OLFM4 is located in specific granules of a subset of human neutrophils.
Keywords: granule proteins, bone marrow, PMN, NGAL
Abstract
OLFM4 was identified initially as a gene highly induced in myeloid stem cells by G-CSF treatment. A bioinformatics method using a global meta-analysis of microarray data predicted that OLFM4 would be associated with specific granules in human neutrophils. Subcellular fractionation of peripheral blood neutrophils demonstrated complete colocalization of OLFM4 with the specific granule protein NGAL, and stimulation of neutrophils with PMA resulted in corelease of NGAL and OLFM4, proving that OLFM4 is a genuine constituent of neutrophil-specific granules. In accordance with this, OLFM4 mRNA peaked at the MY/MM stage of maturation. OLFM4 was, however, present in only 20–25% of peripheral blood neutrophils, as determined by immunocytochemistry and flow cytometry, whereas mRNA for OLFM4 was present in all MY/MM, indicating post-transcriptional regulation as a basis for the heterogeneous expression of OLFM4 protein.
Introduction
OLFM4 is expressed in epithelial cells of the prostate, small intestine, colon, and cells in the bone marrow[1, 2]. It is also expressed in several cancers [3–6] and up-regulated in epithelial cells during inflammation [7]. In accordance with this, OLFM4 expression has been shown to be regulated by NF-κB [8]. These characteristics are to a large extent shared by NGAL, a siderophore-binding glycoprotein [9], identified as a major constituent of neutrophil-specific granules [10]. Little is known about the localization of OLFM4 in cells, but both nuclear and mitochondrial localizations have been proposed [1, 11]. Recently, OLFM4 was suggested to interact with cathepsin c, a protease expressed in PMs and essential for activation of the azurophil granule serine proteases [12]. A bioinformatics method to predict gene function [13], which has performed well on benchmark datasets [14], predicted that OLFM4 would be associated with neutrophil-specific granules. We therefore investigated the subcellular localization of OLFM4 in neutrophils from peripheral blood and the expression during myelopoiesis in the bone marrow.
MATERIALS AND METHODS
Bioinformatics
Full details about the algorithm are published in ref. [13]. Briefly, the approach entails a global meta-analysis of 3,600 human two-color microarray experiments to identify consistently coexpressed genes across different conditions, which imply biological coregulation [15]. Using a literature-mining algorithm (IRIDESCENT) to identify commonalities [16], functional associations were predicted. We queried it to find novel genes predicted to be associated with specific granules. Known specific granule genes served as positive controls.
Antibody generation
Polyclonal rabbit antibodies were generated (Dako, Glostrup, Denmark) against a synthetic peptide (Schafer-N, Copenhagen, DK), representing aa 96–143 of the N-terminal OLFM4 sequence.
Isolation of cells
Bone marrow aspirate or peripheral blood was obtained from healthy volunteers. The study was approved by the local ethics committee. Bone marrow cells were separated as described previously [17]. Nongranulocytic cells were removed by mAb: CD2, CD3, CD10, CD14, CD16, CD19, CD36, CD56, CD61, and glycophorin A using MACS (Miltenyi Biotec, Germany). Peripheral blood neutrophils were isolated as described previously [18].
SDS-PAGE/immunoblotting
Immunoblotting was performed as described previously [19]. Primary antibodies: rabbit anti-human OLFM4 (1:500; antibody 3569, in-house), mouse anti-human cytochrome c (1:1000; antibody 13,575, Abcam, Cambridge, MA, USA); secondary antibodies: HRP-conjugated goat anti-rabbit (1:1000; P0449, Dako) or HRP-conjugated rabbit anit-mouse (1:1000; P0260, Dako).
Quantitative PCR
RNA was isolated using TRIzol (Invitrogen, Carlsbad, CA, USA). cDNA was prepared from 1 μg total RNA using random hexamer primers and Superscript II, as described by the manufacturer (Invitrogen). Real-time PCR was performed using TaqMan gene expression assays (OLFM4 Hs00197437; MPO Hs00165162_m1; LCN2 Hs00194353_m1; MMP9 Hs00234579_m1; ACTB 4326315E, Applied Biosystems, Foster City, CA, USA) on a 3000-P real-time PCR machine (Stratagene, La Jolla, CA, USA). All normalized to ACTB.
Subcellular fractionation
Subcellular fractionation of isolated neutrophils was performed on unstimulated cells and cells stimulated by PMA (Sigma-Aldrich, St. Louis, MO, USA) as described previously [20].
Immunohistochemistry
Cytospins were prepared with 2 × 105 neutrophils/slide. Immunohistochemistry was performed using the REAL EnVision detection system as described by the manufacturer (Dako). Primary antibodies: rabbit anti-human OLFM4 3569 (in-house; 5 ng/μL, 2.5 ng/μL), GC-1(N-20) (Santa Cruz Biotechnology, Santa Cruz, CA, USA; 5 ng/μL, 2.5 ng/μL), PAB0314 (Abnova, Taiwan; 5 ng/μL, 2.5 ng/μL), rabbit Ig X0903 (Dako; 5 ng/μL, 2.5 ng/μL). Cytospins were examined in a BX51 microscope with a DP70 photosystem and analysis 5.0 software (Olympus, Hamburg, Germany). Adobe Photoshop CS5, version 12.0, was used to prepare the images.
Fluorescent immunohistochemistry
Fluorescent immunohistochemistry was performed as described previously [19]. The following primary antisera were used: rabbit anti-human MPO (A0398, Dako), rabbit anti-human NGAL (in-house), rabbit anti-human gelatinase (in-house), all detected with Alexa594-labeled goat anti-rabbit antibody. This was followed by reaction with rabbit anti-human OLFM4 antibody [GC1(N-20), Santa Cruz Biotechnology] at 0.5 μg/mL, using detection with Alexa488-labeled secondary antibodies. Images were acquired in a LSM 700 (Zeiss, Thornwood, NY, USA) microscope equipped with ZENworks (Novell, Provo, UT, USA) software. Adobe Photoshop CS5 Extended, version 12.0, was used to prepare the images.
RNA-FISH
MY/MM were isolated from bone marrow. RNA-FISH was performed as described in ref. [21], except for fixation, which was in 1% paraformaldehyde overnight. The following probes were used for hybridization: OLFM4, 5′FAM-TTGCTATTGTATATAAATGCTCGAGAGTTGCGGATCACC-3′FAM and 5′FAM-TATTGACTTTGCTGTGGATGAGAATGGATTGTGGGTTAT-3′FAM; LCN2, 5′digoxigenin-TCTACGGGAGAACCAAGGAGCTGACTTCGGAACTAAGG-3′digoxigenin; cAMP, 5′digoxigenin-TGGCAAAGAGTTTAAAAGAATTGTCCAGAGAATCAAGG-3′digoxigenin. Slides were analyzed with a Leica DMXRA epifluorescence microscope equipped with a Leica DFC340 FX camera and Leica CW4000 FISH software (Leica Microsystems, Germany).
Flow cytometry
Isolated neutrophils were fixed in 4% formaldehyde for 20 min at room temperature and permeabilized in TBS/1% BSA/1% Triton X-100, 30 min, 4°C. After blocking 10 min in TBS/1% BSA, cells were incubated 30 min with FITC-conjugated antibodies targeting OLFM4 [GC-1(N-20), Santa Cruz Biotechnology; 1:500], gelatinase (in-house; 1:100), or rabbit IgG (BD Biosciences, Franklin Lakes, NJ, USA; 1:1000) and analyzed on FACSsort (BD Biosciences) with CellQuest software.
Sorting of neutrophils in OLFM4 high/OLFM4 low populations
Isolated neutrophils from healthy donors were fixed, permeabilized, and blocked as described above. For depletion of residual nongranulocytic cells, PMNs were stained with a mixture of biotinylated mAb (Supplemental Table 1), together with intracellular staining of OLFM4, gelatinase, and an isotype control, as described above. After staining, cells were washed and incubated with streptavidin, coupled to the phycoerythincyanine 5-fluorochrome (BioLegend, San Diego, CA, USA). Cells were sorted using the FACSAria cell sorter (BD Biosciences), equipped with FACSDiva software. Analysis of data was performed using the FlowJo software package (Version 9.2, MAC).
RESULTS AND DISCUSSION
OLFM4 is a genuine, specific granule protein
OLFM4, situated on chromosome 13q14.3, codes for a 510-aa protein, of which the N-terminal 20 aa fulfill the characteristics for a signal peptide, according to SignalP 3.0 [22]. This indicates that OLFM4 is routed to the secretory pathway. Real-time PCR showed that OLFM4 is expressed during myelopoiesis and that the expression peaks at the MY/MM stage of differentiation and declines as the cells mature (Fig. 1A). This is similar to specific granule proteins, such as NGAL, lactoferrin, and hCAP18, and distinct from MPO, which is maximal in PMs, and from gelatinase (MMP9), which peaks in bm-PMN [23, 24].
Figure 1. OLFM4 is a constituent of neutrophil-specific granules and is maximally transcribed at the MY/MM stage of myeloid differentiation.
(A) Relative expression levels of mRNA for OLFM4, MPO, LCN2 (NGAL), and gelatinase in human bone marrow cells separated into three populations: MB/PM (MB/PM), MY/MM, bm-PMN, and on PMN isolated from peripheral blood (Pb-PMN). Human ACTB was used as a normalizer; performed in triplicates. Bars represent sd. (B) Immunoblot of subcellular neutrophil fractions showing OLFM4 with a MW of ∼64 kDa present in fraction 9-18. (C) Distribution profiles of MPO (marker of azurophil granules), NGAL (marker of specific granules), gelatinase (marker of gelatinase granules), and HSA (marker of secretory vesicles), all measured by ELISA. Distribution profiles for OLFM4 and cytochrome c are based on intensity measurements of bands on immunoblot. y-Axis represents an arbitrary scale where the highest measured value of each protein is given the value 1. (D) Distribution profiles of OLFM4, NGAL, MPO, and cytochrome c from unstimulated neutrophils and neutrophils stimulated with 100 ng/mL PMA prior to nitrogen cavitation and subcellular fractionation. Stimulation of cells with PMA causes mobilization of ∼60% of specific granules and leads to a similar reduction in OLFM4 and in the specific granule protein marker NGAL. The content of the azurophil granule protein MPO and the mitochondrial marker cytochrome c is virtually unchanged by stimulation. Results for MPO and NGAL were measured by ELISA (y-axis represents ng/mL). Results for OLFM4 and cytochrome c are based on density measurements of bands on immunoblots [Supplemental Fig. 1 A and B; y-axis represents intensity (INT)/mm2].
OLFM4 was identified by immunoblotting of subcellular fractions of neutrophils (Fig. 1B). The MW of OLFM4 was 64 kDa but was reduced to the predicted 55 kDa by N-glycanase (data not shown). The identity of the immunoreactive band at 64 kDa as OLFM4 was confirmed by mass spectrometry (Supplemental Fig. 2). ELISA for granule marker proteins confirmed colocalization of OLFM4 with NGAL (Fig. 1C). OLFM4 has previously been claimed to be associated with mitochondria in neutrophils [25]; therefore, we examined the localization of cytochrome c, a marker of mitochondria in the gradient. The distribution profiles indicate that cytochrome c does not colocalize with OLFM4.
NGAL and OLFM4 were comobilized from granules during stimulation with PMA, a potent secretagogue of peroxidase-negative granules of neutrophils [18]. Figure 1D shows that PMA stimulation reduced the amount of OLFM4 and NGAL with >50% in the fractions, where specific granules localize prior to stimulation, whereas the amount of cytochrome c was unchanged by PMA stimulation.
We therefore conclude that OLFM4 is a genuine-specific granule protein and exclude a mitochondrial localization of OLFM4 in neutrophils.
OLFM4 is heterogeneously expressed in neutrophils
Immunocytochemistry showed that OLFM4 is heterogeneously expressed in peripheral blood neutrophils with only 20–25% of neutrophils expressing the protein (Fig. 2A). To ascertain that this expression is independent of the particular source of antibody, the expression was tested using two commercial anti-OLFM4 antibodies: GC-1(N-20) (Santa Cruz Biotechnology) and PAB0314 (Abnova), both giving the same expression. To test this further, we developed an in-house rabbit antibody by immunization with a synthetic peptide covering aa 76–123 of the mature protein. All antibodies showed similar heterogeneous expression by immunocytochemistry (Supplemental Fig. 3).
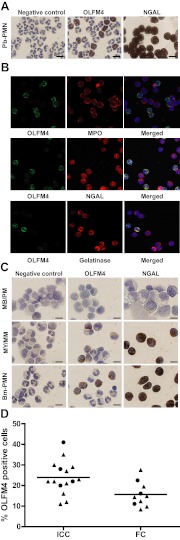
Figure 2. OLFM4 protein is differentially expressed in neutrophils and myeloid precursors.
(A) Immunocytochemistry of OLFM4 and NGAL in mature PMNs isolated from peripheral blood with antibody raised against synthetic OLFM4 peptide (antibody 3569). Original scale bars represent 20 μm. Preabsorbtion of the antibody with synthetic OLFM4 peptide completely abrogated the immunostaining (data not shown). (B) Confocal microscopy of isolated neutrophils stained for OLFM4 [green fluorescence (Alexa488)], in combination with the azurophil granule marker MPO, the specific granule marker NGAL, or the tertiary granule marker gelatinase [all marked with red fluorescence (Alexa594)]. In merged pictures, colocalization (yellow) with NGAL and gelatinase is seen together with DAPI staining (blue) of nuclei. (C) Immunocytochemistry of OLFM4 and NGAL in bone marrow populations enriched in MB/PM, MY/MM, and in bm-PMN shows that OLFM4 appears at the myelocyte stage of maturation. OLFM4 is differentially expressed, whereas NGAL is present in all neutrophils. (D) Isolated neutrophils from healthy donors. Fraction of OLFM4 high cells measured by immunocytochemistry (ICC; n=15) or flow cytometry (FC; n=11). Circles represent female donor; triangles represent male donor; bars represent mean value.
Confocal immunocytochemistry demonstrated that OLFM4 colocalizes with NGAL but not with MPO, present in azurophil granules. Colocalization was also seen with Gelatinase (Fig. 2B). Immunocytochemistry of bone marrow cells showed that OLFM4 appears in myelocytes. Again, only a subset of the neutrophil precursors stains positive for OLFM4 (Fig. 2C). We examined 15 healthy individuals and found a mean fraction of OLFM4-positive neutrophils of 24.9% (95% confidence interval: 19.5–28.4). No gender difference was observed (Fig. 2D).
To further exclude that the heterogeneous OLFM4 expression is a result of antigen masking, we performed flow cytometry on fixed, permeabilized neutrophils labeled with OLFM4 antibody. As seen in Fig. 3A, two populations of cells were distinguished by their expression of OLFM4, again with ∼25% having high OLFM4 expression. This is in contrast to expression Gelatinase, another granule protein present in neutrophil peroxidase-negative granules in all neutrophils. Gelatinase was chosen for practical reasons, as we had a FITC-conjugated antibody available (Fig. 3B). As the number of cells determined by flow cytometry greatly exceeds the number studied by immunocytochemistry, we performed flow cytometry on 11 healthy individuals. This showed that the fraction of OLFM4 high cells was slightly lower than determined by visual counting in the immunocytochemistry slides (Fig. 2D).
Figure 3. FACS of neutrophils confirms the existence of two subsets: OLFM4 high and OLFM4 low.
(A) Isolated neutrophils from a healthy donor unstained or stained with a FITC-conjugated isotype control or with a FITC-conjugated anti-OLFM4 antibody [GC-1(N-20)], clearly showing two distinct populations of OLFM4, whereas staining for gelatinase (B) reveals only one population. (C) Sorting strategy for FACS of OLFM4 high and OLFM4 low populations depicting the gating of the two populations. FITC-A, FITC-area; FSC-A, forward-scatter-area. (D) Western blot of sorted populations. Samples representing 4 × 105 cells were applied in each well. Whereas the OLFM4 low population has no immunoreactivity for OLFM4, the amount of NGAL is equal in the two populations. (E) Cytospins of the sorted populations acquired in a LSM 700 (Zeiss) microscope.
The OLFM4 high and low cells were separated and subjected to Western blotting (Fig. 3D) and confocal fluorescence microscopy (Fig. 3E). In Western blotting, OLFM4 was clearly not detected in the OLFM4 low subset, whereas NGAL was equally detected in the two populations, thus proving the heterogeneous expression of OLFM4 in neutrophils. The fluorescence microscopy on the other hand (Fig. 3E) indicates that the OLFM4 low cells do in fact have some OLFM4 immunereactivity and that the weak signal found by flow cytometry in the OLFM4 low population is not merely a matter of unspecific binding of the anti-OLFM4 antibody.
To test whether the heterogeneity in OLFM4 expression in neutrophils was determined by differences in the expression of the OLFM4 gene, FISH was performed to display mRNA for OLFM4 at the MY/MM stage of cellular differentiation, where the mRNA is at its peak. The expression of OLFM4 was compared with expression of cAMP, which encodes hCAP18, and LCN2, which encodes NGAL, both of which are specific granule proteins present in all neutrophils. We identified OLFM4 mRNA in all cells with no difference in expression of OLFM4 mRNA compared with the mRNA of hCAP18 and NGAL (Fig. 4).
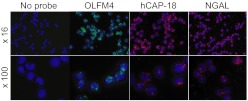
Figure 4. OLFM4 mRNA is present in all cells at the MY/MM stage of differentiation.
Fluorescent in situ hybridization of MY/MM isolated from human bone marrow using no probe, a probe for OLFM4 mRNA, a probe for NGAL mRNA, or a probe for hCAP18 mRNA. mRNA for OLFM4 is present in all cells at the MY/MM stage of differentiation and shows no differential expression compared with NGAL and hCAP18.
The fact that only 20–25% of neutrophils stained positive for OLFM4 in immunocytochemistry is puzzling. To test whether the expression is influenced by G-CSF stimulation in bone marrow, we analyzed the OLFM4 expression in peripheral blood neutrophils from donors treated with G-CSF. In accordance with the results from immunestaining of bm-PMNs (Fig. 2B), we found a heterogeneous OLFM4 expression independent of G-CSF treatment (Table 1).
Table 1. G-CSF-Treated Donors Versus Nontreated Donors.
| Nontreated | G-CSF | |
|---|---|---|
| 15 | 35 | |
| 16 | 8 | |
| 8 | 46 | |
| 11 | 49 | |
| 23 | 19 | |
| 10 | ||
| 12 | ||
| 14 | ||
| 28 | ||
| 20 | ||
| Mean ± sem | 15.67 ± 1.90 | 31.40 ± 7.87 |
| P value | 0.1645 (ns) |
Flow cytometry measurement of OLFM4-positive percentage of peripheral blood neutrophils. The two groups were compared by Mann-Whitney's nonparametric test, resulting in a nonsignificant (ns) P value. Mean of each group is shown together with a sem.
We conclude that OLFM4 identifies a subset of human neutrophils. This is the first demonstration of such, based on the presence or absence of a genuine granule matrix protein. Heterogeneity of neutrophils has been identified before by the GPI-linked neutrophil antigen CD177, localized primarily to the membrane of specific granules and to the plasma membrane [26]. Its expression is determined by polymorphisms of the gene [27]. CD177 is present in 0–100% of neutrophils, but the fraction of positive neutrophils appears to be constant in each individual [28]. This is quite different from the expression of OLFM4, which is present in roughly 20–25% of neutrophils of all individuals tested, and its presence is not regulated at the level of transcription, as all MY/MM express OLFM4 mRNA, as determined by in situ hybridization.
miR-486 was recently shown to target OLFM4 in gastric cancer cells [29], and it is possible that this may also regulate OLFM4 expression during granulopoiesis, but the mechanism regulating miR-486 would then again be active in only a subset of neutrophils.
Our results open for investigation of the differential expression of both coding and non-coding RNAs in the two different neutrophil subsets identified by the expression of OLFM4.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by grants from the Danish Medical Research Council. J.D.W. was supported by a NIH grant (#5 P20 RR020143-07). The expert technical assistance of Mrs. Charlotte Horn and Mrs. Elisabeth Larsen is greatly appreciated.
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
- ACTB
- β-actin
- bm-PMN
- bone marrow PMN cells
- hCAP18
- human cathelicidin antimicrobial protein of 18 kD
- LCN2
- lipocalin 2
- MB
- Myeloblast
- MMP
- matrix metalloproteinase
- MY/MM
- myelocyte/metamyelocyte
- NGAL
- neutrophil gelatinase-associated lipocalin
- OLFM4
- olfactomedin 4
- PM
- promyelocyte
AUTHORSHIP
N.B., J.B.C., N.H.H.H., J.D.W., A.N.S., and S.N.C. designed research. S.N.C., C.T.B., L.C.J., A.G., H.M-J., E.P.C., M.T.L., S.R., and J.T.T. performed experiments. S.N.C., N.B., N.H.H.H., and A.N.S. analyzed data. J.D.W. performed bioinformatics. S.N.C., N.B., J.D.W., and J.T.T. wrote the manuscript.
DISCLOSURES
The authors declare no competing financial interests.
REFERENCES
- 1. Zhang X., Huang Q., Yang Z., Li Y., Li C.Y. (2004) GW112, a novel antiapoptotic protein that promotes tumor growth. Cancer Res. 64, 2474–2481 [DOI] [PubMed] [Google Scholar]
- 2. Zhang J., Liu W. L., Tang D.C., Chen L., Wang M., Pack S. D., Zhuang Z., Rodgers G. P. (2002) Identification and characterization of a novel member of olfactomedin-related protein family, hGC-1, expressed during myeloid lineage development. Gene 283, 83–93 [DOI] [PubMed] [Google Scholar]
- 3. Kobayashi D., Koshida S., Moriai R., Tsuji N., Watanabe N. (2007) Olfactomedin 4 promotes S-phase transition in proliferation of pancreatic cancer cells. Cancer Sci. 98, 334–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Koshida S., Kobayashi D., Moriai R., Tsuji N., Watanabe N. (2007) Specific overexpression of OLFM4(GW112/HGC-1) mRNA in colon, breast and lung cancer tissues detected using quantitative analysis. Cancer Sci. 98, 315–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Oue N., Sentani K., Noguchi T., Ohara S., Sakamoto N., Hayashi T., Anami K., Motoshita J., Ito M., Tanaka S., Yoshida K., Yasui W. (2009) Serum olfactomedin 4 (GW112, hGC-1) in combination with Reg IV is a highly sensitive biomarker for gastric cancer patients. Int. J. Cancer 125, 2383–2392 [DOI] [PubMed] [Google Scholar]
- 6. Yu L., He M., Yang Z., Chen G., Li M., Wang L., Chen S. (2011) Olfactomedin 4 is a marker for progression of cervical neoplasia. Int. J. Gynecol. Cancer 21, 367–372 [DOI] [PubMed] [Google Scholar]
- 7. Shinozaki S., Nakamura T., Iimura M., Kato Y., Iizuka B., Kobayashi M., Hayashi N. (2001) Upregulation of Reg 1α and GW112 in the epithelium of inflamed colonic mucosa. Gut 48, 623–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chin K. L., Aerbajinai W., Zhu J., Drew L., Chen L., Liu W., Rodgers G. P. (2008) The regulation of OLFM4 expression in myeloid precursor cells relies on NF-κB transcription factor. Br. J. Haematol. 143, 421–432 [DOI] [PubMed] [Google Scholar]
- 9. Goetz D. H., Willie S. T., Armen R. S., Bratt T., Borregaard N., Strong R. K. (2000) Ligand preference inferred from the structure of neutrophil gelatinase associated lipocalin. Biochemistry 39, 1935–1941 [DOI] [PubMed] [Google Scholar]
- 10. Kjeldsen L., Bainton D. F., Sengelov H., Borregaard N. (1994) Identification of neutrophil gelatinase-associated lipocalin as a novel matrix protein of specific granules in human neutrophils. Blood 83, 799–807 [PubMed] [Google Scholar]
- 11. Liu W., Lee H. W., Liu Y., Wang R., Rodgers G. P. (2010) Olfactomedin 4 is a novel target gene of retinoic acids and 5-aza-2′-deoxycytidine involved in human myeloid leukemia cell growth, differentiation, and apoptosis. Blood 116, 4938–4947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wenli L., Yan L., Yueqin L., Colemann W. G., Rodgers G. P. Olfactomedin 4 down-regulates neutrophil killing of gram-positive and gram-negative bacteria. (2010) American Society of Hematology Annual Meeting, Abstract no. 3777 [Google Scholar]
- 13. Wren J. D. (2009) A global meta-analysis of microarray expression data to predict unknown gene functions and estimate the literature-data divide. Bioinformatics 25, 1694–1701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Daum J. R., Wren J. D., Daniel J. J., Sivakumar S., McAvoy J. N., Potapova T. A., Gorbsky G. J. (2009) Ska3 is required for spindle checkpoint silencing and the maintenance of chromosome cohesion in mitosis. Curr. Biol. 19, 1467–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee H. K., Hsu A. K., Sajdak J., Qin J., Pavlidis P. (2004) Coexpression analysis of human genes across many microarray data sets. Genome Res. 14, 1085–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wren J. D., Garner H. R. (2004) Shared relationship analysis: ranking set cohesion and commonalities within a literature-derived relationship network. Bioinformatics 20, 191–198 [DOI] [PubMed] [Google Scholar]
- 17. Borregaard N., Sehested M., Nielsen B. S., Sengelov H., Kjeldsen L. (1995) Biosynthesis of granule proteins in normal human bone marrow cells. Gelatinase is a marker of terminal neutrophil differentiation. Blood 85, 812–817 [PubMed] [Google Scholar]
- 18. Borregaard N., Heiple J. M., Simons E. R., Clark R. A. (1983) Subcellular localization of the b-cytochrome component of the human neutrophil microbicidal oxidase: translocation during activation. J. Cell Biol. 97, 52–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Clemmensen S. N., Jacobsen L. C., Rorvig S., Askaa B., Christenson K., Iversen M., Jorgensen M. H., Larsen M. T., van Deurs B., Ostergaard O., Heegaard N. H., Cowland J. B., Borregaard N. (2011) α-1-Antitrypsin is produced by human neutrophil granulocytes and their precursors and liberated during granule exocytosis. Eur. J. Haematol. 86, 517–530 [DOI] [PubMed] [Google Scholar]
- 20. Kjeldsen L., Sengelov H., Lollike K., Nielsen M. H., Borregaard N. (1994) Isolation and characterization of gelatinase granules from human neutrophils. Blood 83, 1640–1649 [PubMed] [Google Scholar]
- 21. Silahtaroglu A. N., Nolting D., Dyrskjot L., Berezikov E., Moller M., Tommerup N., Kauppinen S. (2007) Detection of microRNAs in frozen tissue sections by fluorescence in situ hybridization using locked nucleic acid probes and tyramide signal amplification. Nat. Protoc. 2, 2520–2528 [DOI] [PubMed] [Google Scholar]
- 22. Bendtsen J. D., Nielsen H., von Heijne G., Brunak S. (2004) Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol. 340, 783–795 [DOI] [PubMed] [Google Scholar]
- 23. Cowland J. B., Borregaard N. (1999) The individual regulation of granule protein mRNA levels during neutrophil maturation explains the heterogeneity of neutrophil granules. J. Leukoc. Biol. 66, 989–995 [DOI] [PubMed] [Google Scholar]
- 24. Le Cabec V., Cowland J. B., Calafat J., Borregaard N. (1996) Targeting of proteins to granule subsets is determined by timing and not by sorting: the specific granule protein NGAL is localized to azurophil granules when expressed in HL-60 cells. Proc. Natl. Acad. Sci. USA 93, 6454–6457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu W., Liu Y., Wang R., Li C., Deng C., Rodgers G. P. (2009) Olfactomedin 4 is essential for superoxide production and sensitizes oxidative stress-induced apoptosis in neutrophils. American Society of Hematology Annual Meeting Abstracts 114, 1356 [Google Scholar]
- 26. Goldschmeding R., van Dalen C. M., Faber N., Calafat J., Huizinga T. W., van der Schoot C. E., Clement L. T., von dem Borne A. E. (1992) Further characterization of the NB 1 antigen as a variably expressed 56–62 kD GPI-linked glycoprotein of plasma membranes and specific granules of neutrophils. Br. J. Haematol. 81, 336–345 [DOI] [PubMed] [Google Scholar]
- 27. Caruccio L., Walkovich K., Bettinotti M., Schuller R., Stroncek D. (2004) CD177 polymorphisms: correlation between high-frequency single nucleotide polymorphisms and neutrophil surface protein expression. Transfusion 44, 77–82 [DOI] [PubMed] [Google Scholar]
- 28. Moritz E., Chiba A. K., Kimura E. Y., Albuquerque D., Guirao F. P., Yamamoto M., Costa F. F., Bordin J. O. (2010) Molecular studies reveal that A134T, G156A and G1333A SNPs in the CD177 gene are associated with atypical expression of human neutrophil antigen-2. Vox Sang. 98, 160–166 [DOI] [PubMed] [Google Scholar]
- 29. Oh H. K., Tan A. L., Das K., Ooi C. H., Deng N. T., Tan I. B., Beillard E., Lee J., Ramnarayanan K., Rha S. Y., Palanisamy N., Voorhoeve P. M., Tan P. (2011) Genomic loss of miR-486 regulates tumor progression and the OLFM4 antiapoptotic factor in gastric cancer Clin. Cancer Res. 17, 2657–2667 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.