Abstract
The objectives of this study were to 1) identify the independent effects of exercise (aerobic or resistance training) and weight loss on whole body insulin sensitivity and 2) determine if aerobic or resistance training would be more successful for maintaining improved whole body insulin sensitivity 1 yr following weight loss. Subjects were 97 healthy, premenopausal women, body mass index (BMI) 27–30 kg/m2. Following randomized assignment to one of three groups, diet only, diet + aerobic, or diet + resistance training until a BMI <25 kg/m2 was achieved, body composition, fat distribution, and whole body insulin sensitivity were determined at baseline, in the weight reduced state, and at 1-yr follow up. The whole body insulin sensitivity index (SI) was determined using a frequently sampled intravenous glucose tolerance test. Results of repeated-measures ANOVA indicated a significant improvement in SI following weight loss. However, there were no group or group×time interactions. At 1-yr follow up, there were no significant time or group interactions for SI; however, there was a significant group×time interaction for SI. Post hoc analysis revealed that women in the aerobic training group showed a significant increased SI from weight reduced to 1-yr follow up (P < 0.05), which was independent of intra-abdominal adipose tissue and %fat. No significant differences in SI from weight reduced to 1-yr follow up were observed for diet only or diet + resistance groups. Additionally, multiple linear regression analysis revealed that change in whole body insulin sensitivity from baseline to 1-yr follow up was independently associated with the change in V̇o2max from baseline to 1-yr follow up (P < 0.05). These results suggest that long-term aerobic exercise training may conserve improvements in SI following weight loss and that maintaining cardiovascular fitness following weight loss may be important for maintaining improvements in SI.
Keywords: diet, cardiovascular fitness, visceral fat
obesity and physical inactivity are associated with decreased insulin sensitivity and the development of Type 2 diabetes (15, 20). It has been reported that the more overweight or obese an individual is, the more likely they are to be insulin resistant (6). It is well recognized that weight loss achieved through caloric restriction and/or exercise can improve insulin sensitivity in obese and overweight individuals (5, 7, 8, 26, 27). The improvements in insulin sensitivity following caloric restriction appear to be attributable to changes in body composition (2, 7), whereas the improvement in glucose tolerance and/or insulin sensitivity following exercise training appear to be attributable to changes within the contracting muscle via both a short-term insulin-independent mechanism and long-term insulin-dependent mechanism (9, 11, 25, 30). Therefore, the effects of weight loss and exercise training are often thought to have a synergistic effect on insulin sensitivity.
Weight loss interventions are largely successful at inducing weight loss; the usual course of weight loss interventions show that weight is rapidly lost at the onset of therapy, and the greatest loss occurs 6-mo following treatment (13). However, it has also been shown that approximately one-third of those that successfully lose weight will regain weight within 1 yr following treatment (29). Given that modest weight loss is associated with reduced incidence of Type 2 diabetes (18, 19), hypertension (21), dyslipidemia (14), and improved control of diabetes (23), it is important to assess the effect of different treatments on weight loss and associated risk factors associated with metabolic and cardiovascular diseases.
Current literature regarding the independent effects of exercise on improvement in insulin sensitivity has yielded equivocal results. Some studies suggest that exercise in conjunction with diet provides an additive effect on insulin sensitivity (3, 5, 24), whereas others do not (12, 26). Furthermore, a recent study by Thomas et al. (28) used a novel study design to induce weight regain following weight loss and showed that exercise training preserved several of the improvements in metabolic syndrome parameters that improved following weight loss, demonstrating an independent effect of exercise on these variables even in the presence of weight regain (28). To date, we are unaware of any well-controlled randomized studies that have assessed the independent effects of diet with and without exercise training on whole body insulin sensitivity for 1 yr following weight loss. Therefore, the objectives of this study were to 1) identify the independent effects of exercise (aerobic or resistance training) and weight loss on whole body insulin sensitivity and 2) determine if diet with or without aerobic or resistance training would be more successful for maintaining whole body insulin sensitivity during a 1-yr follow-up period.
METHODS
Subjects.
The study group comprised 213 healthy premenopausal women who volunteered for, and enrolled in, an ongoing study designed to examine metabolic factors that predispose women to weight gain. The sample size included in this study was 97 women from the previously mentioned parent study. This sample size included those subjects that adhered to the diet and exercise requirements for the weight loss phase and remained in the study throughout the 1-yr follow-up assessment. Importantly, there were no significant baseline differences for body mass index (BMI), insulin sensitivity, or body composition measurements between individuals that adhered to or dropped out of the study. Therefore, rather than perform an intent to treat analysis using participants that withdrew from the study, we included only the 97 women that remained in the study through the 1-yr follow-up assessment. Inclusion criteria for the parent study were BMI 27 and 30 kg/m2, premenopausal, age 21–46 yr, sedentary (no more than one time per week regular exercise). All subjects were nonsmokers, of overall good health, and had normal menstrual cycles. Normal glucose tolerance was documented by 2-h postprandial blood glucose levels after an oral glucose load. None of the subjects used oral contraceptives at the time of enrollment in the study or medications known to affect body composition. The Institutional Review Board for Human Subjects-approved informed consent was obtained prior to participation in the study in compliance with the Department of Health and Human Services Regulations for Protection of Human Research Subjects.
Study design.
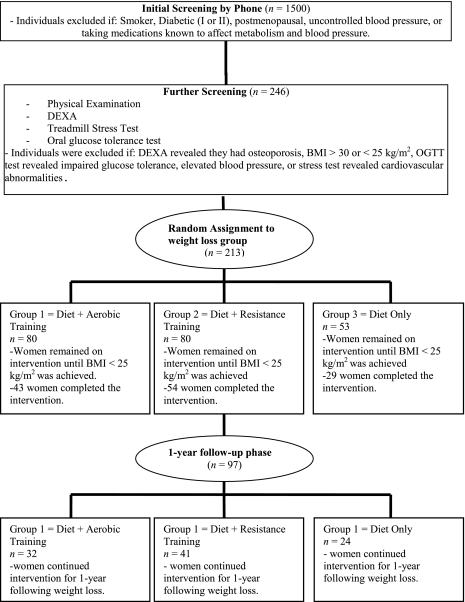
Subjects were randomly assigned to one of three different weight loss groups: diet and aerobic exercise, diet and resistance exercise, and diet only (Fig. 1). All food was furnished during weight loss and consisted of 800 kcal/day that were 20–22% fat, 18–22% protein, and 58–62% carbohydrate. Subjects picked up food at the General Clinical Research Center (GCRC) twice weekly and were instructed to remain on the 800 kcal/day diet until a BMI of <25 kg/m2 was reached. Time needed to reach the goal of a 25 kg/m2 BMI was variable with a mean of 154 ± 61 days. However, no differences between the groups was found (P = 0.2) Subjects were evaluated three times, at baseline in the overweight state (213 subjects were assessed at baseline), following weight loss (BMI <25 kg/m2; 126 subjects reached the target BMI), and 1 yr following the weight loss (97 subjects returned for the 1-yr post weight loss evaluation). During the 1 yr following weight loss, subjects were given instructions on a balanced diet that focused on low-density food intake according to EatRight Weight Management Program principles (31). Data presented in this article are for the 97 subjects (52 African American and 45 European American) that completed the 1-yr evaluation.
Fig. 1.
Flow of participants throughout the study. DEXA, dual-energy x-ray absorptiometry; OGTT, oral glucose tolerance test; BMI, body mass index.
Prior to all evaluations (baseline, weight reduced, and 1-yr follow up), subjects were maintained in a weight-stable state for 4 wk. During those 4 wk, body weight measurements were made at visits to the GCRC 3 days/wk for the first 2 wk and 5 days/wk for the last 2 wk. During the final 2 wk, all meals were provided through the GCRC Research Kitchen to ensure weight stability of <1% variation from initial weight and to maintain daily macronutrient intake within the range of 20–22% of energy as fat, 18–22% as protein, and 58–62% as carbohydrate. Subjects were then admitted to the GCRC for 4 days, during the follicular phase of the menstrual cycle, and underwent assessment of insulin sensitivity, body composition, and fat distribution.
Those subjects assigned to exercise groups were scheduled to train three times each week during weight loss and two times each week during the 1 yr following weight loss. All exercise training was done in an exercise training facility devoted entirely to research and was supervised by exercise physiology study personnel.
Insulin sensitivity.
Whole body insulin sensitivity (SI) was assessed on an in-patient basis in the GCRC after an overnight fast with an insulin-modified, frequently sampled intravenous glucose tolerance test (IVGTT). The IVGTT was performed at least 48 h following the last exercise session for exercise groups. Prior to testing, flexible intravenous catheters were placed in the antecubital spaces of both arms. Three, 2.0-ml blood samples were taken over a 20-min period for determination of basal glucose and insulin (the average of the values was used for basal “fasting” concentrations). At time 0, glucose (50% dextrose; 11.4 g/m2) was administered intravenously. Insulin (0.02 U/kg, Humulin, Eli Lilly, Indianapolis, IN) was injected at 20 min post-glucose injection. Blood samples (2.0 ml) were then collected at the following times (min) relative to glucose administration: 2, 3, 4, 5, 6, 8, 10, 12, 15, 19, 20, 21, 22, 24, 26, 28, 30, 35, 40, 45, 50, 55, 60, 70, 80, 100, 120, 140, and 180. Sera were stored at −85°C until analyzed. Glucose and insulin values were entered into the MINMOD computer program (ver. 3, Richard N. Bergman) for determination of the insulin sensitivity index (1, 22, 32). The acute insulin response (AIR) to intravenous glucose was calculated as the average incremental plasma insulin concentration from the 3rd to 5th min after the glucose bolus. The disposal index was calculated as the product of SI and AIR.
Abdominal adipose tissue.
Cross-sectional area of intra-abdominal adipose tissue (IAAT) and subcutaneous abdominal adipose tissue (SAAT) was determined by computed tomography with the use of a HiLight/HTD Advantage scanner (General Electric, Milwaukee, WI) set at 120 kVp (peak kilovoltage) and 40 mA. Subjects were examined in the supine position with their arms stretched above their heads, taking a 5-mm scan for 2 s at approximately the level of the fourth and fifth lumbar vertebrae. With the use of procedures established by Kvist et al. (16), the attenuation range for adipose tissue was −30 to −190 Hounsfield units. Cross sections of adipose tissue were determined by using a computerized fat tissue-highlighting technique. Tissue cross sections between −30 and −190 Hounsfield units in the respective areas were considered to be IAAT. Both intra- and interobserver test-retest reliability for IAAT and SAAT had an r value of 0.99 with a coefficient of variation of <2% for re-evaluation of 20 scans.
Aerobic training.
Aerobic training entailed continuous walking/jogging on a treadmill, commencing with a warm-up of 3 min, and 3–5 min of stretching. During the first week of training, the subjects performed 20 min of continuous exercise at 67% maximum heart rate. Each week after the 1st week, duration and intensity increased so that by the beginning of the 8th wk, subjects exercised continuously at 80% of maximum heart rate for 40 min. Subjects were encouraged to increase intensity (either speed or grade) when average exercise heart rate was consistently below 80% of maximum. After the exercise session, subjects cooled down for 3–5 min with gradually decreasing exercise intensity.
Resistance training.
After a warm-up on the treadmill or cycle ergometer for 5 min and 3–5 min of stretching, subjects performed the following exercises: squats, leg extension, leg curl, elbow flexion, triceps extension, lateral pull-down, bench press, military press, lower back extension, and bent leg sit-ups. One set of 10 repetitions was performed during the first 4 wk, after which two sets of 10 repetitions were performed for each exercise with 2-min rest between sets. The training was progressive with intensity based on 80% of the maximum weight that an individual lifted one time (1 RM). Strength was evaluated every 3 wk, and adjustments in training resistance were made based on the most current 1 RM in both the weight loss and 1-yr weight maintenance phases. In both the aerobic and resistance exercise groups, subjects were expected to train 3 days/wk during the weight loss and 2 days/wk during 1-yr follow up.
Statistical analysis.
Descriptive statistics were computed for each treatment group (diet only, diet/aerobic, and diet/resistance) at baseline, following weight loss, and at 1-yr follow up. All values are reported as means ± SD. All statistical models were evaluated for residual normality, and logarithmic transformations were performed when appropriate. All data were analyzed using SPSS.
Overall comparisons of the change in insulin sensitivity by intervention group were performed using a 2 (time) × 3 (group) repeated-measures analysis of variance (ANOVA) for the overweight to reduced weight period and the reduced weight to 1-yr follow up. Analysis of covariance with repeated measures was then conducted for insulin sensitivity to adjust for potential confounders, such as changes in body composition (BMI) and fat distribution (IAAT, SAAT, and %fat). Tukey's post hoc analyses were applied when significant group×time interactions occurred.
To further explore potential factors that may be mediating changes in whole body insulin sensitivity; changes between variables (IAAT, SAAT, insulin sensitivity, %fat, and V̇o2max) from baseline to weight reduced, weight reduced to 1-yr follow up, and baseline to 1-yr follow up were calculated. Multiple linear regression analysis was used to identify the independent associations of changes in whole body insulin sensitivity (dependent variable) with changes in IAAT, SAAT, %fat, and V̇o2max (independent variables). Standardized regression coefficients were determined for each independent variable.
RESULTS
Descriptive statistics at baseline and the effects of time, intervention group, and intervention group×time interaction on all variables are shown in Table 1. At baseline there were no significant group differences for any of the variables. A significant time effect was observed for all variables following weight loss. However, there were no group or group×time interactions for any variable from baseline to weight reduction (Table 1).
Table 1.
Body composition and whole body insulin sensitivity with weight loss and 1 yr following weight loss by intervention group
| Aerobic (n = 32) |
Resistance (n = 41) |
Diet (n = 24) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Weight-reduced | 1-yr follow | Baseline | Weight-reduced | 1-yr follow | Baseline | Weight-reduced | 1-yr follow | |
| Body wt, kg | 75 ± 6 | 64 ± 5a | 69 ± 8c | 78 ± 8 | 66 ± 7a | 69 ± 8c | 79 ± 8 | 65 ± 6a | 72 ± 8c |
| BMI, kg/m2 | 28 ± 1 | 24 ± 1a | 26 ± 2c | 28 ± 1 | 24 ± 1a | 26 ± 1c | 28 ± 1 | 24 ± 1a | 26 ± 2c |
| Body fat, % | 46 ± 3 | 35 ± 4a | 41 ± 5c | 45 ± 4 | 34 ± 5a | 39 ± 5c | 45 ± 3 | 34 ± 5a | 41 ± 5c |
| IAAT, cm2 | 81 ± 28 | 46 ± 18a | 54 ± 21c,d | 70 ± 26 | 45 ± 22a | 47 ± 26c,d | 83 ± 32 | 50 ± 21a | 62 ± 28c,d |
| SAAT, cm2 | 202 ± 59 | 141 ± 52a | 168 ± 60c | 187 ± 48 | 132 ± 38a | 146 ± 39c | 184 ± 48 | 121 ± 44a | 158 ± 48c |
| Lean mass, kg | 42 ± 3.3 | 41.9 ± 3.2a,b | 41.7 ± 3.5e | 44.1 ± 4.5 | 44.5 ± 4.5a,b | 44.3 ± 4.7e | 44.7 ± 3.6 | 43.7 ± 3.9a,b | 44 ± 3.9e |
| Vo2max, ml · kg−1 · min−1 | 27.9 ± 4 | 34 ± 5.6a | 32.6 ± 5.6c,e | 28.7 ± 3.4 | 33.1 ± 4.5a | 31.6 ± 5.2c,e | 27.1 ± 3.4 | 31.2 ± 4.5a | 28.9 ± 4.5c,e |
| Fasting glucose, mg/dl | 85 ± 7 | 84 ± 6a | 87 ± 11c | 87 ± 7 | 86 ± 8a | 88 ± 8c | 89 ± 6 | 86 ± 6a | 92 ± 9c |
| Fasting insulin, μIU/ml | 11 ± 4 | 9 ± 3a | 8.5 ± 3c | 12 ± 3 | 9 ± 3a | 10 ± 4c | 11 ± 4 | 8 ± 3a | 9 ± 4c |
| Disposition index (SI × AIR) | 1,846 ± 1,140 | 1,978 ± 1,165a | 2,553 ± 1,280 | 2,175 ± 1,307 | 2,282 ± 1,073a | 2,516 ± 1,556 | 1,335 ± 681 | 2,031 ± 1,695a | 1,996 ± 1,025 |
| SI (× 10−4min−1/μIU/ml) | 2.8 ± 1.7 | 3.9 ± 2.0a | 5.0 ± 2.9d | 3.3 ± 1.8 | 4.4 ± 2.0a | 4.1 ± 1.9d | 2.3 ± 0.89 | 4.5 ± 1.9a | 3.7 ± 1.4d |
All values are reported as mean ± SD. BMI, body mass index; IAAT and SAAT, cross-sectional area of intra-abdominal adipose tissue and subcutaneous abdominal adipose tissue, respectively; SI, sensitivity index; AIR, acute insulin response.
Significant time effect from baseline to weight reduced (P < 0.01).
Significant group × time interaction from baseline to weight reduced. No group interactions were observed from baseline to weight reduced.
Significant time effect from weight reduced to 1-yr follow up.
Significant group × time interaction from weight reduced to 1-yr follow up.
Significant group differences from weight reduced to 1-yr follow up.
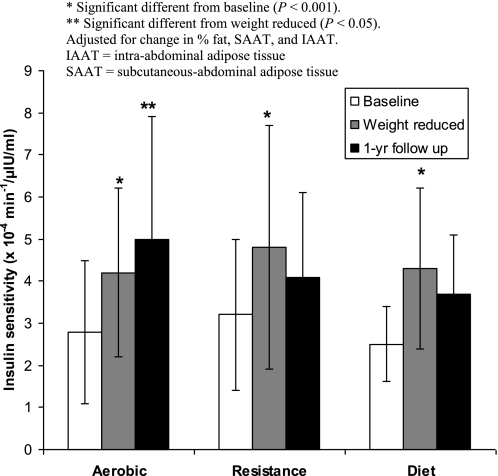
At 1-yr follow up a significant time effect was observed for body weight, BMI, %fat, IAAT, V̇o2max, fasting glucose, and fasting insulin. A significant group effect was observed for V̇o2max, such that V̇o2max was significantly higher in the aerobic training and resistance training group compared with diet only (P < 0.05). Additionally, a significant time×group interaction was observed for whole body insulin sensitivity (Table 1). Post hoc analysis revealed that aerobic training significantly increased whole body insulin sensitivity from weight reduced to 1-yr follow up (P < 0.05), whereas diet only and resistance training showed no statistical difference. Furthermore the improvement in whole body insulin sensitivity with aerobic training was independent of changes in IAAT, SAAT, and %fat (Fig. 2). There were no other group or group×time differences observed for any of the other variables.
Fig. 2.
Changes in whole body insulin sensitivity following weight loss and 1-yr follow up.
In multiple linear regression analysis, the change in whole body insulin sensitivity from baseline to 1-yr follow up was independently associated with change in V̇o2max from baseline to 1-yr follow up (P < 0.05). No other significant associations were observed for any other variables or time periods. These data are presented in Table 2.
Table 2.
A. Multiple linear regression model of whole body insulin sensitivity with IAAT, SAAT, %fat, and Vo2max
| Time | Independent Variables | Standardized β | P |
|---|---|---|---|
| Baseline–Weight Reduced (n = 126) | IAAT | −0.062 | 0.535 |
| Model R2 = 0.011 | SAAT | 0.020 | 0.844 |
| % Fat | −0.064 | 0.522 | |
| Vo2max | 0.052 | 0.600 | |
| Weight Reduced–1 yr Follow Up (n = 97) | IAAT | 0.091 | 0.579 |
| Model R2 = −0.003 | SAAT | −0.070 | 0.655 |
| % Fat | −0.167 | 0.195 | |
| Vo2max | 0.107 | 0.384 | |
| Baseline–1 yr Follow Up (n = 97) | IAAT | 0.066 | 0.600 |
| Model R2 = 0.113 | SAAT | 0.060 | 0.634 |
| % Fat | 0.203 | 0.105 | |
| Vo2max | 0.336 | 0.005* |
P < 0.05.
DISCUSSION
The purpose of this study in healthy overweight premenopausal women was to identify the independent effects of energy restriction alone and energy restriction in combination with aerobic or resistance exercise training on whole body insulin sensitivity during weight loss and during 1-yr follow up. We found that the addition of aerobic or resistance exercise training to the energy restriction paradigm during weight loss did not provide any additive effects on whole body insulin sensitivity; however, during the 1-yr weight maintenance phase aerobic exercise led to a significant improvement in whole body insulin sensitivity, whereas there were no further improvements in whole body insulin sensitivity in the diet or resistance training groups. Furthermore this improvement in whole body insulin sensitivity in the aerobic exercise group was independent of changes in visceral fat and %fat. Lastly, the change in whole body insulin sensitivity from baseline to 1-yr follow up was independently associated with changes in V̇o2max from baseline to 1-yr follow up. These observations suggest that long-term aerobic exercise training may conserve improvements in whole body insulin sensitivity following weight loss, and these improvements transpire independent of modest weight and visceral fat regain. Furthermore, maintaining cardiovascular fitness following weight loss may be important for maintaining improvements in whole body insulin sensitivity.
Prior investigations have not provided a clear picture as to whether there is an independent effect of exercise training on changes in insulin sensitivity during weight loss. Some studies have shown no independent effects of exercise training on insulin sensitivity (4, 26), whereas others have shown an independent effect (3, 5, 24). In this study there was no effect of intervention group (diet only, diet/aerobic, or diet/resistance) on whole body insulin sensitivity, suggesting that the intervention type was not independently related to improvements in insulin sensitivity (Table 1). Instead, time was a significant predictor, suggesting that weight loss was primarily responsible for the improvements in insulin sensitivity. The discrepancy between studies may be attributable to fundamental differences in study design and methodology used to determine insulin sensitivity. For example, the lack of weight stabilization periods in some studies (3, 10) may result in measurements of insulin sensitivity that may reflect negative energy balance rather than the intervention. We used a 4-wk weight stabilization period prior to all pre and post measurements obtained in this study to avoid this confounding factor. The use of different techniques to determine insulin sensitivity may also contribute to the discrepancy among findings.
A novel aspect of this study was the assessment of potential independent effects of aerobic or resistance exercise training on whole body insulin sensitivity during 1-yr post-weight loss. We are unaware of any studies that have assessed independent effects of aerobic or resistance exercise on whole body insulin sensitivity following weight loss. Our results showed that two aerobic exercise sessions per week led to further statistically significant improvements in whole body insulin sensitivity (28% increase). In contrast, the diet only and resistance training groups showed no statistically significant differences in whole body insulin sensitivity (diet = 18% decrease; resistance = 6.8% decrease; Fig. 1). Furthermore, the improvement in whole body insulin sensitivity in the aerobic exercise group was independent of changes in both %fat and IAAT. These findings are unique since the positive effect of exercise on metabolic risk factors is thought to attenuate quickly in the absence of changes in body fat (12).
Although it appears from our study and several others (3, 12, 17) that weight loss is a key component for improving insulin sensitivity in overweight individuals, literature assessing changes in insulin sensitivity during weight regain is lacking. Inasmuch as most individuals that lose weight from diet and/or exercise programs tend to eventually regain the weight, it is important to establish interventions that can maintain these health improvements when relapses in weight loss occur. Our results extend the findings of Thomas et al. (28), in which exercise was protective against metabolic syndrome risk factors during weight regain. These findings highlight the potential protective effect of aerobic exercise to counter the effects of weight regain on whole body insulin sensitivity.
Strengths of this study included robust measures of body composition and body fat distribution. Post-weight loss measures were taken after participants were placed on a 4-wk diet that was designed to ensure an energy balanced state, thereby eliminating the potential confounding effect of negative energy balance. A further strength of the study was the inclusion of a no-exercise dietary control group, which enabled us to assess the independent and combined effects of diet and exercise. A limitation in this study, likely attributable to the rigorous in-patient protocol, was the 40% loss of participants from baseline to weight reduction. However, we were able to retain 97 of the 126 women during the 1-yr follow-up phase of the study. Furthermore, there were no statistically significant differences for any variables at baseline between those that dropped out of the study and those that completed the study, suggesting no confounding bias for individuals that dropped out of the study. Future research should be directed toward understanding the potential mechanisms through which aerobic exercise preserves insulin sensitivity following weight loss.
In conclusion, the overall implication from the present study was that the addition of aerobic or resistance exercise training to the energy restriction paradigm during weight loss did not provide any additive effects on whole body insulin sensitivity. However, following weight loss, aerobic but not resistance exercise training preserved weight loss-induced improvements in whole body insulin sensitivity.
GRANTS
This work was supported by National Institutes of Health RO1DK51684, RO1DK49779, UL 1RR025777, P60DK079626, MO1-RR-00032, P30DK56336, and 2T32DK062710-07.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: G.F. and B.A.G. analyzed data; G.F., G.R.H., and B.A.G. interpreted results of experiments; G.F. prepared figures; G.F. drafted manuscript; G.F., G.R.H., and B.A.G. edited and revised manuscript; G.F., G.R.H., and B.A.G. approved final version of manuscript; G.R.H. and B.A.G. conception and design of research; G.R.H. and B.A.G. performed experiments.
ACKNOWLEDGMENTS
Stouffer's Lean Cuisine and Weight Watchers Smart Ones kindly provided food used during the weight-maintenance periods. We acknowledge David Bryan and Robert Petri for technical assistance; Maryellen Williams and Cindy Zeng conducted laboratory analyses; Paul Zuckerman for project coordination.
REFERENCES
- 1. Bergman R, Phillips L, Cobelli C. Physiologic evaluation of factors controlling glucose tolerance in man: measurement of insulin sensitivity and beta-cell glucose sensitivity from the response to intravenous glucose. J Clin Invest 68: 1456–1467, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Colman E, Katzel LI, Rogus E, Coon P, Muller D, Goldberg AP. Weight loss reduces abdominal fat and improves insulin action in middle-aged and older men with impaired glucose tolerance. Metabolism 44: 1502–1508, 1995 [DOI] [PubMed] [Google Scholar]
- 3. Cox KL, Burke V, Morton AR, Beilin LJ, Puddey IB. Independent and additive effects of energy restriction and exercise on glucose and insulin concentrations in sedentary overweight men. Am J Clin Nutr 80: 308–316, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Dengel DR, Galecki AT, Hagberg JM, Pratley RE. The independent and combined effects of weight loss and aerobic exercise on blood pressure and oral glucose tolerance in older men. Am J Hypertens 11: 1405–1412, 1998 [DOI] [PubMed] [Google Scholar]
- 5. Dengel DR, Pratley RE, Hagberg JM, Rogus EM, Goldberg AP. Distinct effects of aerobic exercise training and weight loss on glucose homeostasis in obese sedentary men. J Appl Physiol 81: 318–325, 1996 [DOI] [PubMed] [Google Scholar]
- 6. Farin HM, Abbasi F, Reaven GM. Body mass index and waist circumference both contribute to differences in insulin-mediated glucose disposal in nondiabetic adults. Am J Clin Nutr 83: 47–51, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Goodpaster BH, Kelley DE, Wing RR, Meier A, Thaete FL. Effects of weight loss on regional fat distribution and insulin sensitivity in obesity. Diabetes 48: 839–847, 1999 [DOI] [PubMed] [Google Scholar]
- 8. Gower BA, Weinsier RL, Jordan JM, Hunter GR, Desmond R. Effects of weight loss on changes in insulin sensitivity and lipid concentrations in premenopausal African American and white women. Am J Clin Nutr 76: 923–927, 2002 [DOI] [PubMed] [Google Scholar]
- 9. Hawley JA, Lessard SJ. Exercise training-induced improvements in insulin action. Acta Physiol (Oxf) 192: 127–135, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Hughes TA, Gwynne JT, Switzer BR, Herbst C, White G. Effects of caloric restriction and weight loss on glycemic control, insulin release and resistance, and atherosclerotic risk in obese patients with type II diabetes mellitus. Am J Med 77: 7–17, 1984 [DOI] [PubMed] [Google Scholar]
- 11. Hughes VA, Fiatarone MA, Fielding RA, Kahn BB, Ferrara CM, Shepherd P, Fisher EC, Wolfe RR, Elahi D, Evans WJ. Exercise increases muscle GLUT-4 levels and insulin action in subjects with impaired glucose tolerance. Am J Physiol Endocrinol Metab 264: E855–E862, 1993 [DOI] [PubMed] [Google Scholar]
- 12. Janssen I, Fortier A, Hudson R, Ross R. Effects of an energy-restrictive diet with or without exercise on abdominal fat, intermuscular fat, and metabolic risk factors in obese women. Diabetes Care 25: 431–438, 2002 [DOI] [PubMed] [Google Scholar]
- 13. Jeffery RW, Drewnowski A, Epstein LH, Stunkard AJ, Wilson GT, Wing RR, Hill DR. Long-term maintenance of weight loss: current status. Health Psychol 19: 5–16, 2000 [DOI] [PubMed] [Google Scholar]
- 14. Klein S, Burke LE, Bray GA, Blair S, Allison DB, Pi-Sunyer X, Hong Y, Eckel RH, and American Heart Association Council on Nutrition PyA, and Metabolism Clinical implications of obesity with specific focus on cardiovascular disease: a statement for professionals from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism: endorsed by the American College of Cardiology Foundation. Circulation 110: 2952–2967, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Kuzuya T, Nakagawa S, Satoh J, Kanazawa Y, Iwamoto Y, Kobayashi M, Nanjo K, Sasaki A, Seino Y, Ito C, Shima K, Nonaka K, Kadowaki T, and Committee of the Japan Diabetes Society on the diagnostic criteria of diabetes mellitus Report of the Committee on the classification and diagnostic criteria of diabetes mellitus. Diabetes Res Clin Pract 55: 65–85, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Kvist H, Chowdhury B, Grangård U, Tylén U, Sjöström L. Total and visceral adipose-tissue volumes derived from measurements with computed tomography in adult men and women: predictive equations. Am J Clin Nutr 48: 1351–1361, 1988 [DOI] [PubMed] [Google Scholar]
- 17. Larson-Meyer DE, Heilbronn LK, Redman LM, Newcomer BR, Frisard MI, Anton S, Smith SR, Alfonso A, Ravussin E. Effect of calorie restriction with or without exercise on insulin sensitivity, beta-cell function, fat cell size, and ectopic lipid in overweight subjects. Diabetes Care 29: 1337–1344, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lindström J, Ilanne-Parikka P, Peltonen M, Aunola S, Eriksson J, Hemiö K, Hämäläinen H, Härkönen P, Keinänen-Kiukaanniemi S, Laakso M, Louheranta A, Mannelin M, Paturi M, Sundvall J, Valle T, Uusitupa M, Tuomilehto J, Group FDPS. Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet 368: 1673–1679, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Lindström J, Louheranta A, Mannelin M, Rastas M, Salminen V, Eriksson J, Uusitupa M, Tuomilehto J, Group FDPS. The Finnish Diabetes Prevention Study (DPS): lifestyle intervention and 3-year results on diet and physical activity. Diabetes Care 26: 3230–3236, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Muoio DM, Newgard CB. Obesity-related derangements in metabolic regulation. Annu Rev Biochem 75: 367–401, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Neter JE, Stam BE, Kok FJ, Grobbee DE, Geleijnse JM. Influence of weight reduction on blood pressure: a meta-analysis of randomized controlled trials. Hypertension 42: 878–884, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Pacini G, Bergman R. MINMOD: a computer program to calculate insulin sensitivity and pancreatic responsivity from the frequently sampled intravenous glucose tolerance test. Comput Methods Programs Biomed 23: 113–122, 1986 [DOI] [PubMed] [Google Scholar]
- 23. Pi-Sunyer X, Blackburn G, Brancati FL, Bray GA, Bright R, Clark JM, Curtis JM, Espeland MA, Foreyt JP, Graves K, Haffner SM, Harrison B, Hill JO, Horton ES, Jakicic J, Jeffery RW, Johnson KC, Kahn S, Kelley DE, Kitabchi AE, Knowler WC, Lewis CE, Maschak-Carey BJ, Montgomery B, Nathan DM, Patricio J, Peters A, Redmon JB, Reeves RS, Ryan DH, Safford M, Van Dorsten B, Wadden TA, Wagenknecht L, Wesche-Thobaben J, Wing RR, Yanovski SZ, Group LAR. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care 30: 1374–1383, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Redman LM, Huffman KM, Landerman LR, Pieper CF, Bain JR, Muehlbauer MJ, Stevens RD, Wenner BR, Kraus VB, Newgard CB, Kraus WE, Ravussin E. Effect of caloric restriction with and without exercise on metabolic intermediates in nonobese men and women. J Clin Endocrinol Metab 96: E312–E321, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Richter EA, Nielsen JN, Jørgensen SB, Frøsig C, Birk JB, Wojtaszewski JF. Exercise signalling to glucose transport in skeletal muscle. Proc Nutr Soc 63: 211–216, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Ross R, Dagnone D, Jones PJ, Smith H, Paddags A, Hudson R, Janssen I. Reduction in obesity and related comorbid conditions after diet-induced weight loss or exercise-induced weight loss in men. A randomized, controlled trial. Ann Intern Med 133: 92–103, 2000 [DOI] [PubMed] [Google Scholar]
- 27. Ross R, Freeman JA, Janssen I. Exercise alone is an effective strategy for reducing obesity and related comorbidities. Exerc Sport Sci Rev 28: 165–170, 2000 [PubMed] [Google Scholar]
- 28. Thomas TR, Warner SO, Dellsperger KC, Hinton PS, WhaleyConnell AT, Rector RS, Liu Y, Linden MA, Chockalingam A, Thyfault JP, Huyette DR, Wang Z, Cox RH. Exercise and the metabolic syndrome with weight regain. J Appl Physiol 109: 3–10, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Turk MW, Yang K, Hravnak M, Sereika SM, Ewing LJ, Burke LE. Randomized clinical trials of weight loss maintenance: a review. J Cardiovasc Nurs 24: 58–80, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wallberg-Henriksson H, Holloszy JO. Contractile activity increases glucose uptake by muscle in severely diabetic rats. J Appl Physiol 57: 1045–1049, 1984 [DOI] [PubMed] [Google Scholar]
- 31. Weinsier RLWN, Morgan SL, Cornwell AR, Craig CB. EatRight lose weight: seven simple steps. Birmingham, AL: Oxmoor House, 1997 [Google Scholar]
- 32. Yang Y, Youn J, Bergman R. Modified protocols improve insulin sensitivity estimation using the minimal model. Am J Physiol Endocrinol Metab 253: E595–E602, 1987 [DOI] [PubMed] [Google Scholar]