K12/SECTM1 is induced by IFN-γ in antigen-presenting cells, and synergizes with anti-CD28 antibody to costimulate CD4 and CD8 T cell proliferation and cytokine production.
Keywords: chromatin immunoprecipitation, graft-versus-host disease, β-galactosidase
Abstract
CD7 is a cell-surface molecule, expressed on T lymphocytes and NK cells, which functions as a costimulatory receptor for T cell proliferation. SECTM1 has been proposed as a ligand for CD7. However, the expression pattern of this molecule in human immune cells and role in human T cell function remain unclear. In the present study, using human rSECTM1, we demonstrate that SECTM1 strongly costimulates CD4 and CD8 T cell proliferation and induces IFN-γ production, likely via a CD7-dependent mechanism. In addition, SECTM1 synergizes with suboptimal anti-CD28 to strongly augment T cell functions. We found a robust induction of IL-2 production when SECTM1 and anti-CD28 signals were present with TCR ligation. Furthermore, addition of SECTM1 into a MLR significantly enhanced proliferation of alloantigen-activated T cells, whereas blockade of SECTM1 inhibited T cell proliferation in a two-way MLR assay. Simultaneously blocking the effect of SECTM1, along with CTLA-4/Fc, diminishes two-way MLR. Finally, we demonstrated that expression of SECTM1 is not detected in monocytes and imMoDCs at the protein level. However, it is strongly induced by IFN-γ in monocytes and imMoDCs, and this induction is STAT1-dependent. These results indicate that SECTM1 is a broadly expressed, IFN-γ-inducible molecule, which functions as a potent costimulatory ligand for T cell activation and is synergistic with anti-CD28.
Introduction
The initial T cell activation occurs by engagement of the antigen-specific TCR with an antigenic peptide in the context of the MHC. However, this signal alone is not sufficient for an effective T cell response and requires a second signal, termed costimulation, for full activation of T cells. T cell costimulation occurs after interaction of receptors on T cells with their ligands on APCs. Absence of a costimulatory signal induces long-term antigen-specific T cell anergy or unresponsiveness.
In addition to well-characterized, costimulatory receptors, such as members of the B7 and the TNFR superfamily [1, 2], CD7 has been proposed to transmit signals that result in T cell proliferation. CD7 is a 40-kD single-domain Ig superfamily molecule expressed on the majority of human mature T and NK cells, as well as on cells in the early stages of T, B, and myeloid cell development. Like CD28, the intracellular domain of CD7 contains a YEEM motif, which activates the PI3K signaling pathway. Anti-CD7 treatment of cells exposed to anti-CD3 or PMA demonstrated that CD7 is comitogenic for human T cells [3] and could also stimulate production of IL-2 [4]; conversely, the antagonist anti-CD7 antibody prevented anti-CD3-induced proliferation but not proliferation induced by PHA, Con A, or PMA [5, 6]. As PBMCs were used in most of the reported studies, it remains to be determined which T cell population is directly responding to anti-CD7 stimulation and whether the proliferation effect is direct or indirect.
Lee et al. [7] reported that CD7 knockout mice showed a defect of antigen-specific CTL activity and induced less IFN-γ in response to tetanus toxoid administration, whereas lymphocytes proliferated normally against PHA, anti-CD3, Con A, and LPS. Bonilla et al. [8], however, detected no measurable defect in T cell functions in CD7 knockout mice. However, CD7 and CD28 double-knockout mice showed decreased numbers of thymocytes and reduced production of IFN-γ and TNF-α in peripheral T cells stimulated by anti-CD3, as compared with CD7 or CD28 single-knockout mice. Interestingly, the aged double-knockout mice developed thyroiditis associated with abnormal numbers and functions of CD4+CD25+ regulatory T cells [9, 10]. These data suggested that CD7 and CD28 act together to regulate T cell functions. However, because of significant differences in the structure of the human and mouse orthologs of CD7, the relevance of the mouse data for human T cells is not clear. For example, unlike human CD7, the extracellular domain of mouse CD7 does not have a stalk region, which may be important for expression on the cell surface [11].
Human SECTM1 is a type I transmembrane glycoprotein that is localized in the Golgi apparatus and exists as a transmembrane and a soluble form [12]. The N-terminus of this molecule contains two Ig-like domains and one N-linked glycosylation motif. Although SECTM1 could be detected on the cell surface in SECTM1 gene-transfected cells, SECTM1 was not located on the surface of nontransfected cell lines that expressed the protein [13, 14] (unpublished data). Nonetheless, the soluble form of SECTM1 can be detected in tissue-culture medium from breast cancer cell lines [12]. Lyman et al. [15] first identified SECTM1 as a ligand for CD7, and a subsequent study confirmed that SECTM1 binds to CD7 [13]. The recombinant soluble extracellular domain of human SECTM1 protein increases expression of human NK cell activation markers, such as CD54, CD69, and CD25 [15], and the mouse SECTM1 protein inhibits Con A but not anti-TCR-induced mouse LN cell proliferation. Interestingly, SECTM1 is adjacent to the human CD7 gene on chromosome 17, where only a 5-kb pair separates them. However, the full biological consequence of SECTM1 interaction with CD7 and the effect of SECTM1 induction on expressing cells remain to be fully defined.
SECTM1 is expressed on thymic epithelial and fibroblast cells, breast cancer and leukemia cell lines, and neutrophils but not in peripheral lymphocytes [12, 13]. The expression of SECTM1 can be up-regulated by IFN-γ in many cell types, including thymic epithelial cells and monocytes [12–14, 16], but the significance of induction of SECTM1 by IFN-γ in immune cells and the mechanisms of the induction and regulation of SECTM1 by IFN-γ remain unknown.
In the present study, we have used recombinant protein representing the soluble N-terminus of human SECTM1 to demonstrate that SECTM1 is a costimulatory ligand for human CD4 and CD8 T cell proliferation and cytokine production and show that this effect is CD7-dependent in human cells. Furthermore, we show that SECTM1 synergizes with anti-CD28 to enhance CD4 and CD8 T cell proliferation and IL-2 production. Consistent with these observations, we demonstrate that SECTM1 protein enhances allogeneic T cell responses, whereas anti-SECTM1-blocking antibody synergized with CTLA-4/Fc to inhibit a two-way MLR. Bioinformatics analysis demonstrated that SECTM1 is widely expressed in human tissues and immune cells at the mRNA level. Moreover, SECTM1 expression is potently induced by IFN-γ in monocytes and imMoDCs, similar to that seen in tissue-culture cell lines [12–14]. The induction of SECTM1 expression by IFN-γ is STAT1-dependent. Collectively, our data definitively establish that human SECTM1 is a potent costimulatory ligand for T cell proliferation and that SECTM1 is expressed in activated APCs induced by IFN-γ. Targeting the SECTM1–CD7 interaction could potentially be used for preventing an allogeneic T cell response, autoimmune diseases, and IFN-γ related diseases.
MATERIALS AND METHODS
Reagents and cell culture
Human rIFN-γ, GM-CSF, IL-4, and CTLA4/Fc were from R&D Systems (Minneapolis, MN, USA). Jurkat and 293T cells were from American Type Culture Collection (Manassas, VA, USA) and cultured in DMEM, supplemented with 10% FBS (Invitrogen, Carlsbad, CA, USA). Human melanoma cell line 1205-Lu cells were cultured in 2% FBS melanoma medium, as described previously [17].
Antibodies
Anti-SECTM1 hybridoma was a gift from Dr. Barton F. Haynes (Duke University, Durham, NC, USA), and antibodies were made at The Wistar Institute Monoclonal Antibody Core Facility (Philadelphia, PA, USA). Anti-SECTM1 was conjugated to the FITC or PE by BioLegend (San Diego, CA, USA). Rabbit anti-SECTM1 2816 antibody was made as described previously and used for Western blot analysis [12]. Florescence-conjugated anti-CD4, -CD8, -CD7, -CD14, -CD25, -CD69, -IL-2, and -IFN-γ were from BD Biosciences (San Diego, CA, USA). Anti-IFN-γ-blocking antibody was from R&D Systems. Anti-CD3 mAb (OKT3), -CD28 (CD28.2), and -SECTM1 isotype control IgG2b were from eBioscience (San Diego, CA, USA). Rabbit anti-actin was from Sigma-Aldrich (St. Louis, MO, USA).
Bioinformatics
Expression of SECTM1 across different tissues and blood cell types was derived from dataset GDS596 from the GEO database (http://www.ncbi.nlm.nih.gov/geo/). Reporter 213716_s_at log2-transformed expression values were used for visualization. Background value was defined at two levels of 5% lowest-expressed probes average [18].
SECTM1-Ig recombinant fusion proteins
The CD5-IgG2b fusion constructs of SECTM1 were made as described previously [13]. Briefly, COS-7 cells at 90–95% confluence were transfected with DNA using Lipofectamine 2000 (Invitrogen). Six hours post-transfection, medium was replaced with 1% low IgG serum medium (Invitrogen) for a further 72 h. Supernatants were harvested and then incubated at 4°C with protein G-Sepharose beads (Invitrogen) for 2 h and eluted with pH 3.0 glycine, followed by dialysis with cold PBS. Proteins were concentrated with Centricon-30 (Millipore, Billerica, MA, USA) and quantified by a BCA protein quantification kit (Pierce, Rockford, IL, USA).
Isolation of monocytes and T cells
This study was reviewed and approved by human subjects review boards at The Wistar Institute and the University of Pennsylvania (Philadelphia, PA, USA). Monocytes were from the AIDS Research Human Immunology Core at the University of Pennsylvania. Monocytes were purified by countercurrent elutriation and seeded in RPMI supplemented with 10% FBS and 100 mM HEPES (R10 medium) at 37°C for 1 h. Nonadhesive cells were removed, and 95% of cells were CD3−CD14+, confirmed by flow cytometric analysis. Human PBMCs were purified from blood of anonymous healthy donors by Histopaque-1077 separation (Amersham Pharmacia, Piscataway, NJ, USA). A T cell-negative selection kit (Invitrogen) was used according to the manufacturer's instruction to enrich resting T cells. The purity of T cells was between 95% and 97%.
Generation of imMoDCs
To generate imMoDCs, monocytes were cultured with GM-CSF (20 ng/ml) and IL-4 (20 ng/ml) in R10 medium for 7 days.
T lymphocyte proliferation assay
T cells (1×105/well) in 200 μl R10 medium were stimulated with combinations of plate-bound anti-CD3 mAb (0.5 μg/ml) plus plate-bound SECTM1-Ig or Ig control proteins and soluble anti-CD28 antibodies in 96-well, round-bottom plates for 72 h at various concentrations. Cells were pulsed with 0.5 μCi [3H]-TdR for the last 18 h, and incorporated [3H]-TdR was measured on a microplate scintillation counter. For a SECTM1-blocking experiment, anti-SECTM1 at various concentrations was added to the cell culture.
Flow cytometric analysis (FACS)
For multicolor staining, cells were incubated with fluorochrome-conjugated mAb for 30 min on ice and washed with 2% FBS PBS buffer (FACS buffer). Anti-CD28, -CD25, -CD69, -CD4, -CD8, and -CD7 were used for T cell experiments. Anti-CD14 was used for monocyte staining. For cytokine intracellular staining, purified T cells were cultured as described above for 6 and 20 h and 4 h in the presence of GolgiPlug. After washing with FACS buffer, cells were stained with surface markers and then introcelluarlly stained with anti-IFN-γ and anti-IL-2 mAb, according to the manufacturer's instruction (BD Biosciences). Samples were analyzed on a FACSCalibur or CyAn, and data were analyzed with FlowJo software (TreeStar, Ashland, OR, USA).
Allogeneic T cell proliferation assays
For the primary allogeneic MLR, 40 Gy-irradiated, allogeneic imMoDCs were cocultured with 1 × 105-purified T cells in 200 μl R10 medium for 5 days in the presence of plate-bound SECTM1-Ig (2 μg/ml) or control Ig (2 μg/ml). The ratio of imMoDCs and T cells was 1:5 and 1:20. [3H]-TdR (0.5 μCi) was added for 18 h before cell harvest.
For two-way MLR, PBMCs from two healthy human volunteers were mixed at the ratio of 1:1 and cultured in R10 medium for 5 days. [3H]-TdR was added 18 h before cell harvest. For blocking experiments, anti-SECTM1 and/or CTLA-4/Fc (2.5 μg/ml) of various concentrations were added in the culture medium, with mouse IgG2b added as an isotype control. Culture cells were also used for FACS analysis of SECTM1 and CD69 expression.
Internalization assay
SECTM1-Ig was conjugated with R-PE using the Zenon mouse IgG2b labeling kit (Invitrogen), according to the manufacturer's instruction. Jurkat cells were incubated with or without 2 μg/ml SECTM1-Ig at 37°C for 5 and 60 min. Cells were washed twice with ice-cold PBS and divided into two parts. One part was left on ice as the no-treatment control, whereas the other part was incubated on ice for 45 s in acid-stripping solution (add HCl to 0.03 M sucrose in 10% FCS PBS, pH=2), which can eliminate 99% of cell-surface-bound proteins [19]. Cells were then washed with R10 medium and analyzed by FACS.
RNA extraction and semiquantitative RT-PCR
Monocytes or imMoDCs were cultured with or without IFN-γ (20 ng/ml) for the indicated time. Total RNA was extracted with the RNA extraction kit (GE Healthcare, Piscataway, NJ, USA) from cells, according to the manufacturer's instructions. RT-PCR was performed with 1 μg/sample using oligo-dT priming with the SuperScript RT-PCR reagent (Invitrogen), and PCR was performed using the following parameters: 5 min at 94°C, 35 cycles of 30 s at 94°C, 30 s at 56°C, 60 s at 72°C, and 10 min at 72°C. Primer sequences used for amplifications were as follows: SECTM1, forward 5′-agagcgaccaagaggatgaa-3′, reverse 5′-cccatgtcaacatcaagctg-3′; β-actin, forward 5′-gtgggccgctctaggcacc-3′, reverse 5′-ctctttgatgtcacgcacgat-3′. PCR products were analyzed on 1% agarose gel containing ethidium bromide.
Western blot analysis
Monocytes or imMoDCs were cultured with or without IFN-γ (20 ng/ml) for the indicated times. Cells were lysed with RIPA buffer (Cell Signaling Technology, Danvers, MA, USA). Protein samples were loaded and separated on 4–12% bis-tris gels (Invitogen) and transferred to PVDF membranes. The proteins were detected with anti-SECTM1 2816 antiserum, and signal was detected using peroxidase-conjugated secondary antibody, followed by development using an enhanced ECL system (Pierce).
Cloning of SECTM1 promoter reporter plasmid
SECTM1 promoter was predicted with the MatInspector program. The SECTM1 promoter region (614 bp) was amplified by RT-PCR from human genomic DNA. The gene was then inserted into the pGL4.10 plasmid to construct a SECTM1 promoter luciferase reporter plasmid. The following primers were used: forward 5′-cgcggtaccttctacccgcaccccaa-3′, reverse 5′-cccaagcttagccccaggaaaatgaaaga-3′. The sequence of construct was verified by sequencing in The Wistar Institute Genomic Core facility.
Luciferase reporter assay
Approximately 2.0 × 105 HEK293 cells were transfected with 1 μg SECTM1 reporter plasmid or control plasmid, with 0.2 μg β-Gal expression plasmid as an internal control for transfection efficiency using Lipofectin 2000 (Invitrogen) for 24 h. Cells were treated with or without IFN-γ (20 ng/ml) for 24 h. Luciferase reporter activity was determined with the Luciferase Assay System (Promega, Madison, WI, USA) and normalized to β-Gal activity.
ChIP assay
1205-Lu cells (1×108) were treated with 20 ng/ml IFN-γ for various times at 37°C with an equal amount of cells left untreated. ChIP assay for the SECTM1 promoter was performed with the kit (Millipore) as recommended. Anti-STAT1 (Cell Signaling Technology) was used to immunoprecipitate the DNA-STAT1 complex, and rabbit serum was used as the control. Primers for amplification of the SECTM1 promoter were as follows: forward 5′- cgcggtaccttgttgtgaacgcgggtg-3′, reverse 5′-cccaagcttagccccaggaaaatgaaaga-3′. PCR was performed using the following parameters: 5 min at 94°C, 38 cycles of 30 s at 94°C, 30 s at 56°C, 30 s at 72°C, and 10 min at 72°C. PCR products were analyzed by gel electrophoresis.
Statistical analysis
Statistical analysis was performed using Student's two-tailed t test. P < 0.05 was considered statistically significant.
RESULTS
Expression of SECTM1 mRNA by human cells
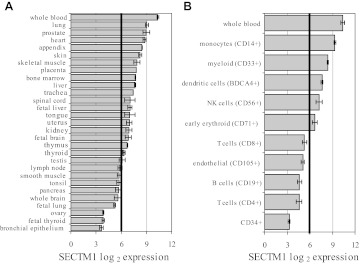
SECTM1 has been reported to be expressed in human breast cancer and leukemia cell lines, neutrophils, thymic epithelium, and fibroblast cells [12, 13]. We further characterized the mRNA expression pattern of SECTM1 on normal human tissues and immune cells by using publicly available gene expression data in GEO databases. Figure 1A shows expression of SECTM1 across major tissues, including whole blood, where the gene was the most highly expressed. Lung, prostate, heart, appendix, and skin are other tissues with expression of SECTM1, whereas bronchial epithelium, fetal thyroid, and ovary express SECTM1 at very low levels.
Figure 1. Bioinformatics analysis of expression of the SECTM1 gene across different human normal tissues.
SECTM1 mRNA expression pattern was obtained by GEO analysis across 29 major tissues (A) and immune cells (B). Vertical black lines indicate background level of expression defined as double the level of 5% lowest-expressed genes average on the array.
Further analysis of immune cells demonstrated that SECTM1 mRNA is expressed in monocytes, CD33-positive myloid cells, positive blood DC antigen 4+ NK cells, as well as CD71 positive early erythroid cells, but not T cells, B cells, CD105 positive endothelial cells, or CD34 positive cells (Fig. 1B).
SECTM1 is a costimulatory ligand for T cell proliferation
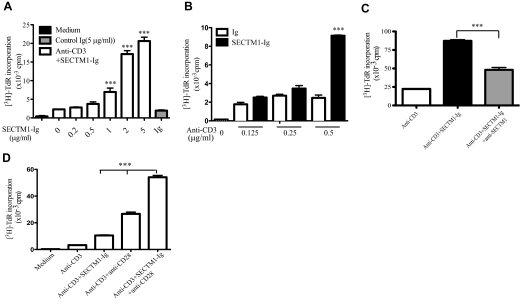
To characterize the effect of SECTM1 on T cell proliferation, we added various amounts of human SECTM1-Ig fusion proteins, ranging from 0.2 μg/ml to 5 μg/ml, into human T cell cultures stimulated with immobilized anti-CD3 (anti-CD3/SECTM1-Ig) and human Ig as control (anti-CD3/Ig). As shown in Fig. 2A, the addition of SECTM1-Ig to immobilized anti-CD3 (anti-CD3/SECTM1-Ig) enhanced T cell proliferation in a dose-dependent manner. A similar effect of SECTM1-Ig on stimulating T cell proliferation was observed on T cells from 12 different healthy donors (Table 1). The stimulatory effect of SECTM1-Ig required the presence of anti-CD3, whereas SECTM1-Ig failed to enhance the proliferation of T cells when anti-CD3 was decreased to 0.25 μg/ml (Fig. 2B). Furthermore, using anti-SECTM1 (10 μg/ml), which specifically blocks the interaction between CD7 and SECTM1 [13], we found that anti-SECTM1 significantly decreased T cell proliferation induced by anti-CD3/SECTM1-Ig, indicating the specificity of rSECTM1-Ig (Fig. 2C).
Figure 2. Costimulatory effect of SECTM1 on T cell proliferation.
(A) Purified T cells were cultured in the presence of various concentrations of immobilized SECTM1-Ig (0.2–5 μg/ml) and a constant amount of immobilized anti-CD3 mAb (0.5 μg/ml) or IgG-fusion protein control (5 μg/ml). Proliferation was determined in a standard [3H]-TdR assay after 3 days of incubation. (B) Purified T cells were cultured in the presence of various concentrations of immobilized anti-CD3 mAb (0–0.5 μg/ml) in the presence of a constant amount of immobilized SECTM1-Ig (5 μg/ml) for 3 days and T cell proliferation determined as in A. (C) Anti-SECTM1-specific mAb blocks SECTM1 costimulatory effect for T cell proliferation. Purified T cells were cultured in the presence of immobilized anti-CD3 (0.5 μg/ml) and/or 5 μg/ml immobilized SECTM1-Ig, with or without anti-SECTM1 (10 μg/ml) for 3 days and T cell proliferation determined as in A. (D) Purified T cells were stimulated with immobilized SECTM1-Ig (2 μg/ml) and/or soluble anti-CD28 mAb (2 μg/ml) or both in the presence of immobilized anti-CD3 mAb (0.5 μg/ml) for 3 days. T cell proliferation was determined as in A. Data reflect one representative of at least two donors from two independent experiments. Error bars indicate sd in triplicate samples. (A and B) ***P < 0.001 versus anti-CD3. (C) ***P < 0.01 vs anti-CD3 (D) ***P < 0.01 versus anti-CD3 + SECTM1-Ig and anti-CD3 + anti-CD28.
Table 1. Anti-CD3-Ig and Anti-CD3/SECTM-Ig Stimulation of Human Peripheral T Cells.
| Anti-CD3-Ig (cpm×10−3) | Anti-CD3 + SECTM1-Ig (cpm×10−3) | Fold increase | |
|---|---|---|---|
| Donor 1 | 2.46 ± 0.53 | 9.14 ± 0.12 | 3.70 |
| Donor 2 | 6.66 ± 00.03 | 203.31 ± 15.5 | 30.5 |
| Donor 3 | 2.30 ± 0.11 | 17.13 ± 1.68 | 7.45 |
| Donor 4 | 3.81 ± 0.71 | 18.83 ± 1.5 | 5.4 |
| Donor 5 | 2.56 ± 0.79 | 23.74 ± 4.34 | 9.3 |
| Donor 6 | 3.29 ± 0.18 | 19.93 ± 4.62 | 6.06 |
| Donor 7 | 2.81 ± 0.52 | 15.38 ± 1.81 | 5.5 |
| Donor 8 | 2.78 ± 0.55 | 15.37 ± 1.71 | 5.52 |
| Donor 9 | 21.46 ± 0.9 | 87.27 ± 2.6 | 4.07 |
| Donor 10 | 16.85 ± 2.19 | 23.94 ± 1.38 | 1.42 |
| Donor 11 | 16.61 ± 0.19 | 42.81 ± 6.98 | 2.58 |
| Donor 12 | 9.08 ± 0.75 | 34.65 ± 8.25 | 3.82 |
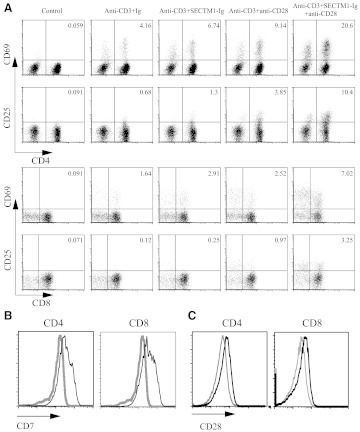
We then analyzed expression of the T cell activation markers of CD69 and CD25 in response to activation with anti-CD3 and SECTM1-Ig. As shown in Fig. 3A, additional SECTM1-Ig (5 μg/ml) significantly increased CD69 and CD25 expression.
Figure 3. SECTM1 enhances T cell activation markers.
(A) Purified T cells were stimulated with immobilized SECTM1-Ig (5 μg/ml) in the presence of anti-CD3 mAb (0.5 μg/ml), with or without soluble anti-CD28 mAb (2 μg/ml) for 48 h. Cells were harvested and stained with anti-CD4, -CD8, -CD69, and -CD25 for FACS analysis gating on live cells. (B) Purified T cells were stimulated with soluble anti-CD28 mAb (2 μg/ml) in the presence of anti-CD3 mAb (0.5 μg/ml) for 48 h. Cells were harvested and stained with anti-CD4, -CD8, and -CD7 for FACS analysis gating on live cells. (C) Purified T cells were stimulated with immobilized SECTM1-Ig (5 μg/ml) in the presence of anti-CD3 mAb (0.5 μg/ml) for 48 h. Cells were harvested and stained with anti-CD4, -CD8, and -CD28 for FACS analysis gating on live cells (gray line: unstimulated T cells; black line: stimulated T cells). Data reflect one representative of four donors from two independent experiments.
Signal transduction pathways for CD28 and CD7 have been well-characterized and appear similar but not identical [2]. To examine the combined effects of SECTM1 and CD28 on T cell proliferation and cytokine production, we added a suboptimal concentration of SECTM1-Ig (2 μg/ml) to purified T cells stimulated with anti-CD3 (0.5 μg/ml) in the presence or absence of a suboptimal concentration of anti-CD28 (2 μg/ml). Addition of SECTM1-Ig, along with anti-CD28, resulted in a significant increase in T cell proliferation (Fig. 2D). Furthermore, addition of SECTM1-Ig to anti-CD3 enhanced CD69 and CD25 expression on the surface of CD4 and CD8 T cells, and the further addition of anti-CD28 resulted in an even greater expression of CD25 and CD69 on CD4 and CD8 T cells (Fig. 3A).
Interestingly, expression of CD7 was up-regulated in CD4 and CD8 T cells stimulated with anti-CD3 (0.5 μg/ml) in the presence of anti-CD28 (2 μg/ml; Fig. 3B). Accordingly, expression of CD28 is also up-regulated in CD4 and CD8 T cells stimulated with anti-CD3 (0.5 μg/ml) in the presence of SECTM1-Ig (2 μg/ml; Fig. 3C). These data partially explain the synergistic effect of CD7 and CD28 on T cell proliferation.
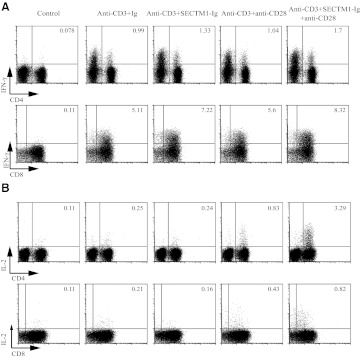
To determine if cytokine responses were modified by the combined effects of SECTM1 and CD28, we characterized the production of cytokines by activated T cells using FACS analysis, which confirmed that SECTM1 enhanced IFN-γ production in CD4 and CD8 T cells, stimulated by anti-CD3 (Fig. 4A). Although anti-CD3/SECTM1-Ig did not increase the production of IL-2 by itself, the addition of anti-CD28 resulted in a large induction of IL-2 production in CD4 T cells and to a lesser extent, in CD8 T cells (Fig. 4B). These results suggest that SECTM1 is a costimulatory ligand that can augment T cell function on its own but can also act in synergy with anti-CD28 to enhance T proliferation and IL-2 production.
Figure 4. SECTM1 costimulates cytokine production in T cells.
(A and B) Purified T cells were stimulated with immobilized SECTM1-Ig (2 μg/ml), with or without soluble anti-CD28 mAb (2 μg/ml) in the presence of anti-CD3 mAb (0.5 μg/ml), and intracellular staining was performed to measure expression of IFN-γ (A) and IL-2 (B). For IFN-γ expression, cells were stimulated for 6 h and IL-2 expression for 18 h (B). Results shown are representative from four donors of two independent experiments.
Synergistic effect of SECTM1 and CTLA-4/Fc in allogeneic T cell response
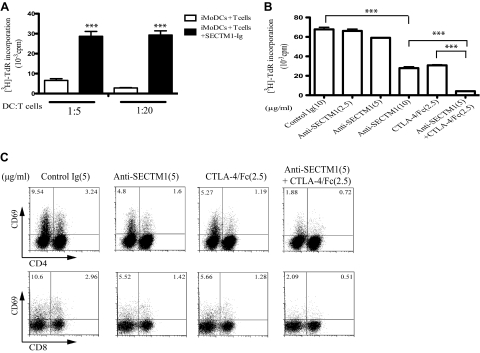
Costimulatory molecules play critical roles in allogeneic T cell response [20]. We used a MLR to investigate the role of SECTM1 in allogeneic T cell proliferation in vitro. We first incubated allogeneic imMoDC with T cells, with or without SECTM1-Ig. The addition of SECTM1-Ig resulted in a strongly increased T cell proliferation when measured by [3H]-TdR incorporation on Day 7 (Fig. 5A). Addition of anti-SECTM1 significantly decreased T cell proliferation in a dose-dependent manner (Fig. 5B). At a concentration of 5 μg/ml, anti-SECTM1 antibody did not have any effect on proliferation. The addition of suboptimal amounts of CTLA-4/Fc to this amount of SECTM1 essentially abolished the proliferation in the MLR (Fig. 5B), further suggesting a synergistic effect between SECTM1 and anti-CD28. Supporting this observation, anti-SECTM1 and CTLA-4/Fc together decreased CD69 expression on CD4, CD8 T cells after 5 days incubation (Fig. 5C).
Figure 5. The effect of SECTM1 on allogeneic T cell responses.
(A) Purified T cells (1×105) were cultured with SECTM1-Ig (5 μg/ml) in the presence of irradiated imMoDCs (iMoDC) at ratios of 5:1 and 20:1 for 5 days and allogeneic T cell proliferation determined as in Fig. 2A. (B) Synergistic effect of anti-SECTM1 and CTLA-4/Fc on two-way MLR. PBMCs from two healthy donors were mixed in 10% FCS RPMI medium for 5 days in the presence of various concentrations of anti-SECTM1 (2.5, 5, 10 μg/ml) and CTLA-4/Fc (2.5 μg/ml) or a combination (anti-SECTM1 5 μg/ml and CTLA-4/Fc 2.5 μg/ml) and cell proliferation determined as in Fig. 2A. (C) Inhibition of CD69 expression in two-way MLR. PBMCs from two healthy donors were mixed and cultured in the presence of anti-SECTM1 mAb (5 μg/ml) or CTLA-4/Fc (2.5 μg/ml) or combined for 5 days. T cell activation was measured by expression of CD69 on CD4 and CD8 T cells. (A) ***P < 0.001 versus imMoDCs + T cells. (B) ***P < 0.001 anti-SECTM1 (10 μg/ml) versus control; ***P < 0.001 anti-SECTM1 (5 μg/ml) + CTLA-4/Fc (2.5 μg/ml) versus anti-SECTM1 (10 μg/ml) or CTLA-4/Fc (2.5 μg/ml).
The costimulatory effect of SECTM1 is CD7-dependent
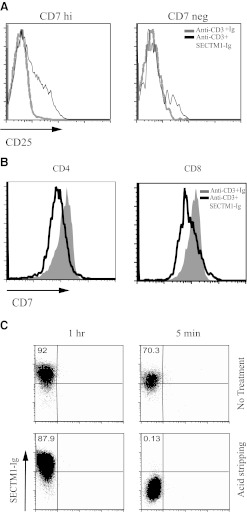
Previous studies have suggested that SECTM1 is a ligand for CD7. We next investigated whether the costimulatory effect of SECTM1 is CD7-dependent. FACS analysis demonstrated that anti-CD3/SECTM1-Ig up-regulated expression of CD25 in CD7-positive cells by Day 3 poststimulation. In contrast, SECTM1 costimulation had a substantially reduced effect on CD7-negative cells (Fig. 6A). This suggested that SECTM1 indeed costimulates CD7-positive cells.
Figure 6. The costimulatory effect of SECTM1 is CD7-dependent.
(A). Expression of CD3 T cell activation marker CD25 was determined by FACS analysis by gating on CD7 high (hi) and negative (neg) T cells after immobilized anti-CD3 antibodies (0.5 μg/ml), with or without SECTM1-Ig (5 μg/ml) stimulation for 3 days. (B) CD7 expression is down-regulated by SECTM1 engagement. Purified T cells were stimulated with immobilized anti-CD3 (0.5 μg/ml), with or without immobilized SECTM1-Ig (5 μg/ml) for 3 days, and CD7 expression was analyzed by FACS analysis. (C) Internalization of SECTM1 after engagement with Jurkat cells, which were incubated with PE-conjugated SECTM1-Ig at 37°C for 5 and 60 min. Cells were treated with or without the acid-stripping method. Internalization of SECTM1 was measured by FACS analysis. Data shown are representative of three independent experiments.
Ligand engagement can down-regulate expression of a receptor by ligand-induced receptor internalization. Anti-CD3/SECTM1-Ig stimulation down-regulates expression of CD7 after 3 days incubation on CD4 and CD8 T cells (Fig. 6B). This suggests that down-regulation of CD7 by SECTM1 might be a result of ligand-induced receptor internalization. To test this, CD7-positive Jurkat cells were incubated with PE-conjugated SECTM1-Ig for 5 and 60 min and treated with or without acid-stripping buffer, which can effectively remove all surface-bound proteins [19]. As shown in Fig. 6C, after 5 min of incubation, acid stripping can remove the surface-bound SECTM1-PE. After 60 min of incubation, however, the SECTM1-PE signal was not diminished by acid stripping, indicating that SECTM1 is indeed internalized by Jurkat cells, presumably by binding to CD7. The data presented above collectively suggest that the costimulatory effect of SECTM1 might be CD7-dependent.
SECTM1 expression is induced by IFN-γ
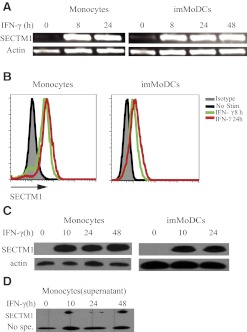
The induction of SECTM1 expression in monocytes and imMoDCs has potentially important implications in regulating T cell functions, and the mechanism of induction has not been determined. Previous studies have demonstrated that SECTM1 is induced by IFN-γ in breast cancer cell lines, and subsequently, in human thymic epithelial cells [13, 14, 16]. We examined the effect of IFN-γ on expression of SECTM1 in monocytes and imMoDCs cells. Addition of IFN-γ rapidly augmented the expression of SECTM1 mRNA in monocytes and imMoDCs by a semiquantitative RT-PCR assay (Fig. 7A). This indicates that regulation of SECTM1 expression by IFN-γ occurs at least at the transcriptional level. Intracellular staining of SECTM1 by FACS analysis demonstrated that expression of SECTM1 protein is also induced as early as 8 h (Fig. 7B) in monocytes and imMoDCs. Western blot analysis further confirmed that SECTM1 is induced by IFN-γ in monocytes and imMoDCs, and this increased expression lasts at least 48 h (Fig. 7C). Finally, Western blot showed that the soluble form of SECTM1 is also induced from a IFN-γ stimulated, monocyte-cultured supernatant (Fig. 7D). Our data demonstrated that SECTM1 is induced by IFN-γ in monocytes and imMoDCs at the mRNA and protein level.
Figure 7. IFN-γ induces expression of SECTM1.
(A) Monocytes (left panel) and imMoDCs (right panel) were stimulated with or without IFN-γ (20 ng/ml) for 0, 8, 24, and 48 h, and SECTM1 mRNA expression was assessed by RT-PCR (top). Analysis of actin levels was used as a loading control (bottom). (B) Monocytes (left panel) and imMoDCs (right panel) were stimulated with or without (No Stim) IFN-γ (20 ng/ml) for 0, 8, and 24 h. SECTM1 protein expression was determined as in Fig. 2A. (C) Western blot analysis of SECTM1 expression in monocytes and imMoDCs. Monocytes and imMoDCs were stimulated with 20 ng/ml IFN-γ. Cells were harvested at each indicated time-point. Whole-cell lysates were analyzed by Western blot using rabbit anti-human SECTM1 2816 polyclonal antibody with actin expression as a loading control. (D) Western blot analysis of SECTM1 expression in monocyte-cultured supernatants. Monocytes were stimulated with 20 ng/ml IFN-γ. Cultured supernatants were harvested at each indicated time.
Induction of SECTM1 expression is dependent on the STAT1 pathway
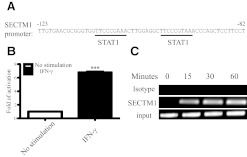
We further investigated the mechanism of IFN-γ induction of the expression of SECTM1. Analysis of the human SECTM1 promoter region by the MatInspector program revealed a number of consensus-binding motifs for known transcription factors, such as STAT1, AP1, PU1, and SP1 in the human SECTM1 promoter region [14]. Among these motifs was a pair of closely spaced STAT1α-binding sites located ∼100 bp from the predicted transcription start site, each of which conformed perfectly to the 9-bp consensus sequence of a STAT-binding element. This motif is also known as a GAS (consensus: TTCNNNGAA) [21]. These tandemly linked GAS elements in the promoter region strongly suggests that SECTM1 expression could be regulated by IFN-γ via STAT1 [22] (Fig. 8A).
Figure 8. Induction of SECTM1 expression is dependent on STAT1 pathway.
(A) MatInspector was used to analyze potential STAT1-binding motifs in the human SECTM1 promoter region. (B) Transcriptional activation of human SECTM1 promoter is induced after IFN-γ treatment. HEK293 cells were transfected with SECTM1 promoter-luciferase. Twenty-four hours later, cells were treated with IFN-γ or left untreated (20 ng/ml) for 24 h. Cells were lysed, and relative luciferase activities were determined with a luminometer. Results were normalized to the β-Gal activity. ***P < 0.001. (C) ChIP assay confirms that induction of SECTM1 by IFN-γ is STAT1-dependent. 1205-Lu cells were stimulated with IFN-γ (20 ng/ml) for 15, 30, and 60 min. Sheared genomic DNA was pulled down with the anti-STAT1 antibody. ChIP was analyzed by PCR using primers specific for the SECTM1 promoter. Data are representative of two independent experiments.
To determine whether IFN-γ treatment resulted in inducible transcriptional activity of the SECTM1 promoter, a plasmid containing the 614-bp 5′-flanking region of the SECTM1 promoter was amplified and cloned into the luciferase reporter plasmid pGL4.10. The plasmids were transfected into the HEK293T cells for 24 h. Cells were then treated with IFN-γ (20 ng/ml) for another 24 h. Luciferase activity of cell lysates was measured. A sevenfold increase in promoter activity was observed in HEK293T cells transfected with the SECTM1 pGL4.10 plasmid after IFN-γ treatment (Fig. 8B).
Using a ChIP assay on the IFN-γ-sensitive melanoma cell line 1205-Lu, we then examined whether STAT1 directly binds to the promoter region of the SECTM1 gene. As shown in Fig. 8C, the binding of STAT1 to the promoter region of the SECTM1 gene increased after 15 min of IFN-γ stimulation. Amplification of the input DNA from the promoter region of the SECTM1 gene was used as a loading control. These data suggest that IFN-γ induces the binding of STAT1 to the promoter region of the SECTM1 gene leading to transcriptional induction of SECTM1.
DISCUSSION
The regulation of T cell activation has been studied intensely, and multiple receptors and ligands have been implicated and characterized. This important process must be tightly regulated, as it is highly dynamic and under positive and negative signal controls. The addition of SECTM1 to the list of costimulatory molecules adds another degree of complexity. Although SECTM1 has been shown to bind to CD7, the functions and expression patterns of SECTM1 in the human immune system have been largely uncharacterized. Here, we have shown that human rSECTM1 is a significant costimulatory ligand and preferentially induces IFN-γ production by CD4 and CD8 T cells, likely via binding to the receptor CD7. The combined effect of SECTM1 and anti-CD28 results in at least an additive effect on T cell proliferation and an apparent synergistic effect on induction of IL-2 production. Consistent with these observations, anti-SECTM1 enhanced CTLA-4/Ig-induced inhibition of an allogeneic T cell response in an in vitro two-way MLR assay. Importantly, SECTM1 is not expressed constitutively by APCs at the protein level but rather, is markedly induced in activated APCs by IFN-γ. Our data suggest that SECTM1 may play an important role in regulating T cell response after encountering antigens.
SECTM1 acts similarly to the agonistic anti-CD7 to costimulate human T cell proliferation and up-regulate CD69 and CD25 expression, as well as IFN-γ production. Our studies demonstrated an important, new difference: whereas anti-CD7 increases IL-2 production [4], SECTM1 alone has little effect on IL-2 production (Fig. 4B). CD28 is a critically important costimulatory molecule for T cell proliferation and IL-2 production, but other costimulators also play critical roles in the development of optimal T cell responses. Indeed, addition of SECTM1 also synergistically enhanced anti-CD3/CD28-induced T cell proliferation (Figs. 2D and Fig. 3A). Interestingly, although SECTM1 alone did not induce IL-2 production, it synergized with anti-CD28 to augment the production of IL-2 up to fourfold in CD4 T cells and twofold in CD8 T cells after 24 h stimulation compared with anti-CD3/CD28 alone (Fig. 4B). The observed synergistic effect of SECTM1/CD7 and CD28 costimulation may have several explanations: first, SECTM1/CD7 and CD28 costimulation up-regulates CD28 and CD7 receptor expression, respectively (Fig. 3B and C). Second, costimulation of CD28 and SECTM1 induces IFN-γ production, thus up-regulating CD80/CD86 and SECTM1 expression in APCs, resulting in an additive effect for T cell proliferation. Another costimulatory ligand, such as 4-1BBL, has also been implicated to enhance CD28 signaling via augmentation of the PI3K pathway [23]. As ligation of CD7 by an agonist antibody also activates the PI3K pathway [2], it appears that SECTM1 might also activate the PI3K and enhance anti-CD28 signaling in T cells. However, unlike other costimulatory molecules, such as activated 4-1BB, which preferentially activates the CD8 T cell [24], SECTM1 costimulates CD4 and CD8 T cells (Figs. 3 and 4). Future work on molecular mechanisms of how SECTM1 synergizes with CD28 signaling pathway is underway, but our data suggest that the synergistic effect between SECTM1-CD7 and -CD28 is critical, especially for initiating T cell activation and IL-2 production.
One critical application of costimulatory receptors is that they regulate allogeneic T cell responses [25, 26], which plays an important role in allograft and GVHD. For example, inhibition of a T cell response after allogeneic bone marrow transplantation by constraining costimulatory signaling is an important strategy to control GVHD. Previous studies demonstrated that blocking CD7 reduced acute MLR in vitro and that the combination of anti-CD3 and anti-CD7 Ricin A significantly inhibits GVHD in preclinical and animal experiments. Anti-CD7 Ricin is being tested for preventing acute GVHD in phase I/II clinical trails via the elimination of allogeneic T cells, as well as inhibition of NK cell functions (ClinicalTrials.gov; identifier: NCT00640497), and our data showed that anti-SECTM1 also inhibits MLR at the dose, which itself has no effect on two-way MLR, synergized with CTLA-4/Fc to diminish allogeneic T cell proliferation. These data suggest that a combination of CTLA-4 with anti-SECTM1 might be more effective and less toxic in the treatment of allogeneic T cell response-related diseases, such as the rejection of transplant organs.
Although SECTM1 protein is not expressed in resting monocytes and imMoDCs, our data indicate that the induction of SECTM1 by IFN-γ occurs at the mRNA level (Fig. 3A). Furthermore, by analyzing the promoter region of human SECTM1 and using luciferase and ChIP assays, we confirmed that induction of SECTM1 by IFN-γ is STAT1-dependent. This expression pattern suggested that the SECTM1-CD7 interaction might be involved in APC-T cell interaction. Interestingly, as the SECTM1 gene is located only 5 Kb from CD7, and both genes are up-regulated by CD28 costimulation in CD4 T cells, this suggests that they may be coordinately regulated during T cell activation, presumably via chromatin remodeling when T cells are activated. In addition, SECTM1 is expressed in nonprofessional APCs and is greatly induced by IFN-γ, such as fibroblasts, endothelial cells (Fig. 1A, and data not shown), suggesting that SECTM1 not only plays an important role in immune cell activation but may also play a role in certain pathological conditions related to IFN-γ, such as inflammatory and autoimmune diseases, as well as nonimmune-related functions.
Our current data and hypotheses are based on in vitro human cell assays, and the manipulation of SECTM1 in vivo must be pursued with caution. Of note, SECTM1 is not the only molecule that binds to CD7. It has been proposed that galectin-1 binds to CD7 and other proteins in a saccharide-dependent manner, inducing T cell apoptosis [27]. Interestingly, galectin-1 induces apoptosis of activated T cells but not resting T cells [28, 29]. In addition, IFN-γ up-regulated galectin-1 expression (unpublished data), and galectin-1 itself can reduce IFN-γ production in an allogeneic T cell response [30], suggesting SECTM1 and galectin-1 may finely regulate T cell activation via CD7 and play a critical role in T cell homeostasis. Future work in vivo using SECTM1 knockout mice will help to address the exact roles of SECTM1 on T cell response.
In summary, our results highlight the importance of human SECTM1/CD7 in human T cell functions, which are distinct and at least additive with CD28. Targeting SECTM1/CD7 and CD28 may provide a novel therapeutic strategy for inflammatory, autoimmune diseases or other IFN-γ-related diseases.
ACKNOWLEDGMENTS
This work was supported by grants from NIH (5P30CA 010,815-42), Commonwealth Universal Research Enhancement Program of the Pennsylvania Department of Health (to E.J.W. and R.E.K.), NIH/National Institute of Allergy and Infectious Diseases (HHSN266200500030C to E.J.W.), and W.W. Smith Charitable Trust for R.E.K. and T.W. We thank Drs. Andrew J. Caton and Hui Hu for discussion and manuscript preparation; members of Drs. Meenhard Herlyn's and Dorothee Herlyn's labs for help with experiments and for discussion; Dr. Ramana Davuluri for helping with analysis of the SECTM1 promoter; Peter Alexander and In-Chul Cha for assisting experiments; The Wistar Institute Cancer Center Flow Cytometry Core Facility for helping with instrument set-up and data analysis; and Genomics and Bioinformatics Core Facility for helping with analysis of SECTM1 expression using existing microarray database.
Footnotes
- anti-SECTM1
- anti-human SECTM1 A1
- β-Gal
- β-galactosidase
- ChIP
- chromatin immunoprecipitation
- GAS
- IFN-γ-activated sequence
- GEO
- Gene Expression Omnibus
- GVHD
- graft-versus-host disease
- HEK
- human embryonic kidney
- imMoDC
- immature monocyte-derived DC
- SECTM1
- K12/SECTM1
AUTHORSHIP
T.W. designed, performed research, and drafted the manuscript. C.H., A.L-C., M.X., and K.A.S-K. performed experiments. E.J. W. helped design experiments and write the paper. R.E.K. designed the experiments and drafted the manuscript.
DISCLOSURES
The authors declare no competing financial interests.
REFERENCES
- 1. Sempowski G. D., Lee D. M., Kaufman R. E., Haynes B. F. (1999) Structure and function of the CD7 molecule. Crit. Rev. Immunol. 19, 331–348 [PubMed] [Google Scholar]
- 2. Stillwell R., Bierer B. E. (2001) T cell signal transduction and the role of CD7 in costimulation. Immunol. Res. 24, 31–52 [DOI] [PubMed] [Google Scholar]
- 3. Carrera A. C., Rincon M., Sanchez-Madrid F., Lopez-Botet M., de Landazuri M. O. (1988) Triggering of co-mitogenic signals in T cell proliferation by anti-LFA-1 (CD18, CD11a), LFA-3, and CD7 monoclonal antibodies. J. Immunol. 141, 1919–1924 [PubMed] [Google Scholar]
- 4. Jung L. K., Roy A. K., Chakkalath H. R. (1992) CD7 augments T cell proliferation via the interleukin-2 autocrine pathway. Cell. Immunol. 141, 189–199 [DOI] [PubMed] [Google Scholar]
- 5. Costantinides Y., Kingsley G., Pitzalis C., Panayi G. S. (1991) Inhibition of lymphocyte proliferation by a monoclonal antibody (RFT2) against CD7. Clin. Exp. Immunol. 85, 164–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Emara M., Carroll R. G. (1992) Signal transduction through crosslinking CD7 and IgM-Fc receptors that inhibits T-cell proliferation. Hum. Immunol. 34, 181–195 [DOI] [PubMed] [Google Scholar]
- 7. Lee D. M., Staats H. F., Sundy J. S., Patel D. D., Sempowski G. D., Scearce R. M., Jones D. M., Haynes B. F. (1998) Immunologic characterization of CD7-deficient mice. J. Immunol. 160, 5749–5756 [PubMed] [Google Scholar]
- 8. Bonilla F. A., Kokron C. M., Swinton P., Geha R. S. (1997) Targeted gene disruption of murine CD7. Int. Immunol. 9, 1875–1883 [DOI] [PubMed] [Google Scholar]
- 9. Heinly C. S., Sempowski G. D., Lee D. M., Patel D. D., McDermott P. M., Scearce R. M., Thompson C. B., Haynes B. F. (2001) Comparison of thymocyte development and cytokine production in CD7-deficient, CD28-deficient and CD7/CD28 double-deficient mice. Int. Immunol. 13, 157–166 [DOI] [PubMed] [Google Scholar]
- 10. Sempowski G. D., Cross S. J., Heinly C. S., Scearce R. M., Haynes B. F. (2004) CD7 and CD28 are required for murine CD4+CD25+ regulatory T cell homeostasis and prevention of thyroiditis. J. Immunol. 172, 787–794 [DOI] [PubMed] [Google Scholar]
- 11. Yoshikawa K., Seto M., Ueda R., Obata Y., Fukatsu H., Segawa A., Takahashi T. (1993) Isolation and characterization of mouse CD7 cDNA. Immunogenetics 37, 114–119 [DOI] [PubMed] [Google Scholar]
- 12. Slentz-Kesler K. A., Hale L. P., Kaufman R. E. (1998) Identification and characterization of K12 (SECTM1), a novel human gene that encodes a Golgi-associated protein with transmembrane and secreted isoforms. Genomics 47, 327–340 [DOI] [PubMed] [Google Scholar]
- 13. Lam G. K., Liao H. X., Xue Y., Alam S. M., Scearce R. M., Kaufman R. E., Sempowski G. D., Haynes B. F. (2005) Expression of the CD7 ligand K-12 in human thymic epithelial cells: regulation by IFN-γ. J. Clin. Immunol. 25, 41–49 [DOI] [PubMed] [Google Scholar]
- 14. Slentz-Kesler K. A. (1997) Characterization of K12, a novel human gene encoding a protein with transmembrane secreted isoforms. Ph.D. thesis, Duke University; [DOI] [PubMed] [Google Scholar]
- 15. Lyman S. D., Escobar S., Rousseau A. M., Armstrong A., Fanslow W. C. (2000) Identification of CD7 as a cognate of the human K12 (SECTM1) protein. J. Biol. Chem. 275, 3431–3437 [DOI] [PubMed] [Google Scholar]
- 16. Huyton T., Gottmann W., Bade-Doding C., Paine A., Blasczyk R. (2011) The T/NK cell co-stimulatory molecule SECTM1 is an IFN “early response gene” that is negatively regulated by LPS in human monocytic cells. Biochim. Biophys. Acta 1810, 1294–1301 [DOI] [PubMed] [Google Scholar]
- 17. Satyamoorthy K., DeJesus E., Linnenbach A. J., Kraj B., Kornreich D. L., Rendle S., Elder D. E., Herlyn M. (1997) Melanoma cell lines from different stages of progression and their biological and molecular analyses. Melanoma Res. 7 (Suppl. 2), S35–S42 [PubMed] [Google Scholar]
- 18. Barrett T., Troup D. B., Wilhite S. E., Ledoux P., Evangelista C., Kim I. F., Tomashevsky M., Marshall K. A., Phillippy K. H., Sherman P. M., Muertter R. N., Holko M., Ayanbule O., Yefanov A., Soboleva A. (2011) NCBI GEO: archive for functional genomics data sets—10 years on. Nucleic Acids Res. 39, D1005–D1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cefai D., Schneider H., Matangkasombut O., Kang H., Brody J., Rudd C. E. (1998) CD28 receptor endocytosis is targeted by mutations that disrupt phosphatidylinositol 3-kinase binding and costimulation. J. Immunol. 160, 2223–2230 [PubMed] [Google Scholar]
- 20. Yang Y. G., Wang H., Asavaroengchai W., Dey B. R. (2005) Role of interferon-γ in GVHD and GVL. Cell. Mol. Immunol. 2, 323–329 [PubMed] [Google Scholar]
- 21. Decker T., Kovarik P., Meinke A. (1997) GAS elements: a few nucleotides with a major impact on cytokine-induced gene expression. J. Interferon Cytokine Res. 17, 121–134 [DOI] [PubMed] [Google Scholar]
- 22. Vinkemeier U., Cohen S. L., Moarefi I., Chait B. T., Kuriyan J., Darnell J. E., Jr. (1996) DNA binding of in vitro activated Stat1 α, Stat1 β and truncated Stat1: interaction between NH2-terminal domains stabilizes binding of two dimers to tandem DNA sites. EMBO J. 15, 5616–5626 [PMC free article] [PubMed] [Google Scholar]
- 23. Zhong X. S., Matsushita M., Plotkin J., Riviere I., Sadelain M. (2010) Chimeric antigen receptors combining 4-1BB and CD28 signaling domains augment PI3kinase/AKT/Bcl-XL activation and CD8+ T cell-mediated tumor eradication. Mol. Ther. 18, 413–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Watts T. H. (2005) TNF/TNFR family members in costimulation of T cell responses. Annu. Rev. Immunol. 23, 23–68 [DOI] [PubMed] [Google Scholar]
- 25. Shlomchik W. D. (2007) Graft-versus-host disease. Nat. Rev. Immunol. 7, 340–352 [DOI] [PubMed] [Google Scholar]
- 26. Welniak L. A., Blazar B. R., Murphy W. J. (2007) Immunobiology of allogeneic hematopoietic stem cell transplantation. Annu. Rev. Immunol. 25, 139–170 [DOI] [PubMed] [Google Scholar]
- 27. Pace K. E., Hahn H. P., Pang M., Nguyen J. T., Baum L. G. (2000) CD7 delivers a pro-apoptotic signal during galectin-1-induced T cell death. J. Immunol. 165, 2331–2334 [DOI] [PubMed] [Google Scholar]
- 28. Rabinovich G. A., Riera C. M., Sotomayor C. E. (1999) Galectin-1, an alternative signal for T cell death, is increased in activated macrophages. Braz. J. Med. Biol. Res. 32, 557–567 [DOI] [PubMed] [Google Scholar]
- 29. Vespa G. N., Lewis L. A., Kozak K. R., Moran M., Nguyen J. T., Baum L. G., Miceli M. C. (1999) Galectin-1 specifically modulates TCR signals to enhance TCR apoptosis but inhibit IL-2 production and proliferation. J. Immunol. 162, 799–806 [PubMed] [Google Scholar]
- 30. Rabinovich G. A., Ramhorst R. E., Rubinstein N., Corigliano A., Daroqui M. C., Kier-Joffe E. B., Fainboim L. (2002) Induction of allogenic T-cell hyporesponsiveness by galectin-1-mediated apoptotic and non-apoptotic mechanisms. Cell Death Differ. 9, 661–670 [DOI] [PubMed] [Google Scholar]