Abstract
The arbuscular mycorrhizal (AM) symbiosis, formed between the majority of land plants and ubiquitous soil fungi of the phylum Glomeromycota, is responsible for massive nutrient transfer and global carbon sequestration. AM fungi take up nutrients from the soil and exchange them against photosynthetically fixed carbon (C) from the host. Recent studies have demonstrated that reciprocal reward strategies by plant and fungal partners guarantee a “fair trade” of phosphorus against C between partners [Kiers ET, et al. (2011) Science 333:880–882], but whether a similar reward mechanism also controls nitrogen (N) flux in the AM symbiosis is not known. Using mycorrhizal root organ cultures, we manipulated the C supply to the host and fungus and followed the uptake and transport of N sources in the AM symbiosis, the enzymatic activities of arginase and urease, and fungal gene expression in the extraradical and intraradical mycelium. We found that the C supply of the host plant triggers the uptake and transport of N in the symbiosis, and that the increase in N transport is orchestrated by changes in fungal gene expression. N transport in the symbiosis is stimulated only when the C is delivered by the host across the mycorrhizal interface, not when C is supplied directly to the fungal extraradical mycelium in the form of acetate. These findings support the importance of C flux from the root to the fungus as a key trigger for N uptake and transport and provide insight into the N transport regulation in the AM symbiosis.
Keywords: arbuscular mycorrhiza, arginine catabolism, carbon transport, Glomus intraradices, urea cycle
The arbuscular mycorrhizal (AM) symbiosis plays a key role in nutrient uptake in the majority of land plants, including such important crop species as corn, soybean, and rice. The AM symbiosis can increase the uptake of phosphate (P) and nitrogen (N), as well as of trace elements such as copper and zinc, and improves the abiotic and biotic stress resistance of the host (2). Previous work has focused primarily on the transport of P in AM symbiosis, but more recent work has highlighted the potential importance of N uptake by fungal symbionts (3, 4). The extraradical mycelium (ERM) of the fungus is able to take up NH4+ (5, 6), NO3− (5–7), and organic N resources (3–5) from the soil and to transfer N to the host. The high mobility of N in the soil has raised the question of whether AM fungi can contribute significantly to the N nutrition of the host (8). It has been suggested that an improved N status of mycorrhizal plants may be simply a consequence of an improved P nutrition (9); however, other studies have demonstrated that AM fungi can deliver substantial amounts of N to the host, with an estimated 21% of total N taken up by the fungal ERM in root organ cultures (10) and 74% of the total N in the leaves of Zea mays coming from the fungal ERM with access to urea (11).
Current models of N transport in the AM symbiosis involve uptake of inorganic N from the soil and N assimilation via the anabolic arm of the urea cycle into arginine (Arg) in the ERM, Arg translocation—likely in connection with polyphosphates (polyP)—to the intraradical mycelium (IRM), and breakdown of Arg via the catabolic arm of the urea cycle into inorganic N, which is subsequently transferred across the mycorrhizal interface to the host (12–14). This model of N transport has been supported by labeling experiments (13, 15, 16) and enzymatic tests (15) and has been further corroborated by molecular data demonstrating the expression of genes putatively involved in Arg biosynthesis in the ERM and Arg breakdown in the IRM (17, 18) and the localization of mycorrhiza-inducible plant NH4+ transporters in the periarbuscular membrane (19).
Nonetheless, what triggers the fungus to transfer N to the host, and whether N acquisition and transport are central aspects of symbiotic function, remain unknown. AM fungi are asexual obligate biotrophs that rely almost exclusively on the host plant's carbon (C) to complete their life cycle, consuming between 4% and 17% of the host's photosynthetically fixed C (20). Recently, Kiers et al. (1) demonstrated that cooperative behavior in the AM symbiosis is stabilized by reciprocal rewarding of P and C resources according to the nutritional benefit provided by the other partner. An increase in C flux from the mycorrhizal host plant to the fungus has been shown to stimulate P uptake and transfer in AM symbiosis (21, 22) and it has been demonstrated that the transcript levels of MST2, a high affinity monosaccharide transporter of Glomus sp., and of PT4, a mycorrhiza-specific plant P transporter, are correlated (23). It has been suggested that polyP play a key role in regulating P transfer to the host (1, 22). A breakdown of polyP in the IRM in response to an increase in C flux from the host (1, 22) also would likely release Arg in the IRM, and thus possibly subsequently increase the N flux to the host.
Here we investigated the effect of C resource availability on fungal N uptake and transfer in the AM symbiosis, to elucidate whether similar reward mechanisms as have been described for P (1) also stimulate N transport and exchange by the mycorrhizal fungus. The ultimate goal is to understand how plant and fungal partners regulate nutrient exchange in the most important and ancient symbiosis of land plants.
Results
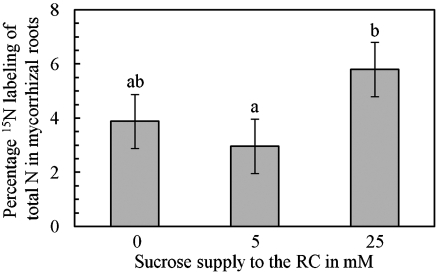
An increase in C availability to the AM fungus via the addition of sucrose to the root compartment (RC) increased the uptake of inorganic and organic N sources by the ERM and transport to the root. After the addition of 25 mM sucrose to the RC, approximately twice as much 15N was detectable in the root tissue (Fig. 1). The uptake of 14C-Arg from the fungal compartment (FC) was ∼2.5 times greater after the addition of sucrose to the RC (Fig. S1). This increase in Arg uptake by the ERM did not result in a detectable increase of 14C count rates in the root tissue, however. In contrast, a time-dependent increase in the 14C count rates in mycorrhizal roots to which no sucrose was supplied was observed after the addition of 14C-Arg to the FC (Fig. S2). Directly supplying the fungus with C by the addition of 4 mM acetate to the fungal ERM produced no significant effect on 15N uptake and transport to the root (Fig. S3). The addition of sucrose to the RC altered the P pool distribution and increased the proportion of short-chain polyP to long-chain polyP in mycorrhizal roots (Fig. S4A), but did not lead to an increase in the P content per dry weight of the roots (Fig. S4C). Neither the P pool distribution nor the P content in the mycorrhizal roots was affected by the addition of acetate to the FC (Fig. S4 B and D). An increase in P availability for the AM fungus did not affect 14C-Arg transport to the roots (Fig. S5).
Fig. 1.
Transport of 15N from the fungal ERM to the roots is dependent on the sucrose supply to the RC. Shown is the percentage of 15N labeling of total N in mycorrhizal roots (mean ± SEM of n = 5). Different letters on the bars indicate statistically significant differences according to the U test (P ≤ 0.05). The lack of a statistically significant difference between 0 mM and 25 mM is caused by one biological replicate in the control treatment with relatively high labeling.
When 15NH4+ was supplied to the FC, Arg and glutamine were the dominant amino acids labeled in the root system (Table S1). Other free amino acids were detectable, but their labeling was relatively low. An increase in the C supply to the AM fungus by the addition of sucrose to the RC or acetate to the FC had no significant effect on the labeling of free amino acids in the mycorrhizal roots.
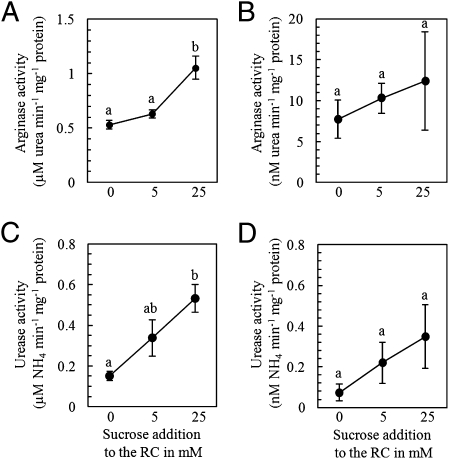
The first increase in arginase and urease activity in the mycorrhizal roots was observed 4 d after the addition of NH4+ or NO3− to the FC (Fig. S6). The addition of 25 mM sucrose to the RC caused a significant increase in arginase and urease activity in mycorrhizal roots compared with control or with lower sucrose treatment (Fig. 2 A and C) and compared with nonmycorrhizal control roots to which the same concentration of sucrose was added. Based on this observation, we can conclude that the enzymatic activity increased, particularly in the IRM of the fungal partner. Arginase and urease were also detected in the ERM, but the activity there was at least two orders of magnitude lower than in the mycorrhizal roots. There was a trend toward increasing arginase and urease activity in the ERM (Fig. 2 B and D), but this activity was not significantly greater than that seen under control conditions. Supplying C directly to the fungus by the addition of acetate to the FC produced no significant changes in arginase or urease activity in the mycorrhizal roots (Fig. S7).
Fig. 2.
Arginase (A and B) and urease (C and D) activities in mycorrhizal roots (A and C) and ERM (B and D) are dependent on the sucrose supply to the RC. Data are given as μM (A and C) or nM (B and D) urea (arginase) or NH4 (urease) min−1 mg−1 of protein (mean ± SEM of n = 6 or 7). Different letters indicate statistically significant differences according to ANOVA and the LSD test (P ≤ 0.05).
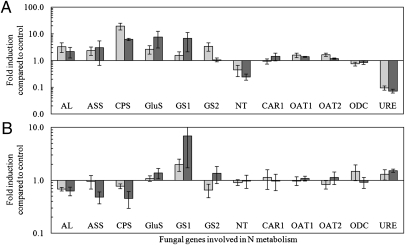
We studied the effect of different C supply conditions on the expression of 12 fungal genes putatively involved in N uptake (nitrate transporter, NT), N assimilation (glutamine synthetase 1 and 2, GS1 and GS2; glutamate synthase, GluS), arginine biosynthesis (carbamoyl-phosphate synthase glutamine chain, CPS; argininosuccinate synthase, ASS; argininosuccinate lyase, AL), arginine breakdown (arginase, CAR1; urease, URE; ornithine aminotransferase 1 and 2, OAT1 and OAT2), and polyamine biosynthesis (ornithine decarboxylase, ODC) (Fig. 3 and Table S2). The addition of sucrose to the RC and the resulting increase in C availability to the AM fungus had an effect on fungal gene expression. Several genes involved in N assimilation and Arg biosynthesis (AL, ASS, CPS, GluS, GS1, and GS2) were induced in the ERM by supplying sucrose to the RC (Fig. 3A). In contrast, NT, a fungal nitrate transporter, was down-regulated in response to the increased C supply. The transcript levels of catabolic Arg genes CAR1, OAT1, OAT2, and ODC were not affected, but the transcript level of URE was down-regulated in the ERM after the addition of sucrose to the RC. The effects of C addition on fungal gene expression were less pronounced in the IRM than in the ERM (Fig. 3B). An increase in C supply led to a slight down-regulation of genes involved in Arg biosynthesis (AL, ASS, and CPS), and increased transcript levels of GS1, but not of GS2. Expression of NT and URE in the IRM was not affected.
Fig. 3.
Fungal gene expression in the ERM (A) or IRM (B) after addition of 5 mM sucrose (light gray) or 25 mM sucrose (dark gray) to the RC. Data are expressed as fold induction compared with control (mean ± SEM of n = 4–6). Values <1 represent a decrease and values >1 an increase in transcript levels relative to control.
The addition of acetate to the FC also produced slight changes in gene expression, but these effects differed from those observed after the addition of sucrose. The transcript levels of the N assimilation and Arg biosynthesis genes (ASS, GluS, and GS2) were slightly lower in the ERM than under control conditions, whereas the fungal arginase (CAR1) was up-regulated in the ERM (Fig. S8A). In the IRM, only a trend toward slight down-regulation of genes involved in N assimilation and Arg biosynthesis (AL, ASS, CPS, GluS, and GS2) was seen (Fig. S8B).
Discussion
AM fungi are able to take up inorganic and organic N from the soil and transfer these N resources to the host (e.g., ref. 6), but whether these fungi are able to contribute significantly to the N nutrition of the plant is unclear (see ref. 8 for a review), and the mechanisms and regulation of N transfer to the host are poorly understood. We examined these mechanisms by manipulating the C supply to the host and fungus and then following the uptake and transport of N sources. We found that the C supply of the host plant triggers the uptake and transport of N in the symbiosis, and that this increase is orchestrated by changes in fungal gene expression. These findings support previous work showing that the AM fungus “rewards” its host with P in response to a higher C supply (1, 21, 22).
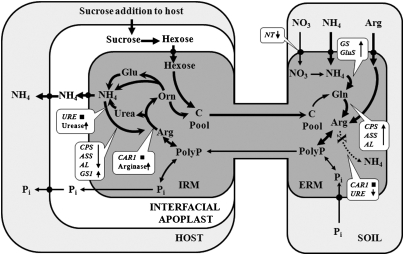
AM fungi are obligate biotrophs; the supply of C from the host is a prerequisite for N transfer across the mycorrhizal interface. This C supply allows the AM fungus to form an extensive ERM, provides the metabolic energy for active uptake processes, and provides the C skeletons required for assimilation of inorganic N into amino acids in the ERM (Fig. 4). The C expenditure for N assimilation is high, and this high C cost has been suggested to be responsible for the decrease in ectomycorrhizal fungal diversity and growth in soils with high N availability (24).
Fig. 4.
Model showing the current model of N transport in the AM symbiosis and effects observed after addition of sucrose to the RC (white boxes). Metabolic pathways that were stimulated by sucrose addition are indicated by bold lines or up-arrows; pathways that were reduced by sucrose addition, by hatched lines or down-arrows; and pathways that were not affected by sucrose addition, by black boxes.
Based on data from similar in vitro experiments, we are confident that the N, P, and C resources in the root-organ cultures were nearly exhausted when the C and N source was added. Under similar conditions, 80–90% of the originally supplied N and C resources were used up, and the P level was undetectable (22, 25). These limiting conditions allowed us to investigate what triggers N uptake and translocation in the symbiosis. Under these conditions, C flux to the mycorrhizal fungus is low, and sucrose supply to the mycorrhizal root increases C transport to the fungal ERM (22), but has no effect on P transport from the ERM to the IRM (Fig. S4C). Conversely, N becomes a limiting resource for the host, and the NH4+ added to the fungal ERM may represent a potentially significant N source for the host.
The results of the present study demonstrate that an increased C supply to the mycorrhizal fungus stimulated the uptake of inorganic and organic N sources by the ERM and transport to the IRM. An increase in C availability approximately doubled 15N transport to the mycorrhizal roots (Fig. 1), and uptake of Arg by the ERM was ∼2.5 times greater than under control conditions (Fig. S1). Despite this increase in 15N-NH4+ and 14C-Arg uptake, 15N labeling in free amino acids and 14C count rates in mycorrhizal roots were not affected by the C supply. This likely can be attributed to the fact that the increase in uptake coincided with a two- to three-fold increase in arginase and urease activity in the mycorrhizal roots in response to the increased C availability (Fig. 2). Greater Arg breakdown in the IRM potentially increases reflux of 14C to the ERM and maintains Arg concentration at a constant level. This idea is supported by the time-dependent accumulation of 14C in mycorrhizal roots observed after the addition of 14C-Arg to the ERM when host and fungus were under C-limiting conditions (Fig. S2). The increase in Arg uptake as a result of increased C availability to the fungus is in contrast to recent results of Hodge and Fitter (4), who reported that shading of the plant had no effect on uptake and the amount of N transferred to the host when the fungal ERM had access to an organic nutrient patch.
The stimulation of N transport in AM symbiosis by an increase in C availability coincided with changes in fungal gene expression. The addition of sucrose to the RC led to increased transcript levels of genes involved in N assimilation (GluS and GS2) and Arg biosynthesis (AL, ASS, and CPS), but decreased transcript levels of a high-affinity nitrate transporter (NT) and fungal urease (URE) in the ERM (Fig. 3A). These observed changes in gene expression are consistent with the predicted model of N transfer in AM symbiosis (12, 15, 17) and the observed stimulation of N transfer to the host. NT encodes a NO3− transporter of Glomus intraradices that is expressed in the ERM in response to an exogenous NO3− supply (17). AM fungi prefer the uptake of NH4+ over NO3−, and a supply of NH4+ reduces the uptake of NO3− by fungal hyphae (10, 16, 26). The uptake of NH4+ is energetically more efficient, because NO3− is more oxidized and its utilization requires more energy. The fact that NH4+ was added to all treatments, including the controls, indicates that the down-regulation of NT in the ERM after the supply of sucrose to the RC was the result of an increase in internal levels of NH4+ or a downstream metabolite, rather than a response to the exogenous NH4+ supply. Interestingly, the NO3− transporter nrt2 of the ectomycorrhizal basidiomycete Hebeloma cylindrosporum is up-regulated in response to a supply with different C sources (27), although a simultaneous supply with NH4+ reduces the effect.
GS1, GS2, and GluS were up-regulated in response to higher C levels. GS catalyzes the reaction of NH3 and glutamate (Glu) to glutamine (Gln), and GluS catalyzes the conversion of Gln and 2-oxoglutarate to two molecules of Glu. Both enzymes are key elements of the GS-GOGAT pathway, and the ERM mainly uses this pathway to assimilate inorganic N (28). Both genes are up-regulated in response to exogenous N sources (17, 28), and the increase in the transcript levels of these enzymes is in line with the greater utilization of NH4+ after sucrose supply to the RC, and with a high GS activity in the ERM of G. intraradices (15). The observed up-regulation of CPS, AL, and ASS and the down-regulation of URE in the ERM are also in agreement with the observed increase in N uptake and transport to the host as more C becomes available to the fungus. CPS catalyzes the formation of carbamoylphosphate from CO2, ATP, and NH3, which is converted by ASS (synthesis of argininosuccinate from citrulline and aspartate) and AL (conversion of l-argininosuccinate to Arg and fumarate) to Arg (Fig. 4). The expression levels of CPS, AL, and ASS in the ERM respond strongly within hours to an exogenous supply of NO3− (17), and it can be assumed that the NH4+ supplied in the present study had a similar effect. The increases in CPS, AL, and ASS expression in combination with the 10-fold down-regulation in transcript levels of URE (hydrolysis of urea to ammonia and CO2) in response to increased C availability will increase the Arg levels in the ERM (stimulation in biosynthesis and reduced catabolic breakdown) and the subsequent transfer of Arg from the ERM to the IRM (Fig. 4).
It has been suggested that Arg could move with polyP from the ERM to the IRM (8, 15). Stimulation of polyP breakdown in the IRM in response to increased C availability (1, 22) would release Arg in the IRM and could facilitate the subsequent breakdown of Arg and transport of NH4+ to the mycorrhizal interface (Fig. 4). The observed increase in short-chain polyP (Fig. S4A) is in line with this hypothesis and suggests increased remobilization of polyP in the IRM after the addition of sucrose to the RC. Short-chain polyP are associated with plant growth benefits and P transport to the host (29). However, under our test conditions, no increase in the P content per dry weight was detected (Fig. S4C), and Arg transport was not affected by the P supply to the FC (Fig. S5). That N and P transport across the mycorrhizal interface are independent processes was recently demonstrated in Medicago truncatula mtpt4 mutants that do not express a mycorrhiza-specific plant P transporter that is essential for P uptake from the mycorrhizal interface (30). In these mutants, arbuscules degenerate prematurely, and a functional symbiosis is not formed under normal conditions. N deficiency rescues the mutant phenotype, however, and a functional symbiosis is established, indicating that N supply by the fungus signals favorable conditions for maintaining symbiosis and regulates the arbuscular lifespan independent of the P transport across the mycorrhizal interface (30).
Gene expression in the fungal IRM was affected only moderately by the addition of sucrose to the RC. Despite the two- to three-fold increase in arginase and urease activity in mycorrhizal roots, the expression of CAR1 and URE remained stable after the addition of sucrose to the RC. Arg is catabolized by arginase (CAR1) and urease (URE), and both genes have been shown to be responsive to the addition of NO3− to the ERM and apparently are the functional genes for Arg breakdown in the IRM. Genes involved in Arg breakdown are highly expressed in the IRM (31). Tian et al. (17) suggested that an increase in Arg level in the IRM after the addition of NO3− to the ERM triggers the up-regulation of CAR1 and URE in the IRM of G. intraradices. Arg has been shown to induce the catabolic genes CAR1 and OAT in Aspergillus nidulans, and Arg catabolism is under the control of N metabolite repression and C catabolite repression systems (32). However, the constant transcript levels of CAR1 and URE, in combination with increased arginase and urease activity after the addition of sucrose to the RC, indicate that both genes are not only transcriptionally, but also posttranscriptionally and/or posttranslationally controlled in G. intraradices. Posttranscriptional and/or posttranslational control mechanisms of Arg catabolism also have been demonstrated for A. nidulans. Posttranscriptional control is often exerted by C source-dependent mRNA turnover (33), and modifications in transcript stability have been shown to play a key role in the posttranscriptional regulation of the transcription factor AreA, which controls the coordinated expression of hundreds of genes involved in Arg catabolism and N metabolism in A. nidulans (34). Arg serves as an important N and C source for fungi such as A. nidulans. Although AM fungi have a different physiology (e.g., they are directly supplied with hexoses via the mycorrhizal interface), it is interesting to speculate that the C supply of the host may be directly involved in transcriptional or posttranscriptional gene regulation in G. intraradices similar to the mechanisms described in A. nidulans.
The transcript levels of all genes involved in Arg biosynthesis (ASS, AL, and CPS), which were up-regulated in the ERM in response to the addition of C, were slightly down-regulated in the IRM. Reduced activity of these enzymes will prevent NH4+, which is released in response to high activity of arginase and urease during Arg breakdown, from being reconverted into Arg when more C becomes available for the fungus, which will increase the flux of NH4+ into the mycorrhizal interface (Fig. 4). However, the slight up-regulation of GS1 in response to increased C availability may indicate that a small percentage of NH4+ and Glu (probably from the conversion of ornithine to Glu; ref. 17) is reassimilated into Gln by the fungus before being transferred into the mycorrhizal interface.
We studied the effect of increased C availability on N transfer by the AM fungus and compared two different supply techniques, indirect supply of C via the mycorrhizal host root by the addition of sucrose to the RC and direct supply by the addition of acetate to the fungal ERM. AM fungi can take up acetate via the ERM and have gluconeogenetic capabilities (35), but are not able to use sucrose. Sucrose must be first hydrolyzed by an acid invertase of plant origin, and the resulting hexoses can be taken up by the AM fungus from the interface. Significant export of C from the IRM to the ERM can be observed 3 d after sucrose is supplied to the mycorrhizal roots (22). The transfer of C across the mycorrhizal interface is the dominant pathway for C uptake by the AM fungus; the uptake of acetate via the ERM after 4 d might be too slow to have any significant effect on N metabolism and the transport of the AM fungus. However, the addition of acetate not only resulted in lower gene expression profiles, but also in a different expression pattern compared with the addition of sucrose (Fig. S8). In the ERM, the transcript levels of genes involved in N assimilation and Arg biosynthesis (ASS, GluS, and GS2) were reduced slightly, but Arg breakdown was induced (CAR1). This finding also might indicate that the AM fungus actively reduces Arg transport through the hyphae from the ERM to the IRM when an exogenous C source is available. This is in agreement with the observation that the addition of acetate did not lead to increased uptake and transport of N to the mycorrhizal roots.
This study provides conclusive evidence that host C transfer stimulates the uptake and transfer of N in the AM symbiosis, and that the increase in N flux is orchestrated by changes in fungal gene expression. Interactions between C and P transfer have been described previously (21, 22), and Kiers et al. (1) demonstrated that cooperation in the AM symbiosis is stabilized by a reciprocal reward mechanism. These findings have challenged the previously held view that the host plant controls the symbiosis. Here we present further support for fungal mediation of the symbiosis by demonstrating that the AM fungus likewise rewards its host with an increase in N transport in response to higher C rewards. These data suggest that although AM fungi will always need a host plant to complete their life cycle, they gain an advantage in symbiosis by adjusting their nutrient transfer, both N and P, in response to the C supply from the host. Our findings are also in agreement with results of Johnson et al. (36), who reported that the AM symbiosis provides the nutrient (P or N) that is most limiting for the host, and that this response is driven by the C-to-nutrient exchange dynamics. This ensures “fair trade” of diverse commodities for one of the most ancient biological markets on earth.
In the present study, we used root-organ cultures to study the interactions between C and N transfer in AM symbiosis. The cultures have drawn criticism for their artificial nature (8); however, they are the system of choice for studies on transport processes (1, 17, 21) and provide the foundation for our current understanding of metabolic processes in AM symbiosis (see ref. 37 for a review). We chose this approach because it (i) allowed us to control the C and nutrient supply for fungal and plant partner separately without changing the whole plant physiology by shading or dark treatment, (ii) provided standardization of the amount of ERM; and (iii) provided easy access to sufficient amounts of ERM, essential for the enzymatic and gene expression studies. Despite the artificial nature of root-organ cultures, they have several characteristics analogous to whole plant systems (37), and recent data on the C exchange characteristics of root-organ cultures are in agreement with data from experiments on whole plant systems (1). Nonetheless, further studies on the interactions between C and N transport in whole plants under natural conditions are needed to critically evaluate the contribution of AM symbiosis to the N nutrition of the host.
Materials and Methods
Experimental System.
The experiments were performed using mycorrhizal root organ cultures of Ri T-DNA–transformed carrot (Daucus carota clone DCI) roots colonized by G. intraradices Schenck & Smith (DAOM 197198; Biosystematics Research Center). According to recent phylogenetic analyses of rDNA sequences, this fungal strain is also now referred to as Glomus irregulare or Rhizophagus irregularis (38). Information on culture conditions is provided in SI Materials and Methods. We used root organ cultures to study the effect of C availability on N transport in AM symbiosis, because this system had several important advantages for these studies. This allowed to separately supply fungal and plant partners with nutrient resources, and to control carbon availability for plant host and fungus. It provided easy access to sufficient quantities of ERM tissue, which was essential for the enzymatic assays and gene expression studies. For all experiments, a time point of 4 d was selected if not stated otherwise, because after 4 d significant transport of C to the ERM after sucrose supply to the RC can be observed (22), and the first increase in arginase and urease activity and Arg contents in the root tissue—after supply of NH4+, NO3−, or Arg to the fungal ERM—was observed (Figs. S2 and S6). This early time point allowed us to analyze fungal responses to different C supply conditions independent of responses that would be expected at later time points, such as differences in mycorrhizal colonization, root growth, fungal ERM development, and fungal strategy.
Experimental Design.
The effect of C resource availability on N uptake and transfer by the AM fungus was tested using two different C sources and supply methods. In the first set, 0 mM, 5 mM, or 25 mM sucrose was added to the RC. Because the AM fungus is not able to use sucrose, the sucrose is first taken up by the mycorrhizal root and then supplied to the AM fungus via the mycorrhizal interface. In the second set of experiments, the fungus was directly supplied with C by the addition of 4 mM acetate to the FC; AM fungi can take up acetate as a C source (35). At the same time, inorganic N in the form of 4 mM NH4Cl (depending on the experiment, either unlabeled or as 15N-NH4Cl) or organic N in the form of 50 μM 14C-labeled Arg (specific activity of 300 mCi mM−1; 0.41 nM as 14C-labeled Arg) was added to the ERM. Four days after supply of the C source and NH4Cl to the FC, the roots and the ERM of the FC were harvested and prepared for further analysis (see below). In addition, arginase and urease activity was examined in a time course experiment (0 h, 2 h, 4 h, 8 h, 24 h, 48 h, and 96 h) after supply of 4 mM NH4Cl or 4 mM KNO3 to the FC. Arg uptake and transport were measured under different P supply conditions by adding 0 μM, 35 μM, 700 μM, or 1.4 mM KH2PO4 to the ERM when the 50 μM 14C-labeled Arg (specific activity of 300 mCi mM−1; 0.41 nM as 14C-labeled Arg) was supplied.
Analysis of Fungal and Root Tissue.
At 4 d after supply of the two different C sources and addition of 15N-NH4Cl to the FC, the total 15N content in the mycorrhizal roots was measured by the analysis of 1H-NMR spectra after extraction of freeze- dried root material according to a method of Wall and Gehrke (39) described in more detail in SI Materials and Methods, and the 15N labeling of free amino acids in mycorrhizal roots was determined by GC-MS. The arginase and urease assays were performed as described by Cruz et al. (15) or Witte and Medina-Escobar (40) with minor modifications. The enzymatic activity in mycorrhizal roots was determined spectrophotometrically, normalized to the protein content (Bradford assay), and compared with the activity in nonmycorrhizal control roots, to which only sucrose was added. The expression of fungal genes putatively involved in N uptake and assimilation and Arg biosynthesis and breakdown was measured after RNA extraction by quantitative PCR. Arg uptake and transport to the root system were determined at different time points (time course experiments) or 4 d after 50 μM 14C-labeled Arg was added to the FC by liquid scintillation counting of the Arg residue in the medium or after digestion of the root material. The P content in the mycorrhizal roots and the P pool distribution were measured spectrophotometrically. Details of all analyses are provided in SI Materials and Methods.
Statistical Analysis.
All experiments were conducted with a minimum of three to seven biological replicates. For the gene expression studies, the root and ERM of two independent plates were pooled to form a single sample. All data were first subjected to ANOVA, and treatment means were tested for significance with the LSD test (P ≤ 0.05) or the U test (P ≤ 0.05), if the data were not normally distributed, using UNISTAT statistical software.
Supplementary Material
Acknowledgments
We thank Chunjie Tian for assistance with RNA extraction and quantitative PCR in the preliminary experiments; David Douds for a critical review of the manuscript; and Jerry A. Mensah, Joseph Lee, and Lindsay Rogers for technical assistance with P analysis and Kjeldahl degradation. Financial support was provided by National Science Foundation IOS Awards 0943338 and 1051397 and a Netherlands Organization for Scientific Research Vidi and Meervoud Grant (to E.T.K.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1118650109/-/DCSupplemental.
References
- 1.Kiers ET, et al. Reciprocal rewards stabilize cooperation in the mycorrhizal symbiosis. Science. 2011;333:880–882. doi: 10.1126/science.1208473. [DOI] [PubMed] [Google Scholar]
- 2.Smith SE, Read DJ. Mycorrhizal Symbiosis. 3rd Ed. Amsterdam: Academic Press; 2008. [Google Scholar]
- 3.Leigh J, Hodge A, Fitter AH. Arbuscular mycorrhizal fungi can transfer substantial amounts of nitrogen to their host plant from organic material. New Phytol. 2009;181:199–207. doi: 10.1111/j.1469-8137.2008.02630.x. [DOI] [PubMed] [Google Scholar]
- 4.Hodge A, Fitter AH. Substantial nitrogen acquisition by arbuscular mycorrhizal fungi from organic material has implications for N cycling. Proc Natl Acad Sci USA. 2010;107:13754–13759. doi: 10.1073/pnas.1005874107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hawkins HJ, Johansen A, George E. Uptake and transport of organic and inorganic nitrogen by arbuscular mycorrhizal fungi. Plant Soil. 2000;226:275–285. [Google Scholar]
- 6.Johansen A, Jakobsen I, Jensen ES. Hyphal transport by a vesicular-arbuscular mycorrhizal fungus of N applied to the soil as ammonium or nitrate. Biol Fertil Soils. 1993;16:66–70. [Google Scholar]
- 7.Tobar R, Azcón R, Barea JM. Improved nitrogen uptake and transport from 15N-labelled nitrate by external hyphae of arbuscular mycorrhiza under water-stressed conditions. New Phytol. 1994;126:119–122. [Google Scholar]
- 8.Smith SE, Smith FA. Roles of arbuscular mycorrhizas in plant nutrition and growth: New paradigms from cellular to ecosystem scales. Annu Rev Plant Biol. 2011;62:227–250. doi: 10.1146/annurev-arplant-042110-103846. [DOI] [PubMed] [Google Scholar]
- 9.Reynolds HL, Hartley AE, Vogelsang KM, Bever JD, Schultz PA. Arbuscular mycorrhizal fungi do not enhance nitrogen acquisition and growth of old-field perennials under low nitrogen supply in glasshouse culture. New Phytol. 2005;167:869–880. doi: 10.1111/j.1469-8137.2005.01455.x. [DOI] [PubMed] [Google Scholar]
- 10.Toussaint JP, St-Arnaud M, Charest C. Nitrogen transfer and assimilation between the arbuscular mycorrhizal fungus Glomus intraradices Schenck & Smith and Ri T-DNA roots of Daucus carota L. in an in vitro compartmented system. Can J Microbiol. 2004;50:251–260. doi: 10.1139/w04-009. [DOI] [PubMed] [Google Scholar]
- 11.Tanaka Y, Yano K. Nitrogen delivery to maize via mycorrhizal hyphae depends on the form of N supplied. Plant Cell Environ. 2005;28:1247–1254. [Google Scholar]
- 12.Govindarajulu M, et al. Nitrogen transfer in the arbuscular mycorrhizal symbiosis. Nature. 2005;435:819–823. doi: 10.1038/nature03610. [DOI] [PubMed] [Google Scholar]
- 13.Jin H, et al. The uptake, metabolism, transport and transfer of nitrogen in an arbuscular mycorrhizal symbiosis. New Phytol. 2005;168:687–696. doi: 10.1111/j.1469-8137.2005.01536.x. [DOI] [PubMed] [Google Scholar]
- 14.Bago B, Pfeffer PE, Shachar-Hill Y. Could the urea cycle be translocating nitrogen in the arbuscular mycorrhizal symbiosis. New Phytol. 2001;149:4–8. doi: 10.1046/j.1469-8137.2001.00016.x. [DOI] [PubMed] [Google Scholar]
- 15.Cruz C, et al. Enzymatic evidence for the key role of arginine in nitrogen translocation by arbuscular mycorrhizal fungi. Plant Physiol. 2007;144:782–792. doi: 10.1104/pp.106.090522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johansen A, Finlay RD, Olsson PA. Nitrogen metabolism of external hyphae of the arbuscular mycorrhizal fungus Glomus intraradices. New Phytol. 1996;133:705–712. [Google Scholar]
- 17.Tian C, et al. Regulation of the nitrogen transfer pathway in the arbuscular mycorrhizal symbiosis: Gene characterization and the coordination of expression with nitrogen flux. Plant Physiol. 2010;153:1175–1187. doi: 10.1104/pp.110.156430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gomez SK, et al. Medicago truncatula and Glomus intraradices gene expression in cortical cells harboring arbuscules in the arbuscular mycorrhizal symbiosis. BMC Plant Biol. 2009;9:10. doi: 10.1186/1471-2229-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guether M, et al. A mycorrhizal-specific ammonium transporter from Lotus japonicus acquires nitrogen released by arbuscular mycorrhizal fungi. Plant Physiol. 2009;150:73–83. doi: 10.1104/pp.109.136390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wright DP, Read DJ, Scholes JD. Mycorrhizal sink strength influences whole plant carbon balance of Trifolium repens L. Plant Cell Environ. 1998;21:881–891. [Google Scholar]
- 21.Hammer EC, Pallon J, Wallander H, Olsson PA. Tit for tat? A mycorrhizal fungus accumulates phosphorus under low plant carbon availability. FEMS Microbiol Ecol. 2011;76:236–244. doi: 10.1111/j.1574-6941.2011.01043.x. [DOI] [PubMed] [Google Scholar]
- 22.Bücking H, Shachar-Hill Y. Phosphate uptake, transport and transfer by the arbuscular mycorrhizal fungus Glomus intraradices is stimulated by increased carbohydrate availability. New Phytol. 2005;165:899–911. doi: 10.1111/j.1469-8137.2004.01274.x. [DOI] [PubMed] [Google Scholar]
- 23.Helber N, et al. A versatile monosaccharide transporter that operates in the arbuscular mycorrhizal fungus Glomus sp. is crucial for the symbiotic relationship with plants. Plant Cell. 2011;23:3812–3823. doi: 10.1105/tpc.111.089813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wallander H. A new hypothesis to explain allocation of dry matter between mycorrhizal fungi and pine seedlings in relation to nutrient supply. Plant Soil. 1995;168-169:243–248. [Google Scholar]
- 25.Jolicoeur M, Williams RD, Chavarie C, Fortin JA, Archambault J. Production of Glomus intraradices propagules, an arbuscular mycorrhizal fungus, in an airlift bioreactor. Biotechnol Bioeng. 1999;63:224–232. doi: 10.1002/(sici)1097-0290(19990420)63:2<224::aid-bit11>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 26.Gachomo E, et al. Germinating spores of Glomus intraradices can use internal and exogenous nitrogen sources for de novo biosynthesis of amino acids. New Phytol. 2009;184:399–411. doi: 10.1111/j.1469-8137.2009.02968.x. [DOI] [PubMed] [Google Scholar]
- 27.Rékangalt D, et al. Expression of the nitrate transporter nrt2 gene from the symbiotic basidiomycete Hebeloma cylindrosporum is affected by host plant and carbon sources. Mycorrhiza. 2009;19:143–148. doi: 10.1007/s00572-008-0221-2. [DOI] [PubMed] [Google Scholar]
- 28.Breuninger M, Trujillo CG, Serrano E, Fischer R, Requena N. Different nitrogen sources modulate activity but not expression of glutamine synthetase in arbuscular mycorrhizal fungi. Fungal Genet Biol. 2004;41:542–552. doi: 10.1016/j.fgb.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 29.Takanishi I, Ohtomo R, Hayatsu M, Saito M. Short-chain polyphosphate in arbuscular mycorrhizal roots colonized by Glomus spp.: A possible phosphate pool for host plants. Soil Biol Biochem. 2009;41:1571–1573. [Google Scholar]
- 30.Javot H, et al. Medicago truncatula mtpt4 mutants reveal a role for nitrogen in the regulation of arbuscule degeneration in arbuscular mycorrhizal symbiosis. Plant J. 2011;68:954–965. doi: 10.1111/j.1365-313X.2011.04746.x. [DOI] [PubMed] [Google Scholar]
- 31.Tisserant E, et al. The transcriptome of the arbuscular mycorrhizal fungus Glomus intraradices (DAOM 197198) reveals functional tradeoffs in an obligate symbiont. New Phytol. 2011;193:755–769. doi: 10.1111/j.1469-8137.2011.03948.x. [DOI] [PubMed] [Google Scholar]
- 32.Dzikowska A, et al. Specific induction and carbon/nitrogen repression of arginine catabolism gene of Aspergillus nidulans—functional in vivo analysis of the otaA promoter. Fungal Genet Biol. 2003;38:175–186. doi: 10.1016/s1087-1845(02)00522-4. [DOI] [PubMed] [Google Scholar]
- 33.Duttagupta R, et al. Global analysis of Pub1p targets reveals a coordinate control of gene expression through modulation of binding and stability. Mol Cell Biol. 2005;25:5499–5513. doi: 10.1128/MCB.25.13.5499-5513.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caddick MX, et al. Opposing signals differentially regulate transcript stability in Aspergillus nidulans. Mol Microbiol. 2006;62:509–519. doi: 10.1111/j.1365-2958.2006.05383.x. [DOI] [PubMed] [Google Scholar]
- 35.Pfeffer PE, Becard G, Shachar-Hill Y, Shachar-Hill Y, Douds DD., Jr Carbon uptake and the metabolism and transport of lipids in an arbuscular mycorrhiza. Plant Physiol. 1999;120:587–598. doi: 10.1104/pp.120.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson NC, Wilson GWT, Bowker MA, Wilson JA, Miller RM. Resource limitation is a driver of local adaptation in mycorrhizal symbioses. Proc Natl Acad Sci USA. 2010;107:2093–2098. doi: 10.1073/pnas.0906710107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fortin JA, et al. Arbuscular mycorrhiza on root-organ cultures. Can J Bot. 2002;80:1–20. [Google Scholar]
- 38.Schüßler A, Walker C. The Glomeromycota. A Species List with New Families and New Genera. Corvallis, OR: Libraries at The Royal Botanic Garden Edinburgh, Edinburgh, UK; The Royal Botanic Garden Kew, Kew, UK; Botanische Staatssammlung Munich, Munich, Germany; and Oregon State University; 2010. pp. 1–56. [Google Scholar]
- 39.Wall LL, Gehrke CW. An automated total protein nitrogen method. J Assoc Off Anal Chem. 1975;58:1221–1226. [Google Scholar]
- 40.Witte CP, Medina-Escobar N. In-gel detection of urease with nitroblue tetrazolium and quantification of the enzyme from different crop plants using the indophenol reaction. Anal Biochem. 2001;290:102–107. doi: 10.1006/abio.2000.4933. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.