Abstract
The Mesozoic is marked by several widespread occurrences of intense organic matter burial. Sediments from the largest of these events, the Cenomanian–Turonian Oceanic Anoxic Event (OAE 2) are characterized by lower nitrogen isotope ratios than are seen in modern marine settings. It has remained a challenge to describe a nitrogen cycle that could achieve such isotopic depletion. Here we use nitrogen-isotope ratios of porphyrins to show that eukaryotes contributed the quantitative majority of export production throughout OAE 2, whereas cyanobacteria contributed on average approximately 20%. Such data require that any explanation for the OAE nitrogen cycle and its isotopic values be consistent with a eukaryote-dominated ecosystem. Our results agree with models that suggest the OAEs were high-productivity events, supported by vigorous upwelling. Upwelling of anoxic deep waters would have supplied reduced N species (i.e., 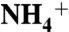 ) to primary producers. We propose that new production during OAE 2 primarily was driven by direct
) to primary producers. We propose that new production during OAE 2 primarily was driven by direct 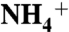 -assimilation supplemented by diazotrophy, whereas chemocline denitrification and anammox quantitatively consumed
-assimilation supplemented by diazotrophy, whereas chemocline denitrification and anammox quantitatively consumed 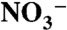 and
and  . A marine nitrogen reservoir dominated by
. A marine nitrogen reservoir dominated by 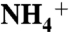 , in combination with known kinetic isotope effects, could lead to eukaryotic biomass depleted in 15N.
, in combination with known kinetic isotope effects, could lead to eukaryotic biomass depleted in 15N.
Keywords: biomarkers, nitrogen fixation, stable isotopes, paleoceanography
Mid-Cretaceous episodes of deposition of organic-rich sediments in the proto-Atlantic and Western Tethys basins known as Oceanic Anoxic Events (OAEs) (1) are attributed to high productivity and/or enhanced organic-matter preservation resulting from increases in nutrient supply and/or decreases in the ventilation of deep waters (2–6). Because OAEs are thought to be associated with enhanced CO2 outgassing during emplacement of large igneous provinces (7, 8), understanding the feedbacks between CO2, anoxia, and nutrient availability may help us understand better the effects of anthropogenic climate change on ocean circulation, oxygen balance, and marine ecology (9).
Basinal anoxia during OAEs would have promoted loss of fixed nitrogen through the processes of denitrification and anammox. The resulting nitrogen deficits in waters returning to the surface via upwelling would have been amended by nitrogen-fixing cyanobacteria, assuming iron and other micronutrients were adequately available (10). Indeed, enhancement of cyanobacterial production during many episodes of ocean anoxia has been proposed based on increased burial of 2-methylhopanoids (11–13), as these compounds are thought to be markers for cyanobacteria (14). Because such biomarker indices are only qualitative indicators of change and cannot provide quantitative estimates of export flux, complementary data generally include isotope ratios of total sedimentary nitrogen (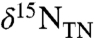 ) (11, 15–17), as diazotrophy also affects the nitrogen isotopic budget of the ocean (18–20).
) (11, 15–17), as diazotrophy also affects the nitrogen isotopic budget of the ocean (18–20).
The modern ocean has several localized regions of anoxia, and in these regions, values of 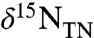 generally are higher than the present deep-water average
generally are higher than the present deep-water average 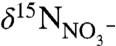 value of +5‰ because of the isotopic fractionation of denitrification expressed in the water column (19, 21, 22). In contrast, sediments from OAE 2 record striking nitrogen isotopic depletion. They are characterized by values of
value of +5‰ because of the isotopic fractionation of denitrification expressed in the water column (19, 21, 22). In contrast, sediments from OAE 2 record striking nitrogen isotopic depletion. They are characterized by values of 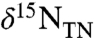 consistently < -1‰, and often < -3‰ (11, 16, 17, 23). Expression of these negative values of
consistently < -1‰, and often < -3‰ (11, 16, 17, 23). Expression of these negative values of 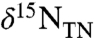 varies consistently by depositional location, with the average value of
varies consistently by depositional location, with the average value of 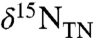 for OAE 2 horizons of the Bonarelli section (Gubbio and Furlo, Italy) being -3.3‰ and the South Ferriby formation (England) being -2.8‰ (16); whereas the average value for the South Atlantic is -1.9‰ (23), for the proto-North Atlantic is -1.8‰ (11, 17, this work), and for the Tarfaya Basin, Morocco is -1.7‰ (between 45–60 m in section) (16). Such differences thus reflect regional heterogeneity of water masses, phototroph ecology, and/or nutrient biogeochemistry; and it has been suggested that patterns of intrabasinal upwelling intensity and nutrient concentrations correspond directly to regional patterns of sedimentation (8, 23, 24).
for OAE 2 horizons of the Bonarelli section (Gubbio and Furlo, Italy) being -3.3‰ and the South Ferriby formation (England) being -2.8‰ (16); whereas the average value for the South Atlantic is -1.9‰ (23), for the proto-North Atlantic is -1.8‰ (11, 17, this work), and for the Tarfaya Basin, Morocco is -1.7‰ (between 45–60 m in section) (16). Such differences thus reflect regional heterogeneity of water masses, phototroph ecology, and/or nutrient biogeochemistry; and it has been suggested that patterns of intrabasinal upwelling intensity and nutrient concentrations correspond directly to regional patterns of sedimentation (8, 23, 24).
When viewed alongside the elevated 2-methylhopanoid ratios, negative values of δ15N have been interpreted as evidence for diazotrophic rebalancing of the nitrogen budget and cyanobacterial dominance of the nitrogen supply for new (export) primary production (6, 11, 15, 25, 26). However, the minimum value of δ15N for the biomass of marine diazotrophs ( ) should be on average approximately -1.3‰, based on the fractionation associated with nitrogenase (εfix = 0-2‰) and the δ15N value of dissolved N2 in seawater (approximately +0.7‰). This number is supported by data that consistently show N-fixing cyanobacteria to have values of
) should be on average approximately -1.3‰, based on the fractionation associated with nitrogenase (εfix = 0-2‰) and the δ15N value of dissolved N2 in seawater (approximately +0.7‰). This number is supported by data that consistently show N-fixing cyanobacteria to have values of 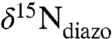 between 0.5‰ and -2‰ (average -1.4 ± 0.9‰) (25, 27–35). Reports of values significantly < -2‰ are from a cultured Trichodesmium sp. (-3.5‰) that was more negative than field samples collected in situ by the same investigators (32) and from experiments on N2-sparged Anabaena spp. (-2.4‰) grown in an artificial-seawater medium (ASP-2) that also contained
between 0.5‰ and -2‰ (average -1.4 ± 0.9‰) (25, 27–35). Reports of values significantly < -2‰ are from a cultured Trichodesmium sp. (-3.5‰) that was more negative than field samples collected in situ by the same investigators (32) and from experiments on N2-sparged Anabaena spp. (-2.4‰) grown in an artificial-seawater medium (ASP-2) that also contained 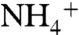 (31). Non-N2-derived N in the culture media may explain these outliers. Given the likelihood that in situ values of
(31). Non-N2-derived N in the culture media may explain these outliers. Given the likelihood that in situ values of 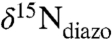 would average approximately -1‰, the prevalence of sedimentary values lower than -2‰ in many OAE sections cannot be explained solely by N supplied via N fixation. These patterns require that additional N-cycling processes be invoked to explain the source of nitrogen driving primary production during OAEs.
would average approximately -1‰, the prevalence of sedimentary values lower than -2‰ in many OAE sections cannot be explained solely by N supplied via N fixation. These patterns require that additional N-cycling processes be invoked to explain the source of nitrogen driving primary production during OAEs.
Nitrogen Isotopic Records of Sediments, Porphyrins, and Kerogen
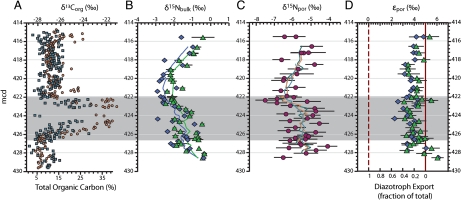
Chlorophyll-derived sedimentary porphyrins can be used to generate records of δ15N values of eukaryotic and prokaryotic phytoplankton that are unaffected by diagenesis (36, 37), as well as to estimate the contribution of cyanobacteria to burial flux (38). We examined nitrogen cycling during OAE 2 using measurements of coeval bulk and porphyrin nitrogen isotopes in sediments from the Ocean Drilling Program Leg 207, Site 1258A (Demerara Rise). A well-defined positive carbon isotope excursion in this section is contained within the high total organic carbon (TOC) interval commonly associated with the OAE (39) (Fig. 1A). Values of 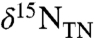 (Fig. 1B) from before the OAE through the first two-thirds of the OAE (428.5–423 m composite depth; mcd) decrease from approximately -0.1‰ to approximately -1.4‰ and fluctuate with greater variance than in the middle and top intervals (p < 0.05). The middle of the analyzed section (423 to 419.5 mcd), which spans the end of the OAE as defined by
(Fig. 1B) from before the OAE through the first two-thirds of the OAE (428.5–423 m composite depth; mcd) decrease from approximately -0.1‰ to approximately -1.4‰ and fluctuate with greater variance than in the middle and top intervals (p < 0.05). The middle of the analyzed section (423 to 419.5 mcd), which spans the end of the OAE as defined by  , is characterized by stable
, is characterized by stable 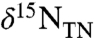 values of -2.1 ± 0.3‰. The top section (419.5 to 415.5 mcd) is characterized by an increase in
values of -2.1 ± 0.3‰. The top section (419.5 to 415.5 mcd) is characterized by an increase in 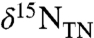 values, returning to -0.7‰ in the uppermost samples. All of these values are lower than the δ15N minima that are observed in modern sediments, even in regions underlying zones of water-column anoxia or intense nitrogen fixation (40, 41). Because diagenesis and interstitial
values, returning to -0.7‰ in the uppermost samples. All of these values are lower than the δ15N minima that are observed in modern sediments, even in regions underlying zones of water-column anoxia or intense nitrogen fixation (40, 41). Because diagenesis and interstitial 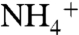 in clays can shift values of
in clays can shift values of 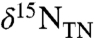 (18, 42), we also measured δ15N values of kerogen. They show an average negative offset of -0.4‰ relative to bulk sediment (Fig. 1B), suggesting the original primary producers had even lower δ15N values than what remains recorded by values of
(18, 42), we also measured δ15N values of kerogen. They show an average negative offset of -0.4‰ relative to bulk sediment (Fig. 1B), suggesting the original primary producers had even lower δ15N values than what remains recorded by values of 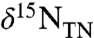 .
.
Fig. 1.
Elemental and isotopic data for site 1258A. The shaded bar represents the 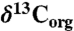 excursion interval that defines the OAE. (A) %TOC (squares) and
excursion interval that defines the OAE. (A) %TOC (squares) and 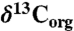 (circles) from (39). (B) δ15N values of bulk sediment (triangles) and kerogen (diamonds), and their 1-m averaged trends. (C) Porphyrin δ15N values, and their 3- (green), 5- (tan), and 9- (blue) point averaged trends. (D) The isotopic offset εpor between bulk sediment and porphyrins (triangles), and kerogen and porphyrins (diamonds), as well as the corresponding fraction of cyanobacterial export based on the endmember values described in the text. The solid vertical line represents a typical algal value of εpor, and the dotted vertical line represents a marine cyanobacterial value of εpor (38). All error bars represent 1σ, and preparative and analytical errors are compounded when possible. Raw data are shown in Table S1.
(circles) from (39). (B) δ15N values of bulk sediment (triangles) and kerogen (diamonds), and their 1-m averaged trends. (C) Porphyrin δ15N values, and their 3- (green), 5- (tan), and 9- (blue) point averaged trends. (D) The isotopic offset εpor between bulk sediment and porphyrins (triangles), and kerogen and porphyrins (diamonds), as well as the corresponding fraction of cyanobacterial export based on the endmember values described in the text. The solid vertical line represents a typical algal value of εpor, and the dotted vertical line represents a marine cyanobacterial value of εpor (38). All error bars represent 1σ, and preparative and analytical errors are compounded when possible. Raw data are shown in Table S1.
Porphyrin values of δ15N (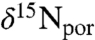 ) also show patterns that are similar to bulk N isotopes, although they exhibit more scatter than the
) also show patterns that are similar to bulk N isotopes, although they exhibit more scatter than the 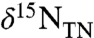 and
and 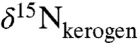 values. The large error ranges are due to full propagation of analytical uncertainty associated with preparation and analysis by the denitrifier method (43). To overcome the scatter, we plotted 3-, 5-, and 9-point moving averages (Fig. 1C). These different resolutions all show similar patterns, indicating that temporal trends in the results are not sensitive to the degree of smoothing and are not dependent on data density. The data are spaced relatively uniformly (0.5 m), although sampling resolution is higher in some horizons surrounding the excursion interval, from 421.9 to 427.5 mcd (0.2 m). The duration of OAE 2 has been estimated to be approximately 400–800 ka (44, 45), corresponding to sampling resolution for the porphyrin data of approximately 20,000–100,000 y per sample, or significantly longer than present-day estimates of N residence time in the ocean (2,000–5,000 y; ref. 9). Trends observed in the smoothed data thus reflect persistent, potentially steady-state perturbations of the marine N cycle. The top and bottom sections of the core have identical values of δ15N por: -5.6 ± 0.7‰ above 419.5 mcd and -5.4 ± 0.7‰ below 423 mcd. However, between 423 and 419.5 mcd, values of
values. The large error ranges are due to full propagation of analytical uncertainty associated with preparation and analysis by the denitrifier method (43). To overcome the scatter, we plotted 3-, 5-, and 9-point moving averages (Fig. 1C). These different resolutions all show similar patterns, indicating that temporal trends in the results are not sensitive to the degree of smoothing and are not dependent on data density. The data are spaced relatively uniformly (0.5 m), although sampling resolution is higher in some horizons surrounding the excursion interval, from 421.9 to 427.5 mcd (0.2 m). The duration of OAE 2 has been estimated to be approximately 400–800 ka (44, 45), corresponding to sampling resolution for the porphyrin data of approximately 20,000–100,000 y per sample, or significantly longer than present-day estimates of N residence time in the ocean (2,000–5,000 y; ref. 9). Trends observed in the smoothed data thus reflect persistent, potentially steady-state perturbations of the marine N cycle. The top and bottom sections of the core have identical values of δ15N por: -5.6 ± 0.7‰ above 419.5 mcd and -5.4 ± 0.7‰ below 423 mcd. However, between 423 and 419.5 mcd, values of 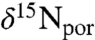 average -6.4 ± 0.6‰ and decrease sharply to -7.5‰ approaching and just after the termination of the OAE. This shift correlates with the phasing observed for
average -6.4 ± 0.6‰ and decrease sharply to -7.5‰ approaching and just after the termination of the OAE. This shift correlates with the phasing observed for 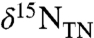 and
and  , but in both cases the N isotopes lag the excursion observed in
, but in both cases the N isotopes lag the excursion observed in 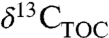 .
.
The εpor Proxy for Eukaryotic vs. Cyanobacterial Burial
The relative fraction of eukaryotic vs. cyanobacterial export production can be estimated from δ15N values of porphyrins and their associated sediments. In previous work we examined the biochemical and physiological basis for fractionation of nitrogen isotopes between biomass and chloropigments (38). The offset, known as εpor (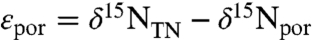 ), differs systematically between eukaryotes and cyanobacteria. Values of εpor for eukaryotes are around 5 ± 2‰; i.e., chlorophyll 5‰ more depleted in 15N than biomass (33, 38, 46). In contrast, cyanobacteria have values of εpor between 0 and -10‰ (i.e., chlorophyll equal to or up to 10‰ enriched in 15N relative to biomass). One example of a value of εpor near -10‰ had been observed previously for Anabaena cylindrica (33). To expand on this finding, we recently reported data showing that among the seven species of cyanobacteria tested to date, freshwater ecotypes cluster around the εpor = -10 ± 2‰ endmember, whereas marine ecotypes cluster around the εpor = 0 ± 2‰ endmember (38).
), differs systematically between eukaryotes and cyanobacteria. Values of εpor for eukaryotes are around 5 ± 2‰; i.e., chlorophyll 5‰ more depleted in 15N than biomass (33, 38, 46). In contrast, cyanobacteria have values of εpor between 0 and -10‰ (i.e., chlorophyll equal to or up to 10‰ enriched in 15N relative to biomass). One example of a value of εpor near -10‰ had been observed previously for Anabaena cylindrica (33). To expand on this finding, we recently reported data showing that among the seven species of cyanobacteria tested to date, freshwater ecotypes cluster around the εpor = -10 ± 2‰ endmember, whereas marine ecotypes cluster around the εpor = 0 ± 2‰ endmember (38).
Because εpor reflects intracellular partitioning of N isotopes downstream of the amino acid glutamate, it is independent of the nitrogen substrate utilized by the organism (N2, 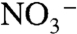 , or
, or 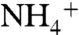 ; ref. 38). Thus, we proposed that εpor would be an excellent proxy for calculating the relative contributions of eukaryotes and cyanobacteria to marine export production. Measured values of εpor would be 5 ± 2‰ in a 100% eukaryotic system and would be 0 ± 2‰ in a 100% marine cyanobacterial system, regardless of the proportion of diazotrophic species among the latter (not all marine cyanobacteria are diazotrophs). Moreover, influx of terrigenous biomass and/or cyanobacteria from fresh waters would lead to values of εpor < 0‰. Indeed, to date the only in situ value of cyanobacterial εpor from the environment is from a freshwater lake in Japan in which εpor was determined to be -13 to -16‰ (47).
; ref. 38). Thus, we proposed that εpor would be an excellent proxy for calculating the relative contributions of eukaryotes and cyanobacteria to marine export production. Measured values of εpor would be 5 ± 2‰ in a 100% eukaryotic system and would be 0 ± 2‰ in a 100% marine cyanobacterial system, regardless of the proportion of diazotrophic species among the latter (not all marine cyanobacteria are diazotrophs). Moreover, influx of terrigenous biomass and/or cyanobacteria from fresh waters would lead to values of εpor < 0‰. Indeed, to date the only in situ value of cyanobacterial εpor from the environment is from a freshwater lake in Japan in which εpor was determined to be -13 to -16‰ (47).
At Site 1258A the observed value of εpor throughout the section averages 4.3 ± 0.8‰ (if calculated vs. 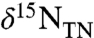 ) or 4.0 ± 0.8‰ (if calculated vs.
) or 4.0 ± 0.8‰ (if calculated vs.  ) (Fig. 1D). The values of εpor reach minima—reflecting maximum burial of cyanobacterial biomass—toward the end of the OAE. There appears to be a qualitative trend of decreasing magnitude of εpor from the beginning of the OAE until approximately 1 mcd below the termination. At this point, εpor increases and fluctuates repeatedly until approximately 4 mcd after termination of the OAE, after which εpor then returns to the starting value near 5‰. This again shows that changes in nitrogen cycle processes are out of phase with the changes in the carbon cycle that define the OAE. Such differences may be expected: in multiple OAE 2 sections the local primary productivity is known to be variable within the overall record of the OAE as defined by δ13C values (24, 48).
) (Fig. 1D). The values of εpor reach minima—reflecting maximum burial of cyanobacterial biomass—toward the end of the OAE. There appears to be a qualitative trend of decreasing magnitude of εpor from the beginning of the OAE until approximately 1 mcd below the termination. At this point, εpor increases and fluctuates repeatedly until approximately 4 mcd after termination of the OAE, after which εpor then returns to the starting value near 5‰. This again shows that changes in nitrogen cycle processes are out of phase with the changes in the carbon cycle that define the OAE. Such differences may be expected: in multiple OAE 2 sections the local primary productivity is known to be variable within the overall record of the OAE as defined by δ13C values (24, 48).
If we assume that only eukaryotic algae and marine cyanobacteria contribute significantly to the burial flux of photosynthetic pigments (i.e., eliminating the possibility of freshwater cyanobacteria), a value of εpor consistently > 4‰ during the OAE indicates that the export flux remained on average ≥80% eukaryotic throughout the event. In contrast with previous interpretations invoking N fixers as the primary source of nutrient N (11, 15, 26), these results indicate that the abundance of cyanobacteria contributing directly to export production during OAE 2 was not large; and it suggests that another N source would have been required to sustain such high rates of eukaryotic export production. However, the data for εpor are consistent with a large relative change in the cyanobacterial population, as observed values of εpor approximately 4‰ within the OAE indicate approximately 20% cyanobacterial biomass, whereas pre- and post-OAE values of εpor nearer 5‰ indicate less burial of cyanobacterial biomass (certainly < 5–10%). The εpor data thus indicate at minimum a doubling to quadrupling of cyanobacterial production, but within a system consistently and overwhelmingly dominated by eukaryotic primary producers. If Site 1258A is representative of OAE 2 in general, the widespread negative values of sedimentary 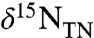 throughout OAE 2 deposits must be attributed to burial of eukaryotes having significant 15N-depletion in their biomass.
throughout OAE 2 deposits must be attributed to burial of eukaryotes having significant 15N-depletion in their biomass.
Nitrogen Cycle in Anoxic Oceans: A Paradox of Nitrification and Denitrification
A modest increase in cyanobacterial production is consistent with expected changes to the nitrogen cycle. During OAE 2, anoxic deep waters of the proto-Atlantic and Western Tethys would have contained nitrogen predominantly in the form of 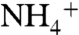 . Upwelling rates were high (8), and
. Upwelling rates were high (8), and 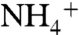 upwelled into oxic surface waters either was assimilated by phytoplankton or oxidized to
upwelled into oxic surface waters either was assimilated by phytoplankton or oxidized to 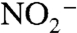 and/or
and/or 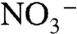 . Reducing conditions impinging on the photic zone likely meant that a greater fraction of this
. Reducing conditions impinging on the photic zone likely meant that a greater fraction of this 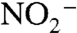 and
and  subsequently was reduced to N2 via denitrification and anammox, causing a modestly greater fixed-nitrogen deficit. Such widespread N deficits suggest it is unlikely that negative values of
subsequently was reduced to N2 via denitrification and anammox, causing a modestly greater fixed-nitrogen deficit. Such widespread N deficits suggest it is unlikely that negative values of 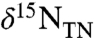 in sediments of OAE 2 could be due to the expression of isotopic discrimination during nutrient uptake, which occurs for eukaryotes only in nutrient-replete systems in which the nitrogen supply is in excess of biological demand (49, 50). The extent to which fixed N was used to completion in OAE 2 surface waters would have determined the ecological niche for N-fixing cyanobacteria, either as free-living cells or as symbionts; but the overall system was N limited, as generally is the case in the marine photic zone (10). Complete utilization of the available nutrient N implies that the total flux must have been isotopically negative.
in sediments of OAE 2 could be due to the expression of isotopic discrimination during nutrient uptake, which occurs for eukaryotes only in nutrient-replete systems in which the nitrogen supply is in excess of biological demand (49, 50). The extent to which fixed N was used to completion in OAE 2 surface waters would have determined the ecological niche for N-fixing cyanobacteria, either as free-living cells or as symbionts; but the overall system was N limited, as generally is the case in the marine photic zone (10). Complete utilization of the available nutrient N implies that the total flux must have been isotopically negative.
A deficit in fixed N during OAEs is not surprising, as anoxia promotes denitrification. What may be surprising is that the deficit was not larger. We suggest that counterintuitively, rates of denitrification may decrease under conditions of extreme basin-wide anoxia. Denitrification and anammox depend on sufficient availability of  and
and 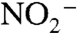 . Because these oxidized N species are produced aerobically, extreme oxygen limitation in the water-column may decrease their rate of formation, leaving a greater fraction of remineralized organic nitrogen to cycle throughout these regionally isolated basins and reenter the photic zone as
. Because these oxidized N species are produced aerobically, extreme oxygen limitation in the water-column may decrease their rate of formation, leaving a greater fraction of remineralized organic nitrogen to cycle throughout these regionally isolated basins and reenter the photic zone as 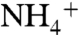 . This in turn would limit the need for compensating N fixation. Evidence for photic-zone sulfide oxidation during OAEs suggests that
. This in turn would limit the need for compensating N fixation. Evidence for photic-zone sulfide oxidation during OAEs suggests that 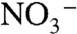 indeed was completely absent beneath the photic zone, at least episodically (5, 51), and that fixed N in these deep waters would have remained in reduced form. We propose that the values of
indeed was completely absent beneath the photic zone, at least episodically (5, 51), and that fixed N in these deep waters would have remained in reduced form. We propose that the values of  found in OAE sediments reflect severe diminishment of the deep-water
found in OAE sediments reflect severe diminishment of the deep-water 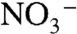 component of the marine N cycle, implying that the deep ocean was a reservoir of
component of the marine N cycle, implying that the deep ocean was a reservoir of 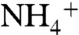 . Upwelled
. Upwelled 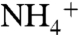 , rather than newly fixed N, was the main N source for primary production. Chemocline impingement on the photic zone would have driven nitrification, denitrification, and anammox into competition with
, rather than newly fixed N, was the main N source for primary production. Chemocline impingement on the photic zone would have driven nitrification, denitrification, and anammox into competition with 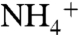 -assimilation. The balance between these processes—which varied regionally—would have set the loss rate of N from the ocean and the compensatory rates of N fixation.
-assimilation. The balance between these processes—which varied regionally—would have set the loss rate of N from the ocean and the compensatory rates of N fixation.
To explain the observed values of 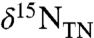 , isotopic mass balance would then require that the newly fixed N (
, isotopic mass balance would then require that the newly fixed N (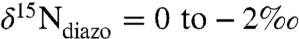 ), plus the upwelled
), plus the upwelled 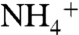 supply, together can yield new production that has values of δ15N < -2‰ (e.g., Bonarelli and South Ferriby sections; ref. 16). This is different from a modern-ocean scenario, in which denitrification associated with the spreading of anoxic zones leads to progressively higher (positive) values of
supply, together can yield new production that has values of δ15N < -2‰ (e.g., Bonarelli and South Ferriby sections; ref. 16). This is different from a modern-ocean scenario, in which denitrification associated with the spreading of anoxic zones leads to progressively higher (positive) values of 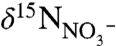 that are then propagated to
that are then propagated to 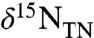 (21, 22). The modern-ocean endmembers are thus near-zero (diazotrophs) and more positive (nitrate assimilation and/or recycling), whereas the OAE endmembers must be near-zero (diazotrophs) and more negative (
(21, 22). The modern-ocean endmembers are thus near-zero (diazotrophs) and more positive (nitrate assimilation and/or recycling), whereas the OAE endmembers must be near-zero (diazotrophs) and more negative (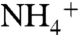 assimilation and recycling). Although required to explain the data, such a scenario is far from intuitive: it requires that the fixed N lost from the ocean by the processes of denitrification plus anammox have a net positive value of δ15N. Below we explore how such a system might be possible.
assimilation and recycling). Although required to explain the data, such a scenario is far from intuitive: it requires that the fixed N lost from the ocean by the processes of denitrification plus anammox have a net positive value of δ15N. Below we explore how such a system might be possible.
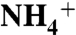 -Upwelling Model
-Upwelling Model
To yield a marine system in which the burial flux of 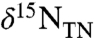 has a negative value, we assume that
has a negative value, we assume that 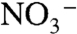 (and
(and 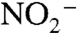 ) are produced only in the aerobic photic zone and are reduced quantitatively to N2 in the chemocline by denitrification and/or anammox. This loss is analogous isotopically to sedimentary denitrification in the modern ocean, which is considered to impart zero fractionation because it proceeds to completion, and by mass balance,
) are produced only in the aerobic photic zone and are reduced quantitatively to N2 in the chemocline by denitrification and/or anammox. This loss is analogous isotopically to sedimentary denitrification in the modern ocean, which is considered to impart zero fractionation because it proceeds to completion, and by mass balance, 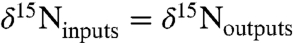 (19).
(19).
The following additional conditions then would be sufficient to achieve a denitrifying flux of N2 that is net isotopically positive. To yield surface waters in which 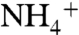 and N2 are the most important bioavailable sources of N, we assume that nitrification of the upwelling flux to
and N2 are the most important bioavailable sources of N, we assume that nitrification of the upwelling flux to 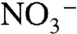 followed by phytoplanktonic assimilation is much less significant than direct assimilation of concomitant upwelling
followed by phytoplanktonic assimilation is much less significant than direct assimilation of concomitant upwelling 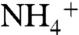 . Where
. Where 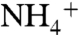 is available,
is available,  is a less favorable nutrient for phytoplankton growth due to the higher energetic costs associated with its reduction (52). Nitrite generally is not believed to be an important source of nutrient N (53), and thus we assume it also is removed by denitrification or, more likely, by anammox. The dominant fractionations and fluxes in the N cycle are then εfix and φfix (N2 fixation), ε1and φ1 (
is a less favorable nutrient for phytoplankton growth due to the higher energetic costs associated with its reduction (52). Nitrite generally is not believed to be an important source of nutrient N (53), and thus we assume it also is removed by denitrification or, more likely, by anammox. The dominant fractionations and fluxes in the N cycle are then εfix and φfix (N2 fixation), ε1and φ1 (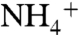 -assimilation), and ε2 and φ2 (ammonium oxidation,
-assimilation), and ε2 and φ2 (ammonium oxidation, 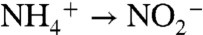 ), whereas the burial flux is small relative to these internal cycles (Fig. 2A). We also specify the flux associated with remineralization of sinking phytoplankton N (φ3), and assume no fractionation for this process. As stated above, all oxidations and reductions downstream of φ2 are quantitative and do not impart further fractionation.
), whereas the burial flux is small relative to these internal cycles (Fig. 2A). We also specify the flux associated with remineralization of sinking phytoplankton N (φ3), and assume no fractionation for this process. As stated above, all oxidations and reductions downstream of φ2 are quantitative and do not impart further fractionation.
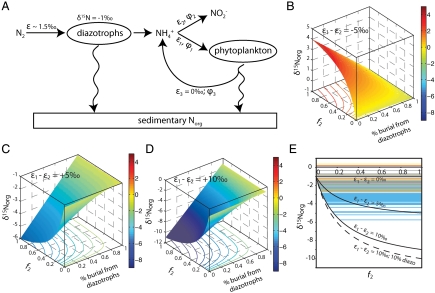
Fig. 2.
Conceptual model for sedimentary values of 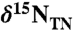 in an ocean in which
in an ocean in which 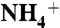 is the dominant fixed N species. (A) System in which the δ15N values of exported eukaryotic biomass depend on the fractional fluxes to ammonium assimilation (φ1), oxidation (φ2), and recycling (φ3), as well as the difference between the associated fractionation factors ε1 and ε2. (B–D) Calculated δ15N values of sedimentary organic matter as a function of percent export from diazotrophs and fractional fluxes φ1 and φ2 for three sets of fractionation factors: (B) ε1 - ε2 = -5‰; (C) ε1 - ε2 = 5‰; (D) ε1 - ε2 = 10‰. (E) Data for
is the dominant fixed N species. (A) System in which the δ15N values of exported eukaryotic biomass depend on the fractional fluxes to ammonium assimilation (φ1), oxidation (φ2), and recycling (φ3), as well as the difference between the associated fractionation factors ε1 and ε2. (B–D) Calculated δ15N values of sedimentary organic matter as a function of percent export from diazotrophs and fractional fluxes φ1 and φ2 for three sets of fractionation factors: (B) ε1 - ε2 = -5‰; (C) ε1 - ε2 = 5‰; (D) ε1 - ε2 = 10‰. (E) Data for 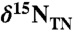 for OAE 2 from the literature [11 (red); 16 (blue, Italy; green, England); 17 (yellow); 23 (orange), and this study (gray)], plotted relative to the range of paired values of ε1 and ε2 solved with the model, assuming 20% export of diazotrophic biomass (solid lines), as well as an ε1/ε2 offset of 10‰ assuming 10% export of diazotrophic biomass (dashed line).
for OAE 2 from the literature [11 (red); 16 (blue, Italy; green, England); 17 (yellow); 23 (orange), and this study (gray)], plotted relative to the range of paired values of ε1 and ε2 solved with the model, assuming 20% export of diazotrophic biomass (solid lines), as well as an ε1/ε2 offset of 10‰ assuming 10% export of diazotrophic biomass (dashed line).
In N-limited surface waters, new production reflects the isotopic signature of the integrated nitrogen budget. The resulting value of 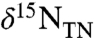 will reflect a weighted average of the δ15N values of diazotrophic cyanobacteria (δdiazo) and of
will reflect a weighted average of the δ15N values of diazotrophic cyanobacteria (δdiazo) and of 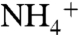 -consuming phytoplankton (δphyto). The former will be equal to
-consuming phytoplankton (δphyto). The former will be equal to 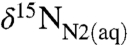 minus εfix, whereas the latter will be equal to the δ15N value of their
minus εfix, whereas the latter will be equal to the δ15N value of their  source minus a fractionation factor ε1. Isotopic mass balance dictates that the source
source minus a fractionation factor ε1. Isotopic mass balance dictates that the source 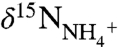 is set by the relative flux of
is set by the relative flux of 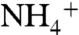 that is utilized (φ1) vs. nitrified (φ2), as well as the flux (φ3) that returns remineralized
that is utilized (φ1) vs. nitrified (φ2), as well as the flux (φ3) that returns remineralized 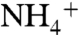 to the surface via upwelling. Ratios of these fluxes, the fractionations associated with
to the surface via upwelling. Ratios of these fluxes, the fractionations associated with 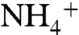 utilization and oxidation (ε1 and ε2, respectively), and the value of δdiazo, together set
utilization and oxidation (ε1 and ε2, respectively), and the value of δdiazo, together set  :
:
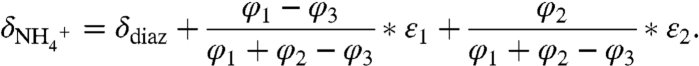 |
Assuming δdiazo = -1‰ and substituting 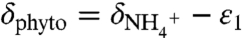 and
and  enables the system to be solved for a range of combinations of ε1 and ε2 (full derivation in SI Text). This model generates negative values for δphyto when ε1 > ε2, and it produces an ocean system in which the major reservoir of dissolved inorganic nitrogen (DIN) accumulates as 15N-depleted
enables the system to be solved for a range of combinations of ε1 and ε2 (full derivation in SI Text). This model generates negative values for δphyto when ε1 > ε2, and it produces an ocean system in which the major reservoir of dissolved inorganic nitrogen (DIN) accumulates as 15N-depleted 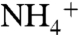 .
.
Biomass having a negative value of δ15N results from the co-occurrence of ammonium oxidation and ammonium assimilation in the photic zone, the competing effects of fractionations associated with these processes on a single 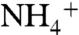 pool, and the upwelling of recycled, 15N-depleted
pool, and the upwelling of recycled, 15N-depleted  . Both assimilation and oxidation fractionate such that their products are more 15N-depleted than the source
. Both assimilation and oxidation fractionate such that their products are more 15N-depleted than the source 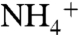 , and therefore the
, and therefore the 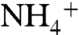 pool in surface waters becomes more 15N-enriched as it is consumed. If the fractionation associated with
pool in surface waters becomes more 15N-enriched as it is consumed. If the fractionation associated with 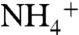 assimilation exceeds the enrichment of the
assimilation exceeds the enrichment of the 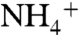 pool that is caused by nitrification/denitrification (i.e., ε1 > ε2), the resulting biomass (φ1) is isotopically negative. Regenerated
pool that is caused by nitrification/denitrification (i.e., ε1 > ε2), the resulting biomass (φ1) is isotopically negative. Regenerated 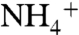 in deep waters isotopically resembles the sinking biomass from which it is remineralized. As this
in deep waters isotopically resembles the sinking biomass from which it is remineralized. As this 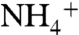 upwells into the photic zone, it again becomes 15N-enriched and the system maintains steady-state.
upwells into the photic zone, it again becomes 15N-enriched and the system maintains steady-state.
The resulting value for total buried organic matter (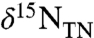 ) is tempered by the percent contribution of diazotrophic biomass (Fig. 2
B–D) such that values of
) is tempered by the percent contribution of diazotrophic biomass (Fig. 2
B–D) such that values of 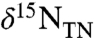 approach -1‰ when there is greater burial of diazotrophs, but decrease as the ratio φ2/φ1 increases and diazotrophic burial decreases. This is consistent with records showing the most negative values of
approach -1‰ when there is greater burial of diazotrophs, but decrease as the ratio φ2/φ1 increases and diazotrophic burial decreases. This is consistent with records showing the most negative values of 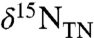 in pelagic locations with lesser apparent bacterial biomass burial (16) and more positive values of
in pelagic locations with lesser apparent bacterial biomass burial (16) and more positive values of 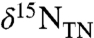 in epicontinental environments with higher apparent bacterial flux (16).
in epicontinental environments with higher apparent bacterial flux (16).
The model thus depends on the relative magnitudes of ε1 and ε2 compared to the N deficit and resulting diazotrophic contribution. It is possible that the fractionation associated with 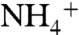 -assimilation (ε1) by the enzyme glutamine synthetase (GS) may exceed that of
-assimilation (ε1) by the enzyme glutamine synthetase (GS) may exceed that of 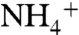 -oxidation (ε2) by the enzyme ammonium monooxygenase (AMO) under some circumstances. The observed value of ε1 (4–27‰) will depend on
-oxidation (ε2) by the enzyme ammonium monooxygenase (AMO) under some circumstances. The observed value of ε1 (4–27‰) will depend on 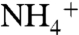 concentration, with larger fractionations expressed under
concentration, with larger fractionations expressed under 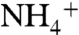 -rich conditions (54). In the modern ocean,
-rich conditions (54). In the modern ocean, 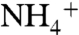 concentrations are low and ε1 is confined to the lower end of this range. Under the
concentrations are low and ε1 is confined to the lower end of this range. Under the 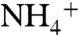 -replete conditions that we propose for OAE 2, assimilation using different enzymatic controls may lead to expression of ε1 with a larger magnitude, although to date very little information is available about fractionation during
-replete conditions that we propose for OAE 2, assimilation using different enzymatic controls may lead to expression of ε1 with a larger magnitude, although to date very little information is available about fractionation during 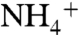 assimilation by natural planktonic assemblages (55).
assimilation by natural planktonic assemblages (55).
The value of ε2 also remains poorly constrained. The relative fraction of aerobic ammonia oxidation by archaea vs. bacteria during OAE 2 is not known, but δ13C and archaeal biomarker data measured in black shales deposited during the Albian OAE1b (approximately 112 Ma) suggest that Crenarchaeota (now called Thaumarchaeota; ref. 56) that are believed to be responsible for most ammonium oxidation in the modern ocean (57), were abundant in the Cretaceous (58). Values of ε2 for bacterial AMO are approximately 14–38‰, for a variety of species grown on 1–2 mM 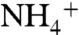 (59). Recent measurements of isotope effects associated with archaeal ammonia oxidation show a similar range of values, from 10–37‰ (60). In all cases, the relative contributions of fractionations associated with transport of
(59). Recent measurements of isotope effects associated with archaeal ammonia oxidation show a similar range of values, from 10–37‰ (60). In all cases, the relative contributions of fractionations associated with transport of 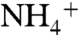 or diffusion of NH3 through membranes and equilibrium of
or diffusion of NH3 through membranes and equilibrium of 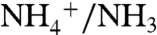 are uncertain. It is thus difficult to extrapolate these cultures to natural systems, except to suggest that bacterial and archaeal AMO results are similar.
are uncertain. It is thus difficult to extrapolate these cultures to natural systems, except to suggest that bacterial and archaeal AMO results are similar.
If ε1 was large due to elevated 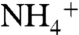 concentrations (54) upwelling to the base of the photic zone from a large, deep
concentrations (54) upwelling to the base of the photic zone from a large, deep 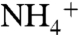 pool, the condition of ε1 > ε2 could be met. For example, if ε2 = 22‰ (average archaeal value) and ε1 = 27‰ (maximum enzymatic effect on
pool, the condition of ε1 > ε2 could be met. For example, if ε2 = 22‰ (average archaeal value) and ε1 = 27‰ (maximum enzymatic effect on 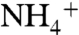 -assimilation), then ε1 - ε2 = 5‰. This results in values of
-assimilation), then ε1 - ε2 = 5‰. This results in values of 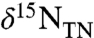 for export production that will be < -2‰ (Fig. 2C) if
for export production that will be < -2‰ (Fig. 2C) if 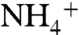 oxidation consumes at least one-tenth of the upwelling
oxidation consumes at least one-tenth of the upwelling 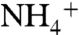 flux (φ2 > 0.1) and the burial contribution of diazotrophs is 20%, the upper limit based on our data for εpor. Other versions of the model that impose larger differences between ε1 and ε2 (e.g., ε1 - ε2 = 10‰, Fig. 2D) also are compatible with some of the data from OAE 2, in particular a few of the very negative values of
flux (φ2 > 0.1) and the burial contribution of diazotrophs is 20%, the upper limit based on our data for εpor. Other versions of the model that impose larger differences between ε1 and ε2 (e.g., ε1 - ε2 = 10‰, Fig. 2D) also are compatible with some of the data from OAE 2, in particular a few of the very negative values of 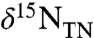 for the sections from Italy and England (Fig. 2
D and E) (16). Analogous models with ε1 < ε2 can produce only positive values of
for the sections from Italy and England (Fig. 2
D and E) (16). Analogous models with ε1 < ε2 can produce only positive values of 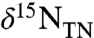 , as would be seen in the modern ocean (Fig. 2B). Using our conceptual model, most data for
, as would be seen in the modern ocean (Fig. 2B). Using our conceptual model, most data for 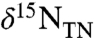 compiled from OAE 2 (11, 16, 17, 23, this paper) fall within isotope space corresponding to ranges of ε1 - ε2 = 5‰ (Fig. 2E).
compiled from OAE 2 (11, 16, 17, 23, this paper) fall within isotope space corresponding to ranges of ε1 - ε2 = 5‰ (Fig. 2E).
We further tested the plausibility of our conceptual framework using a simplified steady-state model that calculates δ15N values of biomass N, 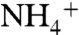 ,
, 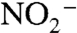 , and
, and 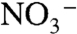 in a two-box (surface and deep) ocean. The model was optimized to reproduce known modern values using estimates of fluxes and fractionation factors from the literature. To run the model subsequently for the OAE, we modified nitrogen-redox partitioning (more
in a two-box (surface and deep) ocean. The model was optimized to reproduce known modern values using estimates of fluxes and fractionation factors from the literature. To run the model subsequently for the OAE, we modified nitrogen-redox partitioning (more 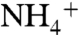 , less
, less 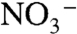 ) and changed the magnitude of associated fluxes proportionally. Rates of upwelling and the total N inventory remained the same in both cases. By changing these parameters, the model generated sedimentary
) and changed the magnitude of associated fluxes proportionally. Rates of upwelling and the total N inventory remained the same in both cases. By changing these parameters, the model generated sedimentary 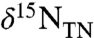 values of -4.4‰ for the OAE and +4.9‰ for modern sediments. For a complete model description, results, and sensitivity analysis, see Supplementary Information.
values of -4.4‰ for the OAE and +4.9‰ for modern sediments. For a complete model description, results, and sensitivity analysis, see Supplementary Information.
Implications
Our model implies a widespread and well-mixed “ammonia ocean” for the proto-Atlantic and Western Tethys because it requires a sustained source of upwelling 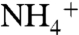 that can be used for biological assimilation. This can be achieved if nitrate production is limited by severe demands on
that can be used for biological assimilation. This can be achieved if nitrate production is limited by severe demands on 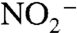 , possibly through enhanced anammox. In such an ocean, ammonia assimilators and N fixers both could out-compete assimilatory
, possibly through enhanced anammox. In such an ocean, ammonia assimilators and N fixers both could out-compete assimilatory 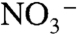 reducers due to the dominance of
reducers due to the dominance of 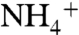 and a limited rate of
and a limited rate of  generation. Postulated high rates of upwelling, combined with nutrient trapping under estuarine circulation in the North Atlantic (8), may explain why these negative δ15N signals are widespread during OAEs, yet are regionally variable (16). The trapping of quantitatively significant levels of
generation. Postulated high rates of upwelling, combined with nutrient trapping under estuarine circulation in the North Atlantic (8), may explain why these negative δ15N signals are widespread during OAEs, yet are regionally variable (16). The trapping of quantitatively significant levels of 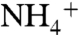 in deep waters during OAEs also helps preserve the total pool of marine N, alleviating the need for excessive rates of nitrogen fixation. Extreme anoxia may therefore exert a natural, negative feedback on the nitrogen cycle by preventing the ocean from denitrifying completely.
in deep waters during OAEs also helps preserve the total pool of marine N, alleviating the need for excessive rates of nitrogen fixation. Extreme anoxia may therefore exert a natural, negative feedback on the nitrogen cycle by preventing the ocean from denitrifying completely.
Our proposed model for the N cycle during OAE 2 also helps to explain why extreme N isotopic depletion is not seen in modern anoxic basins like the Black Sea and the Cariaco Trench, where δ15N values of particulate organic nitrogen are > 0‰ throughout the water column (41). The nutrient sources and circulation patterns in these two systems are not analogous to anoxic oceans. The Cariaco Trench is a silled basin that receives  from the Atlantic, and sedimentary organic nitrogen in the Cariaco basin carries an isotopic signature that reflects a mass balance between Atlantic
from the Atlantic, and sedimentary organic nitrogen in the Cariaco basin carries an isotopic signature that reflects a mass balance between Atlantic 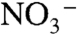 that has been influenced by N2 fixation (approximately 3‰) and N2 (local nitrogen fixation) (61). In the Black Sea, a commonly used analog for anoxic oceans, the supply of N to surface waters is largely sourced from continental rivers, whereas the intense salinity stratification limits the upwelling of deep
that has been influenced by N2 fixation (approximately 3‰) and N2 (local nitrogen fixation) (61). In the Black Sea, a commonly used analog for anoxic oceans, the supply of N to surface waters is largely sourced from continental rivers, whereas the intense salinity stratification limits the upwelling of deep 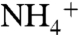 and promotes formation of
and promotes formation of 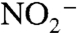 followed by nearly quantitative loss via the anammox process (62). The nutrient N cycle of the modern Black Sea, therefore, primarily is analogous to a large lacustrine system with severe stratification. In contrast, we envision OAE 2 as a time of sustained upwelling.
followed by nearly quantitative loss via the anammox process (62). The nutrient N cycle of the modern Black Sea, therefore, primarily is analogous to a large lacustrine system with severe stratification. In contrast, we envision OAE 2 as a time of sustained upwelling.
The ammonia ocean scenario also may help to explain the temporal evolution of N isotope patterns seen in our data. Values of 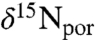 and
and 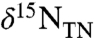 are out of phase with carbon isotopes. They do not begin to decrease until the middle of the OAE interval, and their minimum persists past the traditionally defined termination of the event. This phase lag may reflect the balance of oxidants in the marine system. Enhanced burial of organic carbon during OAEs should be associated with accumulation of oxygen in the ocean and atmosphere. This in turn would increase the rates of ammonium oxidation and nitrification, eventually suppressing anammox and allowing
are out of phase with carbon isotopes. They do not begin to decrease until the middle of the OAE interval, and their minimum persists past the traditionally defined termination of the event. This phase lag may reflect the balance of oxidants in the marine system. Enhanced burial of organic carbon during OAEs should be associated with accumulation of oxygen in the ocean and atmosphere. This in turn would increase the rates of ammonium oxidation and nitrification, eventually suppressing anammox and allowing 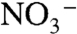 to accumulate. Indeed, our predicted values of
to accumulate. Indeed, our predicted values of  decrease as φ2 increases (Fig. 2
C–E). The predicted isotopic trajectory, therefore, is that
decrease as φ2 increases (Fig. 2
C–E). The predicted isotopic trajectory, therefore, is that 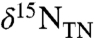 values will decrease during the early stages of ocean reoxidation. Values of
values will decrease during the early stages of ocean reoxidation. Values of 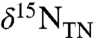 only would “flip” to positive values when the nitrification flux (φ2) was sufficiently high to accumulate excess
only would “flip” to positive values when the nitrification flux (φ2) was sufficiently high to accumulate excess 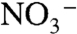 , allowing subsequent denitrification to enrich 15N in the accumulating
, allowing subsequent denitrification to enrich 15N in the accumulating 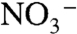 reservoir. These results highlight the importance and promise of using temporal records of εpor in conjunction with
reservoir. These results highlight the importance and promise of using temporal records of εpor in conjunction with  values to examine both the succession of marine ecosystems and the redox state of the ocean.
values to examine both the succession of marine ecosystems and the redox state of the ocean.
In sum, a mid-Cretaceous deep ocean dominated by reduced rather than oxidized nitrogen species, normal rates of ocean circulation (63), and enhanced input of nutrients (5, 6, 8) together could yield negative values of biomass δ15N and sustain a primary producer community that remained rich in eukaryotes. Although the oxidation state and temperature of OAE oceans was very different from the modern ocean, the persistent dominance of eukaryotes and dependence of primary producers on upwelled nutrients suggests that the balance between gross and net production was not greatly dissimilar from the present-day. Our results imply that additional feedbacks act under oxygen-limited conditions to maintain nitrogen balance, thereby limiting the extent of denitrification and the compensatory expansion of diazotrophy during OAEs.
Materials and Methods
Sediments were obtained from Ocean Drilling Program Leg 207, Site 1258A, from the Demerara Rise, offshore from modern Surinam. Samples spanned 415–428 m composite depth (mcd). Forty samples were analyzed for bulk 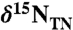 ,
, 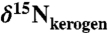 , and
, and  at approximately 0.5-m spacing. Sampling resolution was higher leading into and coming out of the OAE, which spanned approximatley 422–426 mcd (Table S1. Sample preparation and isotopic analysis followed established methods (43); details are given in SI Text.
at approximately 0.5-m spacing. Sampling resolution was higher leading into and coming out of the OAE, which spanned approximatley 422–426 mcd (Table S1. Sample preparation and isotopic analysis followed established methods (43); details are given in SI Text.
Supplementary Material
ACKNOWLEDGMENTS.
We thank Roger Summons, Carolyn Colonero, Amy Kelly, Noreen Tuross, and Kyle McElhoney for assistance with sample preparation and analysis and machine use. We thank Chris Junium and Julian Sachs for helpful discussions and comments, and Don Canfield, the PNAS editorial staff, and two anonymous reviewers for their valuable input. This work was supported by the National Science Foundation Grant OCE-0825269 (to A.P. and R.S.R.) and by the National Aeronautics and Space Administration Astrobiology Institute and the David and Lucille Packard Foundation (A.P.)
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1104313109/-/DCSupplemental.
References
- 1.Schlanger SO, Jenkyns HC. Cretaceous oceanic anoxic events: Causes and consequences. Geol Mijnbouw. 1976;55:179–184. [Google Scholar]
- 2.Jenkyns HC. Cretaceous anoxic events—from continents to oceans. J Geol Soc London. 1980;137:171–188. [Google Scholar]
- 3.Bralower TJ, Thierstein HR. Organic carbon and metal accumulation rates in Holocene and mid-Cretaceous sediments: Palaeoceanographic significance. Geol Soc Spec Publ. 1987;26:345–369. [Google Scholar]
- 4.Pedersen TF, Calvert SE. Anoxia vs productivity—what controls the formation of organic carbon-rich sediments and sedimentary rocks. AAPG Bull. 1990;74:454–466. [Google Scholar]
- 5.Kuypers MMM, Pancost RD, Nijenhuis IA, Damste JSS. Enhanced productivity led to increased organic carbon burial in the euxinic North Atlantic basin during the late Cenomanian oceanic anoxic event. Paleoceanography. 2002;17:3.1–13. [Google Scholar]
- 6.Jenkyns HC. Geochemistry of oceanic anoxic events. Geochem Geophy Geosy. 2010;11:Q03004. [Google Scholar]
- 7.Turgeon SC, Creaser RA. Cretaceous oceanic anoxic event 2 triggered by a massive magmatic episode. Nature. 2008;454:323–329. doi: 10.1038/nature07076. [DOI] [PubMed] [Google Scholar]
- 8.Alexandre JT, et al. The mid-Cretaceous North Atlantic nutrient trap: Black shales and OAEs. Paleoceanography. 2010;25:PA4201. [Google Scholar]
- 9.Brandes JA, Devol AH. A global marine-fixed nitrogen isotopic budget: Implications for Holocene nitrogen cycling. Global Biogeochem Cy. 2002;16:1120. [Google Scholar]
- 10.Falkowski PG. Evolution of the nitrogen cycle and its influence on the biological sequestration of CO2 in the ocean. Nature. 1997;387:272–275. [Google Scholar]
- 11.Kuypers MMM, van Breugel Y, Schouten S, Erba E, Damste JSS. N-2-fixing cyanobacteria supplied nutrient N for Cretaceous oceanic anoxic events. Geology. 2004;32:853–856. [Google Scholar]
- 12.Xie SC, Pancost RD, Yin HF, Wang HM, Evershed RP. Two episodes of microbial change coupled with Permo/Triassic faunal mass extinction. Nature. 2005;434:494–497. doi: 10.1038/nature03396. [DOI] [PubMed] [Google Scholar]
- 13.Sepulveda J, et al. Molecular isotopic evidence of environmental and ecological changes across the Cenomanian–Turonian boundary in the Levant Platform of central Jordan. Org Geochem. 2009;40:553–568. [Google Scholar]
- 14.Summons RE, Jahnke LL, Hope JM, Logan GA. 2-Methylhopanoids as biomarkers for cyanobacterial oxygenic photosynthesis. Nature. 1999;400:554–557. doi: 10.1038/23005. [DOI] [PubMed] [Google Scholar]
- 15.Ohkouchi N, Kashiyama Y, Kuroda J, Ogawa NO, Kitazato H. The importance of diazotrophic cyanobacteria as primary producers during Cretaceous Oceanic Anoxic Event 2. Biogeosciences. 2006;3:467–478. [Google Scholar]
- 16.Jenkyns HC, Matthews A, Tsikos H, Erel Y. Nitrate reduction, sulfate reduction, and sedimentary iron isotope evolution during the Cenomanian–Turonian oceanic anoxic event. Paleoceanography. 2007;22:PA3208. [Google Scholar]
- 17.Junium CK, Arthur MA. Nitrogen cycling during the cretaceous, Cenomanian–Turonian oceanic anoxic event II. Geochem Geophy Geosy. 2007;8:Q03002. [Google Scholar]
- 18.Altabet MA. Variations in nitrogen isotopic composition between sinking and suspended particles—implications for nitrogen cycling and particle transformation in the open ocean. Deep-Sea Res. 1988;35:535–554. [Google Scholar]
- 19.Deutsch C, Sigman DM, Thunell RC, Meckler AN, Haug GH. Isotopic constraints on glacial/interglacial changes in the oceanic nitrogen budget. Global Biogeochem Cy. 2004;18:GB4012. [Google Scholar]
- 20.Casciotti KL, Glover DM, Trull TW, Davies D. Constraints on nitrogen cycling at the subtropical North Pacific Station ALOHA from isotopic measurements of nitrate and particulate nitrogen. Deep Sea Res Part 2 Top Stud Oceanogr. 2008;55:1661–1672. [Google Scholar]
- 21.Brandes JA, Devol AH, Yoshinari T, Jayakumar DA, Naqvi SWA. Isotopic composition of nitrate in the central Arabian Sea and eastern tropical North Pacific: A tracer for mixing and nitrogen cycles. Limnol Oceanogr. 1998;43:1680–1689. [Google Scholar]
- 22.Ganeshram RS, Pedersen TF, Calvert SE, McNeill GW, Fontugne MR. Glacial-interglacial variability in denitrification in the world’s oceans: Causes and consequences. Paleoceanography. 2000;15:361–376. [Google Scholar]
- 23.Rau GH, Arthur MA, Dean WE. 15N/14N variations in Cretaceous Atlantic sedimentary sequences: Implications for past changes in marine nitrogen biogeochemistry. Earth Planet Sci Lett. 1987;82:269–279. [Google Scholar]
- 24.Tsikos H, et al. Carbon-isotope stratigraphy recorded by the Cenomanian–Turonian Oceanic Anoxic Event: Correlation and implications based on three key localities. J Geo Soc London. 2004;161:711–719. [Google Scholar]
- 25.Wada E, Hattori A. Natural abundance of N-15 in particulate organic matter in the North Pacific Ocean. Geochim Cosmochim Acta. 1976;40:249–251. [Google Scholar]
- 26.Kashiyama Y, et al. Diazotrophic cyanobacteria as the major photoautotrophs during mid-Cretaceous oceanic anoxic events: Nitrogen and carbon isotopic evidence from sedimentary porphyrin. Org Geochem. 2008;39:532–549. [Google Scholar]
- 27.Wada E. Nitrogen isotope fractionation and its significance in biogeochemical processes occurring in marine environments. In: Goldberg ED, Horibe Y, Saruhashi K, editors. Isotope Marine Chemistry. Tokyo: Uchida Rokahuko; 1980. pp. 375–398. [Google Scholar]
- 28.Macko SA, Entzeroth L, Parker PL. Regional differences in nitrogen and carbon isotopes on the continental shelf of the Gulf of Mexico. Naturwissenschaften. 1984;71:374–375. [Google Scholar]
- 29.Minagawa M, Wada E. Stepwise enrichment of N-15 along food chains—further evidence and the relation between δ15N and animal age. Geochim Cosmochim Acta. 1984;48:1135–1140. [Google Scholar]
- 30.Minagawa M, Wada E. Nitrogen isotope ratios of red tide organisms in the East China Sea—a characterization of biological nitrogen fixation. Mar Chem. 1986;19:245–259. [Google Scholar]
- 31.Macko SA, Fogel ML, Hare PE, Hoering TC. Isotopic fractionation of nitrogen and carbon in the synthesis of amino acids by microorganisms. Chem Geol. 1987;65(1):79–92. [Google Scholar]
- 32.Carpenter EJ, Harvey HR, Fry B, Capone DG. Biogeochemical tracers of the marine cyanobacterium Trichodesmium. Deep Sea Res Part 1 Oceanogr Res. 1997;44:27–38. [Google Scholar]
- 33.Beaumont VI, Jahnke LL, Des Marais DJ. Nitrogen isotopic fractionation in the synthesis of photosynthetic pigments in Rhodobacter capsulatus and Anabaena cylindrica. Org Geochem. 2000;31:1075–1085. [Google Scholar]
- 34.Zerkle AL, Junium CK, Canfield DE, House CH. Production of N-15-depleted biomass during cyanobacterial N-2-fixation at high Fe concentrations. J Geophys Res Biogeosci. 2008;113:G03014. [Google Scholar]
- 35.Bauersachs T, et al. Nitrogen isotopic fractionation associated with growth on dinitrogen gas and nitrate by cyanobacteria. Limnol Oceanogr. 2009;54:1403–1411. [Google Scholar]
- 36.Sachs JP, Repeta DJ. Oligotrophy and nitrogen fixation during eastern Mediterranean sapropel events. Science. 1999;286:2485–2488. doi: 10.1126/science.286.5449.2485. [DOI] [PubMed] [Google Scholar]
- 37.Higgins MB, Robinson RS, Carter SJ, Pearson A. Evidence from chlorin nitrogen isotopes for alternating nutrient regimes in the Eastern Mediterranean Sea. Earth Planet Sci Lett. 2010;290:102–107. [Google Scholar]
- 38.Higgins MB, et al. Paleoenvironmental implications of taxonomic variation among δ15N values of chloropigments. Geochim Cosmochim Acta. 2011;75:7351–7363. [Google Scholar]
- 39.Erbacher J, Friedrich O, Wilson PA, Birch H, Mutterlose J. Stable organic carbon isotope stratigraphy across Oceanic Anoxic Event 2 of Demerara Rise, western tropical Atlantic. Geochem Geophy Geosy. 2005;6:Q06010. [Google Scholar]
- 40.Bianchi TS, et al. Cyanobacterial blooms in the Baltic Sea: Natural or human-induced? Limnol Oceanogr. 2000;45:716–726. [Google Scholar]
- 41.Thunell RC, Sigman DM, Muller-Karger F, Astor Y, Varela R. Nitrogen isotope dynamics of the Cariaco Basin, Venezuela. Global Biogeochem Cy. 2004;18:GB3001. [Google Scholar]
- 42.Muller PJ. C-N ratios in Pacific deep sea sediments—effect of inorganic ammonium and organic nitrogen compounds sorbed by clays. Geochim Cosmochim Acta. 1977;41:765–776. [Google Scholar]
- 43.Higgins MB, Robinson RS, Casciotti KL, McIlvin MR, Pearson A. A method for determining the nitrogen isotopic composition of porphyrins. Anal Chem. 2009;81:184–192. doi: 10.1021/ac8017185. [DOI] [PubMed] [Google Scholar]
- 44.Sageman BB, Meyers SR, Arthur MA. Orbital time scale and new C-isotope record for Cenomanian–Turonian boundary stratotype. Geology. 2006;34:125–128. [Google Scholar]
- 45.Mitchell RN, et al. Oceanic anoxic cycles? Orbital prelude to the Bonarelli Level (OAE 2) Earth Planet Sci Lett. 2008;267:1–16. [Google Scholar]
- 46.Sachs JP, Repeta DJ, Goericke R. Nitrogen and carbon isotopic ratios of chlorophyll from marine phytoplankton. Geochim Cosmochim Acta. 1999;63:1431–1441. [Google Scholar]
- 47.Katase T, Wada E. Isolation of chlorophyll-a in Microcystis spp. for determination of stable isotopes of carbon and nitrogen, and variation in Suwa Lake. Bunseki Kagaku. 1990;39:451–456. [Google Scholar]
- 48.Kuroda J, Ohkouchi N, Ishii T, Tokuyama H, Taira A. Lamina-scale analysis of sedimentary components in Cretaceous black shales by chemical compositional mapping: Implications for paleoenvironmental changes during the Oceanic Anoxic Events. Geochim Cosmochim Acta. 2005;69:1479–1494. [Google Scholar]
- 49.Altabet MA, Francois R. Sedimentary nitrogen isotopic ratio as a recorder for surface ocean nitrate utilization. Global Biogeochem Cy. 1994;8:103–116. [Google Scholar]
- 50.Waser NA, et al. Nitrogen isotope fractionation during nitrate, ammonium and urea uptake by marine diatoms and coccolithophores under various conditions of N availability. Mar Ecol-Prog Ser. 1998;169:29–41. [Google Scholar]
- 51.Pancost RD, et al. Further evidence for the development of photic-zone euxinic conditions during Mesozoic oceanic anoxic events. J Geol Soc London. 2004;161:353–364. [Google Scholar]
- 52.Eppley RW, Coatsworth JL, Solorzano L. Studies of nitrate reductase in marine phytoplankton. Limnol Oceanogr. 1969;14:194–205. [Google Scholar]
- 53.Casciotti KL, McIlvin MR. Isotopic analyses of nitrate and nitrite from reference mixtures and application to Eastern Tropical North Pacific waters. Mar Chem. 2007;107:184–201. [Google Scholar]
- 54.Hoch MP, Fogel ML, Kirchman DL. Isotope fractionation associated with ammonium uptake by a marine bacterium. Limnol Oceanogr. 1992;37:1447–1459. [Google Scholar]
- 55.Hoch MP, Fogel ML, Kirchman DL. Isotope fractionation during ammonium uptake by marine microbial assemblages. Geomicrobiol J. 1994;12:113–127. [Google Scholar]
- 56.Brochier-Armanet C, Boussau B, Gribaldo S, Forterre P. Mesophilic crenarchaeota: Proposal for a third archaeal phylum, the Thaumarchaeota. Nat Rev Microbiol. 2008;6:245–252. doi: 10.1038/nrmicro1852. [DOI] [PubMed] [Google Scholar]
- 57.Martens-Habbena W, Berube PM, Urakawa H, de la Torre JR, Stahl DA. Ammonia oxidation kinetics determine niche separation of nitrifying Archaea and Bacteria. Nature. 2009;461:976–U234. doi: 10.1038/nature08465. [DOI] [PubMed] [Google Scholar]
- 58.Kuypers MMM, et al. Massive expansion of marine archaea during a mid-Cretaceous oceanic anoxic event. Science. 2001;293:92–94. doi: 10.1126/science.1058424. [DOI] [PubMed] [Google Scholar]
- 59.Casciotti KL, Sigman DM, Ward BB. Linking diversity and stable isotope fractionation in ammonia-oxidizing bacteria. Geomicrobiol J. 2003;20:335–353. [Google Scholar]
- 60.Santoro AE, Casciotti KL. Enrichment and characterization of ammonia-oxidizing archaea from the open ocean: Phylogeny, physiology and stable isotope fractionation. ISME J. 2011 doi: 10.1038/ismej.2011.58. 10.1038/ismej.2011.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meckler AN, et al. Detailed sedimentary N isotope records from Cariaco Basinfor Terminations I and V: Local and global implications. Global Biogeochem Cy. 2007;21:GB4019. [Google Scholar]
- 62.Kuypers MMM, et al. Anaerobic ammonium oxidation by anammox bacteria in the Black Sea. Nature. 2003;422:608–611. doi: 10.1038/nature01472. [DOI] [PubMed] [Google Scholar]
- 63.Meyer KM, Kump LR. Oceanic euxinia in Earth history: Causes and consequences. Annu Rev Earth Pl Sc. 2008;36:251–288. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.