldwide (1, 2) and contributes to >7 million deaths each year (3). In the United States, approximately half of adults with hypertension fail to achieve control of blood pressure (4), and successful treatment commonly requires three or more drugs.
The study of rare Mendelian forms of hypertension has demonstrated the key role of renal salt reabsorption in blood pressure regulation. Mutations in genes resulting in increased net salt reabsorption markedly raise blood pressure, whereas those that reduce salt reabsorption can cause life-threatening low blood pressure (5, 6).
Although in the large majority of hypertensive subjects, the underlying causes are unknown (“essential hypertension”), some cases can be attributed to specific disorders of the kidney and endocrine system (7). Among these, primary aldosteronism is found in ∼10% of patients referred for evaluation of hypertension (8). These patients typically present with hypertension owing to excessive aldosterone secretion that is independent of activity of the renin–angiotensin system and plasma K+ levels. High aldosterone levels increase renal salt reabsorption, leading to hypertension. Hypokalemia and metabolic alkalosis are variable features, resulting from increased renal electrogenic Na+ reabsorption, which provides the electrical driving force for K+ and H+ secretion. Common causes of primary aldosteronism include aldosterone-producing adrenal adenoma (APA; Conn's syndrome) and idiopathic adrenal hyperplasia. Less common causes include the Mendelian disease glucocorticoid-remediable aldosteronism (GRA), which is caused by mutations at the aldosterone synthase locus that result in constitutive expression of this enzyme (9).
We recently described an inherited form of primary aldosteronism in a single family featuring severe hypertension with massive adrenal hyperplasia in the first decade of life and demonstrated that this syndrome is caused by a gain-of-function missense mutation (T158A) in the KCNJ5 gene (10), encoding Kir3.4, a member of the inwardly rectifying K+ channel family (11). In parallel, we showed that either of two somatic mutations (G151R or L168R) in this same channel are a common cause of APAs, found in ∼45% of these tumors. These KCNJ5 mutations lie in and near the highly conserved potassium channel selectivity filter and result in channels that are no longer selective for K+ conductance, but instead also conduct Na+. This new conductance results in Na+ influx, membrane depolarization, and activation of voltage-gated calcium channels, the normal signal for aldosterone production and cellular proliferation in adrenal cortex. When such mutations occur somatically, benign aldosterone-producing adenomas result; when they are inherited, the adrenal cortex becomes hyperplastic and produces large amounts of aldosterone.
To date, a single family with primary aldosteronism due to inherited mutation in KCNJ5 has been reported, raising the question of the prevalence of inherited mutation in KCNJ5 in familial aldosteronism, as well as the phenotypic spectrum of disease in these patients. We report herein four kindreds with early onset primary aldosteronism due to mutation in KCNJ5. The results demonstrate surprising genotype–phenotype correlation, with the most extreme effects on channel selectivity producing the mildest clinical phenotype.
Results
Kindreds with Early Primary Aldosteronism.
We investigated four families comprising 10 individuals diagnosed with early onset hypertension and primary aldosteronism; all but 1 affected subject was diagnosed before age 6, a particularly early age of diagnosis. GRA was excluded by genetic screening in each kindred (9). Pedigrees of the four kindreds are shown in Fig. 1, and summaries of their clinical features are shown in Table 1. Affected members of kindred (K) 767 and K409 had markedly different clinical courses and response to spironolactone compared with subjects in K1486 and K124.
Fig. 1.
KCNJ5 mutations in patients with early onset primary aldosteronism. (Left) Pedigrees are shown, with affected subjects shown as filled symbols and unaffected family members as open symbols; subjects are numbered as in Table 1. The index case is denoted with an arrow. (Right) Sanger DNA sequence traces and corresponding amino acids at KCNJ5 positions 150–152 of tested subjects are shown. In each case, a single-base pair substitution leads to a missense mutation: G151R in affected members of K767 and K409 and G151E in families 1486 and 124. The mutation in subject 409-1 is a de novo mutation.
Table 1.
Clinical features of patients with familial primary aldosteronism
| Subject | KCNJ5 mutation | Age dx, y | Blood pressure, mmHg | PRA,* ng⋅mL−1⋅h−1 | Aldosterone† | K+,‡ mM | Treatment |
| 767-1 | G151R | 1 | 174/85 | <0.2 | 99 μg/24 h | 2.1 | Adrenalectomy |
| 767-2 | G151R | 1 | 120/70 | 0.1 | 68 μg/24 h | 1.4 | Adrenalectomy |
| 767-3 | G151R | 1 | 130/90 | < 0.2 | 88 ng/dL | 3.2 | Adrenalectomy |
| 409-1 | G151R | 4 | 127/80 | <0.1 | 23 ng/dL | 1.7 | No F/U |
| 1486-1 | G151E | 2 | 135/85 | <0.1 | 61 μg/24 h | 3.5 | Spironolactone |
| 1486-2 | G151E | 1 | 153/94 | <0.1 | 91 ng/dL | 2.6 | Spironolactone |
| 1486-3 | G151E | 0.2 | 130/NA | <0.1 | 297 ng/dL | 4.4 | Spironolactone |
| 1486-4 | N/A | 11 | 160/120 | N/A | 18 μg/24 h | 3.0 | 90% adrenalectomy |
| 124-1 | G151E | 4 | High | <0.1 | High | Low | Spironolactone |
| 124-2 | G151E | <6 | High | N/A | High | Low | Adrenalectomy |
Age dx, age of diagnosis; FU, follow up; N/A, not available.
*PRA values < 0.3 are considered suppressed.
†Aldosterone values in μg per 24 h are urinary; values in ng/dL are serum. Normal values for urinary aldosterone on ad libitum diet: < 7 y < 5.7; 8–11 y <10.2. Normal values for serum aldosterone: 1–12 mo: 2–70; 1–5 y: 2–37; 6–12 y: 3–21.
K767.
The index case, subject 767-2, presented at 22 mo with muscular weakness. She had severe hypokalemia (serum K+ 1.4 mmol/L; normal 3.3–4.6) and hypertension (blood pressure of 120/70 mmHg; >99th percentile for age and sex). She was treated with potassium chloride. At 32 mo, she had severe hypertension, with blood pressure of 150/120 mmHg, serum K+ of 2.7 mmol/L (on treatment), and serum HCO3− of 30 mmol/L. Urinary aldosterone was elevated (68 μg/24 h, normal at this age <5.7) despite suppressed plasma renin activity (PRA; 0.1 ng⋅mL−1⋅h−1 supine). Despite treatment with spironolactone, blood pressure remained at 140–160/94–110 mmHg. Adrenal venous sampling and abdominal ultrasound led to a left adrenalectomy, which revealed an enlarged adrenal gland weighing 4.2 g and a small segment of ectopic adrenal tissue [average combined weight of both adrenals in children ages 2–5 y = 4.7 g (12)]. Both adrenal gland and ectopic adrenal tissue were reported as showing hyperplasia of the zona glomerulosa and zona fasciculata. Despite surgery, aldosteronism, difficult-to-control hypertension, and hypokalemia persisted, and at 4 y 4 mo, the right adrenal gland was removed; it weighed 4.3 g and again showed hyperplasia of the zona glomerulosa and fasciculata. The bilateral adrenalectomy normalized blood pressure and K+ levels, and she has since remained healthy on hydrocortisone replacement. She is now 38 y old.
Subject 767-2 has two biological children. Subject 767-1 was found to be severely hypertensive with hypokalemia and alkalosis at 15 mo (Table 1). PRA was suppressed, and aldosterone was elevated. She remained hypertensive despite treatment with combinations of spironolactone, hydrochlorothiazide, amlodipine, atenolol, prazosin, and minoxidil. Computed tomography (CT) initially revealed no adrenal abnormalities; however, repeat study at 23 mo showed bilateral adrenal enlargement. She also had sequential left and right adrenalectomies at ages 3 and 4, demonstrating adrenocortical hyperplasia. The bilateral adrenalectomy normalized blood pressure, serum potassium, and aldosterone levels. She has been treated with glucocorticoids and fludrocortisone. She is now 11 y old.
The second child, subject 767-3, presented at 12 mo with failure to thrive, polydipsia, and polyuria. Evaluation revealed hypertension, hypokalemia, suppressed PRA, and elevated serum aldosterone. Treatment with spironolactone failed to normalize blood pressure. At 18 mo, bilateral laparoscopic adrenalectomy was performed, revealing a 2-g paired adrenal gland weight [average combined weight at ages 1–2 y: 3.56 g (12)], without evidence of adenoma or hyperplasia. Blood pressure and aldosterone level subsequently normalized. The patient has been treated with hydrocortisone. She is now 7 y old.
K1486.
The index case, 1486-1, is a 38-y-old female. Her detailed history has been reported (13). At 26 mo, she had hypertension with blood pressure of 135/85 mmHg (>99th percentile), serum K+ of 3.5 mmol/L, and bicarbonate of 26 mmol/L. PRA was suppressed (<0.1 ng⋅mL−1⋅h−1), and 24-h urinary aldosterone level was elevated (23.5–61.0 μg per 24 h); aldosterone secretion rate was 405 μg/d (normal 50–100). Adrenal venous sampling demonstrated very high aldosterone values from both adrenal veins (5,200 ng/dL on left; 4,600 ng/dL on right). In sharp contrast to subjects in K767, treatment with spironolactone (100 mg/d) normalized her blood pressure; in equally strong contrast, she did not have progression of hypertension or growth of the adrenal glands with age. The patient's blood pressure has been well-controlled with spironolactone for >35 y, with no evidence of hypertensive complications. A CT scan at age 37 revealed no adrenal abnormality.
Her father (subject 1486-4) had early hypertension and aldosteronism. He had removal of the right adrenal and 4/5 of the left adrenal gland at age 14, before the availability of mineralocorticoid antagonists. The glands were described as histologically normal, and he has been normotensive and normokalemic since surgery. Details of the father's history and evaluation were reported by Bartter and Biglieri in 1958 (14).
The index case has two biological children. At 14 mo, subject 1486-2 was hypertensive and hypokalemic, with elevated serum aldosterone despite suppressed PRA. Treatment with spironolactone normalized blood pressure, and he remains well at age 7.
Subject 1486-3 is a 2-y-old female found at 2 mo to have hypertension with suppressed PRA and high aldosterone level. Blood pressure has been successfully treated with spironolactone alone.
K409.
Subject 409-1, a female, presented at age 4 y with hypertension, hypokalemia, and metabolic alkalosis. PRA was suppressed, and serum aldosterone level (23 mg/dL) was considered elevated in the presence of suppressed PRA. Family history was negative for early onset hypertension, and the parents were normotensive adults. Ultrasound of the abdomen was normal. The patient had difficult-to-control hypertension and was subsequently lost to follow-up.
K124.
Subject 124-1 is a 27-y-old male who presented at 4 y with hypertension, undetectable renin, and elevated serum aldosterone. Adrenal scans revealed no masses or abnormalities. Treatment with spironolactone normalized blood pressure.
The index case's father (124-2) presented with hypertension, hypokalemia, and primary aldosteronism in the first years of life. Bilateral adrenalectomy was performed at age 6, before mineralocorticoid receptor antagonists were available. Histologic results are unavailable. He is maintained on cortisol replacement.
Genetic Analysis of KCNJ5.
The recent identification of inherited mutations in KCNJ5 in one family with early severe aldosteronism and massive adrenocortical hyperplasia suggested inherited KCNJ5 mutations as a potential cause of aldosteronism in these subjects.
We directly sequenced KCNJ5 in all available members of each kindred (Fig. 1). Remarkably, all affected members in the four families not only had heterozygous mutations in KCNJ5, but all had mutations that altered the same amino acid, G151, which is one of the two positions showing recurrent somatic mutation in aldosterone-producing adenomas. This amino acid is a universal element of K+ channel selectivity filters from achaebacteria to humans (15, 16). Most interestingly, affected members of two kindreds (767 and 409) had inherited mutations that were identical to one of the recurrent somatic mutations found in APAs, substituting arginine for glycine at position 151 (G151R); one of these mutations was de novo, present in the affected daughter (subject 409-1), but not the unaffected biological parents. In contrast, the other two kindreds (1486 and 124) had a different mutation, resulting in substitution of glutamate for glycine (G151E). This mutation has not been previously reported. Both mutations were absent among 6,000 control chromosomes.
Distinct Clinical Features of Patients with G151R and G151E Mutation.
Review of the clinical features of affected members of families having G151R and G151E mutations illustrates strikingly different presentations. Like the family previously reported with the inherited T158A mutation in KCNJ5, patients with the G151R mutation had severe aldosteronism that was virtually unresponsive to spironolactone and that worsened with age. Among the patients with G151R mutations with follow-up, all have required the radical intervention of bilateral adrenalectomy to achieve control of hypertension and hypokalemia at very young ages (range 1–4 y). Pathology at adrenalectomy in all but one showed adrenal enlargement and hyperplasia of the adrenal cortex. The exception, subject 767-3, underwent adrenalectomy at 18 mo, consistent with the inference that the adrenal cortex in patients with this mutation proliferate postnatally; indeed, there is a strong correlation of adrenal weight and age in patients with G151R and T158A mutations (r2 = 0.83; Fig. S1).
In contrast, patients with the G151E mutation (in K1486 and K124) showed aldosteronism early in life but had no progressive disease. These patients had remarkable responsiveness to single-agent treatment with spironolactone, leading to normalization of blood pressure and K+ in all subjects in whom it has been used and with effectiveness persisting well into adulthood. Moreover, none of these affected subjects showed adrenal enlargement on CT or scintigraphy at ages up to 37 y. In addition, histology of the adrenal glands of subject 1486-4, which were removed before availability of spironolactone, was reported as normal. The response to spironolactone was significantly different between subjects with G151R and G151E mutations (4/4 G151E carriers had blood pressure successfully controlled with spironolactone as a single agent vs. 0/3 G151R carriers, who failed spironolactone and other medical therapies and came to adrenalectomy; P = 0.03).
Consistent with these phenotypic differences, it is noteworthy that, although somatic G151R mutations have been seen in 86 aldosterone-producing adenomas (23% of all APAs), the G151E mutation has never been seen. This difference in frequency in APAs (86 vs. 0) vs. inherited aldosteronism (2 vs. 2) is highly statistically significant (P = 0.001) and consistent with the inference that the G151E mutation cannot support development of increased adrenal cell mass.
KCNJ5G151E Causes Marked Na+-Dependent Lethality.
To understand the molecular mechanisms for the distinctive clinical syndromes resulting from G151R and G151E mutations, we expressed enhanced green fluorescent protein (eGFP)-tagged KCNJ5 bearing wild-type (WT) sequence, the G151R mutation or the G151E mutation in the mammalian 293T-cell line. There were immediately obvious effects of the different mutations on cell survival, because independent preparations and transfections of KCNJ5G151E failed to provide a significant number of viable eGFP-positive cells. Virtually all cells expressing the G151E mutation were dead within 36 h of transfection.
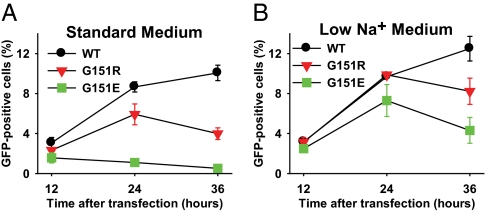
To explore the kinetics of cell survival, we transfected 293T cells with WT or mutant KCNJ5 and determined the percentage of eGFP-positive cells by flow cytometry at 12, 24, and 36 h after transfection (Fig. 2A). Whereas cells transfected with KCNJ5WT showed increasing percentages of eGFP-positive cells over this time period, cells transfected with KCNJ5G151R (associated with adrenocortical hyperplasia) exhibited a smaller increase in the percentage of eGFP-positive cells at 24 h and a subsequent decrease by 36 h (Fig. 2A). The percentage of eGFP-positive cells was significantly lower for KCNJ5G151R than KCNJ5WT at 36 h (4.0% vs. 10.1%; P = 0.003). In further contrast, KCNJ5G151E (associated with no adrenocortical hyperplasia) produced a much more extreme outcome, with very few eGFP-positive cells detected at any time after transfection (0.5% at 36 h; P < 0.0001 vs. KCNJ5WT and 0.003 vs. KCNJ5G151R at 36 h). Whether cell lethality was attributable to apoptosis or necrosis was not determined.
Fig. 2.
Survival of 293T cells transfected with WT and mutant KCNJ5 channels. WT or mutant eGFP-tagged KCNJ5 was transfected into 293T cells, and the percentage of eGFP-positive cells was measured at indicated times by flow cytometry (20,000 events counted per data point). (A) Incubation in physiologic medium (Materials and Methods) shows lower percentage of cells expressing KCNJ5G151R than KCNJ5WT at 36 h (P = 0.003) and lower percentage of cells expressing KCNJ5G151E than either KCNJ5WT (P < 0.0001) or KCNJ5G151R (P = 0.003). (B) Reduction of extracellular Na+ by choline substitution significantly increased survival of cells expressing KCNJ5G151R and KCNJ5G151E at 24 and 36 h, with a more pronounced effect on the G151E mutant (for both mutant channels, P = 0.02 for low Na+ vs. normal medium at 24 h; P = 0.04 at 36 h).
Because the G151R mutation has been previously shown to alter the selectivity of KCNJ5, making it permeable to Na+, we hypothesized that the lethality associated with KCNJ5G151E might be explained by G151E imparting an even larger Na+ conductance, with the resulting increased intracellular Na+ resulting in cell death. If correct, we posited that reducing extracellular Na+ should improve cell survival. We consequently prepared a low-Na+ cell culture medium by substituting choline chloride for sodium chloride and choline bicarbonate for sodium bicarbonate (Materials and Methods). Four hours after transfection, the normal culture medium was replaced with the low Na+ medium, and the percentage of eGFP-positive cells was again recorded (Fig. 2B). There was no substantive impact on the percentage of cells expressing KCNJ5WT at any time point. In contrast, the percentage of eGFP-positive cells after transfection with both mutant constructs showed a significant increase at 24 and 36 h (all P < 0.05). Most strikingly, cells expressing KCNJ5G151E increased nearly sevenfold at 24 h (from 1.1 ± 0.2% to 7.3 ± 1.6%) and more than eightfold at 36 h (0.5% ± 0.1% to 4.3 ± 1.3%). Consistent with a relative increase in survival of cells expressing KCNJ5G151E vs. KCNJ5WT in low Na+ medium, the ratio of KCNJ5WT: KCNJ5G151E cells at 36 h decreased from 22:1 in normal medium to 3:1 in low Na+ medium (P = 0.004).
KCNJ5G151E Has Markedly Higher Na+ Conductance than KCNJ5G151R.
The results above indicate that the dramatically reduced survival of cells expressing the G151E mutation can be mitigated by reducing extracellular Na+, suggesting that the G151E mutation might result in a channel with a larger Na+ conductance. We expressed WT and mutant KCNJ5 channels in 293T cells and measured currents from −100 to +60 mV using the patch-clamp technique with perforated whole-cell recordings. Even brief incubation in physiological solutions with high extracellular Na+ concentrations quickly led to death of cells expressing KCNJ5G151E. To reduce this effect, a low Na+ extracellular solution (40 mM NaCl, 100 mM KCl, and 2 mM MgCl2, pH 7.4, adjusted with KOH) and an intracellular solution containing 140 mM KCl, 4 mM MgCl2, and 5 mM Hepes (pH 7.4, adjusted with KOH) was used.
Representative whole-cell recordings are shown in Fig. 3A, and I–V curves are shown in Fig. 3B. Under these conditions, typical inwardly rectifying barium-sensitive currents were observed for the WT channel, and the reversal potential was close to 0 mV, reflecting the high extracellular K+ level used for measurements; the WT channel was not inhibited when choline was substituted for extracellular Na+ (10). In contrast, KCNJ5G151E channel showed no barium inhibition and increased permeability to barium (a proxy for increased Ca2+ permeability) (Fig. S2), and current was virtually abolished by substituting choline for Na+, indicating that under these conditions the current is predominantly carried by Na+. Although the KCNJ5G151E currents were qualitatively similar to those of KCNJ5G151R (i.e., lack of inhibition by BaCl2 and inhibition by choline substitution), currents were much larger for KCNJ5G151E than those elicited by KCNJ5G151R. For example, at −100 mV, the choline-sensitive Na+ current was nearly fourfold larger with KCNJ5G151E than KCNJ5G151R (P < 0.001) (Fig. 3C).
Fig. 3.
KCNJ5G151E channels have higher Na+ permeability than KCNJ5G151R channels. Whole-cell currents of 293T cells transiently transfected with WT or mutant KCNJ5 channels were measured by using the patch-clamp technique and the voltage protocol indicated in A. Because incubation in solutions with physiological Na+ concentrations led to death of cells expressing KCNJ5G151E, an extracellular solution containing 40 mM NaCl, 100 mM KCl, and 2 mM MgCl2 (pH 7.4, adjusted with KOH) and an intracellular solution containing 140 mM KCl, 4 mM MgCl2, and 5 mM Hepes (pH 7.4, adjusted with KOH) was used (control). In Ba2+ conditions, 1 mM Ba2+ was added to the above extracellular medium. In Ba2+/choline conditions, choline was substituted for Na+ as described in Materials and Methods. Representative whole-cell recordings are shown in A, and I–V curves are shown in B. In B, black, red, and green symbols denote control, Ba2+, and Ba2+/choline conditions, respectively. For better visibility, only one in two data points is plotted where curves overlap. Currents were negligible following transfection with empty vector (Fig. S3). Typical inwardly rectifying currents were observed for the WT channel; barium-sensitivity was abolished in the mutant channels. KCNJ5G151E showed larger currents than KCNJ5G151R, which were abolished by substitution of choline for Na+, indicating a predominant Na+ conductance. (C) Mean current amplitudes at −100 mV for WT and mutant channels using the solutions described in A. Net Na+ current is calculated by subtracting the current after choline substitution from the Ba2+ resistant current. Na+ current is significantly larger in the G151E mutant than in G151R mutant (P < 0.001). n ≥ 3 for each condition.
Discussion
We have described four families with very early onset of primary aldosteronism who have germ-line mutations in KCNJ5 that alter the K+ selectivity of the channel. The increased Na+ conductance introduced by mutant KCNJ5 channels provides a mechanism for aldosteronism (10). From the transmission pattern in these kindreds and the presence of a de novo mutation, we conclude that these heterozygous mutations are sufficient to produce primary aldosteronism. These findings confirm and extend the results from the single small kindred previously reported (10).
These four kindreds collectively harbor two different mutations that are distinct from the inherited T158A mutation previously identified (10). Most surprising is the observation that, although these two mutations both alter G151, an extraordinarily highly conserved element of the K+ channel selectivity filter (15, 16), they resulted in markedly different phenotypes in vivo and in vitro. Patients with KCNJ5G151R mutations had severe hypertension that was poorly controlled by antihypertensives including spironolactone, and all three subjects with follow-up came to the radical intervention of bilateral adrenalectomy. Pathology in surgical specimens showed marked adrenocortical hyperplasia with the exception of the subject who had adrenalectomy at 18 mo of age, consistent with progressive development of hyperplasia with age. These findings are highly similar to those in patients with the previously reported KCNJ5T158A mutation (10).
In contrast, subjects with the KCNJ5G151E mutation had a markedly different clinical course. Although these patients also present early in life, disease is not progressive, and those who have been treated with spironolactone have all had excellent control of blood pressure with this single agent well into adulthood. Moreover, imaging studies have revealed no evidence of adrenal hyperplasia as late as age 37, and the histopathology of the subject with G151E mutation who came to adrenalectomy before the availability of spironolactone showed no adrenocortical hyperplasia.
Studies of cell survival and electrophysiology provide an explanation for this striking phenotypic variation. KCNJ5G151E results in a much larger Na+ conductance than KCNJ5G151R, leading to dramatic lethality in 293T cells, with virtually no cells expressing KCNJ5G151E surviving 36 h in physiologic medium. The mitigation of cell lethality by substitution of choline for Na+ in extracellular medium demonstrates the role of Na+ in lethality. Both the size of the Na+ conductance and the cell lethality are markedly greater in the presence of the G151E mutation compared with G151R.
These striking in vitro effects suggest that the absence of hyperplasia in subjects with G151E mutations is attributable to increased cell death in cells expressing this mutation in vivo, limiting the glomerulosa cell mass and likely accounting for the absence of progressive disease. These cells are nonetheless individually producing large amounts of aldosterone, sufficient to cause hypertension. It is interesting that patients with both G151 mutations present at similar ages, but diverge in severity thereafter, likely due to the increased mass of aldosterone-producing cells among KCNJ5G151R carriers. Whether the observed differential response to mineralocorticoid antagonists is fully explained by differences in aldosterone levels at young age is unclear; mineralocorticoids other than aldosterone, such as 18-oxocortisol, are produced in patients with the T158A mutation (17), and differential production of these unmeasured mineralocorticoids could play a role. Further work will be required to explore these issues.
The rapid lethality of cells expressing the G151E mutation in 293T cells may be partially mitigated in vivo in glomerulosa cells by the presence of constitutively open TASK and TREK K+ channels (18, 19) and by high activity of the Na+/K+-ATPase (20); both would reduce the magnitude of cell depolarization resulting from mutant KCNJ5. The fact that aldosterone-producing cells are not entirely eliminated in vivo in the presence of KCNJ5G151E, resulting in an atretic glomerulosa, may be explained by their continuous replenishment from adrenocortical progenitor or stem cells (21). Provided that these progenitors do not express KCNJ5, mature glomerulosa cells would be continuously born, with their expression of KCNJ5 upon differentiation resulting in constitutive aldosterone production before their demise. This hypothesis could explain the observed dissociation of aldosterone production and cell mass seen in these patients.
Independent evidence that the G151E mutation cannot support increased adrenocortical cell mass comes from the observation that this mutation has never been seen in aldosterone-producing adenomas, unlike the highly prevalent G151R mutation.
The demonstration of increased cell lethality with both G151R and G151E mutations has potential implications for the molecular diagnosis of APAs. With only two mutations in KCNJ5 (G151R and L168R) accounting for ∼45% of APAs, it might be possible to develop specific tests for these somatic mutations in DNA released from dying cells in APAs into the circulation. Identification of these mutations in plasma could be used as a simple noninvasive diagnostic test in patients in whom APA is suspected. The increased turnover of cells harboring these mutations increases the likelihood that these mutations might be of sufficient prevalence in plasma to be detected. This test could be used to identify individuals with high likelihood of APA. Alternatively, it might be possible to develop selective inhibitors of mutant KCNJ5 that would be highly selective and effective in the treatment of patients with these mutations.
Lastly, these findings also have implications for other patients with primary aldosteronism who do not have APA or hyperplasia. The ability of G151E mutations to cause aldosteronism without hyperplasia or APA raises the question of whether somatic, rather than inherited, G151E mutations might frequently contribute to development of hypertension featuring high aldosterone:renin ratios and/or primary aldosteronism in the absence of APA or hyperplasia.
Materials and Methods
PCR and Sequencing of Exon 1 of the KCNJ5 Gene.
Genomic DNA was extracted from peripheral blood cells by using standard methods. KCNJ5 was amplified by PCR using gene-specific primers and genomic DNA as template. Primer sequences were 5′-GACTCTGTACTAGGCACCAGGGATAC-3′ (KCNJ5-F1) and 5′-GAGGGATTGAATGTGTTGCCTGCG-3′ (KCNJ5-R1), resulting in a 1,274-bp PCR product. PCR products were analyzed by agarose gel electrophoresis and directly sequenced by using the forward and reverse primers via Sanger sequencing.
Flow Cytometry.
The 293T cells were seeded on six-well dishes and transfected in triplicate with 0.3 μg of WT, G151R, or G151E pIRES KCNJ5 eGFP using 0.6 μL of Lipofectamine 2000 (Invitrogen). Untransfected cells were used as controls. Four hours after transfection, the medium was changed from OPTI-MEM (Invitrogen) to either standard DMEM (Invitrogen) containing 1.8 mM CaCl2, 0.25 μM Fe(NO3)3·9H2O; 0.8 mM MgSO4, 5 mM KCl, 44 mM NaHCO3, 110 mM NaCl, 1 mM NaH2PO4·H2O, 1 mM sodium pyruvate, 25 mM glucose, vitamins, and amino acids, supplemented with 1% ITS+ (BD Biosciences), but no serum; or modified, low-Na+ medium, in which 44 mM choline bicarbonate was substituted for NaHCO3 and 110 mM choline chloride for NaCl. With the addition of 5.8 mM NaCl via vitamin solution (Invitrogen), this mixture resulted in a calculated total Na+ concentration of 7.7 mM compared with ∼155 mM in standard medium. At 12, 24, and 36 h after transfection, 2 mM EDTA was added to each well, and cells were resuspended. Cells were collected by centrifugation and resuspended in low-Na+ medium, in which 1.8 mM MgCl2 was substituted for 1.8 mM CaCl2, and 2 mM EDTA was added to prevent cell adhesion. 20,000 events were recorded on a BD FACSCalibur, and the percentage of eGFP-positive cells was calculated by using FlowJo software (version 9.2; Tree Star). Untransfected cells were used as controls. Data were plotted by using the SigmaPlot software (Jandel Scientific), and mean ± SEM of three biological replicates of independent DNA preparations is shown.
Electrophysiology.
The 293T cells were transfected with 0.3 μg of plasmid DNA encoding WT or mutant KCNJ5, by using TransIT-293 Transfection Reagent (Mirus). After transfection, the cells were maintained in low-Na+ culture medium as described for flow cytometry experiments, and experiments were typically performed 12 h after transient transfection. Standard whole-cell patch clamp recordings were performed by using an Axon Patch 200A amplifier. Pipettes were pulled from borosilicate glass and had resistances between 2 and 4 MΩ. For measurement of Na+ currents, the bath solution contained 40 mM NaCl, 100 mM KCl, and 2 mM MgCl2 (pH 7.4, adjusted with KOH), and the pipette solution contained 140 mM KCl, 4 mM MgCl2, and 5 mM Hepes (pH 7.4, adjusted with KOH). K+ currents were inhibited by application of 1 mM BaCl2. To determine the Na+ current, choline was substituted for Na+ in the extracellular solution (40 mM choline, 100 mM KCl, 1 mM BaCl2). For measurement of Ba2+ current, the bath solution contained 110 mM BaCl2 and 10 mM Hepes (pH 7.4, adjusted with KOH), and the pipette solution contained 160 mM CsCl, 5 mM EGTA, 1 mM MgCl2, and 5 mM Hepes (pH 7.4, adjusted with KOH). The Ba2+ currents, as an index of Ca2+ currents, were inhibited by application of La3+ (100 μM) at the end of experiments. Data were collected from at least three cells in each condition and were analyzed by a combination of Axon Clampfit 9.2 and SigmaPlot programs.
Supplementary Material
Acknowledgments
We thank our patients and their families for their invaluable contributions to this study and the staff of the Yale Cell Sorter Core Facility. These studies were supported in part by the Leducq Transatlantic Network on Hypertension (R.P.L.), National Institutes of Health Grant NIHDK54983 (to W.-H.W.), and Deutsche Forschungsgemeinschaft Grant SCHO 1386/1-1 (to U.I.S.). R.P.L. is an Investigator for the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1121407109/-/DCSupplemental.
References
- 1.Kearney PM, et al. Global burden of hypertension: Analysis of worldwide data. Lancet. 2005;365:217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 2.Chobanian AV, et al. National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 3.WHO . Global Health Risks - Mortality and Burden of Disease Attributable to Selected Major Risks. Geneva: WHO; 2009. [Google Scholar]
- 4.Yoon SS, Ostchega Y, Louis T. Recent trends in the prevalence of high blood pressure and its treatment and control, 1999-2008. NCHS Data Brief. 2010;(48):1–8. [PubMed] [Google Scholar]
- 5.Lifton RP, Gharavi AG, Geller DS. Molecular mechanisms of human hypertension. Cell. 2001;104:545–556. doi: 10.1016/s0092-8674(01)00241-0. [DOI] [PubMed] [Google Scholar]
- 6.Lifton RP. Genetic dissection of human blood pressure variation: Common pathways from rare phenotypes. Harvey Lect. 2004-2005;100:71–101. [PubMed] [Google Scholar]
- 7.Omura M, Saito J, Yamaguchi K, Kakuta Y, Nishikawa T. Prospective study on the prevalence of secondary hypertension among hypertensive patients visiting a general outpatient clinic in Japan. Hypertens Res. 2004;27:193–202. doi: 10.1291/hypres.27.193. [DOI] [PubMed] [Google Scholar]
- 8.Rossi GP, et al. PAPY Study Investigators. A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J Am Coll Cardiol. 2006;48:2293–2300. doi: 10.1016/j.jacc.2006.07.059. [DOI] [PubMed] [Google Scholar]
- 9.Lifton RP, et al. A chimaeric 11 beta-hydroxylase/aldosterone synthase gene causes glucocorticoid-remediable aldosteronism and human hypertension. Nature. 1992;355:262–265. doi: 10.1038/355262a0. [DOI] [PubMed] [Google Scholar]
- 10.Choi M, et al. K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Science. 2011;331:768–772. doi: 10.1126/science.1198785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krapivinsky G, et al. The G-protein-gated atrial K+ channel IKACh is a heteromultimer of two inwardly rectifying K(+)-channel proteins. Nature. 1995;374:135–141. doi: 10.1038/374135a0. [DOI] [PubMed] [Google Scholar]
- 12.Altman PL, Dittmer DS. Growth, Including Reproduction and Morphological Development. Washington, DC: Federation of American Societies for Experimental Biology; 1962. [Google Scholar]
- 13.Greco RG, Carroll JE, Morris DJ, Grekin RJ, Melby JC. Familial hyperaldosteronism, not suppressed by dexamethasone. J Clin Endocrinol Metab. 1982;55:1013–1016. doi: 10.1210/jcem-55-5-1013. [DOI] [PubMed] [Google Scholar]
- 14.Bartter FC, Biglieri EG. Primary aldosteronism: Clinical staff conference at the National Institutes of Health. Ann Intern Med. 1958;48:647–654. doi: 10.7326/0003-4819-48-3-647. [DOI] [PubMed] [Google Scholar]
- 15.Doyle DA, et al. The structure of the potassium channel: Molecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- 16.Heginbotham L, Lu Z, Abramson T, MacKinnon R. Mutations in the K+ channel signature sequence. Biophys J. 1994;66:1061–1067. doi: 10.1016/S0006-3495(94)80887-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geller DS, et al. A novel form of human mendelian hypertension featuring nonglucocorticoid-remediable aldosteronism. J Clin Endocrinol Metab. 2008;93:3117–3123. doi: 10.1210/jc.2008-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Czirják G, Fischer T, Spät A, Lesage F, Enyedi P. TASK (TWIK-related acid-sensitive K+ channel) is expressed in glomerulosa cells of rat adrenal cortex and inhibited by angiotensin II. Mol Endocrinol. 2000;14:863–874. doi: 10.1210/mend.14.6.0466. [DOI] [PubMed] [Google Scholar]
- 19.Enyeart JJ, Xu L, Danthi S, Enyeart JA. An ACTH- and ATP-regulated background K+ channel in adrenocortical cells is TREK-1. J Biol Chem. 2002;277:49186–49199. doi: 10.1074/jbc.M207233200. [DOI] [PubMed] [Google Scholar]
- 20.Meuli C, Müller J. Characterization of rat capsular adrenal (zona glomerulosa) (Na,K)-ATPase activity. J Steroid Biochem. 1982;16:129–132. doi: 10.1016/0022-4731(82)90154-6. [DOI] [PubMed] [Google Scholar]
- 21.Wood MA, Hammer GD. Adrenocortical stem and progenitor cells: Unifying model of two proposed origins. Mol Cell Endocrinol. 2011;336:206–212. doi: 10.1016/j.mce.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quest Diagnostics . Pediatric Endocrinology Reference Guide. San Juan Capistrano, CA: Nichols Institute; 2004. [Google Scholar]
- 23.Lo FL. In: Nelson Textbook of Pediatrics. Kliegman RM, Stanton BMD, St. Geme J, Schor N, Behrman RE, editors. Philadelphia: Elsevier; 2011. pp. e708-2t–e708-8t. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.