Abstract
The boreal forests, identified as a critical “tipping element” of the Earth's climate system, play a critical role in the global carbon budget. Recent findings have suggested that terrestrial carbon sinks in northern high-latitude regions are weakening, but there has been little observational evidence to support the idea of a reduction of carbon sinks in northern terrestrial ecosystems. Here, we estimated changes in the biomass carbon sink of natural stands throughout Canada's boreal forests using data from long-term forest permanent sampling plots. We found that in recent decades, the rate of biomass change decreased significantly in western Canada (Alberta, Saskatchewan, and Manitoba), but there was no significant trend for eastern Canada (Ontario and Quebec). Our results revealed that recent climate change, and especially drought-induced water stress, is the dominant cause of the observed reduction in the biomass carbon sink, suggesting that western Canada's boreal forests may become net carbon sources if the climate change–induced droughts continue to intensify.
Keywords: global warming, forest aboveground biomass, drought index, northern hemisphere carbon uptake, positive feedback
Global warming and regional droughts may intensify and become more frequent this century as a result of anthropogenic climate change (1). Recent studies (2–4) predicted a weaker northern hemisphere carbon uptake at high latitudes as a result of these changes. One of the greatest uncertainties in global climate change is how to forecast changes in feedback between the biosphere and the atmosphere. Northern high-latitude forests contain about 49% of the carbon stored in forested ecosystems (5) and provide important feedback to the global climate system. Boreal forests, a key but poorly understood component of terrestrial ecosystems, exert strong controls on the global carbon cycle and influence regional hydrology and climatology directly through water and surface energy budgets (6). Previous studies (7–10) indicated that threats to the forest carbon sink as a result of tree mortality caused by rising temperatures and drought around the world have unexpectedly increased in the past decade.
Recent climate changes in this region may have had substantial impact on the carbon balance of Canadian boreal forests as a result of increased fire frequency (11), an unprecedented expansion of insect outbreaks (12), and widespread drought-induced tree mortality (10). Large and long-term forest permanent sampling plots (PSPs) could provide direct estimates of biomass carbon accumulation and possible insights into the future role of forests in the global carbon cycle under a changing climate (13). Recent progress has been made in investigations of the impacts of severe drought on trembling aspen (Populus tremuloides) mortality and associated loss of aboveground biomass in western Canada (10, 14). However, to date, no study has used long-term forest observation plots to directly investigate the spatial distribution of changes in forest biomass carbon in Canadian boreal forests at a national scale as a result of recent climate change.
To assess the potential impacts of climate change-induced drought, we analyzed data from 96 long-term permanent forest observation plots in natural (unmanaged) mature boreal forests that met nine criteria (SI Appendix, Table S1). Our samples included plots in five Canadian provinces in both western Canada (Alberta, Saskatchewan, and Manitoba) and eastern Canada (Ontario and Quebec). The plots spanned 53° of longitude and 9° of latitude, and their elevations ranged from 59 to 2,609 m above sea level (asl) (Fig. 1 and SI Appendix, Table S1). The plots ranged from 0.04 to 0.82 ha ( ha), and collectively contained 22,425 living trees; the plot data included a total of 74,556 observations in these plots. The initial census year ranged from 1963 to 1994 and the final census year ranged from 1990 to 2008. The aboveground stand biomass at each census was estimated by using published allometric models developed for Canadian boreal forests (SI Appendix, ref. S12 and Table S2). We used both the annual climate moisture index (CMI) and the annual moisture index (AMI) to measure climatic water deficits in this study. The CMI value was calculated as the difference between precipitation (PCP) and potential evapotranspiration (PET) (SI Appendix, ref. S10). AMI is defined as the ratio of the annual number of degree days above 5 °C to the mean annual precipitation (SI Appendix, ref. S11).
ha), and collectively contained 22,425 living trees; the plot data included a total of 74,556 observations in these plots. The initial census year ranged from 1963 to 1994 and the final census year ranged from 1990 to 2008. The aboveground stand biomass at each census was estimated by using published allometric models developed for Canadian boreal forests (SI Appendix, ref. S12 and Table S2). We used both the annual climate moisture index (CMI) and the annual moisture index (AMI) to measure climatic water deficits in this study. The CMI value was calculated as the difference between precipitation (PCP) and potential evapotranspiration (PET) (SI Appendix, ref. S10). AMI is defined as the ratio of the annual number of degree days above 5 °C to the mean annual precipitation (SI Appendix, ref. S11).
Fig. 1.
Locations of the 96 forest plots in Canada's boreal forest. The red and black circles represent plots with respectively decreasing and increasing rates of biomass change. The size of the circle is proportional to the plot-specific slope of the ordinary least-squares regression for the rate of biomass change as a function of the calendar year. Thus, the circle size reflects the rate of annual change in biomass. The background colors of green and light green represent the boreal and hemiboreal regions, respectively. In total, 80 plots (83% of the 96 forest plots) were located in the boreal region and 16 (17%) were located in the hemiboreal region. A total of 70 plots were located in the western region (Alberta, Saskatchewan, and Manitoba) and 26 were located in the eastern region (Ontario and Quebec). In total, 89% of the plots (62/70) in the western region and 46% of the plots (12/26) in the eastern region experienced decreasing rate of biomass change. The shapefiles defining Canada's boreal and hemiboreal zones were developed by J. P. Brandt of Natural Resources Canada (SI Appendix, ref. S16) and were obtained from the agency's Web page (http://canadaforests.nrcan.gc.ca/download; accessed December 23 2011).
Results
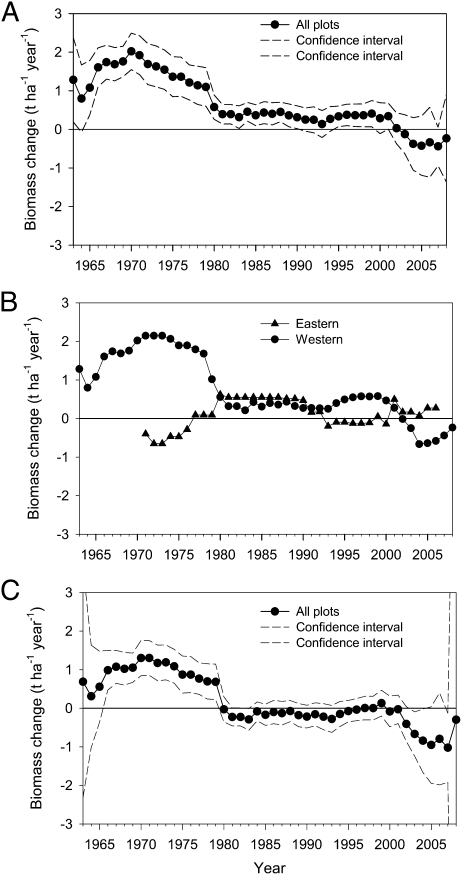
Our analysis showed that the rate of biomass change for all plots combined and for the western region showed significant decreasing trends, but there was no significant change for the eastern region (Fig. 2 A and B and Table 1). In addition to the statistical analysis, we compared the average rates of biomass change for the western and eastern regions between the first and final censuses (SI Appendix, Fig. S1). Consistent with our model results, the biomass in the western region during the last census interval was significantly lower than that during the first interval (P < 0.0001, paired two-sample t test); for the eastern region, there was no significant difference (P = 0.1339, paired two-sample t test). Moreover, the biomass increment decreased significantly during three periods (before 1980, from 1980 to 2000, and after 2000) in the western region, but there was no significant change in the eastern region (SI Appendix, Fig. S2).
Fig. 2.
(A) Annual rate of change in aboveground biomass for all Canadian boreal forest plots combined from 1963 to 2008. The dotted lines represent 95% confidence intervals. (B) Annual rate of change of aboveground biomass for the western and eastern regions. (C) Annual rate of change in stand-age–corrected aboveground biomass for all Canadian boreal forest plots combined from 1963 to 2008. The dotted lines represent 95% confidence intervals.
Table 1.
Fixed effects of the linear mixed models (SI Appendix, Eq. S1) describing trends in the rate of biomass change
| Data | Model | β1 | SE | P | n |
| All plots | Trend in rate of biomass change | −0.0514 | 0.0105 | <0.0001 | 96 |
| Western region | Trend in rate of biomass change | −0.0694 | 0.0117 | <0.0001 | 70 |
| Eastern region | Trend in rate of biomass change | 0.0061 | 0.0229 | 0.7922 | 26 |
β1 is the slope and represents the annual rate of change in biomass (t ha−1 year−1 year−1), n is the number of forest plots used in the model, and P is the significance level for the model's fixed effects based on a t test. The dataset used for fitting the linear mixed models is not the dataset to estimate the average trend dot values in Fig. 2A.
The observed patterns of the rate of biomass change for western and eastern regions may have resulted from changes in tree mortality, growth of surviving trees, or a combination of both factors. To further explore the ecological causes of the rate of biomass change, we analyzed the trends of mortality (SI Appendix, Table S4), stand density (SI Appendix, Table S5), and biomass of the surviving trees (SI Appendix, Table S6). Our analysis revealed that the biomass increment associated with mortality for both regions showed significant increasing trends (SI Appendix, Table S4). These trends were further confirmed by the changes in stand density (SI Appendix, Table S5), which indicated that the number of surviving trees was decreasing significantly for both the western and the eastern regions. However, the rate of biomass change for surviving trees in the western region showed a significant decreasing trend, whereas there was a significant increasing trend for surviving trees in the eastern region (SI Appendix, Table S6). Thus, our analysis indicated that the trend of decreasing biomass change in the western region resulted from the cumulative effects of increased mortality and decreased growth of surviving trees, but mortality had a larger effect (SI Appendix, Table S4; β = 0.0289) than growth of surviving trees (SI Appendix, Table S5; β = −0.0116) on the rate of biomass change. For the eastern region, the simultaneous increase in mortality and growth of surviving trees represented offsetting factors that concealed any significant trend in biomass change.
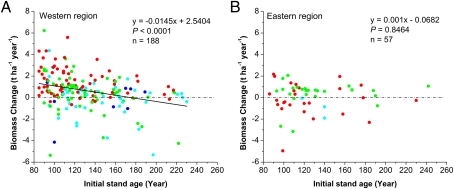
We examined several possible causes for the different trends in the western and eastern regions. One of the best-known causes for a decreasing rate of biomass accumulation in mature forests is an age-related decline (15). Our statistical analysis (SI Appendix, Table S7 and Fig. 3A) showed that the trend for aboveground biomass changed significantly as a function of age for the western region, from an increase at young ages to a decrease in older stands (slope of the regression = −0.011, P = 0.005), but that there was no significant trend for the eastern region (P = 0.8465). We also used the Kolmogorov–Smirnov test and found no significant difference between the age distributions in the western and eastern regions (P = 0.8705). If age is the sole factor responsible for the observed trends, we would expect to see similar trends as a function of age in the two regions. Thus, additional factors must have contributed to the differences in the observed patterns of biomass change. Moreover, we regressed the rate of biomass change as a function of plot size and the length of the census interval to test whether heterogeneity in plot characteristics might have affected the rate of biomass change for the western and eastern regions. Our regressions showed that the rate of biomass change was not significantly correlated with either plot size or the length of the census interval (SI Appendix, Fig. S3), indicating that the heterogeneity of plot characteristics had no significant effect on the rate of biomass change.
Fig. 3.
Plots of the rate of biomass change as a function of stand age for the western and eastern regions. The black line represents the modeled trends from the ordinary least-squares regression model. The red, green, light blue, and blue dots represent the first, second, third, and fourth census, respectively. n is number of censuses for all of the age ranges.
We hypothesized that climate warming may have been the dominant cause of the observed trends. For the western region, the annual mean temperature has increased significantly since 1963 (P < 0.0001), and annual precipitation has decreased significantly (P = 0.0002), and the combination has significantly increased water deficits, whether estimated using CMI or AMI (both P < 0.0001; SI Appendix, Table S8). In contrast, both the annual mean temperature and the annual precipitation increased significantly for the eastern region (both P < 0.0001), resulting in a significantly decreased water deficit (CMI, P = 0.0197; SI Appendix, Table S8). We regressed the rates of biomass change as functions of four climatic variables (temperature, precipitation, CMI, and AMI) and found that all climatic variables were significantly correlated with the rate of biomass change in the western region but not in the eastern region (SI Appendix and Table 2). Scatterplots between the rate of biomass change and the climatic variables also showed consistent results (SI Appendix, Figs. S4–S7).
Table 2.
Fixed effects of the linear mixed models (SI Appendix, Eq. S3) describing the relationships between the rate of biomass change and the climatic variables
| Data | Parameter | β | SE | P | n | β1 + β2Dk* |
| Western region | Average temperature | −1.3608 | 0.3152 | <0.0001 | 70 | AB: −0.2105 |
| Average temperature × AB | 1.1503 | 0.3385 | 0.0008 | SK: −1.3608 | ||
| Average temperature × MB | 1.4756 | 0.3807 | 0.0001 | MB: 0.1148 | ||
| Average precipitation | 0.0240 | 0.0061 | 0.0001 | 70 | AB: 0.0019 | |
| Average precipitation × AB | −0.0221 | 0.0064 | 0.0006 | SK: 0.0240 | ||
| Average precipitation × MB | −0.0155 | 0.0068 | 0.0234 | MB: 0.0850 | ||
| CMI | 0.1397 | 0.0389 | 0.0004 | 70 | AB: 0.0342 | |
| CMI × AB | −0.1055 | 0.0407 | 0.0104 | SK: 0.1397 | ||
| MB: 0.1397 | ||||||
| AMI | −0.0055 | 0.0016 | 0.0009 | 70 | AB: −0.0015 | |
| AMI × AB | 0.0040 | 0.0018 | 0.0279 | SK: −0.0055 | ||
| MB: −0.0055 | ||||||
| Eastern region | Average temperature | −0.0728 | 0.1107 | 0.5135 | 26 | None |
| Average precipitation | 0.0022 | 0.0018 | 0.2211 | 26 | None | |
| CMI | 0.0146 | 0.0117 | 0.2185 | 26 | None | |
| AMI | −0.0008 | 0.0011 | 0.4761 | 26 | None |
β is the estimated model parameter and reflects the association between the rate of biomass change and the climatic variable, n is the number of forest plots used in the model, and P is the significance of the model's fixed effects based on a t test. To simplify, we have only shown the estimated β1 parameters and the significant estimated β2 parameters. SK served as the base/reference category for the western region and QC served as the base/reference category for the eastern region. AB, Alberta; SK, Saskatchewan; MB, Manitoba; QC, Quebec; CMI, climate moisture index; AMI, annual moisture index.
*We calculated the province-specific slope by summing the overall slope β1 and the province's slope adjustment β2. Therefore, the slopes for Alberta, Saskatchewan, and Manitoba represent the relationships between the rate of biomass change and the climatic variables for these provinces.
Because both the stand age (SI Appendix, Table S7) and climatic variables (Table 2) were significantly correlated with the rate of biomass change, we used standardized regression to compare the relative importance of stand age and the climatic variables. By comparing the magnitudes of the estimated coefficients of stand age and the climatic variables from these regressions (Table 3), we found that precipitation, CMI, and AMI had a greater effect than stand age on the rate of biomass change for the western region. The temperature coefficient for Saskatchewan indicated that temperature had a greater impact than stand age on the rate of biomass change in Saskatchewan. Overall, the climatic variables had a stronger effect than stand age on the rate of biomass change in the western region. In addition, we tried to remove the impacts of stand age on the rate of biomass change by regressing stand age on the rate of biomass change to provide the residuals. The trend in the residuals reflects the rate of biomass change without the effects of stand age (Fig. 2C). We also modeled the trends for the rates of stand-age–corrected biomass change (Table 4). The results show that the overall and western trends became slightly weaker than these trends before the correction for stand age. There was no change in the trend for the eastern region. Thus, these results (Fig. 2C and Table 4) further indicated that stand age is not the dominant factor that caused the observed trends for the rate of biomass change.
Table 3.
Fixed effects of the linear mixed models (SI Appendix, Eq. S5) describing the relationships between the rate of biomass change and the combined effects of the climatic variables and stand age
| Data | Parameter | β | SE | P | n | β1 + β2Dk* |
| Western region | Average temperature | −0.9428 | 0.2385 | 0.0001 | 70 | AB: −0.2275 |
| Stand age | −0.2442 | 0.0833 | 0.0038 | SK: −0.9428 | ||
| Average temperature × AB | 0.7153 | 0.2597 | 0.0065 | MB: 0.0235 | ||
| Average temperature × MB | 1.0663 | 0.2860 | 0.0003 | |||
| Average precipitation | 1.3142 | 0.3642 | 0.0004 | 70 | AB: 0.2543 | |
| Stand age | −0.1957 | 0.0820 | 0.0180 | SK: 1.3142 | ||
| Average precipitation × AB | −1.0599 | 0.3838 | 0.0029 | MB: 0.4838 | ||
| Average precipitation × MB | −0.8304 | 0.4028 | 0.0407 | |||
| CMI | 0.9961 | 0.3098 | 0.0015 | 70 | AB: 0.3178 | |
| Stand age | −0.2328 | 0.0796 | 0.0039 | SK: 0.9961 | ||
| CMI × AB | −0.6783 | 0.3260 | 0.0389 | MB: 0.9961 | ||
| AMI | −0.9031 | 0.3032 | 0.0033 | 70 | AB: −0.9031 | |
| Stand age | −0.2382 | 0.0813 | 0.0039 | SK: −0.9031 | ||
| MB: −0.9031 | ||||||
| Eastern region | Average temperature | −0.1261 | 0.1829 | 0.4936 | 26 | None |
| Stand age | −0.0540 | 0.1825 | 0.7684 | |||
| Average precipitation | 0.1739 | 0.1429 | 0.2191 | 26 | None | |
| Stand age | −0.0173 | 0.1430 | 0.9044 | |||
| CMI | 0.1982 | 0.1545 | 0.2052 | 26 | None | |
| Stand age | −0.0615 | 0.1544 | 0.6920 | |||
| AMI | −0.1201 | 0.1662 | 0.4734 | 26 | None | |
| Stand age | −0.0384 | 0.1664 | 0.8183 |
β is the estimated model parameter, n is the number of forest plots used in the model, and P is the significance level of the model's fixed effects based on a t test. To simplify, we only show the estimated β1 parameters and the significant estimated β2 parameters at α = 0.05. SK served as the base/reference category for the western region and QC served as the base/reference category for the eastern region. AB, Alberta; SK, Saskatchewan; MB, Manitoba; ON, Ontario; QC, Quebec; CMI, climate moisture index; AMI, annual moisture index.
*We calculated the province-specific slope by summing the overall slope β1 and the province's slope adjustment β2. Therefore, the slopes for Alberta, Saskatchewan, and Manitoba represent the relationships between the rate of biomass change and the effects of the climatic variables for those provinces.
Table 4.
Fixed effects of the linear mixed models (SI Appendix, Eq. S1) describing trends in the rate of stand-age–corrected biomass change
| Data | Model | β | SE | P | n |
| All plots | Trend in rate of stand-age–corrected biomass change | −0.0473 | 0.0102 | <0.0001 | 96 |
| Western region | Trend in rate of stand-age–corrected biomass change | −0.0599 | 0.0114 | <0.0001 | 70 |
| Eastern region | Trend in rate of stand-age–corrected biomass change | 0.0063 | 0.0229 | 0.7931 | 26 |
β is the slope and represents the annual rate of change in biomass, n is the number of forest plots used in the model, and P is the significance level for the model's fixed effects based on a t test.
Discussion
Our results are consistent with recent findings of large biomass carbon losses caused by a widespread moisture-driven drought in tropical forests in the Amazon basin (8, 16), temperate forests in the western United States (9), and trembling aspen stands in western Canada (10, 14). Western Canada appears to have been more sensitive to drought than eastern Canada. There are a number of reasons that may explain this drought-induced reduction in biomass carbon sink in western Canada: (i) a decline in tree growth (ii); a reduction in net primary production (NPP); and (iii) a widespread increase in tree mortality. Tree-ring studies have shown that the negative effects of recent drought on tree growth are widespread for boreal regions, affecting not only Alaska (17) but also western and central Canada (14, 18). Second, both satellite-based estimates (19, 20) and ground-based measurements (14, 21) showed that summer drought has led to a marked NPP decrease in much of the boreal forest region in North America since the late 1990s. Third, severe climate-change–induced drought has already accelerated tree morality over large areas in western North America (9, 10, 14) in response to the impacts of regional climatic warming and drought. Previous studies have suggested that Canada experienced one of its most serious and extensive droughts on record in 2001 and that the most severely affected areas occurred in the Canadian Prairies, where the 2001 drought followed two to three consecutive years of below-average rainfall. Some areas of central Canada suffered the driest August on record and western Canada suffered the second consecutive year with the most severe drought on record in 2002 (22). For eastern Canada, Girardin et al. (23) showed that climate warming and increases in the amount and frequency of precipitation during the last century had no significant impact on the severity of summer drought. In addition, a long-term reduction in the amount of solar radiation in the Canadian Prairies between 1951 and 2005 (24) may also have contributed to a decline in forest productivity (i.e., net photosynthesis) in western Canada (14).
A combination of the aforementioned factors may therefore explain the regional differences in the biomass carbon sink between western and eastern Canada. Moreover, our results confirmed that water deficits induced by climate warming may be responsible for the decline in the rates of biomass accumulation in the western regions of Canada's boreal forests. The observed rates of biomass accumulation in western regions changed from positive to negative after 2003 (Fig. 2B), with a continuous decrease since the start of the study period, which indicates that climate warming may soon begin to decrease the carbon sink and change these forests into a net carbon source if the climate warming continues to follow the current trends. However, it is important to point out that the precipitation and temperature changes that cause the decreases of the aboveground biomass are large and will undoubtedly have effects on soil respiration that could either amplify or weaken the inferred sink from the aboveground biomass pool (1).
Although the rate of decrease (excluding the effect of stand age) was significant for the western region (Table 4; slope = −0.0599 t ha−1 year−1, P < 0.0001), we estimated that the combined reduction of the carbon biomass sinks for western and eastern regions was 0.0473 ± 0.0204 t ha−1 year−1. This decrease in biomass is equivalent to a net decrease in the carbon sink of 0.0237 ± 0.0102 t C ha−1 year−1. Multiplying this by the estimated area of the Canadian boreal forest (25) (307.14 M ha) produces reduction of the net carbon sink from mature forest equal to 7.28 ± 3.13 million tons of carbon per year (Mt C year−1). The resulting reduction in the biomass carbon of Canadian boreal forests would amount to about 37% of the carbon source in the year 2009 (20 Mt C year−1) projected to be lost due to beetle-caused mortality (12, 26), about 27% of the direct emissions caused by forest fires throughout Canada from 1959 to 1999 (27), and equivalent to about 3.6% of Canada's total annual carbon emission (28). However, extrapolating these 96 representative plots to entire Canadian boreal forests must be done with caution. It is important to note that much of the Canadian boreal forest cannot be represented by the plots used for the current analysis. In addition, we consider our estimate of drought-induced biomass carbon loss to be highly conservative because it excludes carbon emissions caused by increased fire activity (11, 27) and the expansion of mountain pine beetle outbreaks (12). The recent increase in these ecosystem disturbances is positively related to drought (11, 12, 29) and may co-occur with peaks of drought-induced tree mortality (11, 14, 30).
Our results indicate that since 1963, drought-induced water stress has led to a weakening of the biomass carbon sink across a large area of the western Canadian boreal forest, with the largest reduction occurring after 2000. Our results provide observational evidence to support recent studies (2–4) that predicted a weaker northern hemisphere carbon uptake at high latitudes and challenge the traditional view that northern hemisphere carbon sinks will remain dominant over carbon emission sources. Moreover, the recent decrease in biomass may indicate that the boreal forests in western regions will become net carbon emission sources if the stresses resulting from climate-change–induced drought continue to increase in the future, and this will provide a positive feedback that may accelerate future global warming.
Materials and Methods
To assess the impacts of drought on biomass carbon sink of natural stands of boreal forests in Canada, we analyzed data from 96 long-term PSPs that met nine criteria for natural (unmanaged) mature boreal forest stands in five Canadian provinces (Alberta, Saskatchewan, Manitoba, Ontario, and Quebec). The selected plots were censused three to five times ( , SD = 0.6). The length of the census period ranged from 10 to 38 y (
, SD = 0.6). The length of the census period ranged from 10 to 38 y (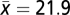 , SD = 6.8). The initial stand age of the plots ranged from 80 to 218 y (
, SD = 6.8). The initial stand age of the plots ranged from 80 to 218 y (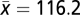 , SD = 34.4). To obtain climatic data for these plots, we used Canada's daily 10-km raster-gridded climate dataset from 1961 to 2003, which contains data on daily maximum and minimum temperatures (Tmax and Tmin) and PCP for latitudes south of 60°N. The climatic data for plots with census data after 2003 were downloaded from the nearest meteorological stations (SI Appendix, Table S3). We calculated the CMI and the AMI from these data. We also calculated the aboveground stand biomass at each census using published Canadian national equations for aboveground tree biomass (SI Appendix, ref. S12).
, SD = 34.4). To obtain climatic data for these plots, we used Canada's daily 10-km raster-gridded climate dataset from 1961 to 2003, which contains data on daily maximum and minimum temperatures (Tmax and Tmin) and PCP for latitudes south of 60°N. The climatic data for plots with census data after 2003 were downloaded from the nearest meteorological stations (SI Appendix, Table S3). We calculated the CMI and the AMI from these data. We also calculated the aboveground stand biomass at each census using published Canadian national equations for aboveground tree biomass (SI Appendix, ref. S12).
Supplementary Material
Acknowledgments
We thank the editor and three anonymous reviewers for their valuable suggestions and comments on the manuscript. We thank the Forest Management Branch of Alberta Ministry of Sustainable Resource Development, Saskatchewan Renewable Resources Forestry Branch, Forestry Branch of Manitoba, Ontario Terrestrial Assessment Program, Ministère des Ressources Naturelles et de la Faune du Québec, and our colleagues (P. Comeau, J. Liu, V. LeMay, S. Huang, J. Parton, and K. Zhou) for providing detailed data. We also thank T. Hogg for his help on the calculations of climate moisture index (CMI), G. Hart for editorial help, and J. Guiot and T. Moore for helpful comments. C.P. acknowledges the support received during his sabbatical leave at Northwest Agriculture and Forestry University. Funding for this study was provided by the Natural Sciences and Engineering Research Council of Canada (NSERC) strategic network (ForValuenet), an NSERC discovery grant, and China's QianRen and 973 programs (2010CB833504-X).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1111576109/-/DCSupplemental.
References
- 1.Denman KL, et al. In: Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. S. Solomon, et al., editors. Cambridge: Univ of Cambridge Press; 2007. pp. 501–587. [Google Scholar]
- 2.Angert A, et al. Drier summers cancel out the CO2 uptake enhancement induced by warmer springs. Proc Natl Acad Sci USA. 2005;102:10823–10827. doi: 10.1073/pnas.0501647102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stephens BB, et al. Weak northern and strong tropical land carbon uptake from vertical profiles of atmospheric CO2. Science. 2007;316:1732–1735. doi: 10.1126/science.1137004. [DOI] [PubMed] [Google Scholar]
- 4.Piao S, et al. Net carbon dioxide losses of northern ecosystems in response to autumn warming. Nature. 2008;451:49–52. doi: 10.1038/nature06444. [DOI] [PubMed] [Google Scholar]
- 5.Dixon RK, et al. Carbon pools and flux of global forest ecosystems. Science. 1994;263:185–190. doi: 10.1126/science.263.5144.185. [DOI] [PubMed] [Google Scholar]
- 6.Bonan GB. Forests and climate change: Forcings, feedbacks, and the climate benefits of forests. Science. 2008;320:1444–1449. doi: 10.1126/science.1155121. [DOI] [PubMed] [Google Scholar]
- 7.Allen CD, et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For Ecol Manage. 2010;259:660–684. [Google Scholar]
- 8.Phillips OL, et al. Drought sensitivity of the Amazon rainforest. Science. 2009;323:1344–1347. doi: 10.1126/science.1164033. [DOI] [PubMed] [Google Scholar]
- 9.van Mantgem PJ, et al. Widespread increase of tree mortality rates in the western United States. Science. 2009;323:521–524. doi: 10.1126/science.1165000. [DOI] [PubMed] [Google Scholar]
- 10.Michaelian M, et al. Massive mortality of aspen following severe drought along the southern edge of the Canadian boreal forest. Glob Change Biol. 2011;17:2084–2094. [Google Scholar]
- 11.Bond-Lamberty B, Peckham SD, Ahl DE, Gower ST. Fire as the dominant driver of central Canadian boreal forest carbon balance. Nature. 2007;450:89–92. doi: 10.1038/nature06272. [DOI] [PubMed] [Google Scholar]
- 12.Kurz WA, et al. Mountain pine beetle and forest carbon feedback to climate change. Nature. 2008;452:987–990. doi: 10.1038/nature06777. [DOI] [PubMed] [Google Scholar]
- 13.Phillips OL, et al. Changes in the carbon balance of tropical forests: Evidence from long-term plots. Science. 1998;282:439–442. doi: 10.1126/science.282.5388.439. [DOI] [PubMed] [Google Scholar]
- 14.Hogg EH, Brandt JP, Michaelian M. Impacts of a regional drought on the productivity, dieback, and biomass of western Canadian aspen forests. Can J For Res. 2008;38:1373–1384. [Google Scholar]
- 15.Gower ST, McMurtrie RE, Murty D. Aboveground net primary production decline with stand age: potential causes. Trends Ecol Evol. 1996;11:378–382. doi: 10.1016/0169-5347(96)10042-2. [DOI] [PubMed] [Google Scholar]
- 16.Lewis SL, Brando PM, Phillips OL, van der Heijden GM, Nepstad D. The 2010 Amazon drought. Science. 2011;331:554. doi: 10.1126/science.1200807. [DOI] [PubMed] [Google Scholar]
- 17.Barber VA, Juday GP, Finney BP. Reduced growth of Alaskan white spruce in the twentieth century from temperature-induced drought stress. Nature. 2000;405:668–673. doi: 10.1038/35015049. [DOI] [PubMed] [Google Scholar]
- 18.Silva LCR, Anand M, Leithead MD. Recent widespread tree growth decline despite increasing atmospheric CO2. PLoS ONE. 2010;5:e11543. doi: 10.1371/journal.pone.0011543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goetz SJ, Bunn AG, Fiske GJ, Houghton RA. Satellite-observed photosynthetic trends across boreal North America associated with climate and fire disturbance. Proc Natl Acad Sci USA. 2005;102:13521–13525. doi: 10.1073/pnas.0506179102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang K, et al. Satellite-based model detection of recent climate driven changes in northern high latitude vegetation productivity. J Geophys Res. 2008;113:G03033. [Google Scholar]
- 21.Schindler DW, Donahue WF. An impending water crisis in Canada's western prairie provinces. Proc Natl Acad Sci USA. 2006;103:7210–7216. doi: 10.1073/pnas.0601568103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonsal BR, Wheaton EE. Atmospheric circulation comparisons between the 2001 and 2002 and the 1961 and 1988 Canadian prairie droughts. Atmos-ocean. 2005;43:163–172. [Google Scholar]
- 23.Girardin MP, et al. Trends and periodicities in the Canadian drought code and their relationships with atmospheric circulation for the southern Canadian boreal forest. Can J For Res. 2004;34:103–119. [Google Scholar]
- 24.Cutforth HW, Judiesch D. Long-term changes to imcoming solar energy on the Canadian prairie. Agric For Meteorol. 2007;145:167–175. [Google Scholar]
- 25.Brandt JP. The extent of the North American boreal zone. Environ Rev. 2009;17:101–161. [Google Scholar]
- 26.Kurz WA, Stinson G, Rampley GJ, Dymond CC, Neilson ET. Risk of natural disturbances makes future contribution of Canada's forests to the global carbon cycle highly uncertain. Proc Natl Acad Sci USA. 2008;105:1551–1555. doi: 10.1073/pnas.0708133105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amiro BD, et al. Direct carbon emissions from Canadian forest fires, 1959-1999. Can J For Res. 2001;31:512–525. [Google Scholar]
- 28.Environment Canada . National Inventory Report: Greenhouse Gas Sources and Sinks in Canada 1990–2005. Gatineau, QC: Government of Canada; 2007. [Google Scholar]
- 29.Girardin MP, Tardif JC, Flannigan MD, Bergeron Y. Forest fire-conducive drought variability in the southern Canadian boreal forest and associated climatology inferred from tree rings. Can Water Resour J. 2006;31:275–296. [Google Scholar]
- 30.Peng C, et al. A drought-induced pervasive increase in tree mortality across Canada's boreal forests. Nature Clim. Change. 2011;1:467–471. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.