Abstract
The 2-kidney, 1-clip (2K1C) model has provided many insights into the pathogenesis of renovascular hypertension. However, studies using the 2K1C model often report low success rates of hypertension, with typical success rates of just 40–60%. We hypothesized that these low success rates are due to fundamental design flaws in the clips traditionally used in 2K1C models. Specifically, the gap widths of traditional silver clips may not be maintained during investigator handling and these clips may also be easily dislodged from the renal artery following placement. Therefore, we designed and tested a novel vascular clip possessing design features to maintain both gap width and position around the renal artery. In this initial study, application of these new clips to the left renal artery produced reliable and consistent levels of hypertension in rats. Nine-day application of clips with gap widths of 0.27, 0.25, and 0.23 mm elicited higher mean arterial blood pressures of 112 ± 4, 121 ± 6, and 135 ± 7 mmHg, respectively (n = 8 for each group), than those of sham-operated controls (95 ± 2 mmHg, n = 8). Moreover, 8 out of 8 rats in each of the 0.23 and 0.25 mm 2K1C groups were hypertensive, whereas 7 out of 8 rats in the 0.27 mm 2K1C group were hypertensive. Plasma renin concentrations were also increased in all 2K1C groups compared with sham-operated controls. In summary, this novel clip design may help eliminate the large degree of unreliability commonly encountered with the 2K1C model.
Keywords: renin-angiotensin-aldosterone system, renovascular hypertension, mean arterial pressure
hypertension is a major cause of morbidity and mortality affecting more than one-third of adults in the United States (10, 14). Renovascular hypertension is a common form of secondary hypertension that results from a decrease in renal blood flow due to renal artery stenosis and subsequent activation of the renin-angiotensin-aldosterone system (RAAS) (1, 8, 20). One of the most commonly used models to study renovascular hypertension is the two-kidney, one-clip (2K1C) model. First described by Goldblatt and colleagues (6, 9), the original 2K1C technique involved application of variable size silver clips to reduce the diameter of renal arteries in dogs and monkeys. Although the 2K1C model has provided significant insights into the pathogenesis of renovascular hypertension, few modifications have been made to the original clip design and there are noteworthy inadequacies in this model (4, 6, 9, 13, 21, 25). Most notably, there is a large failure rate with respect to the number of animals that develop hypertension in the 2K1C model. For example, success rates of 44–80% (6, 9, 21, 25) in rats, and a 45% success rate in mice (23) have been reported, which is costly in terms of animals, supplies, and investigator time. Consequently, although the 2K1C model is associated with many changes similar to those found in patients with renovascular hypertension, its use by researchers may be limited by the unreliability of the clip procedure.
It is likely that the clip design is a major reason for the relatively low success rate of eliciting hypertension in the 2K1C model. Commonly, the clips are made from silver, which is malleable and easily fashioned into a U-shaped clip. However, the very ductile nature of silver may contribute to the unreliability of the clip because it increases the likelihood of investigator-induced changes in gap width while smoothing (deburring) or during clip application, thereby resulting in inconsistent reduction in renal artery diameter. Recently, Lorenz et al. (15) reported promising results in producing reliable 2K1C hypertension in mice by use of a modified polyurethane cuff [internal diameter (ID), 0.27 mm]. However, this modified cuff is also subject to investigator-induced changes due to the ductile nature of polyurethane, which was reflected by the reported variability in cuff size (0.25–0.29 mm), and thus renal artery diameter, upon postmortem examination (15).
Another confounding factor in current 2K1C models is the potential for inflammation or an increased immunological response as a result of the material from which the cuff or clip is made. Silver has been shown to cause substantial perivascular inflammation, tissue granulation, and intimal proliferation (7), especially if silver oxides are formed on the surface during sterilization and handling. This perivascular reaction may further alter renal artery lumen diameter, especially in chronic applications. Kraft et al. (11) demonstrated that silver and stainless-steel implants elicited moderate to severe increases in leukocyte extravasation and venular dilation, respectively, when silver and stainless-steel implants were applied to the dorsal skin-fold in hamsters. Moreover, severe inflammation and edema occurred in 5 out of 6 silver implant preparations 3 days after implantation. In contrast, titanium implants did not cause significant changes in leukocyte leakage or venular dilation. Use of polymers, such as polyurethane-coated silicone implants, has been associated with increased histological staining of vascular endothelial growth factor, transforming growth factor-β, and inflammatory cells at the site of implantation compared with textured-silicone implants in rats (22).
Another confounding factor is that existing clips do not include design elements to prevent displacement from the renal artery. In the aforementioned polyurethane 2K1C cuff method by Lorenz et al. (15), a silk ligature was tied around the cuff after implantation to secure its placement around the renal artery. However, the application of the ligature was not sufficient to prevent all cuffs from becoming dislodged, and the fact that the ligature must be tied around the ductile polyurethane cuff likely contributed to the variability in final cuff diameter (15). The use of a “circumferential constriction” 2K1C method by Lorenz et al. is a novel approach to the 2K1C model, yet due to the method needed to secure the cuff it may be extremely difficult to minimize variations between individual investigators or between research laboratories.
In an attempt to address these problems, we designed a novel titanium vascular clip for use in the 2K1C model. The new clip was designed to eliminate the possibility of deformation during investigator-handling and dislodgement after implantation and allow for repeated use with a material that can be autoclaved. In this initial study, we determined whether the application of these new clips improved the success rate of 2K1C hypertension in rats 9 days following clip placement.
METHODS
This investigation conforms to The Guide for the Care and Use of Laboratory Animals from the National Institutes of Health (NIH Publication No. 85–23, revised 1996), and all protocols were approved by the University of Georgia Animal Care and Use Committee.
Experimental groups.
Thirty-two male Sprague-Dawley rats (380–400 g) were assigned to four study groups (n = 8 per group): sham operated or one of three 2K1C groups (0.27, 0.25, or 0.23 mm gap widths).
Vascular clip design and manufacture.
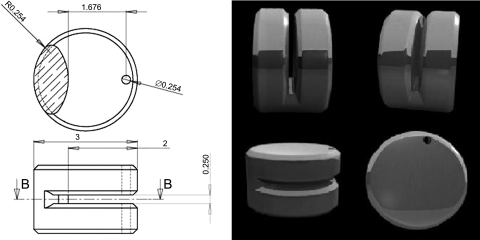
The vascular clip design is shown in Fig. 1. Clips were manufactured by first cutting gap widths of 0.27, 0.25, or 0.23 mm at a depth of 2.0 mm into a medical grade titanium rod (3 mm diameter; M. Vincent & Associates) using carbide saw blades of varying thickness (RobbJack, Lincoln, CA). Once the internal cuts were made, a 0.254-mm hole was drilled using a twist drill (Greenfield Industries, Seneca, SC) through the outer edge of the titanium rod. The edges of all cuts were then deburred using a 90° double-angle milling cutter (Niagara Cutter, Amherst, NY) and individual clips cut from the rod using a 0.79 mm slitting saw (RobbJack). The outer edges of the resultant clips were then chamfered using a lathe (HLV-H Hardinge, Elmira, NY). All clips were manufactured with the use of computer-aided design and manufacturing (CAD/CAM) systems, thus eliminating any clip-to-clip gap width variability (17).
Fig. 1.
Design of the new clip. Left: dimensions are shown in millimeters. B, plane of the cross-section shown on top. Right: illustrations of the new clip design [Patent(s) pending].
Surgical procedures.
All rats were weighed and anesthesia was induced and maintained with 2% isoflurane delivered in 95% O2 and 5% CO2. The left abdominal wall was shaved and coated with 70% alcohol and povidone-iodine. Body temperature was monitored and maintained at 37°C with a digital anal thermometer and heating pad, respectively. A left paracostal celiotomy was performed, the left kidney exteriorized, and the renal artery and vein carefully isolated by blunt dissection. In each of the rats in the 2K1C groups, a clip was placed on the left renal artery with the aid of an Olympus SZ40 dissecting microscope. A nylon suture (5–0 Ethilon, Ethicon) was then passed through the predrilled hole in the clip and tied to prevent clip dislodgement. The kidney was carefully placed back in its original location and the abdominal wall and skin was closed using interrupted sutures (5–0 Ethilon, Ethicon) and 9 mm staples (Reflex9, Cellpoint Scientific). A few drops of local anesthetic (0.1 ml of 2% Lidocaine HCl) and bacitracin, polymixin, and neomycin ointment were applied to the incision site. For rats in the sham group, surgeries were performed as described above, except renal clips were applied to the renal artery then removed prior to the kidney being placed back in its original position. All rats were observed for 4 h following surgery, then individually housed for 24 h and allowed access to standard rat chow and water ad libitum.
Blood pressure measurements.
Nine days following clip application, the rats were weighed, anesthetized as above, and the left medial thigh and inguinal area were shaved and swabbed prior to surgery. The left femoral artery was exposed and isolated by blunt dissection. A polyethylene catheter (PE/3, Scientific Commodities) was advanced inside the femoral artery, such that the tip of the catheter was close to the aortic bifurcation. Mean arterial pressure (MAP) and peak systolic blood pressure (PSBP) were measured via a precalibrated pressure transducer (Radnoti, Monrovia, CA) and recorded using LabChart software (version 7.0, AD Instruments, Colorado Springs, CO). MAP and PSBP were measured for ≥15 min.
Plasma renin concentration.
Immediately following blood pressure measurement, 3 ml of blood was collected in EDTA (7.2 mg, Vacutainer, BD, Franklin Lakes, NJ) via the arterial catheter. Within 30 min of collection, EDTA-plasma samples were prepared by centrifugation, frozen, and stored at −20°C until analysis. Plasma renin concentrations were determined using a modification of a commercial fluorometric kit (SensoLyte 520 Rat Renin Assay Kit, AnaSpec, Fremont, CA) (19). Briefly, stock rat renin substrate and recombinant rat renin were diluted at 1:50 and 1:500 with assay buffer, respectively. Recombinant rat renin was then diluted in series to obtain a nine-point standard curve. All samples, including standards, were analyzed in triplicate. Each well contained 90 μl of serum (or 90 μl of standard) and 10 μl of diluted substrate. The 96-well, flat-bottom, plate was centrifuged at 3,000 rpm for 3 min and incubated at 37°C for 4 h. Fluorescence was measured with a Fluoroskan Ascent 96-well plate reader (Thermo Fisher Scientific) at excitation/emission of 490/520 nm and recorded using Ascent software (version 2.6, Thermo Fisher Scientific). Plasma renin concentration was determined by comparing the plasma samples to the standard curve. There was an excellent linear relationship (R2 = 0.975) between relative fluorescence units and renin concentration in the standard curve.
Organ weight measurements.
Following blood collection, rats were killed by decapitation while still under general anesthesia. Heart weight and clipped and non-clipped kidney weights were recorded, and clip location relative to the renal artery was determined for all rats in the 2K1C groups.
Statistical analysis.
Results are expressed as means ± SE. All data were analyzed using repeated-measures ANOVA with post hoc analysis using the Tukey-Kramer multiple comparison procedure. Comparison of means between sham-operated and 2K1C rat groups were determined by use of Student's paired t-test. A value of P < 0.05 was deemed to be significant (24).
RESULTS
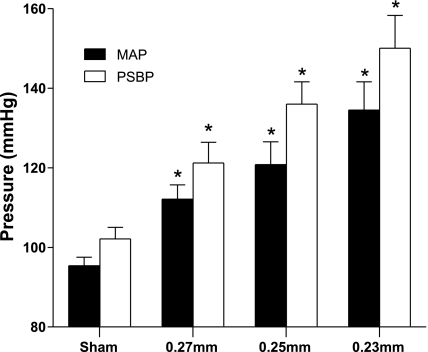
PSBP in all 2K1C groups were significantly higher than in the sham-operated group as determined 9 days after placement of the clips. PSBP in rats with clips of gap widths of 0.27, 0.25, and 0.23 mm were 121 ± 5, 136 ± 6, and 150 ± 8 mmHg, respectively, whereas the PSBP in the sham-operated group was 102 ± 3 mmHg (P < 0.05, for all comparisons between the 2K1C groups and the control group; Fig. 2). Rats with renal clips of widths of 0.27, 0.25, or 0.23 mm had significantly higher MAP values (112 ± 4, 121 ± 6, and 135 ± 7 mmHg, respectively; Fig. 2) than those of the sham-operated rats (95 ± 2 mmHg). Hypertension was observed in 8 of 8 rats in the 0.23 and 0.25 mm clip groups, whereas 7 of 8 rats became hypertensive in the group with the 0.27 mm clips (the MAP of normotensive rat in the 0.27 mm group was 95 mmHg, whereas the other rats had MAP values between 104 and 125 mmHg). Rats with renal clips of widths 0.27, 0.25, or 0.23 mm had significantly higher heart rates (392.5 ± 7.1, 373.0 ± 12.7, and 395.3 ± 6.1 beats/min, respectively) compared with sham-operated rats (343.3 ± 14.3 beats/min). Upon postmortem examination, all clips were found to be in place in all 2K1C rats at the end of the 9-day experimental period.
Fig. 2.
Mean arterial pressure (MAP) and peak systolic blood pressure (PSBP) in sham and 2K1C groups (n = 8) at 9 days. *Significant difference from sham group.
As summarized in Table 1, 9 days after clip placement, body weight changes in all 2K1C rats were significantly lower compared with sham-operated rats (there was no significant difference in initial body weight, day 0, between sham and 2K1C rats). There was no significant difference in non-clipped and clipped kidney weights in the sham-operated group. In contrast, the clipped kidney weights were significantly lower than those of the non-clipped kidney in all 2K1C groups, and non-clipped kidney weights from all 2K1C rats were significantly higher than non-clipped kidney weights from sham-operated rats. Rats in the 0.23 mm 2K1C group had significantly higher heart weights than sham-operated rats (Table 1).
Table 1.
Body and tissue weight parameters in sham and 2K1C groups at 9 days
| Group | ΔBW, g | NCKW, g | NCKW, mg/g Body Wt | CKW, g | CKW, mg/g Body Wt | HW, mg/g Body Wt |
|---|---|---|---|---|---|---|
| Sham | + 8.3 ± 5.1 | 1.3 ± 0.09 | 3.1 ± 0.1 | 1.3 ± 0.06 | 3.0 ± 0.1 | 3.2 ± 0.1 |
| 0.27 mm | −1.1 ± 2.8* | 1.4 ± 0.09 | 3.6 ± 0.1* | 1.1 ± 0.05 | 2.9 ± 0.2† | 3.5 ± 0.1 |
| 0.25 mm | −5.2 ± 3.3* | 1.4 ± 0.04 | 3.6 ± 0.1* | 1.0 ± 0.09 | 2.6 ± 0.2† | 3.5 ± 0.1 |
| 0.23 mm | −12.1 ± 3.8* | 1.5 ± 0.03 | 3.8 ± 0.1* | 1.0 ± 0.08 | 2.7 ± 0.2† | 3.7 ± 0.3* |
Data are presented as means ± SE. ΔBW, change in body weight from day 0; NCKW, non-clipped kidney weight; CKW, clipped kidney weight; HW, heart weight. There were 8 rats in each group. Body and tissue weight in sham and 2K1C groups (n = 8) at 9 days.
P < 0.05, 2 kidney, 1 clip (2K1C) groups vs. sham.
P < 0.05, CKW vs. NCKW.
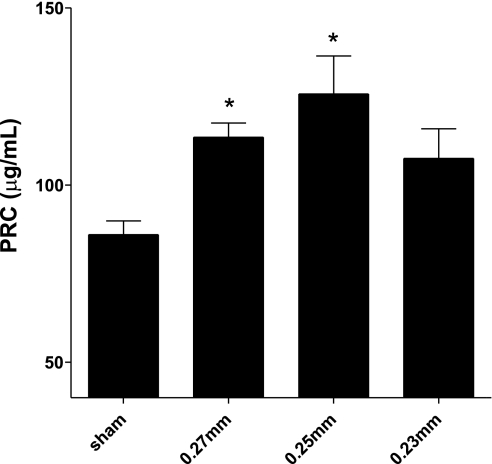
As summarized in Fig. 3, plasma renin concentrations were higher in all 2K1C groups compared with sham-operated rats. Plasma renin concentrations for 0.27, 0.25, and 0.23 mm clip 2K1C rats were 114 ± 4, 126 ± 11, and 108 ± 8 μg/ml, respectively, compared with 86 ± 4 μg/ml for sham-operated rats (P < 0.05, for 0.27 and 0.25 mm clip 2K1C rats compared with sham values; PRC was not significantly increased in the 0.23 mm group, P = 0.057).
Fig. 3.
Plasma renin concentrations (PRC) on day 9 from each group (n = 8); data are presented as means ± SE. *Significant difference from sham group.
DISCUSSION
Although the 2K1C model has provided valuable insights into the pathogenesis of renovascular hypertension, its continued use and adoption by cardiovascular researchers is hindered by the relatively low success rates of development of hypertension in rats (6, 9, 21, 25) and mice (23). The most likely reason for the unreliability of the 2K1C model in eliciting hypertension is that the clip design and materials used in this model have several inherent flaws. Specifically, the traditional use of an open U-shaped clip made from silver leads to inaccurate and inconsistent reduction of renal artery diameter and frequent clip displacement from the renal artery.
The aim of this study was to determine whether consistent levels of hypertension could be produced in 2K1C rats while addressing the flaws in traditionally used clips. We chose an experimental period of 9 days as this is sufficient to detect significant changes in blood pressure (12) and is similar to other studies where novel clip or cuff designs have been evaluated. Initially, our first approach was to use a similar design to traditional clips, but using titanium as the material to eliminate problems with the malleability and the inflammatory effects of silver or stainless-steel. However, when we used open U-shaped titanium clips with gap widths of 0.25 mm, only 2 of 7 rats (29%) became hypertensive 1 wk after clip implantation (data not shown). We determined that the lack of reproducible hypertension produced by our first generation of clips was due to the clips becoming dislodged from the renal artery as clips were no longer in place in 5 of 7 rats (71%) when postmortems were performed. Although these clips were much more robust than similar clips made from silver in terms of maintaining the gap widths during investigator handling, the fine deburring of the titanium appeared to render the surfaces extremely smooth and the clips, therefore, slipped off the artery in the days following placement.
Our next iteration of the design included a 0.254 mm diameter hole at the outer edge of the clip. This allowed the investigator to pass a nonabsorbable suture through the hole and then tie the suture in a knot to prevent the clip from becoming dislodged from the artery. This design element also prevented investigator-induced changes in internal gap width as the application of the suture did not deform the titanium clip. This is in contrast to the methods of Lorenz et al., where a ligature is tied around a ductile piece of polyurethane tubing, which was reported to result in variable cuff widths by these investigators (15). The novel clips produced hypertension in 100% of rats 9 days following placement of clips with gap widths of either 0.25 or 0.23 mm. In the 0.27 mm 2K1C group, 7 of 8 rats developed hypertension.
Since RAAS activation is fundamental to renovascular hypertension, we assayed plasma renin concentration and determined that 0.27 and 0.25 mm 2K1C groups had significantly higher plasma renin concentrations than those in the sham-operated group. However, whereas the 0.23 mm 2K1C group had the highest MAP and PSBP values, rats in this group had lower plasma renin concentrations than either of the other 2K1C groups. The lack of a further increase in plasma renin concentration in the 0.23 mm 2K1C group may be because this level of reduction in renal artery diameter may be too severe and thus damage the kidney, as was demonstrated in one clipped kidney in this group that appeared to develop acute ischemia. This is supported by the literature where gap widths of 0.25 mm are most commonly adopted for rodent 2K1C studies and are associated with consistent increases in RAAS (2, 3, 9). The increases in non-clipped kidney weight over time (i.e., 9 days) and lack of increase in clipped kidney weight over time found in the present study were consistent with previous findings in 2K1C rats (9, 18). While 0.23 mm 2K1C rats were the only 2K1C group that had a significant increase in heart weight compared with sham-operated rats, all 2K1C rats had an increase in heart-to-body weight ratios consistent with the onset of cardiac hypertrophy (5). The difference between body weights of 2K1C rats compared with sham-operated rats on day 9 in this study was similar to that reported by Muller et al. (16).
The results of the current study using the new clip design are consistent with those found in 2K1C models in the rat, namely 1) hypertension, 2) RAAS activation, 3) lack of weight gain in the kidney ipsilateral to the clipped renal artery, and 4) reduced gain in body weight. Cardiac hypertrophy, another common finding in 2K1C hypertension, was only significant in rats from the 0.23 mm gap width group in this initial study. The major difference between this and previous studies using silver and stainless-steel U-shaped clips is the improvement in the reliability of hypertension. Although Lorenz et al. demonstrated improved success with a modified polyurethane cuff in mice, these investigators reported marked variability in cuff diameter (ID 0.25 to 0.29 mm) upon postmortem examination (15). Our experiments directly addressed two main factors that plague studies using traditional silver clips, namely 1) malleability and 2) maintenance of clip placement, which were overcome through the use of titanium and the addition of a tie-in-place hole that resulted in 100% hypertension when clips of gap widths 0.25 or 0.23 mm were used.
GRANTS
Funding for this study was provided by the National Institutes of Health (1RO1NS054117-01A2, Lewis and Robertson) and The University of Georgia Research Foundation (funding for clip design, production, and provisional patent application, Robertson and Chelko).
DISCLOSURES
Chelko and Robertson are listed as the inventors of the new clip in the invention disclosure statement to UGA. Chelko and Robertson have no financial interests in terms of licensing the patents filed for by UGA, but they may receive royalties from any such licensing through UGA.
AUTHOR CONTRIBUTIONS
Author contributions: S.P.C., C.W.S., T.H.L., and T.P.R. performed experiments; S.P.C., C.W.S., T.H.L., and T.P.R. analyzed data; S.P.C., C.W.S., S.J.L., and T.P.R. interpreted results of experiments; S.P.C. prepared figures; S.P.C. and T.P.R. drafted manuscript; S.P.C., C.W.S., S.J.L., and T.P.R. edited and revised manuscript; S.P.C., C.W.S., T.H.L., S.J.L., and T.P.R. approved final version of manuscript; C.W.S., S.J.L., and T.P.R. conception and design of research.
ACKNOWLEDGMENTS
We acknowledge the work of Roger Fortner (UGA Instrument Shop) in the manufacture of the novel titanium renal clips and Steven Arnold (Department of Large Animal Medicine, UGA) for the graphical illustration of the clip design.
REFERENCES
- 1. Berglund G, Andersson O, Wilhelmsen L. Prevalence of primary and secondary hypertension: studies in a random population sample. Br Med J 2: 554, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Braam B, Navar LG, Mitchell KD. Modulation of tubuloglomerular feedback by angiotensin II type 1 receptors during the development of Goldblatt hypertension. Hypertension 25: 1232–1237, 1995 [DOI] [PubMed] [Google Scholar]
- 3. Cervenka L, Wang CT, Mitchell KD, Navar LG. Proximal tubular angiotensin II levels and renal functional responses to AT1 receptor blockade in nonclipped kidneys of Goldblatt hypertensive rats. Hypertension 33: 102–107, 1999 [DOI] [PubMed] [Google Scholar]
- 4. De Jong W. Production of Arneil-Dekanski factor in vitro by plasma of renal hypertensive rats. Eur J Pharmacol 3: 93–95, 1968 [DOI] [PubMed] [Google Scholar]
- 5. Doggrell SA, Brown L. Rat models of hypertension, cardiac hypertrophy and failure. Cardiovasc Res 39: 89–105, 1998 [DOI] [PubMed] [Google Scholar]
- 6. Dussaule J, Michel J, Auzan C, Schwartz K, Corvol P, Menard J. Effect of antihypertensive treatment on the left ventricular isomyosin profile in one-clip, two kidney hypertensive rats. J Pharmacol Exp Ther 236: 512–518, 1986 [PubMed] [Google Scholar]
- 7. Ebina K, Iwabuchi T, Suzuki S. Histological change in permanently clipped or ligated cerebral arterial wall. Acta Neurochir 65: 253–276, 1982 [DOI] [PubMed] [Google Scholar]
- 7a. Goldblatt H. Studies on experimental hypertension. Ann Intern Med 11: 69, 1937 [Google Scholar]
- 7b. Goldblatt H, Lynch J, Hanzel RF, Summerville WW. Studies on experimental hypertension. 1. The production of persistent elevation of systolic blood pressure by means of renal ischemia. J Exp Med 59: 347, 1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Harrison EG, Jr, McCormack LJ. Pathologic classification of renal arterial disease in renovascular hypertension. Mayo Clin Proc 46: 161–167, 1971 [PubMed] [Google Scholar]
- 9. Helle F, Vågnes ØB, Iversen BM. Angiotensin II-induced calcium signaling in the afferent arteriole from rats with two-kidney, one-clip hypertension. Am J Physiol Renal Physiol 291: F140–F147, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Heron M, Hoyert D, Murphy S, Xu J, Kochanek K, Tejada-Vera B. Deaths: Final Data for 2006. National Vital Statistics reports, vol. 57, no. 14, 2009 [PubMed] [Google Scholar]
- 11. Kraft CN, Hansis M, Arens S, Menger MD, Vollmar B. Striated muscle microvascular response to silver implants: a comparative in vivo study with titanium and stainless steel. J Biomed Mater Res 49: 2: 192–199, 2000 [DOI] [PubMed] [Google Scholar]
- 12. Lacolley P, Owen J, Sandock K, Lewis T, Bates J, Robertson T, Lewis S. Occipital artery injections of 5-HT may directly activate the cell bodies of vagal and glossopharyngeal afferent cell bodies in the rat. Neuroscience 143: 1: 289–308, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Leenen F, De Jong W. A solid silver clip for induction of predictable levels of renal hypertension in the rat. J Appl Physiol 31: 142–144, 1971 [DOI] [PubMed] [Google Scholar]
- 14. Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C. Heart disease and stroke statistics–2010 update. A report from the American Heart Association. Circulation 23: 948–54, 2010 [DOI] [PubMed] [Google Scholar]
- 15. Lorenz JN, Lasko VM, Nieman ML, Damhoff T, Prasad V, Beierwaltes WH, Lingrel JB. Renovascular hypertension using a modified two-kidney, one-clip approach in mice is not dependent on the α1 or α2 Na, K-ATPase ouabain-binding site. Am J Physiol Renal Physiol 301: F615–F621, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Muller DN, Klanke B, Feldt S, Cordasic N, Hartner A, Schmieder RE, Luft FC, Hilgers KF. (Pro) renin receptor peptide inhibitor “handle-region” peptide does not affect hypertensive nephrosclerosis in Goldblatt rats. Hypertension 51: 3: 676–681, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Nokar S, Moslehifard E, Bahman T, Bayanzadeh M, Nasirpouri F, Nokar A. Accuracy of implant placement using a CAD/CAM surgical guide: an in vitro study. Int J Oral Maxillofac Implants 26: 520–526, 2011 [PubMed] [Google Scholar]
- 18. Okuniewski R, Davis EA, Jarrott B, Widdop RE. A comparison of the development of renal hypertension in male and female rats. Clin Sci 95: 445–451, 1998 [PubMed] [Google Scholar]
- 19. Schmiedt CW, Hurley KAE, Tong X, Rakhmanova VA, Po CL, Hurley DJ. Measurement of plasma renin concentration in cats by use of a fluorescence resonance energy transfer peptide substrate of renin. Am J Vet Res 70: 1315–1322, 2009 [DOI] [PubMed] [Google Scholar]
- 20. Strandness DE., Jr Natural history of renal artery stenosis. Am J Kidney Dis 24: 630–635, 1994 [DOI] [PubMed] [Google Scholar]
- 21. Tobian L, Janecek J, Tomboulian A. The effect of a high sodium intake on the development of permanent nephrosclerotic hypertension; effect of nephrosclerotic hypertension of the granularity of the juxtaglomerular cells. J Lab Clin Med 53: 842–848, 1959 [PubMed] [Google Scholar]
- 22. Vieira VJ, d'Acampora AJ, Marcos ABW, Di Giunta G, de Vasconcellos ZAA, Bins-Ely J, d'Eça Neves R, Figueiredo CP. Vascular endothelial growth factor overexpression positively modulates the characteristics of periprosthetic tissue of polyurethane-coated silicone breast implant in rats. Plast Reconstr Surg 126: 1899–1910, 2010 [DOI] [PubMed] [Google Scholar]
- 23. Wagenseil JE, Knutsen RH, Li DY, Mecham RP. Elastin-insufficient mice show normal cardiovascular remodeling in 2K1C hypertension despite higher baseline pressure and unique cardiovascular architecture. Am J Physiol Heart Circ Physiol 293: H574–H584, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Wallenstein S, Zucker CL, Fleiss JL. Some statistical methods useful in circulation research. Circ Res 47: 1–9, 1980 [DOI] [PubMed] [Google Scholar]
- 25. Wilson C, Byrom F. Renal changes in malignant hypertension* 1: experimental evidence. 233: 353, 1939 [Google Scholar]