Regulation of gp130 expression in cultured mouse mast cells and role for IL-6 trans-signaling in increasing survival and cytokine production.
Keywords: IL-27, STAT3, SOCS3
Abstract
It is reported that human and mouse mast cells express the IL-27R, which consists of WSX-1 (the IL-27Rα subunit) and the signal-transducing subunit gp130. Although it has been proposed that IL-27 may negatively regulate mast cell-dependent, immediate hypersensitivity responses directly, this has yet to be examined specifically. We found that mouse BMMC and primary peritoneal mast cells are unresponsive to IL-27. Consistent with this, gp130 protein in resting BMMC was not on the cell surface to a measurable degree but was found intracellularly, and data are consistent with incompletely processed N-linked glycosylation. Furthermore, BMMC constitutively expressed SOCS3, a major negative regulator of gp130 signaling. However, BMMC stimulation with IL-10 and consequential STAT3 activation increased gp130 expression, which resulted in a functional gp130 receptor on the BMMC cell surface. IL-10 has not been previously shown to regulate gp130 expression, which on the BMMC surface, permitted IL-6 trans-signaling, found to increase survival under limiting conditions and enhance IL-13 and TNF-α secretion. This study identifies factors that regulate mouse mast cell gp130 expression and signaling and makes conspicuous the limitations of using cultured mouse mast cells to study the effects of the IL-6/IL-12 cytokine family on mast cell biology.
Introduction
Mast cells are noncirculating cells often found near blood vessels and in mucosal and epithelial tissues. Thus, mast cells are sentinels in anatomic sites that often require immune surveillance. This is consistent with their role in host defense against parasites and some bacteria [1–3]. Nonetheless, mast cells are best known for their roles in IgE-dependent immediate hypersensitivity, in which IgER (FcϵRI) aggregation triggers degranulation and secretion of preformed vasoactive mediators and synthesis of proinflammatory mediators.
In addition to roles in host defense and hypersensitivity, mast cells are found with increased frequency in chronically inflamed tissues and are implicated in the pathogenesis of several inflammatory diseases, including rheumatoid arthritis, multiple sclerosis, and atherogenesis [4–8]. The active role of mast cells in tissue homeostasis, immune regulation, and inflammatory diseases is the focus of increasing interest [9, 10]. Furthermore, there is substantial evidence that mast cell responses are influenced by signals integrated from receptors other than FcϵRI, and ligands for these receptors include complement components such as C3a, cytokines such as IL-33, and pathogen-associated molecules such as LPS [11]. This is relevant, as the sites in which mast cells are activated, such as in the skin and lung, are likely to contain multiple soluble factors that influence antigen-mediated mast cell activation or activate mast cells directly.
Several cytokines of the IL-6/12 subfamily of type 1 cytokines share a common signal-transducing receptor known as gp130. Some examples of gp130-using cytokines include IL-6, IL-11, and IL-27. gp130 is ubiquitously expressed in hematopoietic and nonhematopoietic cells, and expression may vary depending on cellular activation status [12, 13]. Once gp130 is activated, downstream signaling involves the JAK/STAT activation pathway, and the STAT3 transcription factor is more potently activated for IL-6 and IL-11 and STAT1 for IL-27. This pathway is under tight control by negative-feedback mechanisms, mediated predominantly by SOCS3, which binds to a gp130 tyrosine residue at position 757 in mice and 759 in human, interfering with STAT activation. Thus, SOCS3 appears to be the dominant mechanism that controls the duration and quality of gp130-mediated signaling, but other mechanisms that regulate gp130 signals in the absence of SOCS3 exist [14, 15].
There are several reports linking allergic inflammation with cytokines that use the gp130 receptor [16–18]. Increased levels of IL-6 and sIL-6Rα are found in the airways of patients with allergic asthma, especially after allergen challenge, and sIL-6Rα levels positively correlate with CD4+ T cell number, IL-5, and IL-13 levels [16]. sIL-6Rα can bind to IL-6, and this complex can signal through gp130 on the cell surface, a process called IL-6 trans-signaling [19]. Studies in asthma models indicate an important role for IL-6 trans-signaling in potentiating Th2 function and allergic inflammation, although it remains unclear if IL-6 trans-signaling is acting directly on T cells or indirectly through other hematopoietic or stromal cells [16, 17]. Furthermore, Th2 and Th17 cells play major roles in persistent asthma, and IL-27 has been shown to inhibit the development of these T cell subsets [20]. Collectively, these findings illustrate the complexity of cytokine networks in allergic inflammation and implicate a role for gp130 as a modifier of allergic disease.
Although there are studies about the effects of gp130-using cytokines in models of allergic asthma and autoimmunity, there is very little information regarding the effects of gp130-using cytokines on mast cells, a key participant in these diseases. It is reported that human and mouse mast cells express the IL-27R, and expression of IL-27Rα and gp130 RNA increases with mast cell activation [21, 22]. Although it has been proposed that IL-27 may negatively regulate immediate hypersensitivity responses by acting on mouse mast cells directly [22], this possibility has yet to be examined specifically. In the studies described herein, we found that mouse BMMC and primary peritoneal mast cells are unresponsive to IL-27. Furthermore, BMMC—the most commonly used source of mast cells for study—do not express gp130 on the cell surface and constitutively express SOCS3. We identify factors in BMMC that regulate gp130 expression and signaling. Lastly, we show that IL-6 trans-signaling supports BMMC survival and increases IL-13 and TNF-α secretion.
MATERIALS AND METHODS
Generation of BMMC
BM cells were cultured (5×105/ml) in RPMI-1640 media containing GlutaMAX and HEPES buffer (Invitrogen, Carlsbad, CA, USA) and completed with 10% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, 50 mM 2-ME, sodium pyruvate 1 mM, and nonessential amino acids (1×), supplemented with IL-3 (10 ng/ml) and SCF (10 ng/ml). For 6–8 weeks, three times/week, nonadherent cells were removed, the media refreshed completely, and cells replated in a new flask. When at least 95% of cells represented a homogenous population of FcϵRI and CD117(Kit)-positive cells by flow cytometry, the cells were maintained at 1 × 106/ml, changed one or two times/week, or used in experiments. Cultures were discarded after 12 weeks. Prior to use in experiments, BMMC cultures were washed to remove SCF and rested for 18–24 h in completed media with IL-3 10 ng/ml alone. In all experiments, unless otherwise indicated, conditions contained IL-3 5 ng/ml to preserve BMMC viability.
Mice
gp130Y757F mice on a mixed 129/BL6 were described previously [23]. Experiments using gp130Y757F mice to generate BMMC also used mixed background WT littermates to generate BMMC for experimental controls. All animals were bred in the animal care facility at the University of Pennsylvania (Philadelphia, PA, USA). Unless otherwise indicated, BMMC and peritoneal cells were derived from C57BL/6 mice. All animal care and work were in accordance with national and institutional guidelines and the Institutional Animal Care and Use Committee at the University of Pennsylvania.
Flow cytometry
Prior to staining with specific antibodies, cells were washed in staining buffer containing PBS, FBS 2%, and sodium azide 0.09% and then blocked in staining buffer with CD16/32 antibody for 15 min at 4°C. Cells were stained with goat biotinylated anti-mouse gp130 (R&D Systems, Minneapolis, MN, USA; catalog number BAF468), 4 μg/ml for 30 min at 4°C, followed by secondary staining with streptavidin-allophycocyanin. Biotinylated normal goat IgG (R&D Systems) was used for isotype control staining. For phospho-STAT3 staining of peritoneal cells, the peritoneum of five mice was lavaged using 5 ml chilled PBS and the transudate cells washed and equally aliquoted in complete medium in 12 × 75 mm round-bottom tubes. The cells were stimulated in 400 μl with the conditions indicated at 37°C for 15 min and then fixed with 2 ml prewarmed BD Phosflow Lyse/Fix buffer for 10 min. After a wash in PBS, the cell pellet was disrupted with light vortexing and the cells permeabilized with 1 ml BD Phosflow Perm Buffer III for 30 min on ice. The cells were washed twice in staining buffer and stained with BD Phosflow PE mouse anti-STAT3 (pY705; BD PharMingen, San Diego, CA, USA), according to the manufacturer's recommendations. Gating events positive for pY-STAT3 were determined by use of BD Phosflow PE isotype control antibodies. All other antibodies used for flow cytometry were purchased from eBioscience (San Diego, CA, USA). FACS was performed on a LSR II flow cytometer and analyzed using FlowJo software.
Mast cell activation assays
To assess for degranulation by β-hexosaminidase release, a typical colorimetric assay was used. BMMC primed with IgE were stimulated with the conditions indicated for 30 min in a 96-well flat-bottom plate (2×105 cells in 200 μl Tyrode's buffer). The plate was centrifuged at 300 g for 10 min, cell-free supernatants were removed, and the pellets were lysed in 200 μl Triton-X-100 0.1% (Sigma-Aldrich, St. Louis, MO, USA). Supernatant and lysate samples (25 μl each) were transferred to a new 96-well plate, mixed with 25 μl 1 mM 4-nitrophenyl 2-acetamido-2-deoxy-β-d-glucopyranoside (catalog number N9376; Sigma-Aldrich) in 0.1 M citrate buffer (pH 5), and incubated at 37°C for 2 h. Thereafter, 200 μl 0.1 carbonate buffer (pH 10.5) was added, and the absorbance was measured at 405 nm. The percent β-hexosaminidase release was determined by dividing the measurements detected in the supernatant by the total measurements detected in the supernatant plus those from the cell pellet. All supernatant cytokine measurements were assayed with ELISA development kits from PeproTech (Rocky Hill, NJ, USA), according to the manufacturer's instructions.
STAT3 inhibition
In a 48-well plate, 5 × 105 BMMC in 500 μl complete media supplemented with IL-3 (5 ng/ml) were incubated at 37°C with increasing concentrations of STAT3 Inhibitor V, Stattic (Santa Cruz Biotechnology, Santa Cruz, CA, USA), as indicated. Stattic was reconstituted with ethanol to a working concentration of 1.5 mM for addition to conditions. After 45 min incubation, IL-10 (10 ng/ml) was added to some conditions. After 24 h, cells were harvested and processed for gp130 staining as described above. In this analysis, gp130 expression was evaluated on Annexin V-negative cells, which stained homogenously for Kit and FcϵRI, thus gating on viable mast cells.
Reverse transcription and real-time quantitative PCR
Total cellular RNA was extracted using the RNeasy mini kit (Qiagen, Valencia, CA, USA) and reverse-transcribed with oligo(dT) primer and the Superscript II First Strand Synthesis reverse transcription kit (Invitrogen). Nonquantitative PCR of mRNA transcripts was performed to detect GAPDH (5′-ACCACAGTCCATGCCATCAC-3′, 5′-TCCACCACCCTGTTGCTGTA-3′), and gp130 (5′-GAGCTTCGAGCCATCCGGGC-3′, 5′-AAGTTCGAGCCGCGCTGGAC-3′). Real-time quantification of mRNA transcripts was performed with Quantitect SYBR Green Mastermix (Qiagen) using the relative standard curve method on the Applied Biosystem's 7900HT Fast PCR machine and analyzed with SDS 2.3 software. Transcript levels were normalized to PPIA mRNA. Primers for gp130, SOCS3, and PPIA were obtained from Qiagen.
Western blot analysis
Cells were rested for 90 min at 37° in RPMI-1640 media with 2% FBS and then lysed in ice-cold Triton-X lysis buffer [50 mM Tris (pH 7.4), 1% Triton-X, 150 mM NaCl] containing a protease inhibitor cocktail (Sigma-Aldrich; P2714) and additional protease inhibitors (dichloroisocoumarin 40 μg/ml and benzamidine 800 μM) and phosphatase inhibitors (sodium orthovanadate 180 μg/ml, sodium fluoride 2.1 mg/ml, and sodium pyrophosphate 13 mg/ml). Protein was electrophoresed under reducing conditions on 10% Bis-Tris or 7% Tris-acetate SDS-PAGE gels (Invitrogen) and transferred to nitrocellulose using the iBlot Dry blotting system (Invitrogen). Membranes were blocked in 5% milk or 5% BSA in TBS-Tween, followed by probing with biotinylated goat anti-gp130 (R&D Systems; catalog number BAF468), 0.3 μg/ml overnight; rabbit anti-gp130 (Santa Cruz Biotechnology; clone M-20; catalogue number sc-656), 1:200 overnight; rabbit anti-pY-STAT3 (Cell Signaling Technology, Beverly, MA, USA), 1:1000 overnight; rabbit anti-pY-STAT1 (Cell Signaling Technology), 1:1000 overnight; rabbit anti-STAT3 (Cell Signaling Technology), 1:1000 for 1 h; rabbit anti-SOCS3 (Cell Signaling Technology), 1:1000 overnight; or rabbit antitubulin (Cell Signaling Technology), 1:1000 for 1 h. Primary antibodies were detected with HRP anti-biotin (Cell Signaling Technology) or HRP anti-rabbit (GE Healthcare, Waukesha, WI, USA) and ECL Plus Western blotting detection reagents (GE Healthcare).
Statistical analysis
Where indicated, one-way ANOVA was used to determine the difference among three or more experimental groups using Prism 4 software. Bonferroni's multiple comparison post-test was used to compare pairs of group means. P < 0.05 was considered significant.
RESULTS
Mouse-cultured and primary peritoneal mast cells are unresponsive to IL-27
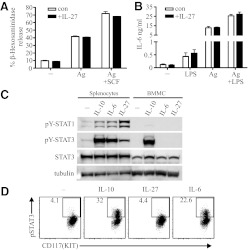
It has been proposed that IL-27 may negatively regulate mast cell activation directly [22]. To examine the direct effects of IL-27 on mouse mast cell activation in vitro, BMMC were cultured with or without IL-27 for 72 h and then sensitized with anti-DNP IgE. Using DNP-HSA to trigger IgE-mediated activation, IL-27 had no effect on BMMC degranulation (Fig. 1A) or cytokine production (Fig. 1B). To determine if BMMC demonstrated any response to IL-27 or IL-6 as the archetype gp130-using cytokine, resting BMMC were exposed to IL-27 or IL-6, and cell lysate was assayed by immunoblot for STAT1 and STAT3 phosphorylation as a measure of receptor signal transduction. Neither IL-27 nor IL-6 exposure resulted in STAT1 or STAT3 phosphorylation in BMMC, consistent with a lack of IL-27R and IL-6R signaling under resting conditions (Fig. 1C).
Figure 1. Mouse cultured and primary peritoneal mast cells are unresponsive to IL-27.
(A) BMMC were cultured with or without IL-27 (50 ng/ml) for 72 h and sensitized with anti-DNP IgE, and then, antigen-dependent degranulation was measured in conditions including multivalent DNP-HSA (Ag; 20 ng/ml) and SCF (50 ng/ml), with or without IL-27 (50 ng/ml). (B) Experiments were performed with conditions similar to A, but LPS (500 ng/ml) was included, and DNP-HSA was used at 100 ng/ml. Cell-free supernatants were taken after 5 h for ELISA. (C) BMMC and splenocytes rested for 90 min were stimulated with the cytokines shown (each 100 ng/ml) for 15 min and then processed for immunoblot. (D) Total peritoneal cells pooled from five mice were stimulated ex vivo with the cytokines shown (each 100 ng/ml) for 15 min and then fixed and processed for flow cytometry. The flow cytometry data depict peritoneal mast cells by gating on FcϵRI-positive and Kit-positive events. Data in all panels are representative of at least three experiments with similar results. pSTAT3, phospho-STAT3.
Mouse BMMC are dependent on IL-3 for survival and are characterized as an immature phenotype [24]. They have biochemical profiles similar to mucosal-type mast cells, which are found in the mucosa of the mouse respiratory tract and gut [24]. In contrast, mast cells in the mouse peritonium are serosal-type mast cells, also similar to those found in mouse skin. To examine whether expression of IL-27R or IL-6R is more characteristic of serosal-type primary mouse mast cells, peritoneal transudate cells were isolated by peritoneal lavage and exposed to IL-27 or IL-6. As a measure of receptor signal transduction, STAT3 phosphorylation was assessed by flow cytometry. Peritoneal mast cells responded to the positive control IL-10, similar to BMMC, but unlike BMMC, peritoneal mast cells demonstrated IL-6 signaling (Fig. 1D). However, as measured by STAT3 (Fig. 1D) and STAT1 (not shown) phosphorylation, peritoneal mast cells failed to respond to IL-27. Taken together, these studies indicate that neither mouse BMMC nor primary peritoneal mast cells are responsive to direct stimulation with IL-27.
gp130 in resting BMMC is incompletely processed for cell-surface expression
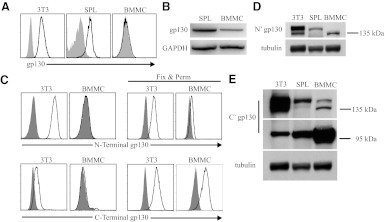
Given that IL-27 and IL-6 are thought to modulate mast cell function [22, 25, 26], it was surprising that BMMC were unresponsive to these cytokines. As gp130 is required for IL-27R and IL-6R signaling, we examined gp130 expression in mouse mast cells. Consistent with our observation that peritoneal mast cells respond to IL-6 ex vivo (Fig. 1D), flow cytometry of freshly isolated peritoneal mast cells detected gp130 expression on the cell surface (Supplemental Fig. 1). In contrast, flow cytometry of resting BMMC revealed that gp130 was undetectable on the cell surface (Fig. 2A). However, using RT-PCR, we indentified that BMMC express gp130 mRNA (Fig. 2B), and flow cytometry of permeabilized BMMC identified that gp130 protein was localized intracellularly (Fig. 2C). We considered that gp130 in BMMC was incompletely processed for the cell surface. Consistent with this idea, immunoblot of BMMC lysate using an antibody raised against recombinant gp130 extracellular domain revealed a gp130 protein form that migrated more quickly on SDS-PAGE than that expressed in splenocytes, suggestive of incompletely processed N-linked glycosylation (Fig. 2D). In contrast, probing BMMC lysate using an antibody raised against the C-terminus of gp130 revealed the preponderant form of gp130 in BMMC to be near 100 kDa, consistent with the predicted MW from the amino acid sequence, further supporting differences in post-translational processing compared with splenocytes (Fig. 2E). These data indicate resting BMMC express gp130 protein that is incompletely processed for cell-surface expression.
Figure 2. gp130 in resting BMMC is incompletely processed for cell-surface expression.
(A) Primary mouse splenocytes (SPL), the 3T3 fibroblast line, and resting BMMC were examined for gp130 on the cell surface using flow cytometry and (B) gp130 mRNA by RT-PCR. (C) Flow cytometry of BMMC and 3T3 cells using intracellular staining (fixed and permeabilized cells) with gp130 antibodies specific for the N-terminal or the C-terminal compares localization of gp130 protein components. (D) Immunoblot of total cell lysate using gp130 antibodies specific for the N-terminal (N′) and the (E) C-terminal (C') components of the gp130 protein. Shaded histograms (A and C) in flow cytometry data represent isotype control antibody fluorescence.
Expression of gp130 on the BMMC cell surface is induced by IL-10
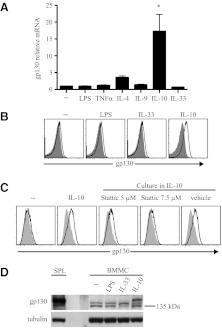
gp130 expression varies in cells or tissues depending on activation and stage of development [12, 13]. In mouse mast cells, expression of gp130 may be modified by cell activation. To evaluate this possibility, BMMC were exposed to soluble mediators known to activate mast cells, and changes in gp130 mRNA were determined by quantitative PCR [26–31]. LPS, TNF-α, IL-9, or IL-33 had no effect on gp130 mRNA, but IL-4 and IL-10 were found to promote gp130 mRNA synthesis (Fig. 3A). BMMC exposure to ionomycin, stimulation of FcϵRI by culture with IgE, or cross-linking FcϵRI with IgE/antigen did not have any effect on gp130 mRNA (not shown). As IL-10 had the greatest significant effect on gp130 mRNA, BMMC were cultured with IL-10 for 72 h, and flow cytometry was performed to evaluate gp130 protein expression on the cell surface. As shown in Fig. 3B, expression of gp130 on the BMMC cell surface was induced by IL-10. Furthermore, IL-10-induced gp130 expression was inhibited by the STAT3 inhibitor Stattic (Fig. 3C), indicating that the effect of IL-10 on gp130 expression was dependent on the cardinal IL-10R pathway. Although IL-10 promoted gp130 receptor expression, IL-6Rα remained undetectable on the BMMC cell surface (Supplemental Fig. 2).
Figure 3. BMMC gp130 receptor expression is induced by IL-10 and dependent on STAT3.
(A) BMMC rested for 90 min were stimulated with LPS 500 ng/ml or the cytokines shown (each 10 ng/ml) for 3 h and then total RNA isolated and quantitative PCR performed. RNA samples were pooled from three independent experiments and run under the same PCR conditions. *P < 0.01 for IL-10 condition compared with all other conditions. (B) BMMC were cultured for 72 h in conditions including LPS (500 ng/ml), IL-33 (10 ng/ml), or IL-10 (10 ng/ml) and then harvested for flow cytometry to examine gp130 expression on the cell surface. (C) BMMC were cultured with IL-10 (10 ng/ml) for 24 h, with or without the addition of Stattic, an inhibitor of STAT3 activation. Stattic was added to conditions 45 min before the addition of IL-10. (D) BMMC were cultured for 72 h in conditions identical to those in B and then processed for immunoblot using antibodies specific for the gp130 N-terminal. Fresh mouse splenocytes were rested for 2 h and used for comparison. (B–D) Data are representative of at least three experiments with similar results. Shaded histograms (B and C) in flow cytometry data represent isotype control antibody fluorescence.
Given that IL-10 brought about gp130 cell-surface expression, we considered that IL-10 would affect gp130 protein processing. As expected, BMMC cultured in IL-10 produced a gp130 protein form that now migrated more slowly upon SDS-PAGE and appeared more similar to that found in the splenocyte cell lysate (Fig. 3D). Taken together, these data show in BMMC that IL-10 promotes synthesis of the gp130 receptor in a manner dependent on STAT3 activation, which increases gp130 transcription and affects post-translational processing.
IL-10 permits IL-6 trans-signaling that is negatively regulated by SOCS3
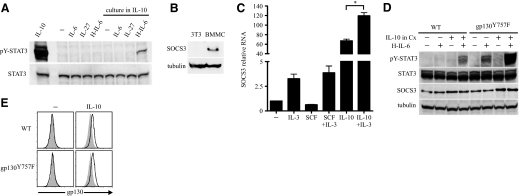
IL-6 binds to IL-6Rα on the surface of target cells, and this complex associates with gp130 molecules initiating downstream signaling. In addition, IL-6 can bind to sIL-6Rα, and this complex then binds gp130 on the cell surface, a process known as IL-6 trans-signaling [32]. Compared with ubiquitously expressed gp130, IL-6Rα is expressed in vivo on only a few cells, such as hepatocytes and some leukocytes [32]. Thus, IL-6 trans-signaling permits IL-6 to affect the development and function of cells that are unresponsive to IL-6 directly but express gp130 on their cell surface, such as in neuronal cells, smooth muscle cells, and endothelial cells [33–35]. Although IL-10 was shown to induce gp130 on the BMMC surface, it was unclear whether this gp130 receptor was functional to transmit signals downstream. To address this question, a well-characterized fusion protein of IL-6 and sIL-6Rα, known as H-IL-6, was used to test IL-6 trans-signaling in BMMC [36], which when cultured in conditions containing IL-10 were exposed to H-IL-6, and cell lysate was assayed by immunoblot for STAT3 phosphorylation. Consistent with the observation that IL-10 induces gp130 on the BMMC cell surface, H-IL-6 resulted in STAT3 activation, only in BMMC that had been cultured in IL-10 (Fig. 4A). Thus, IL-10 promotes a functional gp130 receptor that enables BMMC the capacity for IL-6 trans-signaling.
Figure 4. IL-10 permits IL-6 trans-signaling that is negatively regulated by SOCS3.
(A) BMMC were cultured with or without IL-10 (10 ng/ml) for 72 h. Cells washed and rested for 90 min were stimulated with the cytokines shown (each 100 ng/ml) for 15 min and processed for immunoblot. (B) SOCS3 protein was assessed by immunoblot of lysate from resting BMMC cultured in IL-3 and 3T3 cells. (C) BMMC rested for 90 min were stimulated with the cytokines shown (each 10 ng/ml) for 3 h and then total RNA isolated and quantitative PCR performed. RNA samples were pooled from two independent experiments and run under the same PCR conditions. *P < 0.01. (D) WT and gp130Y757F BMMC were cultured with or without IL-10 (10 ng/ml) for 36 h. Cells washed and rested for 90 min were stimulated with H-IL-6 (100 ng/ml) for 15 min where indicated and processed for immunoblot. Data are representative of two experiments with similar results. (E) WT and gp130Y757F BMMC were cultured with or without IL-10 (10 ng/ml) for 36 h and then harvested for flow cytometry. Unless otherwise indicated, all data represent at least three experiments. Shaded histograms in flow cytometry data represent isotype control antibody fluorescence.
However, the magnitude of STAT3 activation induced by IL-6 trans-signaling was less compared with that resulting from IL-10 signaling (Fig. 4A). This is likely a result, in part, of the greater density of IL-10R on the BMMC cell surface compared with gp130. Nonetheless, although our data show that IL-10 promotes the expression of the gp130 receptor, IL-10 is also known to promote the expression of SOCS3, a negative regulator of gp130 signaling but not IL-10R signaling [37, 38]. Furthermore, we found that resting BMMC cultured in IL-3, which is required to sustain their differentiation and survival, have constitutive SOCS3 protein expression (Fig. 4B). It was considered that the conditions required for maintaining BMMC survival and cell-surface gp130 favored SOCS3 expression. To test this possibility, BMMC rested for 1.5 h without IL-3 were cultured in SCF or IL-10, with or without the addition of IL-3, after which, RNA was isolated, and quantitative PCR for SOCS3 mRNA was performed. IL-3 induced SOCS3 mRNA directly and enhanced SOCS3 mRNA stimulated by IL-10 (Fig. 4C).
These data suggested that the effects of IL-6 trans-signaling on BMMC function would be strongly opposed by SOCS3-negative regulation. Considering this, BMMC were derived from the bone marrow cells of gp130Y757F mutant mice, which have a single point mutation in a gp130 tyrosine residue at position 757, disrupting a domain critical for SOCS3-negative regulation [23, 39]. Mice homozygous for this mutation develop mild aberrant hematopoietic homeostasis and age-dependent splenomegaly and gastric adenoma [23, 40, 41]. We found no evidence of aberrant mast cell homeostasis in gp130Y757F mice, and gp130Y757F BMMC were differentiated in vitro and demonstrated IgE-mediated activation no different than WT BMMC (Supplemental Fig. 3). Thus, gp130Y757F BMMC were used to examine gp130 receptor signals and function in BMMC without the interference of constitutive negative regulation.
gp130Y757F and WT BMMC were cultured in conditions containing IL-10, and then, washed and rested cells were exposed to H-IL-6. As expected, gp130Y757F BMMC conditioned with IL-10 to up-regulate gp130 demonstrated a greater magnitude of STAT3 phosphorylation induced by IL-6 trans-signaling than in WT BMMC conditioned with IL-10 (Fig. 4D). However, for gp130Y757F BMMC, IL-10 exposure to up-regulate gp130 was not required for H-IL-6 to activate STAT3 (Fig. 4D), and we considered two nonmutually exclusive possibilities for this observation: (i) BMMC gp130 receptor levels are down-regulated directly by SOCS3, or (ii) BMMC have a low density of gp130 receptor below our detection limits that receives continuous regulation by SOCS3, such that the gp130Y757F mutant receptor unmasks responsiveness to ligand stimulation. In support of the latter possibility, expression levels of cell-surface gp130 were similar in gp130Y757F and WT BMMC, with and without IL-10 exposure (Fig. 4E). These data show that IL-10-induced gp130 permits IL-6 trans-signaling. Furthermore, BMMC have constitutive SOCS3 expression that negatively regulates gp130 signals.
IL-6 trans-signaling increases BMMC survival under limiting conditions and cytokine secretion
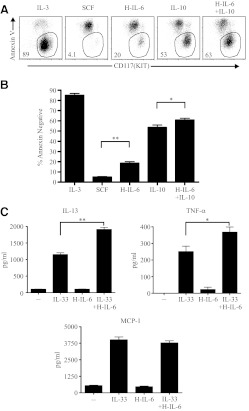
Similar to its role in regulating T cell survival, autocrine IL-6 promotes the survival of human mast cells derived from lung [42–44]. As discussed previously, mouse BMMC are highly dependent on IL-3 for survival and initiate apoptosis when IL-3 is limiting. To test the ability of IL-6 trans-signaling to rescue BMMC from limiting conditions, BMMC were deprived of IL-3 for 5 days, and the effect of IL-6 trans-signaling was examined. As cultured mast cells were found to constitutively express SOCS3, which reduced the potency of IL-6 trans-signaling (Fig. 4D), gp130Y757F BMMC were used in these studies. BMMC, which initiated apoptosis with membrane translocation of phosphatidylserine, were identified using fluorescently labeled Annexin V and flow cytometry. Stimulation of gp130 by IL-6 trans-signaling provided a small but significant increase in BMMC survival compared with stimulation of the tyrosine-protein kinase Kit by SCF (Fig. 5A and B). Although IL-10 up-regulates gp130, the antiapoptotic effect of IL-6 trans-signaling in conditions containing IL-10 was nondescript compared with the stimulatory effects of IL-10 alone (Fig. 5A and B).
Figure 5. IL-6 trans-signaling increases BMMC survival under limiting conditions and cytokine secretion.
(A) gp130Y757F BMMC were cultured for 5 days in the conditions indicated, including IL-3 (5 ng/ml), SCF (10 ng/ml), IL-10 (10 ng/ml), or H-IL-6 (100 ng/ml). The gate shown depicts the percentage of events that is Annexin V-negative and Kit-high viable mast cells, and the dot plots represent one of three experiments. (B) Pooled data from the three experiments depicted in A. *P < 0.05; **P < 0.01. (C) gp130Y757F BMMC were cultured overnight in complete media containing IL-3 (5 ng/ml) and IL-10 (10 ng/ml). After this, washed and rested cells were then stimulated in the conditions shown, including IL-33 (10 ng/ml), H-IL-6 (100 ng/ml), or both. Cell-free supernatants were taken after 3 h for IL-13, TNF-α, and MCP-1 cytokine measurement by ELISA, and ELISA data are from two independent experiments, each with four replicates/condition. *P < 0.05; **P < 0.01.
Next, we examined the effects of gp130 stimulation on BMMC effector responses. gp130Y757F BMMC were cultured overnight in conditions containing IL-10 to up-regulate gp130. These cells were then washed free of IL-10-containing media, and the effects of gp130 stimulation by H-IL-6 on cytokine responses were compared with the effects of IL-33, which directly activates BMMC cytokine responses [30]. BMMC cytokine responses were unaffected by gp130 stimulation alone; however, when gp130 stimulation occurred in conjunction with IL-33 compared with IL-33 alone, BMMC responded with increased IL-13 and TNF-α secretion but not MCP-1 secretion (Fig. 5C). In similar experiments (not shown), it was determined that preconditioning with IL-10 to up-regulate gp130 was required for the synergistic effect of gp130 stimulation on BMMC cytokine production. Additional experiments (not shown) found that BMMC degranulation responses, as measured by β-hexosaminidase release, were unaffected by gp130 stimulation. These data show that in mouse mast cells, at least under conditions favoring gp130 receptor expression and reduced SOCS3 activity, gp130 stimulation by IL-6 trans-signaling increases survival under limiting conditions and enhances IL-13 and TNF-α secretion.
DISCUSSION
In human mast cells, derived from cord blood mononuclear cells, IL-27 is shown to directly stimulate STAT3 phosphorylation and increase IL-1α, TNF-α, and OX40 RNA synthesis [21]. In addition, as mice deficient in IL-27Rα demonstrate increased mast cell-dependent, immediate hypersensitivity in skin, it has been proposed that IL-27 is a negative regulator of mast cell responses [22]. Our study was initiated with the goal of characterizing the direct effects of IL-27 on cultured mouse mast cells. However, our data make conspicuous that mouse BMMC and primary peritoneal mast cells do not respond to IL-27 directly. It remains possible that IL-27R expression and IL-27-mediated responses may differ in primary mast cells in mouse skin compared with mast cells isolated from the peritoneum [22, 45]. Furthermore, it is possible that indirect factors related to exaggerated type 2 immunity in IL-27α–/– mice may regulate the quality and magnitude of mast cell responses in vivo [22, 46].
Our data show that the capacity of mouse mast cells to respond to IL-6 distinguishes primary peritoneal mast cells from BMMC, which lack IL-6Rα and express a gp130 protein form that is incompletely processed for cell-surface receptor use. We demonstrate factors in BMMC that regulate gp130 and show function for gp130 stimulation by IL-6 trans-signaling. Expression of cytokine receptors on the cell surface is a highly regulated and dynamic process. The availability of receptor components is of fundamental importance to transduce exogenous signals. For gp130, signal regulation is not only mediated by molecules that interfere with JAK/STAT activation, such as SOCS3, but also by control of receptor turnover [14, 47, 48]. Radtke and colleagues [14] show that cytokine cross-talk or cellular stress can induce MAPK-activated protein kinase 2-dependent phosphorylation at serine 782 of gp130, which initiates a rapid net loss of cell-surface receptor and reduced IL-6 responses. This is consistent with an earlier finding that phosphorylation of serine 782, which is located in the cytoplasmic domain of gp130, plays a role in controlling cell-surface expression [48]. Furthermore, ligand-induced dimerization of gp130, independent of serine 782 phosphorylation, results in gp130 internalization and endosomal/lysosomal degradation [47]. Given that IL-27 and the IL-6 family cytokines share gp130, a receptor thought to be ubiquitously expressed and constitutively internalized, the need for multiple regulatory mechanisms at the receptor level is understandable [49, 50].
It is likely that the incompletely processed gp130 protein form in resting BMMC is targeted for increased degradation. This idea is consistent with findings by Waetzig and colleagues [51], which demonstrate that the recombinant gp130 protein lacking N-linked glycosylation migrates more quickly on SDS-PAGE and largely remains within the ER-Golgi complex, and its levels increase with proteasome inhibition. In that study, the small amount of N-glycan-deficient gp130, which made it to the cell surface, was capable of signal transduction, as measured by STAT3 phosphorylation, albeit weakly given the decreased receptor density [51]. However, in WT BMMC, the low-density gp130 receptor is antagonized easily by SOCS3, as supported by our findings. SOCS3 expression in BMMC is likely related to their dependence on IL-3-induced STAT5 activation for survival [52]. Similar to STAT3, STAT5 is shown to drive SOCS3 expression by binding the proximal STAT response element on the SOCS3 promoter [53–55].
Our study also shows that IL-10 and consequential STAT3 activation increase gp130 expression, which results in a functional gp130 receptor on the BMMC cell surface. IL-10 is a multifunctional cytokine with a range of effects on immunity and inflammation [56, 57]. In addition to its well-known ability to inhibit macrophage activation and CD4+ T cell effector function, IL-10 directly promotes CD8+ T cell cytotoxicity and enhances B cell survival, proliferation, and antibody production [56, 58–61]. IL-10 synergistically promotes the activity of IL-3 or SCF on mast cell proliferation and differentiation, consistent with its early description as a mast cell growth factor [26, 29, 62]. More recently, studies by Ryan and colleagues [63, 64] show that prolonged exposure to IL-10 decreases mast cell expression of FcϵR1 and the FcϵR1-associated activating kinases, Syk and Fyn. Work from the Ryan group supports a homeostatic model, in which acute inflammation promotes mast cell proliferation, but prolonged inflammatory conditions that include the Th2 cytokine IL-4 and IL-10 suppress mast cell function and reduce survival [63–66]. To the best of our knowledge, IL-10 has not been previously shown to regulate gp130 or gp130 post-translational processing. However, O'Brien and Manolagas confirmed the presence of a STAT3 response element in the gp130 promoter [67]. Thus, consistent with our study on BMMC, robust activation of STAT3 by IL-10 promotes increased gp130 transcription and protein expression.
Given the short duration of IL-10 stimulation used in these experiments, we did not observe IL-10-associated down-regulation of Kit, FcϵR1, or reduced viability—effects reported after BMMC are cultured for 4–6 days with IL-10 [64, 66, 68]. Our data show that when IL-3 is limited, IL-10 and to a lesser extent, gp130 stimulation by IL-6 trans-signaling support BMMC cell survival. gp130 stimulation by IL-6 trans-signaling also had a synergistic effect on IL-33-dependent mast cell cytokine production if gp130 were up-regulated by preconditioning with IL-10. This suggests that in complex inflammatory environments, gp130 receptor expression on mast cells may be modulated by IL-10, providing a pathway for increased IL-6 trans-signaling, which has the potential to affect mast cell activity. However, these findings are mitigated by the need to use gp130Y757F BMMC in these experiments to demonstrate such a potential, given the high level of SOCS3, which we found in resting BMMC, and the fact that IL-10 also increases SOCS3 expression. As mast cells are dependent on STAT5 activation for their development and survival in vitro and in vivo [52], it is possible that resting levels of SOCS3 may be increased in primary tissue mast cells, although this is unlikely given the responsiveness of peritoneal mast cells to IL-6 demonstrated in this study. Given that the physiologic regulation of SOCS3 is currently poorly understood, it is unknown if inflammatory conditions exist, where SOCS3 itself is the target of negative regulation, favoring IL-10-induced gp130 expression and signaling.
Increased levels of IL-6 and sIL-6R have been identified in allergic diseases, such as asthma and allergic conjunctivitis, and other inflammatory diseases in which mast cells participate, such as rheumatoid arthritis [16, 69–71]. It is likely that IL-6 and IL-6 trans-signaling directly affect mast cell homeostasis and effector function in vivo, and our experiments, using cultured mouse mast cells, propose how IL-10 and SOCS3 may regulate these responses. Furthermore, this study makes evident the challenges and limitations of using cultured mouse mast cells to study the effects of the IL-6/IL-12 cytokine subfamily on mast cell biology. In vivo approaches to examine the specific effects of the IL-6/IL-12 cytokine subfamily on mast cell homeostasis and mast cell-mediated inflammation will prove difficult with the current tools available but remain a worthy endeavor, given the increasing potential to target these pathways for therapeutic purposes.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grant K08-AI-070153 and the University of Pennsylvania McCabe Pilot Award to D.F.L.
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
- H-IL-6
- Hyper-IL-6
- PPIA
- peptidylprolyl isomerase A
- pY-STAT
- phosphotyrosine STAT
- SCF
- stem cell factor
- sIL-6Rα
- soluble IL-6Rα
- SOCS3
- suppressor of cytokine signaling 3
AUTHORSHIP
D.T. and D.F.L. designed the research and analyzed the data. D.T., P.T., and D.F.L. performed the research. D.T., D.F.L., S.R-J., and M.E. wrote the paper. J.S., S.R-J., and M.E. produced and provided materials, ideas, and technical assistance necessary to perform the research.
REFERENCES
- 1. Abe T., Nawa Y. (1987) Reconstitution of mucosal mast cells in W/WV mice by adoptive transfer of bone marrow-derived cultured mast cells and its effects on the protective capacity to Strongyloides ratti-infection. Parasite Immunol. 9, 31–38 [DOI] [PubMed] [Google Scholar]
- 2. Kamiya M., Oku Y., Itayama H., Ohbayashi M. (1985) Prolonged expulsion of adult Trichinella spiralis and eosinophil infiltration in mast cell-deficient W/Wv mice. J. Helminthol. 59, 233–239 [DOI] [PubMed] [Google Scholar]
- 3. Echtenacher B., Mannel D. N., Hultner L. (1996) Critical protective role of mast cells in a model of acute septic peritonitis. Nature 381, 75–77 [DOI] [PubMed] [Google Scholar]
- 4. Secor V. H., Secor W. E., Gutekunst C. A., Brown M. A. (2000) Mast cells are essential for early onset and severe disease in a murine model of multiple sclerosis. J. Exp. Med. 191, 813–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Metz M., Grimbaldeston M. A., Nakae S., Piliponsky A. M., Tsai M., Galli S. J. (2007) Mast cells in the promotion and limitation of chronic inflammation. Immunol. Rev. 217, 304–328 [DOI] [PubMed] [Google Scholar]
- 6. Eklund K. K. (2007) Mast cells in the pathogenesis of rheumatic diseases and as potential targets for anti-rheumatic therapy. Immunol. Rev. 217, 38–52 [DOI] [PubMed] [Google Scholar]
- 7. Hueber A. J., Asquith D. L., Miller A. M., Reilly J., Kerr S., Leipe J., Melendez A. J., McInnes I. B. (2010) Mast cells express IL-17A in rheumatoid arthritis synovium. J. Immunol. 184, 3336–3340 [DOI] [PubMed] [Google Scholar]
- 8. Kovanen P. T. (2007) Mast cells: multipotent local effector cells in atherothrombosis. Immunol. Rev. 217, 105–122 [DOI] [PubMed] [Google Scholar]
- 9. Weller K., Foitzik K., Paus R., Syska W., Maurer M. (2006) Mast cells are required for normal healing of skin wounds in mice. FASEB J. 20, 2366–2368 [DOI] [PubMed] [Google Scholar]
- 10. Lu L. F., Lind E. F., Gondek D. C., Bennett K. A., Gleeson M. W., Pino-Lagos K., Scott Z. A., Coyle A. J., Reed J. L., Van Snick J., Strom T. B., Zheng X. X., Noelle R. J. (2006) Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature 442, 997–1002 [DOI] [PubMed] [Google Scholar]
- 11. Gilfillan A. M., Tkaczyk C. (2006) Integrated signalling pathways for mast-cell activation. Nat. Rev. Immunol. 6, 218–230 [DOI] [PubMed] [Google Scholar]
- 12. Silver J. S., Hunter C. A. (2010) gp130 at the nexus of inflammation, autoimmunity, and cancer. J. Leukoc. Biol. 88, 1145–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Saito M., Yoshida K., Hibi M., Taga T., Kishimoto T. (1992) Molecular cloning of a murine IL-6 receptor-associated signal transducer, gp130, and its regulated expression in vivo. J. Immunol. 148, 4066–4071 [PubMed] [Google Scholar]
- 14. Radtke S., Wuller S., Yang X. P., Lippok B. E., Mutze B., Mais C., de Leur H. S., Bode J. G., Gaestel M., Heinrich P. C., Behrmann I., Schaper F., Hermanns H. M. (2010) Cross-regulation of cytokine signalling: pro-inflammatory cytokines restrict IL-6 signalling through receptor internalisation and degradation. J. Cell Sci. 123, 947–959 [DOI] [PubMed] [Google Scholar]
- 15. Yasukawa H., Ohishi M., Mori H., Murakami M., Chinen T., Aki D., Hanada T., Takeda K., Akira S., Hoshijima M., Hirano T., Chien K. R., Yoshimura A. (2003) IL-6 induces an anti-inflammatory response in the absence of SOCS3 in macrophages. Nat. Immunol. 4, 551–556 [DOI] [PubMed] [Google Scholar]
- 16. Doganci A., Eigenbrod T., Krug N., De Sanctis G. T., Hausding M., Erpenbeck V. J., Haddad el B., Lehr H. A., Schmitt E., Bopp T., Kallen K. J., Herz U., Schmitt S., Luft C., Hecht O., Hohlfeld J. M., Ito H., Nishimoto N., Yoshizaki K., Kishimoto T., Rose-John S., Renz H., Neurath M. F., Galle P. R., Finotto S. (2005) The IL-6R α chain controls lung CD4+CD25+ Treg development and function during allergic airway inflammation in vivo. J. Clin. Invest. 115, 313–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Finotto S., Eigenbrod T., Karwot R., Boross I., Doganci A., Ito H., Nishimoto N., Yoshizaki K., Kishimoto T., Rose-John S., Galle P. R., Neurath M. F. (2007) Local blockade of IL-6R signaling induces lung CD4+ T cell apoptosis in a murine model of asthma via regulatory T cells. Int. Immunol. 19, 685–693 [DOI] [PubMed] [Google Scholar]
- 18. Miyazaki Y., Inoue H., Matsumura M., Matsumoto K., Nakano T., Tsuda M., Hamano S., Yoshimura A., Yoshida H. (2005) Exacerbation of experimental allergic asthma by augmented Th2 responses in WSX-1-deficient mice. J. Immunol. 175, 2401–2407 [DOI] [PubMed] [Google Scholar]
- 19. Rose-John S., Scheller J., Elson G., Jones S. A. (2006) Interleukin-6 biology is coordinated by membrane-bound and soluble receptors: role in inflammation and cancer. J. Leukoc. Biol. 80, 227–236 [DOI] [PubMed] [Google Scholar]
- 20. Stumhofer J. S., Hunter C. A. (2008) Advances in understanding the anti-inflammatory properties of IL-27. Immunol. Lett. 117, 123–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pflanz S., Hibbert L., Mattson J., Rosales R., Vaisberg E., Bazan J. F., Phillips J. H., McClanahan T. K., de Waal Malefyt R., Kastelein R. A. (2004) WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27. J. Immunol. 172, 2225–2231 [DOI] [PubMed] [Google Scholar]
- 22. Artis D., Villarino A., Silverman M., He W., Thornton E. M., Mu S., Summer S., Covey T. M., Huang E., Yoshida H., Koretzky G., Goldschmidt M., Wu G. D., de Sauvage F., Miller H. R., Saris C. J., Scott P., Hunter C. A. (2004) The IL-27 receptor (WSX-1) is an inhibitor of innate and adaptive elements of type 2 immunity. J. Immunol. 173, 5626–5634 [DOI] [PubMed] [Google Scholar]
- 23. Tebbutt N. C., Giraud A. S., Inglese M., Jenkins B., Waring P., Clay F. J., Malki S., Alderman B. M., Grail D., Hollande F., Heath J. K., Ernst M. (2002) Reciprocal regulation of gastrointestinal homeostasis by SHP2 and STAT-mediated trefoil gene activation in gp130 mutant mice. Nat. Med. 8, 1089–1097 [DOI] [PubMed] [Google Scholar]
- 24. Galli S. J., Dvorak A. M., Marcum J. A., Ishizaka T., Nabel G., Der Simonian H., Pyne K., Goldin J. M., Rosenberg R. D., Cantor H., Dvorak H. F. (1982) Mast cell clones: a model for the analysis of cellular maturation. J. Cell Biol. 95, 435–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hunter C. A. (2005) New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nat. Rev. Immunol. 5, 521–531 [DOI] [PubMed] [Google Scholar]
- 26. Hu Z. Q., Zhao W. H., Shimamura T. (2007) Regulation of mast cell development by inflammatory factors. Curr. Med. Chem. 14, 3044–3050 [DOI] [PubMed] [Google Scholar]
- 27. Townsend J. M., Fallon G. P., Matthews J. D., Smith P., Jolin E. H., McKenzie N. A. (2000) IL-9-deficient mice establish fundamental roles for IL-9 in pulmonary mastocytosis and goblet cell hyperplasia but not T cell development. Immunity 13, 573–583 [DOI] [PubMed] [Google Scholar]
- 28. Conti P., Kempuraj D., Kandere K., Di Gioacchino M., Barbacane R. C., Castellani M. L., Felaco M., Boucher W., Letourneau R., Theoharides T. C. (2003) IL-10, an inflammatory/inhibitory cytokine, but not always. Immunol. Lett. 86, 123–129 [DOI] [PubMed] [Google Scholar]
- 29. Thompson-Snipes L., Dhar V., Bond M. W., Mosmann T. R., Moore K. W., Rennick D. M. (1991) Interleukin 10: a novel stimulatory factor for mast cells and their progenitors. J. Exp. Med. 173, 507–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ho L. H., Ohno T., Oboki K., Kajiwara N., Suto H., Iikura M., Okayama Y., Akira S., Saito H., Galli S. J., Nakae S. (2007) IL-33 induces IL-13 production by mouse mast cells independently of IgE-FcϵRI signals. J. Leukoc. Biol. 82, 1481–1490 [DOI] [PubMed] [Google Scholar]
- 31. Qiao H., Andrade M. V., Lisboa F. A., Morgan K., Beaven M. A. (2006) FcϵR1 and Toll-like receptors mediate synergistic signals to markedly augment production of inflammatory cytokines in murine mast cells. Blood 107, 610–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jones S. A., Richards P. J., Scheller J., Rose-John S. (2005) IL-6 transsignaling: the in vivo consequences. J. Interferon Cytokine Res. 25, 241–253 [DOI] [PubMed] [Google Scholar]
- 33. Klouche M., Bhakdi S., Hemmes M., Rose-John S. (1999) Novel path to activation of vascular smooth muscle cells: up-regulation of gp130 creates an autocrine activation loop by IL-6 and its soluble receptor. J. Immunol. 163, 4583–4589 [PubMed] [Google Scholar]
- 34. Marz P., Otten U., Rose-John S. (1999) Neural activities of IL-6-type cytokines often depend on soluble cytokine receptors. Eur. J. Neurosci. 11, 2995–3004 [DOI] [PubMed] [Google Scholar]
- 35. Romano M., Sironi M., Toniatti C., Polentarutti N., Fruscella P., Ghezzi P., Faggioni R., Luini W., van Hinsbergh V., Sozzani S., Bussolino F., Poli V., Ciliberto G., Mantovani A. (1997) Role of IL-6 and its soluble receptor in induction of chemokines and leukocyte recruitment. Immunity 6, 315–325 [DOI] [PubMed] [Google Scholar]
- 36. Peters M., Blinn G., Solem F., Fischer M., Meyer zum Buschenfelde K. H., Rose-John S. (1998) In vivo and in vitro activities of the gp130-stimulating designer cytokine Hyper-IL-6. J. Immunol. 161, 3575–3581 [PubMed] [Google Scholar]
- 37. Yoshimura A., Naka T., Kubo M. (2007) SOCS proteins, cytokine signalling and immune regulation. Nat. Rev. Immunol. 7, 454–465 [DOI] [PubMed] [Google Scholar]
- 38. Qin H., Roberts K. L., Niyongere S. A., Cong Y., Elson C. O., Benveniste E. N. (2007) Molecular mechanism of lipopolysaccharide-induced SOCS-3 gene expression in macrophages and microglia. J. Immunol. 179, 5966–5976 [DOI] [PubMed] [Google Scholar]
- 39. El Kasmi K. C., Holst J., Coffre M., Mielke L., de Pauw A., Lhocine N., Smith A. M., Rutschman R., Kaushal D., Shen Y., Suda T., Donnelly R. P., Myers M. G., Jr., Alexander W., Vignali D. A., Watowich S. S., Ernst M., Hilton D. J., Murray P. J. (2006) General nature of the STAT3-activated anti-inflammatory response. J. Immunol. 177, 7880–7888 [DOI] [PubMed] [Google Scholar]
- 40. Jenkins B. J., Roberts A. W., Najdovska M., Grail D., Ernst M. (2005) The threshold of gp130-dependent STAT3 signaling is critical for normal regulation of hematopoiesis. Blood 105, 3512–3520 [DOI] [PubMed] [Google Scholar]
- 41. Jenkins B. J., Grail D., Nheu T., Najdovska M., Wang B., Waring P., Inglese M., McLoughlin R. M., Jones S. A., Topley N., Baumann H., Judd L. M., Giraud A. S., Boussioutas A., Zhu H. J., Ernst M. (2005) Hyperactivation of Stat3 in gp130 mutant mice promotes gastric hyperproliferation and desensitizes TGF-β signaling. Nat. Med. 11, 845–852 [DOI] [PubMed] [Google Scholar]
- 42. Jones S. A. (2005) Directing transition from innate to acquired immunity: defining a role for IL-6. J. Immunol. 175, 3463–3468 [DOI] [PubMed] [Google Scholar]
- 43. Teague T. K., Marrack P., Kappler J. W., Vella A. T. (1997) IL-6 rescues resting mouse T cells from apoptosis. J. Immunol. 158, 5791–5796 [PubMed] [Google Scholar]
- 44. Cruse G., Cockerill S., Bradding P. (2008) IgE alone promotes human lung mast cell survival through the autocrine production of IL-6. BMC Immunol. 9, 2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Xing W., Austen K. F., Gurish M. F., Jones T. G. (2011) Protease phenotype of constitutive connective tissue and of induced mucosal mast cells in mice is regulated by the tissue. Proc. Natl. Acad. Sci. USA 108, 14210–14215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bancroft A. J., Humphreys N. E., Worthington J. J., Yoshida H., Grencis R. K. (2004) WSX-1: a key role in induction of chronic intestinal nematode infection. J. Immunol. 172, 7635–7641 [DOI] [PubMed] [Google Scholar]
- 47. Blanchard F., Wang Y., Kinzie E., Duplomb L., Godard A., Baumann H. (2001) Oncostatin M regulates the synthesis and turnover of gp130, leukemia inhibitory factor receptor α, and oncostatin M receptor β by distinct mechanisms. J. Biol. Chem. 276, 47038–47045 [DOI] [PubMed] [Google Scholar]
- 48. Gibson R. M., Schiemann W. P., Prichard L. B., Reno J. M., Ericsson L. H., Nathanson N. M. (2000) Phosphorylation of human gp130 at Ser-782 adjacent to the Di-leucine internalization motif. Effects on expression and signaling. J. Biol. Chem. 275, 22574–22582 [DOI] [PubMed] [Google Scholar]
- 49. Thiel S., Behrmann I., Dittrich E., Muys L., Tavernier J., Wijdenes J., Heinrich P. C., Graeve L. (1998) Internalization of the interleukin 6 signal transducer gp130 does not require activation of the Jak/STAT pathway. Biochem. J. 330, 47–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Thiel S., Dahmen H., Martens A., Müller-Newen G., Schaper F., Heinrich P. C., Graeve L. (1998) Constitutive internalization and association with adaptor protein-2 of the interleukin-6 signal transducer gp130. FEBS Lett. 441, 231–234 [DOI] [PubMed] [Google Scholar]
- 51. Waetzig G. H., Chalaris A., Rosenstiel P., Suthaus J., Holland C., Karl N., Valles Uriarte L., Till A., Scheller J., Grotzinger J., Schreiber S., Rose-John S., Seegert D. (2010) N-linked glycosylation is essential for the stability but not the signaling function of the interleukin-6 signal transducer glycoprotein 130. J. Biol. Chem. 285, 1781–1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shelburne C. P., McCoy M. E., Piekorz R., Sexl V., Roh K. H., Jacobs-Helber S. M., Gillespie S. R., Bailey D. P., Mirmonsef P., Mann M. N., Kashyap M., Wright H. V., Chong H. J., Bouton L. A., Barnstein B., Ramirez C. D., Bunting K. D., Sawyer S., Lantz C. S., Ryan J. J. (2003) Stat5 expression is critical for mast cell development and survival. Blood 102, 1290–1297 [DOI] [PubMed] [Google Scholar]
- 53. Magrangeas F., Boisteau O., Denis S., Jacques Y., Minvielle S. (2001) Negative cross-talk between interleukin-3 and interleukin-11 is mediated by suppressor of cytokine signalling-3 (SOCS-3). Biochem. J. 353, 223–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Barclay J. L., Anderson S. T., Waters M. J., Curlewis J. D. (2007) Regulation of suppressor of cytokine signaling 3 (SOC3) by growth hormone in pro-B cells. Mol. Endocrinol. 21, 2503–2515 [DOI] [PubMed] [Google Scholar]
- 55. Story D. J., Stephens J. M. (2006) Modulation and lack of cross-talk between signal transducer and activator of transcription 5 and suppressor of cytokine signaling-3 in insulin and growth hormone signaling in 3T3–L1 adipocytes. Obesity (Silver Spring) 14, 1303–1311 [DOI] [PubMed] [Google Scholar]
- 56. Moore K. W., de Waal Malefyt R., Coffman R. L., O'Garra A. (2001) Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 19, 683–765 [DOI] [PubMed] [Google Scholar]
- 57. Mocellin S., Panelli M. C., Wang E., Nagorsen D., Marincola F. M. (2003) The dual role of IL-10. Trends Immunol. 24, 36–43 [DOI] [PubMed] [Google Scholar]
- 58. Chen W. F., Zlotnik A. (1991) IL-10: a novel cytotoxic T cell differentiation factor. J. Immunol. 147, 528–534 [PubMed] [Google Scholar]
- 59. Santin A. D., Hermonat P. L., Ravaggi A., Bellone S., Pecorelli S., Roman J. J., Parham G. P., Cannon M. J. (2000) Interleukin-10 increases Th1 cytokine production and cytotoxic potential in human papillomavirus-specific CD8(+) cytotoxic T lymphocytes. J. Virol. 74, 4729–4737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rousset F., Garcia E., Defrance T., Peronne C., Vezzio N., Hsu D. H., Kastelein R., Moore K. W., Banchereau J. (1992) Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. Proc. Natl. Acad. Sci. USA 89, 1890–1893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Burdin N., Van Kooten C., Galibert L., Abrams J. S., Wijdenes J., Banchereau J., Rousset F. (1995) Endogenous IL-6 and IL-10 contribute to the differentiation of CD40-activated human B lymphocytes. J. Immunol. 154, 2533–2544 [PubMed] [Google Scholar]
- 62. Rennick D., Hunte B., Holland G., Thompson-Snipes L. (1995) Cofactors are essential for stem cell factor-dependent growth and maturation of mast cell progenitors: comparative effects of interleukin-3 (IL-3), IL-4, IL-10, and fibroblasts. Blood 85, 57–65 [PubMed] [Google Scholar]
- 63. Kennedy Norton S., Barnstein B., Brenzovich J., Bailey D. P., Kashyap M., Speiran K., Ford J., Conrad D., Watowich S., Moralle M. R., Kepley C. L., Murray P. J., Ryan J. J. (2008) IL-10 suppresses mast cell IgE receptor expression and signaling in vitro and in vivo. J. Immunol. 180, 2848–2854 [DOI] [PubMed] [Google Scholar]
- 64. Gillespie S. R., DeMartino R. R., Zhu J., Chong H. J., Ramirez C., Shelburne C. P., Bouton L. A., Bailey D. P., Gharse A., Mirmonsef P., Odom S., Gomez G., Rivera J., Fischer-Stenger K., Ryan J. J. (2004) IL-10 inhibits Fc ϵ RI expression in mouse mast cells. J. Immunol. 172, 3181–3188 [DOI] [PubMed] [Google Scholar]
- 65. Bouton L. A., Ramirez C. D., Bailey D. P., Yeatman C. F., Yue J., Wright H. V., Domen J., Rosato R. R., Grant S., Fischer-Stenger K., Ryan J. J. (2004) Costimulation with interleukin-4 and interleukin-10 induces mast cell apoptosis and cell-cycle arrest: the role of p53 and the mitochondrion. Exp. Hematol. 32, 1137–1145 [DOI] [PubMed] [Google Scholar]
- 66. Yeatman C. F., 2nd, Jacobs-Helber S. M., Mirmonsef P., Gillespie S. R., Bouton L. A., Collins H. A., Sawyer S. T., Shelburne C. P., Ryan J. J. (2000) Combined stimulation with the T helper cell type 2 cytokines interleukin (IL)-4 and IL-10 induces mouse mast cell apoptosis. J. Exp. Med. 192, 1093–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. O'Brien C. A., Manolagas S. C. (1997) Isolation and characterization of the human gp130 promoter. Regulation by STATS. J. Biol. Chem. 272, 15003–15010 [DOI] [PubMed] [Google Scholar]
- 68. Mirmonsef P., Shelburne C. P., Fitzhugh Yeatman C., 2nd, Chong H. J., Ryan J. J. (1999) Inhibition of Kit expression by IL-4 and IL-10 in murine mast cells: role of STAT6 and phosphatidylinositol 3′-kinase. J. Immunol. 163, 2530–2539 [PubMed] [Google Scholar]
- 69. Shoji J., Kawaguchi A., Gotoh A., Inada N., Sawa M. (2007) Concentration of soluble interleukin-6 receptors in tears of allergic conjunctival disease patients. Jpn. J. Ophthalmol. 51, 332–337 [DOI] [PubMed] [Google Scholar]
- 70. Klimiuk P. A., Sierakowski S., Latosiewicz R., Cylwik J. P., Cylwik B., Skowronski J., Chwiecko J. (2003) Interleukin-6, soluble interleukin-2 receptor and soluble interleukin-6 receptor in the sera of patients with different histological patterns of rheumatoid synovitis. Clin. Exp. Rheumatol. 21, 63–69 [PubMed] [Google Scholar]
- 71. Yokoyama A., Kohno N., Sakai K., Kondo K., Hirasawa Y., Hiwada K. (1997) Circulating levels of soluble interleukin-6 receptor in patients with bronchial asthma. Am. J. Respir. Crit. Care Med. 156, 1688–1691 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.