Abstract
Bdelloid rotifers, a class of freshwater invertebrates, are extraordinarily resistant to ionizing radiation (IR). Their radioresistance is not caused by reduced susceptibility to DNA double-strand breakage for IR makes double-strand breaks (DSBs) in bdelloids with essentially the same efficiency as in other species, regardless of radiosensitivity. Instead, we find that the bdelloid Adineta vaga is far more resistant to IR-induced protein carbonylation than is the much more radiosensitive nematode Caenorhabditis elegans. In both species, the dose–response for protein carbonylation parallels that for fecundity reduction, manifested as embryonic death. We conclude that the great radioresistance of bdelloid rotifers is a consequence of an unusually effective system of anti-oxidant protection of cellular constituents, including those required for DSB repair, allowing bdelloids to recover and continue reproducing after doses of IR causing hundreds of DSBs per nucleus. Bdelloid rotifers therefore offer an advantageous system for investigation of enhanced anti-oxidant protection and its consequences in animal systems.
Keywords: cell death, Deinococcus radiodurans, desiccation, DNA breakage
Bdelloid rotifers, small freshwater invertebrates known for their remarkable ability to survive and resume reproduction after desiccation at any life stage are also extraordinarily resistant to ionizing radiation (1, 2). The association of radioresistance with resistance to desiccation, seen also in Deinococcus radiodurans and other desiccation-resistant bacteria (3), may be explained as an evolutionary adaptation to survive exposure to oxidative molecular species generated by the disruption of electron-transport chains during desiccation (4, 5). The radioresistance of D. radiodurans has been variously ascribed to an unusual chromosome alignment that keeps homologous regions together (6), a special mechanism for the repair of double-strand breaks (DSBs) in DNA (7), and protection of protein against radiolytically produced oxidative species (8). Here we show (i) that the principal factor responsible for the extreme radioresistance of bdelloid rotifers is their possession of a greatly enhanced system of anti-oxidant protection as assayed by protection against ionizing radiation (IR)-induced protein carbonylation and (ii) that irradiation of bdelloid oocytes and Caenorhabditis elegans germline mitotic cells results not in their own death but rather in the death of the embryos descended from them.
Results and Discussion
Radioresistance and Protection Against Protein Carbonylation.
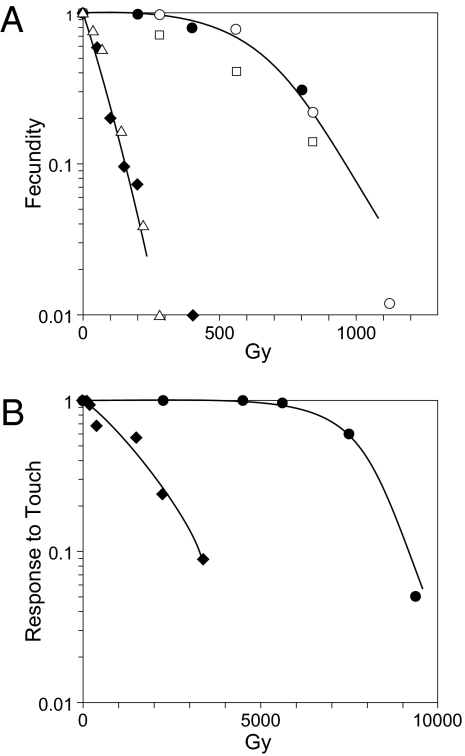
The extreme radioresistance of bdelloid rotifers, greater than that known for any other animals, is exemplified by the dose–response for fecundity reduction in the bdelloids Adineta vaga and Philodina roseola compared with that for the nematode C. elegans and the monogonont rotifer Euchlanis dilatata (Fig. 1A). Similarly, the ability to respond to touch, which is much more radioresistant than reproduction, is more radioresistant in A. vaga than in C. elegans (Fig. 1B).
Fig. 1.
(A) Dose–response for fecundity. ●, A. vaga; ◆, C. elegans. Dose rate was 26–38 Gy/min. ○, A. vaga; □, P. roseola; △, E. dilatata. Dose rate was 2.3 Gy/min; data represented by unfilled symbols are from ref. 1. Curve is drawn to fit data for A. vaga. (B) Dose–response for motion in response to touch 24 h after irradiation. Dose rate was 38 Gy/min.
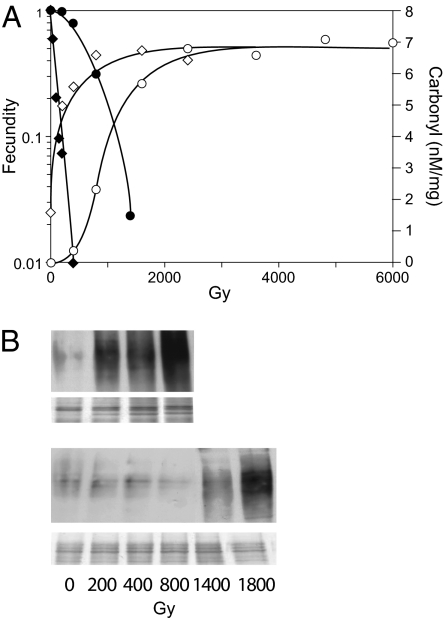
The cause of bdelloid radioresistance is not to be sought in reduced susceptibility to IR-induced DNA breakage or in an unusually small genome because γ-radiation produces essentially the same number of DSBs per megabase per gray (∼0.005 Gy−1Mb−1) in A. vaga as in other eukaryotes, and its genome is not smaller than that of C. elegans (1). Instead, it has been proposed that a major contributor to bdelloid radiation resistance is an enhanced capacity for scavenging reactive molecular species generated by IR and that the proteins and other cellular components thereby protected include those essential for the repair of DSBs but not DNA itself (1). In agreement with this explanation, we find that A. vaga is far more resistant than C. elegans to IR-induced protein carbonylation, a reaction of hydroxyl radicals with accessible side chains of certain amino acids (9, 10), and that the dose–response for fecundity reduction is paralleled by that for carbonylation in both species (Fig. 2 A and B). Thus, the pronounced shoulder in the dose–response for bdelloid fecundity is accompanied by a corresponding lag in carbonylation whereas the nematode displays little if any shoulder or lag and the sharp decrease in its fecundity with dose is accompanied by a correspondingly steep rise in carbonylation. At the highest doses, carbonylation reaches a plateau that is the same in both species (Figs. 2B and 3), indicating that the difference in their susceptibility to IR-induced carbonylation is not caused by a difference of their proteins but results instead from greater protection against carbonylation in the bdelloid.
Fig. 2.
(A) Dose–response for fecundity and protein carbonylation assayed by ELISA. Dose rate was 26–38 Gy/min. Fecundity: ●, A. vaga; ◆, C. elegans. Carbonylation: ○, A. vaga; ◇, C. elegans. The indicated degree of carbonylation was obtained by comparison with the provided albumin standards and should be regarded as only proportional to the actual value because the composition of the experimental samples differs from that of the standard. No difference in carbonylation is found between extracts made soon after irradiation and extracts from animals kept on ice an additional 3 h, whereas, if kept 3 h at room temperature, nearly half of the carbonylated protein is lost. It may therefore be concluded that proteolysis of carbonylated protein during irradiation is negligible. (B) Dose dependence for protein carbonylation visualized on Western blots with silver-stain loading controls beneath.
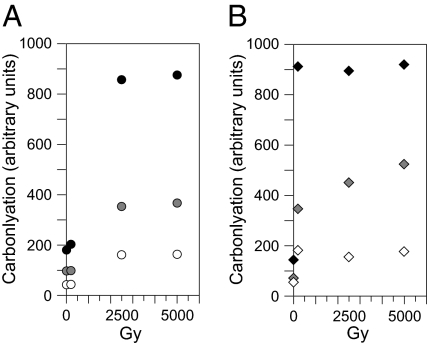
Fig. 3.
Saturation of IR-induced protein carbonylation at high dose observed by Western blotting. (A) A. vaga. (B) C. elegans. Relative amounts of extract assayed: 1, solid symbols; 1/2, shaded symbols; 1/4, open symbols. Doses: 0, 200, 2,500 and 5,000 Gy. The observation of plateaus by quantitative Western blotting shows that they are not an artifact peculiar to OxyELISA.
Not only does protein carbonylation parallel fecundity reduction over the dose range in which fecundity was measured, it further appears that the quantitative relation between IR-induced carbonylation and fecundity reduction is approximately the same in A. vaga and C. elegans. Thus, a 10-fold reduction in fecundity is accompanied by a like degree of IR-induced carbonylation in both species (Fig. 2A). Similarly, the quantitative relation between carbonylation and colony-forming ability in the radioresistant bacteria D. radiodurans is the same as that for Escherichia coli (11). The dose–response for reproductive killing is therefore predictable from that for protein carbonylation in both cases, implying that essentially all of the enhanced radioresistance of A. vaga and D. radiodurans may be accounted for by their high levels of anti-oxidant protection and that no unrelated factor such as an unusual mechanism of DSB repair (7) makes a significant contribution.
Nature of the Bdelloid Anti-Oxidant System.
Although the biochemical identity of the bdelloid anti-oxidant system remains to be established and may or may not be like that of D. radiodurans (11, 12), some of its features may be specified on the basis of present knowledge: (i) As irradiation was done on ice, the system is present constitutively, consistent with the lack of a dose-rate effect for fecundity reduction (Fig. 1A). (ii) The steep rise in A. vaga carbonylation beyond a dose of ∼400 Gy indicates that the system is largely depleted beyond that dose. (iii) The lack of any evident lag in C. elegans carbonylation indicates that the relevant anti-oxidant capacity of the bdelliod is much greater than that of the nematode. (iv) The anti-oxidant system is unable to protect DNA against breakage (1), consistent with electron track modeling showing that the compaction of chromatin in chromosomes renders DNA inaccessible to hydroxyl radicals radiolytically produced in the surrounding medium, leaving direct energy deposition in DNA as the principal cause of DSBs (13) and accounting for the uniform susceptibility of chromosomal DNA to IR-induced breakage across a diversity of species, regardless of their radiosensitivity.
Delayed IR-Induced Killing and the Involvement of DNA Damage in IR-Induced Cell Killing.
Even at the highest doses at which fecundity was measured, irradiation had no effect on the number of eggs deposited by A. vaga (1) and no more than a modest effect, if any, on the number of eggs deposited by C. elegans, but instead gave rise to eggs that failed to hatch. Unlike the embryonic structures seen in non-irradiated eggs, such eggs contain numerous cells but no recognizable embryonic structures although, in the case of C. elegans, there is a fertilization envelope, indicating sperm penetration. Thus, IR reduces fecundity not by killing the germline cells present at the time of irradiation—primary oocytes in A. vaga and premeiotic cells in C. elegans—but rather by causing lethality in the embryos descended from them. Such delayed reproductive death is distinct from the apoptotic death of C. elegans oocytes irradiated at pachtytene (14). Instead, irradiation of premeiotic C. elegans germ cells and somatic cells causes cell cycle delay followed by necrotic death generations later, resembling the IR-induced clonogenic death of mammalian cells (15, 16). An extreme example of IR-induced delayed reproductive death occurs in bdelloid and monogonont rotifers where a dose of IR causing a given reduction in fecundity of irradiated parents causes nearly the same reduction in the fecundity of their progeny (1).
The delayed manifestation of IR-induced lethality, especially across generations, must mean that there are IR-induced alterations with the ability to replicate or to initiate self-sustaining processes that persist through numerous germline and somatic cell divisions, including meiosis in the nematode (the bdelloid being ameiotic), eventually causing lethality in a dose-dependent manner. Diverse mechanisms, some requiring DNA breakage and some not, have been proposed to explain IR-induced delayed reproductive lethality. Those requiring DNA breakage include progressively increasing aneuploidy and gene loss via the breakage-fusion-bridge cycle or, for holocentrics, via recurring mis-segregation of chromosome fragments (17). Certainly, IR-induced DNA breakage can kill cells without any requirement for damaged protein. This is seen, for example, in yeast, where G1 haploids, lacking homologous templates for DSB repair, are far more radiosensitive than diploids (18) even though a haploid lethal dose must cause correspondingly less damage to protein. Moreover, there appear to be no species so radiosensitive, not even the extremely radiosensitive anaerobic bacterium Shewanella oneidensis, that it is killed by a dose clearly too small to cause at least one DSB (19). Nor is it ruled out that DNA breakage is required to cause the delayed reproductive death of non-irradiated cells in the presence of irradiated cells—the bystander effect (20).
Thus, in the present case, IR-induced cell killing may require DNA breakage, and the correlation with protein carbonylation may simply reflect the inactivation of cellular components required for successful DNA repair. There is, however, a necessary qualification. Consider, for example, the case in which fecundity is reduced 10-fold in both A. vaga and C. elegans. Although the degree of IR-induced carbonylation is essentially the same for both species, there will be many more DSBs in the bdelloid. Equal survival in the two cases then requires that A. vaga be able to repair a large number of DSBs with the same probability as that with which C. elegans repairs a much smaller number, possibly through the operation of checkpoints in both species that delay the cell cycle until repair is complete (14). The decrease of fecundity with dose would then be caused not by the increasing number of initial DSBs, but rather by the increasing likelihood that the DNA repair system is inactivated. The situation also arises in bacteria where, as mentioned above, the degree of IR-induced protein carbonylation bears the same quantitative relation to survival in both D. radiodurans and E. coli (11), raising the question of whether bacteria have such checkpoints (21).
In some cases, however, IR-induced delayed reproductive death may depend on heritable effects not requiring DNA breakage (22). Effects that have been proposed in this regard include altered gene expression resulting from aberrant DNA cytosine methylation (23), centrosome amplification leading to multipolar mitosis (24), and self-sustaining production of hydrogen peroxide and elevated oxidative stress associated with mitochondrial dysfunction (25). Indeed, the question of how IR kills cells, with its important implications for radiotherapy, is a matter of continuing debate (26, 27). The parallel that we find between radiosensitivity and anti-oxidant status may well extend to diverse animal species and cell types. Bdelloid rotifers, with their greatly enhanced anti-oxidant status, therefore provide a unique system for investigation of the consequences of oxidative damage and anti-oxidant protection in animal systems generally.
Materials and Methods
A. vaga (gift of Claudia Ricci, University of Milan, Milan, Italy) was cultured at 25 °C in spring water with E. coli grown in LB broth and harvested as described (1), washed with spring water on a 10-μM Nitex screen under mild vacuum, and rinsed with 2 mL of spring water supplemented with 1.25 mM KH2PO4/K2HPO4 (pH 6), 0.05 mM M MgSO4 into 2-cm plastic petri dishes for irradiation. Rotifers from each dose group were individually placed in separate wells of a 96-well microtiter dish with a small amount of E. coli and kept 10 d to allow completion of egg deposition. Hatchlings, identified by their small size, were counted and removed daily. Dead parents and dead hatchlings were rare, and the number of eggs produced (hatchlings plus unhatched eggs) was independent of dose. Relative fecundity at each dose was taken as the total number of hatchlings normalized to the number produced by nonirradiated animals. The mean number of eggs deposited per animal was 12.
C. elegans wild-type N2 was cultured at 25 °C on nematode growth medium (NGM) agar plates seeded with E. coli OP50 grown in LB broth. Young (nongravid) L4 larvae from age-synchronized cultures were rinsed three times by gravity sedimentation through spring water supplemented with 1.25 mM KH2PO4/K2HPO4 (pH 6), 0.05 mM MgSO4, transferred to microcentrifuge tubes containing 2 mL of the same solution, and irradiated. For measuring fecundity, nematodes from each dose group were poured onto nonseeded NGM plates, individually transferred to wells of 12-well dishes containing agar seeded with E. coli, and removed after 24 h, at which time nearly all animals were alive with no indication of excess lethality up to 800 Gy, the highest dose at which C. elegans fecundity was measured. Live larvae were counted in each well after an additional 24 h. Relative fecundity at each dose was determined from cohorts of 12–48 animals in three separate experiments as the average of the number of live larvae produced per live parent, normalized to the number produced at zero dose. Dead larvae and unfertilized eggs (oocysts) were rare, and their frequency was unrelated to dose. In contrast, the number of unhatched eggs increased with dose and accounted for most of the decrease in live larvae. The principal effect of irradiation was therefore to prevent normal development and hatching of deposited eggs rather than to decrease the total number deposited. The mean number of live larvae produced by non-irradiated animals was 71.
Irradiation was done on ice with a 137Cs source delivering 26–38 Gy/min, depending on the sample position. Protein carbonylation was determined by ELISA (OxyELISA, Millipore) and by Western blotting (OxyBlot, Millipore). For carbonylation assays, ∼1,000 animals were centrifuged for 15 min at 4,000 × g, resuspended in 150 μL PBS (OxyBlot) or the provided lysis buffer (OxyELISA), in both cases supplemented with one mini tablet of Roche Complete Protease Inhibitor without EDTA per 10 mL, followed by two liquid nitrogen freeze–thaw cycles, homogenization in a microtissue grinder, and centrifugation 20 min at 12,000 × g. The supernatant was diluted 10-fold or more with PBS without protease inhibitor to a protein concentration of 10 μg/mL (Lowry). All steps up to this point were done on ice or at 4 °C. Samples were then supplemented with 10 mg/100 μL lipid removal agent (Sigma; 13360-U), kept 1 h at room temperature, and centrifuged 15 min at 10,000 × g. For Western blots, the supernatant was derivatized with 2,4-dinitrophenylhydrazine (DNPH), run on SDS/PAGE gels, and the derivatized proteins were transferred to PVDF membranes and treated with the provided primary and secondary antibodies as specified in the Millipore OxyBlot Protein Oxidation Dectection Kit. Carbonylated protein was visualized on autoradiographic film using the Amersham ECL Advance chemiluminescence detection system (Fig. 2B). Relative values of protein carbonylation (Fig. 3) were obtained by scanning comparable sections of individual lanes with an HP Scan Jet model 4850, followed by background subtraction and analysis with Image J software (28). To verify that equal amounts of protein were run in each lane, half of each derivatized sample was run in parallel on SDS/PAGE gel followed by silver staining. For OxyELISA, the 10,000 g supernate was assayed for carbonylated protein as described in the Millipore OxyELISA Oxidized Protein Quantitation kit, and values of nM carbonyl per mg protein (Fig. 2A) were obtained by comparison with a calibration series using the provided albumin standards.
Acknowledgments
Jacqueline Brooks generously rendered advice and assistance. This work was supported by the Ellison Medical Foundation and the National Science Foundation (M.M.), the Nestlé Foundation (M.L.), and the Institut National de la Santé et de la Recherche Médicale (A.K. and M.R.).
Footnotes
The authors declare no conflict of interest.
References
- 1.Gladyshev E, Meselson M. Extreme resistance of bdelloid rotifers to ionizing radiation. Proc Natl Acad Sci USA. 2008;105:5139–5144. doi: 10.1073/pnas.0800966105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mark Welch DB, Ricci C, Meselson M. Bdelloid rotifers: Progress in understanding the success of an evolutionary scandal. In: Schön I, Martens K, van Dijk P, editors. Lost Sex: The Evolutionary Biology of Parthenogenesis. New York: Springer; 2009. pp. 259–279. [Google Scholar]
- 3.Fredrickson JK, et al. Protein oxidation: Key to bacterial desiccation resistance? ISME J. 2008;2:393–403. doi: 10.1038/ismej.2007.116. [DOI] [PubMed] [Google Scholar]
- 4.França MB, Panek AD, Eleutherio ECA. Oxidative stress and its effects during dehydration. Comp Biochem Physiol A Mol Integr Physiol. 2007;146:621–631. doi: 10.1016/j.cbpa.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 5.Leprince O, Atherton NM, Deltour R, Hendry GAF. The involvement of respiration in free-radical processes during loss of desiccation tolerance in germinating Zea mays L. Plant Physiol. 1994;104:1333–1339. doi: 10.1104/pp.104.4.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daly MJ, Minton KW. Resistance to radiation. Science. 1995;270:1318. doi: 10.1126/science.270.5240.1318. [DOI] [PubMed] [Google Scholar]
- 7.Zahradka K, et al. Reassembly of shattered chromosomes in Deinococcus radiodurans. Nature. 2006;443:569–573. doi: 10.1038/nature05160. [DOI] [PubMed] [Google Scholar]
- 8.Daly MJ, et al. Protein oxidation implicated as the primary determinant of bacterial radioresistance. PLoS Biol. 2007;5:e92. doi: 10.1371/journal.pbio.0050092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu G, Chance MR. Radiolytic modification and reactivity of amino acid residues serving as structural probes for protein footprinting. Anal Chem. 2005;77:4549–4555. doi: 10.1021/ac050299+. [DOI] [PubMed] [Google Scholar]
- 10.Stadtman ER. Oxidation of free amino acids and amino acid residues in proteins by radiolysis and by metal-catalyzed reactions. Annu Rev Biochem. 1993;62:797–821. doi: 10.1146/annurev.bi.62.070193.004053. [DOI] [PubMed] [Google Scholar]
- 11.Krisko A, Radman M. Protein damage and death by radiation in Escherichia coli and Deinococcus radiodurans. Proc Natl Acad Sci USA. 2010;107:14373–14377. doi: 10.1073/pnas.1009312107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daly MJ, et al. Small-molecule antioxidant proteome-shields in Deinococcus radiodurans. PLoS ONE. 2010;5:e12570. doi: 10.1371/journal.pone.0012570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valota A, et al. Modelling study on the protective role of OH radical scavengers and DNA higher-order structures in induction of single- and double-strand break by gamma-radiation. Int J Radiat Biol. 2003;79:643–653. doi: 10.1080/09553000310001596977. [DOI] [PubMed] [Google Scholar]
- 14.Gartner A, Milstein S, Ahmed S, Hodgkin J, Hengartner MO. A conserved checkpoint pathway mediates DNA damage-induced apoptosis and cell cycle arrest in C. elegans. Mol Cell. 2000;5:435–443. doi: 10.1016/s1097-2765(00)80438-4. [DOI] [PubMed] [Google Scholar]
- 15.Weidhaas JB, Eisenmann DM, Holub JM, Nallur SV. A Caenorhabditis elegans tissue model of radiation-induced reproductive cell death. Proc Natl Acad Sci USA. 2006;103:9946–9951. doi: 10.1073/pnas.0603791103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Puck TT, Marcus PI. Action of x-rays on mammalian cells. J Exp Med. 1956;103:653–666. doi: 10.1084/jem.103.5.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carpenter JE, Bloem S, Marek F. Inherited sterility in insects. In: Dyck VA, Hendrichs J, Robinson AS, editors. Sterile Insect Technique. Berlin; Heidelberg; London; New York: Springer; 2005. pp. 115–146. [Google Scholar]
- 18.Mortimer RK. Radiobiological and genetic studies on a polyploid series (haploid to hexaploid) of Saccharomyces cerevisiae. Radiat Res. 1958;9:312–326. [PubMed] [Google Scholar]
- 19.Daly MJ, et al. Accumulation of Mn(II) in Deinococcus radiodurans facilitates gamma-radiation resistance. Science. 2004;306:1025–1028. doi: 10.1126/science.1103185. [DOI] [PubMed] [Google Scholar]
- 20.Mothersill C, Seymour CB. Radiation-induced bystander effects and the DNA paradigm: An “out of field” perspective. Mutat Res. 2006;597:5–10. doi: 10.1016/j.mrfmmm.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 21.Autret S, Levine A, Holland IB, Séror SJ. Cell cycle checkpoints in bacteria. Biochimie. 1997;79:549–554. doi: 10.1016/s0300-9084(97)82002-0. [DOI] [PubMed] [Google Scholar]
- 22.Aypar U, Morgan WF, Baulch JE. Radiation-induced genomic instability: Are epigenetic mechanisms the missing link? Int J Radiat Biol. 2011;87:179–191. doi: 10.3109/09553002.2010.522686. [DOI] [PubMed] [Google Scholar]
- 23.Rugo RE, et al. Methyltransferases mediate cell memory of a genotoxic insult. Oncogene. 2011;30:751–756. doi: 10.1038/onc.2010.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inanç B, Dodson H, Morrison CG. A centrosome-autonomous signal that involves centriole disengagement permits centrosome duplication in G2 phase after DNA damage. Mol Biol Cell. 2010;21:3866–3877. doi: 10.1091/mbc.E10-02-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dayal D, et al. Mitochondrial complex II dysfunction can contribute significantly to genomic instability after exposure to ionizing radiation. Radiat Res. 2009;172:737–745. doi: 10.1667/RR1617.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daly MJ. A new perspective on radiation resistance based on Deinococcus radiodurans. Nat Rev Microbiol. 2009;7:237–245. doi: 10.1038/nrmicro2073. [DOI] [PubMed] [Google Scholar]
- 27.Brown M, Wilson G. Apoptosis genes and resistance to cancer therapy: What does the experimental and clinical data tell us? Cancer Biol Ther. 2003;2:477–490. doi: 10.4161/cbt.2.5.450. [DOI] [PubMed] [Google Scholar]
- 28.Collins TJ. ImageJ for microscopy. Biotechniques. 2007;43(1, Suppl):25–30. doi: 10.2144/000112517. [DOI] [PubMed] [Google Scholar]