Abstract
Cognitive deficits in older adults attributable to Alzheimer's disease (AD) pathology are featured early on by hippocampal impairment. Among these individuals, deterioration in spatial navigation, manifested by poor hippocampus-dependent allocentric navigation, may occur well before the clinical onset of dementia. Our aim was to determine whether allocentric spatial navigation impairment would be proportional to right hippocampal volume loss irrespective of general brain atrophy. We also contrasted the respective spatial navigation scores of the real-space human Morris water maze with its corresponding 2D computer version. We included 42 cognitively impaired patients with either amnestic mild cognitive impairment (n = 23) or mild and moderate AD (n = 19), and 14 cognitively intact older controls. All participants underwent 1.5T MRI brain scanning with subsequent automatic measurement of the total brain and hippocampal (right and left) volumes. Allocentric spatial navigation was tested in the real-space version of the human Morris water maze and in its corresponding computer version. Participants used two navigational cues to locate an invisible goal independent of the start position. We found that smaller right hippocampal volume was associated with poorer navigation performance in both the real-space (β = −0.62, P < 0.001) and virtual (β = −0.43, P = 0.026) versions, controlling for demographic variables, total brain and left hippocampal volumes. In subsequent analyses, the results were significant in cognitively impaired (P ≤ 0.05) but not in cognitively healthy (P > 0.59) subjects. The respective real-space and virtual scores strongly correlated with each other. Our findings indicate that the right hippocampus plays a critical role in allocentric navigation, particularly when cognitive impairment is present.
Persons with Alzheimer's disease (AD) (1, 2) and with amnestic mild cognitive impairment (MCI) (3), who are known to be at higher risk for developing AD, experience difficulties with spatial navigation. Based on animal research, two basic navigation types were distinguished (4). Egocentric navigation is route or body centered and is dependent mainly on parietal cortices and caudate nucleus (4–8). The more flexible and complex allocentric type is world centered and it is dependent mainly on the hippocampus (5, 9). In humans, medial temporal lobe function is highly lateralized with the right hippocampus predominantly associated with spatial navigation and topographical memory (10). Recent research has underscored the importance of the hippocampus for spatial navigation in cognitively impaired subjects (11, 12). For example, a case study of a patient with early AD (13) reported a distinct navigation deficit indicative of hippocampal atrophy. However, structural background of the allocentric navigation impairment has not yet been entirely elucidated, particularly in the real-space environment.
The navigation disability in AD and MCI patients has been found to involve selective impairment of spatial cognition associated with atrophy of the right-lateralized navigational network (11). However, this study did not differentiate between allocentric and egocentric types of navigation. A recent study using virtual reality environment reported impaired egocentric and allocentric spatial navigation and reduced right/left hippocampal volumes in amnestic MCI (aMCI) patients compared with cognitively healthy controls (12). However, a significant association was not found between the allocentric navigation performance and hippocampal volume, and navigation in real space was not assessed.
In our previous study, we found allocentric spatial navigation impairment in subjects with aMCI and AD in both the real-space and computerized versions of the test (3). However, these results were not supported with structural data. In the present study, we extend these previous findings by evaluating whether impairment in allocentric spatial navigation may be proportional to atrophy in the right hippocampus, an area crucial for performance of such tasks. Accordingly, we hypothesized that allocentric navigation performance would be dependent on the right hippocampal volume.
Specifically, we hypothesized that
i) Allocentric navigation performance would be associated with right hippocampal volume independent of total brain volume, suggesting that the result is not a function of total brain atrophy but is specific to the right hippocampus.
ii) The association between the right hippocampal volume and allocentric navigation would be more pronounced in cognitively impaired older adults.
iii) The 2D computerized test results would be consistent with results from a real-space setting.
Virtual reality environments do not fully reflect navigation in the real space, whereas a real-space setting has a better ability to mimic real-life situations. In our study, allocentric navigation was investigated in a real-space human analog of the Morris water maze (hMWM) (3, 14) and its corresponding 2D computerized version (3, 14). The subject had to find a hidden goal inside a circular arena using orientation cues on the arena circumference. The results of both versions of the hMWM test were regressed on volumes of both the right and left hippocampi, controlling for total brain volume and differences in age, sex, and education, to evaluate proportion of brain atrophy in relation to navigational errors.
Results
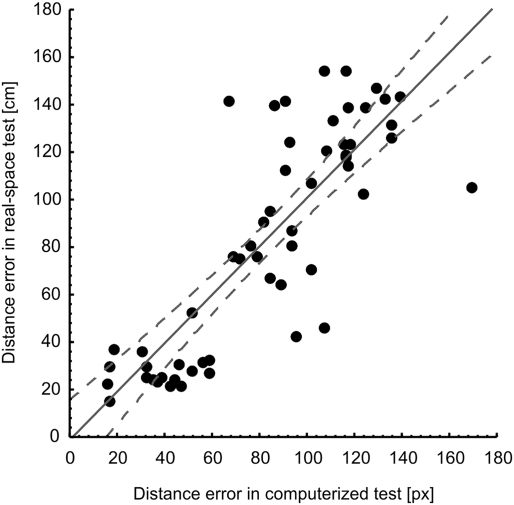
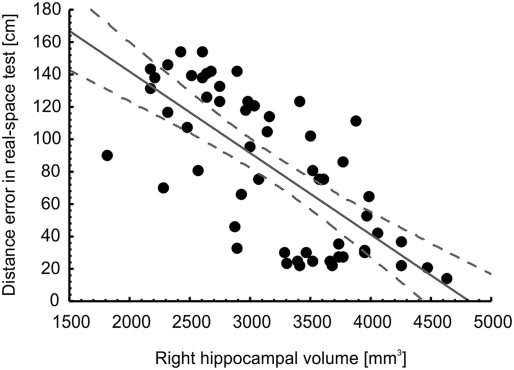
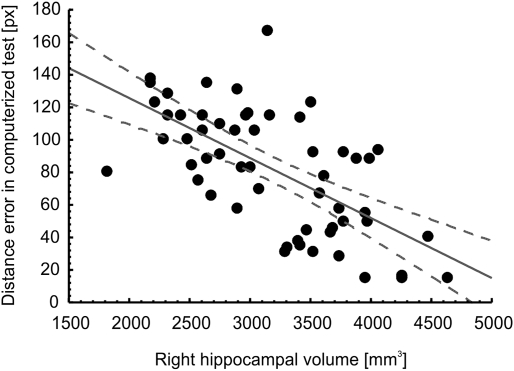
In correlational analyses with the entire sample (Table 1), age correlated negatively with hippocampal volume but not with total brain volume, and positively with the magnitude of errors in the real-space version of the hMWM but not in the virtual version. Women had lower total brain volume, but not lower hippocampal volume (potentially due to the lower likelihood to detect significant differences in such a relatively small structure) than men. Years of education correlated positively with hippocampal volume and negatively with the extent of errors on the spatial navigation tests. Positive correlations between brain and hippocampal volumes were strong as expected, as were negative correlations between brain/hippocampal volumes and the magnitude of errors on the spatial navigation tests. Finally, there was a strong correlation between navigation performance in the real-space and in the 2D computerized version, which is illustrated in Fig. 1. Figs. 2 and 3 illustrate the observed correlations between right hippocampal volume and performance on the real-space and 2D computerized versions of the navigation test, respectively.
Table 1.
Correlational matrix (n = 56)
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| 1) Age | – | |||||||
| 2) Female sex | −0.09 | – | ||||||
| 3) Years of education | −0.08 | −0.27* | – | |||||
| 4) Total brain volume | −0.02 | −0.50*** | 0.26 | – | ||||
| 5) Right hippocampal volume | −0.31* | −0.23 | 0.34* | 0.54*** | – | |||
| 6) Left hippocampal volume | −0.31* | −0.01 | 0.34* | 0.46*** | 0.81*** | – | ||
| 7) hMWM real version | 0.41** | −0.03 | −0.42** | −0.46*** | −0.71*** | −0.63*** | – | |
| 8) hMWM virtual version | 0.26 | 0.05 | −0.48*** | −0.40** | −0.64*** | −0.61*** | 0.83*** | – |
| Mean | 72.0 | – | 13.7 | 1,483,036 | 3,182 | 3,251 | 82.4 | 82.1 |
| SD | 8.2 | – | 3.5 | 125,957 | 659 | 693 | 46.3 | 37.7 |
| Range | 53–87 | – | 9–22 | 1,257,139–1,815,347 | 1,814–4,622 | 1,759–4,742 | 15.3–154.6 | 15.5–168.6 |
*P < 0.05, **P < 0.01, ***P < 0.001.
Fig. 1.
Correlation between allocentric spatial navigation accuracy obtained from the real-space and 2D computerized versions of the hMWM in the entire sample (r = 0.83, P < 0.001).
Fig. 2.
Correlation between right hippocampal volume in mm3 and allocentric spatial navigation accuracy evaluated as an average of distance error in centimeters between the participant's choice and the correct result across eight trials of each participant in the real-space version of the hMWM in the entire sample.
Fig. 3.
Correlation between right hippocampal volume in mm3 and allocentric spatial navigation accuracy evaluated as an average of distance error in pixels between the participant's choice and the correct result across eight trials of each participant in the 2D computerized version of the hMWM in the entire sample.
In multivariate regression analyses (Table 2) adjusted for age, sex, and years of education, the initial model (model 1), which included the covariates plus total brain volume score, yielded a significant association between smaller total brain volume and poorer performance (i.e., greater average distance error) on both the real-space and computerized version of the spatial navigation test. When the measure of the right hippocampal volume was added to variables from model 1 (model 2), there was a significant association between smaller right hippocampal volume and poorer performance on both versions of the spatial navigation test, whereas the association between total brain volume and spatial navigation was reduced to nonsignificant. The addition of right hippocampal volume into the regression model also led to a significant improvement in model fit, as indicated by the significant change in the R2 value. Finally, the addition of left hippocampal volume to the variables from model 2 in the final model (model 3), presumed to reflect verbal ability (and the potential bias by the level of understanding instructions), did not affect the results and did not contribute to model fit. Next, we assessed potential mediation of the link between total brain volume and spatial navigation by the right hippocampal volume using the Sobel test (15). The test was significant for the real-space (z = −3.62, P < 0.001) and 2D computerized (z = −3.35, P < 0.001) spatial navigation test.
Table 2.
Associations between spatial navigation performance and right hippocampal volume
| Model 1 |
Model 2 |
Model 3 |
||||
| β | P value | β | P value | β | P value | |
| Navigation in real space | ||||||
| Total brain volume | −0.54 | <0.001 | −0.30 | 0.008 | −0.33 | 0.004 |
| Right hippocampal volume | −0.48 | <0.001 | −0.62 | <0.001 | ||
| Left hippocampal volume | 0.19 | 0.225 | ||||
| Adjusted R2 | 0.51*** | 0.65*** | 0.65 | |||
| Navigation in virtual space | ||||||
| Total brain volume | −0.40 | <0.001 | −0.16 | 0.225 | −0.15 | 0.280 |
| Right hippocampal volume | −0.48 | <0.001 | −0.43 | 0.026 | ||
| Left hippocampal volume | −0.07 | 0.715 | ||||
| Adjusted R2 | 0.35*** | 0.48*** | 0.48 | |||
β, standardized regression coefficient; age, sex, and years of education are controlled. Model 1: covariates plus total brain volume; model 2: variables in model 1 plus right hippocampal volume; model 3: variables in model 2 plus left hippocampal volume. ***P < 0.001. P values reported with adjusted R2 indicate whether the addition of each variable led to a significant improvement in model fit compared with the previous model, adjusting for the change in the number of variables in the model.
We also estimated regression models for those with and without cognitive impairment separately (Table 3). Because model 2 was superior with respect to model fit in analyses with the entire sample, only results from this model were reported. The pattern of results was retained for those with but not those without cognitive impairment. The Sobel test assessing mediation of the associations between total brain volume and spatial navigation in cognitively impaired participants by right hippocampal volume was significant in the real space (z = −2.42, P = 0.015), but only approached significance in the virtual space (z = −1.75, P = 0.080).
Table 3.
Associations between spatial navigation performance and right hippocampal volume estimated separately by cognitive impairment
| With CI, n = 42 |
Without CI, n = 14 |
|||
| β | P value | β | P value | |
| Navigation in real space | ||||
| Total brain volume | −0.28 | 0.063 | 0.27 | 0.430 |
| Right hippocampal volume | −0.45 | 0.004 | 0.03 | 0.933 |
| R2 | 0.39*** | 0.08 | ||
| Navigation in virtual space | ||||
| Total brain volume | −0.23 | 0.168 | −0.11 | 0.760 |
| Right hippocampal volume | −0.33 | 0.054 | −0.22 | 0.521 |
| R2 | 0.21** | 0.08 | ||
β, standardized regression coefficient. ***P < 0.001, **P < 0.01. P values indicate whether the variance in spatial navigation explained by the independent variables is significant.
Discussion
We found that allocentric navigation impairment in a real-space setting and in its corresponding 2D computerized versions was proportional to the right hippocampal volume. These results appeared to reflect a link between the extent of right hippocampal atrophy and spatial navigation performance, particularly in those with cognitive impairment represented by aMCI or AD. We concluded that smaller right hippocampal volume irrespective of a total brain atrophy, as well as age, sex, education, and left hippocampal atrophy, is responsible for decline in allocentric navigation performance. Our results are consistent with previous studies indicating that the hippocampus is a key structure for allocentric navigation in animals (9, 16, 17) and humans (9, 18, 19).
DeIpolyi et al. (11) reported an association between overall navigation impairment (without distinguishing between egocentric and allocentric types of navigation) and atrophy of the right hippocampus in cognitively impaired subjects (aMCI and AD). We build on these findings by presenting allocentric navigation in relation to hippocampal volume in older cognitively impaired and cognitively healthy subjects examined within one study, and using both real-space and 2D computerized settings. One advantage of our study is that our test is a direct analogy of MWM, which is widely used to study animal spatial navigation (16, 17).
A recent study (12) reported impairment of allocentric navigation in the virtual reality park and smaller right and left hippocampal volumes in aMCI patients than in controls. However, hippocampal volumes did not correlate with allocentric navigation accuracy neither in the main analysis (with all subjects together) nor in the subanalyses (with aMCI and control subjects separately). Conversely, we found an association between right hippocampal volume and allocentric navigation that was demonstrated in the real-space and its corresponding 2D computerized version.
We found the associations between right hippocampal atrophy and spatial navigation performance to be independent of total brain atrophy. In fact, we found that right hippocampal atrophy served as a mediator of the initially observed association between total brain atrophy and navigation performance, providing further evidence for the importance of right hippocampal volume for navigation performance. Our hMWM test was shown to reflect hippocampal impairment. Given that the hippocampus is impaired early on during the course of AD and aMCI, our test might be used as an early marker of aMCI and AD, despite the relatively large heterogeneity within aMCI patients with respect to clinical progression (20). Future research that includes follow-up data should explore the utility of the test to predict the conversion from aMCI to AD and the onset of aMCI.
We also tested whether left hippocampal volume may partially explain the association between right hippocampal volume and navigation performance. We found essentially no effect of left hippocampus, indicating that the results were not confounded by abilities associated with this brain structure.
We found a parallel pattern of results in the real-space and computerized versions, confirming our previous findings (3). Although the two versions may not be fully interchangeable, including some reliance of computer skills of the subject, this finding still points to the utility of the relatively simple-to-administer computer-based test to assess potential spatial navigation deficits to identify early signs of incipient AD, as was suggested previously (3).
We should mention that we were unable to evaluate the association between allocentric navigation and brain structures other than the hippocampus. Neuroimaging studies using patients with MCI (21) indicate significant differences in volumes of multiple brain regions, including, for example, entorhinal cortical thickness and volume and parahippocampal gyrus volume. There are human functional MRI studies suggesting that allocentric navigation may be dependent on parahippocampal activity during exploration of a virtual-reality maze (22).Thus, we might expect that hippocampus-adjacent structures such as parahippocampal gyrus (predominantly on the right) may also relate to allocentric navigation. However, structural correlates of allocentric navigation in MCI and AD subjects are still underexplored. Future studies should focus on more complex structural correlates of allocentric navigation, especially in cognitively impaired individuals.
In conclusion, our results indicate that the right hippocampus plays a critical role in allocentric navigation in older adults in the real space and virtual space, especially in those with cognitive impairment. Together, the results can serve as a basis for future research to ascertain the ability of spatial navigation testing to identify patients in the preclinical stage of AD, where hippocampal deficits are among the primary symptoms. In addition, the findings indicate that our computer version of the human analog of the Morris water maze can reasonably imitate navigation in the real world and serve as a useful, inexpensive, and reliable screening tool for early detection of hippocampal dysfunction in older adults.
Methods
Subjects.
We recruited all participants at the Memory Clinic, Department of Neurology, Motol University Hospital and 2nd Faculty of Medicine, Charles University in Prague. Cognitively intact controls were caregivers and/or family members of the patients or volunteers from among students of the University of the Third Age, associated with 2nd Faculty of Medicine, Charles University in Prague. All participants signed an informed consent on the study approved by the university hospital ethical committee. Only right-handed persons were included. Participants underwent standard neurological and laboratory evaluation, followed by a semistructured interview and extended neuropsychological testing. The test battery included Clinical Dementia Rating, Activities of Daily Living, Hachinski Ischemic Scale, Geriatric Depression Scale (GDS), Mini Mental State Examination (MMSE). The neuropsychological test battery was comprised of the Clock Drawing Test, Auditory Verbal Learning Test (AVLT), Free and Cued Selective Reminding Test with Immediate Recall (FCSRT-IR), digit span forward and reversed, Initial Letter Fluency, Trail-Making Tests (TMT) A and B, and Rey–Osterrieth complex figure. Participants with depression (GDS > 7) were excluded.
Participants were classified using established clinical criteria and the results of neuropsychological tests (Table 4). The group with cognitive impairment (n = 42) included 16 patients with mild probable AD and three with moderate probable AD, and 23 patients with aMCI (see Table 5 for demographic information for study participants). Subjects with AD met the Diagnostic and Statistical Manual of Mental Disorders IV-TR criteria for dementia (23) and the National Institute of Neurological and Communicative Disorders and Stroke and Alzheimer Disease and Related Disorders Association criteria for probable AD (24). Patients with aMCI met the clinical criteria for MCI established by Petersen and colleagues (20, 25). The threshold for memory impairment was derived from the same literature as scoring >1.5 SD below the mean of age- and education-adjusted norms on any memory test. We also included 14 older controls who did not meet the criteria for cognitive impairment.
Table 4.
Neuropsychological results
| Cognitively intact, mean (SD) | Cognitively impaired, mean (SD)* | |
| AVLT1-6 | 62.0 (11.0) | 32.5 (13.1) |
| AVLT1-5 | 52.7 (10.9) | 28.8 (10.5) |
| AVLT after 30 min | 11.6 (3.02) | 2.4 (3.1) |
| TMT A | 17.6 (4.2) | 30.7 (17.3) |
| TMT B | 69.3 (26.9) | 253.1 (158.9) |
| FAS total | 43.2 (6.2) | 31.1 (12.4) |
| Digit span forward numbers | 6.7 (1.3) | 5.3 (1.1) |
| Digit span reversed numbers | 5.1 (1.0) | 3.9 (1.1) |
| FCSRT-IR total score (free + cued) | 15.9 (0.3) | 12.3 (4.1) |
| FCSRT-IR free recall score | 10.9 (2.1) | 4.6 (3.3) |
| Benton A errors | 3.2 (2.1) | 13.1 (4.6) |
| Benton C errors | 0.6 (1.4) | 2.5 (1.9) |
*In independent t tests, the groups differed significantly in all neuropsychological test scores (P < 0.001).
Table 5.
Demographic information for the participants in this study
| Cognitively intact, mean (SD) | Cognitively impaired, mean (SD) | P value | |
| N | 14 | 42 | – |
| Age (y) | 68.1 (7.1) | 73.3 (8.2) | 0.038 |
| Years of education | 15.4 (3.9) | 13.1 (3.5) | 0.033 |
| % female | 85.2 | 59.5 | 0.078 |
| MMSE | 29.4 (0.7) | 24.8 (3.6) | <0.001 |
P values are based on independent t tests for differences in means and a χ2 test for a difference in frequencies.
Spatial Navigation Testing: The Hidden Goal Task.
The Hidden Goal Task (HGT) is a human analog of the MWM (14), where the allocentric vs. egocentric types of navigation are tested separately. Allocentric navigation is independent of individual's position on the start; it is the prominent distal cues in the subject's environment that are used to navigate toward the goal. However, egocentric navigation is dependent on individual's body position on the start. The start-goal distance and the start-goal direction are used to find the goal. Following our hypothesis, in this study we evaluated allocentric subtask. The main task of the participant was to find the hidden goal, using allocentric strategy. We used two versions of the HGT. In brief, it was the real-space version, represented by circular velvet arena, 2.9 m in diameter, and virtual 2D computerized test. First, the 2D computerized version was administered. A map-like view of the arena was projected on a 17-inch computer touch-screen. The arena was depicted as a large white circle with the start position and two distinct, red and green, orientation cues on its circumference. The red circle inside the arena represented the goal. In the beginning of the 2D computerized test, the correct goal position and mutual relationship with the orientation cues was presented to the participant. This feedback was provided before the first trial of the task and after each trial of eight (to facilitate learning), but not during the real-space version testing. The participant was requested to move a touch-screen pen from the start to the supposed goal and draw the entire route. Similarly, in the real-space version, the goal position on the floor was pointed out with a handheld pointer stick. The entire procedure is described in detail elsewhere (3).
The mutual relationship of the hidden goal and the orientation cues was stable across all trials. The navigation performance was measured as the average distance error between goal position determined by participant and the correct goal position that was programmed for each trial of eight. There was no time limit.
MRI Acquisition.
All participants were examined in a 1.5 T MRI scanner (Gyroscan; Philips Medical Systems). A 3D T1-weighted fast field echo sequence was obtained with the following scanning variables: coronal acquisition, 1.0-mm slice thickness; total scanning time, 14 min; TR = 25 ms; TE = 5 ms; flip angle = 30°; field of view, 256 mm; and matrix 256 × 256. Initial visual assessment was performed by an experienced neuroradiologist to discard any other relevant brain pathology and to ensure appropriate study quality.
MRI Volumetry.
Automatic cortical parcellation and labeling of total brain, right and left hippocampal volumes was performed by free experimental software FreeSurfer package (26) (v4.4.0; http://surfer.nmr.mgh.harvard.edu), implemented into a Mac OS X (Apple) workstation. The original raw Digital Imaging and Communications in Medicine data were converted to FreeSurfer's appropriate .mgz format. FreeSurfer provides a fully automatic cortical parcellation and segmentation of subcortical structures. The program calculates brain subvolumes by assigning a neuroanatomical label to each voxel based on probabilistic information estimated automatically from a manually labeled training set. In brief, FreeSurfer's processing includes motion correction; removal of nonbrain tissue using a hybrid watershed/surface deformation procedure; multiple intensity and spatial normalization; Talairach transformation; and segmentation of the subcortical white matter and deep gray matter structures (27, 28). The overall process and analysis pipeline has been described elsewhere (http://surfer.nmr.mgh.harvard.edu). FreeSurfer was evaluated as a reasonable substitute for manual tracing (29) and is commonly used in the studies (21). The analysis outputs were checked by knowledgeable operator. Finally, the total brain, right and left hippocampal volumes were calculated in cubic centimeters and millimeters, respectively.
Data Analysis.
For analysis, the average distance error across eight trials between the participant's choice and the correct goal position was evaluated. The average distance error was measured in centimeters in the real-space and in pixels in the 2D computerized versions. Initially, we calculated Pearson correlation coefficients to explore bivariate relationships within the data. We also inspected score distribution for all continuous variables, and found both skewness and kurtosis within acceptable range (±0.5). In the main analyses, we used multivariate linear regression models to estimate associations between spatial navigation performance and the right hippocampal volume. Participants with and without cognitive impairment differed significantly with respect to age [t(54) = 2.12, P < 0.05] and education [t(54) = 2.19, P < 0.05], and the result for sex differences approached significance [χ2(1) = 3.21, P = 0.073]. These variables have also been found to relate to spatial navigation ability in previous studies (30–33) and therefore were controlled in the analyses. Total brain (model 1), right (model 2), and left (model 3) hippocampal volume measures were added sequentially. We report standardized regression coefficients, which allow a direct comparison of the magnitude of the associations by standardizing the variance of each variable to the same metric. Model fit was assessed by the R2 value adjusted for the number of variables in the model. In subsequent analyses, we also estimated the same associations separately in cognitively impaired and cognitively intact participants.
We performed the Sobel test (15) to assess whether the apparent mediation of the association between total brain volume and spatial navigation performance by right hippocampal volume would reach statistical significance. The Sobel test was estimated in a mediation analysis proposed by Preacher and Hayes (34), which extends the work of Baron and Kenny (35); the advantages include estimation of all three hypothesized pathways (predictor to outcome; predictor to mediator, and mediator to outcome) simultaneously and incorporating a bootstrapping technique, which reduces sample size demands (36). The result is expressed as a Z test, where the absolute value >1.96 corresponds to statistical significance at P < 0.05. SAS software version 9 was used in all analyses, with statistical significance set at a two-tailed 0.05 level.
Acknowledgments
Support for this work was provided by Grant Agency of the Czech Republic Grants 309/09/1053 and 309/09/0286; European Regional Development Fund Project St. Anne's University Hospital, Brno, International Clinical Research Center CZ.1.05/1.1.00/02.0123; Ministry of Education, Youth and Sports of the Czech Republic Grants 1M0517 and LC554; Research Project AV0Z50110509; and Internal Grant Agency of the Ministry of Health Grant IGA NS 10331.
Footnotes
The authors declare no conflict of interest.
References
- 1.McShane R, et al. Getting lost in dementia: A longitudinal study of a behavioral symptom. Int Psychogeriatr. 1998;10:253–260. doi: 10.1017/s1041610298005365. [DOI] [PubMed] [Google Scholar]
- 2.Pai MC, Jacobs WJ, Int J. Topographical disorientation in community-residing patients with Alzheimer's disease. Int J Geriatr Psychiatry. 2004;19:250–255. doi: 10.1002/gps.1081. [DOI] [PubMed] [Google Scholar]
- 3.Hort J, et al. Spatial navigation deficit in amnestic mild cognitive impairment. Proc Natl Acad Sci USA. 2007;104:4042–4047. doi: 10.1073/pnas.0611314104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Keefe J, Nadel L. The Hippocampus as a Cognitive Map. New York: Oxford Univ Press; 1978. [Google Scholar]
- 5.Maguire EA, et al. Knowing where and getting there: A human navigation network. Science. 1998;280:921–924. doi: 10.1126/science.280.5365.921. [DOI] [PubMed] [Google Scholar]
- 6.Packard MG, Knowlton BJ. Learning and memory functions of the basal ganglia. Annu Rev Neurosci. 2002;25:563–593. doi: 10.1146/annurev.neuro.25.112701.142937. [DOI] [PubMed] [Google Scholar]
- 7.White NM, McDonald RJ. Multiple parallel memory systems in the brain of the rat. Neurobiol Learn Mem. 2002;77:125–184. doi: 10.1006/nlme.2001.4008. [DOI] [PubMed] [Google Scholar]
- 8.Weniger G, Ruhleder M, Wolf S, Lange C, Irle E. Egocentric memory impaired and allocentric memory intact as assessed by virtual reality in subjects with unilateral parietal cortex lesions. Neuropsychologia. 2009;47:59–69. doi: 10.1016/j.neuropsychologia.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 9.O'Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34:171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- 10.Spiers HJ, et al. Unilateral temporal lobectomy patients show lateralized topographical and episodic memory deficits in a virtual town. Brain. 2001;124:2476–2489. doi: 10.1093/brain/124.12.2476. [DOI] [PubMed] [Google Scholar]
- 11.deIpolyi AR, Rankin KP, Mucke L, Miller BL, Gorno-Tempini ML. Spatial cognition and the human navigation network in AD and MCI. Neurology. 2007;69:986–997. doi: 10.1212/01.wnl.0000271376.19515.c6. [DOI] [PubMed] [Google Scholar]
- 12.Weniger G, Ruhleder M, Lange C, Wolf S, Irle E. Egocentric and allocentric memory as assessed by virtual reality in individuals with amnestic mild cognitive impairment. Neuropsychologia. 2011;49:518–527. doi: 10.1016/j.neuropsychologia.2010.12.031. [DOI] [PubMed] [Google Scholar]
- 13.Burgess N, Trinkler I, King J, Kennedy A, Cipolotti L. Impaired allocentric spatial memory underlying topographical disorientation. Rev Neurosci. 2006;17:239–251. doi: 10.1515/revneuro.2006.17.1-2.239. [DOI] [PubMed] [Google Scholar]
- 14.Kalová E, Vlcek K, Jarolímová E, Bures J. Allothetic orientation and sequential ordering of places is impaired in early stages of Alzheimer's disease: Corresponding results in real space tests and computer tests. Behav Brain Res. 2005;159:175–186. doi: 10.1016/j.bbr.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 15.Sobel ME. In: Sociological Methodology. Leinhardt S, editor. Washington, DC: Am Soc Assoc; 1982. pp. 290–312. [Google Scholar]
- 16.Morris RG, Garrud P, Rawlins JN, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 17.Morris RG. Spatial localization does not require the presence of local cues. Learn Motiv. 1981;12:239–261. [Google Scholar]
- 18.Burgess N, Jeffery KJ, O'Keefe J. The Hippocampal and Parietal Foundations of Spatial Cognition. Oxford: Oxford Univ Press; 1999. [Google Scholar]
- 19.Astur RS, Taylor LB, Mamelak AN, Philpott L, Sutherland RJ. Humans with hippocampus damage display severe spatial memory impairments in a virtual Morris water task. Behav Brain Res. 2002;132:77–84. doi: 10.1016/s0166-4328(01)00399-0. [DOI] [PubMed] [Google Scholar]
- 20.Petersen RC. Mild cognitive impairment. Continuum (N Y) 2004;10:9–28. [Google Scholar]
- 21.Desikan RS, et al. Alzheimer's Disease Neuroimaging Initiative Automated MRI measures identify individuals with mild cognitive impairment and Alzheimer's disease. Brain. 2009;132:2048–2057. doi: 10.1093/brain/awp123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aguirre GK, Detre JA, Alsop DC, D'Esposito M. The parahippocampus subserves topographical learning in man. Cereb Cortex. 1996;6:823–829. doi: 10.1093/cercor/6.6.823. [DOI] [PubMed] [Google Scholar]
- 23.American Psychiatric Association . 2000. Diagnostic and Statistical Manual of Mental Disorders (Am Psych Assoc, Washington, DC), 4th Ed, text rev. [Google Scholar]
- 24.McKhann G, et al. Clinical diagnosis of Alzheimer's disease: Report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer's disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 25.Petersen RC, et al. Mild cognitive impairment: Clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 26.Fischl B, et al. Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 27.Ségonne F, et al. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22:1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 28.Fischl B, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- 29.Morey RA, et al. A comparison of automated segmentation and manual tracing for quantifying hippocampal and amygdala volumes. Neuroimage. 2009;45:855–866. doi: 10.1016/j.neuroimage.2008.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iaria G, Palermo L, Committeri G, Barton JJ. Age differences in the formation and use of cognitive maps. Behav Brain Res. 2009;196:187–191. doi: 10.1016/j.bbr.2008.08.040. [DOI] [PubMed] [Google Scholar]
- 31.Moffat SD, Resnick SM. Effects of age on virtual environment place navigation and allocentric cognitive mapping. Behav Neurosci. 2002;116:851–859. doi: 10.1037//0735-7044.116.5.851. [DOI] [PubMed] [Google Scholar]
- 32.Astur RS, Tropp J, Sava S, Constable RT, Markus EJ. Sex differences and correlations in a virtual Morris water task, a virtual radial arm maze, and mental rotation. Behav Brain Res. 2004;151:103–115. doi: 10.1016/j.bbr.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 33.Rahman Q, Koerting J. Sexual orientation-related differences in allocentric spatial memory tasks. Hippocampus. 2008;18:55–63. doi: 10.1002/hipo.20375. [DOI] [PubMed] [Google Scholar]
- 34.Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods Instrum Comput. 2004;36:717–731. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- 35.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 36.MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test the significance of the mediated effect. Psychol Methods. 2002;7:83–10. doi: 10.1037/1082-989x.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]