Abstract
We report our studies on the use of two catalyst systems, based on the ligands BrettPhos (1) and RuPhos (2), which provide the widest scope for Pd-catalyzed C–N cross-coupling reactions to date. Often low catalyst loadings and short reaction times can be used with functionalized aryl and heteroaryl coupling partners. The reactions are highly robust and can be set up and performed without the use of a glovebox. These catalysts should find wide application in the synthesis of complex molecules including pharmaceuticals, natural products and functional materials.
1. Introduction
The Pd-catalyzed amination of aryl halides has become a valuable tool in industrial and academic research for the synthesis of substituted anilines.1–7 Such aromatic amines appear frequently in biologically active molecules,8 including a number of pharmaceuticals currently on the market, as well as in materials with useful physical properties.9–12 This cross-coupling methodology allows the conceptually simple, yet powerful, disconnection of an aromatic amine to an aryl halide or pseudo halide and a nitrogen nucleophile. The development of these Pd-catalyzed methods has been influential in the design of synthetic routes to novel pharmaceuticals, increasing both the efficiency of synthesis and the speed with which analogues for biological assay may be accessed. As a result, since the discovery13–15 of this process in 1995 there has been great interest in this area by numerous groups.1, 7, 16–25 Recently, significant improvements in substrate scope and catalyst loadings have been realized.2, 26–32 Unfortunately, this process is yet to approach generality and the goal of being able to couple any nitrogen nucleophile with any aryl or heteroaryl halide is far from accomplished.
In a notable recent contribution, Hartwig showed that palladium complexes generated from the Josiphos ligand CyPF-tBu are efficient catalysts for the coupling of aryl halides with 1° aliphatic amines and 1° anilines.26–28 These authors focused on the coupling of heteroaryl halides or functionalized aryl halides with simple amines, only a handful of examples of the coupling of heteroaryl amines with heteroaryl halides were presented and these required higher Pd loadings. In a typical synthetic application, however, both the nucleophilic and electrophilic components are functionalized. It is therefore an important goal to address such substrate combinations at lower catalyst loadings. The ability to use relatively low catalyst loading is most salient on process development and manufacturing scales in order to reduce the cost of the catalyst and ease the removal of Pd residues from the product. Unfortunately, despite these recent improvements in the ability to perform Pd-catalyzed cross-coupling reactions at lower catalyst loadings, the beneficial effects are significantly mitigated on manufacturing scale by the prolonged reactions times (typically 24 – 48 h). On these larger scales the cost of materials (reagents, catalysts, solvent, starting materials) usually only contributes 20 – 45% of the overall cost.33 In this setting the conversion costs become dominant, hence throughput (including reactor residence time and resource intensity) makes a substantial contribution to the economic viability of a process. For these reactions to become more industrially relevant, both low catalyst loadings and short reaction times should be attained.
Furthermore, the excellent mono- to diarylation selectivities observed when using Josiphos-type ligands stems from the poor ability of these metal complexes to catalyze the arylation of secondary amines.26 Indeed it was found that this transformation could only be performed for a small subset of secondary amines and at relatively high catalyst loading. In contrast, through the use of N-heterocyclic carbene ligands Nolan has shown that secondary amines can be coupled with unfunctionalized aryl halides at very low catalyst loadings, however, this method has only been demonstrated with simple amines such as morpholine and di-n-butylamine.2, 29, 31, 32 A more general means to carry out this reaction at low catalyst loading would be of great significance.
In addition to these issues of catalyst loading, another concern is that a number of practically important nitrogen nucleophiles lack efficient catalytic systems for their arylation. Other than N-methyl aniline, which is an especially easy substrate for most catalysts, acyclic N-alkyl anilines can be challenging.34 Very few studies have been devoted to this group of substrates and such as have been disclosed proceed in modest yields and with relatively high catalyst loadings. Furthermore, in the course of synthesis of biologically-active molecules, the arylation of heteroaryl amines is often desired, such transformations have been described both by Cu-35, 36 and Pd-catalyzed methods, however, systematic studies are limited.37–50
Similarly, there are also conspicuous deficiencies in the scope of suitable electrophiles for Pd-catalyzed amination. The amination of 6-membered ring heteroaryl halides bearing a single heteroatom is becoming increasingly well developed,51–54 however, other classes of heteroaryl halides have remained recalcitrant. The use of 5-membered ring heteroaryl halides has also been limited, especially those containing multiple heteroatoms.37–39, 55
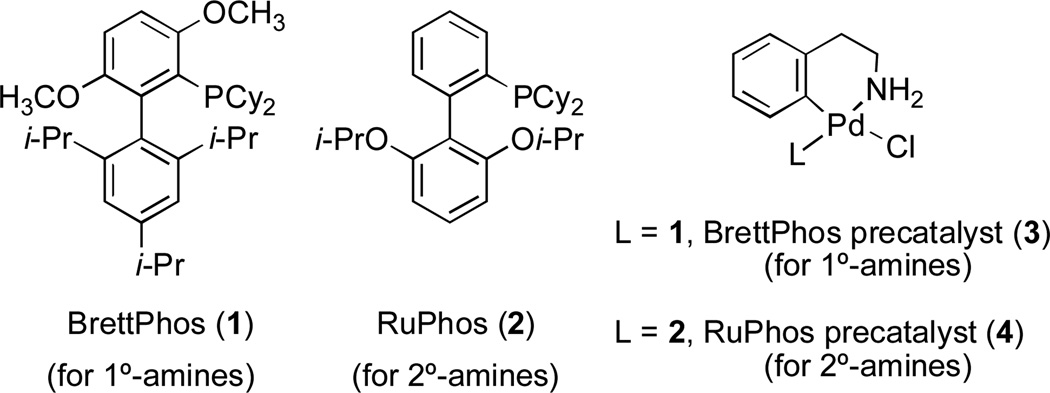
Herein, we report our studies on the use of two Pd precatalyst systems (Figure 1), based on the ligands BrettPhos (1) and RuPhos (2), who’s use have been shown to quantitatively form the active monoligated Pd(0) complex under the reaction conditions.56 These catalysts provide the widest scope for C–N cross-coupling reactions to date and go a significant way towards addressing the above-described limitations in Pd-catalyzed amination.
Fig. 1.
Ligands and precatalysts used in these studies for Pd-catalyzed C–N bond formation (1 and 3 are used for 1°-amines, 2 and 4 are used for 2°-amines).
The use of these catalysts promotes the cross-coupling of a wide range of 1° and 2° alkyl amines and anilines with aryl halides and heteroaryl halides allowing the cross-coupling of complex substrate combinations, often at low catalyst loadings and in short reaction times.
2. Results and Discussion
2.1.1. Catalytic amination of functionalized aryl chlorides with primary alkylamines
In a previous communication57 we showed that a catalyst system based on BrettPhos is highly active in the arylation of simple primary amines with a variety of simple aryl chlorides using 0.01 – 0.05 mol% Pd. Therefore, we were interested in exploring the scope of these systems in greater depth. Previously, we have shown that the use of LHMDS as base in conjunction with a Pd catalyst based on a dialkylbiaryl-phosphine ligand allowed arylation of amines to be performed in the presence of a range of protic functional groups, including hydroxyls, amides and enolizable ketones.54, 58 Hartwig has shown that unfunctionalized primary amines can be coupled with a range of functionalized aryl halides at catalyst loadings of 0.005 – 0.5 mol% Pd using a Josiphos–type ligand employing this base.26–28 Similarly, using 0.05 – 0.5 mol% Pd in conjunction with BrettPhos (Table 1) we were able to couple simple primary aliphatic amines with a range of functionalized aryl chlorides that contain phenolic OH (entries 1a, 1b and 1d) or aliphatic OH groups (entry 1c and 1e), amides (entries 1f, 1g and 1i), carboxylic acids (entries 1h and 1l), enolizable ketones (entry 1j) and esters (entry 1k). It is noteworthy that in the examples given in entries 1a, 1b, 1d, 1e, 1f and 1g the use of BrettPhos affords higher yields in shorter reaction times with equal or in some cases lower Pd loadings than previously reported.26
Table 1.
Cross-coupling reactions of functionalized aryl chlorides and simple primary aliphatic aminesa
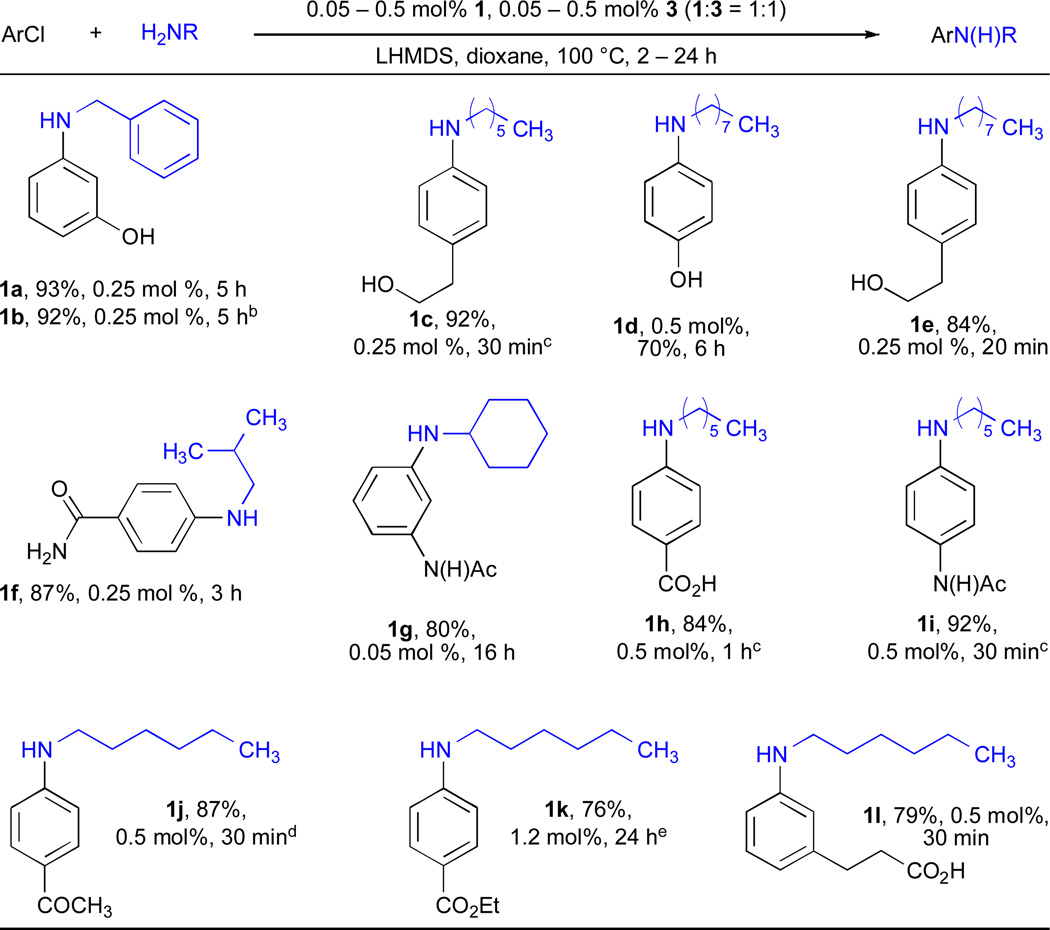 |
ArCl (1 mmol), amine (1.2 mmol).
ArBr.
90 °C.
80 °C.
K2CO3, t-BuOH
2.1.2. Functionalized aliphatic primary amines as the nucleophiles using a BrettPhos ligated Pd-catalyst
Despite the outstanding results described with unfunctionalized 1° amines, no examples of similar reactions with functionalized 1° amines were described. We thus decided to ascertain whether 3 could be used with such substrates. Indeed we found that it was also possible to couple functionalized primary amines with functionalized aryl chlorides (Table 2). Unprotected non–α–amino acids can be employed successfully under these conditions (entry 2c–2e). These substrates have rarely been employed in Pd-catalyzed amination reactions,59 despite the utility of N-aryl amino acid products. These methods present a complimentary approach to the Cu-catalyzed arylation of amino acids, which are only effective for aryl bromides and iodides.60–62 The ability to use 2-chloro-p-xylene (entries 2c and 2d) is also important, as ortho-substituted aryl halides are often challenging substrates for Cu-catalyzed amination reactions. We have recently shown that 3 presents an effective system for the Pd-catalyzed amination of aryl iodides.63 Not surprisingly, under the reaction conditions reported here it was possible to effect the amination of 4-chloroiodobenzene with complete selectivity for coupling of the aryl iodide (entry 2g). Notably, functionalized coupling partners also produce the desired C–N coupling product in good to excellent yield (entries 2a, 2b, 2f–2k).
Table 2.
Pd-catalyzed cross-coupling reactions involving functionalized primary aliphatic aminesa
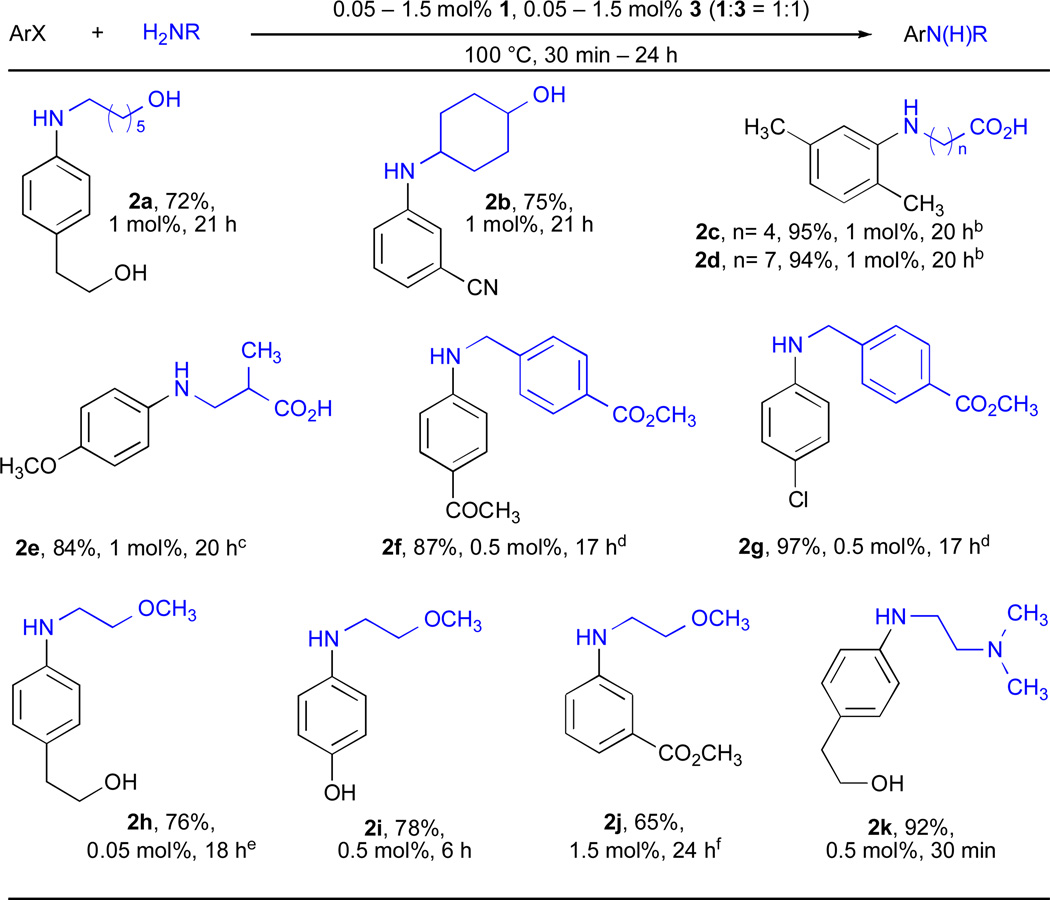 |
ArCl (1 mmol), amine (1.2 mmol), LHMDS, dioxane.
NaOt-Bu, dioxane.
KOt-Bu, toluene.
ArI, 110 °C, Cs2CO3, toluene.
90 °C.
K2CO3, t-BuOH.
A BrettPhos-based catalyst also proved useful in the N-arylation of primary anilines. Although a number of Pd-based catalyst systems have been disclosed for this transformation, these systems have typically not been challenged with the type of functionalized substrates that are often encountered in practical applications. In order to accommodate the greatest range of functional groups, the use of weak inorganic bases is often necessary. With a system based on 1, it was possible to perform coupling reactions of functionalized aryl iodides with functionalized anilines (Table 3) at catalyst levels as low as 0.1 mol% Pd.63 To our knowledge these are the most efficient coupling reactions reported that employ a weak (carbonate or phosphate) base. In order to utilize substrates bearing protic functional groups, LHMDS was employed and its use proved successful (entries 3g and 3j). It was possible to achieve the selective amination of aryl iodides in the presence of both aryl chlorides (entry 3a) and bromides (entries 3b and 3k), thus generating products suitable for further functionalization.
Table 3.
Cross-coupling reactions of functionalized aryl halides and functionalized aryl aminesa
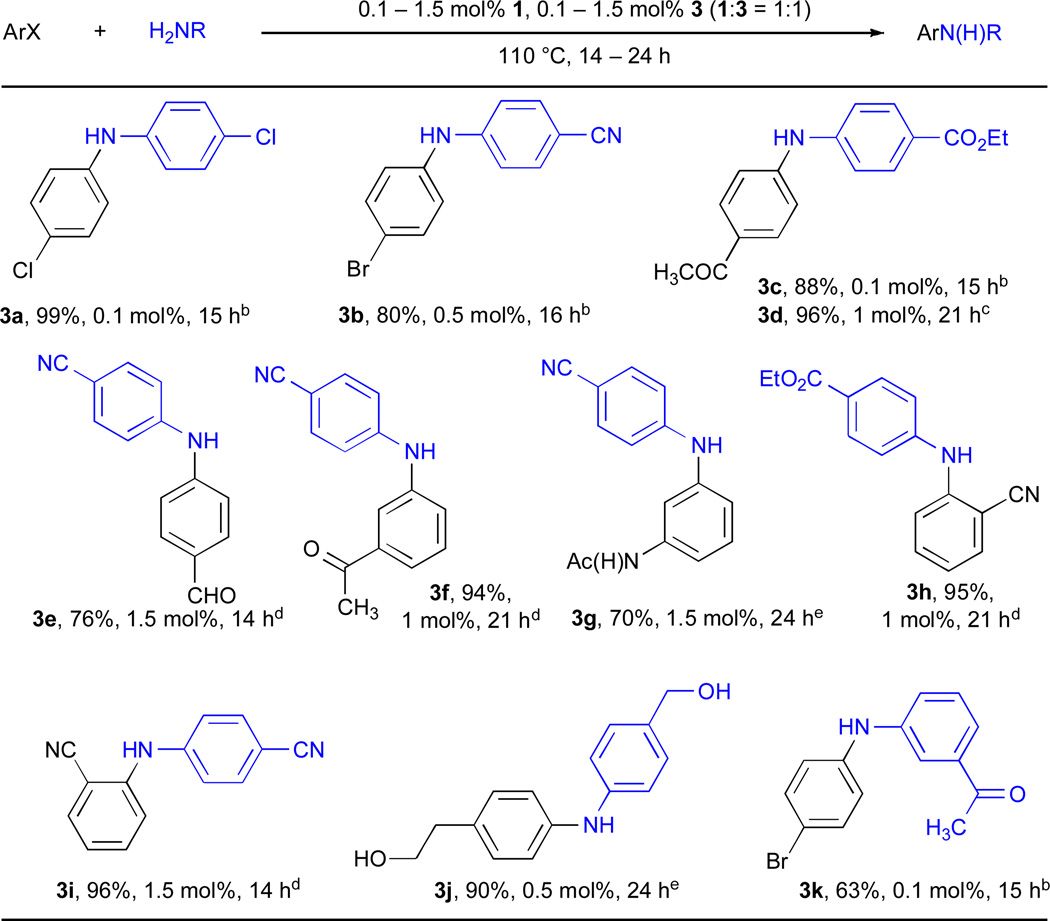 |
ArX (1 mmol), amine (1.2 mmol).
ArI, Cs2CO3, toluene.
ArBr, 1 mol%, K2CO3, t-BuOH.
ArCl, K2CO3, t-BuOH.
ArCl, 100 °C, LHMDS, dioxane.
2.1.3. Synthesis of heteroaryl secondary amines
The coupling of heteroaryl halides is typically more challenging than aryl halides. A number of hypotheses have been proposed to explain this phenomenon.28, 34, 55, 64 These substrates not only present an academic challenge, but are arguably of the highest importance in industrial settings. For example, the majority of applications of Pd-catalyzed amination in pharmaceutical research make use of heteroaryl halides as substrates. These factors have prompted a great deal of research for the utilization of these substrates in C–N cross-coupling reactions.1 Some success in developing catalyst systems that allow the amination of heteroaryl halides at relatively low catalyst loadings has been reported.26–30 However, these studies have been largely limited to 6-membered ring heterohalides. Further, these reactions have generally employed strong bases such as MOt-Bu (M = Na, K) or LHMDS, which limit the functional groups that can be tolerated.
2.1.3.1. The amination of six-membered ring heteroaryl halides under weak base conditions
Using 3 and the combination of K2CO3 and t-BuOH we were able to successfully employ a number of heteroaryl halides in Pd–catalyzed amination reactions (Table 4). Few examples of the amination of 5-halopyrimidines have been reported26, 54 and we were able to efficiently couple this substrate with both aliphatic amines (entries 4a and 4b) and anilines (entry 4c). The combination of 6-membered heteroaryl amines using the K2CO3/t-BuOH also proved feasible (entries 4d–4h). This class of substrates has been more challenging in the past because of the weaker nucleophilicity of the amine nitrogen, as well as their ability to chelate the Pd. We have previously demonstrated the coupling of these compounds with aryl iodides using 1.63 We have now extended this process to aryl bromides and chlorides. Amino–pyridines (entries 4e, 4f, 5h and 5m), –pyrazines (entries 4d and 5g), –pyrazolyls (entries 5k and 5l) and –pyrimidines (entries 4g, 4h, 5i and 5j) could all be successfully transformed to the desired product (Table 4 and Table 5).
Table 4.
Synthesis of heteroaryl secondary amines from C–N coupling of aryl halides and primary aminesa
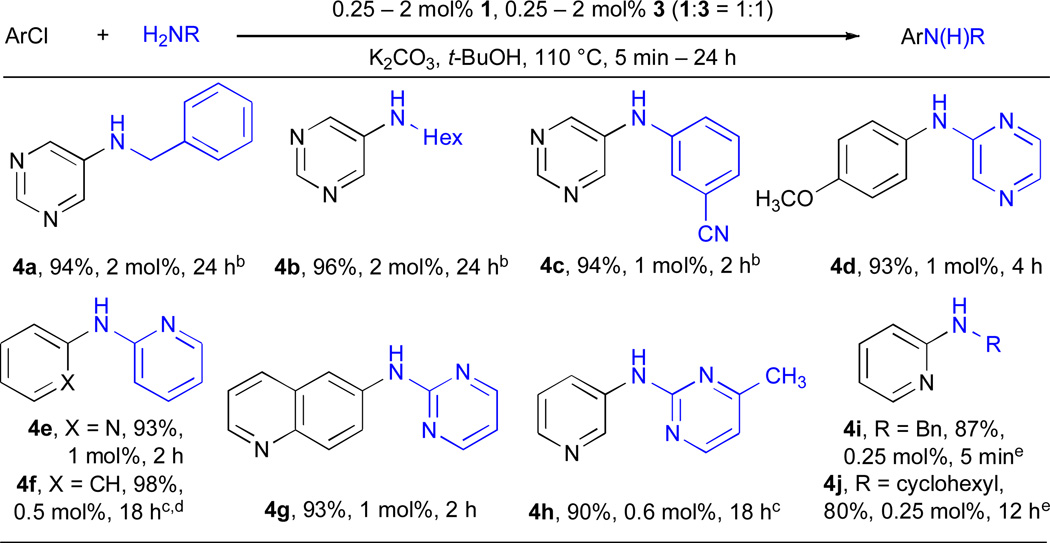 |
ArCl (1 mmol), amine (1.2 mmol).
ArBr.
ArBr, LHMDS, dioxane.
100 °C.
100 °C, LHMDS, dioxane.
Table 5.
Cross-coupling reactions of functionalized aryl halides and heteroaryl aminesa
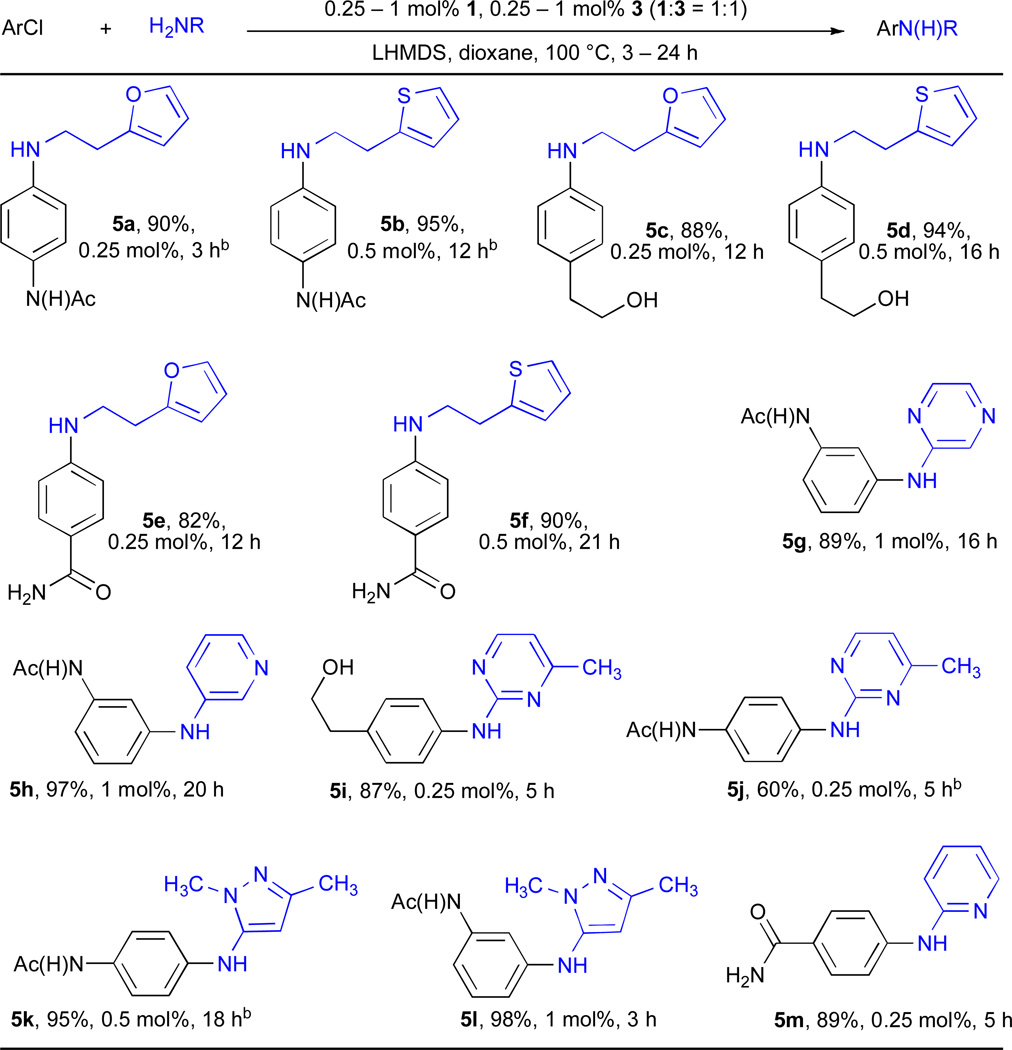 |
ArCl (1 mmol), amine (1.2 mmol).
ArBr.
When LHMDS was used as the base, we could couple furan– (entries 5a, 5c and 5e) and thiophene– (entries 5b, 5d and 5f) containing aliphatic amines with aryl chlorides bearing functional groups with active protons. Also, 2-aminopyridine was successfully combined with 4-chlorobenzamide in 89% yield using only 0.25 mol% Pd in 5 h (entry 5m).
2.1.4. Pd-catalyzed cross-coupling reactions involving 5-membered heterocycles with multiple heteroatoms
Five-membered heteroaryl halides have been problematic substrates in Pd-catalyzed C–N cross-coupling reactions. It has been demonstrated that reductive elimination of the corresponding Pd(II) amido complexes (generated from secondary amine) to form the heteroaryl amines is slow and low yielding with dppf as the supporting ligand.53 More recently we have disclosed that catalysts based on 2 and SPhos could effectively couple chlorothiophenes, as well as 2-chlorobenzothiazoles, with primary and secondary amines in high yields.54 Five-membered ring heteroaryl halides containing multiple heteroatoms are especially challenging. Systematic studies of Pd-catalyzed amination of this class of substrates have only employed benzofused 5-membered heteroaryl halides. Furthermore, all previous examples of this class of transformation have used secondary amines; no examples with primary amines have been reported.51, 54 Therefore, we investigated the use of 3 for the coupling of 5-membered heterocycles with multiple heteroatoms with primary amines, and in particular, with anilines (Table 6).
Table 6.
Pd-catalyzed cross-coupling reactions involving 5-membered heterocycles with multiple heteroatomsa
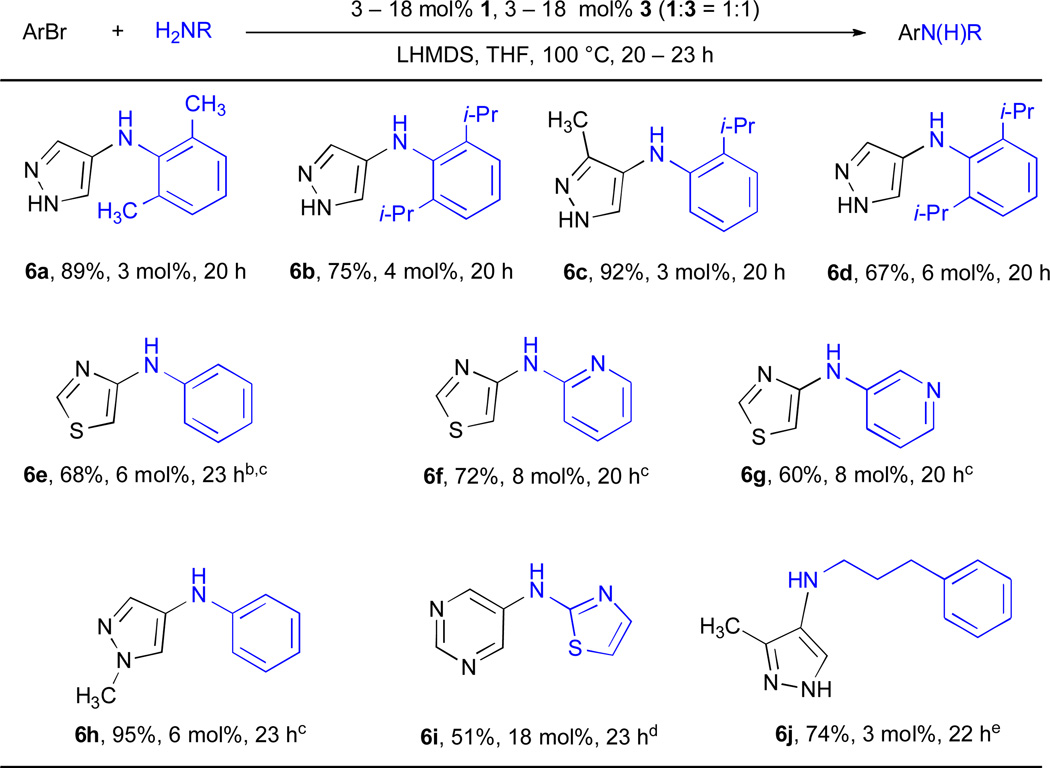 |
ArBr (1.0 mmol), amine (1.2 mmol).
65 °C.
NaOt-Bu, dioxane.
K2CO3, t-BuOH, 110 °C.
ArCl.
90 °C.
The use of 3 in combination with LHMDS as the base allowed the amination of substituted halopyrazoles (entries 6a–6d, 6j) in good yields. Unfortunately, relatively high Pd loadings were required. When using LHMDS as base it was not necessary to use a protecting group for the heterocyclic NH (Table 6), in accord with our previous findings for the amination of haloindoles.54 We were particularly interested to examine the reaction of 5–membered ring heteroaryl halides with heteroaryl amines, as this difficult substrate combination is frequently encountered in the synthesis of biologically active molecules.40–50 Xantphos has received the most use for the cross-coupling of 5-membered heteroaryl amines bearing multiple heteroatoms;37–41 although, in some circumstances other ligands have been employed to give the desired product in moderate yields.44–46, 65 Unfortunately, these reactions exploiting Xantphos have been largely limited to activated aryl bromides; electron neutral aryl bromides have been noted as having a narrower substrate scope and typically benzofused amines must be employed. By employing 1 as the supporting ligand, however, it was possible to effect the coupling of pyridyl amines with 4-bromothiazole (entries 6f and 6g), as well as 2-aminothiazole with 5-bromopyrimidine (entry 6i). Certain other heteroaryl halides remain recalcitrant; we were not able to obtain any coupling product from the reaction of bromoisoxazoles, bromoimidazoles or 3- or 5-halopyrazoles.
2.2.1. Scope of the amination of aryl chlorides with secondary amines
To date little success in the cross-coupling of 2° amines with aryl chlorides at low catalyst loadings (<0.5 mol% Pd) has been obtained. For the coupling of acyclic secondary aliphatic amines, Nolan reported two examples of the arylation of di-n-butylamine at 0.01 mol% Pd using a NHC carbene ligand.31 The use of cyclic secondary amines is also rare; Beller reported a single example of the cross-coupling of piperidine at 0.1 mol% Pd of an activated aryl chloride66 and Hartwig reported two examples with morpholine,26 one at 0.1 mol% Pd and the other at 0.05 mol% Pd (although with reaction times of 48 hours). For the coupling of N–substituted anilines at low Pd loadings, Beller has reported a handful of examples for the arylation of N-methyl aniline with aryl chlorides at 0.01 mol% Pd.67, 68 We have previously demonstrated that the use of the dialkylbiaryl phosphine ligand RuPhos permits the arylation of a range of secondary amines with aryl iodides at low catalyst loadings (0.01 – 0.1 mol% Pd).63 We, therefore, elected to study the scope of this system in the amination of aryl chlorides (Table 7). The combination of NaOt-Bu in THF proved efficient for these reactions. Both morpholine and N′-methylpiperazine underwent arylation with unactivated aryl chlorides at 0.05 mol% Pd (entries 7c and 7d). This catalyst system was also suitable for the cross-coupling of acyclic secondary amines at 0.05 mol% Pd (entry 7j). When using N-methylaniline arylation of 4-chloroanisole could be performed at 0.01 mol% Pd (entry 7a). Although these are encouraging results, we were interested in investigating more challenging substrate combinations.34 It was found that larger N-substituents present a greater challenge; the reactions of N-ethyl, N-benzyl and N-isopropyl anilines progressively required higher catalysts loadings. The coupling of N-methyl anilines bearing substituents at the 2–position could also be achieved by increasing the catalyst loading to 0.3 mol% Pd (entries 7h and 7i). ortho-Substituted aryl chlorides could also be employed, although once again this necessitated a slight increase in catalyst loading (compare 7a and 7b). Indeed it was even possible to bring about the coupling of ortho-substituted anilines with ortho-substituted aryl chlorides (entry 7i). Notably, by increasing the catalyst loading to 0.5 mol% Pd, the coupling of N-methyl-2,4,6-trimethylaniline with 2-chlorotoluene could be effected in an excellent yield (entry 7n).
Table 7.
RuPhos as the ligand for Pd-catalyzed C–N coupling reactions of secondary amines and aryl chloridesa
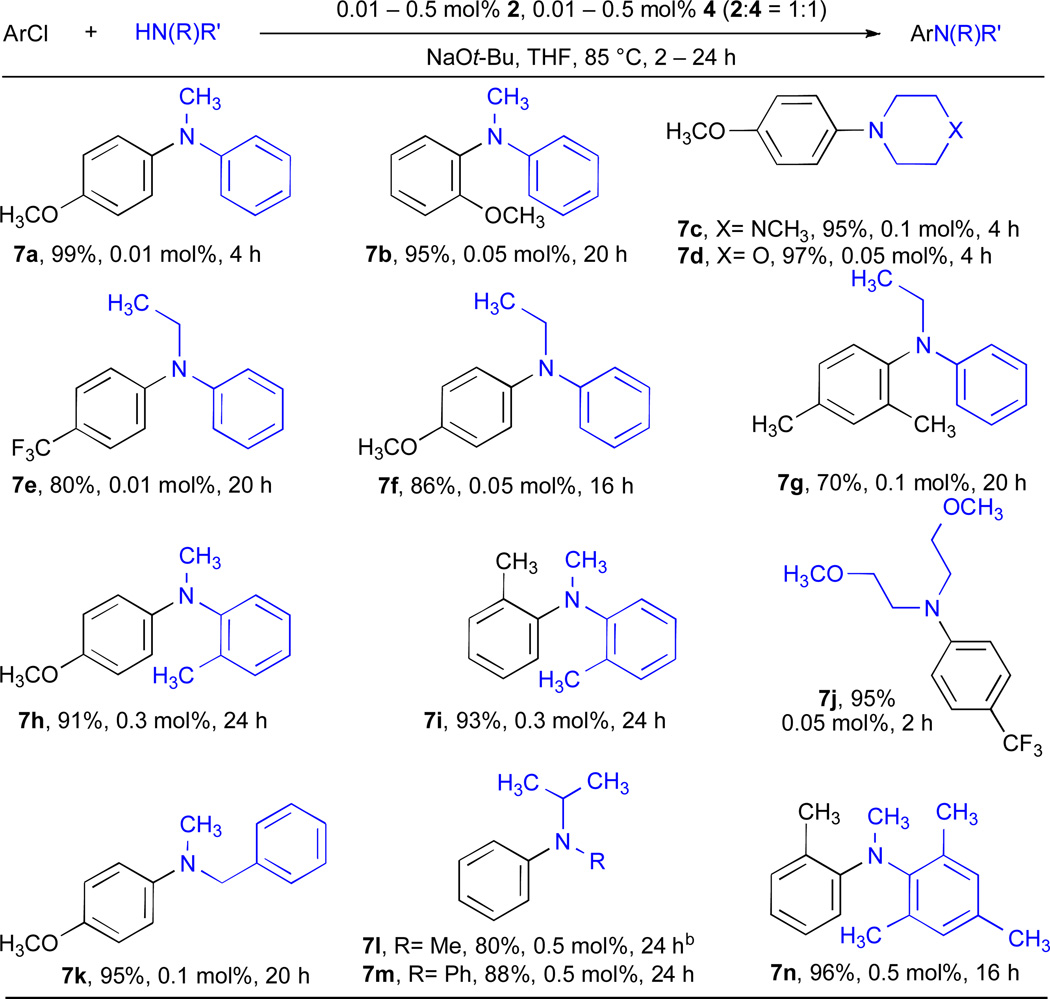 |
ArCl (1 mmol), amine (1.2 mmol).
amine (2 mmol).
2.2.2. Scope of amination reactions of heteroaryl chlorides with secondary amines
We proceeded to investigate the coupling of secondary amines with heteroaryl chlorides (Table 8). Of the heteroaryl chlorides examined, 2-chloropyridine proved to be the least challenging – cyclic secondary amines could be coupled with this substrate with 0.025 – 0.1 mol% Pd (entries 8a–8c and 8e). The cross-coupling of 3-chloropyridine proved more difficult, requiring 1 mol% Pd to achieve efficient coupling with pyrrolidine (entry 8d). The use of Cs2CO3 allowed the coupling of a chloropyridine to be performed in the presence of a nitrile (entry 8j) or of a 2-methylbenzothiazole (entry 8o). The ability to couple acyclic aliphatic amines with heteroaryl chlorides is especially noteworthy (entry 8i). Furthermore, employing LHMDS as base facilitated the coupling of unprotected haloheterocycle containing a free NH group such as 4-chloropyrazole (entry 8m) with N-methylaniline. Although the catalyst loadings are high (4 – 6 mol% Pd) and N-methylaniline is among the easiest nucleophile to use in such reactions, such electrophiles have not previously been employed in Pd-catalyzed C–N cross-coupling reactions. It appears that a general trend for the efficiency of the coupling of secondary heteroaryl chlorides with secondary amines can be discerned: 5- and 6-membered secondary cyclic alkylamines (e.g., pyrrolidine, piperidine) ~ N-methylanilines > N-ethylanilines > N-methylbenzylamine > N-methyl-o-tolylamine > secondary acyclic alkylamines.34
Table 8.
Cross-coupling reactions of (hetero)aryl chlorides and secondary aminesa
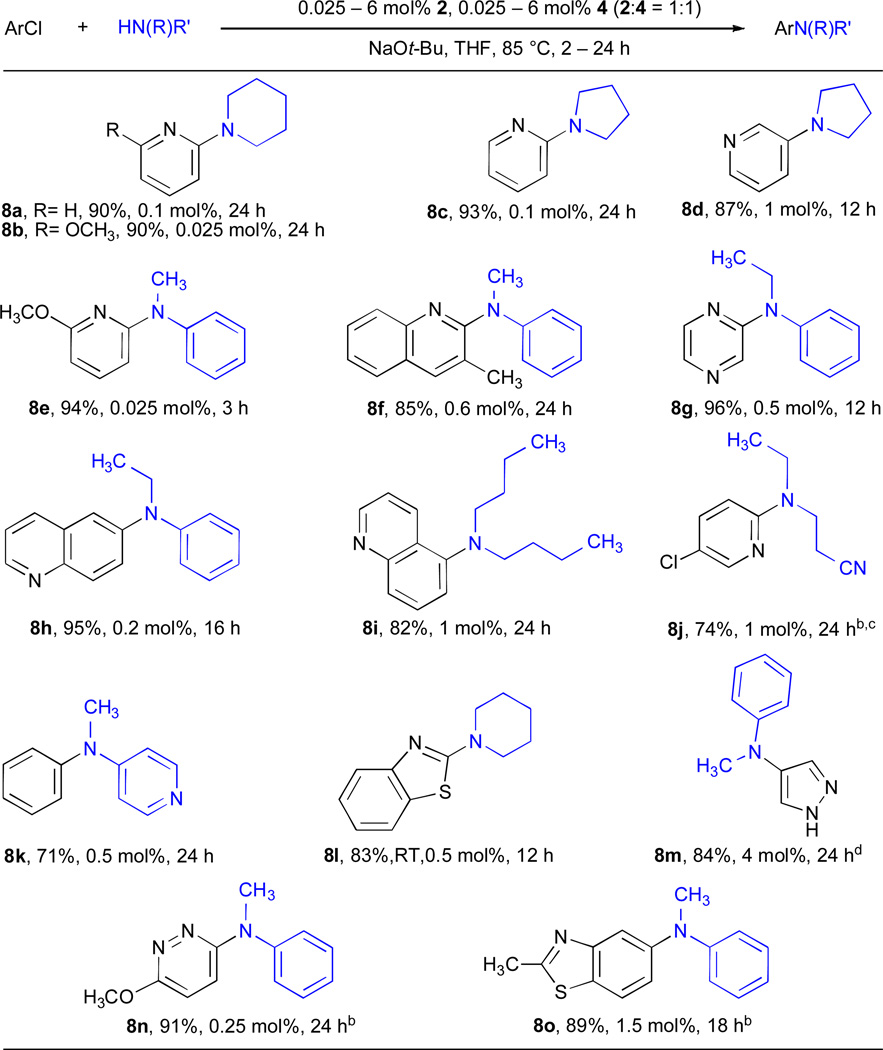 |
ArCl (1 mmol), amine (1.2 mmol).
Cs2CO3/t-BuOH.
amine (2 mmol).
LHMDS/THF, L = SPhos, 100 °C.
2.2.3. Generation of functionalized tertiary amines
The use of Cs2CO3 as base in Pd-catalyzed amination processes allows a wider range of electrophilic functional groups to be incorporated into the substrates than if NaOt-Bu is employed (Table 9). Unfortunately, reaction rates are typically lower with a weaker base, necessitating higher catalyst loadings. There are no reported examples of Cs2CO3 as the base in the arylation of secondary amines with aryl chlorides employing <0.5 mol% Pd. By the use of a RuPhos-based catalyst system it was possible to effect the cross-coupling of a number of substrate combinations bearing electrophilic functional groups, such as esters (entries 9b and 9f), nitriles (entry 9a) and enolizable ketones (entry 9c), at only 0.2 mol% Pd loading. Aliphatic amines require slightly higher catalyst loadings (entries 9e, 9h, 9i–9m) than anilines. For example, the arylation of N-methylisopropyl amine can also be achieved using Cs2CO3 as the base (entry 9g).
Table 9.
RuPhos ligated Pd-catalyzed amination of aryl chlorides by using Cs2CO3/t-BuOHa
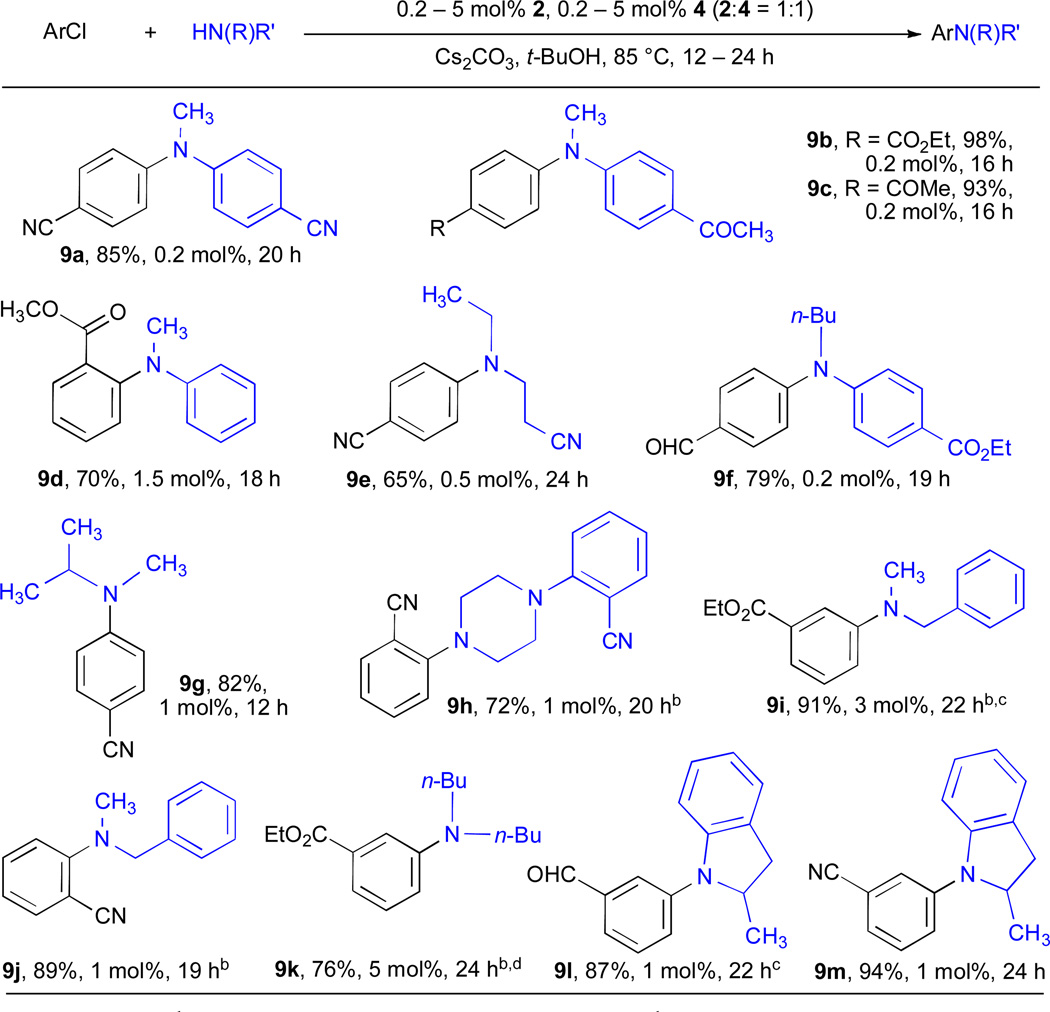 |
ArCl (1 mmol), amine (1.2 mmol).
THF/Cs2CO3.
3% reduction of ArCl.
6% reduction of ArCl.
As in the case of primary amines, the use of LHMDS as base allows the incorporation of protic functional groups into the reactants (Table 10). Under these conditions cyclic secondary amines could be coupled at 0.15 mol% Pd (entry 10a); however, acyclic secondary amines such as di-n-butylamine (entries 10b and 10c) typically required higher catalyst loadings (1.5 mol%). For the coupling of these substrates with ortho-substituted aryl chlorides SPhos proved to be a superior ligand. For example, di-n-butylamine was combined with 2-chlorotoluene in only 12 h with a catalyst loading of 2 mol% Pd (entry 10d).
Table 10.
Pd-catalyzed amination of aryl chlorides by using LHMDS/THFa
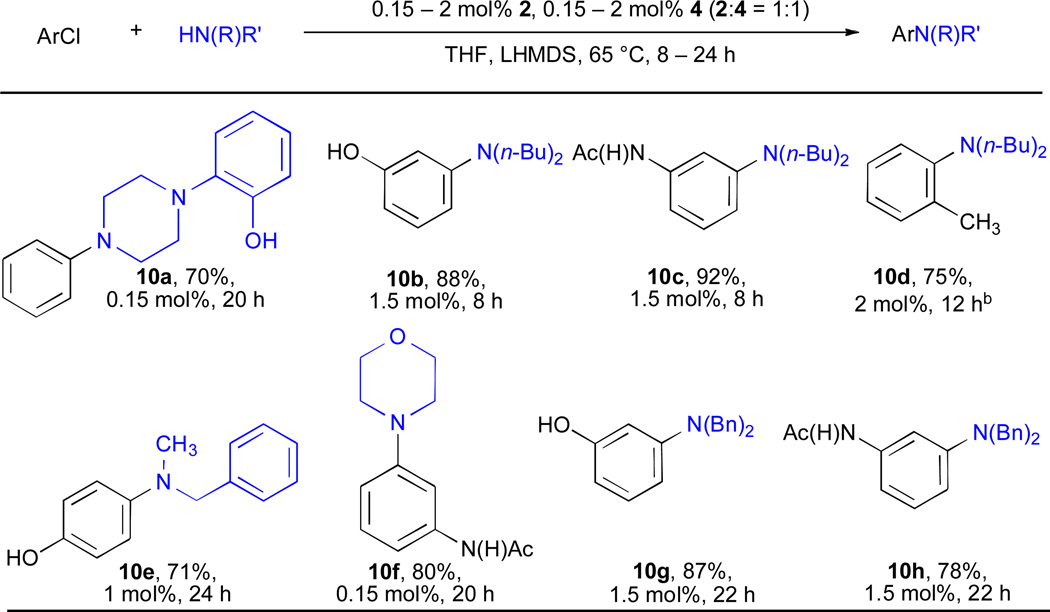 |
ArCl (1 mmol), amine (1.2 mmol).
RT, L= SPhos.
2.2.4. Catalytic amination reactions of heteroaryl chlorides and heteroaryl secondary amines
Despite advances in the Pd-catalyzed amination reactions of heteroaryl chlorides with primary amines, the use of secondary amines has presented challenges and is less studied. Further, reports of the coupling of secondary heteroaryl amines with heteroaryl chlorides are rare.69, 70 We were, therefore, interested to investigate the feasibility of this transformation due to the importance of the resulting products in pharmaceuticals and the appearance of heteroaryl triarylamines in materials science applications.11 In the presence of a RuPhos-based catalyst this challenging transformation could be achieved for a number of substrate combinations (Table 11), including pyridyl (entries 11a, 11c and 11i), pyrazinyl (entries 11b, 11d and 11j), quinoxinyl (entry 11g) and quinolyl chlorides (entry 11e, 11f, 11h and 11k). Despite the relatively high catalyst loadings (0.5 – 4 mol% Pd) required, the ability to achieve the cross-coupling of these substrates in such high yield is of significant importance. Moreover, it was also possible to use the combination of Cs2CO3/t-BuOH to effect these transformations (entries 11d, 11i and 11j), allowing even greater functional group tolerance. Using these conditions the somewhat acidic 2-methyl benzothiazole moiety (entries 11i and 11j) was not problematic. We note that reduction of the aryl halides could not be suppressed completely in some cases (entries 11e and 11f); however, the products were still isolated in good to excellent yields.
Table 11.
Cross-coupling reactions of heteroaryl chlorides and heteroaryl secondary aminesa
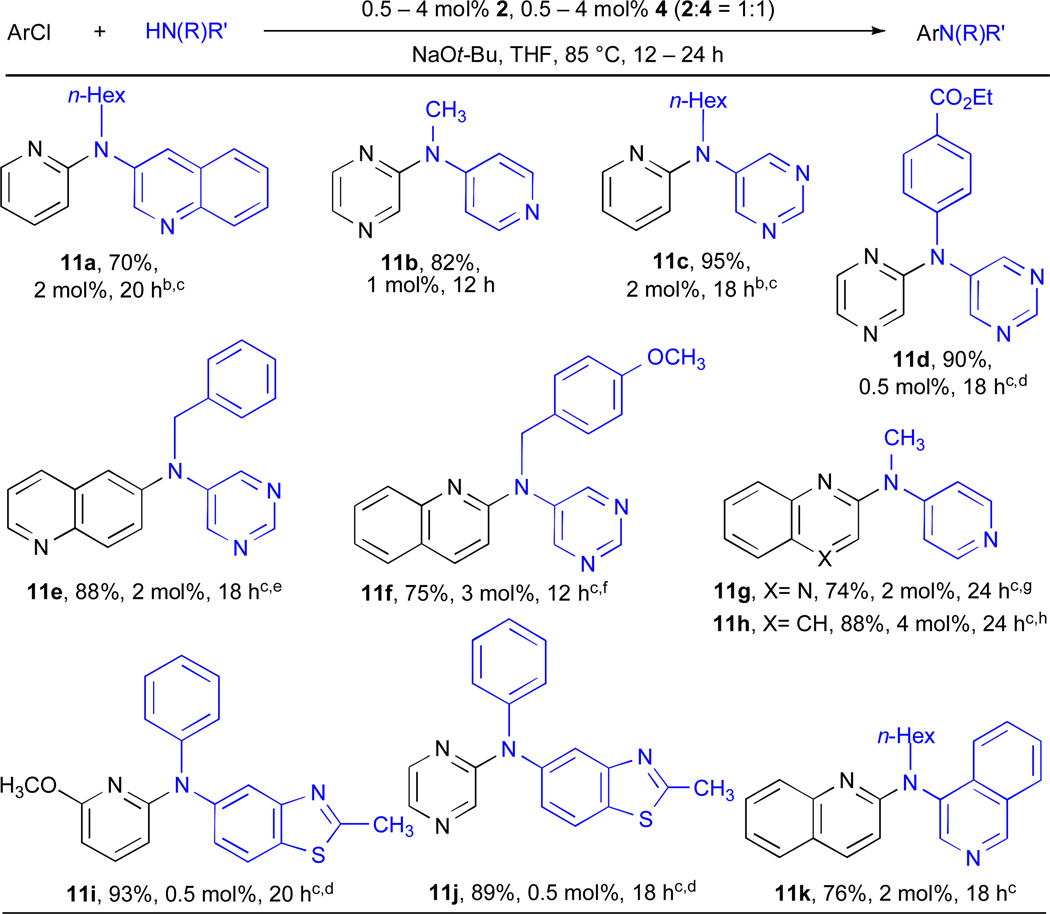 |
ArCl (1.0 mmol), amine (1.2 mmol).
amine (2 mmol).
0.5 mmol scale.
Cs2CO3/t-BuOH.
3% reduction of ArCl.
4% reduction of ArCl.
45 °C.
45 °C, SPhos.
2.2.5. Catalytic amination of (mono) Boc and Cbz protected secondary diamines
N-Aryl piperazines appear frequently in biologically active molecules,8 but the Pd-catalyzed arylation of these substrates can be more challenging than other cyclic amines.71 There have only been a limited number of studies of the arylation of piperazines with aryl chlorides and these have typically used relatively large amounts of catalyst (>1 mol% Pd) and have employed activated substrates as coupling partners.72, 73 By using a RuPhos-based catalyst system, we were able to arylate Boc-protected piperazine with a range of aryl chlorides in good yields (Table 12, entries 12a, 12b and 12e–12g). Under the same conditions Cbz-protected piperazine gave lower yields for these reactions. However, by switching the base and solvent combination to K3PO4 in t-BuOH we are able to obtain the products of these reactions in improved yields (entries 12c and 12d).
Table 12.
Cross-coupling reactions of aryl chlorides and Boc and Cbz protected aminesa
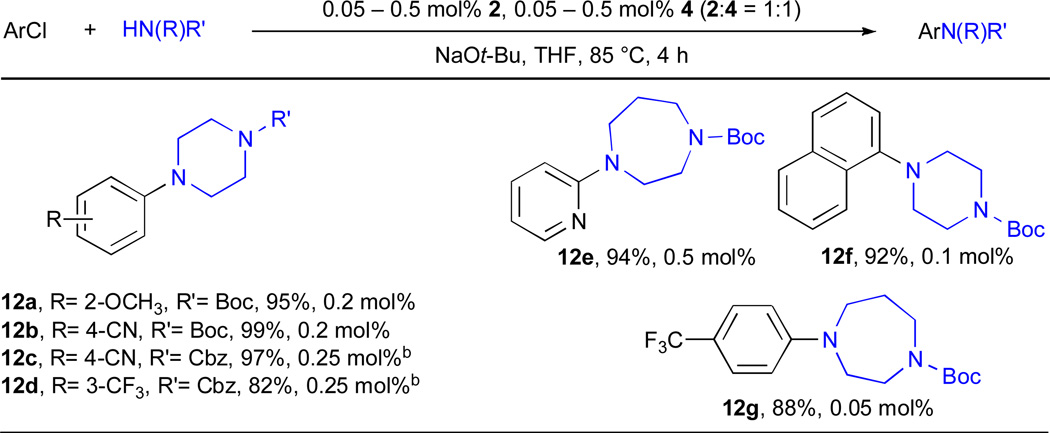 |
ArCl (1 mmol), amine (1.2 mmol).
K3PO4, t-BuOH, 110 °C, 16 h.
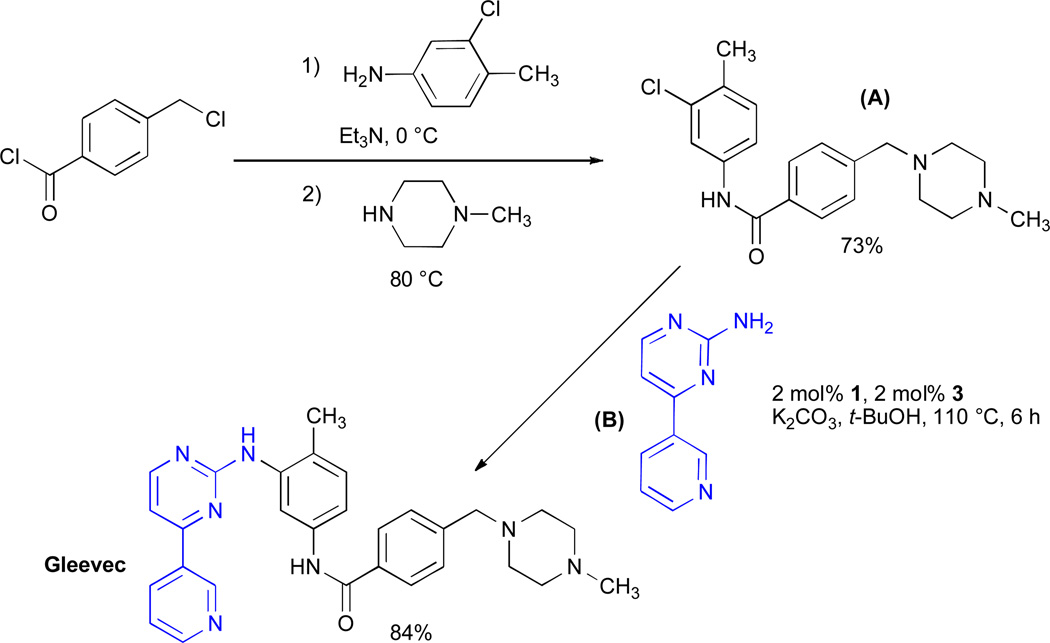
Synthesis of Gleevec®
Above, we have shown that catalyst based on 1 can successfully couple a range of heteroaryl substrates. We wanted to further highlight the utility of our system by applying it to the synthesis of an important biologically active molecule. Imatinib base (Gleevec®) is a small molecule protein kinase inhibitor that is used in the treatment of chronic myelogenous leukemia and was the first of a growing class of kinase inhibitors that specifically target various tumor cells.74–76 A key feature of many of this revolutionary class of drugs is the diarylamine subunit.42 This molecule also possesses a number of features typical of modern pharmaceutical agents including multiple heteroatoms and electrophilic functional groups, as well as having high polarity.77 This, therefore, seemed to be an ideal target on which to test the applicability of a BrettPhos-based catalyst. A number of syntheses of imatinib have been previously reported,78–83 including approaches involving both Pd–78, 83 and Cu–catalyzed82 cross-coupling. These, however were inefficient with respect to the reactivity of catalyst required and could not use aryl chloride substrates as coupling partners. For our approach to the synthesis of Gleevec®, we envisioned a C–N cross-coupling reaction between a 2-aminopyrimidine B and fragment A (Scheme 1). The 2-aminopyrimidine fragment can be made in a single step and is commercially available. The required aryl chloride coupling partner A was prepared in a 2-step, one pot procedure in 73% yield from commercially available precursors. The cross-coupling reaction between A and commercially available B proceeded efficiently in the presence of 2 mol% 3 to furnish imatinib base in 84% yield. The ability to perform this challenging cross-coupling reaction allows a very concise and efficient 2-pot synthesis of imatinib base.
Scheme 1.
Synthesis of Imatinib base
3. Conclusions
In summary, we have shown that a broad range of 1° and 2° amines can be arylated with functionalized aryl and heteroaryl halides. Further, the examples described show that the presence of multiple heteroatoms in either (or both) coupling partners has little deleterious effect on the reaction. It is notable that despite the wide variety of coupling partners only two ligand systems are required, BrettPhos, 1 for 1° amines and RuPhos, 2 for 2° amines. Often low catalyst loadings and short reaction times can be used and these results compare favorably with previously reported catalyst systems. The reactions are highly robust and can be set up and performed without the use of a glovebox. As a result of this unique combination of desirable features, we expect these catalysts to be widely employed in synthetic contexts where these characteristics are prerequisites for applicability, such as in the synthesis of pharmaceuticals, natural products and functional materials.
Supplementary Material
Acknowledgments
This activity is supported by an educational donation provided by Amgen and by funds from the National Institutes of Health (Grant GM-58160). B.P.F. thanks Boehringer Ingelheim and The American Chemical Society for a fellowship. We are grateful to Dr. David S. Surry for comments and help with this manuscript. The NMR instruments used for this study were furnished by funds from the National Science Foundation (CHE 9808061 and DBI 9729592).
Footnotes
Electronic Supplementary Information (ESI) available: Synthetic methods and spectral data.. See DOI: 10.1039/b000000x/
Notes and references
- 1.Surry DS, Buchwald SL. Angew. Chem., Int. Ed. 2008;47:6338–6361. doi: 10.1002/anie.200800497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marion N, Nolan SP. Acc. Chem. Res. 2008;41:1440–1449. doi: 10.1021/ar800020y. [DOI] [PubMed] [Google Scholar]
- 3.Hartwig JF. Acc. Chem. Res. 2008;41:1534–1544. doi: 10.1021/ar800098p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tasler S, Mies J, Langa M. Adv. Synth. Catal. 2007;349:2286–2300. [Google Scholar]
- 5.Torborg C, Beller M. Adv. Synth. Catal. 2009;351:3027–3043. [Google Scholar]
- 6.Buchwald SL, Mauger C, Mignani G, Scholz U. Adv. Synth. Catal. 2006;348:23–39. [Google Scholar]
- 7.Kantchev EAB, O'Brien CJ, Organ MG. Angew. Chem. Int. Ed. 2007;46:2768–2813. doi: 10.1002/anie.200601663. [DOI] [PubMed] [Google Scholar]
- 8.Horton DA, Bourne GT, Smythe ML. Chem. Rev. 2003;103:893–930. doi: 10.1021/cr020033s. [DOI] [PubMed] [Google Scholar]
- 9.Eelkema R, Anderson HL. Macromolecules. 2008;41:9930–9933. [Google Scholar]
- 10.Kawabe Y, Ikeda H, Sakai T, Kawasaki K. J. Mater. Chem. 1992;2:1025–1031. [Google Scholar]
- 11.Ning ZJ, Tian H. Chem. Commun. 2009:5483–5495. doi: 10.1039/b908802d. [DOI] [PubMed] [Google Scholar]
- 12.Lygaitis R, Getautis V, Grazulevicius JV. Chem. Soc. Rev. 2008;37:770–788. doi: 10.1039/b702406c. [DOI] [PubMed] [Google Scholar]
- 13.Guram AS, Rennels RA, Buchwald SL. Angew. Chem. Int. Ed. Engl. 1995;34:1348–1350. [Google Scholar]
- 14.Wolfe JP, Buchwald SL. J. Org. Chem. 1996;61:1133–1135. [Google Scholar]
- 15.Louie J, Hartwig JF. Tetrahedron Lett. 1995;36:3609–3612. [Google Scholar]
- 16.Wolfe JP, Wagaw S, Buchwald SL. J. Am. Chem. Soc. 1996;118:7215–7216. [Google Scholar]
- 17.Driver MS, Hartwig JF. J. Am. Chem. Soc. 1996;118:7217–7218. [Google Scholar]
- 18.Guari Y, van Es DS, Reek JNH, Kamer PCJ, van Leeuwen P. Tetrahedron Lett. 1999;40:3789–3790. [Google Scholar]
- 19.Hamann BC, Hartwig JF. J. Am. Chem. Soc. 1998;120:7369–7370. [Google Scholar]
- 20.Ehrentraut A, Zapf A, Beller M. J. Mol. Catal. A. 2002;182:515. [Google Scholar]
- 21.Hill LL, Moore LR, Huang RC, Craciun R, Vincent AJ, Dixon DA, Chou J, Woltermann CJ, Shaughnessy KH. J. Org. Chem. 2006;71:5117–5125. doi: 10.1021/jo060303x. [DOI] [PubMed] [Google Scholar]
- 22.Nishiyama M, Yamamoto T, Koie Y. Tetrahedron Lett. 1998;39:617–620. [Google Scholar]
- 23.Kataoka N, Shelby Q, Stambuli JP, Hartwig JF. J. Org. Chem. 2002;67:5553–5566. doi: 10.1021/jo025732j. [DOI] [PubMed] [Google Scholar]
- 24.Ackermann L. Synthesis. 2006:1557–1571. [Google Scholar]
- 25.Urgaonkar S, Xu JH, Verkade JG. J. Org. Chem. 2003;68:8416–8423. doi: 10.1021/jo034994y. [DOI] [PubMed] [Google Scholar]
- 26.Shen Q, Ogata T, Hartwig JF. J. Am. Chem. Soc. 2008;130:6586–6596. doi: 10.1021/ja077074w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen QL, Hartwig JF. Org. Lett. 2008;10:4109–4112. doi: 10.1021/ol801615u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen QL, Shekhar S, Stambuli JP, Hartwig JF. Angew. Chem. Int. Ed. Engl. 2005;44:1371–1375. doi: 10.1002/anie.200462629. [DOI] [PubMed] [Google Scholar]
- 29.Marion N, Ecarnot EC, Navarro O, Amoroso D, Bell A, Nolan SP. J. Org. Chem. 2006;71:3816–3821. doi: 10.1021/jo060190h. [DOI] [PubMed] [Google Scholar]
- 30.Navarro O, Marion N, Mei JG, Nolan SP. Chem.-Eur. J. 2006;12:5142–5148. doi: 10.1002/chem.200600283. [DOI] [PubMed] [Google Scholar]
- 31.Marion N, Navarro O, Mei JG, Stevens ED, Scott NM, Nolan SP. J. Am. Chem. Soc. 2006;128:4101–4111. doi: 10.1021/ja057704z. [DOI] [PubMed] [Google Scholar]
- 32.Broggi J, Clavier H, Nolan SP. Organometallics. 2008;27:5525–5531. [Google Scholar]
- 33.Zhang TY. Chem. Rev. 2006;106:2583–2595. doi: 10.1021/cr040677v. [DOI] [PubMed] [Google Scholar]
- 34.Muci AR, Buchwald SL. Top. Curr. Chem. 2002;219:131–209. [Google Scholar]
- 35.Liu Y, Bai Y, Zhang J, Li Y, Jiao J, Qi X. Eur. J. Org. Chem. 2007:6084–6088. [Google Scholar]
- 36.Zhang H, Cai Q, Ma D. J. Org. Chem. 2005;70:5164–5173. doi: 10.1021/jo0504464. [DOI] [PubMed] [Google Scholar]
- 37.Yin JJ, Zhao MM, Huffman MA, McNamara JM. Org. Lett. 2002;4:3481–3484. doi: 10.1021/ol0265923. [DOI] [PubMed] [Google Scholar]
- 38.Schulte JP, Tweedie SR. Synlett. 2007:2331–2336. [Google Scholar]
- 39.Bonala R, Torres MC, Iden CR, Johnson F. Chem. Res. Toxicol. 2006;19:734–738. doi: 10.1021/tx0600191. [DOI] [PubMed] [Google Scholar]
- 40.Zhao M, Yin JJ, Huffman MA, McNamara JM. Tetrahedron. 2006;62:1110–1115. [Google Scholar]
- 41.Shen ZL, Hong YM, He XF, Mo WM, Hu BX, Sun N, Hu XQ. Org. Lett. 2010;12:552–555. doi: 10.1021/ol902759k. [DOI] [PubMed] [Google Scholar]
- 42.Quintas-Cardama A, Kantarjian H, Cortes J. Nat. Rev. Drug. Discov. 2007;6:834–848. doi: 10.1038/nrd2324. [DOI] [PubMed] [Google Scholar]
- 43.Andersen CB, Wan Y, Chang JW, Riggs B, Lee C, Liu Y, Sessa F, Villa F, Kwiatkowski N, Suzuki M, Nallan L, Heald R, Musacchio A, Gray NS. Acs Chem. Biol. 2008;3:180–192. doi: 10.1021/cb700200w. [DOI] [PubMed] [Google Scholar]
- 44.Schoffers E, Olsen PD, Means JC. Org. Lett. 2001;3:4221–4223. doi: 10.1021/ol016900h. [DOI] [PubMed] [Google Scholar]
- 45.Wang ZW, Rizzo CJ. Org. Lett. 2001;3:565–568. doi: 10.1021/ol006968h. [DOI] [PubMed] [Google Scholar]
- 46.Bauer D, Whittington DA, Coxon A, Bready J, Harriman SP, Patel VF, Polverino A, Harmange JC. Bioorg. Med. Chem. Lett. 2008;18:4844–4848. doi: 10.1016/j.bmcl.2008.07.080. [DOI] [PubMed] [Google Scholar]
- 47.Queiroz M, Begouin A, Ferreira I, Kirsch G, Calhelha RC, Barbosa S, Estevinho LM. Eur. J. Org. Chem. 2004:3679–3685. [Google Scholar]
- 48.Vidal B, Nueda A, Esteve C, Domenech T, Benito S, Reinoso RF, Pont M, Calbet M, Lopez R, Cadavid MI, Loza MI, Cardenas A, Godessart N, Beleta J, Warrellow G, Ryder H. J. Med. Chem. 2007;50:2732–2736. doi: 10.1021/jm061333v. [DOI] [PubMed] [Google Scholar]
- 49.Knight RL, Allen DR, Birch HL, Chapman GA, Galvin FC, Jopling LA, Lock CJ, Meissner JWG, Owen DA, Raphy G, Watson RJ, Williams SC. Bioorg. Med. Chem. Lett. 2008;18:629–633. doi: 10.1016/j.bmcl.2007.11.075. [DOI] [PubMed] [Google Scholar]
- 50.Hong YP, Tanoury GJ, Wilkinson HS, Bakale RP, Wald SA, Senanayake CH. Tetrahedron Lett. 1997;38:5607–5610. [Google Scholar]
- 51.Hooper MW, Utsunomiya M, Hartwig JF. J. Org. Chem. 2003;68:2861–2873. doi: 10.1021/jo0266339. [DOI] [PubMed] [Google Scholar]
- 52.Ogawa K, Radke KR, Rothstein SD, Rasmussen SC. J. Org. Chem. 2001;66:9067–9070. doi: 10.1021/jo016195q. [DOI] [PubMed] [Google Scholar]
- 53.Hooper MW, Hartwig JF. Organometallics. 2003;22:3394–3403. [Google Scholar]
- 54.Charles MD, Schultz P, Buchwald SL. Org. Lett. 2005;7:3965–3968. doi: 10.1021/ol0514754. [DOI] [PubMed] [Google Scholar]
- 55.Anderson KW, Tundel RE, Ikawa T, Altman RA, Buchwald SL. Angew. Chem. Int. Ed. 2006;45:6523–6527. doi: 10.1002/anie.200601612. [DOI] [PubMed] [Google Scholar]
- 56.Biscoe MR, Fors BP, Buchwald SL. J. Am. Chem. Soc. 2008;130:6686–6687. doi: 10.1021/ja801137k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fors BP, Watson DA, Biscoe MR, Buchwald SL. J. Am. Chem. Soc. 2008;130:13552–13554. doi: 10.1021/ja8055358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Harris MC, Huang XH, Buchwald SL. Org. Lett. 2002;4:2885–2888. doi: 10.1021/ol0262688. [DOI] [PubMed] [Google Scholar]
- 59.Cheung WS, Calvo RR, Tounge BA, Zhang SP, Stone DR, Brandt MR, Hutchinson T, Flores CM, Player MR. Bioorg. Med. Chem. Lett. 2008;18:4569–4572. doi: 10.1016/j.bmcl.2008.07.035. [DOI] [PubMed] [Google Scholar]
- 60.Ma DW, Cai QA. Acc. Chem. Res. 2008;41:1450–1460. doi: 10.1021/ar8000298. [DOI] [PubMed] [Google Scholar]
- 61.Ma DW, Zhang YD, Yao JC, Wu SH, Tao FG. J. Am. Chem. Soc. 1998;120:12459–12467. [Google Scholar]
- 62.Lu Z, Twieg RJ. Tetrahedron Lett. 2005;46:2997–3001. [Google Scholar]
- 63.Fors BP, Davis NR, Buchwald SL. J. Am. Chem. Soc. 2009;131:5766–5768. doi: 10.1021/ja901414u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shen QL, Hartwig JF. J. Am. Chem. Soc. 2007;129:7734–7735. doi: 10.1021/ja0722473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baeza A, Burgos C, Alvarez-Builla J, Vaquero JJ. Tetrahedron Lett. 2007;48:2597–2601. [Google Scholar]
- 66.Beller M, Riermeier TH, Reisinger CP, Herrmann WA. Tetrahedron Lett. 1997;38:2073–2074. [Google Scholar]
- 67.Rataboul F, Zapf A, Jackstell R, Harkal S, Riermeier T, Monsees A, Dingerdissen U, Beller M. Chem.-Eur. J. 2004;10:2983–2990. doi: 10.1002/chem.200306026. [DOI] [PubMed] [Google Scholar]
- 68.Tewari A, Hein M, Zapf A, Beller M. Tetrahedron. 2005;61:9705–9709. [Google Scholar]
- 69.Bolm C, Frison JC, Le Paih J, Moessner C, Raabe G. J. Organomet. Chem. 2004;689:3767–3777. [Google Scholar]
- 70.Patriciu OI, Pillard C, Finaru AL, Sandulescu L, Guillaumet G. Synthesis. 2007:3868–3876. [Google Scholar]
- 71.Hepperle M, Eckert J, Gala D, Shen L, Evans CA, Goodman A. Tetrahedron Lett. 2002;43:3359–3363. [Google Scholar]
- 72.Michalik D, Kumar K, Zapf A, Tillack A, Arlt M, Heinrich T, Beller M. Tetrahedron Lett. 2004;45:2057–2061. [Google Scholar]
- 73.Schon U, Messinger J, Buckendahl M, Prabhu MS, Konda A. Tetrahedron. 2009;65:8125–8131. [Google Scholar]
- 74.Capdeville R, Buchdunger E, Zimmermann J, Matter A. Nat. Rev. Drug. Discov. 2002;1:493–502. doi: 10.1038/nrd839. [DOI] [PubMed] [Google Scholar]
- 75.Arora A, Scholar EM. J. Pharmacol. Exp. Ther. 2005;315:971–979. doi: 10.1124/jpet.105.084145. [DOI] [PubMed] [Google Scholar]
- 76.Deininger M, Buchdunger E, Druker BJ. Blood. 2005;105:2640–2653. doi: 10.1182/blood-2004-08-3097. [DOI] [PubMed] [Google Scholar]
- 77.Nagar B, Bornmann WG, Pellicena P, Schindler T, Veach DR, Miller WT, Clarkson B, Kuriyan J. Cancer Res. 2002;62:4236–4243. [PubMed] [Google Scholar]
- 78.O. K. Loiseleur D, Abel S, Buerger HM, Meisenbach M, Schmitz B, Sedelmeier G. 03/066613 WO Patent. 2003
- 79.Leonetti F, Capaldi C, Carotti A. Tetrahedron Lett. 2007;48:3455–3458. [Google Scholar]
- 80.Ivanov AS, Shishkov SV. Monatsh. Chem. 2009;140:619–623. [Google Scholar]
- 81.Zimmermann J, Buchdunger E, Mett H, Meyer T, Lydon NB, Traxler P. Bioorg. Med. Chem. Lett. 1996;6:1221–1226. [Google Scholar]
- 82.Liu YF, Wang CL, Bai YJ, Han N, Jiao JP, Qi XL. Org. Process Res. Dev. 2008;12:490–495. [Google Scholar]
- 83.Hopkin MD, Baxendale IR, Ley SV. Chem. Commun. 2010;46:2450–2452. doi: 10.1039/c001550d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.