Abstract
Purpose of review
Exercise-induced bronchoconstriction (EIB) refers to acute airflow obstruction that is triggered by a period of physical exertion. Here we review recent findings about the epidemiology of EIB, immunopathology leading to EIB, and the latest understanding of the pathogenesis of EIB.
Recent findings
Longitudinal studies demonstrated that airway hyperresponsiveness to exercise or cold air at an early age are among the strongest predictors of persistent asthma. Patients that are susceptible to EIB have epithelial disruption and increased levels of inflammatory eicosanoids such as cysteinyl leukotrienes (CysLT)s. The leukocytes implicated in production of eicosanoids in the airways include both a unique mast cell population as well as eosinophils. A secreted phospholipase A2 (sPLA2) enzyme that serves as a regulator of CysLT formation is present in increased quantities in asthma. Transglutaminase 2 (TGM2) is expressed at increased levels in asthma and serves as a regulator of sPLA2-X. Further, sPLA2-X acts on target cells such as eosinophils to initiate cellular eicosanoid synthesis.
Summary
Recent studies have advanced our understanding of EIB as a syndrome that is caused by the increased production of inflammatory eicosanoids. The airway epithelium may be an important regulator of the production of inflammatory eicosanoids by leukocytes. Abstract word count: 199
Keywords: Asthma, Eicosanoid, Eosinophil, Exercise-induced bronchoconstriction, Leukotriene, Mast Cell, Phospholipase, Prostaglandin, and Transglutaminase 2
Introduction
Exercise-induced bronchoconstriction (EIB) is a syndrome where a brief period of exercise or increase in ventilation triggers airflow obstruction that lasts 30 to 90 minutes in the absence of treatment. Although EIB occurs predominantly among patients with established asthma, there is evidence from cross sectional studies that only a portion of patients with asthma have EIB when tested with a specific challenge test. For example, in Algeria the prevalence of EIB was 47% among children with established asthma (1). These data are consistent with the largest prior study that established a prevalence of EIB of 46% out of 164 asthmatic children (2). Also consistent with prior studies (3), the Algerian study also found that 13.9% of children without a history of asthma had EIB (1). In accord with smaller studies showing that EIB identifies children at risk for chronic asthma, two recent large cohort studies have extended these findings. Parent reported exercise induced wheeze and a history of atopy were the strongest predictors of asthma over at least 6 years of longitudinal follow-up among 628 children who were evaluated prior to the age of 5 (4). In a longitudinal birth cohort that included follow-up data on 849 children, airway hyperresponsiveness (AHR) to cold dry air hyperpnea was associated with a increased odds ratio of 4.5 of asthma at 22 years of age (5).
EIB is a prototypical manifestation of indirect AHR similar to the airway response to hypertonic aerosols, eucapnic voluntary hyperpnea (EVH) and adenosine, but is only weakly related to baseline lung function or direct airway responsiveness to histamine or methacholine (6). However, several recent population-based studies in patients with symptoms of asthma have found that tests of indirect and direct AHR hyperresponsiveness perform similarly as screening tests (7, 8). Among 509 adolescents and adults with signs and symptoms of asthma, the sensitivity of mannitol to identify EIB was 59% and for methacholine was 56% (7); the prevalence of EIB in the study population was 43.5%. In a population of 99 children with suspected asthma, 21% of whom had EIB, the positive and negative predicted value of mannitol challenge for EIB were 68% and 89% (8).
The abnormal distribution of alveolar ventilation (Va) and perfusion (Q) that occurs during EIB can lead to arterial hypoxemia during exercise. Images of the airways during EIB obtained by hyperpolarized helium demonstrate areas of closure or near closure of segmental airways of the lungs during EIB (9). Of interest is a recent comparison of exercise- and mannitol-induced bronchoconstriction demonstrating that Va/Q imbalance was more pronounced in EIB than mannitol-induced bronchoconstriction, but there was less hypoxemia because of the residual increase in ventilation after exercise (10). The danger of bronchoconstriction triggered by exercise was highlighted several years ago by a population-based study that found 61 of 263 sports-related fatalities in young adults were caused by asthma exacerbation (11).
Pathophysiological determinants of EIB
Collectively, the epidemiology of EIB indicates that patients with this disorder represent a discrete phenotype. Whether or not this phenotype is a durable clinical phenotype awaits further longitudinal epidemiological studies. One recent study found that increased bronchodilator response is associated with asthma symptoms during exercise suggesting that subjects with more variability in airway tone may be more susceptible to EIB (12). In another very provocative study, the authors related pilocarpine-induced sweat secretion with methacholine reactivity and found that subjects with a negative methacholine challenge had more salivary secretion and higher sweat rate (13). Since prior studies have found that the rate of water transfer out of the airways is a major determinant of the severity of bronchoconstriction after exercise challenge, these findings suggest that the there may be an alteration in water handling by the airway epithelial surface. It is also recognized both humans and in animal models that dietary salt is a modifier of the severity of EIB (14), also possibly indicating a alteration in water handling by the epithelium. In line with evidence that the epithelium may play a major role in this disorder is that the number of airway epithelial cells shed into induced sputum is substantially higher among asthmatics with EIB compared to asthmatics without EIB (15).
The intensity of cellular airway inflammation and the generation of inflammatory mediators, particularly eicosanoids (i.e products of arachidonic acid) such as leukotrienes have been associated with the susceptibility to EIB (15, 16). Although sputum eosinophilia per se does not appear to be required for EIB, several prior studies have associated the degree of sputum eosinophilia with the severity of EIB. A recent study of the inhaled corticosteroid (ICS) ciclesonide further refined these observations by demonstrating that the magnitude and onset of the suppression of EIB in response to high dose but not low dose ICS therapy was associated with the degree of sputum eosinophilia (17). Patients without sputum eosinophilia were less likely to have an improvement in EIB on an ICS (17). Mast cell infiltration of the airways has also been implicated in EIB. In a genome-wide expression study of airway cells, the expression of the mast cell genes tryptase and carboxypeptidase A3 (CPA3) were significantly increased in EIB positive asthmatics (18). This intraepithelial mast cell phenotype with high expression of tryptase and CPA3, but low expression of chymase was recently described in the Th2 high molecular phenotype of asthma (19, 20). Since this Th2 high phenotype is IL-13 driven (21), it is interesting to note that a genetic study found an association between IL-13 gene polymorphisms and the severity of EIB, and with the response to a leukotriene receptor antagonist (LTRA) among these subjects with EIB (22).
Several studies have noted an increase in the concentration of cysteinyl leukotrienes (CysLTs) in the airways of patients with EIB (15, 16), particularly the ratio of CysLTs to prostaglandin E2 (PGE2) (15). A recent study also found that patients with EIB relative to asthmatics without EIB have a reduction in the levels of the protective eicosanoid lipoxin A4 (23). The levels of 8-isoprostanes, non-enzymatic products of phospholipid oxidation, were increased in exhaled breath condensate (EBC) of asthmatics with EIB and correlated with the severity of EIB (24). As in prior studies (25) a recent study found that the fraction of exhaled nitric oxide (FENO) is elevated in asthmatics with EIB, and furthered the understanding of this relationship by showing that the relationship between FENO and EIB was restricted to subjects with atopy (26). Angiopoetin 2, a mediator that enhances microvascular permeability, is increased the airways in asthma and is correlated with the severity of EIB (27). Among children with asthma, including obese children, the severity of EIB was positively correlated with serum leptin and negatively with serum adiponectin (28). In addition, the levels of 25-hydroxy-vitamin D were reduced among subjects with asthma and were lower in asthmatics with EIB relative to those without EIB (29).
Inflammatory mediator release in the airways during EIB
Although the precise nature of the underlying stimulus for mediator release in the airways is not known with certainty, there is strong evidence that mediators from mast cells and eosinophils are released into the airways during EIB, and that this mediator release is the predominant cause of EIB (14, 30). During exercise, heat and water are transferred out of the airways to equilibrate the inspired air to the temperature and humidity of the lower airways. At the epithelial surface there is a transfer of water from the osmotically sensitive epithelial cells via the tight junctions, as well as a thermal gradient. Though osmotic stimuli are also known to directly activate inflammatory cells such as mast cells (31), it is reasonable to also consider that the stimulus of exercise or hyperpnea is predominant sensed by the airway epithelium leading to the activation of inflammatory mediator release from leukocytes residing in close contact to the epithelium. Two recent studies indicate that epithelial stress occurs during exercise in vivo by demonstrating that clara cell secretory protein (CC16) measured in the urine following challenge is increased after exercise and isocapnic hyperpnea challenge (32, 33).
The non-invasive method of exhaled breath condensate (EBC) has been used recently to better understand the nature of mediator release in the airways following exercise challenge. The levels of CysLTs in EBC were higher in the asthma group with EIB and increased after exercise challenge most notably in the EIB positive group; further, the change in CysLTs in EBC following challenge was correlated with the severity of EIB (34). These findings are consistent with the findings identified in induced sputum demonstrating a sustained increase in CysLTs and other bronchoconstrictive eicosanoids such as PGD2 in the airways following exercise challenge to induce EIB (14, 30). Mast cells and eosinophils are strongly implicated as the cellular sources of CysLTs and other eicosanoids in EIB. The eosinophil product eosinophilic cationic protein (ECP) is released into the airways following exercise challenge in patients with EIB (14). Mast cell degranulation occurs during exercise challenge as evidenced by histamine and tryptase release into the airways following challenge (30).
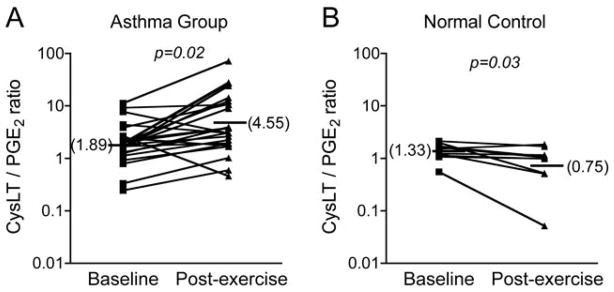
The connection between inflammatory eicosanoid release by leukocytes and epithelial stress is not initially obvious, since the epithelium itself is thought to have relatively limited capacity to synthesize CysLTs. The epithelium may directly lead to eicosanoid release through 15-lipoxygenase-1 (15-LO-1) since the levels of the 15-LO product 15S-hydroxyeicosatetranoic acid (15S-HETE) are increased after exercise challenge in subjects with EIB (35). The epithelium is also a major source of PGE2 that is known to inhibit EIB when given by inhalation. The production of PGE2 actually decreases post exercise challenge among asthmatics with EIB (30), and the ratio of CysLTs to PGE2 increases in asthmatics post challenge, while there is a decrease in this ratio in normal subjects (35) (Figure 1). A unifying explanation for these findings is that the epithelium serves as a key regulator of the balance of eicosanoids in the airways by activating the release of bronchoconstrictive eicosanoids in inflammatory cells in close contact and by alterations that reduce the synthesis of PGE2. The epithelium has reduced in vitro capacity for PGE2 synthesis when treated with IL-13 through a reduction in the synthetic enzymes cyclooxygenase-2 (COX-2) and PGE synthase 1 (36).
Figure 1.
Opposing effects of exercise challenge on the CysLT to PGE2 ratio in induced sputum. Asthmatics with EIB have an increase in the CysLT to PGE2 ratio in response to exercise (A), while normal controls have a decrease in the CysLT to PGE2 ratio following exercise challenge (B). Adapted from reference 30.
Recent provocative data from a single research group contradicts older studies that have generally failed to demonstrate a cellular influx into the airways following exercise challenge (37, 38), or an increase in airway hyperresponsiveness (39, 40). An increase in high sensitivity C-reactive protein (CRP) was identified only in asthmatics with EIB following exercise challenge (41). In addition, the FENO, serum ECP and AHR to inhaled histamine were all increased following exercise challenge in asthmatics with EIB (41). Further they found that RANTES and eotaxin were increased in EBC in asthmatics relative to controls, and that the levels of RANTES and eotaxin were increased in EBC after exercise challenge only in the group with EIB, but not in asthmatics without EIB (42, 43). These are provocative findings that require further confirmation, but suggest that exercise challenge may trigger chemokines involved in leukocyte recruitment and AHR in subjects that are susceptible to this disorder.
Regulation of eicosanoid synthesis by secreted phospholipase A2 (sPLA2)
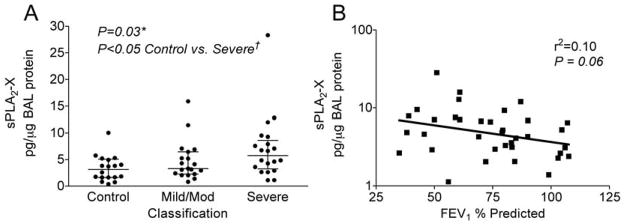
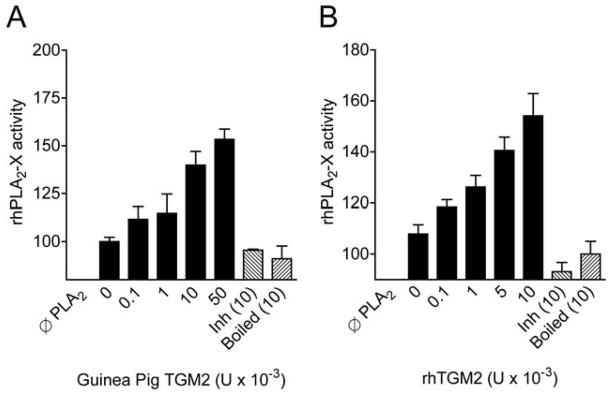
The first rate-limiting step in the formation of the CysLTs and other eicosanoids is the release of arachidonic acid from membrane phospholipids that is regulated by the PLA2 enzymes. In addition to the cytosolic PLA2α (cPLA2α), several secreted PLA2s (sPLA2)s have been implicated in eicosanoid synthesis, and may preferentially direct eicosanoid production toward LT synthesis. In humans, increased sPLA2 activity has been identified in nasal lavage fluid and in bronchoalveolar lavage (BAL) fluid following allergen challenge, but the identities of the sPLA2s were not characterized. We characterized sPLA2 gene expression in induced sputum cells of asthmatics with EIB, and found significant expression of sPLA2 group II, X and IIA enzymes (35). In a subsequent study examining the identities of sPLA2s in BAL fluid of asthmatic and non-asthmatic subjects, the sPLA2 groups IIA and X predominated, but only sPLA2-X was elevated in association with lung function and eicosanoid formation in the airways (44) (Figure 2). It is notable that sPLA2-X is expressed predominantly in the airway epithelium (44) as we recently found that transglutaminase 2 (TGM2) is increase in the airways of patients with EIB and serves as a regulator of sPLA2-X activity (18) (Figure 3). Since sPLA2-X is secreted and can act on other target cells such as eosinophils to initiate eicosanoid formation, we examined the effects of exogenous sPLA2-X on human eosinophils and found that sPLA2-X rapidly initiated CysLT formation in eosinophils in a manner that was dependent upon the enzymatic activity of the enzyme, but occurred via activation of p38 and c-Jun MAPK (JNK) and cPLA2α (45). In vivo, genetic deficiency of either sPLA2-V or sPLA2-X in a murine model of asthma attenuates the development of allergen-induced inflammation, mucus release, and AHR (46, 47), as does inhibition of human sPLA2-X that was inserted under the endogenous promoter in a mouse model (48). These findings suggest that sPLA2-X may serve as a key regulator of eicosanoid formation in the airways and that this enzyme is strongly implicated in features of AHR such as EIB.
Figure 2.
Levels of sPLA2-X protein in BAL fluid in relation to asthma severity and lung function. The levels of sPLA2-X in BAL fluid normalized to total protein were higher in asthmatics relative to controls, with the largest difference in the severe asthma group relative to controls (A). The figure shows the medians and interquartile ranges. The levels of sPLA2-X were associated with lung function among asthmatics by regression analysis (B). Adapted from reference 44.
Figure 3.
Increase in sPLA2-X enzyme activity mediated by TGM2. Pre-incubation of recombinant human sPLA2-X with purified TGM2 from guinea pig liver (A) or with recombinant human TGM2 (B) causes an increase in the PLA2 activity of the sPLA2-X enzyme. Denaturing the TGM2 with heat (boiled) or inhibiting the activity of the enzyme by saturating the enzyme with N-carbobenzoxy-Gln-Gly (Inh) demonstrate that the in vitro findings are due to the enzymatic activity of TGM2. Adapted from reference 18.
Sensory nerve involvement in EIB
An important study using isolated capsaicin-sensitive neurons demonstrated that these neurons respond directly to the CysLT LTD4 via the CysLT1 receptor, and increased the excitability of these neurons to other electrical and chemical stimuli (49). This study is important because two studies conducted in animal models of EIB indicate that the mechanism of bronchoconstriction is mediated through the sensory nerve activation with retrograde axonal transmission via the release of neurokinins (50, 51). These data are also consistent with our findings in humans that goblet cell mucin 5AC (MUC5AC) is released into the airways during EIB in association with the levels of CysLTs and neurokinin A (NKA) suggesting that CysLTs mediate the activation of sensory nerves and mucus release during EIB in humans (52).
Conclusions
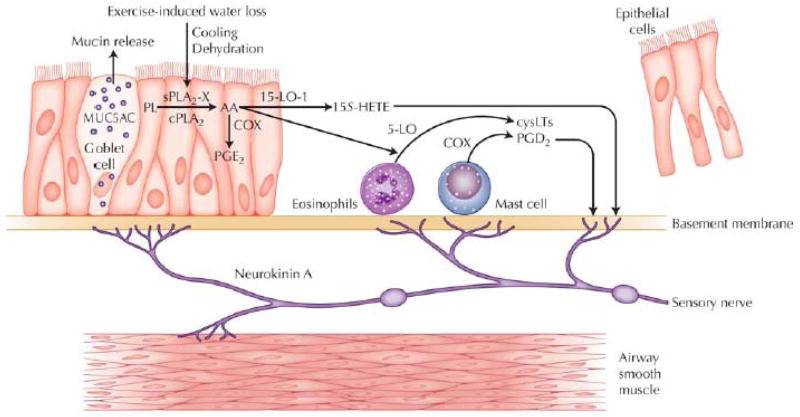
Recent studies have advanced our understanding of EIB as a distinct syndrome in asthma that is related to indirect AHR, and is notable for increased production of CysLTs and shedding of epithelial cells into the airway lumen (Figure 4). Exercise challenge serves as a stimulus to the airway epithelium and adjacent leukocytes resulting in sustained CysLT and PGD2 release in association with smooth muscle contraction and the release of MUC5AC that may be the consequence of sensory nerve activation. Several lines of evidence indicate that mast cells and eosinophils serve as the principal sources of inflammatory eicosanoids in this disorder. Recent work has identified the strong expression of sPLA2-X in the airway epithelium and elevated levels of sPLA2-X protein in BAL fluid of patients with asthma. A genome-wide expression study identified TGM2 with increased expression in asthma and that found TGM2 serves as a regulator of sPLA2-X. The sPLA2-X enzyme acts on target cells such as eosinophils to initiate cellular eicosanoid synthesis. These studies suggest that the airway epithelium serves as an important regulator of the production of inflammatory eicosanoids by leukocytes.
Figure 4.
Disease model of exercise-induced bronchoconstriction (EIB) pathogenesis. Asthmatics with EIB have increased concentrations of shed epithelial cells, CysLTs, and CysLT/PGE2 ratio in induced sputum. Exercise challenge initiates the production of CysLTs, PGD2, and 15S-HETE, and a reduction in PGE2. The release of sPLA2-X by the airway epithelium may initiate CysLT production in adjacent leukocytes. Contraction of the airway smooth muscle and mucin release occurs in part through retrograde axonal transmission in sensory nerves that release neurokinin A. 15-LO-1 - 15-lipoxygenase-1; 5-LO - 5-lipoxygenase; COX - cyclooxygenase; cPLA2 - cytosolic phospholipase A2; MUC5AC - mucin 5AC; PL -phospholipids. Adapted from reference 53.
Key Points.
Exercise-induced bronchoconstriction (EIB) is an asthma phenotype with epithelial shedding and increased production of inflammatory mediators such as leukotrienes.
EIB in childhood is a risk factor for persistent asthma in adulthood.
Following exercise challenge, mediators are released into the airways from mast cells and eosinophils.
A regulator of leukotriene formation call secreted phospholipase A2 group X (sPLA2-X) has been identified in the airways of patients with asthma.
Transglutaminase 2 is overexpressed in the epithelium of patients with asthma and serves as a regulator of sPLA2-X activity.
Acknowledgments
Supported by National Institutes of Health grant HL089215.
Acknowledgements/Disclosures
Dr Hallstrand has received research funding from the NIH (HL089215) and is on the speaker’s bureau for Merck and Co., and Genentech.
Abbreviations
- 15-LO-1
15-Lipoxygenase-1
- 15S-HETE
15S-Hydroxyeicosatetranoic Acid
- AHR
Airway Hyperresponsiveness
- BAL
Bronchoalveolar Lavage
- COX-2
Cyclooxygenase-2
- CPA3
Carboxypeptidase A3
- CRP
C-reactive protein
- cPLA2α
Cytosolic Phospholipase A2α
- CysLT
Cysteinyl Leukotrienes
- EBC
Exhaled breath condensate
- ECP
Eosinophilic Cationic Protein
- EIB
Exercise-induced Bronchoconstriction
- FENO
Fraction of Exhaled Nitric Oxide
- JNK
c-Jun MAPK
- ICS
Inhaled Corticosteroid
- IL-13
Interleukin-13
- LT
Leukotriene
- LTRA
Leukotriene receptor antagonist
- MUC5AC
Mucin 5AC
- PG
Prostaglandin
- Q
Perfusion
- sPLA2
Secreted Phospholipase A2
- TGM2
Transglutaminase 2
- Va
Alveolar ventilation
References and Recommended Reading
Papers of particular interest published recently, have been highlighted as:
* Of importance
** Of major importance
- 1.Benarab-Boucherit Y, Mehdioui H, Nedjar F, Delpierre S, Bouchair N, Aberkane A. Prevalence rate of exercise-induced bronchoconstriction in Annaba (Algeria) schoolchildren. J Asthma. 2011;48:511–516. doi: 10.3109/02770903.2011.578315. [DOI] [PubMed] [Google Scholar]
- 2.Cabral AL, Conceicao GM, Fonseca-Guedes CH, Martins MA. Exercise-induced bronchospasm in children: effects of asthma severity. Am J Respir Crit Care Med. 1999;159:1819–1823. doi: 10.1164/ajrccm.159.6.9805093. [DOI] [PubMed] [Google Scholar]
- 3.Hallstrand TS, Curtis JR, Koepsell TD, Martin DP, Schoene RB, Sullivan SD, Yorioka GN, Aitken ML. Effectiveness of screening examinations to detect unrecognized exercise-induced bronchoconstriction. J Pediatr. 2002;141:343–348. doi: 10.1067/mpd.2002.125729. [DOI] [PubMed] [Google Scholar]
- 4**.Frank PI, Morris JA, Hazell ML, Linehan MF, Frank TL. Long term prognosis in preschool children with wheeze: longitudinal postal questionnaire study 1993–2004. BMJ. 2008;336:1423–1426. doi: 10.1136/bmj.39568.623750.BE. This study with longitudinal follow-up data provides evidence that symptoms of wheeze during exercise are associated with persistent asthma in children. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5**.Stern DA, Morgan WJ, Halonen M, Wright AL, Martinez FD. Wheezing and bronchial hyper-responsiveness in early childhood as predictors of newly diagnosed asthma in early adulthood: a longitudinal birth-cohort study. Lancet. 2008;372:1058–1064. doi: 10.1016/S0140-6736(08)61447-6. Airway hyperresponsiveness to cold air at a young age was associated with asthma at age 22 years in a longitudinal study indicating that EIB is an influential phenotype in the asthma syndrome. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson SD. Indirect challenge tests: Airway hyperresponsiveness in asthma: its measurement and clinical significance. Chest. 2010;138:25S–30S. doi: 10.1378/chest.10-0116. [DOI] [PubMed] [Google Scholar]
- 7.Anderson SD, Charlton B, Weiler JM, Nichols S, Spector SL, Pearlman DS. Comparison of mannitol and methacholine to predict exercise-induced bronchoconstriction and a clinical diagnosis of asthma. Respir Res. 2009;10:4. doi: 10.1186/1465-9921-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barben J, Kuehni CE, Strippoli MP, Schiller B, Hammer J, Trachsel D. Mannitol dry powder challenge in comparison with exercise testing in children. Pediatr Pulmonol. 2011 doi: 10.1002/ppul.21453. [DOI] [PubMed] [Google Scholar]
- 9.Samee S, Altes T, Powers P, de Lange EE, Knight-Scott J, Rakes G, Mugler JP, 3rd, Ciambotti JM, Alford BA, Brookeman JR, et al. Imaging the lungs in asthmatic patients by using hyperpolarized helium-3 magnetic resonance: assessment of response to methacholine and exercise challenge. J Allergy Clin Immunol. 2003;111:1205–1211. doi: 10.1067/mai.2003.1544. [DOI] [PubMed] [Google Scholar]
- 10*.Munoz PA, Gomez FP, Manrique HA, Roca J, Barbera JA, Young IH, Anderson SD, Rodriguez-Roisin R. Pulmonary gas exchange response to exercise- and mannitol-induced bronchoconstriction in mild asthma. J Appl Physiol. 2008;105:1477–1485. doi: 10.1152/japplphysiol.00108.2008. This study highlights the potential of bronchoconstriction during exercise to cause hypoxemia, although the persistent increase in ventilation following exercise actually protected against hypoxemia. [DOI] [PubMed] [Google Scholar]
- 11.Becker JM, Rogers J, Rossini G, Mirchandani H, D’Alonzo GE., Jr Asthma deaths during sports: report of a 7-year experience. J Allergy Clin Immunol. 2004;113:264–267. doi: 10.1016/j.jaci.2003.10.052. [DOI] [PubMed] [Google Scholar]
- 12.Galant SP, Morphew T, Newcomb RL, Hioe K, Guijon O, Liao O. The relationship of the bronchodilator response phenotype to poor asthma control in children with normal spirometry. J Pediatr. 2011;158:953–959. e951. doi: 10.1016/j.jpeds.2010.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park C, Stafford C, Lockette W. Exercise-induced asthma may be associated with diminished sweat secretion rates in humans. Chest. 2008;134:552–558. doi: 10.1378/chest.08-0366. [DOI] [PubMed] [Google Scholar]
- 14.Mickleborough TD, Lindley MR, Ray S. Dietary salt, airway inflammation, and diffusion capacity in exercise-induced asthma. Med Sci Sports Exerc. 2005;37:904–914. [PubMed] [Google Scholar]
- 15.Hallstrand TS, Moody MW, Aitken ML, Henderson WR., Jr Airway immunopathology of asthma with exercise-induced bronchoconstriction. J Allergy Clin Immunol. 2005;116:586–593. doi: 10.1016/j.jaci.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carraro S, Corradi M, Zanconato S, Alinovi R, Pasquale MF, Zacchello F, Baraldi E. Exhaled breath condensate cysteinyl leukotrienes are increased in children with exercise-induced bronchoconstriction. J Allergy Clin Immunol. 2005;115:764–770. doi: 10.1016/j.jaci.2004.10.043. [DOI] [PubMed] [Google Scholar]
- 17*.Duong M, Subbarao P, Adelroth E, Obminski G, Strinich T, Inman M, Pedersen S, O’Byrne PM. Sputum eosinophils and the response of exercise-induced bronchoconstriction to corticosteroid in asthma. Chest. 2008;133:404–411. doi: 10.1378/chest.07-2048. This study further refined the relationship between airway eosinophilia and EIB by demonstrating that patients with EIB who had eosinophilia were more likely to have improvement in EIB during therapy with an inhaled steroid. [DOI] [PubMed] [Google Scholar]
- 18**.Hallstrand TS, Wurfel MM, Lai Y, Ni Z, Gelb MH, Altemeier WA, Beyer RP, Aitken ML, Henderson WR. Transglutaminase 2, a novel regulator of eicosanoid production in asthma revealed by genome-wide expression profiling of distinct asthma phenotypes. PLoS One. 2010;5:e8583. doi: 10.1371/journal.pone.0008583. Using a genome-wide expression design, this study identified the increased expression of mast cell genes and TGM2 in patients with EIB, and further demonstrated that TGM2 serves as a regulator of sPLA2-X identifying important regulators of eicosanoid formation in asthma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19*.Dougherty RH, Sidhu SS, Raman K, Solon M, Solberg OD, Caughey GH, Woodruff PG, Fahy JV. Accumulation of intraepithelial mast cells with a unique protease phenotype in T(H)2-high asthma. J Allergy Clin Immunol. 2010;125:1046–1053. e1048. doi: 10.1016/j.jaci.2010.03.003. Although this study did not specifically examine patients with EIB, it demonstrates the increased expression of tryptase and CPA3 positive mast cells in the airway epithelium using quantitative morphometry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woodruff PG, Boushey HA, Dolganov GM, Barker CS, Yang YH, Donnelly S, Ellwanger A, Sidhu SS, Dao-Pick TP, Pantoja C, et al. Genome-wide profiling identifies epithelial cell genes associated with asthma and with treatment response to corticosteroids. Proc Natl Acad Sci U S A. 2007;104:15858–15863. doi: 10.1073/pnas.0707413104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woodruff PG, Modrek B, Choy DF, Jia G, Abbas AR, Ellwanger A, Koth LL, Arron JR, Fahy JV. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. 2009;180:388–395. doi: 10.1164/rccm.200903-0392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang MJ, Lee SY, Kim HB, Yu J, Kim BJ, Choi WA, Jang SO, Hong SJ. Association of IL-13 polymorphisms with leukotriene receptor antagonist drug responsiveness in Korean children with exercise-induced bronchoconstriction. Pharmacogenet Genomics. 2008;18:551–558. doi: 10.1097/FPC.0b013e3282fe94c5. [DOI] [PubMed] [Google Scholar]
- 23.Tahan F, Saraymen R, Gumus H. The role of lipoxin A4 in exercise-induced bronchoconstriction in asthma. J Asthma. 2008;45:161–164. doi: 10.1080/02770900701847068. [DOI] [PubMed] [Google Scholar]
- 24.Barreto M, Villa MP, Olita C, Martella S, Ciabattoni G, Montuschi P. 8-Isoprostane in exhaled breath condensate and exercise-induced bronchoconstriction in asthmatic children and adolescents. Chest. 2009;135:66–73. doi: 10.1378/chest.08-0722. [DOI] [PubMed] [Google Scholar]
- 25.Scollo M, Zanconato S, Ongaro R, Zaramella C, Zacchello F, Baraldi E. Exhaled nitric oxide and exercise-induced bronchoconstriction in asthmatic children. Am J Respir Crit Care Med. 2000;161:1047–1050. doi: 10.1164/ajrccm.161.3.9905043. [DOI] [PubMed] [Google Scholar]
- 26.Malmberg LP, Pelkonen AS, Mattila PS, Hammaren-Malmi S, Makela MJ. Exhaled nitric oxide and exercise-induced bronchoconstriction in young wheezy children - interactions with atopy. Pediatr Allergy Immunol. 2009;20:673–678. doi: 10.1111/j.1399-3038.2009.00858.x. [DOI] [PubMed] [Google Scholar]
- 27.Kanazawa H, Tochino Y, Asai K. Angiopoietin-2 as a contributing factor of exercise-induced bronchoconstriction in asthmatic patients receiving inhaled corticosteroid therapy. J Allergy Clin Immunol. 2008;121:390–395. doi: 10.1016/j.jaci.2007.09.026. [DOI] [PubMed] [Google Scholar]
- 28.Baek HS, Kim YD, Shin JH, Kim JH, Oh JW, Lee HB. Serum leptin and adiponectin levels correlate with exercise-induced bronchoconstriction in children with asthma. Ann Allergy Asthma Immunol. 2011;107:14–21. doi: 10.1016/j.anai.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 29.Chinellato I, Piazza M, Sandri M, Peroni DG, Cardinale F, Piacentini GL, Boner AL. Serum vitamin D levels and exercise-induced bronchoconstriction in children with asthma. Eur Respir J. 2011;37:1366–1370. doi: 10.1183/09031936.00044710. [DOI] [PubMed] [Google Scholar]
- 30.Hallstrand TS, Moody MW, Wurfel MM, Schwartz LB, Henderson WR, Jr, Aitken ML. Inflammatory basis of exercise-induced bronchoconstriction. Am J Respir Crit Care Med. 2005;172:679–686. doi: 10.1164/rccm.200412-1667OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gulliksson M, Palmberg L, Nilsson G, Ahlstedt S, Kumlin M. Release of prostaglandin D2 and leukotriene C4 in response to hyperosmolar stimulation of mast cells. Allergy. 2006;61:1473–1479. doi: 10.1111/j.1398-9995.2006.01213.x. [DOI] [PubMed] [Google Scholar]
- 32.Romberg K, Bjermer L, Tufvesson E. Exercise but not mannitol provocation increases urinary Clara cell protein (CC16) in elite swimmers. Respir Med. 2011;105:31–36. doi: 10.1016/j.rmed.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 33.Bolger C, Tufvesson E, Sue-Chu M, Devereux G, Ayres JG, Bjermer L, Kippelen P. Hyperpnea-Induced Bronchoconstriction and Urinary CC16 Levels in Athletes. Med Sci Sports Exerc. 2011;43:1207–1213. doi: 10.1249/MSS.0b013e31820750d8. [DOI] [PubMed] [Google Scholar]
- 34.Bikov A, Gajdocsi R, Huszar E, Szili B, Lazar Z, Antus B, Losonczy G, Horvath I. Exercise increases exhaled breath condensate cysteinyl leukotriene concentration in asthmatic patients. J Asthma. 2010;47:1057–1062. doi: 10.1080/02770903.2010.512690. [DOI] [PubMed] [Google Scholar]
- 35.Hallstrand TS, Chi EY, Singer AG, Gelb MH, Henderson WR., Jr Secreted phospholipase A2 group X overexpression in asthma and bronchial hyperresponsiveness. Am J Respir Crit Care Med. 2007;176:1072–1078. doi: 10.1164/rccm.200707-1088OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trudeau J, Hu H, Chibana K, Chu HW, Westcott JY, Wenzel SE. Selective downregulation of prostaglandin E2-related pathways by the Th2 cytokine IL-13. J Allergy Clin Immunol. 2006;117:1446–1454. doi: 10.1016/j.jaci.2006.01.049. [DOI] [PubMed] [Google Scholar]
- 37.Gauvreau GM, Ronnen GM, Watson RM, O’Byrne PM. Exercise-induced bronchoconstriction does not cause eosinophilic airway inflammation or airway hyperresponsiveness in subjects with asthma. Am J Respir Crit Care Med. 2000;162:1302–1307. doi: 10.1164/ajrccm.162.4.2001054. [DOI] [PubMed] [Google Scholar]
- 38.Jarjour NN, Calhoun WJ. Exercise-induced asthma is not associated with mast cell activation or airway inflammation. J Allergy Clin Immunol. 1992;89:60–68. doi: 10.1016/s0091-6749(05)80041-7. [DOI] [PubMed] [Google Scholar]
- 39.Foresi A, Mattoli S, Corbo GM, Verga A, Sommaruga A, Ciappi G. Late bronchial response and increase in methacholine hyperresponsiveness after exercise and distilled water challenge in atopic subjects with asthma with dual asthmatic response to allergen inhalation. J Allergy Clin Immunol. 1986;78:1130–1139. doi: 10.1016/0091-6749(86)90262-9. [DOI] [PubMed] [Google Scholar]
- 40.Zawadski DK, Lenner KA, McFadden ER., Jr Effect of exercise on nonspecific airway reactivity in asthmatics. J Appl Physiol. 1988;64:812–816. doi: 10.1152/jappl.1988.64.2.812. [DOI] [PubMed] [Google Scholar]
- 41.Zietkowski Z, Skiepko R, Tomasiak-Lozowska MM, Mroczko B, Szmitkowski M, Bodzenta-Lukaszyk A. Changes in high-sensitivity C-reactive protein in serum and exhaled breath condensate after intensive exercise in patients with allergic asthma. Int Arch Allergy Immunol. 2010;153:75–85. doi: 10.1159/000301582. [DOI] [PubMed] [Google Scholar]
- 42.Zietkowski Z, Skiepko R, Tomasiak-Lozowska MM, Mroczko B, Szmitkowski M, Bodzenta-Lukaszyk A. RANTES in exhaled breath condensate of allergic asthma patients with exercise-induced bronchoconstriction. Respiration. 2010;80:463–471. doi: 10.1159/000264923. [DOI] [PubMed] [Google Scholar]
- 43.Zietkowski Z, Skiepko R, Tomasiak-Lozowska MM, Zietkowska E, Bodzenta-Lukaszyk A. Eotaxin in Exhaled Breath Condensate of Allergic Asthma Patients with Exercise-Induced Bronchoconstriction. Respiration. 2011 doi: 10.1159/000323180. [DOI] [PubMed] [Google Scholar]
- 44**.Hallstrand TS, Lai Y, Ni Z, Oslund RC, Henderson WR, Jr, Gelb MH, Wenzel SE. Relationship between levels of secreted phospholipase A groups IIA and X in the airways and asthma severity. Clin Exp Allergy. 2011;41:801–810. doi: 10.1111/j.1365-2222.2010.03676.x. This study identified sPLA2-IIA and sPLA2-X as the predominant sPLA2s in BAL fluid and further assocaited sPLA2-X with clinical and cellular parameters in patients with asthma. The study also identified the airway epithelium as the major source of sPLA2-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45*.Lai Y, Oslund RC, Bollinger JG, Henderson WR, Jr, Santana LF, Altemeier WA, Gelb MH, Hallstrand TS. Eosinophil cysteinyl leukotriene synthesis mediated by exogenous secreted phospholipase A2 group X. J Biol Chem. 2010;285:41491–41500. doi: 10.1074/jbc.M110.153338. By demonstrating the activation of eosinophils by sPLA2-X, this study demonstrates a mechanism by which an epithelial derived enzyme can activate adjacent leukocytes to generate CysLTs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Henderson WR, Jr, Chi EY, Bollinger JG, Tien YT, Ye X, Castelli L, Rubtsov YP, Singer AG, Chiang GK, Nevalainen T, et al. Importance of group X-secreted phospholipase A2 in allergen-induced airway inflammation and remodeling in a mouse asthma model. J Exp Med. 2007;204:865–877. doi: 10.1084/jem.20070029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Munoz NM, Meliton AY, Arm JP, Bonventre JV, Cho W, Leff AR. Deletion of secretory group V phospholipase A2 attenuates cell migration and airway hyperresponsiveness in immunosensitized mice. J Immunol. 2007;179:4800–4807. doi: 10.4049/jimmunol.179.7.4800. [DOI] [PubMed] [Google Scholar]
- 48.Hendersen WR, Jr, Oslund RC, Bollinger JG, Ye X, Tien YT, Xue J, Gelb MH. Blockade of human group X secreted phospholipase A2-induced airway inflammation and hyperresponsiveness in a mouse asthma model by a selective group X secreted phospholipase A2 inhibitor. J Biol Chem. 2011 doi: 10.1074/jbc.M111.235812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taylor-Clark TE, Nassenstein C, Undem BJ. Leukotriene D4 increases the excitability of capsaicin-sensitive nasal sensory nerves to electrical and chemical stimuli. Br J Pharmacol. 2008;154:1359–1368. doi: 10.1038/bjp.2008.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lai YL, Lee SP. Mediators in hyperpnea-induced bronchoconstriction of guinea pigs. Naunyn Schmiedebergs Arch Pharmacol. 1999;360:597–602. doi: 10.1007/s002109900090. [DOI] [PubMed] [Google Scholar]
- 51.Freed AN, McCulloch S, Meyers T, Suzuki R. Neurokinins modulate hyperventilation-induced bronchoconstriction in canine peripheral airways. Am J Respir Crit Care Med. 2003;167:1102–1108. doi: 10.1164/rccm.200201-055OC. [DOI] [PubMed] [Google Scholar]
- 52.Hallstrand TS, Debley JS, Farin FM, Henderson WR., Jr Role of MUC5AC in the pathogenesis of exercise-induced bronchoconstriction. J Allergy Clin Immunol. 2007;119:1092–1098. doi: 10.1016/j.jaci.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hallstrand TS, Henderson WR., Jr Role of leukotrienes in exercise-induced bronchoconstriction. Curr Allergy Asthma Rep. 2009;9:18–25. doi: 10.1007/s11882-009-0003-8. [DOI] [PubMed] [Google Scholar]