Abstract
Adenosine A1 receptor antagonists have diuretic/natriuretic activity and may be useful for treating sodium-retaining diseases, many of which are associated with increased renal sympathetic tone. Therefore, it is important to determine whether A1 receptor antagonists alter renal sympathetic neurotransmission. In isolated, perfused rat kidneys, renal vasoconstriction induced by renal sympathetic nerve simulation was attenuated by 1) 1,3-dipropyl-8-p-sulfophenylxanthine (xanthine analog that is a nonselective adenosine receptor antagonist, but is cell membrane impermeable and thus does not block intracellular phosphodiesterases), 2) xanthine amine congener (xanthine analog that is a selective A1 receptor antagonist), 3) 1,3-dipropyl-8-cyclopentylxanthine (xanthine analog that is a highly selective A1 receptor antagonist), and 4) FK453 (nonxanthine analog that is a highly selective A1 receptor antagonist). In contrast, FR113452 (enantiomer of FK453 that does not block A1 receptors), MRS-1754 (selective A2B receptor antagonist), and VUF-5574 (selective A3 receptor antagonist) did not alter responses to renal sympathetic nerve stimulation, and ZM-241385 (selective A2A receptor antagonist) enhanced responses. Antagonism of A1 receptors did not alter renal spillover of norepinephrine. 2-Chloro-N6-cyclopentyladenosine (highly selective A1 receptor agonist) increased renal vasoconstriction induced by exogenous norepinephrine, an effect that was blocked by 1,3-dipropyl-8-cyclopentylxanthine, U73122 (phospholipase C inhibitor), GF109203X (protein kinase C inhibitor), PP1 (c-src inhibitor), wortmannin (phosphatidylinositol 3-kinase inhibitor), and OSU-03012 (3-phosphoinositide-dependent protein kinase-1 inhibitor). These results indicate that adenosine formed during renal sympathetic nerve stimulation enhances the postjunctional effects of released norepinephrine via coincident signaling and contributes to renal sympathetic neurotransmission. Likely, the coincident signaling pathway is: phospholipase C → protein kinase C → c-src → phosphatidylinositol 3-kinase → 3-phosphoinositide-dependent protein kinase-1.
Keywords: kidney, norepinephrine, adenosine receptors, A1 receptor antagonists
renal adenosine A1 receptors importantly regulate kidney function. For example, in opossum kidney cells (proximal tubular phenotype), activation of A1 adenosine receptors increases Na+-phosphate and Na+-glucose symport (7), and in rabbit proximal convoluted tubules, stimulation of A1 receptors accelerates Na+-3HCO3− symport in the basolateral membrane (56). It is now firmly established that activation of A1 receptors augments proximal tubular sodium reabsorption (30, 31, 59, 63).
Because A1 receptor activation stimulates sodium reabsorption, it is not surprising that blockade of A1 receptors decreases sodium reabsorption and increases sodium excretion (i.e., A1 antagonists are diuretics). Indeed, selective blockade of renal A1 receptors rapidly (within minutes) and markedly (3- to 10-fold) increases urinary sodium excretion in animals and humans with little or no effect on potassium excretion (30, 31, 63). For this reason, A1 receptor antagonists represent a new class of diuretics that may prove useful for the treatment of a variety of cardiovascular disorders. Indeed, A1 receptor antagonists are in development as diuretics for the management of chronic and acute heart failure (27) and may be useful for other renal indications such as treatment of liver cirrhosis (28), hepatorenal syndrome (27), and prevention of radiocontrast media-induced nephropathy (27).
A concern, however, with regard to using A1 antagonists as diuretics or in renal diseases in general is that blockade of A1 receptors theoretically could augment renal sympathetic neurotransmission, an effect that could reduce renal blood flow, glomerular filtration, and electrolyte excretion. In many organ systems and tissues, prejunctional A1 receptors attenuate norepinephrine (NE) release from sympathetic nerve varicosities (16). Importantly, sympathetic nerve stimulation not only releases NE, but also ATP (a cotransmitter with NE and a precursor of adenosine) as well as enzymes involved in the metabolism of ATP to adenosine (64). Thus, renal sympathetic nerve stimulation (RSNS) would likely increase both NE and adenosine in neuroeffector junctions of the renal microcirculation, and A1 receptor antagonists, by blocking prejunctional A1 receptors, could possibly prevent prejunctional inhibition of NE release by endogenous adenosine formed in the neuroeffector junction and therefore might augment renal sympathetic neurotransmission.
Counter to the aforementioned deduction, one can construct a line of reasoning leading to the diametrically opposite conclusion that A1 receptor antagonists might attenuate, rather than augment, renal sympathetic neurotransmission. In this regard, early studies suggest that adenosine augments renal vasoconstriction induced by NE. Hedqvist and Fredholm (24) reported in 1976 that in the isolated, perfused rabbit kidney adenosine caused a concentration-dependent increase in vasoconstrictor responses to RSNS and exogenous NE, yet decreased the release of NE evoked by nerve stimulation. In 1978, these investigators extended their findings by demonstrating that the effects of adenosine in the rabbit kidney were blocked by theophylline (25) (a low-potency, nonselective adenosine receptor antagonist) and that theophylline per se markedly inhibited (by ∼60%) vasoconstrictor responses to RSNS, yet caused only a modest increase (by ∼25%) in RSNS-induced NE release. These results suggested a dual role for endogenous adenosine in sympathetic neurotransmission in the rabbit kidney, i.e., modest prejunctional inhibition of NE release but profound augmentation of postjunctional responses to released (and exogenous) NE. Yoneda et al. (65) using an in vivo dog kidney preparation, similarly concluded that endogenous adenosine inhibits RSNS-induced NE release and facilitates NE-induced renal vasoconstriction. However, neither administration of exogenous adenosine nor blockade of adenosine receptors with theophylline affected RSNS-induced renal vasoconstriction, suggesting offsetting prejunctional vs. postjunctional effects of exogenous and endogenous adenosine. Ekas and co-workers in 1981 (12) and 1983 (13) reported that in isolated, perfused rat kidneys exogenous adenosine inhibited RSNS-induced vasoconstriction and NE release, but had little effect on responses to exogenous NE.
Surprisingly, in the last 20 years, the role of endogenous adenosine in renal sympathetic neurotransmission has received little attention, and therefore our knowledge in this area has remained stagnant. However, since these early pioneering studies, there have been three profound changes: 1) it is now widely appreciated that adenosine can act on multiple receptor subtypes that have disparate effects, 2) a broad array of highly potent and selective adenosine receptor antagonists and agonists for the adenosine receptor subtypes is now available, and 3) A1 receptor antagonists are now being developed as a novel class of diuretics and renal drugs. Therefore, it seems appropriate to revisit the role of adenosine in renal sympathetic neurotransmission using modern pharmacological tools to more precisely define in particular the role of A1 adenosine receptors in renal sympathetic neurotransmission.
METHODS
Animals.
Kidneys for isolation and perfusion were obtained from male Sprague-Dawley rats weighing ∼400 g. Animals were purchased from Charles River (Wilmington, MA). The Institutional Animal Care and Use Committee approved all procedures. The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23, revised 1996).
Drugs.
1,3-Dipropyl-8-p-sulfophenylxanthine [DPSPX; xanthine analog that is a nonselective adenosine receptor antagonist that does not penetrate cell membranes (57)], xanthine amine congener [XAC; xanthine analog that is a selective A1 receptor antagonist (37)], 1,3-dipropyl-8-cyclopentylxanthine [DPCPX; xanthine analog that is a highly selective A1 receptor antagonist (37)], ZM-241385 [nonxanthine analog that is a highly selective A2A receptor antagonist (37)], MRS-1754 [nonxanthine analog that is a highly selective A2B receptor antagonist (37)], VUF-5574 [nonxanthine analog that is a highly selective A3 receptor antagonist (60)], 2-chloro-N6-cyclopentyladenosine [CCPA; a highly selective A1 receptor agonist (37)], U73122 [phospholipase (PLC) inhibitor (3)], U73343 [inactive analog of U73122 (50)], GF109203X [protein kinase C (PKC) inhibitor (38)], PP1 [c-src inhibitor (22)], wortmannin [phosphatidylinositol 3-kinase (PI3K) inhibitor (61)], and apocynin [antioxidant (26)] were obtained from Sigma (St. Louis, MO). FK453 [nonxanthine analog that is a highly selective A1 receptor antagonist (39, 40)] and FK113452 [enantiomer of FK453 that does not block A1 receptors (39, 40)] were kindly provided by Fujisawa Pharmaceutical (Osaka, Japan). OSU-03012 [3-phosphoinositide-dependent protein kinase-1 (PDK1) inhibitor] was obtained from Selleck Chemicals (Houston, TX).
Isolated, perfused rat kidney.
Rat kidneys were isolated and perfused at a constant rate (5 ml/min) with Tyrode's solution as previously described by us (46). All stated concentrations are the final concentrations in the perfusate entering the kidney. Kidneys were allowed to stabilize for 1 h before the protocols were initiated as described below.
RSNS.
RSNS was accomplished by placing a platinum bipolar electrode around the renal artery close to the kidney and connecting the electrode to a Grass stimulator (model SD9E; Grass Instruments, Quincy, MA) as previously described by us (35). The tissues around the electrode were kept moist with Tyrode's solution and the stimulation parameters were biphasic pulses, 1-ms pulse duration; 35 V at indicated frequencies.
Analysis of NE.
NE was quantified using high-performance liquid chromatography with electrochemical detection as previously described by us (58).
Analysis of adenosine.
Adenosine was quantified using ultraperformance liquid chromatography-tandem mass spectrometry as previously described by us (52).
Protocol 1.
Many xanthine derivatives inhibit intracellular phosphodiesterases (1) and thereby increase intracellular 3′,5′-cAMP levels and reduce vascular responses to most vasoconstrictors. Therefore, as an initial test of the role of adenosine in renal sympathetic neurotransmission, we examined the effects of DPSPX [an adenosine receptor antagonist that does not penetrate cell membranes (57) and therefore does not inhibit intracellular phosphodiesterases] on responses to RSNS. Perfused kidneys were subjected to two periods of RSNS (9 Hz for 5 min) separated by 35 min. In one group, DPSPX (300 μmol/l) was added to the perfusate 15 min before the second stimulation period (DPSPX group) and in another group DPSPX was not added (control group).
Protocol 2.
To confirm and extend the results from protocol 2, the concentration-dependent effects of DPSPX (30, 100, and 300 μmol/l) on renal sympathetic neurotransmission were assessed by eliciting perfusion pressure responses to RSNS during four stimulation periods (9 Hz for 5 min) separated by 15 min. In the control group, no treatments were added to the perfusate, whereas in the treatment group DPSPX was added to the perfusate between the first and second, second and third, and third and fourth periods to provide the indicated concentrations in the perfusate.
Protocol 3.
Although DPSPX is a useful pharmacological tool to assess the role of cell surface adenosine receptors, DPSPX is not selective for the A1 receptor, but instead it antagonizes all four adenosine receptor subtypes. To ascertain whether the effects of DPSPX were mediated by blockade of A1 receptors, we next examined the effects of XAC [xanthine derivative that is selective for the A1 receptor (37)], DPCPX [xanthine derivative that is highly selective for the A1 receptor (37)], FK453 [nonxanthine derivative that is highly selective for the A1 receptor (39, 40)], and FR113452 [the enantiomer of FK453 that does not block the A1 receptor (39, 40)]. The effects of these agents on RSNS responses were assessed at concentrations of 10, 100, and 1,000 nmol/l using the same experimental design as described for protocol 2.
Protocol 4.
To determine whether the adenosine receptor antagonists augmented NE release, renal venous perfusate was collected before and during RSNS (9 Hz for 5 min) in control kidneys or kidneys pretreated for 15 min with 300 μmol/l of DPSPX or 1,000 nmol/l of XAC. A similar experiment was performed comparing the effects of 1,000 nmol/l of FR113452 vs. FK453 on NE release.
Protocol 5.
To determine whether other adenosine receptor subtypes contribute to renal sympathetic neurotransmission, we compared the effects of DPCPX [highly selective A1 receptor antagonist (37)], ZM-241385 [selective A2A receptor antagonist (37)], MRS-1754 [selective A2B receptor antagonist (37)], and VUF-5574 [selective A3 receptor antagonist (60)] on responses to RSNS. To detect subtle changes in RSNS responses, we employed a paired experimental design in which both kidneys from the same rat were removed and perfused separately but simultaneously. After a rest period (30 min), a test response (5 Hz RSNS) was elicited. For each pair of kidneys, 15 min before initiating experimental RSNS, one kidney received vehicle only and the other kidney received one of the four antagonists (1,000 nmol/l), and then a frequency-response relationship was elicited by stimulating at 2, 3, 4, 5, 6, 7, 8, 9, and 10 Hz for 2 min at 15-min intervals. (Two-minute stimulations, rather than 5-min stimulations, were employed in this protocol to prevent degradation of the preparation due to repeated stimulations.) By using a paired design and by normalizing experimental responses to the test response in each kidney, between-animal and between-kidney variability was reduced.
Protocol 6.
Adenosine can be formed in the neuroeffector junction due to release of the cotransmitter ATP, which can be rapidly metabolized by nucleotidases to adenosine (64). If this is the main source of adenosine that participates in renal sympathetic neurotransmission, then blockade of A1 receptors should attenuate vasoconstrictor responses to RSNS (which would release ATP) but might not attenuate vasoconstrictor responses to exogenous NE (which would not release cotransmitters and therefore adenosine levels might not be elevated). To test this prediction, perfused kidneys were subjected to RSNS at 3, 5, and 7 Hz (5-min stimulation periods) and to exogenous NE (5-min infusions) at 100, 175, and 275 nmol/l in the absence and then presence of DPCPX (100 nmol/l). Responses were elicited at 10-min intervals.
Protocol 7.
Because we did not observe any effects of the A1 antagonists on NE release, we hypothesized that the A1 receptor participates in renal sympathetic neurotransmission via coincident signaling with NE (i.e., we postulated that A1 receptor activation enhances NE-induced renal vasoconstriction). To test this hypothesis, renovascular responses to exogenous NE were elicited with 5-min intrarenal artery infusions of increasing concentrations of NE to find a concentration of NE that would increase perfusion pressure ∼20 mmHg. The average concentration of NE required to achieve basal responses in this range was 199 ± 11 nmol/l. Next, the concentration of NE that increased renal perfusion pressure to the desired target range was administered to the kidneys six times (5-min infusions with 10-min rest periods between responses). The first three responses were basal responses in the absence of any treatments to establish the reproducibility of the NE-induced renal vasoconstriction. Next, in two groups of kidneys, CCPA [a highly selective A1 receptor agonist (37)] was infused into the renal artery to achieve increasing concentrations of CCPA of 1, 3, and 10 nmol/l, and responses to NE were elicited 10 min into the infusion of each concentration of CCPA. One of these two groups was cotreated with DPCPX (1,000 nmol/l; CCPA + DPCPX group), whereas the other only received CCPA (CCPA group). In a third group, neither CCPA nor DPCPX was administered, yet responses to NE were elicited every 10 min (time control group).
Protocol 8.
Our previous work indicates that in the renal microcirculation, the mechanism by which Gi pathway activators augment vasoconstrictor responses to Gq pathway activators is via coincident signaling at the level of PLC leading to activation of the PKC → c-src → PI3K pathway (33). To test whether this mechanism of coincident signaling mediates the ability of A1 receptors [which are coupled to Gi (49)] to enhance renovascular responses to NE [which are mediated by α1-adrenoceptors which are coupled to Gq (9)], kidneys were pretreated with either U73122 [1 μmol/l; phospholipase C inhibitor (3)], U73343 [1 μmol/l; inactive analog of U73122 (50)], GF109203X [3 μmol/l; protein kinase C inhibitor (38)], PP1 [1 μmol/l; c-src inhibitor (22)], or wortmannin [0.2 μmol/l; PI3K inhibitor (61)]. Because 3-phosphoinositide-dependent protein kinase-1 (PDK1) is activated by PI3K (48), we also examined the effects of OSU-03012 [0.5 μmol/l; inhibitor of PDK1 (66)]. After 15 min of pretreatment, renovascular responses to exogenous NE were elicited with 5-min intrarenal artery infusions of increasing concentrations of NE to find a concentration of NE that would increase perfusion pressure ∼20 mmHg. Next, the concentration of NE that increased renal perfusion pressure to the desired target range was administered to the kidneys (5-min infusion). This response was a basal response in the presence of pretreatment with the inhibitors but in the absence of CCPA. Next, CCPA was infused into the renal artery to achieve a concentration of CCPA of 3 nmol/l and responses to NE were again elicited 10 min into the infusion of CCPA.
Protocol 9.
Our previous work demonstrates that in both the isolated, perfused rat kidney (46) and mouse kidney (52), RSNS increases the release of adenosine into the renal venous perfusate. However, we have not yet examined the effects of exogenous NE on adenosine release from isolated, perfused kidneys. Therefore, in protocol 9, NE we infused into the renal artery for 5 min at 175 nmol/l, and renal venous perfusate was collected during the last min of the treatment. After a rest period of 10 min, this protocol was repeated with a higher concentration of NE (275 nmol/l).
Statistics.
Data were analyzed by one- or two-factor ANOVA, with post hoc comparisons using a Fisher's least significant difference (LSD) test or by Student's unpaired or paired t-test as appropriate. The criterion of significance was P < 0.05. All values in text and figures are means ± SE.
RESULTS
Protocol 1.
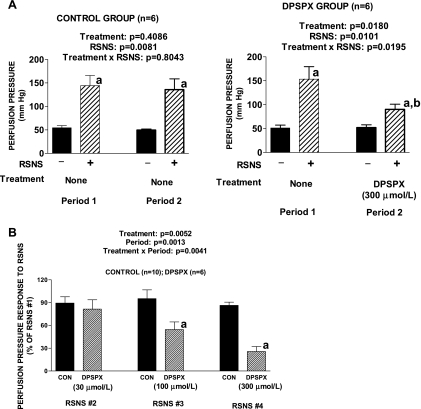
DPSPX is an adenosine receptor antagonist that does not penetrate cell membranes and therefore does not inhibit intracellular phosphodiesterases. In the DPSPX group, the perfusion pressure response to RSNS was significantly reduced in the presence of DPSPX (Fig. 1A) and this effect was not due to degradation in the responsiveness of the preparation as evidenced by the fact that responses in the control group were maintained. DPSPX did not affect baseline perfusion pressure.
Fig. 1.
A: bar graphs show increases in renal perfusion pressure in response to renal sympathetic nerve stimulation (RSNS) during 2 experimental periods. Left graph: both RSNS periods were in the absence of any treatment. Right graph: 1,3-dipropyl-8-p-sulfophenylxanthine (DPSPX; 300 μmol/l) was added to the perfusate between the first and second periods. aP < 0.05 for − vs. + in the same period; bP < 0.05 for + in period 1 vs. + in period 2. B: bar graph shows effects of increasing concentrations of DPSPX on perfusion pressure responses to RSNS. The experiment entailed 4 periods of RSNS, and in the DPSPX group, but not the control (CON) group, DPSPX was added to the perfusate between the first and second periods, second and third periods, and third and fourth periods. The responses to RSNS during the second, third, and fourth periods are expressed as a percentage of the basal response during the first period in the absence of any treatments. Basal perfusion pressures (before treatments) were 58 ± 4 and 64 ± 5 mmHg for the CON group and DPSPX group, respectively. Basal perfusion pressures were not affected by the DPSPX. RSNS responses during the first period (before treatments) were 71 ± 5 and 76 ± 13 mmHg for the CON group and DPSPX group, respectively. aP < 0.05 compared with CON group in corresponding RSNS period. A and B: P values in panels are from 2-factor ANOVA and all values represent means ± SE for the indicated number of experiments (n).
Protocol 2.
Responses to RSNS (expressed as a percentage of the initial response) were stable in the control group, whereas a concentration-dependent reduction in responses to RSNS was observed in the DPSPX group (Fig. 1B). DPSPX did not affect baseline perfusion pressures.
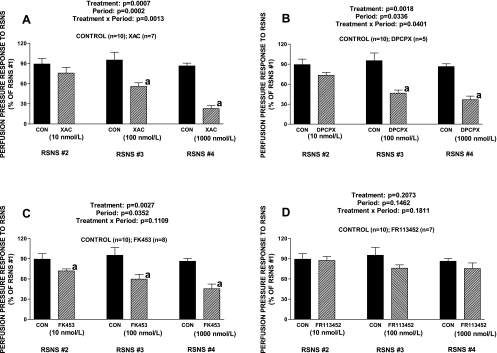
Protocol 3.
XAC (xanthine derivative selective for the A1 receptor), DPCPX (xanthine derivative highly selective for the A1 receptor), and FK453 (nonxanthine derivative highly selective for the A1 receptor) inhibited responses to RSNS (Fig. 2, A, B, and C, respectively) and the inhibition was similar to that observed for DPSPX. In contrast to FK453, FR113452 (enantiomer of FK453 that does not block A1 receptors) did not significantly alter vasoconstrictor responses to RSNS (Fig. 2D). Neither XAC, DPCPX, FK453, nor FR113452 affected baseline perfusion pressures.
Fig. 2.
Bar graphs show effects of increasing concentrations of xanthine amine congener (XAC; A), 1,3-dipropyl-8-cyclopentylxanthine (DPCPX; B), FK453 (C), or FR113452 (D) on perfusion pressure responses to RSNS. The experiment entailed 4 periods of RSNS, and in the XAC, DPCPX, FK453, or FR113452 groups, but not the CON group, XAC, DPCPX, FK453, or FR113452 was added to the perfusate between the first and second periods, second and third periods, and third and fourth periods. The responses to RSNS during the second, third, and fourth periods are expressed as a percentage of the basal response during the first period in the absence of any treatments. Basal perfusion pressures (before treatments) were 58 ± 4, 52 ± 3, 53 ± 1, 55 ± 3, and 55 ± 2 mmHg for the CON group, XAC group, DPCPX group, FK453 group, and FR113452 group, respectively. Basal perfusion pressures were not affected by either XAC, DPCPX, FK453, or FR113452. RSNS responses during the first period (before treatments) were 71 ± 5, 69 ± 16, 101 ± 9, 86 ± 7, and 87 ± 9 mmHg for the CON group, XAC group, DPCPX group, FK453 group, and FR113452 group, respectively. aP < 0.05 compared with CON group in corresponding RSNS period. The P values in the figure are from 2-factor ANOVA. Values represent means ± SE for the indicated number of experiments (n).
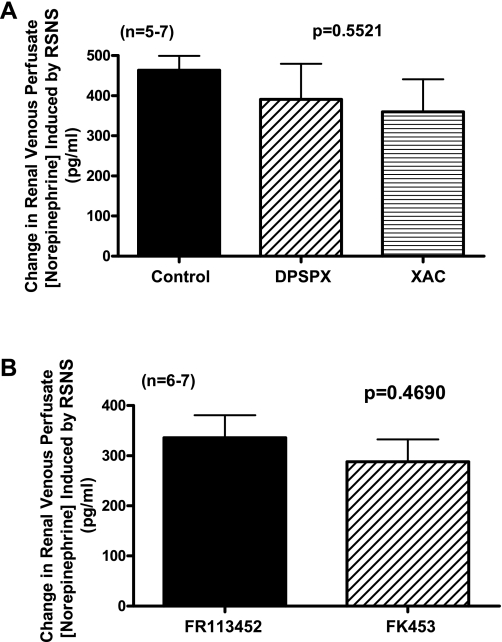
Protocol 4.
RSNS caused a robust increase in the concentration of NE in the renal venous perfusate of control kidneys, and this response was not altered by high concentrations of either DPSPX or XAC (Fig. 3A). As shown in Fig. 3B, the increase in NE concentration in the renal venous perfusate induced by RSNS was similar and not significantly different in FR113452-treated vs. FK453-treated kidneys.
Fig. 3.
A: bar graph shows effects of RSNS on the renal venous perfusate concentration of norepinephrine in nontreated kidneys (CON) and in kidneys treated with either DPSPX (300 μmol/l) or XAC (1,000 nmol/l). The P value is from 1-factor ANOVA comparing CON vs. DPSPX vs. XAC groups. Basal levels of norepinehprine before RSNS in the CON, DPSPX-treated, and XAC-treated groups were not significantly different (55 ± 23, 93 ± 24, and 164 ± 68 pg/ml, respectively). B: bar graph shows effects of RSNS on the renal venous perfusate concentration of norepinephrine in kidneys treated with either FR113452 (1,000 nmol/l) or FK453 (1,000 nmol/l). Basal levels of norepinehprine before RSNS in the FR113452-treated and FK452-treated groups were not significantly different (96 ± 19 and 52 ± 20 pg/ml, respectively). The P value is from Student's unpaired t-test comparing FR113452 vs. FK453 groups. For both panels, values represent means ± SE for the indicated number of experiments (n).
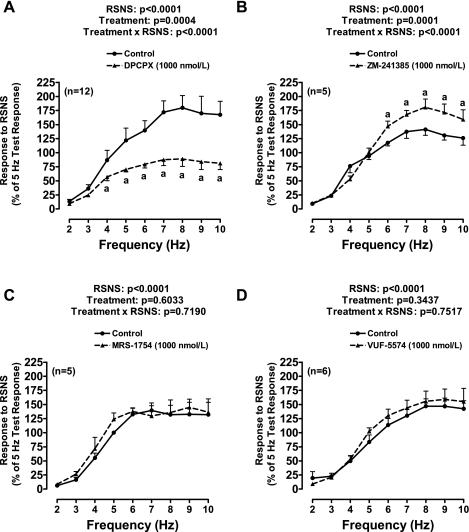
Protocol 5.
DPCPX (Fig. 4A) significantly attenuated responses to RSNS; in contrast, ZM-241385 (selective A2A receptor antagonist) increased responses to RSNS at high frequencies of stimulation (Fig. 4B). Neither MRS-1754 (selective A2B receptor antagonist) nor VUF-5574 (selective A3 receptor antagonist) significantly affected vasoconstrictor responses induced by RSNS (Fig. 4, C and D, respectively). Neither DPCPX, ZM-241385, MRS-1754, nor VUF-5574 affected baseline perfusion pressures.
Fig. 4.
Line graphs show effects of DPCPX (A), ZM-241385 (B), MRS-1754 (C), or VUF-5574 (D) on perfusion pressure responses to RSNS. These experiments employed a paired experimental design in which both kidneys from the same rat were removed and perfused separately but simultaneously. Of each pair, 15 min before initiating RSNS, one kidney received vehicle only (DMSO) and the other kidney received one of the four antagonists (1,000 nmol/l). A frequency-response relationship was elicited by stimulating at 2, 3, 4, 5, 6, 7, 8, 9, and 10 Hz for 2 min at 15-min intervals. Responses were normalized to a 5-Hz test response that was elicited before drug treatments. A: basal perfusion pressures (before treatments) were 51 ± 2 and 53 ± 2 mmHg for the CON group and DPCPX group, respectively. Basal perfusion pressures were not affected by DPCPX. Test RSNS responses (before treatments) were 75 ± 6 and 85 ± 10 mmHg for the CON group and DPCPX group, respectively. B: basal perfusion pressures (before treatments) were 50 ± 2 and 46 ± 4 mmHg for the CON group and ZM-241385 group, respectively. Basal perfusion pressures were not affected by ZM-241385. Test RSNS responses (before treatments) were 82 ± 12 and 83 ± 15 mmHg for the CON group and ZM-241385 group, respectively. C: basal perfusion pressures (before treatments) were 45 ± 2 and 48 ± 3 mmHg for the CON group and MRS-1754 group, respectively. Basal perfusion pressures were not affected by MRS-1754. Test RSNS responses (before treatments) were 97 ± 4 and 111 ± 6 mmHg for the CON group and MRS-1754 group, respectively. D: basal perfusion pressures (before treatments) were 47 ± 2 and 44 ± 2 mmHg for the CON group and VUF-5574 group, respectively. Basal perfusion pressures were not affected by VUF-5574. Test RSNS responses (before treatments) were 105 ± 20 and 102 ± 20 mmHg for the CON group and VUF-5574 group, respectively. aP < 0.05 compared with CON group at corresponding frequency of RSNS. The P values in the figure are from repeated-measures 2-factor ANOVA. Values represent means ± SE for the indicated number of experiments (n).
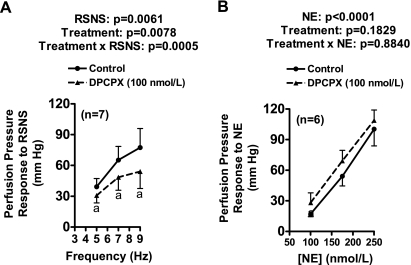
Protocol 6.
As shown in Fig. 5, DPCPX significantly inhibited responses to RSNS (Fig. 5A) but not to exogenous NE (Fig. 5B). DPCPX did not affect baseline perfusion pressure.
Fig. 5.
Line graphs show effects of DPCPX on frequency-dependent perfusion pressure responses to RSNS (A) and concentration-related perfusion pressure responses to exogenous norepinephrine (NE; B). Basal perfusion pressure was 63 ± 3 mmHg and was unaffected by DPCPX. aP < 0.05 compared with CON group at corresponding RSNS frequency. The P values in panels are from 2-factor ANOVA. Values represent means ± SE for the indicated number of experiments (n).
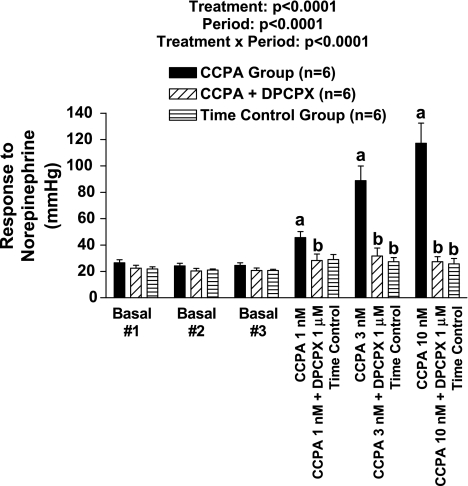
Protocol 7.
As shown in Fig. 6, basal responses to exogenous NE were stable during the first three basal periods in all three groups. In the CCPA (highly selective A1 receptor agonist) group, CCPA concentration dependently enhanced the vasoconstrictor response to NE such that at 10 nmol/l, the NE response was augmented fivefold. CCPA per se did not alter baseline perfusion pressure. In the presence of DPCPX, CCPA did not affect the responses to NE, and the responses to NE were stable in the time control group.
Fig. 6.
Bar graph shows perfusion pressure responses to exogenous norepinephrine before (basal) and during treatment with increasing concentrations of 2-chloro-N6-cyclopentyladenosine (CCPA) with or without cotreatment with DPCPX. Some kidneys were not treated with either CCPA or DPCPX (time control). Basal perfusion pressures (before treatments) were 57 ± 4, 61 ± 2, and 60 ± 3 mmHg in the CCPA group, CCPA + DPCPX group, and time control group, respectively, and were not affected by the treatments. aP < 0.05 compared with the third baseline response (basal #3) of CCPA group. bP < 0.05 compared with responses in CCPA group during corresponding period. The P values in graph are from 2-factor ANOVA. Values represent means ± SE for the indicated number of experiments (n).
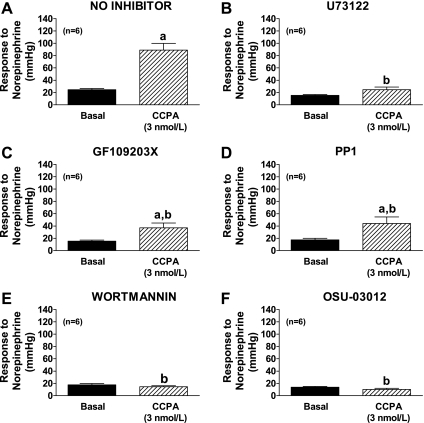
Protocol 8.
CCPA (3 nmol/l) in the absence of inhibitors enhanced the vasoconstrictor response to NE (Fig. 7A). CCPA per se did not alter baseline perfusion pressure. The ability of CCPA to enhance the vasoconstrictor response to NE was attenuated in the presence of U73122 (phospholipase C inhibitor), GF109203X (protein kinase C inhibitor), PP1 (c-src inhibitor), wortmannin (PI3K inhibitor), or OSU-03012 (inhibitor of PDK1; Fig. 7, B, C, D, E, and F, respectively). In contrast to these inhibitors, neither U73343 (inactive analog of U73122) nor the antioxidant apocynin (10 μmol/l) attenuated the ability of CCPA to enhance NE-induced vasoconstriction [perfusion pressure response to NE: in absence of inhibitors, 24 ± 2 and 89 ± 11 mmHg before and during CCPA, respectively (n = 6); in U73343-treated kidneys; 21 ± 3 and 96 ± 8 mmHg before and during CCPA, respectively (n = 3); in apocynin-treated kidneys; 27 ± 1 and 116 ± 19 mmHg before and during CCPA, respectively (n = 6)].
Fig. 7.
Bar graphs show perfusion pressure responses to exogenous norepinephrine before (basal) and during treatment with CCPA (3 nmol/l) without (A) or with pretreatment with either U73122 (1 μmol/l; B), GF109203X (3 μmol/l; C), PP1 (1 μmol/l; D), wortmannin (0.2 μmol/l; E), or OSU-03012 (0.5 μmol/l; F). Basal perfusion pressures (before treatments) were 54 ± 4, 47 ± 3, 51 ± 2, 49 ± 2, 43 ± 1, and 40 ± 2 mmHg in the no inhibitor group, U73122 group, GF109203X group, PP1 group, wortmannin group, and OSU-03012 group, respectively, and were not affected by the treatments. aP < 0.05 compared with basal response in same group. bP < 0.05 compared with responses in presence of CCPA in no inhibitor group. Values represent means ± SE for the indicated number of experiments (n).
Protocol 9.
NE at 175 and 275 nmol/l increased perfusion pressure from 50 ± 4 to 59 ± 7 and to 90 ± 9 mmHg, respectively (n = 6). However, NE did not significantly (P = 0.2400) increase adenosine levels (basal levels of adenosine were 22 ± 4 ng/ml; in the presence of 175 ng/l of NE, adenosine levels were 27 ± 5 ng/ml; in the presence of 275 nmol/l of NE, adenosine levels were 30 ± 5 ng/ml).
DISCUSSION
The present study supports the conclusion that endogenous adenosine, via agonism of A1 receptors, contributes to renal sympathetic neurotransmission. The evidence for this conclusion is that nonselective blockade of cell surface adenosine receptors with DPSPX and selective antagonism of A1 receptors with three different antagonists (XAC, DPCPX, and FK453) attenuates vasoconstrictor responses to RSNS. DPSPX, XAC, and DPCPX are xanthine derivatives, and therefore it is conceivable that they share off-target effects due to the xanthine component of their chemical structure that accounts for their ability to attenuate responses to RSNS. However, the fact that FK453, a nonxanthine drug, also inhibits RSNS responses makes this possibility remote. Moreover, the possibility of off-target effects of FK453 is remote because FR113452, the enantiomer of FK453 that is inactive at A1 receptors, does not attenuate vasoconstrictor responses to RSNS. Finally, because DPSPX does not penetrate cell membranes, it is unlikely that inhibition of intracellular phosphodiesterases contributes to the observed effects of the antagonists.
The mechanism by which endogenous adenosine via the A1 receptor facilitates renal sympathetic neurotransmission does not involve prejunctional effects. This conclusion is based on our findings that neither DPSPX nor XAC nor FK453 alters the spillover of endogenous NE into the renal venous perfusate. Although activation of prejunctional A1 receptors can attenuate NE release from sympathetic nerve varicosities (16), the role of endogenous adenosine in this regard in intact organ systems is controversial. For example, our previous studies demonstrate that although exogenous adenosine can inhibit noradrenergic neurotransmission in the in situ blood-perfused rat mesentery, antagonism of adenosine receptors with DPSPX does not alter noradrenergic neurotransmission (32, 41). Therefore, with regard to the peripheral sympathetic nervous system, the prejunctional effect of A1 receptor activation is likely more of pharmacological interest, rather than physiological importance.
Most likely the mechanism by which endogenous adenosine participates in renal sympathetic neurotransmission involves coincident signaling at the postjunctional membrane. Coincident signaling is the convergence of signaling pathways such that one pathway augments the effects of the other pathway because of synergistic actions on a protein coincident detector (54). In this regard, A1 receptor activation is known to augment angiotensin II-induced renal vasoconstriction (42, 47). Since both NE and angiotensin II signal via Gq-coupled receptors [α1-adrenoceptors (9) and angiotensin II AT1 receptors (44), respectively], the A1 receptor would also likely enhance NE-induced renal vasoconstriction. Also, A1 receptors are Gi-coupled receptors (49), and studies by our lab demonstrate coincident signaling between Gq-coupled receptors (for example, AT1 receptors and vasopressinV1 receptors) and Gi-coupled receptors (for example, Y1 receptors and α2-adrenoceptors) in the renal microcirculation resulting in potentiation of Gq-induced renal vasoconstriction by the Gi signal transduction pathway (10, 11, 17). Thus, taken together, this line of reasoning would predict coincident signaling in the renal microcirculation between released adenosine, via A1 receptor-induced activation of Gi, and released NE, via α1-adrenoceptor-induced activation of Gq. Therefore, blockade of A1 receptors would be expected to inhibit RSNS-induced renal vasoconstriction by inhibiting coincident signaling by postjunctional receptors. In support of this conclusion, our results show that CCPA, a highly potent and highly selective A1 receptor agonist, causes a profound increase in the vasoconstrictor response to exogenous NE. Also, our previous work indicates that in the renal microcirculation, the mechanism by which Gi pathway activators augment vasoconstrictor responses to Gq pathway activators is via coincident signaling at the level of PLC leading to activation of the PKC → c-src → PI3K pathway (33). The present study demonstrates that inhibition of each of the steps in this pathway (PLC, PKC, c-src, and PI3K) blocks the CCPA-induced augmentation of renovascular responses to NE. Moreover, blockade of PDK1, the major downstream transducer for PI3K (48), also abolishes CCPA-induced enhancement of renovascular responses to NE. These findings are highly supportive of the following mechanism for the coincident signaling pathway between A1 receptors and NE: PLC → PKC → c-src → PI3K → PDK1. Also, these findings are entirely consistent with the conclusions of Hansen et al. (23) that A1-induced renal vasoconstriction in mouse afferent arterioles is mediated by Gi pathway-induced activation of PLC.
The results of the present study support the concept that adenosine participates in renal sympathetic neurotransmission via A1 receptors primarily by augmenting NE-induced vasoconstriction via coincident signaling, rather than by “direct” vasoconstriction independent of NE. This conclusion is based on the observations that CCPA, a potent and selective A1 receptor agonist, does not per se affect baseline perfusion pressure in the isolated, perfused rat kidney at 1, 3, or 10 nmol/l; yet CCPA remarkably enhances the vasoconstrictor response to NE at these same concentrations. In support of this concept, our previous work demonstrates that in the isolated, perfused rat kidney blockade of α1-adrenoceptors with either prazosin or phentolamine eliminates vasoconstrictor responses to RSNS at 3 to 9 Hz (for 3 min) (46). If adenosine in the neuroeffector junction were causing direct vasoconstriction, then RSNS at these frequencies should induce a response, albeit reduced, in the presence of α1-adrenoceptor blockade.
Interestingly, in isolated, perfused mouse arterioles, A1 receptor activation causes profound vasoconstriction (23), suggesting “direct” vasoconstriction. Importantly, even in this model system, A1-induced renal vasoconstriction is mediated by βγ-subunit-induced activation of PLC (23). As reviewed by Selbie and Hill (54), β subunits released by Gi-coupled receptors can synergize with αq subunits released by Gq-coupled receptors to cause a more robust activation of PLC (most likely PLC-β isoforms) and its downstream transducer molecules including PKC. Although in reconstituted systems (55) or overexpressing systems (67) β subunit directly stimulates PLC-β, in tissues and cells under physiological conditions, Gi-coupled receptors generally exert minimal “direct” PLC activation per se, but rather significantly enhance the effects of Gq-coupled receptors on PLC activation via a mechanism that is inhibited by pertussis toxin and β subunit scavengers (54). The mechanism of this interaction may in part be due to β subunits inhibiting the ability of PLC-β to activate the GTPase activity of αq (5).
We hypothesize that A1 receptors require coincident signaling to cause potent vasoconstriction and that the partners in the coincident signaling process can be many different endogenous agonists that also activate PLC depending on the physiological context. So the point is not that the interaction is specific, but that it is important in the renal sympathetic neuroeffector junction. With regard to renal sympathetic neurotransmission, NE would be the primary coincident signaling partner simply because NE is released into and achieves high concentrations in the same biophase in which adenosine is formed. Also, although we did not measure or control angiotensin II in the present study, the fact that α1-adrenoceptor blockade abolishes RSNS responses eliminates the possibility that adenosine is enhancing neurotransmission in the isolated, perfused rat kidney by coincident signaling with angiotensin II. This is expected since in the isolated, perfused rat kidney renin substrate is not provided and any released renin would not recirculate to the preglomerular microcirculation. However, in vivo it is entirely possible that coincident signaling occurs between A1 receptors and AT1 receptors due to sympathetically driven renin release and accumulation of renin with subsequent formation of angiotensin II.
Adenosine in the neuroeffector junction could arise from adenosine production triggered by NE acting on α-adrenoceptors or β-adrenoceptors on postjunctional cell surfaces. Indeed, in the isolated, perfused rat kidney blockade of adrenergic receptors reduces RSNS-induced renal venous adenosine and inosine secretion (46). Alternatively, as Westfall and co-workers (64) elegantly demonstrated, adenosine can be formed in the neuroeffector junction due to release of the cotransmitter ATP, which can be rapidly metabolized by releasable nucleotidases (which undergo exocytosis along with ATP) to adenosine (the Westfall mechanism). If the main source of the adenosine that participates in renal sympathetic neurotransmission is due to NE-induced activation of postjunctional adrenergic receptors, then blockade of A1 receptors might be expected to attenuate vasoconstrictor responses to both exogenous NE and RSNS. On the other hand, if the main source of adenosine is via exocytosis of ATP and nucleotidases followed by metabolism of ATP to adenosine, then blockade of A1 receptors would attenuate vasoconstrictor responses to RSNS but would not attenuate vasoconstrictor responses to exogenous NE (which would not release cotransmitters and therefore not engage the Westfall mechanism). Our results are consistent with the Westfall mechanism because DPCPX attenuates vasoconstrictor responses to RSNS, but not to exogenous NE, and exogenous NE does not siginificantly increase adenosine production by the isolated, perfused kidney. However, it is also conceivable that the differential effects of A1 receptor antagonism on responses to exogenous NE vs. RSNS could be due to relatively selective expression of vasoconstrictor A1 receptors in the postjunctional surface of the neuroeffector junction vs. relatively selective expression of vasodilatory A2A (4) and A2B (15) receptors (which would counteract the effects of A1 receptors) in the noninnervated vascular muscle cell membranes in the kidney microcirculation. Because exogenously administered NE would mostly engage noninnervated vascular smooth muscle cell surfaces, whereas NE released from sympathetic nerve terminals would stimulate mostly innervated vascular smooth muscle cell surfaces, this too could explain the fact that DPCPX attenuates responses to RSNS, but not to exogenous NE. Finally, it is conceivable that NE stimulates postjunctional release of adenosine in the renal neuroeffector junction via α-adrenoceptors or β-adrenoceptors, but does so to a much lesser extent outside of the neuroeffector junction. These three possible explanations for why A1 antagonism reduces responses to RSNS, but not responses to exogenous NE, are not mutually exclusive.
The present study also shows that blockade of A2B and A3 receptors has little if any effect on renal sympathetic neurotransmission. In contrast, antagonism of A2A receptors augments responses to RSNS. This is not unexpected because renal A2A receptors are vasodilatory (4) and blockade of A2A receptors would be expected to enhance responses to RSNS.
In addition to adenosine/A1 receptor interactions, it is conceivable that ATP/P2 receptor interactions also participate in renal sympathetic neurotransmission; the present study does not address this possibility. In this regard, ATP directly activates P2 receptors, and agonism of some P2 receptor subtypes elicits intense renal vasoconstriction (20, 21). For example, by contracting renal vascular smooth muscle cells in afferent arterioles, ATP participates in renal autoregulation via activation of P2X1 receptors (29). Presently, however, the literature regarding the role of ATP/P2 receptor interactions in renal sympathetic neurotransmission is sparse and contradictory, with some investigators concluding that ATP, via P2 receptors, is a direct cotransmitter in the renal sympathetic nervous system (62), while others conclude that ATP/P2 receptor interactions do not participate in renal sympathetic neurotransmission (14, 53).
The advantage of the isolated, Tyrode's-perfused kidney system is the absence of variables that could be affected by treatments and cloud data interpretation. Nonetheless, there are caveats with this model system. First, basal perfusion pressures are lower than arterial pressure because the kidneys are perfused with Tyrode's solution, which has much lower viscosity compared with blood (2). Despite this, the isolated, perfused rat kidney is characterized by high vascular sensitivity to vasoconstrictors (17, 36, 51) and the vascular response of the preparation is similar to that observed in vivo (34). Second, Tyrode's solution is a poor oxygen carrier. However, the preparation is stable with regard to reproducible responses and the basal secretion of purines is low (46), suggesting that impaired oxygen delivery is not influencing vascular responses or adenosine production. Third, reactive oxygen species may be formed in the perfusate or by the kidney; however, in the present study addition of the antioxidant apocynin did not alter the ability of CCPA to enhance vasoconstrictor response to NE, suggesting that the results were not an artifact of oxidative stress.
In two phase II clinical trials, the A1 receptor antagonist rolofylline (KW-3902) demonstrated promise as a diuretic in acute decompensated heart failure with renal impairment or diuretic resistance (6, 19). Similarly, in a third phase II trial in patients with chronic congestive heart failure, rolofylline exerted beneficial effects (8). Surprisingly, in a large phase III clinical trial in patients with acute heart failure and renal impairment, rolofylline did not significantly affect the primary endpoint, although the percentage of patients experiencing treatment success was significantly increased (43), and a subsequent analysis of this study (45) showed that rolofylline administration was associated with an increase in the proportion of patients showing early relief from dyspnea and with a numerically lower mortality at 14 and 30 days, largely because of reduced heart failure mortality. These studies speak to the need of being able to identify patients most likely to respond to A1 antagonists. This would improve the likelihood of a positive outcome in clinical trials and help select individual patients most likely to benefit from these drugs if and when they are clinically available. The present study suggests that biomarkers of increased renal sympathetic tone may usefully serve this purpose. Finally, the present results suggest that A1 antagonists may be beneficial in renal diseases and hypertension associated with increased renal sympathetic tone.
GRANTS
This study was supported by National Institutes of Health Grants DK091190, HL069846, DK068575, and DK079307.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: E.K.J. and Z.M. conception and design of research; E.K.J., D.C., S.P.T., and Z.M. analyzed data; E.K.J. and S.P.T. interpreted results of experiments; E.K.J. prepared figures; E.K.J. drafted manuscript; E.K.J., D.C., S.P.T., and Z.M. edited and revised manuscript; E.K.J., D.C., S.P.T., and Z.M. approved final version of manuscript; D.C., S.P.T., and Z.M. performed experiments.
Supplementary Material
REFERENCES
- 1. Beavo JA, Reifsnyder DH. Primary sequence of cyclic nucleotide phosphodiesterase isozymes and the design of selective inhibitors. Trends Pharmacol Sci 11: 150–155, 1990 [DOI] [PubMed] [Google Scholar]
- 2. Bekersky I. Use of the isolated perfused kidney as a tool in drug disposition studies. Drug Metab Rev 14: 931–960, 1983 [DOI] [PubMed] [Google Scholar]
- 3. Bleasdale JE, Thakur NR, Gremban RS, Bundy GL, Fitzpatrick FA, Smith RJ, Bunting S. Selective inhibition of receptor-coupled phospholipase C-dependent processes in human platelets and polymorphonuclear neutrophils. J Pharmacol Exp Ther 255: 756–768, 1990 [PubMed] [Google Scholar]
- 4. Carroll MA, Doumad AB, Li J, Cheng MK, Falck JR, McGiff JC. Adenosine2A receptor vasodilation of rat preglomerular microvessels is mediated by EETs that activate the cAMP/PKA pathway. Am J Physiol Renal Physiol 291: F155–F161, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Chidiac P, Ross EM. Phospholipase C-β1 directly accelerates GTP hydrolysis by Gαq and acceleration is inhibited by Gβγ subunits. J Biol Chem 274: 19639–19643, 1999 [DOI] [PubMed] [Google Scholar]
- 6. Cotter G, Dittrich HC, Weatherley BD, Bloomfield DM, O'Connor CM, Metra M, Massie BM. The PROTECT pilot study: a randomized, placebo-controlled, dose-finding study of the adenosine A1 receptor antagonist rolofylline in patients with acute heart failure and renal impairment. J Card Fail 14: 631–640, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Coulson R, Johnson RA, Olsson RA, Cooper DR, Scheinman SJ. Adenosine stimulates phosphate and glucose transport in opossum kidney epithelial cells. Am J Physiol Renal Fluid Electrolyte Physiol 260: F921–F928, 1991 [DOI] [PubMed] [Google Scholar]
- 8. Dittrich HC, Gupta DK, Hack TC, Dowling T, Callahan J, Thomson S. The effect of KW-3902, an adenosine A1 receptor antagonist, on renal function and renal plasma flow in ambulatory patients with heart failure and renal impairment. J Card Fail 13: 609–617, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Docherty JR. Subtypes of functional α1-adrenoceptor. Cell Mol Life Sci 67: 405–417, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dubinion JH, Mi Z, Jackson EK. Role of renal sympathetic nerves in regulating renovascular responses to angiotensin II in spontaneously hypertensive rats. J Pharmacol Exp Ther 317: 1330–1336, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Dubinion JH, Mi Z, Zhu C, Gao L, Jackson EK. Pancreatic polypeptide-fold peptide receptors and angiotensin II-induced renal vasoconstriction. Hypertension 47: 545–551, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Ekas RD, Jr, Steenberg ML, Eikenburg DC, Lokhandwala MF. Presynaptic inhibition of sympathetic neurotransmission by adenosine in the rat kidney. Eur J Pharmacol 76: 301–307, 1981 [DOI] [PubMed] [Google Scholar]
- 13. Ekas RD, Jr, Steenberg ML, Lokhandwala MF. Increased norepinephrine release during sympathetic nerve stimulation and its inhibition by adenosine in the isolated perfused kidney of spontaneously hypertensive rats. Clin Exp Hypertens 5: 41–48, 1983 [DOI] [PubMed] [Google Scholar]
- 14. Eppel GA, Ventura S, Denton KM, Evans RG. Lack of contribution of P2X receptors to neurally mediated vasoconstriction in the rabbit kidney in vivo. Acta Physiol 186: 197–207, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Feng MG, Navar LG. Afferent arteriolar vasodilator effect of adenosine predominantly involves adenosine A2B receptor activation. Am J Physiol Renal Physiol 299: F310–F315, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fredholm BB, Duner-Engstrom M, Fastbom J, Hu PS, van der Ploeg I. Role of G proteins, cyclic AMP, and ion channels in the inhibition of transmitter release by adenosine. Ann NY Acad Sci 604: 276–288, 1990 [DOI] [PubMed] [Google Scholar]
- 17. Gao L, Zhu C, Jackson EK. α2-Adrenoceptors potentiate angiotensin II- and vasopressin-induced renal vasoconstriction in spontaneously hypertensive rats. J Pharmacol Exp Ther 305: 581–586, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Givertz MM, Massie BM, Fields TK, Pearson LL, Dittrich HC. The effects of KW-3902, an adenosine A1 receptor antagonist,on diuresis and renal function in patients with acute decompensated heart failure and renal impairment or diuretic resistance. J Am Coll Cardiol 50: 1551–1560, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Guan Z, Osmond DA, Inscho EW. P2X receptors as regulators of the renal microvasculature. Trends Pharmacol Sci 28: 646–652, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Guan Z, Osmond DA, Inscho EW. Purinoceptors in the kidney. Exp Biol Med (Maywood) 232: 715–726, 2007 [PubMed] [Google Scholar]
- 22. Hanke JH, Gardner JP, Dow RL, Changelian PS, Brissette WH, Weringer EJ, Pollok BA, Connelly PA. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. J Biol Chem 271: 695–701, 1996 [DOI] [PubMed] [Google Scholar]
- 23. Hansen PB, Castrop H, Briggs J, Schnermann J. Adenosine induces vasoconstriction through Gi-dependent activation of phospholipase C in isolated perfused afferent arterioles of mice. J Am Soc Nephrol 14: 2457–2465, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Hedqvist P, Fredholm BB. Effects of adenosine on adrenergic neurotransmission; prejunctional inhibition and postjunctional enhancement. Naunyn Schmiedebergs Arch Pharmacol 293: 217–223, 1976 [DOI] [PubMed] [Google Scholar]
- 25. Hedqvist P, Fredholm BB, Olundh S. Antagonistic effects of theophylline and adenosine on adrenergic neuroeffector transmission in the rabbit kidney. Circ Res 43: 592–598, 1978 [DOI] [PubMed] [Google Scholar]
- 26. Heumuller S, Wind S, Barbosa-Sicard E, Schmidt HHHW, Busse R, Schroder K, Brandes RP. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension 51: 211–217, 2008 [DOI] [PubMed] [Google Scholar]
- 27. Hocher B. Adenosine A1 receptor antagonists in clinical research and development. Kidney Int 78: 438–445, 2010 [DOI] [PubMed] [Google Scholar]
- 28. Hocher B, Heiden S, von Websky K, Arafat AM, Rahnenführer J, Alter M, Kalk P, Ziegler D, Fischer Y, Pfab T. Renal effects of the novel selective adenosine A1 receptor blocker SLV329 in experimental liver cirrhosis in rats. PLos One 6: e17891, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Inscho EW, Cook AK, Clarke A, Zhang S, Guan Z. P2X1 receptor-mediated vasoconstriction of afferent arterioles in angiotensin II-infused hypertensive rats fed a high-salt diet. Hypertension 57: 780–787, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jackson EK. A1 receptor antagonists as diuretic/natriuretic agents. Drugs Future 27: 1057–1069, 2002 [Google Scholar]
- 31. Jackson EK. P1 and P2 receptors in the renal system. In: Handbook of Experimental Pharmacology Volume 151/II; Purinergic and Pryimidinergic Signalling II: Cardiovascular, Respiratory, Immune, Metabolic and Gastrointestinal Tract Function, edited by Abbrachio M, Williams M. Berlin: Springer-Verlag, 2001, p. 33–71 [Google Scholar]
- 32. Jackson EK. Role of adenosine in noradrenergic neurotransmission in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 253: H909–H918, 1987 [DOI] [PubMed] [Google Scholar]
- 33. Jackson EK, Gao L, Zhu C. Mechanism of the vascular angiotensin II/α2-adrenoceptor interaction. J Pharmacol Exp Ther 314: 1109–1116, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Jackson EK, Gillespie DG, Zhu C, Ren J, Zacharia LC, Mi Z. α2-Adrenoceptors enhance angiotensin II-induced renal vasoconstriction: role for NADPH oxidase and RhoA. Hypertension 51: 719–726, 2008 [DOI] [PubMed] [Google Scholar]
- 35. Jackson EK, Mi ZC. Sitagliptin augments sympathetic enhancement of the renovascular effects of angiotensin II in genetic hypertension. Hypertension 51: 1637–1642, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jackson EK, Zhang M, Liu W, Mi Z. Inhibition of renal dipeptidyl peptidase IV enhances peptide YY1–36-induced potentiation of angiotensin II-mediated renal vasoconstriction in spontaneously hypertensive rats. J Pharmacol Exp Ther 323: 431–437, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Jacobson KA, Knutsen LJS. P1 and P2 purine and pyrimidine receptor ligands. In: Purinergic and Pyrmidinergic Signalling I, edited by Abbracchio MP, Williams M. Berlin: Springer-Verlag, 2001, p. 129–175 [Google Scholar]
- 38. Ku WC, Cheng AJ, Wang TC. Inhibition of telomerase activity by PKC inhibitors in human nasopharyngeal cancer cells in culture. Biochem Biophys Res Commun 241: 730–736, 1997 [DOI] [PubMed] [Google Scholar]
- 39. Kuan CJ, Herzer WA, Jackson EK. Cardiovascular and renal effects of blocking A1 adenosine receptors. J Cardiovasc Pharmacol 21: 822–828, 1993 [DOI] [PubMed] [Google Scholar]
- 40. Kuan CJ, Herzer WA, Jackson EK. An experimental paradigm for investigating the role of endogenous adenosine/A1 receptor interactions in vivo. J Pharmacol Exp Ther 263: 657–662, 1992 [PubMed] [Google Scholar]
- 41. Kuan CJ, Jackson EK. Role of adenosine in noradrenergic neurotransmission. Am J Physiol Heart Circ Physiol 255: H386–H393, 1988 [DOI] [PubMed] [Google Scholar]
- 42. Lai EY, Patzak A, Steege A, Mrowka R, Brown R, Spielmann N, Persson PB, Fredholm BB, Persson AEG. Contribution of adenosine receptors in the control of arteriolar tone and adenosine-angiotensin II interaction. Kidney Int 70: 690–698, 2006 [DOI] [PubMed] [Google Scholar]
- 43. Massie BM, O'Connor CM, Metra M, Ponikowski P, Teerlink JR, Cotter G, Weatherley BD, Cleland JGF, Givertz MM, Voors A, DeLucca P, Mansoor GA, Salerno CM, Bloomfield DM, Dittrich HC, Committees PIa. Rolofylline, an adenosine A1 receptor antagonist, in acute heart failure. N Engl J Med 363: 1419–1428, 2010 [DOI] [PubMed] [Google Scholar]
- 44. Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol 292: C82–C97, 2007 [DOI] [PubMed] [Google Scholar]
- 45. Metra M, O'Connor CM, Davison BA, Cleland JGF, Ponikowski P, Teerlink JR, Voors AA, Givertz MM, Mansoor GA, Bloomfield DM, Jia G, DeLucca P, Massie B, Dittrich H, Cotter G. Early dyspnoea relief in acute heart failure: prevalence, association with mortality, and effect of rolofylline in the PROTECT Study. Eur Heart J 32: 1519–1534, 2011 [DOI] [PubMed] [Google Scholar]
- 46. Mi Z, Jackson EK. Effects of alpha- and beta-adrenoceptor blockade on purine secretion induced by sympathetic nerve stimulation in the rat kidney. J Pharmacol Exp Ther 288: 295–301, 1999 [PubMed] [Google Scholar]
- 47. Modlinger PS, Welch WJ. Adenosine A1 receptor antagonists and the kidney. Curr Opin Nephrol Hypertens 12: 497–502, 2003 [DOI] [PubMed] [Google Scholar]
- 48. Mora A, Komander D, van Aalten DMF, Alessi DR. PDK1, the master regulator of AGC kinase signal transduction. Sem Cell Dev Biol 15: 161–170, 2004 [DOI] [PubMed] [Google Scholar]
- 49. Munshi R, Pang IH, Sternweis PC, Linden J. A1 adenosine receptors of bovine brain couple to guanine nucleotide-binding proteins Gi1, Gi2, and Go. J Biol Chem 266: 22285–22289, 1991 [PubMed] [Google Scholar]
- 50. Muto Y, Nagao T, Urushidani T. The putative phospholipase C inhibitor U73122 and its negative control, U73343, elicit unexpected effects on the rabbit parietal cell. J Pharmacol Exp Ther 282: 1379–1388, 1997 [PubMed] [Google Scholar]
- 51. Oellerich WF, Malik KU. Neuropeptide Y modulates the vascular response to periarterial nerve stimulation primarily by a postjunctional action in the isolated perfused rat kidney. J Pharmacol Exp Ther 266: 1321–1329, 1993 [PubMed] [Google Scholar]
- 52. Ren J, Mi ZC, Jackson EK. Assessment of nerve stimulation-induced release of purines from mouse kidneys by tandem mass spectrometry. J Pharmacol Exp Ther 325: 920–926, 2008 [DOI] [PubMed] [Google Scholar]
- 53. Sehic E, Ruan Y, Malik KU. Attenuation by α,β-methylenadenosine-5′-triphosphate of periarterial nerve stimulation-induced renal vasoconstriction is not due to desensitization of purinergic receptors. J Pharmacol Exp Ther 271: 983–992, 1994 [PubMed] [Google Scholar]
- 54. Selbie LA, Hill SJ. G protein-coupled receptor cross-talk: the fine-tuning of multiple receptor signalling pathways. Trends Pharmacol Sci 19: 87–93, 1998 [DOI] [PubMed] [Google Scholar]
- 55. Smrcka AV, Sternweis PC. Regulation of purified subtypes of phosphatidylinositol-specific phospholipase C β by G protein α and βγ subunits. J Biol Chem 268: 9667–9674, 1993 [PubMed] [Google Scholar]
- 56. Takeda M, Yoshitomi K, Imai M. Regulation of Na+-3HCO3− cotransport in rabbit proximal convoluted tubule via adenosine A1 receptor. Am J Physiol Renal Fluid Electrolyte Physiol 265: F511–F519, 1993 [DOI] [PubMed] [Google Scholar]
- 57. Tofovic SP, Branch KR, Oliver RD, Magee WD, Jackson EK. Caffeine potentiates vasodilator-induced renin release. J Pharmacol Exp Ther 256: 850–860, 1991 [PubMed] [Google Scholar]
- 58. Tofovic SP, Kusaka H, Pfeifer CA, Jackson EK. Central effects of caffeine on renal renin secretion and norepinephrine spillover. J Cardiovasc Pharmacol 28: 302–313, 1996 [DOI] [PubMed] [Google Scholar]
- 59. Vallon V, Miracle C, Thomson S. Adenosine and kidney function: potential implications in patients with heart failure. Eur J Heart Fail 10: 176–187, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. van Muijlwijk-Koezen JE, Timmerman H, van der Goot H, Menge WM, Frijtag Von Drabbe Kunzel J, de Groote M, Ijzerman AP. Isoquinoline and quinazoline urea analogs as antagonists for the human adenosine A3 receptor. J Med Chem 43: 2227–2238, 2000 [DOI] [PubMed] [Google Scholar]
- 61. Vanhaesebroeck B, Leevers SJ, Ahmadi K, Timms J, Katso R, Driscoll PC, Woscholski R, Parker PJ, Waterfield MD. Synthesis and function of 3-phosphorylated inositol lipids. Annu Rev Biochem 70: 535–602, 2001 [DOI] [PubMed] [Google Scholar]
- 62. Vonend O, Stegbauer J, Sojka J, Habbel S, Quack I, Robaye B, Boeynaems JM, Rump LC. Noradrenaline and extracellular nucleotide cotransmission involves activation of vasoconstrictive P2X1,3- and P2Y6-like receptors in mouse perfused kidney. Br J Pharmacol 145: 66–74, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Welch WJ. Adenosine type 1 receptor antagonists in fluid retaining disorders. Expert Opin Investig Drugs 11: 1553–1562, 2002 [DOI] [PubMed] [Google Scholar]
- 64. Westfall DP, Todorov LD, Mihaylova-Todorova ST. ATP as a cotransmitter in sympathetic nerves and its inactivation by releasable enzymes. J Pharmacol Exp Ther 303: 439–444, 2002 [DOI] [PubMed] [Google Scholar]
- 65. Yoneda H, Hisa H, Satoh S. Effects of adenosine on adrenergically induced renal vasoconstriction in dogs. Eur J Pharmacol 176: 109–116, 1990 [DOI] [PubMed] [Google Scholar]
- 66. Zhu J, Huang JW, Tseng PH, Yang YT, Fowble J, Shiau CW, Shaw YJ, Kulp SK, Chen CS. From the cyclooxygenase-2 inhibitor celecoxib to a novel class of 3-phosphoinositide-dependent protein kinase-1 inhibitors. Cancer Res 64: 4309–4318, 2004 [DOI] [PubMed] [Google Scholar]
- 67. Zhu X, Birnbaumer L. G protein subunits and the stimulation of phospholipase C by Gs- and Gi-coupled receptors: lack of receptor selectivity of Galpha(16) and evidence for a synergic interaction between Gbeta gamma and the alpha subunit of a receptor activated G protein. Proc Natl Acad Sci USA 93: 2827–2831, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.