Abstract
Defective structural and neural upper airway properties both play a pivotal role in the pathogenesis of obstructive sleep apnea. A more favorable structural upper airway property [pharyngeal critical pressure under hypotonic conditions (passive Pcrit)] has been documented for women. However, the role of sex-related modulation in compensatory responses to upper airway obstruction (UAO), independent of the passive Pcrit, remains unclear. Obese apneic men and women underwent a standard polysomnography and physiological sleep studies to determine sleep apnea severity, passive Pcrit, and compensatory airflow and respiratory timing responses to prolonged periods of UAO. Sixty-two apneic men and women, pairwise matched by passive Pcrit, exhibited similar sleep apnea disease severity during rapid eye movement (REM) sleep, but women had markedly less severe disease during non-REM (NREM) sleep. By further matching men and women by body mass index and age (n = 24), we found that the lower NREM disease susceptibility in women was associated with an approximately twofold increase in peak inspiratory airflow (P = 0.003) and inspiratory duty cycle (P = 0.017) in response to prolonged periods of UAO and an ∼20% lower minute ventilation during baseline unobstructed breathing (ventilatory demand) (P = 0.027). Thus, during UAO, women compared with men had greater upper airway and respiratory timing responses and a lower ventilatory demand that may account for sex differences in sleep-disordered breathing severity during NREM sleep, independent of upper airway structural properties and sleep apnea severity during REM sleep.
Keywords: obstructive sleep apnea, pharyngeal critical pressure, compensatory responses to upper airway obstruction
obstructive sleep apnea (OSA) is a common chronic disorder with a higher prevalence and greater disease severity in adult men compared with women (14, 21, 36, 40, 41). Inspiratory collapse of the upper airway musculature is the predominant factor causing OSA (3, 30, 31). Structural upper airway properties and neural activation of the upper airway musculature in response to upper airway obstruction (4, 9, 15, 20, 24) play a pivotal role in the pathogenesis of OSA (18, 20, 24). However, it is currently unclear if differences in sleep apnea disease severity between men and women are due to structural differences of the upper airway or impairments in compensatory neural responses to upper airway obstruction.
Sex differences also exist in ventilatory control mechanisms that may contribute to the expression of sleep apnea. Differential responses to upper airway obstruction have been identified for chemoresponsiveness (26, 42), arousal effects on ventilation (11, 12), timing responses to acute periods of upper airway obstruction (28), and airflow demand (28, 38). These studies suggest that ventilatory compensatory mechanisms in response to upper airway obstruction may protect women from developing sleep-disordered breathing, although the mechanisms for stabilizing breathing in the face of upper airway obstruction remain unclear.
We hypothesized that 1) women have greater compensatory ventilatory responses to upper airway obstruction independent of upper airway structural properties, and that 2) these responses produce differences in the expression of sleep disordered breathing pattern in women compared with men. We matched obese men and women by body mass index (BMI), age and their pharyngeal critical pressure (Pcrit), which is an index for the structural upper airway properties (4, 9, 15, 20, 24). We first determined sleep-disordered breathing severity during non-rapid eye movement (NREM) and rapid eye movement (REM) sleep in these pairwise matched men and women. We then experimentally produced brief and prolonged periods of upper airway obstruction during stable NREM sleep to investigate whether upper airway responses and ventilatory responses differ between sexes. Some of the data of this study have been presented in abstract form (2).
METHODS
Subjects
We assessed passive and active upper airway properties in a total of 80 subjects, consisting of 34 apneic men, 39 apneic women, and 7 nonapneic obese women, who all participated in previous protocols examining upper airway properties during sleep. OSA was defined as a NREM apnea-hypopnea index (AHI) of ≥5 events/h. The protocols were approved by the Johns Hopkins Institutional Review Board, and informed, written consent was obtained from each volunteer.
Experimental Procedures
Baseline polysomnography.
A standard full-night baseline sleep study, including infrared video cameras monitoring (Somologica, Medcare, Buffalo, NY), was performed to stage sleep and score respiratory events, according to the American Academy of Sleep Medicine criteria (7).
Experimental setup for determining upper airway and ventilatory responses.
In a separate night, responses of the upper airway and respiratory timing were assessed by adding a pneumotachograph (Hans Rudolph, Kansas City, MO) attached to a differential pressure transducer, in series with a tightly fitting nasal mask and a modified continuous positive airway pressure (CPAP) device designed to deliver negative and positive pressures over a range of −20 to +20 cmH2O (ResMed, Bella Vista, NSW, Australia) to the standard polysomnographic sleep study, the setup (23) and performance characteristics (24) of which are previously described. Respiratory effort was monitored via a Hyatt-type esophageal balloon (Ackrad Laboratories, Cranford, NJ), an alae nasi EMG, and/or thoracic and abdominal inductive plethysmography. Subjects were instructed to sleep in supine position and were monitored visually with infrared video cameras continuously during measurement periods. All signals were recorded and displayed on a computer software system (Windag/200, Datag, Akron, OH; or Somologica, Medcare, Buffalo, NY).
Experimental Protocols
A series of experimental protocols during NREM sleep were performed to determine ventilatory responses of the upper airway and overall respiratory timing responses. Our experimental approach is illustrated in Fig. 1 and described in detail below.
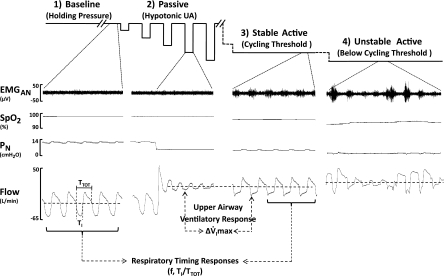
Fig. 1.
Schematic diagram of the experimental protocols (top traces) and polysomnographic responses during non-rapid eye movement (NREM) sleep in an apneic male subject. 1) Baseline (far left): nasal pressure (PN) was adjusted to effective continuous positive airway pressure (CPAP) and held for at least 3 min (holding pressure) to establish a stable nonflow-limited breathing pattern (see flow trace bottom far left). 2) Passive upper airway obstruction (UAO; middle left): a series of brief (5 breaths) drops in PN from holding pressure were performed without concomitant activation of EMG. During these drops, UAO ensued, as indicated by inspiratory flow limitation as in the flow trace below. 3) Active stable UAO (middle right) without arousal: PN was reduced stepwise by 1–2 cmH2O for at least 10 min to a level that produced a stable flow-limited breathing pattern with maximal concomitant cyclic inspiratory activation of EMG, but no intermittent hypoxia. This pressure is referred to as the cycling threshold, because any further reduction in PN was associated with an unstable breathing pattern, as characterized by the presence of either hypopneas, apneas, intermittent hypoxia, or arousal (see panel 4 far right: active unstable UAO). Our analytic approach is also shown. The difference in peak inspiratory airflow (V̇imax) between cycling threshold and passive drop at same pressure level was defined as upper airway ventilatory response (ΔV̇imax). Comparisons in timing parameters [respiratory rate (f), inspiratory duty cycle (Ti/Ttot)] between holding pressure and cycling threshold were taken as respiratory timing responses to UAO. EMGAN, electromyogram of alae nasi; SpO2, oxyhemoglobin saturation from pulse oximetry.
Passive condition: brief periods of upper airway obstruction.
We first titrated nasal CPAP to a holding pressure that attenuated neuromuscular activity, as reported previously (1, 23, 30). Thereafter, nasal pressure was repeatedly reduced from the holding pressure in 1- to 2-cmH2O increment to lower levels each for five breaths until complete upper airway closure (zero airflow) was induced (1, 23, 29). At least two series of stepwise reductions in nasal pressure were collected for subsequent analysis of passive Pcrit and airway resistance from pressure-flow relationship (23, 31) in each subject. If arousals occurred, the protocol was resumed after subjects returned to stable stage 2 NREM sleep for at least 3 min.
Active condition: sustained periods of upper airway obstruction.
Nasal pressure was reduced stepwise from holding pressure by 1–2 cmH2O for periods of at least 10 min during NREM sleep. As nasal pressure was sequentially reduced, initial stable airflow became progressively more flow limited, until the development of periodic hypopneas and/or apneas (>3 consecutive events in the last 5 min of the 10-min trial). The nasal pressure just before the development of periodic hypopneas and apneas was determined as the maximally active condition in the absence of arousal and referred to as the cycling threshold (20). If prolonged periods of awakening occurred, the protocol was resumed at a slightly higher nasal pressure after patients reinitiated 3 min of stable stage 2 NREM sleep.
Data Analyses
Analysis of primary breathwise outcome parameters was performed at three experimental conditions. 1) We first identified periods of nonflow-limited breathing during stable NREM sleep (baseline condition) for determining baseline minute ventilation when the individuals were set on holding pressure. 2) We then selected flow-limited breaths at the cycling threshold (cycling threshold condition) as follows: in the absence of any arousal during the cycling threshold, the last 10 flow-limited breaths before the end of this pressure level were selected. If arousal had occurred during the cycling threshold, flow-limited breaths were taken from the second half of the 10-min periods that were out of the vicinity of an cortical arousal (at least 1 min after or before an arousal). If a prolonged awakening (>60 s) occurred at the cycling threshold, we resumed the nasal pressure to holding pressure and reinitiated the protocol, as mentioned above. 3) Finally, we selected flow-limited breaths 2–5 from each acute pressure drop (passive condition), which matched the nasal pressure of the cycling threshold. Flow limitation was determined using the established criteria (6, 19, 24). These breaths were used to measure the primary outcome parameters, as detailed below and as illustrated in Fig. 1. Apneas standard American Academy of Sleep Medicine criteria (7), and hypopneas were defined as a discernable reduction in airflow that was either followed by an arousal, or associated with a ≥3% fall in oxyhemoglobin saturation.
Upper airway responses.
In the present study, we have defined an upper airway response as the difference in mean peak inspiratory airflow (V̇imax) of the breaths between the cycling threshold (n = 30–40 breaths/individual) and passive pressure drops (n = 7–12 breaths/individual) (20). In addition, the transmural pressure across the pharynx is defined as the mask pressure difference between cycling threshold and passive Pcrit, which was assessed to determine the intensity of the experimental stimulus at the cycling threshold in men and women.
Ventilatory responses.
For each subject, we determined respiratory rate, inspiratory duty cycle (Ti/Tt), and minute ventilation from at least 30–40 breaths at holding pressure and at cycling threshold. Differences in the Ti/Tt from holding pressure to cycling threshold were defined as the compensatory respiratory timing responses to upper airway obstruction. The minute ventilation at prolonged periods of upper airway obstruction (cycling threshold) compared with baseline nonflow-limited breathing (holding pressure) was used to determine the overall strength of the upper airway flow (V̇imax) and Ti/Tt responses.
Statistical Analysis
Multidimensional exact matching of subjects based on the major risk factors for sleep apnea, passive upper airway collapsibility, BMI, and age, was used to reducing the between-individual variability due to these factors. Power calculation for the change (Δ) in V̇imax as an outcome parameter paired group demonstrated a sample size of 12 achieve 95% power to detect a difference of 37 ml/s between the null hypothesis that both group means are 102 ml/s and the alternative hypothesis that the mean in the men is 65 ml/s with known group standard deviations of 25 ml/s, with a significance level (α) of 0.05 using a two-sided, two-sample t-test.
Primary outcomes in the study were the differences in V̇imax between the passive and active (cycling threshold) conditions, and the changes in ventilatory parameters (respiratory rate, Ti/Tt, and minute ventilation) between baseline and cycling threshold conditions, which were presented in absolute values and in percent change from baseline condition. Secondary outcomes included cycling threshold pressure level, transmural pressure, and polysomnographic parameters in all subjects. Data were confirmed to have a normal distribution (test for skewness and kurtosis). All comparisons of outcomes between conditions within groups and between pairwise matched groups were performed with paired, two-tailed Student's t-tests with Bonferroni adjustment. Values are expressed as means ± SD, unless otherwise stated, and P < 0.05 was considered significant.
RESULTS
Subjects Characteristics
From the original 80 subjects, we identified 31 pairs of Pcrit-matched (±1.0 cmH2O) apneic men and women (Table 1, left two columns) for comparing polysomnographic indexes in NREM and REM sleep. In these pairs, nine patients could not complete an “active” protocol, as outlined in Fig. 1. In an additional five patients, we could not identify sufficient durations of nasal pressures with stable flow-limited breathing (cycling threshold) due to either a low arousal threshold (n = 2) or an active Pcrit that was similar (n = 3) to the passive Pcrit. The mean passive Pcrit values (0.3 ± 3.3 cmH2O) of these five individuals were similar to those of the group as a whole.
Table 1.
Anthropometric data for matched apneic men and women
| Matched by Pcrit |
Matched by Pcrit, BMI, and Age |
|||
|---|---|---|---|---|
| Variable | Apneic Men | Apneic Women | Apneic Men | Apneic Women |
| n | 31 | 31 | 12 | 12 |
| Anthropometry | ||||
| Age, yr | 40.8 ± 9.9 | 44.0 ± 10.6 | 38.9 ± 6.8 | 40.7 ± 11.6 |
| Height, cm | 177.7 ± 8.5 | 164.8 ± 5.0* | 179.8 ± 4.3 | 167.8 ± 5.5* |
| Weight, kg | 122.0 ± 38.7 | 128.3 ± 31.8 | 151.6 ± 26.6 | 132.1 ± 22.2* |
| BMI, kg/m2 | 38.4 ± 11.3 | 46.9 ± 10.5* | 46.9 ± 8.7 | 46.7 ± 6.7 |
| Waist, cm | 125.7 ± 29.1 | 124.9 ± 20.5 | 146.9 ± 16.4 | 127.4 ± 17.0* |
| Hip, cm | 126.4 ± 22.3 | 139.3 ± 20.4* | 142.6 ± 19.6 | 139.1 ± 14.0 |
| Waist/hip ratio | 0.99 ± 0.11 | 0.90 ± 0.08* | 1.04 ± 0.08 | 0.92 ± 0.07* |
| Passive Pcrit, cmH2O | 0.2 ± 2.1 | 0.1 ± 2.0 | 0.9 ± 2.3 | 0.7 ± 2.1 |
| Passive RUS, cmH2O · l−1 · s | 20.2 ± 8.5 | 22.9 ± 11.0 | 21.3 ± 8.7 | 28.7 ± 12.8 |
Values are means ± SD; n, no. of subjects. BMI, body mass index; Pcrit, pharyngeal critical pressure; RUS, upstream resistance.
P < 0.05 vs. matched apneic men.
Of the 17 remaining pairs of patients, a total of 12 pairs could be further matched by BMI (±5 kg/m2). Results of the matching process of these pairs are presented in Table 1 (right two columns), demonstrating that men and women did not differ in their age, BMI, and passive Pcrit.
Polysomnographic Characteristics
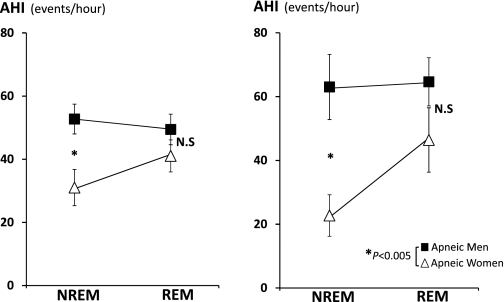
Figure 2 shows the AHI for NREM and REM sleep in the group of apneic men and women matched for passive Pcrit (Fig. 2, left) and the subgroup matched for BMI, age, and passive Pcrit (Fig. 2, right). As can be seen, while there was no difference in the AHI of REM sleep between men and women, the AHI during NREM sleep was approximately one-half in women compared with men. The lower severity of sleep-disordered breathing in women was also associated with a higher percentage of hypopneas, higher oxygen levels, and greater percentage of stage 3 sleep (see Table 2). A lower NREM AHI was also found in a subgroup of women (n = 5) matched to men (n = 5) by waist-to-hip ratio (0.89 in both groups) and passive Pcrit (−0.6 ± 2.6 vs. 0.7 ± 2.7 cmH2O). The neck circumference was also similar between men (41.6 ± 5.8 cm) and women (40.5 ± 1.4 cm). The NREM AHI in women was 12.0 ± 2.4 vs. 37.6 ± 29.5 events/h in men, despite a markedly higher BMI in women (51.4 ± 7.8 vs. 37.8 ± 12.1 kg/m2) and slightly older age (40.8 ± 12.8 vs. 35.6 ± 2.0 yr, women vs. men).
Fig. 2.
Mean (SE) values of apnea-hypopnea index (AHI) during NREM and rapid eye movement (REM) sleep. Left: 62 pairwise matched apneic men and women by passive pharyngeal critical pressure (Pcrit). Right: 24 pairwise matched apneic men and women by body mass index, age, and passive Pcrit. NS, not significant. *P < 0.005.
Table 2.
Polysomnographic data for matched apneic men and women
| Matched by Pcrit |
Matched by Pcrit, BMI, and Age |
|||
|---|---|---|---|---|
| Variable | Apneic Men | Apneic Women | Apneic Men | Apneic Women |
| n | 31 | 31 | 12 | 12 |
| TST, min | 385.4 ± 91.1 | 377.6 ± 77.2 | 367.8 ± 97.7 | 426.3 ± 42.0 |
| Sleep efficiency, %TST | 88.5 ± 8.5 | 82.7 ± 13.7 | 86.9 ± 13.3 | 88.7 ± 5.1 |
| NREM, %TST | 85.0 ± 6.9 | 87.1 ± 7.2 | 87.3 ± 7.4 | 84.1 ± 6.1 |
| Stage 1 | 24.7 ± 18.9 | 15.7 ± 13.5* | 25.1 ± 23.9 | 12.2 ± 7.5 |
| Stage 2 | 58.5 ± 15.4 | 61.3 ± 12.0 | 59.8 ± 20.2 | 60.1 ± 8.8 |
| Stage 3 | 1.8 ± 3.1 | 10.2 ± 8.9* | 2.4 ± 3.6 | 11.7 ± 8.7* |
| REM, %TST | 15.0 ± 6.9 | 12.9 ± 7.2 | 12.7 ± 7.4 | 15.9 ± 6.1 |
| Total AHI, events/h | 52.5 ± 25.1 | 33.6 ± 30.3* | 64.3 ± 33.5 | 26.7 ± 22.2* |
| NREM AHI, events/h | 52.7 ± 26.3 | 31.0 ± 31.9* | 63.0 ± 35.4 | 22.7 ± 22.5* |
| REM AHI, events/h | 49.4 ± 25.7 | 41.1 ± 26.8 | 64.7 ± 26.2 | 46.4 ± 34.9 |
| Supine NREM AHI | 51.7 ± 28.0 | 33.3 ± 34.3 | 67.2 ± 33.4 | 30.7 ± 33.5 |
| Supine REM AHI | 46.4 ± 26.5 | 41.0 ± 31.2 | 64.9 ± 19.8 | 48.9 ± 37.9 |
| Hypopnea, % | ||||
| NREM | 70.2 ± 29.1 | 82.9 ± 20.3* | 65.8 ± 24.3 | 76.0 ± 26.3 |
| REM | 69.5 ± 34.4 | 73.3 ± 33.9 | 52.7 ± 24.4 | 56.0 ± 36.2 |
| Arterial SaO2, % | ||||
| Average baseline value | ||||
| NREM | 95.9 ± 2.4 | 95.9 ± 1.7 | 95.1 ± 2.0 | 96.2 ± 2.0 |
| REM | 95.6 ± 2.9 | 95.1 ± 2.6 | 93.8 ± 3.7 | 95.1 ± 2.4 |
| Average low value | ||||
| NREM | 90.0 ± 4.7 | 91.4 ± 2.9 | 88.0 ± 4.5 | 92.1 ± 3.2* |
| REM | 87.8 ± 7.1 | 88.9 ± 6.4 | 84.6 ± 7.5 | 89.0 ± 4.9 |
Values are means ± SD; n, no. of subjects. TST, total sleep time; NREM, nonrapid eye movement; REM, rapid eye movement; AHI, apnea-hypopnea index; SaO2, oxyhemoglobin saturation.
P < 0.05 vs. matched apneic men.
Upper Airway Mechanical Load
The nasal pressure level at the cycling threshold and the transmural pressure (nasal pressure at cycling threshold minus passive Pcrit) were similar between men and women (nasal pressure: 4.9 ± 3.1 vs. 6.1 ± 2.3 cmH2O, P = 0.11, transmural pressure 4.1 ± 1.7 vs. 5.2 ± 1.7 cmH2O, P = 0.13, women vs. men).
Upper Airway Responses to Sustained Periods of Upper Airway Obstruction
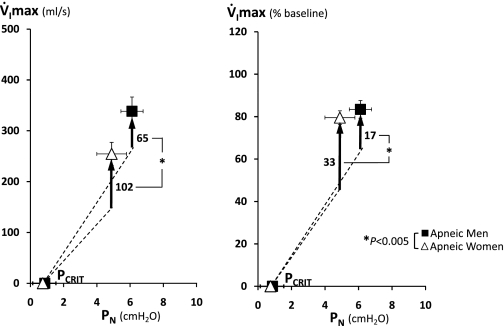
To determine compensatory ventilatory responses of the upper airway to sustained periods of upper airway obstruction, we compared V̇imax between passive condition and the cycling threshold. V̇imax at cycling threshold was lower in apneic women than men (Fig. 3 left), but this difference disappeared if V̇imax was adjusted for baseline V̇imax. As can be seen in Fig. 3, right, V̇imax at cycling threshold was 83% in men and 80% in women compared with each baseline inspiratory flow. In contrast, apneic women had greater V̇imax increases from passive to active condition compared with apneic men (ΔV̇imax, see upward vertical arrows, Fig. 3, left and right). Our data show that 1) men and women had similar percent reductions in inspiratory airflow at cycling threshold, but that 2) compensatory ventilatory responses of the upper airway (ΔV̇imax) were greater in women than men.
Fig. 3.
Mean passive pressure-flow relationships (see dashed line) were constructed from the passive pharyngeal Pcrit and upstream resistance values for 24 pairwise matched apneic men and women given in Table 1. Mean (SE) values of V̇imax are shown for the cycling threshold, as well as the magnitude of increase in V̇imax at cycling threshold from the passive pressure-flow relationship (see upward vertical arrows). Left: absolute values of V̇imax vs. PN. Right: percent changes in V̇imax relative to baseline (100%) vs. PN. *P < 0.005.
Respiratory Timing Responses to Sustained Periods of Upper Airway Obstruction (Baseline vs. Cycling Threshold)
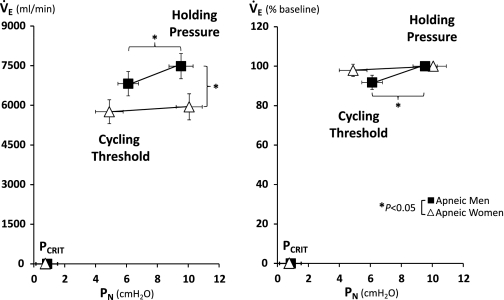
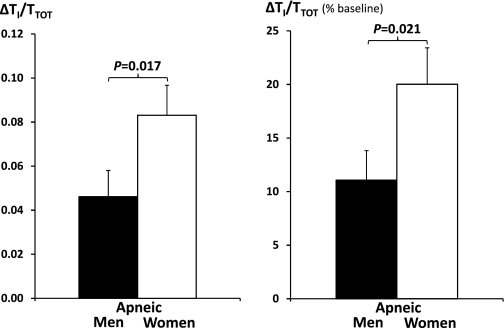
As shown in Fig. 4, women had lower minute ventilation at baseline nonflow-limited condition (holding pressure), indicating a lower ventilatory demand that is independent of BMI and age. While men demonstrated a significant decline of minute ventilation from baseline to cycling threshold, women maintained minute ventilation during inspiratory flow limitation at cycling threshold. As shown in Fig. 5, the preservation of ventilation at cycling threshold in women was associated with an approximately twofold increase in Ti/Tt (percent change from baseline; P = 0.021) from baseline, which was similar in men and women (0.41 ± 0.05 vs. 0.41 ± 0.05). There was no sex difference in respiratory rate at both baseline (17.5 ± 4.7 vs. 17.7 ± 3.6) and cycling threshold (18.9 ± 5.6 vs. 18.2 ± 4.25), for women vs. men.
Fig. 4.
Mean (SE) values of minute ventilation (V̇e) are shown for the holding pressure, cycling threshold, and passive pharyngeal Pcrit in 24 pairwise matched apneic men and women. Left: absolute values of V̇e vs. PN. Right: percent changes in V̇e relative to baseline (100%) vs. PN. *P < 0.05.
Fig. 5.
Mean (SE) values of increase in inspiratory duty cycle (ΔTI/TTOT) from baseline to the cycling threshold in absolute values (left) and in percent changes from baseline (right) in 24 pairwise matched apneic men and women.
DISCUSSION
In the present study, we found that apneic women compared with men with comparable passive upper airway mechanical properties had a markedly lower sleep apnea disease severity in NREM but not REM sleep. These differences were associated with greater compensatory responses of the upper airway (increase in inspiratory airflow), as well as greater increases of the Ti/Tt in women compared with men. Moreover, ventilatory demand (minute ventilation during unobstructed breathing) was lower in women, further decreasing the challenge of preserving ventilation in the face of upper airway obstruction during NREM sleep. In summary, women have a lower ventilatory demand and greater compensatory upper airway and respiratory timing responses to sustained upper airway obstruction, independent of the passive upper airway mechanical property, BMI, and age during NREM sleep. These differences may serve as physiological traits protecting women from development of sleep-disordered breathing and explain the sex differences in prevalence and clinical presentation of OSA.
Sex Differences in Sleep Apnea Severity Are Independent of Passive Pcrit
After having men and women matched for the passive upper airway mechanical properties, we found apneic women had similar sleep apnea disease severity compared with men during REM sleep, but not NREM sleep. The similarity of sleep-disordered breathing severity in REM sleep is best explained by the low muscular activity in REM sleep, which may approximate the hypotonic state of the passive Pcrit measurement. In fact, previously, Eastwood et al. (4) demonstrated that the REM, but not NREM, AHI correlated with the passive Pcrit obtained during general anesthesia. In contrast, despite similar upper airway mechanical properties, women had lower apnea and hypopnea rate and higher proportion of hypopneas during NREM sleep than men matched for BMI and age (see data in Table 2). Sex-related differences in sleep apnea severity have been previously demonstrated in some studies (21, 33, 36), but they did not adjust for both upper airway mechanical properties and anthropometric characteristics. We now extend the findings of these studies by showing that sex differences in sleep apnea still persist during NREM sleep when men and women are matched by their mechanical upper airway properties (passive Pcrit). Moreover, a lower AHI in NREM sleep existed even in women with a markedly higher BMI (∼15 kg/m2 heavier than men) but matched for truncal body composition and Pcrit. Our findings suggest that women have better mechanisms for preserving ventilation that is sleep stage dependent, leading to a lesser degree of sleep-disordered breathing in NREM but not REM sleep.
Airflow and Respiratory Timing Responses to Sustained Periods of Upper Airway Obstruction
Upper airway neuromuscular responses provide one mechanism for compensating for reductions in airflow during upper airway obstruction. After controlling for mechanical loads on the upper airway, we found that women had a greater V̇imax response than the men. These responses could be due to greater levels of neural input (10, 17, 25, 37) or differences in neural-mechanical efficiency (34, 35) of upper airway muscles. Further studies are required to determine which of these mechanisms are responsible for the greater upper airway response in women compared with men.
Ventilation can also be preserved during periods of upper airway obstruction by increasing the Ti/Tt (32 28, 5). Previously, we showed that increases in the duty cycle in response to brief upper airway obstruction (passive hypotonic condition) were independent of sex (28). In the present study, we also did not have a sex difference in the immediate Ti/Tt responses, but show that Ti/Tt during prolonged periods are sex dependent. While the passive condition during sleep is void of changes in arterial blood-gas changes, the prolonged period was associated with lower oxyhemoglobin levels. Alterations in blood gases are known to increase respiratory drive, thereby increasing Ti/Tt and inspiratory effort. Thus Ti/Tt increases during prolonged period of upper airway responses may be due to both chemical and mechanical reflex activation.
Mechanisms for Ventilatory Responses
We found that women tolerated a lower CPAP and thereby lower transmural pressure than men. This increased tolerance could be due to the increase in inspiratory airflow and/or Ti/Tt, both of which increase minute ventilation independent of changes in cortical activity (13, 28, 39). Alternative explanations are that women might have a lower ventilatory demand, higher arousal threshold, or generate less respiratory effort at a given reduction in ventilation (lower loop gain). Our finding that women have a lower eupneic ventilation on optimum CPAP (holding pressure) led us to conclude that a lower ventilatory demand may contribute to tolerating lower CPAP and hence to a better compensation during NREM sleep. In contrast, a higher arousal threshold or lower loop gain cannot be accounted for in explaining our findings, because we found that, unlike men, women did not tolerate any reduction in ventilation. This means that they either had a lower arousal threshold than men or a higher loop gain (or a combination of the two), which is the opposite of what one would expect for someone who adequately compensates during NREM sleep. In summary, our findings indicate that women can better preserve ventilation during NREM sleep than men by stronger upper airway and Ti/Tt responses and a lower ventilatory demand.
These greater upper airway and respiratory timing responses may depend on the level of sex hormones, or may be related to elevation in leptin, which has been shown to increase inspiratory muscle activity in response to hypercapnia (8), Ti/Tt in response to hyperinflation (8), and hypoxic and hypercapnic ventilatory drive during sleep (16, 22). Although we did not measure leptin levels in our study population, obese women are known to have higher leptin levels than BMI-matched men (27), which may contribute to greater ventilatory drive of the upper airway and greater Ti/Tt response in women.
Limitations
Several considerations may limit the interpretation of our finding. First, we recognize that our findings are limited to a severely obese population and cannot be generalized to lean to moderate obese cohorts. Nevertheless, our experimental paradigm allowed us to determine sex-related differences in compensatory ventilatory responses of the upper airway and Ti/Tt responses, independent of potential confounding by upper airway structural loads. Second, we did not control for the menstrual cycle and menopausal status in women. It is possible that hormonal changes may have influenced our upper airway and respiratory timing responses, and the inclusion of postmenopausal women may have led us to underestimate potential sex-related differences between men and women. Third, differential responses could potentially be due to a different experimental stimulus. Because there was no difference in the holding pressure, CPAP at the passive drop, and the percent decline in inspiratory airflow from holding pressure to the passive drop, we do not think that greater Ti/Tt responses were a result of differential stimulus to the upper airway. Fourth, although it was not investigated in this study, changes in the shape of inspiratory curve could be a factor that contributes to an increase in the mean airflow during airflow limitation at the cycling threshold. Fifth, obesity can alter the mechanical properties of the respiratory system and chemosensitivity, which may have influenced our findings. We did not determine pulmonary function tests and arterial blood gases in most of our patients. However, as shown in Table 2, we did not find a significant reduction in the baseline oxyhemoglobin saturation during REM and NREM sleep, indicating that oxygenation was not significantly impaired, and that potential obesity related mechanical and chemosensitive impairments were similar between men and women. Finally, we could not determine respiratory effort at a given reduction in ventilation (lower loop gain), because more than one-half of our population did not accept esophageal pressure measurements.
Implications
Our study has several methodological and clinical implications. First, unlike EMG measurement of upper airway dilator muscle, airflow (V̇imax) measurements are an absolute rather than relative measure of upper airway neuromuscular responses (20). Thus airflow measures offer a robust and feasible method for quantifying ventilatory responses of the upper airway musculature to a given stimulus. Second, mild reductions in airflow (∼12–20%) precipitated periodicity in breathing pattern in our obese population. Thus thresholds of reductions in airflow for defining hypopneas may be lower in obese compared with the general population, in which a flow reduction threshold of 30% defines periodic events such as hypopneas. Third, neural responses may differ in each sleep stage and account for differences in sleep-disordered breathing severity. Women had greater compensatory upper airway and timing responses in NREM sleep, which might wane in REM sleep. If neural compensatory drive is sleep stage dependent, a loss of these responses in REM sleep may explain the greater preponderance of REM sleep apnea in women. Finally, although the mechanisms for a greater upper airway and Ti/Tt response in women remain unclear, further research is warranted to uncover potential neural and chemical pathways of this response. Discovering agents that can modulate these pathways may lead to the development of specific pharmacological treatment for OSA.
Summary
Our findings demonstrate that women have a lower ventilatory demand and greater compensatory upper airway and respiratory timing responses to sustained upper airway obstruction independent of the passive upper airway mechanical property, BMI, and age during NREM sleep. These differences may serve as physiological traits protecting women from development of sleep-disordered breathing and explain the sex differences in prevalence and clinical presentation of OSA.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute (Washington, DC) Grants HL-72126, HL-50381, HL-37379, HL-077137, and P50 HL-084945-01.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: C.-H.C., J.P.K., S.P.P., P.L.S., A.R.S., and H.S. conception and design of research; C.-H.C., J.P.K., S.P.P., B.M.M., and H.S. performed experiments; C.-H.C., J.P.K., S.P.P., B.M.M., and H.S. analyzed data; C.-H.C., J.P.K., S.P.P., P.L.S., A.R.S., and H.S. interpreted results of experiments; C.-H.C., J.P.K., S.P.P., and H.S. prepared figures; C.-H.C., J.P.K., S.P.P., B.M.M., and H.S. drafted manuscript; C.-H.C., J.P.K., S.P.P., B.M.M., P.L.S., A.R.S., and H.S. edited and revised manuscript; C.-H.C., J.P.K., S.P.P., B.M.M., P.L.S., A.R.S., and H.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Steven Shapiro for contributions to this study, which included technical support, data analysis, and help in the preparation of tables and figures.
REFERENCES
- 1. Boudewyns A, Punjabi N, Van de Heyning PH, De Backer WA, O'Donnell CP, Schneider H, Smith PL, Schwartz AR. Abbreviated method for assessing upper airway function in obstructive sleep apnea. Chest 118: 1031–1041, 2000 [DOI] [PubMed] [Google Scholar]
- 2. Chin CH, Kirkness JP, Patil SP, McGinley BM, Smith PL, Schwartz AR, Schneider H. Neuromuscular response to upper airway obstruction in apneic men and women (Abstract). Am J Respir Crit Care Med 181: A2198, 2010 [Google Scholar]
- 3. Deegan PC, McNicholas WT. Pathophysiology of obstructive sleep apnoea. Eur Respir J 8: 1161–1178, 1995 [DOI] [PubMed] [Google Scholar]
- 4. Eastwood PR, Szollosi I, Platt PR, Hillman DR. Comparison of upper airway collapse during general anaesthesia and sleep. Lancet 359: 1207–1209, 2002 [DOI] [PubMed] [Google Scholar]
- 5. Hoshino Y, Ayuse T, Kurata S, Ayuse T, Schneider H, Kirkness JP, Patil SP, Schwartz AR, Oi K. The compensatory responses to upper airway obstruction in normal subjects under propofol anesthesia. Respir Physiol Neurobiol 166: 24–31, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hosselet JJ, Norman RG, Ayappa I, Rapoport DM. Detection of flow limitation with a nasal cannula/pressure transducer system. Am J Respir Crit Care Med 157: 1461–1467, 1998 [DOI] [PubMed] [Google Scholar]
- 7. Iber C, Ancoli-Israel S, Chesson AL, Jr, Quan SF. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Westchester, IL: American Academy of Sleep Medicine, 2007 [Google Scholar]
- 8. Inyushkina EM, Merkulova NA, Inyushkin AN. Mechanisms of the respiratory activity of leptin at the level of the solitary tract nucleus. Neurosci Behav Physiol 40: 707–713, 2010 [DOI] [PubMed] [Google Scholar]
- 9. Isono S, Tanaka A, Remmers JE, Nishino T. Comparison of static mechanics of passive pharynx between patients with obstructive sleep apnea and normal subjects (Abstract). Am J Respir Crit Care Med 151: A667, 1995 [Google Scholar]
- 10. Jordan AS, Catcheside PG, O'Donoghue FJ, Saunders NA, McEvoy RD. Genioglossus muscle activity at rest and in response to brief hypoxia in healthy men and women. J Appl Physiol 92: 410–417, 2002 [DOI] [PubMed] [Google Scholar]
- 11. Jordan AS, Eckert DJ, Catcheside PG, McEvoy RD. Ventilatory response to brief arousal from non-rapid eye movement sleep is greater in men than in women. Am J Respir Crit Care Med 168: 1512–1519, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Jordan AS, McEvoy RD, Edwards JK, Schory K, Yang CK, Catcheside PG, Fogel RB, Malhotra A, White DP. The influence of gender and upper airway resistance on the ventilatory response to arousal in obstructive sleep apnoea in humans. J Physiol 558: 993–1004, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jordan AS, Wellman A, Heinzer RC, Lo YL, Schory K, Dover L, Gautam S, Malhotra A, White DP. Mechanisms used to restore ventilation after partial upper airway collapse during sleep in humans. Thorax 62: 861–867, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kapsimalis F, Kryger MH. Gender and obstructive sleep apnea syndrome, part 1: clinical features. Sleep 25: 412–419, 2002 [PubMed] [Google Scholar]
- 15. Kirkness JP, Schwartz AR, Schneider H, Punjabi NM, Maly JJ, Laffan AM, McGinley BM, Magnuson T, Schweitzer M, Smith PL, Patil SP. Contribution of male sex, age, and obesity to mechanical instability of the upper airway during sleep. J Appl Physiol 104: 1618–1624, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Makinodan K, Yoshikawa M, Fukuoka A, Tamaki S, Koyama N, Yamauchi M, Tomoda K, Hamada K, Kimura H. Effect of serum leptin levels on hypercapnic ventilatory response in obstructive sleep apnea. Respiration 75: 257–264, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Malhotra A, Huang Y, Fogel RB, Pillar G, Edwards JK, Kikinis R, Loring SH, White DP. The male predisposition to pharyngeal collapse: importance of airway length. Am J Respir Crit Care Med 166: 1388–1395, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Malhotra A, White DP. Obstructive sleep apnoea. Lancet 360: 237–245, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Mansour KF, Rowley JA, Badr MS. Noninvasive determination of upper airway resistance and flow limitation. J Appl Physiol 97: 1840–1848, 2004 [DOI] [PubMed] [Google Scholar]
- 20. McGinley BM, Schwartz AR, Schneider H, Kirkness JP, Smith PL, Patil SP. Upper airway neuromuscular compensation during sleep is defective in obstructive sleep apnea. J Appl Physiol 105: 197–205, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. O'Connor C, Thornley KS, Hanly PJ. Gender differences in the polysomnographic features of obstructive sleep apnea. Am J Respir Crit Care Med 161: 1465–1472, 2000 [DOI] [PubMed] [Google Scholar]
- 22. O'Donnell CP, Schaub CD, Haines AS, Berkowitz DE, Tankersley CG, Schwartz AR, Smith PL. Leptin prevents respiratory depression in obesity. Am J Respir Crit Care Med 159: 1477–1484, 1999 [DOI] [PubMed] [Google Scholar]
- 23. Patil SP, Punjabi NM, Schneider H, O'Donnell CP, Smith PL, Schwartz AR. A simplified method for measuring critical pressures during sleep in the clinical setting. Am J Respir Crit Care Med 170: 86–93, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Patil SP, Schneider H, Marx JJ, Gladmon E, Schwartz AR, Smith PL. Neuromechanical control of upper airway patency during sleep. J Appl Physiol 102: 547–556, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Pillar G, Malhotra A, Fogel R, Beauregard J, Schnall R, White DP. Airway mechanics and ventilation in response to resistive loading during sleep: influence of gender. Am J Respir Crit Care Med 162: 1627–1632, 2000 [DOI] [PubMed] [Google Scholar]
- 26. Regensteiner JG, Woodard WD, Hagerman DD, Weil JV, Pickett CK, Bender PR, Moore LG. Combined effects of female hormones and metabolic rate on ventilatory drives in women. J Appl Physiol 66: 808–813, 1989 [DOI] [PubMed] [Google Scholar]
- 27. Saad MF, Damani S, Gingerich RL, Riad-Gabriel MG, Khan A, Boyadjian R, Jinagouda SD, El-Tawil K, Rude RK, Kamdar V. Sexual dimorphism in plasma leptin concentration. J Clin Endocrinol Metab 82: 579–584, 1997 [DOI] [PubMed] [Google Scholar]
- 28. Schneider H, Krishnan V, Pichard LE, Patil SP, Smith PL, Schwartz AR. Inspiratory duty cycle responses to flow limitation predict nocturnal hypoventilation. Eur Respir J 33: 1068–1076, 2009 [DOI] [PubMed] [Google Scholar]
- 29. Schwartz AR, O'Donnell CP, Baron J, Schubert N, Alam D, Samadi SD, Smith PL. The hypotonic upper airway in obstructive sleep apnea: role of structures and neuromuscular activity. Am J Respir Crit Care Med 157: 1051–1057, 1998 [DOI] [PubMed] [Google Scholar]
- 30. Schwartz AR, Smith PL, Wise RA, Gold AR, Permutt S. Induction of upper airway occlusion in sleeping individuals with subatmospheric nasal pressure. J Appl Physiol 64: 535–542, 1988 [DOI] [PubMed] [Google Scholar]
- 31. Smith PL, Wise RA, Gold AR, Schwartz AR, Permutt S. Upper airway pressure-flow relationships in obstructive sleep apnea. J Appl Physiol 64: 789–795, 1988 [DOI] [PubMed] [Google Scholar]
- 32. Tagaito Y, Schneider H, O'Donnell CP, Smith PL, Schwartz AR. Ventilating with tracheal gas insufflation and periodic tracheal occlusion during sleep and wakefulness. Chest 122: 1742–1750, 2002 [DOI] [PubMed] [Google Scholar]
- 33. Vagiakis E, Kapsimalis F, Lagogianni I, Perraki H, Minaritzoglou A, Alexandropoulou K, Roussos C, Kryger M. Gender differences on polysomnographic findings in Greek subjects with obstructive sleep apnea syndrome. Sleep Med 7: 424–430, 2006 [DOI] [PubMed] [Google Scholar]
- 34. Van Lunteren E, Dick TE. Intrinsic properties of pharyngeal and diaphragmatic respiratory motoneurons and muscles. J Appl Physiol 73: 787–800, 1992 [DOI] [PubMed] [Google Scholar]
- 35. Van Lunteren E, Strohl KP. The muscles of the upper airways. Clin Chest Med 7: 171–188, 1986 [PubMed] [Google Scholar]
- 36. Ware JC, McBrayer RH, Scott JA. Influence of sex and age on duration and frequency of sleep apnea events. Sleep 23: 165–170, 2000 [PubMed] [Google Scholar]
- 37. White DP, Edwards JK, Shea SA. Local reflex mechanisms: influence on basal genioglossal muscle activation in normal subjects. Sleep 21: 719–728, 1998 [DOI] [PubMed] [Google Scholar]
- 38. Younes M. Contributions of upper airway mechanics and control mechanisms to severity of obstructive apnea. Am J Respir Crit Care Med 168: 645–658, 2003 [DOI] [PubMed] [Google Scholar]
- 39. Younes M. Role of arousals in the pathogenesis of obstructive sleep apnea. Am J Respir Crit Care Med 169: 623–633, 2004 [DOI] [PubMed] [Google Scholar]
- 40. Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 328: 1230–1235, 1993 [DOI] [PubMed] [Google Scholar]
- 41. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med 165: 1217–1239, 2002 [DOI] [PubMed] [Google Scholar]
- 42. Zhou XS, Shahabuddin S, Zahn BR, Babcock MA, Badr MS. Effect of gender on the development of hypocapnic apnea/hypopnea during NREM sleep. J Appl Physiol 89: 192–199, 2000 [DOI] [PubMed] [Google Scholar]