Regulatory T cells that are induced by intraocular antigens require exogenous IFN-γ for their suppressive function but not for their induction.
Keywords: anterior chamber, eye, immune privilege, interferon-gamma, local adoptive transfer
Abstract
Introduction of alloantigens into the AC induces a form of immune tolerance known as ACAID, which induces antigen-specific CD8+ Tregs, contributing to ocular immune privilege by down-regulating immune responses. Recent evidence suggests IFN-γ is needed for the suppressive function of CD8+ ACAID Tregs. This study tested the hypothesis that IFN-γ is needed for alloantigen-specific ACAID CD8+ Tregs to execute their suppressive function but is not required for the establishment of ACAID CD8+ Tregs. To address this hypothesis, ACAID was induced by injecting BALB/c spleen cells into the AC of WT C57BL/6 mice, IFN-γ−/− C57BL/6 mice, or anti-IFN-γ-treated WT C57BL/6 mice. LAT assays using C57BL/6 APCs as stimulators, CD4+ T cells from C57BL/6 mice previously immunized toward BALB/c alloantigens as effector cells, and IFN-γ-competent, IFN-γ−/−, or IFN-γR−/− CD8+ Tregs were used to evaluate the suppressive function of CD8+ ACAID Tregs in response to IFN-γ. IFN-γ−/− mice or mice treated with anti-IFN-γ antibody prior to AC injection of alloantigen failed to develop ACAID. The suppressive function of IFN-γ−/− ACAID CD8+ Tregs was restored through the administration of exogenous IFN-γ. This suppressive responsiveness toward IFN-γ was CD8+ Treg-intrinsic, as CD8+ Tregs from IFN-γR−/− mice, which were primed in the AC with alloantigens, were not able to suppress alloantigen-specific DTH responses. These results indicate that IFN-γ is not needed for the induction of CD8+ ACAID Tregs but is required for ACAID Tregs to exert the suppression of allospecific DTH responses.
Introduction
Ocular immune privilege is an adaptation that protects the eye from immune-mediated inflammation and prevents irreparable damage to nonregenerative tissues that are vital for vision. The term “immune privilege” was first proposed by Peter Medawar in the 1940s [1], when he noted that foreign tissues placed into the AC of the eye did not undergo rejection. Ocular immune privilege is also extended to corneal allografts, which enjoy an unusually high acceptance rate in comparison with allografts transplanted to other sites. Corneal allografts survive even without the administration of systemic immunosuppressive drugs or MHC matching [2, 3]. It was first believed that the immune privilege of the eye was solely a result of corneal avascularity, which promoted sequestration of corneal antigens from the immune system, a phenomenon akin to the more contemporary term “immunological ignorance” [4]. However, research over the past 30 years has demonstrated that the immune privilege of the eye is a dynamic immunosuppressive phenomenon [5]. Ocular immune privilege involves three different mechanisms: (1) the unique anatomical features of the eye; (2) the expression of soluble and membrane-bound immunosuppressive factors, such as TGF-β, IL-10, FasL, and PD-L1 within the eye, which disable activated T cells; and (3) the induction of a unique antigen-specific tolerance known as ACAID [6].
ACAID is the deviant systemic immune response evoked by introducing antigens into the AC of the eye and involves a unique cellular mechanism, in which noncomplement-fixing antibody responses are preserved, but destructive cellular reactions, such as DTH and CTL responses, are suppressed [7]. ACAID is the culmination of a complex series of cellular interactions that leads to the generation of two different Treg populations; CD4+CD25+ Tregs block the afferent phase of the immune response, and CD8+ Tregs suppress the efferent phase of the immune response by blocking the effector responses of previously sensitized CD4+ T cells [8].
Antigens introduced into the AC are processed by ocular APCs, which migrate to the spleen. B cells within the marginal zone of the spleen present these cognate antigens to CD8+ T cells in the context of Qa-1, a nonconventional MHC class molecule [9, 10]. The induction of CD8+ ACAID Tregs in the spleen requires the presence of IL-10 derived from γδ T cells and CD4+CD25+ T cells [11, 12] and active TGF-β [13, 14]. CD8+ ACAID Tregs promote their suppressive effects by the up-regulation of FasL expression and the production of IL-10 and TGF-β, but they do not require the expression of CTL-associated molecule granzyme B or perforin to suppress DTH responses [15, 16]. Recent evidence shows that the suppressive function of ACAID CD8+ Tregs requires the presence of the Th1 cytokine IFN-γ [15]. Accordingly, the present study was conducted to elucidate the requirement of IFN-γ in the generation and function of CD8+ ACAID Tregs.
MATERIALS AND METHODS
Mice
C57BL/6 (H-2b) and BALB/c (H-2d) mice were purchased from UT Southwestern Mouse Breeding Facility (Dallas, TX, USA). IFN-γR−/− and IFN-γ−/− mice on the C57BL/6 background were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). Mice were 8–12 weeks in age. The animal studies were approved by the Institutional Animal Care and Use Committee at UT Southwestern Medical Center at Dallas. All animals used in these experiments were housed and cared for in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research.
AC and s.c. injection of alloantigenic cells
Mice were anesthetized with 80 mg/kg ketamine hydrochloride (Fort Dodge Laboratories, Fort Dodge, IA, USA) and 0.006 mg/kg xylazine (Bayer, Shawnee Mission, KS, USA) given i.p. A glass micropipette (∼80 μm diameter) was fitted onto a sterile No. 5 French infant-feeding tube (Professional Medical Products, Greenwood, SC, USA) and mounted onto a 0.1-ml syringe (Hamilton, Whittier, CA, USA). A Hamilton automatic dispensing apparatus was used to inject 1 × 105 plastic nonadherent BALB/c spleen cells suspended in 4 μl HBSS into the AC of C57BL/6 mice. Positive control mice and all CD4+ T cell donors were sensitized for BALB/c allospecific DTH by one s.c. injection of 1 × 106 BALB/c splenocytes in 100 μl HBSS.
DTH assay
DTH was measured using a conventional ear-swelling assay. An eliciting dose of 1 × 106 mitomycin C-treated (400 μg/ml) BALB/c spleen cells in 25 μl HBSS was injected into the s.c. tissue of the left ear. The right ear served as a negative control and was injected with 25 μl HBSS without cells. Results were expressed as alloantigen-specific ear-swelling response = (experimental ear 24 h measurement−experimental ear 0 h measurement) − (negative control ear 24 h measurement−negative ear 0 h measurement).
Isolation of CD8+T cells, CD4+ T cells, and alloantigen-pulsed adherent APCs
CD8+ T cells and CD4+ T cells were isolated by using CD8 Ly-2 or CD4 L3T4 MACS beads, respectively (Miltenyi Biotec, Auburn, CA, USA). APCs were isolated from naïve C57BL/6 mice. Single-cell suspensions were incubated with NH4Cl erythrocyte lysis solution for 5 min and then washed three times with HBSS. C57BL/6 APCs were isolated by incubating spleen cell suspensions onto two, 100 mm BD Primaria plates (BD Biosciences, Franklin Lakes, NJ, USA) at 37°C for 1.5 h. Nonadherent cells were removed by vigorous washing. Adherent APCs were removed by scraping the plate with a cell scraper. APCs were cultured in 4 ml complete RPMI and 1 ml sonicated BALB/c cell lysate. Cells were incubated at 37°C overnight before use in assays.
LAT assay
LAT assays were used to test the suppressive function of putative ACAID CD8+ Tregs [12, 17]. CD8+ T cells (1×106 cells), isolated from C57BL/6 mice previously primed in the AC with nonadherent BALB/c splenocytes, CD4+ T cells (1×106 cells) collected from s.c.-immunized donors, and BALB/c alloantigen-pulsed adherent C57BL/6 splenocytes (1×106 cells), were mixed in 25 μl HBSS and injected into the left ear pinna of a naïve C57BL/6 mouse. The suppressive function of CD8+ T cells was demonstrated by the inhibition of the ear-swelling responses mediated by immune CD4+ T cells.
mAb and rmIFN-γ treatment
Anti-IFN-γ mAb was purified from RA-6A2 (American Type Culture Collection, Mansassas, VA, USA) culture supernatants. A dose of 500 μg anti-IFN-γ was administered i.p. on Day −1 before and + 7 after AC injection of alloantigens. Anti-IFN-γ (12 μg/ml) or rat IgG isotype antibody (12 μg/ml) was coinjected with the mixed cell populations in the LAT assay. rmIFN-γ (8 μg/ml; Sigma-Aldrich, St. Louis, MO, USA) was coinjected with the mixed cell population in the LAT assay.
Cytokine analysis
C57BL/6 WT or IFN-γ−/− APCs, CD4+ T cells, and CD8+ T cells (1×106 cells each) were cultured for 5 days in a 24-well plate at a 1:1:1 ratio. Supernatants were collected, and IFN-γ production was quantified using Quantikine ELISA kits (R&D Systems, Minneapolis, MN, USA). To assess cytokine gene expression, total RNA was isolated from 5× 106 cells using the RNeasy mini kit, followed by on-column DNA digestion (Qiagen, Valencia, CA, USA). Complementary DNA was synthesized from 1 μg total RNA using the RT2 miRNA First Strand Kit (SABiosciences, Frederick, MD, USA). Quantitative real-time PCR was performed on a MyiQ thermocycler (Bio-Rad, Hercules, CA, USA) using SYBR Green SuperArray RT2 qPCR Master Mix (SABiosciences). The 2−ΔΔcomparative threshold method of quantification was used to assess the fold change of IFN-γ (normalized to GAPDH) compared with IFN-γ−/− spleen cells.
Flow cytometric analysis
Expression of CD11b and F4/80 on adherent APCs and expression of CD103 and TGF-βRII on CD4+ cells were assessed by flow cytometry. Single-cell suspensions were prepared and washed in HBSS containing 2% FBS. Cells were incubated with anti-F4/80-PE antibody (BD Biosciences), anti-CD11b-FITC antibody (BD Biosciences), anti-CD103 antibody (BD Biosciences), anti-TGF-βRII antibody (R&D Systems), rat IgG2a κ-FITC (BD Biosciences), rat IgG2b κ-PE (BD Biosciences), and goat IgG-PE (R&D Systems) for 30 min at 4°C, washed three times, and fixed in 2% formalin. Cell-surface expression was assessed for fluorescence using a FACScalibur flow cytometer (BD Biosciences).
Statistical analysis
Results for DTH assays were evaluated by ANOVA with a Tukey post-test. Data results are expressed as mean ± sem. Differences in all experiments were considered to be statistically significant if the P values were <0.05.
Online Supplemental Material
P values for comparisons between various experiment and control groups are listed in Supplemental Table 1.
RESULTS
IFN-γ is needed for alloantigen-induced ACAID
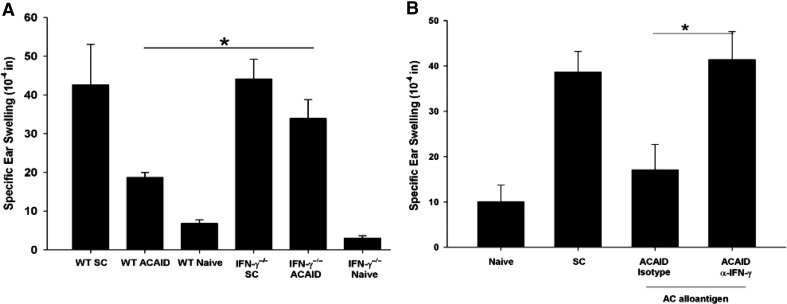
To test the hypothesis that IFN-γ is required for alloantigen-induced ACAID, C57BL/6 IFN-γ−/− mice were primed in the AC with nonadherent BALB/c splenocytes prior to s.c. immunization with BALB/c splenocytes. Seven days after s.c. immunization, the AC-primed mice, as well as control mice, were tested for the suppression of allospecific DTH responses using an ear-swelling assay. Unlike WT C57BL/6 mice, IFN-γ−/− mice primed in the AC with BALB/c alloantigens did not develop ACAID and were unable to suppress DTH responses (Fig. 1A). To confirm that IFN-γ was required for the expression of ACAID, WT mice were treated with 500 μl anti-IFN-γ or 500 μl of an isotype control antibody administered i.p. 1 day before and 7 days after AC priming with nonadherent BALB/c splenocytes. Mice treated with the isotype control antibody developed ACAID, as shown by their suppressed ear-swelling responses to BALB/c alloantigens (Fig. 1B). By contrast, C57BL/6 mice treated with anti-IFN-γ antibody did not develop ACAID and instead, mounted positive ear-swelling responses to BALB/c alloantigens. This confirms that IFN-γ is required for the development of ACAID induced by alloantigens.
Figure 1. IFN-γ is needed for alloantigen-induced ACAID.
(A) BALB/c nonadherent splenocytes were injected into the AC of WT or IFN-γ−/− C57BL/6 mice, which were then immunized s.c. (SC) with BALB/c splenocytes 7 days later. WT SC = WT mice that were immunized s.c. once and served as the positive DTH control. DTH responses toward BALB/c alloantigens were determined 7 days after s.c. immunization using an ear-swelling assay. This experiment was performed twice with similar results; n = 5 mice/group/experiment. WT SC versus WT ACAID, *P = 0.047; WT ACAID versus IFN-γ−/− ACAID, *P = 0.037; IFN-γ−/− SC versus IFN-γ−/− ACAID, *P = 0.078. (B) WT C57BL/6 mice were treated with 500 μg anti-IFN-γ or an isotype control antibody on Day −1 and Day +7. BALB/c nonadherent splenocytes cells were injected into the AC on Day 0. Mice were immunized s.c. with BALB/c splenocytes on Day +7. DTH responses to BALB/c alloantigens were determined 7 days after s.c. immunization using an ear-swelling assay. SC versus ACAID isotype antibody-treated, *P = 0.36; ACAID α-IFN-γ versus ACAID isotype antibody-treated, *P = 0.038. This experiment was conducted three times ; n = 5 mice/group/experiment. Results are expressed as mean ± sem.
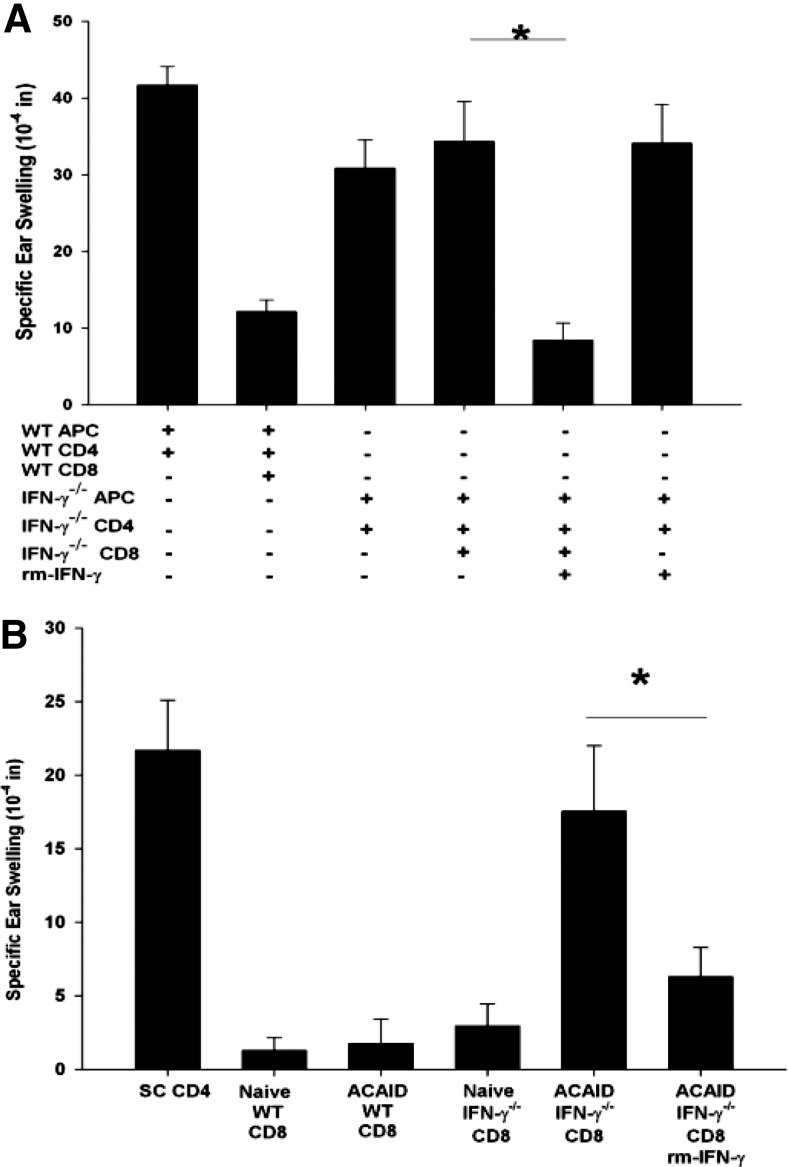
Ancillary cells from IFN-γ-competent donors restore the function of ACAID CD8+ Tregs from C57BL/6 IFN-γ−/− mice
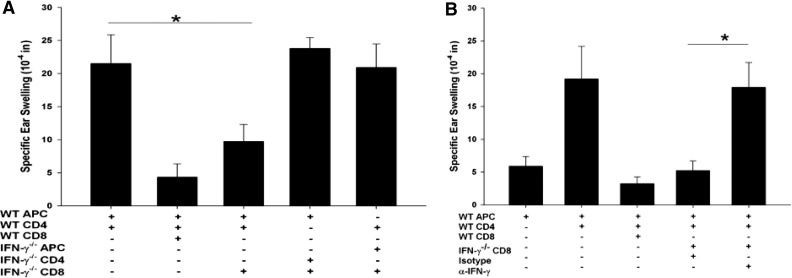
CD8+ ACAID Treg activity is detected when antigen-specific CD4+ immune T cells and CD8+ ACAID Tregs are confronted with antigen-pulsed APCs. Sensitized CD4+ T cells mediate DTH, which is observed by an ear-swelling response; however, in the presence of CD8+ ACAID Tregs, no significant ear swelling occurs. Experiments were performed to determine if IFN-γ was needed for the induction and the expression of CD8+ ACAID Treg suppression of DTH ear-swelling responses. Accordingly, C57BL/6 IFN-γ−/− mice were primed in the AC with nonadherent BALB/c splenocytes, and CD8+ T cells from the spleen were collected 7 days after s.c. immunization and tested for their capacity to suppress DTH responses against BALB/c alloantigens. LAT assays were performed by coinjecting the following cells into the ears of naïve mice: 1) C57BL/6 APCs pulsed in vitro with BALB/c alloantigens; 2) CD4+ T cells, which were isolated from the spleens of C57BL/6 mice that had been immunized 7 days earlier; and 3) CD8+ T cells from mice that were primed in the AC with nonadherent BALB/c splenocytes. The results showed that CD8+ T cells from AC-primed C57BL/6 IFN-γ−/− mice displayed ACAID T regulatory function and suppressed allospecific DTH responses if the LAT assays included APC and CD4+ T cells from IFN-γ-competent WT mice (Fig. 2A). The requirement of IFN-γ in the function of ACAID CD8+ Tregs was confirmed by performing a LAT assay using: 1) antigen-pulsed WT APCs; 2) antialloantigen immune WT CD4+ T cells; 3) CD8+ T cells from AC-primed IFN-γ−/− mice; and 4) anti-IFN-γ antibody or an isotype control antibody. In these experiments, the aforementioned restoration of Treg activity by CD8+ T cells from AC-primed mice was ablated when the anti-IFN-γ antibody was coinjected into the experimental ears (Fig. 2B). By contrast, coinjection of an isotype control antibody did not block the suppressive activity of the CD8+ ACAID Tregs.
Figure 2. Suppressive response of ACAID CD8+ Tregs in response to ancillary IFN-γ.
CD8+ T cells were isolated from WT or IFN-γ−/− mice that were primed in the AC with nonadherent BALB/c splenocytes. CD4+ T cells were isolated from WT or IFN-γ−/− mice that were immunized s.c. with BALB/c alloantigen. WT and IFN-γ−/− adherent APCs were pulsed in vitro with BALB/c alloantigens. (A) The cell populations were mixed together to test CD8+ T cell-suppressive capacity in response to IFN-γ, provided by CD4+ T cells, adherent APCs, or CD4+ T cells and APCs. This experiment was conducted three times; n = 5 mice/group/experiment; *P = 0.048. (B) The suppressive function of CD8+ T cells in response to IFN-γ was examined in a LAT assay by coinjecting isotype control or anti-IFN-γ antibody (12 μg) into the ear with the mixed cell populations. This experiment was conducted three times; n = 5 mice/group/experiment; *P = 0.048. Results are expressed as mean ± sem.
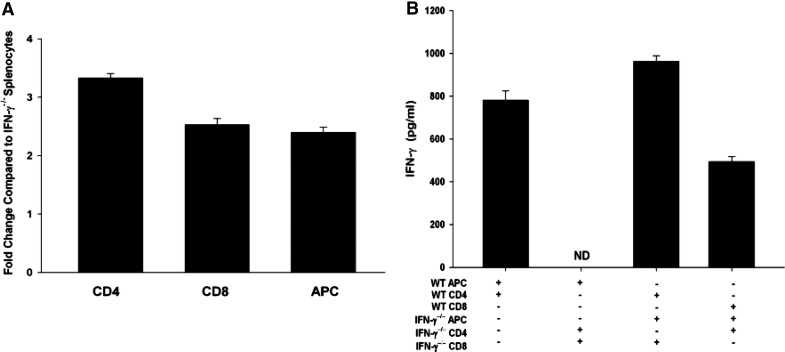
Additional experiments sought to determine whether the antigen-pulsed APC or the CD4+ anti-BALB/c immune T cells used in the LAT assay were the source of IFN-γ that restored the suppressive function of CD8+ ACAID T cells from IFN-γ−/− mice. As expected, CD4+ T cells from s.c.-immunized WT mice expressed the IFN-γ gene and produced significant quantities of IFN-γ protein when confronted with BALB/c alloantigens. CD8+ T cells from C57BL/6 WT ACAID mice demonstrated low constitutive IFN-γ gene expression, which was up-regulated significantly following exposure to BALB/c alloantigens (Fig. 3A and B).
Figure 3. Ancillary immune cells produce IFN-γ, which “licenses” CD8+ ACAID Tregs to exert their regulatory function.
(A) mRNA isolated from ACAID CD8+ Tregs, immunized CD4+ T cells, and adherent cells was quantified for IFN-γ expression by quantitative RT-PCR. This experiment was performed twice with similar results; n = 3 for each experiment. Differences among CD4+ T cells, CD8+ T cells, and APC were significant; P < 0.001. (B) ACAID CD8+ Tregs, CD4+ T cells, and adherent APCs were isolated from WT or C57BL/6 IFN-γ−/− mice. APCs were pulsed with BALB/c alloantigens prior to culturing with CD8+ T cells and CD4+ T cells. Cell cultures were then incubated for 5 days. Supernatants were quantified for IFN-γ production by ELISA. This experiment was conducted twice; n = 3. Results are expressed as mean ± sem. ND, Not detected.
IFN-γ licenses CD8+ ACAID Tregs to suppress allospecific DTH
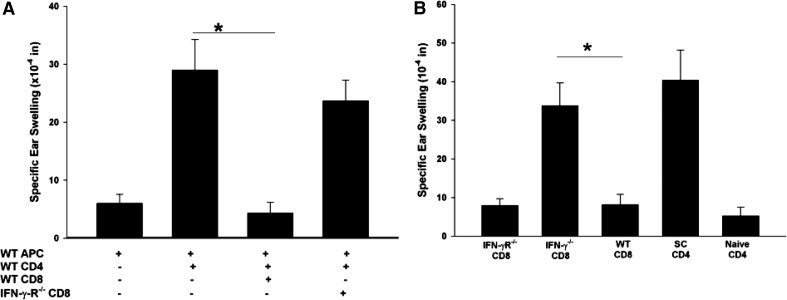
CD8+ ACAID Tregs from IFN-γ−/− mice suppressed allospecific DTH responses if the LAT assays included APCs and CD4+ T cells from IFN-γ-competent WT mice. Additional experiments were performed to confirm that IFN-γ was needed for CD8+ ACAID Treg-suppressive functions. Accordingly, a LAT assay was used to compare ACAID CD8+ T cell populations derived from WT mice and IFN-γR−/− mice primed in the AC with BALB/c alloantigens. Although IFN-γR−/− mice immune cells can produce IFN-γ, they cannot respond to this cytokine [18]. By contrast, immune cells from WT mice can produce and respond to IFN-γ. WT and IFN-γR−/− mice were primed in the AC with BALB/c alloantigens prior to s.c. immunization with BALB/c spleen cells. CD8+ spleen cells from AC-primed WT mice and IFN-γR−/− mice were tested for their capacity to suppress DTH responses in a LAT assay. The results clearly demonstrated that CD8+ T cells from IFN-γR−/− mice failed to suppress DTH when coinjected with alloantigen-pulsed APCs and alloimmunized CD4+ T cells from IFN-γ-competent WT mice (Fig. 4A) and suggest that CD8+ ACAID Tregs require IFN-γ to mediate suppression.
Figure 4. CD8+ ACAID Tregs need to respond to IFN-γ to express their regulatory function.
(A) CD8+ T cells were isolated from WT or C57BL/6 IFN-γR−/− mice, which were injected with nonadherent BALB/c spleen cells in the AC. DTH-positive control mice received CD4+ effectors from immunized mice. C57BL/6 APCs pulsed with BALB/c alloantigens in vitro were added to all cell mixtures. This experiment was performed twice with similar results; n = 5 mice/group/experiment; *P < 0.001. (B) CD8+ T cells were isolated from WT, IFN-γR−/−, or IFN-γ−/− mice, which were primed in the AC with nonadherent BALB/c splenocytes on Day 0 and immunized s.c. with nonadherent BALB/c splenocytes 7 days later. Positive control mice were not primed in the AC but were immunized s.c. with nonadherent BALB/c splenocytes. CD4+ T cells and CD8+ T cells were isolated from naïve, negative control mice and immunized mice 7 days following s.c. immunizations and coinjected with APCs pulsed with BALB/c alloantigens into the ears of naïve C57BL/6 mice. Specific ear swelling was assessed 24 h later. Results are expressed as mean ± sem. This experiment was conducted twice with five mice/group/experiment; *P = 0.018. Differences in the means between the IFN-γ−/− CD8 and SC CD4 groups were not significantly different (P>0.05).
However, it might also be argued that the presence of a positive DTH response using putative CD8+ Tregs was not a result of an absence of suppression but rather, the generation of positive DTH by CD8+ T cells from IFN-γ−/− and IFN-γR−/− mice. This was examined in additional experiments, in which WT, IFN-γ−/−, and IFN-γR−/− C57BL/6 mice were primed in the AC with nonadherent BALB/c splenocytes on Day 0. On Day 7, mice were immunized s.c. with BALB/c spleen cells. CD8+ cells were isolated from the spleens 7 days after s.c. immunization and tested in a LAT assay, in which CD8+ cells were mixed with APCs pulsed with BALB/c alloantigens and coinjected into the ears of naïve C57BL/6 mice. Interestingly, the results demonstrated that CD8+ T cells from AC-primed IFN-γ−/− mice mounted positive DTH to the BALB/c alloantigens (Fig. 4B). However, CD8+ T cells from IFN-γR−/− mice did not generate positive DTH responses. Thus, ACAID CD8+ Tregs cannot suppress alloantigen-specific DTH, unless IFN-γ is present, and the Tregs express the IFN-γR.
Role of IFN-γ in the induction of ACAID CD8+ Tregs
The results described above indicate that IFN-γ is required by CD8+ ACAID Tregs to mediate their suppressive function. However, it is not known whether IFN-γ is needed for the initial induction of CD8+ ACAID Tregs. It is important to note that although IFN-γ−/− CD8+ cells cannot produce IFN-γ, they are capable of responding to IFN-γ [19, 20]. Thus, AC-primed C57BL/6 IFN-γ−/− mice might generate CD8+ ACAID Tregs that suppress DTH responses if they are provided exogenous IFN-γ. This was addressed by injecting BALB/c nonadherent spleen cells into the AC of IFN-γ−/− mice, followed by a s.c. immunization 7 days after AC injection. Seven days following s.c. immunization, CD8+ T cells were isolated from AC-primed IFN-γ−/− mice and were coinjected into the ears of naïve mice, along with alloantigen-pulsed APCs and CD4+ effector T cells from s.c.-immunized C57BL/6 IFN-γ−/− mice. As expected, CD8+ cells from AC-primed IFN-γ−/− mice failed to suppress DTH responses when combined with APCs and CD4+ effector cells from IFN-γ−/− donors (i.e., in the absence of IFN-γ). However, coinjection of exogenous IFN-γ in these assays restored AC-primed IFN-γ−/− CD8+ T cell suppression of allospecific DTH responses (Fig. 5A). Injection of the same amount of exogenous IFN-γ with APC and immune CD4+ effector T cells in the absence of CD8+ T cells did not suppress DTH responses, which confirmed that the restoration of the suppressive properties of CD8+ T cells from AC-primed IFN-γ−/− mice was not a result of a nonspecific, anti-inflammatory effect of exogenous IFN-γ. The expression of positive DTH by CD8+ T cells from IFN-γ−/− mice in the absence of IFN-γ (Fig. 5A) but suppression of DTH when IFN-γ was present (Fig. 2B) suggested that two populations of CD8+ T cells were present. One population was capable of mediating DTH, and the other was suppressive (i.e., ACAID Tregs). The data suggest that suppression dominates over positive DTH if IFN-γ is present. To test this, a LAT assay similar to that performed in Fig. 4B was performed. CD8+ T cells were isolated from IFN-γ−/− mice that had been primed in the AC with nonadherent BALB/c splenocytes and along with WT APCs and PBS or exogenous rmIFN-γ, were coinjected into the ears of naïve mice. Ear- swelling responses were assessed 24 h later. The results showed that the presence of exogenous IFN-γ prevented the expression of positive ear swelling by the CD8+ T cells from AC-primed IFN-γ−/− mice (Fig. 5B). Together, these results indicate that IFN-γ is not required for the generation of ACAID CD8+ Tregs, which down-regulate DTH responses by CD4+ T cells from WT mice and CD8+ T cells from IFN-γ−/− mice.
Figure 5. IFN-γ licenses CD8+ ACAID Tregs, whereas the absence of IFN-γ promotes the expression of CD8+ T cells that mediate DTH.
(A) IFN-γ licenses ACAID CD8+ Tregs. CD8+ T cells were isolated from WT or IFN-γ−/− mice that had been primed in the AC with nonadherent BALB/c splenocytes. CD4+ T cells were isolated from WT or IFN-γ−/− mice that had been immunized s.c. with BALB/c alloantigen. WT and IFN-γ−/− adherent APCs were pulsed in vitro with BALB/c alloantigens. rmIFN-γ was injected into the ear (8 μg) with the mixed cell populations; *P < 0.05. (B) In the absence of IFN-γ, CD8+ ACAID T cells mediate DTH. CD8+ T cells were isolated from WT or IFN-γ−/− mice that had been primed in the AC with nonadherent BALB/c splenocytes. The positive control consisted of CD4+ T cells that were isolated from WT mice that had been immunized s.c. with BALB/c alloantigen. WT adherent APCs were pulsed in vitro with BALB/c alloantigens and were coinjected into the ears along with CD8+ T cells that were collected from AC-primed or naïve mice. rmIFN-γ was injected into the ear (8 μg) with the mixed cell populations. Results are expressed as mean ± sem. Each of these experiments was performed twice with similar results; n = 5 mice/group/experiment; *P = 0.004.
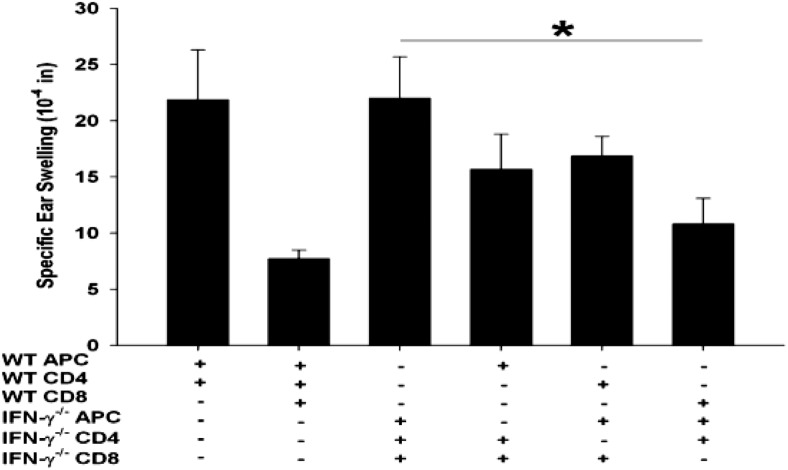
IFN-γ produced by ACAID CD8+ Tregs is sufficient to license their suppressive activity
The results to this point indicated that IFN-γ was necessary for the expression but not the induction of CD8+ ACAID Tregs. The present findings also demonstrated that providing exogenous IFN-γ licenses CD8+ ACAID Tregs from C57BL/6 IFN-γ−/− hosts to perform their suppressive function. Therefore, it was important to determine if CD8+ ACAID Tregs produced the IFN-γ needed to mediate suppression of DTH. Accordingly, this was tested in a LAT assay using CD8+ ACAID Tregs, CD4+ T cells, and APCs from WT or C57BL/6 IFN-γ−/− mice. The LAT assays recapitulated previous experiments and demonstrated that CD4+ T cells and APCs from WT but not IFN-γ−/− mice could license the suppressive activity of CD8+ ACAID Tregs from IFN-γ−/− mice (Fig. 6). Interestingly, CD8+ ACAID Tregs from WT mice suppressed DTH responses even when coinjected with APCs and CD4+ T cells from IFN-γ−/− mice. Thus, CD8+ ACAID Tregs are capable of producing sufficient quantities of IFN-γ to license their suppressive activity.
Figure 6. CD8+ ACAID Tregs can produce IFN-γ, which licenses them to be suppressive.
ACAID CD8+ T cells were isolated from WT or C57BL/6 IFN-γ−/− mice that were primed in the AC with nonadherent BALB/c splenocytes. CD4+ T cells were isolated from WT or IFN-γ−/− mice that were immunized s.c. with BALB/c alloantigen. WT and IFN-γ−/− adherent APCs were pulsed in vitro with BALB/c alloantigen. The source of IFN-γ, which induces the licensing of CD8+ Tregs, was tested in a LAT assay. Results are expressed as mean ± sem. This experiment was performed twice with similar results; n = 5 mice/group/experiment; *P = 0.048.
DISCUSSION
ACAID is a systemic form of tolerance that is believed to protect the eye from immune-mediated inflammatory damage. The complex sequence of events that culminates in the generation of end-stage CD8+ Tregs is initiated when antigens are processed by F4/80+ APCs in the AC. Within 24 h, the F4/80+ ocular APCs migrate to the thymus [21] and the spleen [22]. After the ocular APCs enter the spleen, a series of complex and poorly understood cellular interactions involving CD4+ T cells, NKT cells, B cells, and γδ T cells culminates in the generation of CD8+ Tregs that suppress DTH responses in an antigen-specific manner. Cone and colleagues [15] have previously shown that although IFN-γ was not needed for the induction of ACAID against haptenated BSA (TNP-BSA), it was essential for licensing an ACAID CD8+ Treg-suppressive function, that is, for the expression of ACAID. The present study sought to expand on these previous studies and determine: 1) if IFN-γ affected alloantigen-induced ACAID; 2) at what stage of ACAID IFN-γ was needed; and 3) which cells produced IFN-γ.
These results confirm that IFN-γ is needed for the alloantigen-induced ACAID and further characterize the role of IFN-γ by demonstrating that IFN-γ is not needed for the generation of CD8+ ACAID Tregs but is required for these Tregs to exert their suppressive effects on DTH responses. The results of the LAT assays revealed that IFN-γ generated by CD4+ alloimmune effector cells restores the suppressive activity of CD8+ ACAID Tregs from IFN-γ−/− mice. Thus, CD8+ ACAID Tregs can be generated in the absence of IFN-γ, but these Tregs cannot suppress DTH responses, unless exogenous IFN-γ is provided. It is noteworthy that CD8+ T cells from IFN-γ−/− mice can mediate DTH responses, unless exogenous IFN-γ is provided. IFN-γ acts as a switch that licenses the CD8+ Treg population, which suppresses DTH responses [23]. IFN-γR−/− mice with collagen-induced arthritis have an even more pronounced Th1 cytokine profile than WT mice. This suggests that responsiveness to IFN-γ acts as a counter-regulator of Th1 differentiation and blocks excessive Th1-mediated pathology [20, 24]. Together, these results show that CD8+ ACAID Tregs are poised to suppress antigen-specific DTH but must be licensed by IFN-γ.
The present observations showed that CD8+ ACAID Tregs are capable of providing endogenous IFN-γ to suppress alloantigenic inflammatory responses. However, CD8+ Tregs from IFN-γ−/− mice that are unable to produce IFN-γ can still mediate suppression when provided IFN-γ from ancillary cell populations. We sought to determine which coinjected cell population in the LAT assay provides IFN-γ to activate ACAID CD8+ Treg suppression. We initially hypothesized that IFN-γ, produced by immune CD4+ T cells, induced CD8+ ACAID Treg suppression, as CD4+ T cells, not APCs, produced IFN-γ. Surprisingly, our results demonstrated that CD8+ ACAID Tregs from IFN-γ−/− mice were able to suppress DTH responses only when CD4+ T cells and APCs from IFN-γ-competent donors were present. One possibility why IFN-γ produced by CD4+ T cells was not sufficient to promote CD8+ ACAID Treg suppression may be a result of an alteration of the functional activity of APCs from IFN-γ−/− donors. In response to IFN-γ, APCs up-regulate the expression of MHC class I and II and costimulatory factors [25]. Previous functional analysis showed that bone marrow-derived DCs from IFN-γ−/− mice had altered cytokine secretion, costimulatory factor expression, allostimulatory activity, and antigen-presentation capacity [26]. Therefore, an altered APC phenotype may not provide the proper signals to license CD8+ ACAID Treg-suppressive function. Alternatively, APCs have been shown to support suppression by the expression of immunosuppressive factors such as IDO [27], which can support CD8+ ACAID Treg suppression by directly inhibiting CD4+ T cell responses. The reliance of APC responsiveness toward IFN-γ in supporting ACAID Treg-suppressive function will be determined in future studies.
On first blush, our results and the previously published findings of Cone et al. [15] seem counterintuitive, as IFN-γ was characterized originally as a proinflammatory cytokine that enhanced Th1-mediated inflammatory reactions by promoting CTL, DTH, and allograft rejection [28–30]. Despite its recognized effects in promoting immune responses, IFN-γ also has tolerogenic effects [31]. IFN-γ has been shown to ameliorate disease in autoimmune models, mitigate graft-versus-host disease, and prevent allograft rejection [32–34]. Moreover, depletion of IFN-γ robs the corneal allograft of its immune privilege and results in accelerated corneal allograft rejection [35, 36].
Although IFN-γ was once viewed as strictly a proinflammatory cytokine, it is now clear that it also has important immunoregulatory functions by promoting the suppressive response of Tregs [37]. Mice deficient in IFN-γ signaling have exacerbated EAE and experimental autoimmune uveitis [38–41]. Moreover, adoptive transfer of Tregs treated with IFN-γ alleviates EAE symptoms [38]. IFN-γ conditioning can enhance the population of T cells with suppressive potential by up-regulating the expression of the regulatory transcription factor FoxP3 [42] and the immunoregulatory cytokines TGF-β and IL-10 [43]. IFN-γ up-regulates FoxP3 expression in CD4+CD25+ Tregs [44, 45], and it has also been associated with suppression mediated by CD8+ Tregs [15, 43, 46, 47]. In models in which suppression of immune responses was IFN-γ-dependent, induction of TGF-β production [43] or the expression of fibrinogen-like 2 [48] was up-regulated in CD8+ Tregs in the presence of IFN-γ.
An additional role of IFN-γ in exerting suppression during ACAID may be its ability to enhance the susceptibility of CD4+ effector cells to be suppressed by CD8+ Tregs. In studies on murine autoimmunity models [49, 50] and human autoimmune diseases [51], it has been reported that T effector cells are resistant to Treg-mediated inhibition. CD4+ effector cells exposed to IFN-γ may become more susceptible to ACAID CD8+ Treg-mediated suppression by up-regulating TGF-βRII or conversion to a suppressive population by expressing a Treg-associated marker CD103 [52]. When CD4+ effector cells were exposed to exogenous IFN-γ, we noted that the expression level of TGF-βR or CD103 was not enhanced in comparison with CD4+ T effector cells cultured alone (data not shown). However, this does not discount other possible mechanisms that may promote CD4+ T effector cell susceptibility to Treg-mediated suppression, such as regulation mediated by FasL, CTLA-4, or PD-L1.
It is noteworthy that IFN-γ is necessary for the expression of ACAID and for sustaining the immune privilege of corneal allografts [35, 36, 53]. Orthotopic corneal allografts are in direct contact with the AC of the eye, and it has been suggested that corneal alloantigens are sloughed into the AC during and after keratoplasty [54]. The notion that orthotopic corneal allografts induce ACAID is consistent with findings from rodent models of keratoplasty, which have reported that mice with long-term corneal allografts display suppressed DTH responses toward donor alloantigens, which mimic the down-regulation of DTH that occurs following injection of alloantigens into the AC [54, 55]. Moreover, AC injection of donor cells prior to orthotopic corneal transplantation results in a significant increase in the acceptance of corneal allografts in rats and mice [56–58]. However, there is reason to suspect that the Tregs induced by AC injection of alloantigens (i.e., ACAID) are different from the Tregs induced by corneal allografts. For example, in vivo administration of anti-CD8 antibody abolishes ACAID but has no effect on the immune privilege of corneal allografts. By contrast, in vivo treatment with an anti-IL-17 antibody promotes corneal allograft rejection but does not adversely affect alloantigen-induced ACAID [33].
The presented results add to a growing list of reports demonstrating the plasticity of the immune response and support the concept that cytokines and T cells can exert opposite effects depending on the timing and location of their actions. In the case of IFN-γ, it can promote inflammation or immunosuppression under different conditions. The once widely held notion that ACAID and corneal allograft survival were the result of suppressed IFN-γ production is no longer tenable and in fact, appears to be the opposite.
Supplementary Material
ACKNOWLEDGMENTS
Support for this work was provided by NIH grants EY005631 and EY 020799 and an unrestricted grant from Research to Prevent Blindness (New York, NY, USA).
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
- −/−
- deficient
- AC
- anterior chamber
- ACAID
- anterior chamber-associated immune deviation
- EAE
- experimental autoimmune encephalitis
- FasL
- Fas ligand
- FoxP3
- forkhead box P3
- LAT
- local adoptive transfer
- m
- murine
- PD-L1
- programmed cell death ligand 1
- Treg
- T regulatory cell
- UT
- The University of Texas
REFERENCES
- 1. Medawar P. B. (1948) Immunity to homologous grafted skin; the fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye. Br. J. Exp. Pathol. 29, 58–69 [PMC free article] [PubMed] [Google Scholar]
- 2. Fink N., Stark W. J., Maguire M. G., Stulting D., Meyer R., Foulks G., Smith R. E., Rapoza P. (1994) Effectiveness of histocompatibility matching in high-risk corneal transplantation: a summary of results from the Collaborative Corneal Transplantation Studies. Cesk. Oftalmol. 50, 3–12 [PubMed] [Google Scholar]
- 3. The Collaborative Corneal Transplantation Studies Research Group (1992) The collaborative corneal transplantation studies (CCTS). Effectiveness of histocompatibility matching in high-risk corneal transplantation. Arch. Ophthalmol. 110, 1392–1403 [PubMed] [Google Scholar]
- 4. Barker C. F., Billingham R. E. (1977) Immunologically privileged sites. Adv. Immunol. 25, 1–54 [PubMed] [Google Scholar]
- 5. Niederkorn J. Y., Streilein J. W. (1982) Induction of anterior chamber-associated immune deviation (ACAID) by allogeneic intraocular tumors does not require splenic metastases. J. Immunol. 128, 2470–2474 [PubMed] [Google Scholar]
- 6. Niederkorn J. Y. (2007) The induction of anterior chamber-associated immune deviation. Chem. Immunol. Allergy 92, 27–35 [DOI] [PubMed] [Google Scholar]
- 7. Streilein J. W. (2003) Ocular immune privilege: therapeutic opportunities from an experiment of nature. Nat. Rev. Immunol. 3, 879–889 [DOI] [PubMed] [Google Scholar]
- 8. Wilbanks G. A., Streilein J. W. (1990) Characterization of suppressor cells in anterior chamber-associated immune deviation (ACAID) induced by soluble antigen. Evidence of two functionally and phenotypically distinct T-suppressor cell populations. Immunology 71, 383–389 [PMC free article] [PubMed] [Google Scholar]
- 9. D'Orazio T. J., Mayhew E., Niederkorn J. Y. (2001) Ocular immune privilege promoted by the presentation of peptide on tolerogenic B cells in the spleen. II. Evidence for presentation by Qa-1. J. Immunol. 166, 26–32 [DOI] [PubMed] [Google Scholar]
- 10. Chattopadhyay S., O'Rourke J., Cone R. E. (2008) Implication for the CD94/NKG2A-Qa-1 system in the generation and function of ocular-induced splenic CD8+ regulatory T cells. Int. Immunol. 20, 509–516 [DOI] [PubMed] [Google Scholar]
- 11. Ashour H. M., Niederkorn J. Y. (2006) γδ T cells promote anterior chamber-associated immune deviation and immune privilege through their production of IL-10. J. Immunol. 177, 8331–8337 [DOI] [PubMed] [Google Scholar]
- 12. Skelsey M. E., Mayhew E., Niederkorn J. Y. (2003) CD25+, interleukin-10-producing CD4+ T cells are required for suppressor cell production and immune privilege in the anterior chamber of the eye. Immunology 110, 18–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. D'Orazio T. J., Niederkorn J. Y. (1998) A novel role for TGF-β and IL-10 in the induction of immune privilege. J. Immunol. 160, 2089–2098 [PubMed] [Google Scholar]
- 14. Sonoda K. H., Nakamura T., Young H. A., Hart D., Carmeliet P., Stein-Streilein J. (2007) NKT cell-derived urokinase-type plasminogen activator promotes peripheral tolerance associated with eye. J. Immunol. 179, 2215–2222 [DOI] [PubMed] [Google Scholar]
- 15. Cone R. E., Chattopadhyay S., O'Rourke J. (2008) Control of delayed-type hypersensitivity by ocular- induced CD8+ regulatory T cells. Chem. Immunol. Allergy 94, 138–149 [DOI] [PubMed] [Google Scholar]
- 16. Ren Y., Yang P., Li B., Gao Y., Zhou H., Huang X., Zhu L., Kijlstra A. (2006) OVA-specific CD8+ T cells do not express granzyme B during anterior chamber associated immune deviation. Graefes Arch. Clin. Exp. Ophthalmol. 244, 1315–1321 [DOI] [PubMed] [Google Scholar]
- 17. D'Orazio T. J., Niederkorn J. Y. (1998) Splenic B cells are required for tolerogenic antigen presentation in the induction of anterior chamber-associated immune deviation (ACAID). Immunology 95, 47–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Haring J. S., Badovinac V. P., Olson M. R., Varga S. M., Harty J. T. (2005) In vivo generation of pathogen-specific Th1 cells in the absence of the IFN-γ receptor. J. Immunol. 175, 3117–3122 [DOI] [PubMed] [Google Scholar]
- 19. Yoneda Y., Hirota R., Tashiro J., Okada M., Sakurai K., Lee K., Ueda K., Kubota T., Yoshida R. (2003) Cellular origin of IFN-γ essential for hair cycle in normal skin. J. Interferon Cytokine Res. 23, 299–305 [DOI] [PubMed] [Google Scholar]
- 20. Matthys P., Vermeire K., Billiau A. (2001) Mac-1(+) myelopoiesis induced by CFA: a clue to the paradoxical effects of IFN-γ in autoimmune disease models. Trends Immunol. 22, 367–371 [DOI] [PubMed] [Google Scholar]
- 21. Wang Y., Goldschneider I., O'Rourke J., Cone R. E. (2001) Blood mononuclear cells induce regulatory NK T thymocytes in anterior chamber-associated immune deviation. J. Leukoc. Biol. 69, 741–746 [PubMed] [Google Scholar]
- 22. Wilbanks G. A., Streilein J. W. (1992) Macrophages capable of inducing anterior chamber associated immune deviation demonstrate spleen-seeking migratory properties. Reg. Immunol. 4, 130–137 [PubMed] [Google Scholar]
- 23. Koch M. A., Tucker-Heard G., Perdue N. R., Killebrew J. R., Urdahl K. B., Campbell D. J. (2009) The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat. Immunol. 10, 595–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Matthys P., Vermeire K., Mitera T., Heremans H., Huang S., Schols D., De Wolf-Peeters C., Billiau A. (1999) Enhanced autoimmune arthritis in IFN-γ receptor-deficient mice is conditioned by mycobacteria in Freund's adjuvant and by increased expansion of Mac-1+ myeloid cells. J. Immunol. 163, 3503–3510 [PubMed] [Google Scholar]
- 25. Farrar M. A., Schreiber R. D. (1993) The molecular cell biology of interferon-γ and its receptor. Annu. Rev. Immunol. 11, 571–611 [DOI] [PubMed] [Google Scholar]
- 26. Wu X., Hou W., Sun S., Bi E., Wang Y., Shi M., Zang J., Dong C., Sun B. (2006) Novel function of IFN-γ: negative regulation of dendritic cell migration and T cell priming. J. Immunol. 177, 934–943 [DOI] [PubMed] [Google Scholar]
- 27. Hwu P., Du M. X., Lapointe R., Do M., Taylor M. W., Young H. A. (2000) Indoleamine 2,3-dioxygenase production by human dendritic cells results in the inhibition of T cell proliferation. J. Immunol. 164, 3596–3599 [DOI] [PubMed] [Google Scholar]
- 28. Bugeon L., Cuturi M. C., Hallet M. M., Paineau J., Chabannes D., Soulillou J. P. (1992) Peripheral tolerance of an allograft in adult rats—characterization by low interleukin-2 and interferon-γ mRNA levels and by strong accumulation of major histocompatibility complex transcripts in the graft. Transplantation 54, 219–225 [DOI] [PubMed] [Google Scholar]
- 29. Nickerson P., Steiger J., Zheng X. X., Steele A. W., Steurer W., Roy-Chaudhury P., Strom T. B. (1997) Manipulation of cytokine networks in transplantation: false hope or realistic opportunity for tolerance? Transplantation 63, 489–494 [DOI] [PubMed] [Google Scholar]
- 30. Chan S. Y., DeBruyne L. A., Goodman R. E., Eichwald E. J., Bishop D. K. (1995) In vivo depletion of CD8+ T cells results in Th2 cytokine production and alternate mechanisms of allograft rejection. Transplantation 59, 1155–1161 [PubMed] [Google Scholar]
- 31. Zhang J. (2007) Yin and yang interplay of IFN-γ in inflammation and autoimmune disease. J. Clin. Invest. 117, 871–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang H., Asavaroengchai W., Yeap B. Y., Wang M. G., Wang S., Sykes M., Yang Y. G. (2009) Paradoxical effects of IFN-γ in graft-versus-host disease reflect promotion of lymphohematopoietic graft-versus-host reactions and inhibition of epithelial tissue injury. Blood 113, 3612–3619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cunnusamy K., Paunicka K., Reyes N., Yang W., Chen P. W., Niederkorn J. Y. (2010) Two different regulatory T cell populations that promote corneal allograft survival. Invest. Ophthalmol. Vis. Sci. 51, 6566–6574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sis B., Famulski K. S., Allanach K. L., Zhu L. F., Halloran P. F. (2007) IFN-γ prevents early perforin-granzyme-mediated destruction of kidney allografts by inducing donor class I products in the kidney. Am. J. Transplant. 7, 2301–2310 [DOI] [PubMed] [Google Scholar]
- 35. Hargrave S. L., Hay C., Mellon J., Mayhew E., Niederkorn J. Y. (2004) Fate of MHC-matched corneal allografts in Th1-deficient hosts. Invest. Ophthalmol. Vis. Sci. 45, 1188–1193 [DOI] [PubMed] [Google Scholar]
- 36. Cunnusamy K., Chen P. W., Niederkorn J. Y. (2010) IL-17 promotes immune privilege of corneal allografts. J. Immunol. 185, 4651–4658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sawitzki B., Kieselbach B., Fisser M., Meisel C., Vogt K., Gaestel M., Lehmann M., Risch K., Grutz G., Volk H. D. (2004) IFN-γ regulation in anti-CD4 antibody-induced T cell unresponsiveness. J. Am. Soc. Nephrol. 15, 695–703 [DOI] [PubMed] [Google Scholar]
- 38. Nishibori T., Tanabe Y., Su L., David M. (2004) Impaired development of CD4+ CD25+ regulatory T cells in the absence of STAT1: increased susceptibility to autoimmune disease. J. Exp. Med. 199, 25–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang Z., Hong J., Sun W., Xu G., Li N., Chen X., Liu A., Xu L., Sun B., Zhang J. Z. (2006) Role of IFN-γ in induction of Foxp3 and conversion of CD4+ CD25− T cells to CD4+ Tregs. J. Clin. Invest. 116, 2434–2441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Caspi R. R., Chan C. C., Grubbs B. G., Silver P. B., Wiggert B., Parsa C. F., Bahmanyar S., Billiau A., Heremans H. (1994) Endogenous systemic IFN-γ has a protective role against ocular autoimmunity in mice. J. Immunol. 152, 890–899 [PubMed] [Google Scholar]
- 41. Jones L. S., Rizzo L. V., Agarwal R. K., Tarrant T. K., Chan C. C., Wiggert B., Caspi R. R. (1997) IFN-γ-deficient mice develop experimental autoimmune uveitis in the context of a deviant effector response. J. Immunol. 158, 5997–6005 [PubMed] [Google Scholar]
- 42. Feng G., Wood K. J., Bushell A. (2011) Regulatory T cell enrichment by IFN-γ conditioning. Methods Mol. Biol. 677, 281–301 [DOI] [PubMed] [Google Scholar]
- 43. Myers L., Croft M., Kwon B. S., Mittler R. S., Vella A. T. (2005) Peptide-specific CD8 T regulatory cells use IFN-γ to elaborate TGF-β-based suppression. J. Immunol. 174, 7625–7632 [DOI] [PubMed] [Google Scholar]
- 44. Feng G., Gao W., Strom T. B., Oukka M., Francis R. S., Wood K. J., Bushell A. (2008) Exogenous IFN-γ ex vivo shapes the alloreactive T-cell repertoire by inhibition of Th17 responses and generation of functional Foxp3+ regulatory T cells. Eur. J. Immunol. 38, 2512–2527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jiang X. F., Zhu L., Cui Z. M., Guo D. W., Sun W. Y., Lin L., Tang Y. F., Wang X. F., Liang J. (2011) Transplant long-surviving induced by CD40-CD40 ligand costimulation blockade is dependent on IFN-γ through its effect on CD4(+)CD25(+) regulatory T cells. Transpl. Immunol. 24, 113–118 [DOI] [PubMed] [Google Scholar]
- 46. Tang X., Maricic I., Purohit N., Bakamjian B., Reed-Loisel L. M., Beeston T., Jensen P., Kumar V. (2006) Regulation of immunity by a novel population of Qa-1-restricted CD8αα+TCRαβ+ T cells. J. Immunol. 177, 7645–7655 [DOI] [PubMed] [Google Scholar]
- 47. Seo S. K., Choi J. H., Kim Y. H., Kang W. J., Park H. Y., Suh J. H., Choi B. K., Vinay D. S., Kwon B. S. (2004) 4-1BB-mediated immunotherapy of rheumatoid arthritis. Nat. Med. 10, 1088–1094 [DOI] [PubMed] [Google Scholar]
- 48. Li X. L., Menoret S., Bezie S., Caron L., Chabannes D., Hill M., Halary F., Angin M., Heslan M., Usal C., Liang L., Guillonneau C., Le Mauff B., Cuturi M. C., Josien R., Anegon I. (2010) Mechanism and localization of CD8 regulatory T cells in a heart transplant model of tolerance. J. Immunol. 185, 823–833 [DOI] [PubMed] [Google Scholar]
- 49. Monk C. R., Spachidou M., Rovis F., Leung E., Botto M., Lechler R. I., Garden O. A. (2005) MRL/Mp CD4+,CD25− T cells show reduced sensitivity to suppression by CD4+,CD25+ regulatory T cells in vitro: a novel defect of T cell regulation in systemic lupus erythematosus. Arthritis Rheum. 52, 1180–1184 [DOI] [PubMed] [Google Scholar]
- 50. You S., Belghith M., Cobbold S., Alyanakian M. A., Gouarin C., Barriot S., Garcia C., Waldmann H., Bach J. F., Chatenoud L. (2005) Autoimmune diabetes onset results from qualitative rather than quantitative age-dependent changes in pathogenic T-cells. Diabetes 54, 1415–1422 [DOI] [PubMed] [Google Scholar]
- 51. Venigalla R. K., Tretter T., Krienke S., Max R., Eckstein V., Blank N., Fiehn C., Ho A. D., Lorenz H. M. (2008) Reduced CD4+,CD25− T cell sensitivity to the suppressive function of CD4+,CD25high,CD127−/low regulatory T cells in patients with active systemic lupus erythematosus. Arthritis Rheum. 58, 2120–2130 [DOI] [PubMed] [Google Scholar]
- 52. Zhao D., Zhang C., Yi T., Lin C. L., Todorov I., Kandeel F., Forman S., Zeng D. (2008) In vivo-activated CD103+CD4+ regulatory T cells ameliorate ongoing chronic graft-versus-host disease. Blood 112, 2129–2138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cone R. E., Li X., Sharafieh R., O'Rourke J., Vella A. T. (2007) The suppression of delayed-type hypersensitivity by CD8+ regulatory T cells requires IFN-γ. Immunology 120, 112–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Niederkorn J. Y. (2006) Anterior chamber-associated immune deviation and its impact on corneal allograft survival. Curr. Opin. Organ Transpl. 11, 360–365 [Google Scholar]
- 55. Sonoda Y., Streilein J. W. (1993) Impaired cell-mediated immunity in mice bearing healthy orthotopic corneal allografts. J. Immunol. 150, 1727–1734 [PubMed] [Google Scholar]
- 56. Niederkorn J. Y., Mellon J. (1996) Anterior chamber-associated immune deviation promotes corneal allograft survival. Invest. Ophthalmol. Vis. Sci. 37, 2700–2707 [PubMed] [Google Scholar]
- 57. She S. C., Moticka E. J. (1993) Ability of intracamerally inoculated B- and T-cell enriched allogeneic lymphocytes to enhance corneal allograft survival. Int. Ophthalmol. 17, 1–7 [DOI] [PubMed] [Google Scholar]
- 58. She S. C., Steahly L. P., Moticka E. J. (1990) Intracameral injection of allogeneic lymphocytes enhances corneal graft survival. Invest. Ophthalmol. Vis. Sci. 31, 1950–1956 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.