Abstract
We previously demonstrated that there is a paucity of brush-border membrane NHE3 in neonates, the predominant Na+/H+ exchanger in the adult proximal tubule, while NHE8 is relatively highly expressed in neonates compared with adults. We recently showed that metabolic acidosis in neonatal rodents can increase brush-border membrane NHE8 protein expression and Na+/H+ exchange activity. To further examine the regulation of NHE8 by acid, we incubated NRK cells, which express NHE8 but not NHE3, with either acid or control media (6.6 vs. 7.4). There was an increase in Na+/H+ exchanger activity within 6 h of incubation with acid media assessed as the rate of sodium-dependent recovery of pH from an acid load (dpHi/dt). The acid stimulation persisted for at least 24 h. The increase in Na+/H+ exchange activity was paralleled by an increase in surface expression of NHE8, assessed by surface biotinylation and streptavidin precipitation. The increase in both apical membrane NHE8 protein expression and Na+/H+ exchange activity with pH 6.6 media compared with 7.4 media was not affected by actinomycin D or cycloheximide consistent with an increase in surface expression independent of mRNA or protein synthesis. Furthermore, there was no increase in total cellular NHE8 protein abundance or mRNA abundance with acid media. Finally, we demonstrate that the increase in surface expression of NHE8 with acid media was blocked by colchicine and cytochalasin D and mediated by acid increasing the rate of exocytosis. In conclusion, NHE8 surface expression and activity are regulated by acid media by increasing the rate of trafficking to the apical membrane.
Keywords: proximal tubule, acidification, neonate, Na+/H+ exchange
the proximal tubule reabsorbs the majority of filtered bicarbonate and two-thirds of proximal tubule bicarbonate reabsorption is mediated by luminal Na+/H+ exchange. The predominant Na+/H+ exchanger on the apical membrane in adult proximal tubules is NHE3 (9, 26), which is upregulated in response to metabolic acidosis (1, 13, 19, 22, 26). However, not all proximal tubule luminal Na+/H+ activity is due to NHE3. In the neonatal proximal tubule, there is Na+/H+ exchange activity despite a paucity of NHE3 (21). Most importantly, NHE3 null mice have apical membrane Na+/H+ exchange activity demonstrating that another isoform is present on the luminal membrane of the proximal tubule (12).
NHE8 was cloned by Goyal et al. (16) and localized to the apical membrane of the proximal tubule (9, 16, 23). NHE8 is a sodium-dependent proton exchanger that is more sensitive to EIPA than is NHE3 (26, 28). The Na+/H+ exchange activity that is present in neonates at a time of very low NHE3 expression is likely due to NHE8 since NHE8 is highly expressed on the apical membrane of neonatal proximal tubules (9, 23). We showed that NHE8 is a developmental isoform in both mice and rats, where brush-border membrane expression of NHE8 decreases as NHE3 increases during postnatal maturation (9, 23). Adult levels of apical membrane NHE3 and NHE8 expression occur at about the time of weaning.
The neonatal proximal tubule has a lower rate of Na+/H+ exchange activity and bicarbonate transport than the adult proximal tubule (4, 5, 8, 20). We recently showed in both neonatal mice and rats that the rate of Na+/H+ exchange activity can be increased by metabolic acidosis (23). Metabolic acidosis in neonatal rats and mice was accompanied by an increase in both brush-border membrane NHE3 as well as NHE8 expression. However, while there was an increase in total cellular NHE3 protein abundance, that was not true for NHE8 suggesting that trafficking may regulate NHE8's response to metabolic acidosis. The purpose of the present in vitro study was to examine directly whether acid media increases activity and surface expression of NHE8 and whether acid media increases NHE8 exocytosis.
METHODS
Cell culture.
Normal rat kidney cells (NRK cells) were purchased from American Type Culture Collection (Manassas, VA). Cells were cultured in high-glucose DMEM (GIBCO, Grand Island, NY) supplemented with 5% fetal calf serum and 1 mM sodium pyruvate at 37°C in a 95% O2-5% CO2 environment and supplemented with penicillin (100 U/ml) and streptomycin (100 U/ml) as previously described by our laboratory (28). The cells were rendered quiescent when confluent by incubation in serum-free DMEM/Ham's F12 (Sigma, St. Louis, MO) adjusted to a pH of 7.4 for 24 h. They were then washed extensively to remove the serum and incubated in the serum-free DMEM/Ham's F12 that was adjusted to pH 6.6 or maintained at a pH of 7.4. The media was adjusted to an osmolality of 295 mosmol/kgH2O. The bicarbonate concentration of the pH 6.6 media was 5 mM and the 7.4 had a bicarbonate concentration of 19 mM. Unless otherwise specified, all chemicals were obtained from Sigma.
cDNA synthesis and real-time PCR.
Total cellular RNA was isolated from NRK cells using GenElute Mammalian Total RNA Miniprep Kit per the manufacturer's instructions (Sigma). RNA (2 μg) was treated with DNAse I and the product was used to synthesize cDNA using random hexamer primers and reverse transcriptase (Stratagene, La Jolla, CA) at an annealing temperature of 25°C for 10 min, extension at 42°C for 50 min, and termination at 70°C for 15 min.
Real-time PCR was performed using an iCycler PCR Thermal Cycler (Bio-Rad, Hercules, CA) to quantify relative mRNA abundance. Primers for NHE8 were mixed with cDNA and SYBR green master mix (Bio-Rad) using the manufacturer's instructions. The PCR conditions were denaturation at 94°C for 30 s, annealing at 62°C for 30 s, and extension at 72°C for 30 s for 40 cycles. 28s was used as housekeeping gene used to normalize the relative expression of NHE8 using the method described by Vandesompele et al. (24). NHE8 primers were (forward) 5′- AAGCCTATTCTTCCGGTGCAGACA-3′, (reverse) 5′- AGAGAAACAACAGCCACGCTCTCA-3′, and 28S (forward) 5′-TTG AAA ATC CGG GGG AGA G-3′, (reverse) 5′-ACA TTG TTC CAA CAT GCC AG-3′.
SDS-PAGE and immunoblotting.
NRK cells were rinsed with PBS three times followed by lysis with RIPA buffer [150 mM NaCl, 50 mM Tris·HCl (pH 7.4), 5 mM EDTA, 1% Triton X-100, 0.5% deoxycholate, 1% SDS, and 0.5% deoxycholate containing protease inhibitors]. The protein lysates were centrifuged at 14,000 g for 30 min at 4°C and the protein content was determined using the Bradford reaction. Proteins were heated to 37°C for 2 min, separated on a 8% polyacrylamide gel using SDS-PAGE as previously described (14), and transferred to a polyvinylidene diflouride membrane (Immobilon, Millipore, Billerica, MA) at 400 mA at 4°C for 1 h (7, 10, 21). The blots were blocked with Blotto (1% nonfat milk and 0.05% Tween-20 in PBS, pH 7.4) for at least 1 h. The blots were then incubated with a monoclonal NHE8 antibody (a gift from Orson Moe) overnight at 4°C at a 1:5 dilution. The blots were washed in Blotto followed by the addition of the secondary antibody, a horseradish peroxidase-conjugated donkey anti-mouse antibody at 1:10,000 dilution (Santa Cruz Biotechnology). Enhanced chemiluminescence was used to detect bound antibody. In blots where total protein was assessed, equal loading of the samples was verified using an antibody to β-actin at a 1:15,000 dilution (Sigma). Relative protein abundance was assessed using densitometry.
Measurement of NHE8 in total cell lysates.
Confluent NRK cells in 100-mm plates were washed twice with ice-cold PBS and lysed with RIPA lysis buffer [150 mM NaCl, 50 mM Tris·HCl (pH 7.4), 5 mM EDTA, 1% Triton X-100, 0.5% deoxycholate, and 0.1% SDS] containing a protease inhibitor protease cocktail (1:1,000 dilution) and 100 μg/ml phenyl-methane-sulfonyl fluoride. The samples were centrifuged at 13,000 g for 30 min at 4°C and the supernatant was used for immunoblotting using 50 μg of protein per sample. The protein was heated to 60°C for 2 min before SDS-PAGE as described above.
Cell surface NHE8 expression.
The protocol for cell surface biotinylation has previously been described from our laboratory (10). Confluent NRK cells were rinsed with ice-cold PBS. The cells were biotinylated by adding 1.5 mg/ml sulfo-NHS-SS-biotin (Pierce, Rockford, IL) in 10 mM triethanolamine (pH 7.4), 150 mM NaCl, and 2 mM CaCl2 and incubated on a horizontal rotator for 60 min at 4°C. Plates were washed twice with 6 ml of quenching buffer (PBS with 100 mM glycine, 1 mM MgCl2, and 0.1 mM CaCl2,) for 20 min at 4°C. Cells were then washed once more with ice-cold PBS. NRK cells were then lysed with RIPA buffer containing protease inhibitors, centrifuged at 13,000 g, and the supernatant was diluted to 2.5 mg/ml protein with RIPA buffer containing protease inhibitors. The cell lysates containing equal amounts of protein were then incubated overnight with streptavidin-agarose beads (Pierce) at 4°C. Streptavidin-agarose beads were then washed as previously described (10, 28) and biotinylated proteins were heated to 85°C in loading buffer to release the proteins that were then assayed by immunoblot.
In experiments designed to determine whether RNA or protein synthesis was necessary for acid to increase surface NHE8, confluent NRK cells were preincubated with serum-free media containing actinomycin D (5 × 10−6 M) or cycloheximide (10 μg/ml) for 1 h (10, 25). Similarly, in experiments designed to examine whether microtubules or the actin cytoskeleton were involved in trafficking of NHE8, cells were preincubated for 1 h with 10−4 M colchicine or 10−6 M cytochalasin D at pH 7.4 (18, 27). Cells were then exchanged into serum-free media at either pH 7.4 or pH 6.6 containing either actinomycin D, cycloheximide, colchicine, or cytochalasin D and incubated for 24 h. Surface NHE8 was then assayed as above.
NHE8 exocytosis.
The assays used to assess exocytosis have been described previously by our laboratory (10). NRK cells were rinsed with PBS and incubated at 4°C with 1.5 mg/ml sulfo-NHS-SS-acetate (Pierce) in 0.1 M sodium phosphate (pH 7.5) and 0.15 M NaCl three times at 30-min intervals (4°C) on a horizontal rotator. Sulfo-NHS-SS-acetate blocks all cell surface NHS-reactive sites so that NHE8 on the cell surface is blocked. The cells were warmed to 37°C for 24 h in either pH 6.6 or 7.4 media. The newly inserted NHE8, which was previously intracellular, was detected by biotinylation of the new surface proteins, followed by binding to streptavidin-agarose beads and immunoblotting. This assay was also performed in the presence of actinomycin D (5 × 10−6 M).
NHE8 activity assay in NRK cells.
NHE8 activity was measured using the pH-sensitive dye 2′,7′-bis(2-carboxyethyl)-5(6)-carboxyfluorescein (BCECF-AM; Molecular Probes, Eugene, OR), as described previously (15). NRK cells were incubated with 10 μM BCECF-AM (BCECF-acetoxymethyl ester) for 20 min at 37°C, and intracellular pH (pHi) was determined from the ratio of fluorescence (excitation: 500 and 450 nm, emission: 530 nm) in a computer-controlled spectrofluorometer (Photon Technology International, Birmingham, NJ). The 500/450 fluorescence ratio was calibrated to pHi using the K+/nigericin technique (6, 17). Na+/H+ exchange activity was determined as the initial rate of change in pHi after an acid load upon addition of sodium (15, 28). Briefly, NRK cells were initially incubated in sodium-free solution with nigericin (10 μg/ml) in the absence of CO2/HCO3− resulting in cell acidification due to the absence of sodium and the cell-to-bath potassium gradient. The sodium-free solution consisted of (in mM) 115 choline chloride, 5 KCl, 1.54 MgCl2, 1.1 CaCl2, and 30 HEPES. All solutions had pH 7.40 and osmolality 295 mosmol/kgH2O. After a steady-state pH was reached, solution was replaced by sodium-free solution containing albumin (1g/dl) to remove the nigericin. This solution was then replaced by a sodium-free solution without albumin. There was no change in cell pH until the sodium-free solution was replaced by a sodium-containing solution (in mM) 25 NaCl, 90 choline Cl, 5 KCl, 1.54 MgCl2, 1.1 CaCl2, and 30 HEPES. We used choline as a sodium replacement and lowered the sodium concentration to 25 mM NaCl because higher concentrations yielded very fast pH recoveries limiting our ability to discern differences. The resulting sodium-dependent increase in pH was due to Na+/H+ exchange. In some experiments, 10−6 M EIPA was added to the incubation solution to determine whether there was comparable inhibition of Na+/H+ exchange activity in the pH 6.6 and pH 7.4 group.
To validate our findings using another technique, we incubated some cells with the same sodium-containing solution as above except that 20 mM NH4Cl replaced 20 mM NaCl. Addition of NH4Cl led to an increase in intracellular pH. After steady state was achieved, this solution was replaced with a sodium-free solution and pHi decreased rapidly due to dissociation of the NH4+ to NH3 and H+. This solution was then replaced with 25 mM sodium-containing solution and the rate of recovery of pH was determined as above.
Measurement of basal pHi.
NRK cells on coverslips were rendered quiescent after they became confluent in serum-free media with a pH of 7.4 for 24 h after which they were incubated in media with pH of 7.4 or 6.6 for 24 h. The coverslips were incubated with BCECF-containing solution for 20 min after which they were transferred to a cuvette containing serum-free media containing (in mM) 130 NaCl, 30 HEPES, 5 KCl, 5 glucose, 5 alanine, 1.1 calcium chloride, 1.54 MgCl2, 1.5 Na2HPO4, and adjusted to pH 6.6 or 7.4 with hydrochloric acid or sodium hydroxide. The pHi of the cells was then measured using the spectrophotometer.
Determination of buffer capacity.
The buffer capacity was calculated as described previously by Boyarsky et al. (11) in explicit detail. We determined the buffer capacity by measuring the change in pHi in response to a change in intracellular NH4+ using the following equation: β = Δ[NH4+]i/ΔpHi.
Specifically, we assessed the change in pHi after removing 10 mM NH4Cl (NaCl replacement) from the bathing solution initially containing 20 mM NH4Cl.
Statistical analysis.
All data are expressed as means ± SE. Comparisons were made using an unpaired Student's t-test. A P value of ≤0.05 was considered significant.
RESULTS
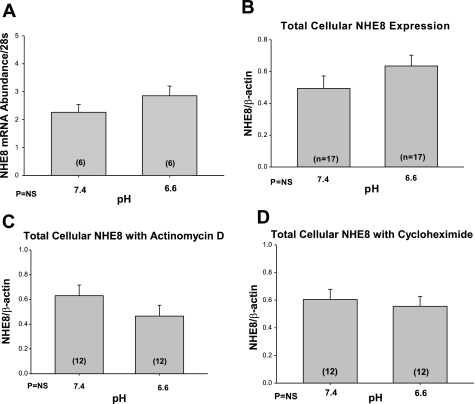
In the first series of experiments, we examined whether NHE8 mRNA in NRK cells was affected by the pH of the media after incubation for 24 h. The pH of the media did cause a change in pHi. After incubation with 7.4 media for 24 h, the pHi was 7.63 ± 0.07 and 6.97 ± 0.10 in cells incubated at pH 6.6 (P < 0.01). As shown in Fig. 1A, there was no difference in NHE8 mRNA expression compared with 28s in NRK cells incubated for 24 h at pH 6.6 compared with 7.4. There was also no effect of media pH on total cellular NHE8 protein abundance (Fig. 1B) in NRK cells incubated for 24 h. This was also true for cells incubated in the presence of actinomycin D or cycloheximide (Fig. 1, C and D, respectively).
Fig. 1.
Effect of acid media on NHE8 mRNA and NHE8 total cellular protein abundance. The effect of media pH (7.4 vs. 6.6) was assayed in normal rat kidney (NRK) cells. NRK cells were incubated at either of the 2 pHs for 24 h. A: effect of media pH on mRNA abundance was assessed using real-time PCR. B: we show that pH did not affect the total cellular NHE8 expression. C and D: cells were incubated at either pH 6.6 or 7.4 in the presence of 5 × 10−6 M actinomycin D or 10 μg/ml cycloheximide. There was no effect of pH on total cellular NHE8 expression in the presence of either actinomycin D or cycloheximide. β-Actin as a loading control for these immunoblots.
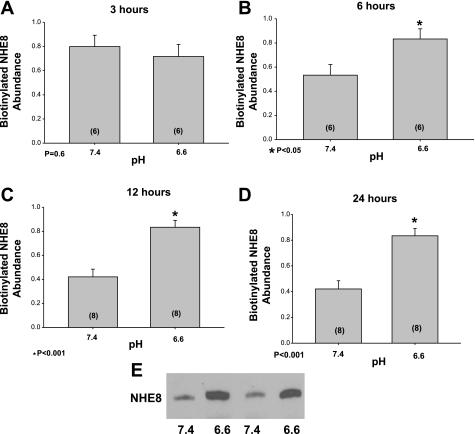
The total cellular NHE8 does not necessarily correlate with the amount of NHE8 on the apical membrane. We used surface biotinylation followed by streptavidin precipitation and immunoblotting to determine whether acid incubation increased surface expression of NHE8. As shown in Fig. 2, there was a time-dependent increase in NHE8 expression on the apical membrane of NRK cells incubated at pH 6.6 compared with media that had a pH of 7.4. The expression was comparable at 3 h, but NHE8 expression was higher at pH 6.6 than 7.4 at 6, 12, and 24 h of incubation.
Fig. 2.
Effect of acid incubation on apical membrane NHE8 protein expression in vitro. NRK cells were incubated for 3, 6, 12, and 24 h in media that was adjusted to either a pH of 7.4 or 6.6 (A: 3 h; B: 6 h; C: 12 h; D: 24 h). Cells were then surface biotinylated and proteins were retrieved using streptavidin precipitation from whole cell lysates and measured using immunoblot analysis. The figure shows the summary of the results showing an increase in NHE8 surface expression with acid incubation starting at 6 h compared with control. E: typical immunoblot at 24 h where there is greater expression of NHE8 in cells incubated at 6.6 compared with 7.4. All blots were relative expression using 1.0 as the highest density band on an individual immunoblot. *Denotes 6.6 different than 7.4.
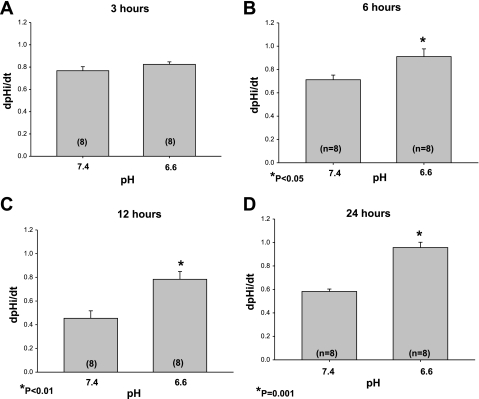
In the next series of experiments, we assessed Na+/H+ exchanger activity by measuring the sodium-dependent recovery from an acid load. As shown in Fig. 3A, there was no difference in dpHi/dt at 3 h in cells incubated at pH 6.6 and 7.4. There was a small but statistically significant increase in Na+/H+ exchanger activity in cells incubated with an acid pH compared with cells incubated at pH 7.4 at 6 h and a more pronounced increase at 12 h which was sustained in cells incubated in an acid medium for 24 h (Fig. 3, B-D). In cells incubated for 24 h, the dpHi/dt was 0.06 ± 0.01 in the 7.4 group and 0.05 ± 0.01 in the presence of 10−6 M EIPA (P = NS), which is comparable to the inhibition we previously found for NHE8 (28). Typical tracings of pH recovery from cell acidification using the nigericin technique are shown in Fig. 4. To validate our findings using another technique, we assessed the rate of recovery from an acid load induced by incubation and removal of 20 mM NH4Cl. The dpHi/dt was 0.04 ± 0.01 in cells incubated at pH 7.4 for 24 h and 0.14 ± 0.05 pH U/min for cells incubated at pH 6.6 (P = 0.01). The difference in rates of recovery from an acid load between the 7.4 and 6.6 group was not due to a difference in buffer capacity as this was comparable in the two groups (3.6 ± 0.3 and 3.3 ± 0.5 for the 7.4 and 6.6 groups, respectively).
Fig. 3.
Effect of acid incubation on Na+/H+ exchange activity in NRK cells. Na+/H+ exchange activity was assessed in NRK cells using the K+/nigericin technique. There was no pH recovery in the absence of sodium. The rate of cell pH was assessed after the addition of 25 mM sodium. The dpHi/dt was comparable at pH 6.6 and 7.4 at 3 h as shown in A. In these experiments, there was no difference in the initial cell pH nor the intracellular pH (pHi) nadir from which pH recovery was measured after addition of sodium. The initial cell pH in the 7.4 group was 7.64 ± 0.01 in the control group and 7.66 ± 0.01 in the 6.6 group. The pHi nadir was 6.64 ± 0.01 and 6.63 ± 0.01 in the 7.4 and 6.6 pH groups, respectively. The rate of recovery from an acid load was faster in cells incubated at pH 6.6 compared with 7.4 for 6, 12, and 24 h as shown in B, C, and D, respectively.
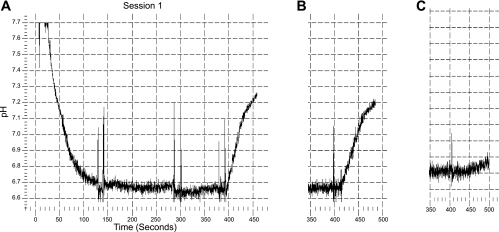
Fig. 4.
Typical tracing of pH recovery after cell acidification. Cells were acidified using a sodium-free solution with nigericin (25–150 s; the technique used in Figs. 3 and 5–8). Cell acidification resulted from the absence of sodium and the cell-to-bath potassium gradient. After a steady-state pH was reached, the solution was replaced by sodium-free solution containing albumin (1 g/dl) to remove the nigericin (150–300 s). This solution was then replaced by a sodium-free solution without albumin (300–400 s). There was no pH recovery in the absence of sodium (300–400 s in A). At 400 s, sodium was added and the rate of recovery of cell pH was measured. dpHi/dt was measured as the initial rate of change in cell pH. A: tracing from cells incubated at pH 6.6. B: recovery from cells at 7.4 which is slower. C: result of a tracing of cells incubated at pH 7.4 where 10−6 M EIPA was in the 0 sodium bathing solution and in the solution containing sodium.
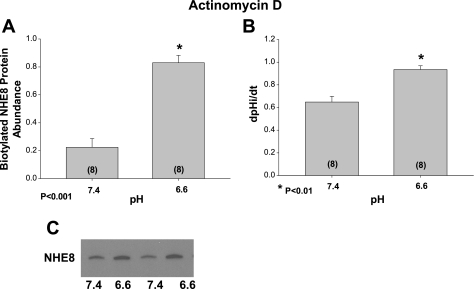
In the next series of experiments, we examined the effect of actinomycin D on surface NHE8 expression and Na+/H+ exchanger activity. NRK cells were incubated at pH 7.4 and 6.6 for 24 h in the presence of actinomycin D to inhibit mRNA synthesis. The stimulation of Na+/H+ exchanger activity and increase in surface NHE8 expression in cells incubated at the lower pH media remained in the presence of actinomycin D. These results are shown in Fig. 5.
Fig. 5.
Effect of acid incubation on apical membrane NHE8 protein abundance and Na+/H+ exchange activity with inhibition of RNA synthesis. Apical membrane NHE8 was assayed using surface biotin labeling and streptavidin precipitation in the presence of either 5 × 10−6 M actinomycin D to inhibit mRNA synthesis or vehicle. Actinomycin D was added 1 h before incubation of cells with the different pHs for 24 h. Apical membrane NHE8 was increased in cells incubated with an acidic media in the presence of actinomycin D as shown in A. A typical immunoblot is shown below. B: effect of actinomycin D on Na+/H+ exchanger activity. Cells incubated at pH 6.6 in the presence of actinomycin D still had a faster dpHi/dt in response to the addition of sodium after cell acidification than cells incubated at pH 7.4. A typical immunoblot is shown in C where the abundance of NHE8 is greater in cells incubated at pH 6.6 compared with 7.4.
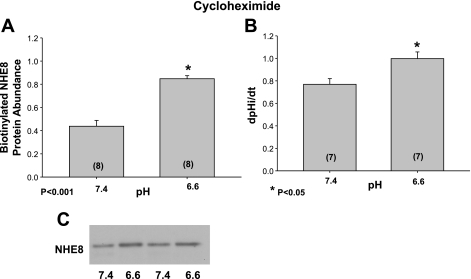
We next examined the effect of media pH on the surface expression of NHE8 as well as Na+/H+ exchanger activity when protein synthesis was inhibited with cycloheximide. These results are shown in Fig. 6. After incubation at pH 6.6 for 24 h in the presence of cycloheximide, the expression of NHE8 was greater than cells incubated at 7.4. Similarly, the Na+/H+ exchanger activity was also faster in cells incubated at pH 6.6 in the presence of cycloheximide compared with that at 7.4. Thus, the effect of acid pH on surface expression of NHE8 and Na+/H+ exchanger activity was not dependent on mRNA or protein synthesis.
Fig. 6.
Effect of acid incubation on apical membrane NHE8 protein abundance and Na+/H+ exchange activity with inhibition of protein synthesis. NRK cells were incubated with 10 μg/ml cycloheximide to inhibit protein synthesis for 1 h and then an additional 24 h at pH 6.6 or 7.4 also in the presence of cycloheximide. A: effect of incubation with the 2 pHs on surface NHE8 expression. A typical immunoblot is shown below. B: effect of the different pH media on Na+/H+ exchanger activity in the presence of cycloheximide. C: typical immunoblot where the protein expression was greater in cells incubated at pH 6.6 compared with cells incubated at 7.4 (both in the presence of cycloheximide).
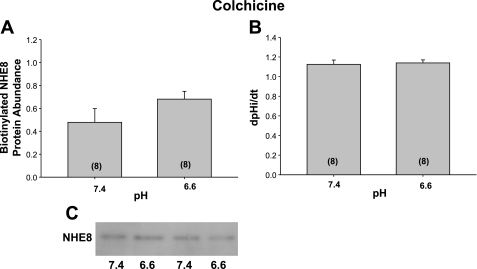
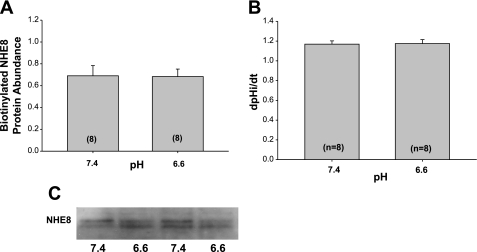
The effect of colchicine, an inhibitor of cytoskeleton microtubule-mediated trafficking, on NHE8 by acid pH was next examined. As shown in Fig. 7, the surface expression of NHE8 and Na+/H+ exchanger activity was comparable after 24 h of incubation at pH 6.6 and 7.4 in the presence of colchicine. This indicates that microtubules are likely involved in the acid-mediated increase in exocytosis by acid pH. The effect of acid on NHE8 surface expression and Na+/H+ exchanger activity was also affected by cytochalasin D, which disrupts the actin cytoskeleton as shown in Fig. 8.
Fig. 7.
Effect of acid pH on NHE8 surface expression and Na+/H+ exchanger activity in the presence of colchicine. NRK cells were incubated with 10−4 M colchicine, which inhibits microtubule polymerization, for 1 h and then an additional 24 h at pH 6.6 or 7.4 also in the presence of colchicine. A: colchicine inhibits the increase in surface expression and B: Na+/H+ exchanger activity by acid media. C: immunoblot showing comparable expression with the 2 pHs in the presence of colchicine.
Fig. 8.
Effect of acid pH on NHE8 surface expression (A) and Na+/H+ exchanger activity (B) in the presence of cytochalasin D. NRK cells were incubated with 1 μM cytochalasin D, which inhibits actin polymerization, for 24 h at pH 6.6 or 7.4. As shown, the acid-induced increase in NHE8 surface expression and Na+/H+ exchanger activity was absent with cytochalasin D. C: immunoblot showing comparable expression with the two pHs wing cytochalasin D in the media.
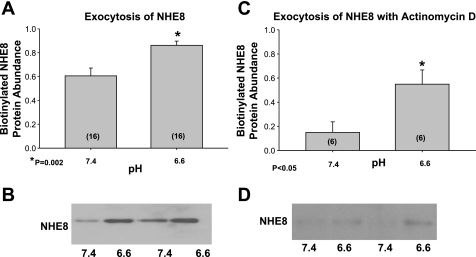
The increased surface expression of NHE8 after incubation with acidic media in the presence of actinomycin D and cycloheximide without an increase in total cellular NHE8 is consistent with an increase in trafficking of NHE8 to the apical membrane. We next examined whether acid incubation affected exocytosis by first labeling the NRK cells with sulfo-NHS-acetate to saturate all the NHS-reactive sites on the apical membrane. We then incubated the cells in either pH 6.6 or 7.4 media and performed surface biotinylation as in methods. The biotinylated surface NHE8 is that which was intracellular at the beginning of the experiment and not accessible to sulfo-NHS-acetate. The NHE8 retrieved by biotinylation is that which was inserted to the apical membrane during the 24-h incubation at pH 7.4 or 6.6. As shown in Fig. 9, A and B, there was greater surface NHE8 in the cells incubated at pH 6.6 than 7.4 consistent with acid media increasing exocytosis of NHE8 at 24 h. As shown in Fig. 9, C and D, there was also an increase in surface expression of NHE8 when cells were incubated in pH 6.6 compared with 7.4 when the exocytosis assay was performed in the presence of actinomycin D.
Fig. 9.
Effect of acid pH on exocytosis of NHE8 in NRK cells. NRK cells were rinsed with sulfo-NHS-SS-acetate to saturate all cell surface NHS-reactive sites. This blocks NHE8 on the apical membrane. The cells were rinsed and then incubated at 37°C for 24 h at either pH 6.6 or 7.4. The newly inserted NHE8 from the intracellular pool was detected by biotinylation of the apical membrane proteins, followed by binding to streptavidin-agarose beads and immunoblotting. A: the new surface NHE8 representing exocytosis is greater at pH 6.6. than 7.4. B: typical immunoblot showing increase in surface expression which is a measure of exocytosis. C: increase in surface expression of NHE8 when cells were incubated in pH 6.6 compared with 7.4 when the exocytosis assay was performed in the presence of actinomycin D. D: typical immunoblot.
DISCUSSION
There are a number of protective mechanisms that occur in response to metabolic acidosis including an increase in proximal tubule bicarbonate reabsorption mediated in large part by an increase in Na+/H+ exchange activity (1, 13, 19, 22, 26). In the adult proximal tubule, apical Na+/H+ exchange is predominantly via NHE3 (9, 23, 26). Na+/H+ exchange in the neonatal proximal tubule is likely predominantly mediated by NHE8, which is highly expressed on the neonatal proximal tubule brush-border membrane and which decreases in abundance at the time of weaning in the rat and mouse concordant with the increase in NHE3 expression (9, 23).
Neonates have a lower serum bicarbonate concentration than adults and are prone to metabolic acidosis due to diseases such as diarrhea. We recently examined whether neonatal mice and rats could respond to a metabolic acidosis with an increase in proximal tubule acidification (23). Both mice and rats were studied at a time when NHE8 was highly expressed on the apical membrane and there was a paucity of NHE3. Three days of acid loading resulted in a 10-meq/l decrease in the serum bicarbonate concentration. There was an increase in Na+/H+ exchanger activity with acid loading in both mice and rats. In rats brush-border membrane vesicles were studied and the increase in Na+/H+ exchange activity was highly EIPA sensitive consistent with an upregulation of NHE8 and not NHE3 by acidosis. As in the adult rat, there was no change in NHE3 mRNA abundance in mice and actually a decrease in NHE3 mRNA abundance in the neonatal rats with acidosis (1, 23). Acid loading did not affect NHE8 mRNA abundance in either neonatal mice or rats. In both neonatal mice and rats, there was an increase in total protein and brush-border membrane NHE3 protein abundance. However, NHE8 expression was increased on the brush border without a change in the total cellular content suggesting that trafficking may be involved in the regulation of NHE8 by acid. In this study, we used NRK cells that express NHE8 and we induced intracellular acidification by incubation with a media of pH 6.6 compared with pH 7.4 to study the mechanism for the increase in NHE8 by acid.
Previous studies examined the mechanism for the stimulation in Na+/H+ caused by metabolic acidosis using OKP cells, a cell line that like adult proximal tubules expresses NHE3 but does not express NHE8 (3, 27). Incubation of OKP cells in acid compared with control media for 24 h resulted in an increase in Na+/H+ exchanger activity, NHE3 mRNA and NHE3 protein abundance (2, 3, 27). Incubation of OKP cells with an acid media resulted in an increase in exocytosis of NHE3 to the apical membrane (27). In OKP cells incubation in acid media resulted in an increase in NHE3 mRNA after 12 h of incubation that was comparable to the increase at 24 h (3). This is in contrast to the present study where there was no increase in NHE8 mRNA abundance in NRK cells even after 24 h of incubation of cells in acid media compared with control 7.4 pH media. Furthermore, in the present study the acid-induced effect on NHE8 was still found in the presence of actinomycin D.
There were other differences in the regulation of NHE3 and NHE8 by acid media. Incubation with acid media increased NHE3 total protein abundance at 24 h but not before that time (27). This is in contrast to the present findings examining the effect of NHE8 in NRK cells where NHE8 total protein abundance was not affected by the pH of the media even after 24 h. In OKP cells, there was an increase in surface NHE3 protein abundance as early as 6 h after incubation in acid media compared with control which was reflected by an increase in Na+/H+ exchanger activity at this time point (3, 27). The increase in Na+/H+ exchanger activity in OKP cells was not inhibited by cycloheximide, suggesting that mechanisms other than mRNA and protein synthesis were involved in the acute stimulation of Na+/H+ exchange activity by acid (27). In the present study, there was an acid-induced increase in Na+/H+ exchange activity and surface NHE8 expression in the presence of cycloheximide. Thus, the effect of acid on NHE8 surface protein expression does not require protein synthesis.
The effect of acid on trafficking of NHE3 to the apical membrane has been studied previously (27). Using the same exocytosis assay as in the present study, NHE3 trafficking to the apical membrane was stimulated by acid media at 6 h of incubation; well before the increase in mRNA and total protein abundance by acid. There was a concordant increase in Na+/H+ exchange activity in OKP cells at 6 h as well. The increase in Na+/H+ exchange activity was blocked by both colchicine and latrunculin B consistent with microtubules and actin cytoskeleton being involved in trafficking. Thus, in OKP cells acid incubation increases NHE3 and Na+/H+ exchange activity by both trafficking and mRNA and protein synthesis. The effect of acid on trafficking to the apical membrane occurs before the increase in NHE3 mRNA and protein abundance.
In the present study, we also demonstrate a concordant time-dependent increase in surface NHE8 protein expression and Na+/H+ exchange activity. We demonstrate that this occurs in the presence of cycloheximide and actinomycin D. The acid-induced increase in surface NHE8 protein abundance and Na+/H+ exchanger activity was blocked by both colchicine and cytochalasin D. Thus, microtubules and the actin cytoskeleton are necessary for the increase in surface NHE8 expression. Finally, we show that the acid-induced increase in NHE8 is mediated by an increase in exocytosis.
In summary, the present study examined the mechanism for the increase in NHE8 with acid media. In response to acid media, NRK cells had a time-dependent increase in Na+/H+ exchange activity and surface NHE8 protein abundance with no change in NHE8 total cellular protein or mRNA. Our results are consistent with our previous in vivo studies showing that brush-border membrane NHE8 and Na+/H+ exchange activity increase with metabolic acidosis, but there was no change in NHE8 mRNA and total cellular NHE8 protein abundance. Furthermore, the increase in apical membrane NHE8 protein abundance and Na+/H+ exchange activity occurred in the presence of actinomycin D and cycloheximide demonstrating that the acid-induced increase in NHE8 surface expression occurred without mRNA or protein synthesis. The increase in NHE8 surface activity by acid was mediated by an increase in exocytosis. These data demonstrate that trafficking is the predominant and likely sole mechanism for mediating the acid-induced regulation of NHE8.
GRANTS
This work was supported by National Institutes of Health Grants DK41612 (to M. Baum) and DK078596 (to M. Baum), T32 DK07257 (to Peter Igarashi and M. Baum), and the O'Brien Center P30DK079328 (to Peter Igarashi).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: C.J., K.T., J.G., Q.Z., and V.D. performed experiments; C.J., K.T., J.G., Q.Z., and M.B. analyzed data; C.J., J.G., and M.B. interpreted results of experiments; C.J. and M.B. prepared figures; C.J., K.T., J.G., Q.Z., V.D., and M.B. approved final version of manuscript; M.B. conception and design of research; M.B. drafted manuscript; M.B. edited and revised manuscript.
REFERENCES
- 1. Ambuhl PM, Amemiya M, Danczkay M, Lotscher M, Kaissling B, Moe OW, Preisig PA, Alpern RJ. Chronic metabolic acidosis increases NHE3 protein abundance in rat kidney. Am J Physiol Renal Fluid Electrolyte Physiol 271: F917–F925, 1996 [DOI] [PubMed] [Google Scholar]
- 2. Ambuhl PM, Yang X, Peng Y, Preisig PA, Moe OW, Alpern RJ. Glucocorticoids enhance acid activation of the Na+/H+ exchanger 3 (NHE3). J Clin Invest 103: 429–435, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Amemiya M, Yamaji Y, Cano A, Moe OW, Alpern RJ. Acid incubation increases NHE-3 mRNA abundance in OKP cells. Am J Physiol Cell Physiol 269: C126–C133, 1995 [DOI] [PubMed] [Google Scholar]
- 4. Baum M. Neonatal rabbit juxtamedullary proximal convoluted tubule acidification. J Clin Invest 85: 499–506, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baum M. Developmental changes in rabbit juxtamedullary proximal convoluted tubule acidification. Pediatr Res 31: 411–414, 1992 [DOI] [PubMed] [Google Scholar]
- 6. Baum M, Cano A, Alpern RJ. Glucocorticoids stimulate Na+/H+ antiporter in OKP cells. Am J Physiol Renal Fluid Electrolyte Physiol 264: F1027–F1031, 1993 [DOI] [PubMed] [Google Scholar]
- 7. Baum M, Dwarakanath V, Alpern RJ, Moe OW. Effects of thyroid hormone on the neonatal renal cortical Na+/H+ antiporter. Kidney Int 53: 1254–1258, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baum M, Quigley R. Prenatal glucocorticoids stimulate neonatal juxtamedullary proximal convoluted tubule acidification. Am J Physiol Renal Fluid Electrolyte Physiol 261: F746–F752, 1991 [DOI] [PubMed] [Google Scholar]
- 9. Becker AM, Zhang J, Goyal S, Dwarakanath V, Aronson PS, Moe OW, Baum M. Ontogeny of NHE8 in the rat proximal tubule. Am J Physiol Renal Physiol 293: F255–F261, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bobulescu IA, Dwarakanath V, Zou L, Zhang J, Baum M, Moe OW. Glucocorticoids acutely increase cell surface Na+/H+ exchanger-3 (NHE3) by activation of NHE3 exocytosis. Am J Physiol Renal Physiol 289: F685–F691, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Boyarsky G, Ganz MB, Sterzel RB, Boron WF. pH regulation in single glomerular mesangial cells. I. Acid extrusion in absence and presence of HCO3−. Am J Physiol Cell Physiol 255: C844–C856, 1988 [DOI] [PubMed] [Google Scholar]
- 12. Choi JY, Shah M, Lee MG, Schultheis PJ, Shull GE, Muallem S, Baum M. Novel amiloride-sensitive sodium-dependent proton secretion in the mouse proximal convoluted tubule. J Clin Invest 105: 1141–1146, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cogan MG, Rector FC., Jr Proximal reabsorption during metabolic acidosis in the rat. Am J Physiol Renal Fluid Electrolyte Physiol 242: F499–F507, 1982 [DOI] [PubMed] [Google Scholar]
- 14. Dagan A, Gattineni J, Cook V, Baum M. Prenatal programming of rat proximal tubule Na+/H+ exchanger by dexamethasone. Am J Physiol Regul Integr Comp Physiol 292: R1230–R1235, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gattineni J, Sas D, Dagan A, Dwarakanath V, Baum M. Effect of thyroid hormone on the postnatal renal expression of NHE8. Am J Physiol Renal Physiol 294: F198–F204, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goyal S, Vanden Heuvel G, Aronson PS. Renal expression of novel Na+/H+ exchanger isoform NHE8. Am J Physiol Renal Physiol 284: F467–F473, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Moe OW, Miller RT, Horie S, Cano A, Preisig PA, Alpern RJ. Differential regulation of Na/H antiporter by acid in renal epithelial cells and fibroblasts. J Clin Invest 88: 1703–1708, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Peng Y, Amemiya M, Yang X, Fan L, Moe OW, Yin H, Preisig PA, Yanagisawa M, Alpern RJ. ET(B) receptor activation causes exocytic insertion of NHE3 in OKP cells. Am J Physiol Renal Physiol 280: F34–F42, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Preisig PA, Alpern RJ. Chronic metabolic acidosis causes an adaptation in the apical membrane Na/H antiporter and basolateral membrane Na(HCO3)3 symporter in the rat proximal convoluted tubule. J Clin Invest 82: 1445–1453, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schwartz GH, Evan AP. Development of solute transport in rabbit proximal tubule. I. HCO3 and glucose absorption. Am J Physiol Renal Fluid Electrolyte Physiol 245: F382–F390, 1983 [DOI] [PubMed] [Google Scholar]
- 21. Shah M, Gupta N, Dwarakanath V, Moe OW, Baum M. Ontogeny of Na+/H+ antiporter activity in rat proximal convoluted tubules. Pediatr Res 48: 206–210, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tsai CJ, Ives HE, Alpern RJ, Yee VJ, Warnock DG, Rector FC., Jr Increased Vmax for Na+/H+ antiporter activity in proximal tubule brush-border vesicles from rabbits with metabolic acidosis. Am J Physiol Renal Fluid Electrolyte Physiol 247: F339–F343, 1984 [DOI] [PubMed] [Google Scholar]
- 23. Twombley K, Gattineni J, Bobulescu IA, Dwarakanath V, Baum M. Effect of metabolic acidosis on neonatal proximal tubule acidification. Am J Physiol Regul Integr Comp Physiol 299: R1360–R1368, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3: RESEARCH0034, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang D, Zhang H, Lang F, Yun CC. Acute activation of NHE3 by dexamethasone correlates with activation of SGK1 and requires a functional glucocorticoid receptor. Am J Physiol Cell Physiol 292: C396–C404, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wu MS, Biemesderfer D, Giebisch G, Aronson PS. Role of NHE3 in mediating renal brush border Na+-H+ exchange. Adaptation to metabolic acidosis. J Biol Chem 271: 32749–32752, 1996 [DOI] [PubMed] [Google Scholar]
- 27. Yang X, Amemiya M, Peng Y, Moe OW, Preisig PA, Alpern RJ. Acid incubation causes exocytic insertion of NHE3 in OKP cells. Am J Physiol Cell Physiol 279: C410–C419, 2000 [DOI] [PubMed] [Google Scholar]
- 28. Zhang J, Bobulescu IA, Goyal S, Aronson PS, Baum MG, Moe OW. Characterization of Na+/H+ exchanger NHE8 in cultured renal epithelial cells. Am J Physiol Renal Physiol 293: F761–F766, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]