Abstract
Heart growth is tightly controlled so that the heart reaches a predetermined size. Fetal heart growth occurs through cardiomyocyte proliferation, whereas postnatal heart growth involves primarily physiological cardiomyocyte hypertrophy. The Hippo kinase cascade is an important regulator of organ growth. A major target of this kinase cascade is YAP1, a transcriptional coactivator that is inactivated by Hippo kinase activity. Here, we used both genetic gain and loss of Yap1 function to investigate its role in regulating proliferative and physiologic hypertrophic heart growth. Fetal Yap1 inactivation caused marked, lethal myocardial hypoplasia and decreased cardiomyocyte proliferation, whereas fetal activation of YAP1 stimulated cardiomyocyte proliferation. Enhanced proliferation was particularly dramatic in trabecular cardiomyocytes that normally exit from the cell cycle. Remarkably, YAP1 activation was sufficient to stimulate proliferation of postnatal cardiomyocytes, both in culture and in the intact heart. A dominant negative peptide that blocked YAP1 binding to TEAD transcription factors inhibited YAP1 proliferative activity, indicating that this activity requires YAP1–TEAD interaction. Although Yap1 was a critical regulator of cardiomyocyte proliferation, it did not influence physiological hypertrophic growth of cardiomyocytes, because postnatal Yap1 gain or loss of function did not significantly alter cardiomyocyte size. These studies demonstrate that Yap1 is a crucial regulator of cardiomyocyte proliferation, cardiac morphogenesis, and myocardial trabeculation. Activation of Yap1 in postnatal cardiomyocytes may be a useful strategy to stimulate cardiomyocyte expansion in therapeutic myocardial regeneration.
Keywords: heart development, physiological hypertrophy
Between the early heart tube stage and adulthood, the murine heart increases by >300-fold in mass (Fig. S1) (1). The ∼18-fold increase in mass achieved during fetal life occurs mainly through cardiomyocyte proliferation, whereas the remaining ∼18-fold growth achieved postnatally largely involves increased cardiomyocyte size (physiological hypertrophy), plus expansion of nonmyocyte populations. Growth of the heart is precisely regulated so that there is little variability in the final size of the adult heart. Derangements of these regulatory pathways likely contribute to congenital heart malformations, the leading form of major birth defect. Moreover, understanding of these pathways will be highly relevant for regenerative approaches to postnatal heart disease.
The Hippo signaling cascade was discovered in Drosophila as a potent mechanism that regulates cell proliferation and organ size (2, 3). The core kinases of this signaling pathway, Hippo (Hpo) and Warts (Wts), and their regulatory subunits Salvador (Sav) and Mats, phosphorylate Yorkie (Yki) (4–7). In the absence of phosphorylation by the Hpo kinases cascade, Yki coactivates transcription in conjunction with specific transcription factors, such as the TEAD family transcription factor Scalloped (Sd), stimulating organ growth by increasing cell proliferation and reducing apoptosis (8, 9). Incompletely understood upstream molecular signals activate the Hpo kinase cascade, leading to Yki phosphorylation, nuclear export, and reduced organ growth. The core of the Hippo pathway is highly conserved in mammals, where Mst1/2, Sav1, Lats1/2, Yap1, and Tead1-4 are the orthologs of Hpo, Sav, Wts, Yki, and Sd, respectively (3). Yap1 gain and loss of function in liver and skin suggest that Yap1 plays an important role in mammalian organ size regulation, as in Drosophila (10–12).
In this study, we investigated the function of Yap1 in regulating heart growth. Yap1 loss of function impaired cardiomyocyte proliferation and caused lethal myocardial hypoplasia. Meanwhile, Yap1 gain of function enhanced cell-cycle activity in cardiomyocytes both in vitro and in intact fetal and infant hearts. However, Yap1 gain and loss of function did not substantially alter cell size, indicating that Yap1 influences organ size primarily by regulating proliferation. Collectively, our data demonstrate that Yap1 is a powerful regulator of cardiomyocyte proliferation.
Results
YAP1 Expression in Fetal and Postnatal Heart.
We measured YAP1 expression at different stages of heart development. YAP1 protein was robustly detected in neonatal and juvenile mouse heart and declined with age, so that it was nearly undetectable by 12 wk of age (Fig. S2A). To distinguish expression in myocytes compared with nonmyocytes, we dissociated fetal, neonatal, and adult hearts and separated cells into myocyte and nonmyocyte fractions. By Western blotting, YAP1 was detected in both myocytes and nonmyocytes, with predominantly cardiomyocyte expression in fetal heart (Fig. S2B). In neonatal and 1.5-mo-old heart, expression levels in cardiomyocytes and nonmyocytes were comparable (Fig. S2B).
Fetal Cardiomyocyte-Restricted Loss of Yap1 Causes Lethal Cardiac Hypoplasia.
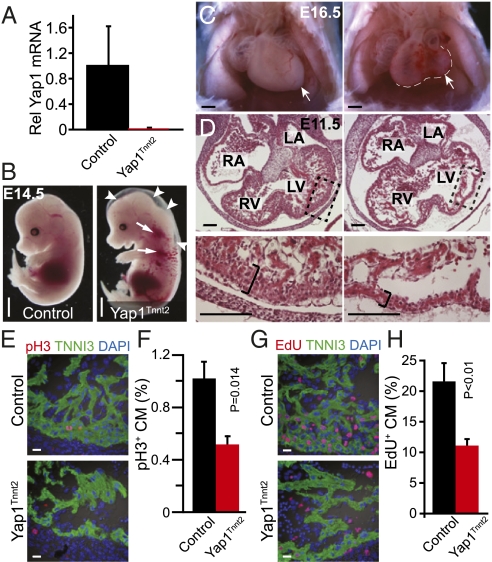
To test the hypothesis that Yap1 is required for fetal cardiomyocyte proliferation, we inactivated a conditional Yap1 allele (Yap1flox) specifically in cardiomyocytes early in heart development using Tnnt2–Cre (13). To measure the extent of Yap1 inactivation, we used FACS to isolate pure populations of cardiomyocytes from Yap1fl/+::Tnnt2–Cre::Rosa26mTmG/+ (Yap1 heterozygous control) or Yap1fl/fl::Tnnt2–Cre::Rosa26mTmG/+ (Yap1 mutant) heart at embryonic day (E) 12.5. In these embryos, Tnnt2–Cre activated membrane-localized GFP (mGFP) from the Cre-activated Rosa26mTmG reporter and inactivated the conditional Yap1 allele specifically in cardiomyocytes. By quantitative RT-PCR (qRT-PCR), Yap1 transcripts were depleted by >95% at E12.5 in mutant compared with heterozygous control cardiomyocytes (Fig. 1A), confirming effective gene inactivation.
Fig. 1.
Fetal cardiomyocyte-restricted inactivation of YAP1. (A) YAP1 mRNA levels in control (Yap1fl/+::Tnnt2–Cre::Rosa26mTmG/+) and Yap1Tnnt2 (Yap1fl/fl::Tnnt2–Cre::Rosa26mTmG/+) mutant cardiomyocytes, isolated at E12.5 by FACS for GFP. (B) Hydrops fetalis and peripheral hemorrhage in mutant embryo at E13.5. (Scale bar: 500 μm.) (C) Whole-mount image of E16.5 embryos after removal of the ventral chest wall, showing severe hypoplasia of mutant hearts. Hypoplasia was more severe in the left ventricle. For clarity, the white line indicates the edge of the heart. (Scale bar: 500 μm.) (D) Histological sections of E11.5 hearts. Magnifications show dramatic hypoplasia of compact myocardium in mutants (black brackets). (Scale bar: 100 μm.) (E and F) Reduced pH3 immunoreactivity in Yap1Tnnt2 mutant hearts. n = 3. (Scale bar in E: 10 μm.) (G and H) Reduced EdU staining in Yap1Tnnt2 mutant hearts. n = 3. (Scale bar: 10 μm.)
Yap1fl/fl::Tnnt2–Cre (abbreviated Yap1Tnnt2) embryos were present at a sub-Mendelian ratio by E12.5 and were not recovered beyond E16.5. The embryos displayed peripheral edema and pericardial effusion, consistent with heart failure (Fig. 1B and Fig. S3A). Overall cardiac patterning was preserved, but ventricular chamber size was severely reduced (Fig. 1C and Fig. S3B). In some cases, the hypoplasia affected both ventricles equally, whereas in other cases the left ventricle was more severely affected. Histological sectioning demonstrated a four-chambered heart with two atrioventricular and two outflow tract valves (Fig. 1D and Fig. S3C). The myocardium was markedly hypoplastic (Fig. 1D). Expression of MLC2A (MGI:MYL7) and MLC2V (MGI:MYL2), markers of atria and ventricular chamber specification, was unperturbed (Fig. S3D), indicating normal chamber specification. Rare survivors to E16.5 had ventricular septal defects (Fig. S3C).
To investigate the cellular mechanism underlying the myocardial hypoplasia, we performed immunostaining for markers of proliferation and apoptosis. Staining for histone H3 phosphorylated on serine 10 (pH3), a marker of M phase of the cell cycle, showed substantially reduced cardiomyocyte proliferation. Quantitative analysis showed that the fraction of cardiomyocytes positive for pH3 was reduced by twofold (P < 0.05; Fig. 1 E and F). This result was confirmed by analysis of cardiomyocyte uptake of 5-ethynyl-2’-deoxyuridine (EdU), a nucleotide analog that labels cells passing through S phase. EdU+ cardiomyocytes were reduced by twofold in Yap1Tnnt2 mutant heart (P < 0.05; Fig. 1 G and H). Although YAP1 regulates apoptosis in other settings (14), apoptosis was not elevated in Yap1Tnnt2 cardiomyocytes, as assessed by TUNEL staining (Fig. S3E). Collectively, these data indicate that Yap1 is essential for fetal cardiomyocyte proliferation.
To confirm that reduced cardiomyocyte proliferation led to decreased cardiomyocyte number, we used FACS to quantitate cardiomyocyte number in dissociated fetal hearts (Fig. S3 F and G). We took advantage of the Rosa26mTmG (15) Cre reporter allele, in which Cre activity switches off baseline mRFP (membrane-bound RFP) and activates mGFP. GFP+ cardiomyocyte number was reduced in Yap1fl/fl::Tnnt2–Cre::Rosa26mTmG/+ mutants compared with Yap1fl/+::Tnnt2–Cre::Rosa26mTmG/+ heterozygous controls. On the other hand, cardiomyocyte size, as measured by FACS forward scatter, did not differ between groups, suggesting that Yap1 is not required for fetal cardiomyocyte size regulation (Fig. S3H). Collectively, these data indicate that Yap1 is required to promote normal cardiomyocyte proliferation in the fetal heart.
Cardiomyocyte Hypertrophic Growth Does Not Require Yap1.
The fetal heart primarily grows through cardiomyocyte proliferation, but by postnatal day (P) 4, cardiomyocytes stop increasing in number and postnatal heart growth occurs by increased cardiomyocyte size (physiological hypertrophy) (16). Loss of Yap1 in the fetal heart impaired cardiomyocyte proliferation but did not alter size. Therefore, we hypothesized that Yap1 is dispensable for physiological cardiomyocyte hypertrophy.
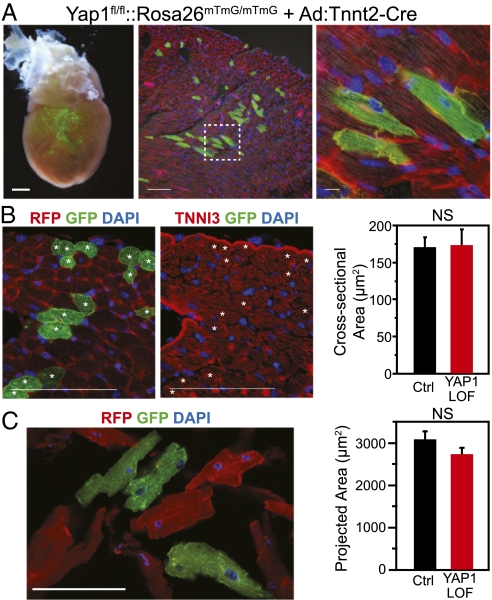
To test this hypothesis, we asked whether loss of Yap1 has a cell-autonomous effect on postnatal cardiomyocyte growth. We sought to inactivate Yap1 postnatally in a small fraction of cardiomyocytes, to avoid potentially triggering secondary effects that may result from widespread myocardial Yap1 inactivation. A previous report indicated that retro-orbital administration of adenovirus to newborn pups leads to mosaic cardiomyocyte gene transfer (17). Retro-orbital delivery of Ad:Tnnt2–Cre to Yap1fl/fl::Rosa26mTmG/mTmG pups activated the Cre-dependent mGFP reporter (and inactivated the baseline mRFP reporter) in a small fraction of cardiomyocytes, confirming that this technique is an effective means to achieve mosaic Cre-mediated recombination in the heart (Fig. 2A). We did not observe GFP expression outside of the heart.
Fig. 2.
YAP1 is not cell autonomously required for postnatal hypertrophic cardiomyocyte growth. (A) Mosaic Cre-mediated recombination after retro-orbital delivery of Ad:Tnnt2–Cre to P3 neonatal Yap1fl/fl::Rosa26mTmG/mTmG mice. Boxed area is magnified in the Right panel. [Scale bars (from left): 500, 100, 10 μm.] (B and C) Hearts were examined at 4 wk (B) or 6 wk (C). Sizes of YAP1-deficient (mGFP+ and mRFP−) and control (mGFP− and mRFP+) cardiomyocytes were measured in histological sections (cross-sectional area, B) or in dissociated preparations (projected area, C). No significant difference was detected between groups (NS; n = 4). (Scale bars: 100 μm.)
To investigate the cell-autonomous role of Yap1 in postnatal cardiomyocyte growth, we delivered Ad:Tnnt2–Cre to Yap1fl/fl::Rosa26mTmG/mTmG pups at 3 d of life. At 4–6 wk of life, we compared the size of GFP+ (Yap1-deficient) and GFP− (control) cardiomyocytes. In tissue sections, the cross-sectional areas of GFP+ and GFP− cardiomyocytes did not differ significantly (Fig. 2B). Likewise, in dissociated cardiomyocyte preparations, cardiomyocyte projected area was not significantly altered (Fig. 2C). Thus, we conclude that Yap1 is not required in a cell-autonomous manner for physiological cardiac hypertrophy.
Cardiomyocytes also increase in size in response to biomechanical stress, a process known as pathological hypertrophy. To determine whether YAP1 is essential for pathological hypertrophy, we performed ascending aortic constriction (AAC) on adult mice with mosaic inactivation of Yap1. The cross-sectional area of GFP− control cardiomyocytes was larger in AAC compared with unoperated hearts, consistent with activation of the hypertrophic response (Fig. S4). GFP+ mutant cardiomyocytes were not significantly different in size compared with GFP− cardiomyocytes in either unoperated or AAC hearts (Fig. S4). Thus, YAP1 was dispensable for pathological cardiomyocyte hypertrophy.
Yap1 Gain of Function Stimulates Proliferation of Cultured Cardiomyocytes.
Given the essential role of Yap1 in sustaining normal cardiomyocyte proliferation, we next asked whether increased Yap1 is sufficient to drive cardiomyocyte proliferation. We developed an adenoviral vector to express activated YAP1 with an N-terminal triple FLAG epitope (Ad:FLAG–aYap1), where “activated YAP1” contained a serine 127 to alanine substitution that blocked Hippo pathway-mediated YAP1 inactivation (18). We confirmed expression in cultured neonatal rat cardiomyocytes (Fig. S5A). We used this tool to ask whether YAP1 gain of function enhances proliferation of fetal cardiomyocytes, which are normally actively proliferating. We transduced wild-type E16.5 fetal rat cardiomyocytes with Ad:FLAG–aYap1 or control (LacZ-expressing adenovirus, Ad:LacZ) adenovirus and measured the fraction of cells undergoing DNA synthesis [5′-bromodeoxyuridine (BrdU) uptake and mitosis (pH3 staining)]. Activated YAP1 significantly increased both measures of cardiomyocyte cell-cycle activity (Fig. S5B). Consistent with active fetal cardiomyocyte proliferation, the number of cardiomyocytes after 2 d of culture was greater in the activated YAP1 group compared with controls (Fig. S5B).
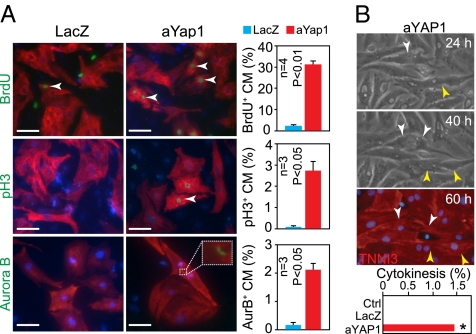
Having established that activated YAP1 was sufficient to stimulate proliferation of cardiomyocytes that were already actively engaged in the cell cycle, we next investigated whether Yap1 gain of function could sustain proliferation in postnatal cardiomyocytes, which normally exit the cell cycle. Prior studies demonstrated that rat cardiomyocytes cease increasing in number by P4, although DNA synthesis and karyokinesis were detected above background levels for another 1–2 wk (16). Therefore, we prepared cardiomyocyte cultures from P4 neonatal rats. As expected, control neonatal rat ventricular cardiomyocytes treated with LacZ adenovirus rarely stained for pH3 or Aurora B, markers of M phase and cytokinesis, respectively, confirming that nearly all neonatal cardiomyocytes had exited the cell cycle (Fig. 3A). A small fraction (2%) of control neonatal cardiomyocytes were labeled with BrdU, consistent with low-level, persistent DNA synthesis activity (Fig. 3A). In contrast to control neonatal cardiomyocytes , aYAP1 neonatal cardiomyocytes exhibited far greater cell-cycle activity, with labeling indexes of 31%, 2.7%, and 2.1% for BrdU, pH3, and AuroraB, respectively (Fig. 3A). aYAP1 neonatal cardiomyocytes were observed at all stages of the cell cycle (Fig. S5C). Sequential imaging of the same fields over time demonstrated completion of cytokinesis in a subset of aYAP1-expressing but not control cardiomyocytes (Fig. 3B and Fig. S5D). We detected cell division events in 0/366 control cells, 0/267 LacZ cells, and 8/566 aYAP1 cells (P = 0.015; Fig. 3B). However, there was ongoing apoptosis in these cultures, which was reduced, but not eliminated, with FLAG–aYap1 expression (Fig. S5 E and F). As a result, there was an overall decline in cardiomyocyte number over the course of the experiment, although this decline was least in the aYAP1 group as a result of both increased proliferation and reduced apoptosis (Fig. S5G). Collectively, these data indicate that activated YAP1 continues to drive the cell cycle in postnatal cardiomyocytes, which normally have very little cell-cycle activity.
Fig. 3.
Activated YAP1 stimulated proliferation of cultured cardiomyocytes. (A) aYAP1 stimulated cell-cycle activity of P4 neonatal rat cardiomyocytes, as measured by immunostaining for BrdU, pH3, and Aurora B (AurB). (Scale bars: 50 μm.) (B) Sequential imaging of cardiomyocytes at the indicated times after aYAP1 transduction. Two cardiomyocytes underwent cytokinesis in this field (arrowheads). At 60 h, cultures were immunostained for the cardiomyocyte marker TNNI3. Full imaging series is in Fig. S5D. *P = 0.015.
Activated Yap1 Stimulated Fetal Cardiomyocyte Proliferation in Vivo.
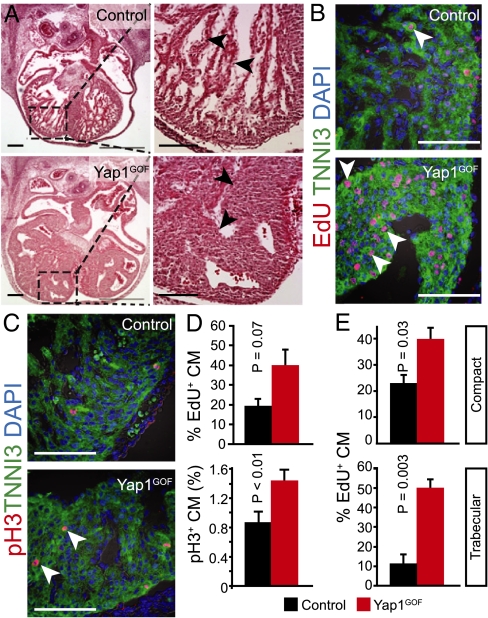
Next, we asked whether YAP1 gain of function stimulates cardiomyocyte proliferation in intact fetal heart. We used a reported transgene (TetO–aYap1) (10) that expresses activated YAP1 (YAP1–[S127A]) from a doxycycline (Dox)-dependent promoter. In Tnnt2–Cre::Rosa26fs-rtTA::TetO-aYap1 (abbreviated Yap1GOF) mice, Dox induced expression of activated YAP1 selectively in cardiomyocytes (Fig. S6 A and B). Administration of Dox to YAP1GOF fetuses starting at E8.5 resulted in fetal demise by E15.5. At E12.5, mutant fetuses exhibited peripheral hemorrhage, hepatic congestion, and cardiomegaly (Fig. S6C). Histological examination revealed dramatic myocardial overgrowth with moderate thickening of the compact myocardium and marked expansion of the trabecular myocardium, causing near chamber obliteration (Fig. 4A, arrowheads).
Fig. 4.
Fetal YAP1 gain of function stimulated cardiomyocyte proliferation in vivo. (A) Histological sections of Yap1GOF heart at E12.5 revealed marked hypertrabeculation that nearly obliterated the cardiac chambers. Black arrowheads indicate trabecular myocardium. (B and C) pH3 and EdU staining showed elevated cardiomyocyte proliferation, particularly in trabecular myocardium (white arrowheads). (D) Quantitation of B and C for ventricular myocardium (compact and trabecular pooled). (E) Quantitation of cardiomyocyte EdU uptake in compact vs. trabecular myocardium. (Scale bars: 100 μm.)
To evaluate the cellular mechanism for myocardial expansion, we performed immunostaining for proliferation markers. Cardiomyocyte proliferation was significantly increased in Yap1GOF fetal heart and control mice exposed to Dox starting at E8.5, as measured by the M-phase marker pH3 (Fig. 4 C and D). The S-phase marker EdU also increased by twofold, which approached, but did not achieve, statistical significance (P = 0.07, n = 3; Fig. 4 B and D). Thus, Yap1 gain of function in fetal heart was sufficient to enhance cardiomyocyte proliferation, causing marked cardiomyocyte hyperplasia.
In control heart, the proliferation rate of cardiomyocytes was substantially lower in trabecular compared with compact myocardium (Fig. 4 B and C), indicating that, even in fetal heart, cardiomyocytes in specific compartments are exiting the cell cycle. This finding was particularly evident by pH3 staining, where it was very rare to observe pH3+ trabecular cardiomyocytes. In contrast, proliferating mutant trabecular cardiomyocytes were readily observed in Yap1GOF (Fig. 4 B and C). Quantitation of EdU uptake showed that Yap1GOF stimulated trabecular myocardial proliferation fivefold, to levels comparable with compact myocardium (Fig. 4E). These data indicate that Yap1 gain of function is sufficient to sustain cell-cycle activity in trabecular cardiomyocytes, causing tremendous expansion of trabecular myocardium.
Cardiomyocyte proliferation is often linked to differentiation. Trabecular and compact cardiomyocytes have distinct gene expression programs. One marker expressed in trabecular, but not compact, myocardium is Nppa. In situ hybridization showed that Nppa expression was strongly down-regulated in trabecular myocardium expressing activated YAP1 (Fig. S6D), consistent with impaired activation of the trabecular myocardial gene program.
Activated YAP1 Stimulated Postnatal Cardiomyocyte Proliferation in Vivo.
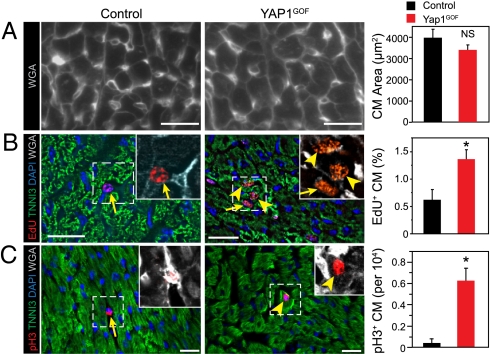
In both P4 neonatal cardiomyocytes and fetal trabecular cardiomyocytes, YAP1 gain of function was sufficient to drive proliferation of cardiomyocytes that normally have exited the cell cycle. Therefore, we asked whether YAP1 gain of function was sufficient to drive proliferation of cardiomyocytes in the intact infant heart, in which cardiomyocytes are normally exiting from the cell cycle. We treated Yap1GOF mice with Dox from P5 and analyzed hearts at P15. Heart weight was increased in YAP1GOF mice compared with the controls (heart weight/body weight, 9.0 ± 0.1 vs. 7.5 ± 0.5 mg/g; P = 0.03; n = 3). This result was not due to cardiomyocyte hypertrophy, because cardiomyocyte size did not differ significantly between Yap1GOF and control groups (Fig. 5A). EdU labeling index was increased nearly twofold in YAP1GOF cardiomyocytes, indicative of a higher fraction of cardiomyocytes passing through S phase during the 24-h EdU pulse (Fig. 5B). This finding was corroborated by staining for the M-phase marker pH3, which showed 15-fold increased cell-cycle activity in Yap1GOF cardiomyocytes (Fig. 5C). Collectively, these data indicate that YAP1 gain of function increased postnatal cardiomyocyte proliferation.
Fig. 5.
Postnatal YAP1 gain of function stimulated cardiomyocyte proliferation in vivo. YAP1GOF or control mice were treated with Dox from P5 to P15, when they were analyzed for cardiomyocyte proliferation. (A) The size of YAP1 gain-of-function cardiomyocytes was indistinguishable from control. Wheat germ agglutinin (WGA) staining outlined the edges of cardiomyocytes. (B and C) Cardiomyocyte proliferation was increased based on EdU labeling index (B, deconvolution) and pH3 staining (C, confocal). Arrowheads indicate cardiomyocytes and arrows nonmyocytes. Insets show magnifications of boxed regions, with cardiomyocyte borders highlighted by WGA staining. n = 3. *P < 0.05. (Scale bars: 20 μm.)
YAP1 Regulates Expression of Cell-Cycle Genes in Cardiomyocytes.
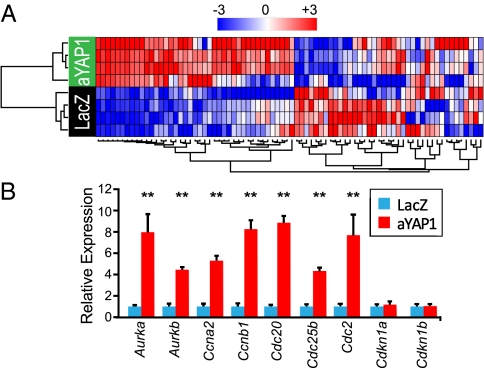
To begin to identify genes downstream of Yap1 that stimulate cardiomyocyte proliferation, we performed microarray expression profiling in Yap1 gain of function. We used the Affymetrix Rat Gene 1.0 ST microarray to compare gene expression between P4 neonatal cardiomyocyte transduced with FLAG-aYap1 or LacZ (control). Gene Set Enrichment Analysis (19) of the expression profiles using gene sets for manually curated canonical pathways indicated that the large majority of the sets significantly enriched by activated YAP1 related to cell proliferation and DNA synthesis (Fig. S7 A and B). Hierarchical clustering using cell cycle-related genes clustered samples into control or aYAP1 groups (Fig. 6A and Fig. S7C). Comparison of individual gene expression levels between groups identified 1,263 differentially expressed probe sets corresponding to 1,057 known genes (n = 4; P < 0.005 and fold change >50%; Dataset S1). We validated differential expression of a subset of cell cycle-regulated genes by qRT-PCR. Of eight genes tested that were differentially expressed by microarray, six were validated by qRT-PCR (Fig. 6B). Among up-regulated cell-cycle genes were Cyclin A2 (CcnA2), Cyclin B1 (CcnB1), and Cyclin-dependent kinase 1 (Cdc2), which have been shown to be sufficient to drive limited cardiomyocyte proliferation in postnatal hearts (20–22). Cyclins D1 and D2, which also promote cardiomyocyte proliferation (23, 24), approached but did not reach our cutoff thresholds for differential expression by microarray (P = 0.003–0.004 and fold change 1.4–1.5). These data indicate that YAP1 stimulates cardiomyocyte proliferation through the coordinate activation of a number of cell-cycle genes.
Fig. 6.
YAP1 promotes expression of cell-cycle genes through interaction with TEAD1. (A) Heat map displaying two-way hierarchical clustering of cell-cycle genes in P4 neonatal cardiomyocytes expressing either aYAP1 or LacZ (control). Expression of cell-cycle genes segregated samples into treatment groups. (B) Validation of differential expression of cell-cycle genes by qRT-PCR. **P < 0.001. n = 4.
YAP1 Stimulation of Cardiomyocyte Proliferation Requires TEAD Interaction.
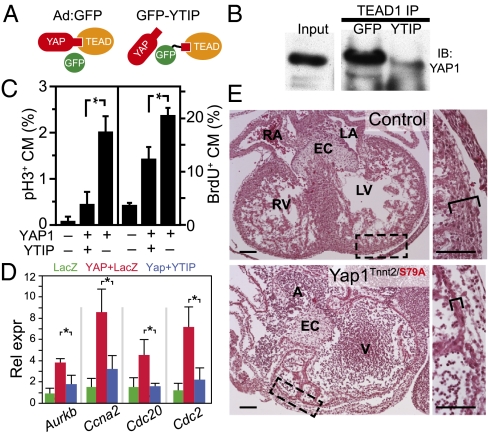
In many contexts, coactivator YAP1 binds to TEAD family factors to stimulate cell proliferation (2). However, in some cases YAP1 regulation of cell growth is independent of TEAD factors (8). To assess the role of YAP1–TEAD interactions in promoting cardiomyocyte proliferation, we expressed GFP fused to a region of YAP1 sufficient to bind TEAD (25). We reasoned that this peptide, corresponding to amino acid residues 47–155 of YAP1, would interfere with YAP1–TEAD interaction (Fig. 7A) and named the peptide YTIP (YAP1–TEAD Interfering Peptide). In coimmunoprecipitation experiments, we confirmed that GFP–YTIP impaired YAP1–TEAD1 interaction (Fig. 7B).
Fig. 7.
YAP1 stimulation of cardiomyocyte proliferation requires TEAD interaction. (A) YAP1-TEAD inhibitory peptide (YTIP) strategy. (B) Endogenous TEAD1 and YAP1 interaction was blocked by Ad:GFP–YTIP in MES13 cells. (C) aYAP1 stimulation of P4 neonatal cardiomyocyte proliferation was attenuated by GFP–YTIP, as measured by BrdU and pH3 immunostaining. *P < 0.01. n = 3. (D) Inhibition of TEAD interaction reduced expression of YAP1-activated cell-cycle genes, as measured by qRT-PCR. *P < 0.05. n = 3. (E) Cardiomyocyte YAP1S79A, deficient in TEAD interaction, did not support normal fetal myocardial growth. Yap1Tnnt2/S79A indicates the genotype Yap1fl/S79A::Tnnt2–Cre. H&E-stained sections of E12.5 heart are shown. (Right) Boxed areas are enlarged. Brackets indicate compact myocardial thickness. (Scale bars: 100 μm.)
We next measured the effect of GFP–YTIP on activated YAP1 stimulation of cardiomyocyte proliferation. GFP–YTIP strongly attenuated the stimulatory effect of activated YAP1 on proliferation of P4 neonatal cardiomyocytes, as measured by BrdU uptake and pH3 immunoreactivity (Fig. 7C). The antiproliferative effect of GFP–YTIP was paralleled by decreased activation of cell-cycle genes Cdk1 and Cyclin A2 by aYAP1 (Fig. 7D). These data indicate that YAP1 mitogenic activity in cardiomyocytes requires YAP1–TEAD interaction.
Substitution of YAP1 serine 79 by alanine selectively abolished TEAD interaction, and a Yap1S79A allele was unable to restore proliferation defects caused by ablation of wild-type Yap1 (11). We generated Yap1fl/S79A::Tnnt2–Cre embryos, in which only the mutant, and not the wild-type, YAP1 protein would be expressed in the heart. These mutant mice exhibited cardiomyocyte hypoplasia at least comparable in severity to that caused by cardiomyocyte Yap1 ablation (Fig. 7E). These data indicate that YAP1 stimulation of cardiomyocyte proliferation requires TEAD interaction, both in vitro and in vivo.
Discussion
Growth of the mammalian heart occurs through both cardiomyocyte proliferation and hypertrophy, with proliferation and hypertrophy primarily driving fetal and postnatal heart growth, respectively. YAP1 has been shown to promote cellular proliferation and organ growth in multiple systems in flies and mammals (2). Although YAP1 regulation of organ growth through control of cellular proliferation has been studied in depth, little has been reported about YAP1 regulation of hypertrophic organ growth. Our results indicate that YAP1 is both necessary and sufficient for fetal cardiomyocyte proliferation. However, YAP1 is neither necessary nor sufficient for postnatal hypertrophic growth of the heart. Thus, our data indicate that YAP1 regulates organ size predominantly by controlling cell number.
Normal heart development requires precisely regulated regional heart growth. Selective hypoplasia of heart structures are seen in congenital heart disease such as hypoplastic left heart syndrome. Our study showed that proper regulation of YAP1 activity is critical for normal growth of the fetal heart. Interestingly, overall size of the left ventricular chamber was more severely affected in some YAP1TNT loss-of-function mutants (e.g., Fig. 1C and Fig. S3D). Moreover, it is likely that YAP1 activity is regionally controlled, so that localized disruption of YAP1 regulation could lead to selective chamber hypoplasia. Thus, mutation of genes in the YAP pathway may participate in pathogenesis of congenital heart disease involving abnormalities of myocardial growth.
Myocardial trabeculation is essential for fetal heart growth and function. Cardiomyocytes in trabeculae exit the cell cycle through unknown mechanisms, suggesting that growth of trabeculae occurs from addition of cardiomyocytes from compact myocardium. Neuregulin signaling to ErbB2/ErbB4 is essential for myocardial trabeculation (26, 27), and recent studies suggest that ErbB2 acts by promoting compact myocardial proliferation and directional cardiomyocyte migration into trabeculae (28). Our data indicate that YAP1 is essential for regulating myocardial trabeculation, because trabeculation was reduced in YAP1TNT mutants. This reduction of trabeculation may be a consequence of reduced proliferation of cardiomyocytes in compact myocardium. YAP1 activation in cardiomyocytes prevented trabecular cardiomyocyte cell-cycle exit, suggesting that negative regulation of the YAP1 pathway is essential for limiting trabecular myocardial expansion. Interestingly, YAP1 activation also prevented expression of the trabecular cardiomyocyte marker Nppa, suggesting that YAP1-induced trabecular proliferation is linked to impaired differentiation. Abnormalities of YAP1 regulation may contribute to myocardial noncompaction, which is seen in some forms of human cardiomyopathy.
The Hippo pathway is the best-known regulator of YAP1 activity. However, these kinases have multiple downstream targets and influence diverse processes such as apoptosis and autophagy (29). Indeed, the roles of Hippo pathway kinases LATS and MST in regulating these later processes have been explored by cardiomyocyte-restricted transgenic gain and inhibition of function (30, 31). Recently, cardiac-restricted inactivation of the Hippo pathway components Sav1, Lats2, or Mst1/2 was shown to cause cardiac overgrowth (32). Thus, the findings of that study combined with those of the present study indicate that Hippo kinases restrain the proliferative activity of YAP1 on fetal cardiomyocytes.
Gene expression profiling showed that activated YAP1 potently and coordinately activated gene programs that promote cell-cycle activity. YAP1 activates transcription by binding to DNA-binding transcription factors, with TEAD1-4, orthologs of Drosophila Scalloped, being the best known (2). However, not all proliferative activities of YAP1 or its Drosophila ortholog Yorkie are mediated by TEAD/Scalloped. For example, Yorkie but not Scalloped, is required for growth of Drosophila imaginal discs (8). Using a dominant negative peptide that blocks YAP1–TEAD and a YAP1 point mutation-defective TEAD interaction, we showed that YAP1 stimulation of cardiomyocyte proliferation requires its interaction with TEAD.
Activated YAP1 potently promoted proliferation of fetal trabecular and neonatal ventricular cardiomyocytes that normally exit the cell cycle. These data show that activation of YAP1 can overcome signals that normally lead cardiomyocytes to exit the cell cycle. However, a limitation of the current study is that we cannot distinguish whether YAP1 is preventing cell-cycle withdrawal or reinitiating cell-cycle reentry. This limitation will need to be addressed further to test the hypothesis that activated YAP1 can induce cell-cycle reentry in adult, fully mature cardiomyocytes, with important potential ramifications for regenerative approaches to heart injury.
Materials and Methods
Please see SI Materials and Methods for details.
All animal procedures were approved by the Children's Hospital Boston Institutional Animal Care and Use Committee. TetO-YAP1 (10), Yap1flox (11), Yap1S79A (11), Rosa26mTmG (15), Rosa26fs-rtTA (33), and Tnnt2-Cre (13) alleles were described.
Tnnt2–Cre adenovirus was constructed using the rat Tnnt2 promoter (34) and the AdEasy system (Stratagene). 3xFlag–YAP1 adenovirus expressed S127A-mutated human YAP1 cDNA with an N-terminal triple FLAG epitope tag. The GFP–YTIP construct contained eGFP fused to residues 47–155 of human YAP1. Cesium chloride gradient-purified viruses were titered with the AdEasy titer kit (Stratagene). Retro-orbital adenoviral injection to neonatal pups was performed as described (17).
Fetal and neonatal rat cardiomyocyte culture was performed by using the Neomyts cardiomyocyte dissociation kit (Cellutron). Adult cardiomyocytes were isolated by antegrade collagenase perfusion and purified by differential centrifugation.
Antibody sources are listed in Table S1. Primers for quantitative RT-PCR are in Table S2. Total RNA was isolated by using the RNeasy kit (Qiagen) and hybridized to Affymetrix microarrays (Rat Gene 1.0 ST).
Results are expressed as mean ± SEM. Two group comparisons were performed using Student t test.
Supplementary Material
Acknowledgments
This work was supported by National Heart, Lung, and Blood Institute Grants HL095712 and HL100401 and by charitable contributions from Gail Federici Smith, Edward Marram, and Karen Carpenter.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE33019).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1116136109/-/DCSupplemental.
References
- 1.Ishiwata T, Nakazawa M, Pu WT, Tevosian SG, Izumo S. Developmental changes in ventricular diastolic function correlate with changes in ventricular myoarchitecture in normal mouse embryos. Circ Res. 2003;93:857–865. doi: 10.1161/01.RES.0000100389.57520.1A. [DOI] [PubMed] [Google Scholar]
- 2.Pan D. The hippo signaling pathway in development and cancer. Dev Cell. 2010;19:491–505. doi: 10.1016/j.devcel.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao B, Lei QY, Guan KL. The Hippo-YAP pathway: New connections between regulation of organ size and cancer. Curr Opin Cell Biol. 2008;20:638–646. doi: 10.1016/j.ceb.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harvey KF, Pfleger CM, Hariharan IK. The Drosophila Mst ortholog, hippo, restricts growth and cell proliferation and promotes apoptosis. Cell. 2003;114:457–467. doi: 10.1016/s0092-8674(03)00557-9. [DOI] [PubMed] [Google Scholar]
- 5.Lai ZC, et al. Control of cell proliferation and apoptosis by mob as tumor suppressor, mats. Cell. 2005;120:675–685. doi: 10.1016/j.cell.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 6.Pantalacci S, Tapon N, Léopold P. The Salvador partner Hippo promotes apoptosis and cell-cycle exit in Drosophila. Nat Cell Biol. 2003;5:921–927. doi: 10.1038/ncb1051. [DOI] [PubMed] [Google Scholar]
- 7.Wu S, Huang J, Dong J, Pan D. hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell. 2003;114:445–456. doi: 10.1016/s0092-8674(03)00549-x. [DOI] [PubMed] [Google Scholar]
- 8.Wu S, Liu Y, Zheng Y, Dong J, Pan D. The TEAD/TEF family protein Scalloped mediates transcriptional output of the Hippo growth-regulatory pathway. Dev Cell. 2008;14:388–398. doi: 10.1016/j.devcel.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 9.Zhang L, et al. The TEAD/TEF family of transcription factor Scalloped mediates Hippo signaling in organ size control. Dev Cell. 2008;14:377–387. doi: 10.1016/j.devcel.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Camargo FD, et al. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol. 2007;17:2054–2060. doi: 10.1016/j.cub.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 11.Schlegelmilch K, et al. Yap1 acts downstream of α-catenin to control epidermal proliferation. Cell. 2011;144:782–795. doi: 10.1016/j.cell.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong J, et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–1133. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiao K, et al. An essential role of Bmp4 in the atrioventricular septation of the mouse heart. Genes Dev. 2003;17:2362–2367. doi: 10.1101/gad.1124803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halder G, Johnson RL. Hippo signaling: Growth control and beyond. Development. 2011;138:9–22. doi: 10.1242/dev.045500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- 16.Li F, Wang X, Capasso JM, Gerdes AM. Rapid transition of cardiac myocytes from hyperplasia to hypertrophy during postnatal development. J Mol Cell Cardiol. 1996;28:1737–1746. doi: 10.1006/jmcc.1996.0163. [DOI] [PubMed] [Google Scholar]
- 17.Jerome LA, Papaioannou VE. DiGeorge syndrome phenotype in mice mutant for the T-box gene, Tbx1. Nat Genet. 2001;27:286–291. doi: 10.1038/85845. [DOI] [PubMed] [Google Scholar]
- 18.Zhao B, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Subramanian A, et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chaudhry HW, et al. Cyclin A2 mediates cardiomyocyte mitosis in the postmitotic myocardium. J Biol Chem. 2004;279:35858–35866. doi: 10.1074/jbc.M404975200. [DOI] [PubMed] [Google Scholar]
- 21.Cheng RK, et al. Cyclin A2 induces cardiac regeneration after myocardial infarction and prevents heart failure. Circ Res. 2007;100:1741–1748. doi: 10.1161/CIRCRESAHA.107.153544. [DOI] [PubMed] [Google Scholar]
- 22.Bicknell KA, Coxon CH, Brooks G. Forced expression of the cyclin B1-CDC2 complex induces proliferation in adult rat cardiomyocytes. Biochem J. 2004;382:411–416. doi: 10.1042/BJ20031481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pasumarthi KB, Nakajima H, Nakajima HO, Soonpaa MH, Field LJ. Targeted expression of cyclin D2 results in cardiomyocyte DNA synthesis and infarct regression in transgenic mice. Circ Res. 2005;96:110–118. doi: 10.1161/01.RES.0000152326.91223.4F. [DOI] [PubMed] [Google Scholar]
- 24.Soonpaa MH, et al. Cyclin D1 overexpression promotes cardiomyocyte DNA synthesis and multinucleation in transgenic mice. J Clin Invest. 1997;99:2644–2654. doi: 10.1172/JCI119453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vassilev A, Kaneko KJ, Shu H, Zhao Y, DePamphilis ML. TEAD/TEF transcription factors utilize the activation domain of YAP65, a Src/Yes-associated protein localized in the cytoplasm. Genes Dev. 2001;15:1229–1241. doi: 10.1101/gad.888601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee KF, et al. Requirement for neuregulin receptor erbB2 in neural and cardiac development. Nature. 1995;378:394–398. doi: 10.1038/378394a0. [DOI] [PubMed] [Google Scholar]
- 27.Meyer D, Birchmeier C. Multiple essential functions of neuregulin in development. Nature. 1995;378:386–390. doi: 10.1038/378386a0. [DOI] [PubMed] [Google Scholar]
- 28.Liu J, et al. A dual role for ErbB2 signaling in cardiac trabeculation. Development. 2010;137:3867–3875. doi: 10.1242/dev.053736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Radu M, Chernoff J. The DeMSTification of mammalian Ste20 kinases. Curr Biol. 2009;19:R421–R425. doi: 10.1016/j.cub.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto S, et al. Activation of Mst1 causes dilated cardiomyopathy by stimulating apoptosis without compensatory ventricular myocyte hypertrophy. J Clin Invest. 2003;111:1463–1474. doi: 10.1172/JCI17459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsui Y, et al. Lats2 is a negative regulator of myocyte size in the heart. Circ Res. 2008;103:1309–1318. doi: 10.1161/CIRCRESAHA.108.180042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heallen T, et al. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science. 2011;332:458–461. doi: 10.1126/science.1199010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Belteki G, et al. Conditional and inducible transgene expression in mice through the combinatorial use of Cre-mediated recombination and tetracycline induction. Nucleic Acids Res. 2005;33:e51. doi: 10.1093/nar/gni051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang G, Yeh HI, Lin JJ. Characterization of cis-regulating elements and trans-activating factors of the rat cardiac troponin T gene. J Biol Chem. 1994;269:30595–30603. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.