Abstract
The G protein-coupled free fatty acid receptor-1 (FFA1/GPR40) plays a major role in the regulation of insulin secretion by fatty acids. GPR40 is considered a potential therapeutic target to enhance insulin secretion in type 2 diabetes; however, its mode of regulation is essentially unknown. The aims of this study were to test the hypothesis that glucose regulates GPR40 gene expression in pancreatic β-cells and to determine the mechanisms of this regulation. We observed that glucose stimulates GPR40 gene transcription in pancreatic β-cells via increased binding of pancreas-duodenum homeobox-1 (Pdx-1) to the A-box in the HR2 region of the GPR40 promoter. Mutation of the Pdx-1 binding site within the HR2 abolishes glucose activation of GPR40 promoter activity. The stimulation of GPR40 expression and Pdx-1 binding to the HR2 in response to glucose are mimicked by N-acetyl glucosamine, an intermediate of the hexosamine biosynthesis pathway, and involve PI3K-dependent O-GlcNAcylation of Pdx-1 in the nucleus. We demonstrate that O-GlcNAc transferase (OGT) interacts with the product of the PI3K reaction, phosphatidylinositol 3,4,5-trisphosphate (PIP3), in the nucleus. This interaction enables OGT to catalyze O-GlcNAcylation of nuclear proteins, including Pdx-1. We conclude that glucose stimulates GPR40 gene expression at the transcriptional level through Pdx-1 binding to the HR2 region and via a signaling cascade that involves an interaction between OGT and PIP3 at the nuclear membrane. These observations reveal a unique mechanism by which glucose metabolism regulates the function of transcription factors in the nucleus to induce gene expression.
The pancreatic β-cell plays a central role in the control of glucose homeostasis, and β-cell dysfunction is key to the pathogenesis of type 2 diabetes (1). Insulin secretion from the β-cell is tightly regulated by the coordinated action of hormonal, metabolic, and neural factors, ensuring its maintenance within a narrow physiological range. Among these factors, circulating fatty acids do not initiate insulin secretion but strongly potentiate glucose-stimulated insulin secretion (GSIS) (reviewed in ref. 2). The G protein-coupled free fatty acid receptor-1 (FFA1/GPR40) is specifically expressed in pancreatic β-cells and activated by medium- to long-chain fatty acids (3–5). GPR40 plays a major role in fatty-acid amplification of GSIS (3, 4, 6–10) and, as such, is being considered as a potential therapeutic target in type 2 diabetes (11–13). Indeed, a recent report from a phase II clinical trial of the first orally available, selective GPR40 agonist, TAK-875 (13), identified the agonist as a highly effective drug for the treatment of type 2 diabetes (14, 15). However, the mode of regulation of GPR40 in pancreatic β-cells remains essentially unknown.
We previously characterized the proximal promoter of the GPR40 gene and showed that the 5′-upstream region contains two evolutionary conserved regions, HR2 and HR3, which display DNase I hypersensitivity in β-cells (16). HR3 contains the core promoter region and displays features of a TATA-less promoter, including a GC-rich region and two putative Sp1 binding sites. It also contains an A-box, a putative binding site for the transcription factor pancreas-duodenum homeobox-1 (Pdx-1). HR2 is a strong transcriptional enhancer mediating β cell-specific expression of GPR40 and contains functional binding sites for Pdx-1 and BETA2. Because Pdx-1 and BETA2 are glucose-responsive transcription factors implicated in glucose regulation of insulin gene expression (17), these observations prompted us to ascertain whether GPR40 expression is regulated by glucose in islets and, if so, to determine the mechanisms of such regulation.
Results
Glucose Induces GPR40 Expression.
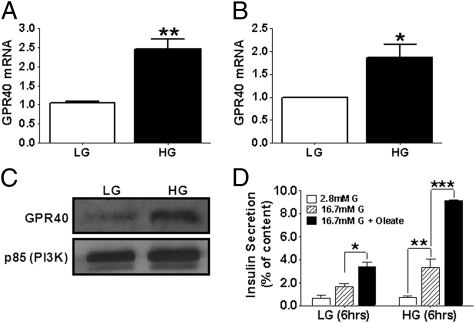
Islets isolated from C57BL/6 mice or human cadaveric donors were incubated at either 2.8 or 16.7 mmol/L glucose for 6 or 24 h, respectively, and mRNA levels of GPR40 were measured by RT-PCR (Fig. 1). High glucose treatment increased GPR40 mRNA levels by 2.5 ± 0.3-fold (n = 17; P < 0.01) in C57BL/6 islets (Fig. 1A) and by 1.9 ± 0.3-fold (n = 8; P < 0.05) in human islets (Fig. 1B) compared with low glucose concentrations. This stimulation was reflected in an increase in GPR40 protein levels (Fig. 1C) and was associated with a greater ability of the fatty acid oleate to potentiate GSIS after a 6-h incubation of islets in the presence of elevated glucose (Fig. 1D).
Fig. 1.
Isolated mouse (A; n = 17) or human (B; n = 8) islets were treated with 2.8 mmol/L low glucose (LG) or 16.7 mmol/L high glucose (HG) for 6 (mouse islets) or 24 (human islets) h, and GPR40 mRNA levels measured by RT-PCR. *P < 0.05; **P < 0.01. (C) Representative immunoblots for GPR40 in human islets exposed to 2.8 or 16.7 mmol/L glucose for 24 h (n = 3). The p85 subunit of PI3K was used as a loading control. (D) Mouse islets were treated with 2.8 or 16.7 mmol/L glucose for 6 h. Insulin secretion was assessed in 1-h static incubations in response to 2.8 or 16.7 mmol/L glucose ± 0.5 mmol/L oleate. Data are expressed as insulin secretion and normalized to insulin content (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001.
Induction of GPR40 Expression by Glucose Is Transcriptional and Requires an Intact Pdx-1 Binding Site in the HR2 Region of the Promoter.
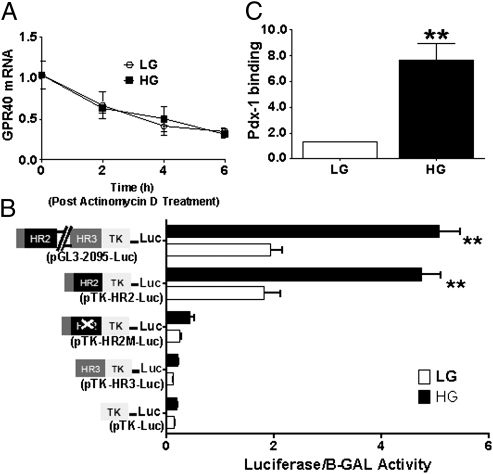
To determine whether glucose induction of GPR40 expression was due to an increase in mRNA stability, we measured the decay of GPR40 mRNA after transcriptional inhibition with 5 μg/mL actinomycin-D in islets treated with either 2.8 or 16.7 mmol/L glucose for 6 h. As shown in Fig. 2A, there was no effect of glucose on GPR40 mRNA decay, suggesting a transcriptional level of regulation. To examine this possibility, MIN6 cells were transfected with the pGL3-2095-Luc reporter construct containing the conserved regions HR2 and HR3 of the GPR40 promoter (16). As shown in Fig. 2B, high glucose increased the activity of pGL3-2095-Luc plasmid by 2.5 ± 0.2-fold (n = 8; P < 0.01), indicating that the main effect of glucose is on GPR40 gene transcription. We then examined whether glucose responsiveness of the promoter was conferred by the HR2 or HR3 regions. We found that high glucose increased the activity of the pTK-HR2-Luc reporter construct but not that of the pTK-HR3-Luc reporter (Fig. 2B), suggesting that HR2, but not HR3, confers glucose responsiveness of the promoter. The HR2 region contains a Pdx-1 binding site (16), which could be regulated by glucose. As shown in Fig. 2B, mutation of the Pdx-1 site on the HR2 strongly diminished both the basal activity of the reporter and its response to glucose, suggesting that this site mediates, at least in part, glucose responsiveness of the GPR40 promoter. Accordingly, Pdx-1 occupancy of the endogenous GPR40 promoter was markedly increased in islets treated with 16.7 mmol/L glucose for 6 h, as assessed by chromatin immunoprecipitation (Fig. 2C).
Fig. 2.
(A) GPR40 mRNA levels in islets exposed to 2.8 (LG) or 16.7 (HG) mmol/L glucose ± 5 μg/mL of actinomycin-D for 0, 2, 4, and 6 h (n = 3). (B) MIN6 cells were cotransfected with 1 μg of a pTK-Luc control, pGL3-2095-Luc, pTK-HR2-Luc, pTK-HR3-Luc, or a pTK-HR2-Luc plasmid in which the Pdx-1 site is mutated (pTK-HR2M-Luc) and 0.1 μg of internal control pGL4-74-RLuc. Transfected cells were treated with either 1 (LG) or 11.1 (HG) mmol/L glucose for 24 h (n = 8). **P < 0.01. (C) Chromatin immunoprecipitation analysis showing the effect of 16.7 (HG) mmol/L glucose on the recruitment of Pdx-1 to the GPR40 promoter compared with islets treated with 2.8 (LG) mmol/L glucose (n = 3). **P < 0.01.
Glucose Stimulation of GPR40 Expression Requires PI3K Activity but Not Secreted Insulin.
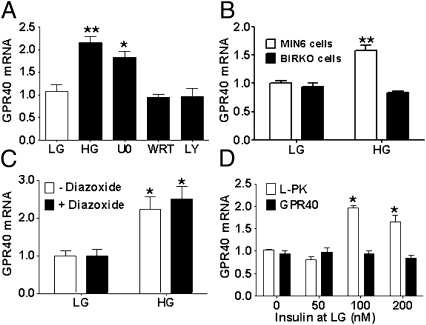
We asked whether glucose regulation of GPR40 gene transcription was mediated by a direct effect of glucose or indirectly via GSIS. We found that the PI3K inhibitors LY294002 (20 μmol/L) and wortmannin (10 μmol/L) blocked glucose induction of GPR40 mRNA, whereas the MEK1/2 inhibitor U0126 (10 μmol/L) had no effect (Fig. 3A). As a complementary approach, we used the βIRKO cell line, derived from mice with β cell-specific deletion of the insulin receptor, in which glucose regulation of several kinases of the insulin signaling cascade, including PI3K, is compromised (18). Consistent with a role for PI3K activation in glucose stimulation of GPR40 mRNA expression, glucose had no effect on GPR40 mRNA levels in βIRKO cells (Fig. 3B).
Fig. 3.
(A) GPR40 mRNA levels in mouse islets treated with 2.8 (LG) or 16.7 (HG) mmol/L glucose ± 10 μmol/L U0126, 10 μmol/L wortmannin (WRT), or 20 μmol/L LY294002 (LY) for 6 h (n = 3). **P < 0.01, *P < 0.05. (B) GPR40 mRNA levels in βIRKO and MIN6 cells treated with 1 (LG) or 11.1 (HG) mmol/L glucose for 6 h. (C) GPR40 mRNA levels in mouse islets treated with 2.8 (LG) or 16.7 (HG) mmol/L glucose ± 625 μmol/L diazoxide. (D) GPR40 and L-type pyruvate kinase (l-PK) mRNA levels in mouse islets treated with 2.8 (LG) mmol/L glucose ± insulin (n = 3). *P < 0.01.
To examine whether PI3K-dependent stimulation of GPR40 mRNA by glucose was mediated by secreted insulin, we first blocked endogenous insulin secretion with the ATP-sensitive K+-channel opener diazoxide. As expected, diazoxide (625 μmol/L) completely blocked the accumulation of insulin in the culture medium (Fig. S1); however, inhibiting insulin secretion did not prevent glucose stimulation of GPR40 mRNA (Fig. 3C). Second, we tested whether exogenous insulin could mimic the effect of glucose on GPR40 mRNA. As shown in Fig. 3D, exogenous insulin did not induce GPR40 mRNA, although it did stimulate expression of the liver isoform of pyruvate kinase, as shown (19).
Glucose Stimulation of GPR40 Expression Is Mediated by the Hexosamine Biosynthesis Pathway.
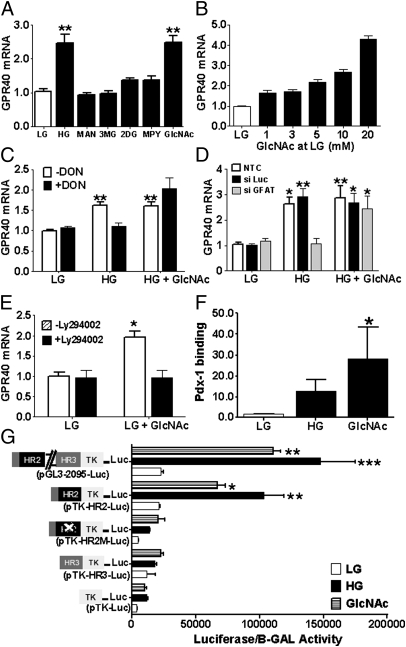
To identify the signals derived from glucose metabolism that stimulate GPR40 mRNA expression, we first examined the effects of nonmetabolizable glucose analogs (Fig. 4A). Neither the metabolically inactive monosaccharide mannitol, nor 3-O-methylglucose, which is transported into the cell but cannot be phosphorylated, nor 2-deoxyglucose, which is phosphorylated by glucokinase but not further metabolized, could induce GPR40 mRNA expression. Thus, metabolism of glucose beyond phosphorylation is necessary for stimulation of GPR40 mRNA expression.
Fig. 4.
(A) GPR40 mRNA levels in mouse islets treated with 2.8 (LG) ± 13.9 mmol/L mannitol (MAN), 3-O-methyl-d-glucose (3MG), 2-deoxy-d-glucose (2DG), 5 mmol/L methyl-pyruvate (MPY), or 20 mmol/L N-acetylglucosamine (GlcNAc). (B) Effect of GlcNAc on GPR40 mRNA levels of islets cultured with 2.8 (LG) mmol/L glucose for 6 h (n = 2–5). **P < 0.01. (C) GPR40 mRNA levels in MIN6 cells treated with 1 (LG) or 11.1 (HG) mmol/L glucose ± 10 mmol/L GlcNAc and ± 20 μmol/L DON for 6 h (n = 3). *P < 0.05, **P < 0.01. (D) GPR40 mRNA levels in nontransfected MIN6 cells or in MIN6 cells transfected with either control or GFAT-1 siRNA. Twenty-four hours after transient transfection, cells were treated with 1 (LG) or 11.1 (HG) mmol/L glucose ± 10 mmol/L GlcNAc for 6 h (n = 3). *P < 0.05, **P < 0.01. (E) GPR40 mRNA levels in mouse islets treated with 2.8 mmol/L glucose ± 20 mmol/L GlcNAc ± 20 μmol/L LY294002 for 6 h (n = 3). *P < 0.05. (F) Chromatin immunoprecipitation analysis showing the effect of 20 mmol/L GlcNAc on the recruitment of Pdx-1 to the GPR40 promoter (n = 3). *P < 0.05. (G) MIN6 cells were cotransfected with 1 μg of either a pTK-Luc control, pGL3-2095-Luc, pTK-HR2-Luc, pTK-HR3-Luc, or a pTK-HR2-Luc plasmid in which the Pdx-1 site is mutated (pTK-HR2M-Luc) and 300 ng of internal control pCMV-β-gal. Transfected cells were treated with either 1 mmol/L glucose (LG) ± 10 mmol/L GlcNAc or 11.1 (HG) mmol/L glucose for 24 h. Luciferase activity was normalized to β-gal activity of the internal control (n = 3). *P < 0.05, **P < 0.01.
Once glucose is phosphorylated, glucose-6-phosphate can enter several metabolic pathways that are involved in intracellular signaling and regulation of gene expression. Methyl-pyruvate (5 mmol/L), an efficient mitochondrial substrate in the β-cell, increased insulin secretion (Fig. S2) but did not induce GPR40 mRNA (Fig. 4A). Alternatively, glucose can induce gene transcription via its metabolism through the hexosamine biosynthesis pathway (HBP) (20–23). To determine the contribution of the HBP to the induction of GPR40 mRNA by glucose, we measured GPR40 mRNA levels in islets treated with the HBP intermediate N-acetyl glucosamine (GlcNAc; 20 mmol/L), which promotes O-GlcNAc modification of proteins (also known as O-GlcNAcylation) by increasing UDP-GlcNAc (24). We found that GlcNAc stimulated GPR40 mRNA expression in mouse islets to a level similar to that observed with high glucose (Fig. 4A) and in a dose-dependent manner (Fig. 4B). Next, we examined whether inhibiting glucose entry into the HBP would attenuate glucose stimulation of GPR40 mRNA. To this aim we first used the glutamine analog 6-diazo-5-oxo-l-norleucine (DON), an inhibitor of l-glutamine d-fructose 6-phosphate amidotransferase (GFAT), the rate-limiting enzyme that metabolizes fructose-6-phosphate to glucosamine-6-phosphate. Consistent with a role of HBP in glucose stimulation of GPR40 mRNA expression, DON (20 μmol/L) completely blocked glucose induction of GPR40 mRNA in MIN6 cells, and these inhibitory effects were rescued by GlcNAc (Fig. 4C). As a complementary approach, we silenced GFAT-1 expression in MIN6 cells by using siRNA (Fig. S3). GFAT-1 knockdown blocked the stimulation of GPR40 mRNA expression by glucose, and this inhibition was rescued by GlcNAc (Fig. 4D). The PI3K inhibitor LY294002 (20 μmol/L) blocked GlcNAc induction of GPR40 mRNA in islets (Fig. 4E). Finally, GlcNAc increased Pdx-1 binding to the HR2 region (Fig. 4F) and GPR40 promoter activity (Fig. 4G).
Pdx-1 Is O-GlcNAcylated in Response to Glucose in a PI3K-Dependent Manner.
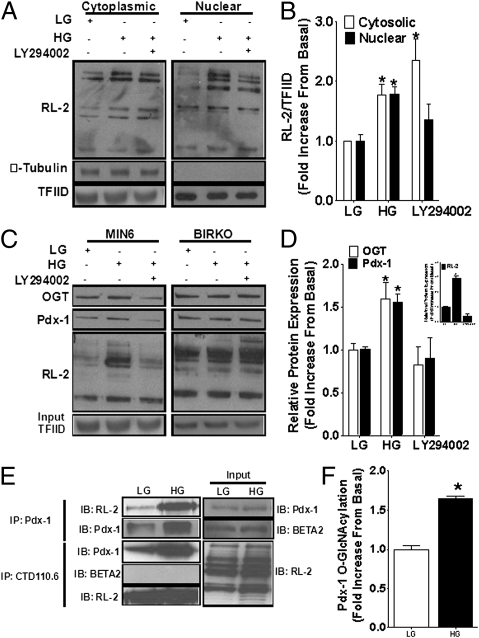
The end product of the HBP (UDP-GlcNAc) is a substrate for O-GlcNAcylation of cytoplasmic and nuclear proteins including transcription factors (24). Pdx-1 contains at least two O-GlcNAcylation sites, and O-GlcNAcylation of Pdx-1 increases its DNA binding activity (25). We first investigated whether O-GlcNAcylation of cytoplasmic and nuclear proteins is regulated by glucose in MIN6 cells and, if so, whether this regulation depends on PI3K activity (Fig. 5). We found that overall protein O-GlcNAcylation, as detected by the antibody RL-2, was increased in both cytoplasmic and nuclear extracts of cells treated with high glucose (Fig. 5 A and B). The PI3K inhibitor LY294002 (20 μmol/L) blocked glucose-induced O-GlcNAcylation of nuclear but not cytosolic proteins (Fig. 5 A and B). To further analyze the O-GlcNAcylation profile of nuclear proteins, we used succinylated wheat germ agglutinin (sWGA)-conjugated beads, which specifically bind to O-GlcNAc and precipitate O-GlcNAc–modified proteins. Immunoblotting of sWGA-pulled down proteins with RL-2 revealed minimal O-GlcNAcylation of nuclear protein in MIN6 cells treated with 1 mmol/L glucose (Fig. 5 C and D). In contrast, high glucose treatment increased sWGA binding by 2.9 ± 0.2-fold (n = 3; P < 0.05) in MIN6 cells (Fig. 5 C and D, Inset), confirming that O-GlcNAcylation of nuclear proteins is modulated by glucose. In agreement with the data in Fig. 5 A and B, the presence of LY294002 significantly blocked high glucose-induced O-GlcNAcylation of nuclear proteins. Immunoblotting using an anti–Pdx-1 antibody confirmed that Pdx-1 is O-GlcNAcylated (22, 25) and showed that the levels of O-GlcNAcylated Pdx-1 increase in MIN6 cells treated with high glucose by 1.6 ± 0.1-fold (Fig. 5 C and D; n = 3; P < 0.05). In addition, the PI3K inhibitor LY294002 significantly blocked glucose-induced O-GlcNAcylation of Pdx-1 in the nucleus (Fig. 5 C and D). Immunoblotting with an anti-OGT antibody (Fig. 5C) confirmed that O-GlcNAc transferase (OGT), the enzyme that modifies proteins by O-GlcNAcylation, is also O-GlcNAcylated (26, 27) in response to glucose, a modification that has been suggested to enhance its activity (27). Glucose induction of O-GlcNAcylation of nuclear proteins including Pdx-1 and OGT was not observed in βIRKO cells (Fig. 5C, Right), confirming the pharmacological approach with LY294002 and demonstrating that PI3K activity is required for O-GlcNAcylation of nuclear proteins, including Pdx-1. O-GlcNAcylation of Pdx-1 in the presence of high glucose was confirmed in coimmunoprecipitation assays in which immunoprecipitated Pdx-1 was immunoblotted with RL-2 (Fig. 5 E and F) or, conversely, when immunoprecipitated CTD110.6 (an antibody against O-GlcNAcylated proteins) was immunoblotted with Pdx-1 (Fig. 5E). In contrast, we did not detect coimmunoprecipitation of BETA2 with CTD110.6 in nuclear extracts from MIN6 cells (Fig. 5E).
Fig. 5.
(A) MIN6 cells were treated with 1 (LG) or 11.1 (HG) mmol/L glucose ± 20 μmol/L LY294002 for 6 h. Cytoplasmic and nuclear proteins were immunoblotted using anti–RL-2 antibody. (B) Quantification of blots presented in A (n = 3). *P < 0.05. (C) MIN6 and βIRKO cells were treated with 1 (LG) or 11.1 (HG) mmol/L glucose ± 20 μmol/L LY294002 for 6 h. Nuclear proteins were pulled down by using sWGA-conjugated beads, and analyzed by immunoblotting. Input proteins were immunoblotted with anti-TFIID antibody as a loading control. (D) Quantification of the blots presented in C (n = 3). *P < 0.05. Quantification of the RL-2 blot is shown in Inset (n = 3). (E) MIN6 cells were treated with 1 (LG) or 11.1 (HG) mmol/L glucose for 6 h. Nuclear proteins were immunoprecipitated by using an anti–Pdx-1 or anti-CTD11.6 antibody and immunoblotted using anti–RL-2, anti–Pdx-1, or anti-BETA2 antibody. Input proteins were immunoblotted with anti-Pdx1, anti-BETA2, or anti–RL-2 antibody. (F) Quantification of Pdx-1 blots presented in E (n = 3). *P < 0.05.
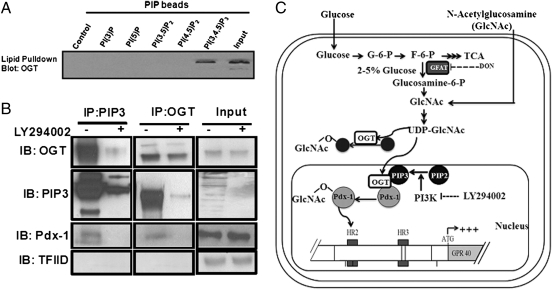
O-GlcNAc Transferase Interacts with Phosphatidylinositol 3,4,5-Trisphosphate in the Nucleus.
The observation that the PI3K pathway is required for both HBP-mediated regulation of GPR40 gene expression and glucose-induced O-GlcNAcylation of nuclear proteins prompted us to examine whether OGT interacts with the product of the PI3K reaction, phosphatidylinositol 3,4,5-trisphosphate (PIP3), in the nucleus. Sequence analysis of OGT identified a PIP3 binding region adjacent to its catalytic domain at the carboxyl terminus, and upon activation of PI3K signaling, PIP3 recruits OGT to the plasma membrane (28). To investigate whether the interaction between PIP3 and OGT also occurs in the nucleus, we performed affinity pull-down assays by using phosphoinositide-conjugated affinity beads and nuclear extracts from MIN6 cells. Pulled-down proteins were separated on SDS/PAGE and analyzed by immunoblotting using anti-OGT antibody. As shown in Fig. 6A, OGT was only detected in pulled-down proteins with PIP3-conjugated beads but not with unconjugated control or other PIP-conjugated beads. Similar results were obtained by coimmunoprecipitation assays in which immunoprecipitated PIP3 was immunoblotted with OGT (Fig. 6B, Left) or, conversely, when immunoprecipitated OGT was immunoblotted with PIP3 (Fig. 6B, Middle). Furthermore, we found that the interaction between OGT and PIP3 in the nucleus was blocked in cells treated with LY294002 (Fig. 6B). We next sought to determine whether Pdx-1 is also recruited to the PIP3–OGT complex in the nucleus, where it is O-GlcNAcylated. Immunoblotting with Pdx-1 of nuclear extracts immunoprecipitated with either PIP3 (Fig. 6B, Left) or OGT (Fig. 6B, Middle) revealed that Pdx-1 interacts with both OGT and PIP3 in the nucleus in a PI3K-dependent manner, suggesting the existence of a PIP3–OGT–Pdx-1 complex.
Fig. 6.
(A) Nuclear extracts from MIN6 cells were pulled down by phosphoinositide-conjugated affinity beads followed by immunoblotting with an anti-OGT antibody (n = 2). (B) MIN6 cells cultured at HG were treated ± 20 μmol/L LY294002 for 24 h. Nuclear proteins were immunoprecipitated with either an anti-PIP3 or anti-OGT antibody and immunoblotted with anti-OGT, anti-PIP3, or anti–Pdx-1 antibodies. Input protein was immunoblotted with an anti-OGT, anti-PIP3, anti–Pdx-1, or anti-TFIID antibody as loading controls (n = 3). (C) A model for glucose regulation of GPR40 gene expression in pancreatic β-cells.
Discussion
This study aimed to identify the mode of regulation of the gene encoding the fatty-acid receptor GPR40 in pancreatic β-cells. We show that GPR40 mRNA is stimulated by glucose and that this effect is associated with an enhancement of the insulin secretory response of the islets to fatty acids. The main effect of glucose on GPR40 expression does not lie at the level of mRNA stability but, rather, at the transcriptional level. Thus, glucose stimulates the activity of a GPR40 promoter reporter construct in transient transfection assays. This effect is essentially recapitulated by using a construct containing only the HR2 region, demonstrating the critical role of this region for glucose regulation of the gene. Previously, we showed that β cell-specific expression of GPR40 is also due in large part to the HR2 region of the promoter (16). This region was shown to bind the β cell-specific transcription factors Pdx-1 and BETA2. We now demonstrate that most of the effect of glucose on GPR40 promoter activity depends on the Pdx-1 binding site in the HR2 region, because a 10-bp mutation spanning the A-box in the pTK-HR2-Luc reporter essentially abolished glucose responsiveness. These findings are consistent with a previous report showing that conditional inactivation of Pdx-1 in mice leads to a decrease in GPR40 expression in islets (29).
The complete blockade of glucose induction of GPR40 mRNA in the presence of wortmannin or LY294002 and in βIRKO cells indicates that this process depends on PI3K activity. The inability of exogenous insulin to induce GPR40 expression and the preserved effect of glucose upon inhibition of endogenous insulin secretion with diazoxide, however, indicate that it is not mediated in an autocrine manner by insulin release. Although this conclusion might seem in contradiction with the results obtained in βIRKO cells, it is important to point out that βIRKO cells are not only devoid of the insulin receptor but also display dysregulation of the downstream insulin signaling cascade, including PI3K (18). Therefore, we attribute the inability of glucose to stimulate GPR40 expression in βIRKO cells to defective PI3K activity rather than to the absence of the insulin receptor.
To identify the glucose-derived metabolite mediating stimulation of GPR40 mRNA expression, we used several glucose analogs and showed that glucose metabolism through the HBP is required for its effect. Thus, the stimulatory effect of glucose on GPR40 mRNA is mimicked by the HBP intermediate GlcNAc and is blocked by pharmacological inhibition or RNA interference against GFAT. Importantly, adding back GlcNAc rescues GPR40 expression upon inhibition of GFAT, as would be expected because GlcNAc is downstream of GFAT in the HBP. O-GlcNAcylation is an important posttranslational modification of nucleo-cytoplasmic proteins, including transcription factors (24). It is in large part regulated by the flux of glucose through the HBP and generation of UDP-GlcNAc, which in β-cells is proportional to the extracellular glucose concentration (30). In β-cells O-GlcNAcylation mediate the induction of MafA expression by glucose (21) and the subcellular distribution of BETA2 (31). Here, we show that glucose increases O-GlcNAcylation of Pdx-1, but not BETA2, in the nucleus and its recruitment to the GPR40 promoter.
The question then arises as to what is the link between the PI3K and the HB pathways in the regulation of GPR40 gene expression by glucose. One important finding derived from sequence analysis of OGT was the identification of a C-terminal PIP-binding region adjacent to its catalytic domain. This domain interacts with PIP3 with greater affinity than other phosphatidylinositols. Although this interaction does not modulate the activity of OGT directly (28), it may regulate its subcellular localization and act to anchor and target activated OGT to signaling and transcriptional complexes where PIP3 is abundant, such as the plasma and nuclear membranes (32–36). Accordingly, Corrillo et al. (37) demonstrated the existence of a PI3K pathway-dependent and cellular compartment-specific activation of O-GlcNAc. Our data suggest that in the presence of high glucose, glucose metabolism through the HBP activates OGT by increasing its donor substrate UDP-GlcNAc, and PIP3 generation through PI3K activity anchors activated OGT at the nuclear membrane. This interaction will consequently increase OGT's access to substrates such as transcription factors, a number of which have been shown to be activated by O-GlcNAcylation (38, 39) (Fig. 6C). In fact, we show by using coimmunoprecipitation assays that Pdx-1 interacts with both OGT and PIP3 in the nucleus, suggesting the existence of a PIP3–OGT–Pdx1 O-GlcNAcylation complex. It is important to note that the interaction between OGT and Pdx-1 is abolished by PI3K inactivation, strongly emphasizing the requirement for PIP3 to anchor OGT in the nucleus where it recruits and O-GlcNAcylates Pdx-1.
The observation that OGT interacts with PIP3 in the nucleus provides an interesting link between the regulation of gene expression by glucose and the insulin signaling pathway. In the liver, the presence of insulin is required for glucose stimulation of glycolytic and lipogenic gene expression, an effect that has been attributed to the stimulation of hepatic glucokinase by insulin-enabling intracellular glucose flux (40). Given that the transcription factor carbohydrate-responsive element-binding protein was recently shown to be stabilized and activated by O-GlcNAcylation (41), our results provide a potential additional mechanism underlying the requirement for insulin, namely the permissive effect of PI3K activity and PIP3 generation for O-GlcNAcylation of transcription factors in the nucleus. However, whether the mechanisms identified herein in β-cells also operate in hepatocytes remains to be determined.
In conclusion, these findings uniquely demonstrate that expression of the gene encoding the fatty-acid receptor GPR40 is transcriptionnally regulated by glucose via a mechanism that involves glucose flux through the HBP and O-GlcNAcylation of Pdx-1 and depends on a direct interaction between OGT and PIP3 in the nucleus. Because increased fatty-acid signaling in the β-cell is an important mechanism of β-cell compensation in the face of nutrient excess, we propose that stimulation of GPR40 expression under conditions of hyperglycemia results in enhanced GPR40 signaling, thereby contributing to normalizing blood glucose levels. Several GPR40 agonists are at various stages of drug development for the treatment of type 2 diabetes. Our findings therefore provide important insight into the biology of the receptor and identify a unique mechanism of regulation of gene expression via PI3K-dependent O-GlcNAcylation.
Experimental Procedures
Detailed methods for mouse islets isolation and culture, use of human islets, protein extraction and immunoblotting, RT-PCR, transient transfection and small interfering RNA (siRNA) knock down, Chromatin immunoprecipitation (Chip) assays, subcellular fractionations, co-immunoprecipitations, and sWGA-pull down and affinity-pull down assays can be found in SI Experimental Procedures.
Expression of Data and Statistical Analysis.
Data are expressed as mean ± SEM and were analyzed by Student's t tests or one-way ANOVA with Bonferroni or Dunnett's multiple comparison tests, as appropriate, using GraphPad Instat (GraphPad Software); P < 0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank Drs. James Shapiro and Tatsuya Kin from the University of Alberta and the Coordinating Center of the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases-supported Integrated Islet Distribution Program for providing isolated human islets. This study was supported by the Canadian Institutes of Health Research Grant MOP-86545 (to V.P.) and National Institutes of Health Grant R01-DK67536 (to R.N.K.). M.K. is the recipient of a postdoctoral fellowship from the Canadian Diabetes Association. M.F. is the recipient of postdoctoral fellowships from Diabète Québec and from the Centre de Recherche du Centre Hospitalier de l'Universite de Montreal. V.P. holds the Canada Research Chair in Diabetes and Pancreatic Beta-cell Function.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1114350109/-/DCSupplemental.
References
- 1.Prentki M, Nolan CJ. Islet beta cell failure in type 2 diabetes. J Clin Invest. 2006;116:1802–1812. doi: 10.1172/JCI29103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poitout V. Lipid partitioning in the pancreatic beta-cell: Physiologic and pathophysiologic implications. Curr Opin Endocrinol Diabetes. 2002;9:152–159. [Google Scholar]
- 3.Itoh Y, et al. Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature. 2003;422:173–176. doi: 10.1038/nature01478. [DOI] [PubMed] [Google Scholar]
- 4.Briscoe CP, et al. The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids. J Biol Chem. 2003;278:11303–11311. doi: 10.1074/jbc.M211495200. [DOI] [PubMed] [Google Scholar]
- 5.Kotarsky K, Nilsson NE, Flodgren E, Owman C, Olde B. A human cell surface receptor activated by free fatty acids and thiazolidinedione drugs. Biochem Biophys Res Commun. 2003;301:406–410. doi: 10.1016/s0006-291x(02)03064-4. [DOI] [PubMed] [Google Scholar]
- 6.Steneberg P, Rubins N, Bartoov-Shifman R, Walker MD, Edlund H. The FFA receptor GPR40 links hyperinsulinemia, hepatic steatosis, and impaired glucose homeostasis in mouse. Cell Metab. 2005;1:245–258. doi: 10.1016/j.cmet.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 7.Latour MG, et al. GPR40 is necessary but not sufficient for fatty acid stimulation of insulin secretion in vivo. Diabetes. 2007;56:1087–1094. doi: 10.2337/db06-1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kebede M, et al. The fatty acid receptor GPR40 plays a role in insulin secretion in vivo after high-fat feeding. Diabetes. 2008;57:2432–2437. doi: 10.2337/db08-0553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagasumi K, et al. Overexpression of GPR40 in pancreatic beta-cells augments glucose-stimulated insulin secretion and improves glucose tolerance in normal and diabetic mice. Diabetes. 2009;58:1067–1076. doi: 10.2337/db08-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alquier T, et al. Deletion of GPR40 impairs glucose-induced insulin secretion in vivo in mice without affecting intracellular fuel metabolism in islets. Diabetes. 2009;58:2607–2615. doi: 10.2337/db09-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bharate SB, Nemmani KV, Vishwakarma RA. Progress in the discovery and development of small-molecule modulators of G-protein-coupled receptor 40 (GPR40/FFA1/FFAR1): An emerging target for type 2 diabetes. Expert Opin Ther Pat. 2009;19:237–264. doi: 10.1517/13543770802665717. [DOI] [PubMed] [Google Scholar]
- 12.Kebede MA, Alquier T, Latour MG, Poitout V. Lipid receptors and islet function: Therapeutic implications? Diabetes Obes Metab. 2009;11(Suppl 4):10–20. doi: 10.1111/j.1463-1326.2009.01114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsujihata Y, et al. TAK-875, an orally available GPR40/FFA1 agonist enhances glucose-dependent insulin secretion and improves both postprandial and fasting hyperglycemia in type 2 diabetic rats. J Pharmacol Exp Ther. 2011;1:228–237. doi: 10.1124/jpet.111.183772. [DOI] [PubMed] [Google Scholar]
- 14.Araki T, Hirayama M, Hiroi S, Kaku K. GPR40-induced insulin secretion by the novel agonist TAK-875: First clinical findings in patients with type 2 diabetes. Diabetes Obes Metab. December 22, 2011 doi: 10.1111/j.1463-1326.2011.01525.x. [DOI] [PubMed] [Google Scholar]
- 15.Yashiro H, et al. The effects of TAK-875, a selective GPR40/FFA1 agonist, on insulin and glucagon in isolated rat and human islets. J Pharmacol Exp Ther. November 21, 2011 doi: 10.1124/jpet.111.187708. [DOI] [PubMed] [Google Scholar]
- 16.Bartoov-Shifman R, Ridner G, Bahar K, Rubins N, Walker MD. Regulation of the gene encoding GPR40, a fatty acid receptor expressed selectively in pancreatic beta cells. J Biol Chem. 2007;282:23561–23571. doi: 10.1074/jbc.M702115200. [DOI] [PubMed] [Google Scholar]
- 17.Poitout V, et al. Regulation of the insulin gene by glucose and fatty acids. J Nutr. 2006;136:873–876. doi: 10.1093/jn/136.4.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Assmann A, Ueki K, Winnay JN, Kadowaki T, Kulkarni RN. Glucose effects on beta-cell growth and survival require activation of insulin receptors and insulin receptor substrate 2. Mol Cell Biol. 2009;29:3219–3228. doi: 10.1128/MCB.01489-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.da Silva Xavier G, Varadi A, Ainscow EK, Rutter GA. Regulation of gene expression by glucose in pancreatic beta -cells (MIN6) via insulin secretion and activation of phosphatidylinositol 3′-kinase. J Biol Chem. 2000;275:36269–36277. doi: 10.1074/jbc.M006597200. [DOI] [PubMed] [Google Scholar]
- 20.Rumberger JM, Wu T, Hering MA, Marshall S. Role of hexosamine biosynthesis in glucose-mediated up-regulation of lipogenic enzyme mRNA levels: Effects of glucose, glutamine, and glucosamine on glycerophosphate dehydrogenase, fatty acid synthase, and acetyl-CoA carboxylase mRNA levels. J Biol Chem. 2003;278:28547–28552. doi: 10.1074/jbc.M302793200. [DOI] [PubMed] [Google Scholar]
- 21.Vanderford NL, Andrali SS, Ozcan S. Glucose induces MafA expression in pancreatic beta cell lines via the hexosamine biosynthetic pathway. J Biol Chem. 2007;282:1577–1584. doi: 10.1074/jbc.M605064200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akimoto Y, et al. Elevation of the post-translational modification of proteins by O-linked N-acetylglucosamine leads to deterioration of the glucose-stimulated insulin secretion in the pancreas of diabetic Goto-Kakizaki rats. Glycobiology. 2007;17:127–140. doi: 10.1093/glycob/cwl067. [DOI] [PubMed] [Google Scholar]
- 23.Wells L, Whelan SA, Hart GW. O-GlcNAc: A regulatory post-translational modification. Biochem Biophys Res Commun. 2003;302:435–441. doi: 10.1016/s0006-291x(03)00175-x. [DOI] [PubMed] [Google Scholar]
- 24.Issad T, Kuo M. O-GlcNAc modification of transcription factors, glucose sensing and glucotoxicity. Trends Endocrinol Metab. 2008;19:380–389. doi: 10.1016/j.tem.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 25.Gao Y, Miyazaki J, Hart GW. The transcription factor PDX-1 is post-translationally modified by O-linked N-acetylglucosamine and this modification is correlated with its DNA binding activity and insulin secretion in min6 beta-cells. Arch Biochem Biophys. 2003;415:155–163. doi: 10.1016/s0003-9861(03)00234-0. [DOI] [PubMed] [Google Scholar]
- 26.Konrad RJ, et al. Purification of the O-glycosylated protein p135 and identification as O-GlcNAc transferase. Biochem Biophys Res Commun. 2001;288:1136–1140. doi: 10.1006/bbrc.2001.5902. [DOI] [PubMed] [Google Scholar]
- 27.Kreppel LK, Blomberg MA, Hart GW. Dynamic glycosylation of nuclear and cytosolic proteins. Cloning and characterization of a unique O-GlcNAc transferase with multiple tetratricopeptide repeats. J Biol Chem. 1997;272:9308–9315. doi: 10.1074/jbc.272.14.9308. [DOI] [PubMed] [Google Scholar]
- 28.Yang X, et al. Phosphoinositide signalling links O-GlcNAc transferase to insulin resistance. Nature. 2008;451:964–969. doi: 10.1038/nature06668. [DOI] [PubMed] [Google Scholar]
- 29.Edfalk S, Steneberg P, Edlund H. Gpr40 is expressed in enteroendocrine cells and mediates free fatty acid stimulation of incretin secretion. Diabetes. 2008;57:2280–2287. doi: 10.2337/db08-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hanover JA, Lai Z, Lee G, Lubas WA, Sato SM. Elevated O-linked N-acetylglucosamine metabolism in pancreatic beta-cells. Arch Biochem Biophys. 1999;362:38–45. doi: 10.1006/abbi.1998.1016. [DOI] [PubMed] [Google Scholar]
- 31.Andrali SS, Qian Q, Ozcan S. Glucose mediates the translocation of NeuroD1 by O-linked glycosylation. J Biol Chem. 2007;282:15589–15596. doi: 10.1074/jbc.M701762200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bacqueville D, et al. Characterization of a G protein-activated phosphoinositide 3-kinase in vascular smooth muscle cell nuclei. J Biol Chem. 2001;276:22170–22176. doi: 10.1074/jbc.M011572200. [DOI] [PubMed] [Google Scholar]
- 33.Cocco L, Maraldi NM, Capitani S, Martelli AM, Manzoli FA. Nuclear localization and signalling activity of inositol lipids. Ital J Anat Embryol. 2001;106(2) Suppl 1:31–43. [PubMed] [Google Scholar]
- 34.Okada M, Ye K. Nuclear phosphoinositide signaling regulates messenger RNA export. RNA Biol. 2009;6:12–16. doi: 10.4161/rna.6.1.7439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ye K, Ahn JY. Nuclear phosphoinositide signaling. Front Biosci. 2008;13:540–548. doi: 10.2741/2699. [DOI] [PubMed] [Google Scholar]
- 36.Boronenkov IV, Loijens JC, Umeda M, Anderson RA. Phosphoinositide signaling pathways in nuclei are associated with nuclear speckles containing pre-mRNA processing factors. Mol Biol Cell. 1998;9:3547–3560. doi: 10.1091/mbc.9.12.3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carrillo LD, Froemming JA, Mahal LK. Targeted in vivo O-GlcNAc sensors reveal discrete compartment-specific dynamics during signal transduction. J Biol Chem. 2011;286:6650–6658. doi: 10.1074/jbc.M110.191627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Comer FI, Hart GW. O-GlcNAc and the control of gene expression. Biochim Biophys Acta. 1999;1473:161–171. doi: 10.1016/s0304-4165(99)00176-2. [DOI] [PubMed] [Google Scholar]
- 39.Yang X, Zhang F, Kudlow JE. Recruitment of O-GlcNAc transferase to promoters by corepressor mSin3A: Coupling protein O-GlcNAcylation to transcriptional repression. Cell. 2002;110:69–80. doi: 10.1016/s0092-8674(02)00810-3. [DOI] [PubMed] [Google Scholar]
- 40.Dentin R, et al. Hepatic glucokinase is required for the synergistic action of ChREBP and SREBP-1c on glycolytic and lipogenic gene expression. J Biol Chem. 2004;279:20314–20326. doi: 10.1074/jbc.M312475200. [DOI] [PubMed] [Google Scholar]
- 41.Guinez C, et al. O-GlcNAcylation increases ChREBP protein content and transcriptional activity in the liver. Diabetes. 2011;60:1399–1413. doi: 10.2337/db10-0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.