Abstract
The skeletal response to short-term exercise training remains poorly described. We thus studied the lower limb skeletal response of 723 Caucasian male army recruits to a 12-wk training regime. Femoral bone volume was assessed using magnetic resonance imaging, bone ultrastructure by quantitative ultrasound (QUS), and bone mineral density (BMD) using dual-energy X-ray absorptiometry (DXA) of the hip. Left hip BMD increased with training (mean ± SD: 0.85 ± 3.24, 2.93 ± 4.85, and 1.89 ± 2.85% for femoral neck, Ward's area, and total hip, respectively; all P < 0.001). Left calcaneal broadband ultrasound attenuation rose 3.57 ± 0.5% (P < 0.001), and left and right femoral cortical volume by 1.09 ± 4.05 and 0.71 ± 4.05%, respectively (P = 0.0001 and 0.003), largely through the rise in periosteal volume (0.78 ± 3.14 and 0.59 ± 2.58% for right and left, respectively, P < 0.001) with endosteal volumes unchanged. Before training, DXA and QUS measures were independent of limb dominance. However, the dominant femur had higher periosteal (25,991.49 vs. 2,5572 mm3, P < 0.001), endosteal (6,063.33 vs. 5,983.12 mm3, P = 0.001), and cortical volumes (19,928 vs. 19,589.56 mm3, P = 0.001). Changes in DXA, QUS, and magnetic resonance imaging measures were independent of limb dominance. We show, for the first time, that short-term exercise training in young men is associated not only with a rise in human femoral BMD, but also in femoral bone volume, the latter largely through a periosteal response.
Keywords: skeleton, exercise, training, bone remodelling, density, quantitative ultrasound, bone mineral density, military recruits, magnetic resonance imaging, dual-energy X-ray absorptiometry
the human skeleton is far from inert: bone structure and composition are dynamically maintained through simultaneous deposition and resorption. Altering the balance of these processes allows rapid architectural remodeling, whether favorably (e.g., fracture healing), or unfavorably (e.g., disuse osteoporosis). Mechanistic investigations of remodeling in healthy humans have been hampered by the lengthy timescales of population-based studies (such as those of progression of osteoporosis). The study of the human skeletal response to exercise offers one potential means of exploring the skeletal adaptive process.
Through cell mechanotransduction, exercise causes skeletal remodeling through a simultaneous increase in both resorption and deposition of bone tissue (99, 113). In the long term, this elevates bone mass (111, 114), a finding confirmed by prospective studies (13). The same may hold true with shorter training period bone mass rising with 14–15 wk training in army recruits (15, 66, 76). However, resorption may exceed deposition in early training (113): lumbar spine bone mineral density (BMD) has been observed to fall (15), and calcaneal trabecular separation increase (25) with 15 and 10 wk (respectively) of military training.
Meanwhile, the geometry and the long-axis distribution of bone mass are key determinants of bone strength (49). Changes in bone geometry with exercise have been poorly studied, due in part to a paucity of appropriate available technologies. Although used to assess change in bone geometry (36, 73), dual-energy X-ray absorptiometry (DXA) cannot allow for bone's hollow asymmetric form, nor readily differentiate endosteal and periosteal changes (118). Concerns over ionizing radiation exposure have limited the application of peripheral quantitative computed tomography (pQCT) in young cohorts (35, 43). However, those data, which do exist (69), support the fact that changes in bone turnover with training are also associated with changes in bone volume and density. Magnetic resonance imaging (MRI) overcomes such problems and has helped describe the effects of long-term activity on bone geometry (8, 19, 22, 35, 42, 118). However, while rodent bone volume increases with only 10 wk of endurance training (52), MRI has yet to be applied to the detailed study of the early human skeletal response with bone (cortical or endosteal) volume.
Thus data describing the early response of the human skeleton to exercise (and bone morphological change, in particular) are sparse and conflicting. Furthermore, no large prospective study has ever simultaneously assessed changes in bone mineralization, microstructure, and macroscopic form in young adults. Such data have clinical relevance, given the demonstrable benefits of short-term intervention in elevating bone mass, even in those of teenage years. First, an understanding of bone remodeling in the young may help inform the public health agenda with regards to exercise in this age group as a means of augmenting peak bone mass and preventing longer term skeletal ill health. Second, a comprehensive study such as this would improve our understanding of the mechanisms through which bone strength is preserved, especially in terms of the relative contributions to changes in geometry and BMD. Third, such studies may help identify those most at risk of stress fracture with training, allowing appropriate adjustment of training loads. Such descriptive data would also allow for association with further genetic or biochemical markers, such that the cellular mechanisms regulating the bone response can be elucidated. This would allow novel therapeutic targets to be identified. Finally, given that some core bone remodeling processes may be fundamental to disease pathogenesis (e.g., stress fracture, osteoporosis), observations may have value beyond athletes and military recruits.
We have thus performed the first large prospective study to simultaneously assess exercise-induced changes in bone mineralization, microstructure, and macroscopic form in young adult men.
MATERIALS AND METHODS
This study had appropriate ethics approval (Defence Medical Services Clinical Research Committee) and was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. Written, informed consent was obtained from all subjects.
Study Subjects
Subjects were consecutive Caucasian men recruited to the Army Training Regiment, Lichfield, UK, over a 21-mo period. All were free from metabolic bone, cardiovascular, and renal diseases, and were taking no medications. Those with contraindications to MRI (such as implanted metal or electronic devices or claustrophobia) were deemed ineligible for study. At entry, subject height and weight were recorded. While leg dominance may be defined by stance or kicking, these may be considered largely synonymous (41). Leg dominance was thus recorded by asking the recruits whether they were right, left, or both-footed, with regards to ball kicking.
Training Regimen
All recruits underwent the same 12-wk program of physical training. Described in more detail elsewhere (115, 116), this involved multiple 40- to 80-min periods of strength training (∼28 periods), endurance training (∼15 periods), agility (∼8 periods), material handling (∼6 periods), circuit training (∼4 periods), and sports (∼6 periods). Strength exercises included assisted pull-up, leg press, bench press, seated row, dead lift, high curl, and upright row. The endurance period consisted of interval running, followed by loaded marching with progressively increasing loads. Circuit training generally consisted of high-repetition, low-force exercise using all major muscle groups, and sports periods were mainly ball games in a small area. In addition, training included other physical exercise, such as prolonged marching with various loads while on military exercise, and many 40- to 80-min periods of drill that averaged about one 40-min period/day. Total energy expenditure is calculated to be 3,590 kcal/day (91).
Assessment of Skeletal Phenotype
Both before and after completion of the 12-wk military training program, skeletal phenotype of the lower limbs was examined using DXA, quantitative ultrasound (QUS) and MRI. These modalities were selected on the basis of accuracy and reproducibility, but also for cost, safety (minimizing radiation exposure), accessibility (imaging having to be performed at the training center), and speed (important, given severe restrictions on available scanning time during the training program).
Application of Phenotyping Modalities
DXA.
Widely used in both clinical and research environments, DXA is a highly characterized method of noninvasively measuring bone density with application to all areas of the skeleton. The Hologic QDR-1000/W (Hologic, Bedford, MA) system with analysis protocols and edge detection algorithms designed by VERTEC Scientific (Reading, UK) was used. This system offers acceptable precision; coefficient of variation is <1.5% for neck of femur in vitro repeat measurements, while in vivo the overall precision for the femoral neck and trochanter is 2–3%, and that for Ward's triangle is >3% (62, 102). Effective subject radiation dose is 0.07 μSv. For these reasons, this same system has been previously used in the study of BMD in young men (15, 81).
All data were obtained by a single operator (K. Eleftheriou), using a Hologic QDR-1000 machine (Hologic, Bedford, MA). A standardized positioning protocol was employed throughout the investigative period, thereby avoiding movement artifact and ensure repeatability at the posttraining 12-wk scan. Subjects were positioned supine, with the left foot braced and strapped to a plastic triangular frame, ensuring fixed internal rotation of 60°, thereby fixing the femoral neck angle. Imaging an 8-cm segment of the proximal femur immediately distal to the base of the lesser trochanter provided proximal femoral BMD (PFBMD). Other regional and net average BMD measurements obtained were for the total hip (THBMD), femoral neck (FNBMD), greater trochanter (TRBMD), and intertrochanteric region (ITBMD). Although the value of measurements in Ward's triangle (WTBMD) is questioned (70), these were included so as to attain a more complete picture of the response of bone to exercise in this group of young men (very different from the more elderly population to whom DXA is usually applied). To ensure data quality, a quality control (QC) program that includes use of an anthropomorphic phantom was performed at the beginning of each scan session.
QUS systems.
QUS systems assess broadband ultrasound attenuation (BUA; in dB/MHz) (61), offering information related to bone ultrastructure: the more complex the structure of the bone, the greater the “block” to sound transmission. Normal bone thus has a higher attenuation than osteoporotic bone. QUS also measures the velocity of sounds (VOS; in ms−1), which rises with increased bone connectivity. QUS is normally applied to the calcaneus, a bone with limited soft tissue coverage and a (high) proportion of trabecular bone (12, 86). The same system has been previously applied to a smaller study of UK army recruits (25).
Employing a single-operator technique, a single CUBA Clinical QUS was used (McCue Ultrasonics PLC, Winchester, UK) throughout the study period. At standard room temperature, the subject's heel was measured for the appropriate sized insert and positioned in the foot well, according to the manufacturer's protocols. This device uses silicone pads brought into contact with each side of the patient's heel by means of a motorized mechanism with acoustic coupling by means of ultrasound gel. QUS parameters (BUA and VOS) were then measure. The left heel was studied so as to allow for comparison of ipsilateral bone ultrastructure with DXA scanning of the left proximal femur. BUA is influenced by trabecular orientation, and precision is thus increased by repositioning the heel twice (and taking two separate measures). Further scans cause cutaneous pitting and increased measurement error. Using such a protocol, sensitivity is entirely acceptable for a study such as ours: changes in BUA of ∼2.5%, and in VOS of 0.3% (121), can be detected; coefficient of variation for calcaneal BUA was 1.1–1.2% and for VOS 0.17–0.30% (37). A manufacturer QC program utilizing an artificial phantom was performed before each investigative day.
MRI.
MRI has been successfully applied in the assessment of bone geometry (22, 42, 43, 59, 118) and has been validated in the measurement of femoral volumes; repeatability and accuracy (118) far exceed those of DXA and are comparable to those obtained using pQCT and computed tomography (94, 98). For intraobserver variability, coefficients of variation range from 0.5 ± 0.5% [for total volume (TV)] to 3.1 ± 3.1% [for cortical width (CW)]. For interobserver variability, values are 0.55 ± 0.5% (TV) and 3.6 ± 3.6% (CW), respectively. MRI accuracy is excellent for TV (3.3 ± 6.4%) and cortical volume (CV) (3.5 ± 4.0%) (118).
With the use of methodologies similar to those of past studies (20, 22, 42, 118), proximal femoral bone volumes were determined on site using a mobile 1.5-T Siemens Sonata MR scanner (Sonata, Siemens Medical Systems, Erlangen, Germany). A standardized positioning protocol was used, whereby recruits were in a supine position, with arms held across the chest. Legs were held together and in position using Velcro strapping so as to prevent movement artifact. A pilot coronal scan was obtained to identify the greater trochanter as a fixed reference point. Subsequently, five transaxial spin echo images (TR669, field of view 45 cm × 45 cm, slice thickness 10 mm) of both femurs were obtained at a fixed distance from the greater trochanter, both before and after training. The slices were at 10-mm intervals, with slice 1 in the proximal shaft of the femur below the level of the lesser trochanter, and slice 10 located distally.
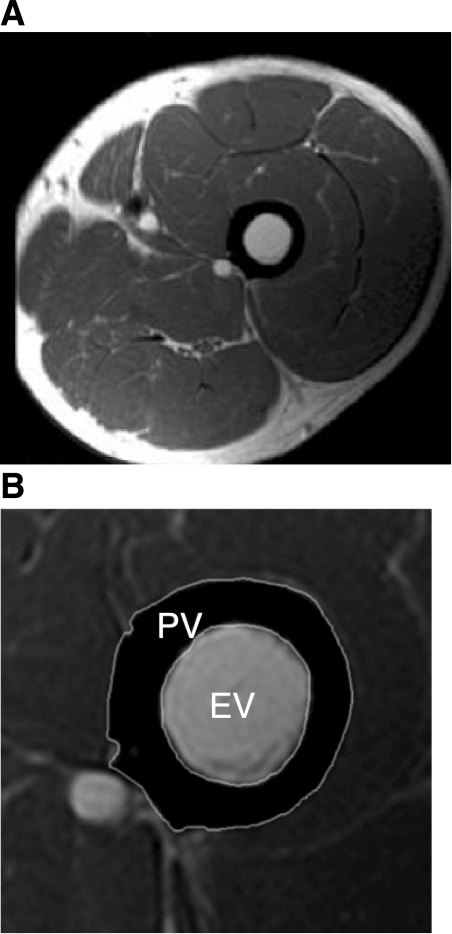
Image analysis was performed using CMR tools (Cardiovascular Imaging Solutions, London, UK) by the same clinician. As previously reported (20), this allows image magnification and measurement of femoral cortical bone cross-sectional area (CSA), as well as that of periosteal and endosteal bone cross sections (Fig. 1) for both left and right femurs. Measurements were then used to calculate periosteal volume (PV), CV and endosteal volume (EV) for the 50-mm section of each of the left and right proximal femurs. Our intraobserver error (5%) was consistent with previous observations (20).
Fig. 1.
MRI image analysis was carried out using CMR-Tools. A: example of unmagnified image of left thigh in cross section. B: magnified image of the same left thigh cross section. The operator is able to magnify each bone image and outline the region of interest (ROI) free hand (for greater free-hand bone-edge contour accuracy, ROIs are only placed once the image has been greatly magnified). This process is repeated three times so as to obtain the three cross-sectional values described below. 1) Endostial volume (EV): analysis of this ROI alone gives the cross-sectional value for the femur within the boundaries of the inner bony cortex, referred to here as the EV when in series with other slices, or “endostial cross section” when considered for individual sections. 2) Cortical volume: with two ROIs in place, the software will calculate the cross section of cortical bone, referred to here as the “cortical volume” when in series with other slices, or “cortical cross section” when considered for individual sections. 3) Periostial volume (PV): analysis of this ROI alone gives the cross-sectional value for the femur within the boundaries of the outer bony cortex, referred to here as the PV when in series with other slices, or “periostial cross section” when considered for individual sections.
Reproducibility
At the beginning of the study, reproducibility of the various scanning methodologies was assessed. Coefficients of variation were calculated as previously described (23). All scanning and MRI analysis was performed by the senior author. Intraobserver reproducibility of DXA measures was assessed in 30 subjects who had two repeat scans in the same day (2–3 h between repeat scans). The coefficient of variation ranged from 1.21 to 1.62%, similar to previous reports (67). QUS intraobserver reproducibility was assessed in a similar way to DXA. The coefficient of variation was calculated as 2.7% for BUA and 0.9% for VOS, similar to previous reports (24). MRI intraobserver reproducibility was assessed in 20 scans from 20 subjects. Contours were drawn twice 1 wk apart on the same images by a single operator (K. Eleftheriou). The coefficient of variation was calculated at 2.9, 3.1, and 3.9% for CV, PV, and EV, respectively. There are no previous comparable studies using the same MRI scanner and analysis software, although similar methods have produced similar repeatability measurements (118).
Statistical Analysis
Statistical analysis was conducted using Intercooled STATA 10.0 (StataCorp). Outliers (defined as those 3 SD away from the mean) comprised only 0–0.75% of all measurements and were excluded in analysis. All DXA measurements except PFBMD required natural log transformation to change their distribution to the normal to allow appropriate statistical analysis. PV, EV, CV, BUA, and VOS did not require transformation. Differences in baseline skeletal measures between those who did not complete training and those who did, and therefore had a completion scan, were analyzed using an unpaired T-test.
The influence of age upon skeletal parameters was investigated using linear regression, while the comparison of lifestyle factors, such as smoking, pre-basic military training weight-bearing sports activity levels, and alcohol consumption, between those who completed training and those who did not, was investigated using Fisher's exact test.
The main endpoint of the study was to investigate the longitudinal change in skeletal parameters with exercise. As such, the difference in baseline and completion measures was analyzed using a paired t-test.
Values are presented as means ± SD, unless otherwise stated. Throughout, a P-value of <0.01 was considered significant.
RESULTS
Of 1,430 recruits available, 923 volunteered. Of these, 740 were randomly selected to take part, all but 17 of whom were studied (due to restricted timings of recruit availability). Thus 723 recruits were initially assessed. Time restrictions prevented assessment of all three phenotypes in all recruits. Pretraining DXA measures were made in 651 recruits, MRI measures in 650, and QUS measures in 572. Lack of availability of the necessary specific algorithm for PFBMD assessment meant that baseline measures were only recorded in 529 recruits, with paired data being available for 380.
Not all recruits complete basic training in this 12-wk period. While some opt out or are discharged for disciplinary reasons, others leave (or are set back in training) on medical grounds. Of these, one third have preexisting (but unidentified or concealed) conditions, which preclude military service. The remainder suffer injuries, with many being “overuse” injuries, such as stress fractures and anterior knee pain (10, 30, 74). For such reasons, and due to other military duties, some 290 recruits were unavailable for follow-up. In those available, time constraints meant that not all subjects obtained every follow-up scan.
Follow-up investigation at the end of training lead to paired pre- and posttraining measures in 382 with DXA, 399 with MRI, and 372 with QUS. Follow-up investigation at the end of training lead to paired pre- and posttraining measures in 382 with DXA, 399 with MRI, and 372 with QUS. Lack of availability of the necessary specific algorithm for PFBMD assessment meant that baseline measures were only recorded in 529 recruits, with paired data being available for 380.
The total 433 subjects examined posttraining by MRI, DXA, or QUS (age 19.87 ± 2.32 yr, height 177.97 ± 6.14 cm, weight 73.8 ± 9.8 kg) were similar to those who only had baseline measures in most regards (age 20.00 ± 2.29 yr, height 177.13 ± 6.54 cm, weight 72.0 ± 9.4 kg; P = 0.34, P = 0.28, and P = 0.4, respectively), but were slightly heavier (73.8 ± 8.00 vs. 72.2 ± 12.3 kg, P = 0.04). However, body mass index was similar in those that did and did not have repeat scans (23.25 ± 2.52 vs. 22.96 ± 2.52 kg/m; P = 0.15).
Comparison (by Fisher's exact test) between those with paired data and those with only baseline data for smoking group (current smokers, recent ex-smokers within 6 mo, ex-smokers for greater than 6 mo, or nonsmokers), alcohol intake (no units per week, 1–9 units/wk, 10–21 units/wk, and >21 units/wk), and weight-bearing sporting activity yielded no difference in these lifestyle factors (P = 0.121, 0.644, 0.064, respectively). Imaging data were also similar for those reexamined compared with those with only baseline data. At entry, MRI data did not differ for the 251 who did not complete training to be scanned again, and the 399 who did (PV: 2,595.57 ± 342.15 vs. 2,653.57 ± 322.95 mm3, P = 0.072; CV: 2,029.93 ± 250.59 vs. 1,978.50 ± 277.37 mm3, P = 0.015; EV: 613.64 ± 187.64 vs. 617.07 ± 200.86 mm3; P = 0.826, respectively). The same was true for the BMD measures of the 382 individuals with paired data and the 269 without (THBMD: 1.083 ± 0.135 vs. 1.068 ± 0.141 g/cm2, P = 0.170; FNBMD: 0.975 ± 0.129 vs. 0.957 ± 0.134 g/cm2, P = 0.080; TRBMD: 0.840 ± 0.117 vs. 0.822 ± 0.116 g/cm2, P = 0.055; ITBMD: 1.241 ± 0.156 vs. 1.222 ± 0.163 g/cm2, P = 0.152; PFBMD: 1.781 ± 0.182 vs. 1.786 ± 0.196 g/cm2, P = 0.050; WTBMD: 0.886 ± 0.149 vs. 0.876 ± 0.167 g/cm2, P = 0.445) and for QUS (completed = 307, not complete = 265; BUA: 88.10 ± 14.60 vs. 90.134 ± 15.24 dB/MHz, P = 0.10; VOS: 1,662.98 ± 36.28 vs. 1,663.38 vs. 37.78 ms−1, P = 0.81).
DXA Measurements
Derived DXA measurements (Table 1) were comparable to those obtained using identical equipment in healthy UK Caucasian males of a similar age (55). All baseline anthropometric measures, with the exception of weight, were similar for those who had repeat scans (weight 73.9 ± 13.3 kg, age 19.90 ± 3.06 yr, height 178.05 ± 8.16 cm) and those who did not (weight 72.4 ± 12.3 kg; age 19.94 ± 1.97 yr, height 177.22 ± 5.9 cm; P values 0.04, 0.77, and 0.08, respectively). Similarly, BMD for all regions of interest were similar in those who did and did not complete training (P values 0.07–0.30), with the exception of PFBMD and TRBMD, which were slightly lower (but of borderline significance; P = 0.045 and P = 0.05) in the group that did not have repeat scans (1.769 ± 0.167 vs.1.781 ± 0.182 g/cm2 for PFBMD, and 0.831 ± 0.123 vs. 0.813 ± 0.135 g/cm2 for TRBMD).
Table 1.
DXA measurements at the left femur for army recruits before and after the 12-wk training for the various regions of interest: THBMD, FNBMD, TRBMD, ITBMD, WTBMD, as well as PFBMD
| Pretraining (All Data) | Pretraining (Paired Data) | Posttraining (Paired Data) | Mean of %Changes | Paired t-test | |
|---|---|---|---|---|---|
| n | 651 (for PFBMD, n = 529) | 382 | 382 | ||
| THBMD | 1.077 ± 0.137 | 1.083 ± 0.135 | 1.102 ± 0.128 | 1.89 ± 2.85 | <0.001 |
| FNBMD | 0.967 ± 0.131 | 0.975 ± 0.129 | 0.982 ± 0.127 | 0.85 ± 3.24 | <0.001 |
| TRBMD | 0.832 ± 0.117 | 0.840 ± 0.116 | 0.855 ± 0.113 | 1.98 ± 2.71 | <0.001 |
| ITBMD | 1.233 ± 0.159 | 1.240 ± 0.156 | 1.261 ± 0.150 | 1.76 ± 3.14 | <0.001 |
| WTBMD | 0.882 ± 0.157 | 0.886 ± 0.149 | 0.910 ± 0.147 | 2.93 ± 4.85 | <0.001 |
| PFBMD | 1.771 ± 0.187 | 1.781 ± 0.182 | 1.803 ± 0.175 | 1.36 ± 3.65 | <0.001 |
Values are means ± SD in g/cm2; n, no. of measurements. DXA, dual-energy X-ray absorptiometry; THBMD, total hip bone mineral density (BMD); FNBMD, femoral neck BMD; TRBMD, trochanter BMD; ITBMD, intertrochanteric region BMD; WTBMD, Ward's triangle BMD; PFBMD, femoral shaft subregion (8-cm segment of the proximal femur immediately distal to the base of the lesser trochanter) BMD.
With training, BMD rose significantly in all areas assessed (P < 0.001 for all regions of interest; paired t-test). The lowest mean of relative changes was for FNBMD (0.85 ± 3.24%) and the highest for WTBMD (2.93 ± 4.85%).
QUS Measurements
Results are shown in Table 2. Both BUA and VOS values were in the range expected for UK men of this age group, based on previously published normative data using the same equipment (60). The group that did not have a repeat scan after training differed slightly from those who did in terms of weight (72.5 ± 7.8 vs. 74.0 ± 13.6 kg, P = 0.04) and height (177.19 ± 5.37 vs. 178.26 ± 8.37 cm, P = 0.03), but not age (19.97 ± 1.79 vs. 19.85 ± 3.11 yr, P = 0.48) or pretraining QUS measurements (P = 0.81 and 0.20 for BUA and VOS, respectively).
Table 2.
QUS measurements at the left calcaneus for army recruits before and after the 12-wk training
| Pretraining (All Data) | Pretraining (Paired Data) | Posttraining (Paired Data) | Mean of %Changes | Paired t-test | |
|---|---|---|---|---|---|
| n | 572 | 307 | 307 | ||
| BUA, dB/MHz | 89.04 ± 14.92 | 88.10 ± 14.60 | 90.60 ± 13.93 | 3.57 ± 10.06 | <0.001 |
| VOS, ms−1 | 1,663.0 ± 36.28 | 1,662.6 ± 35.00 | 1,663.5 ± 33.80 | 0.06 ± 1.60 | 0.58 |
Values are means ± SD; n, no. of measurements. QUS, quantitative ultrasound; BUA, broadband ultrasound attenuation; VOS, velocity of sounds. Mean BUA increased significantly after training (P < 0.001; paired t-test), with a mean relative change of 3.57 ± 0.57%. No significant change was noted for VOS (P = 0.58).
Mean BUA increased significantly with training (paired data: pretraining, 88.10 ± 14.60; posttraining, 90.60 ± 13.93 dB/MHz, P < 0.001; paired t-test), but no significant change was noted for VOS (pretraining, 1,662.6 ± 53.88; posttraining, 1,663.5 ± 33.8 ms−1; P = 0.58; paired t-test) (Table 2).
MRI Measurements
See Table 3. Although no direct comparable data were available, the range of overall periosteal CSAs used to calculate volumes (296.16–757.96 mm2) is comparable to published data (11, 42). Anthropomorphic measures were similar in those who had repeat scans (age 19.87 ± 2.40 yr, height 178.06 ± 4.99 cm, weight 73.7 ± 10.0 kg) and those who had not (age 19.98 ± 2.06 yr, height 177.17 ± 5.86 cm, weight 72.5 ± 8.7 kg; P = 0.52, 0.07, and 0.12, respectively). Those lacking a posttraining scan had smaller pretraining PV and CV, but not EV on both the right (25,607.9 ± 2,934.4, 19,767.8 ± 2,619.2, 5,839.3 ± 1,843.6 mm3; P values 0.01, 0.02, and 0.5, respectively) and left leg (24,841.5 ± 2,857.4, 19,096.0 ± 2,595.5, 5,725.0 ± 1,823.6 mm3; P values 0.002, 0.002, and 0.53, respectively).
Table 3.
MRI measurements on both left and right femurs for army recruits before and after their 12-wk training
| Pretraining (All Data) | Pretraining (Paired Data) | Posttraining (Paired Data) | Mean of %Changes | Paired t-test | |
|---|---|---|---|---|---|
| n | 650 | 399 | 399 | ||
| PV (right) | 26,250.3 ± 3,330.6 | 26,435.7 ± 3,229.5 | 2,6617.0 ± 3,132.2 | 0.78 ± 3.14 | <0.001 |
| EV (right) | 6,149.6 ± 1,927.0 | 6,136.4 ± 1,876.4 | 6,121.7 ± 1,801.2 | 0.44 ± 10.26 | 0.66 |
| CV (right) | 20,100.7 ± 2,622.5 | 20,299.3 ± 2,505.9 | 20,495.3 ± 2,449.7 | 1.09 ± 4.05 | <0.001 |
| PV (left) | 25,547.7 ± 3,189.0 | 25,781.0 ± 3,134.9 | 25,915.7 ± 3,066.4 | 0.59 ± 2.58 | <0.001 |
| EV (left) | 6,026.9 ± 1,850.3 | 6,019.0 ± 1,843.9 | 6,034.0 ± 1,767.3 | 1.13 ± 11.75 | 0.68 |
| CV (left) | 19,520.9 ± 2,588.7 | 19,762.0 ± 2,527.8 | 19,881.7 ± 2,501.2 | 0.71 ± 4.05 | 0.003 |
Values are means ± SD in mm3; n, no. of measurements. Similar changes were seen on both legs, with periostial (PV) and cortical volume (CV) increasing significantly after the 12-wk training program (P ≤ 0.003), with no change seen in the endostial volume (EV) (P ≥ 0.66).
PV and CV rose significantly during the 12-wk training program (P ≤ 0.003) and to a similar degree in both legs (Table 3), with no change seen in EV (P ≥ 0.66). These data suggest that the measured increases in CV occurred by increasing the periosteal envelope without change in the endosteal measurements. The changes in CV were (mean ± SD) 0.71 ± 4.05% on the left and 1.09 ± 4.05% on the right femur.
Influence of Age
Age did not significantly correlate with MRI measures (PV R2 = 3.0%, P = 0.273; CV R2 = 3.6%, P = 0.1277; EV R2 = 1.0%, P = 0.8495), QUS (BUA, R2 = 3.6%, P = 0.152, VOS R2 = 3.5%, P = 0.1,579), nor DXA (THBMD, R2 = 0.4%, P = 0.6118; FNBMD, R2 = 1.6%, P = 0.3137, TRBMD, R2 = 2.9%, P = 0.1743; ITBMD, R2 = 2.4%, P = 0.2168), although baseline log WTBMD and PFBMD did regress with age (R2 = 1.87%, P = 0.0075, and R2 = 3.15%, P < 0.0001, respectively).
Associations With Dominance
See Tables 4 and 5. Both legs underwent MRI scanning, while only left leg DXA/QUS measurement was performed. The majority of subjects were right-leg dominant.
Table 4.
DXA, QUS, and MRI measurements at baseline according to dominance
| Dominant Side |
Nondominant Side |
||||
|---|---|---|---|---|---|
| Means ± SD | n | Means ± SD | n | P Value | |
| DXA measurements | |||||
| THBMD, g/cm2 | 1.064 ± 0.133 | 85 | 1.079 ± 0.136 | 499 | 0.35 |
| FNBMD, g/cm2 | 0.952 ± 0.131 | 85 | 0.971 ± 0.129 | 499 | 0.20 |
| TRBMD, g/cm2 | 0.826 ± 0.118 | 85 | 0.834 ± 0.115 | 499 | 0.55 |
| ITBMD, g/cm2 | 1.222 ± 0.154 | 85 | 1.234 ± 0.157 | 499 | 0.51 |
| WTBMD, g/cm2 | 0.86739 ± 0.143 | 85 | 0.886 ± 0.157 | 499 | 0.30 |
| PFBMD*, g/cm2 | 1.759 ± 0.163 | 85 | 1.76938 ± 0.188 | 499 | 0.63 |
| QUS measurements | |||||
| BUA, dB/MHz | 88.79 ± 17.68 | 77 | 88.86 ± 14.31 | 436 | 0.76 |
| VOS, ms−1 | 1,660.93 ± 39.64 | 77 | 1,663.17 ± 35.57 | 436 | 0.89 |
| MRI volume measurements | |||||
| PV, mm3 | 25,991.49 ± 3,207.8 | 525 | 25,572.68 ± 3,176.6* | 525 | <0.001 |
| EV, mm3 | 6,063.33 ± 1,875.19 | 525 | 5,983.12 ± 1,814.0* | 525 | 0.001 |
| CV, mm3 | 19,928.22 ± 2,616.6 | 525 | 19,589.56 ± 2,598.1* | 525 | <0.001 |
Values are means ± SD; n, no. of measurements.
Only MRI measures were found to be statistically different between the dominant and nondominant sides (P ≤ 0.004).
Table 5.
Changes in DXA, QUS, and MRI measures after training according to dominance
| Measure | Dominance of Measurement | n | Pretraining | Posttraining | P Value | P Value for the Effect of Dominance on Training Response |
|---|---|---|---|---|---|---|
| DXA measurements | ||||||
| THBMD, g/cm2 | Dominant | 42 | 1.075 ± 0.126 | 1.103 ± 0.120 | <0.001 | 0.06 |
| Nondominant | 300 | 1.082 ± 0.136 | 1.101 ± 0.129 | <0.001 | ||
| FNBMD, g/cm2 | Dominant | 42 | 0.965 ± 0.128 | 0.978 ± 0.128 | 0.008 | 0.21 |
| Nondominant | 300 | 0.976 ± 0.129 | 0.982 ± 0.127 | <0.001 | ||
| TRBMD, g/cm2 | Dominant | 42 | 0.846 ± 0.115 | 0.867 ± 0.113 | <0.001 | 0.11 |
| Nondominant | 300 | 0.838 ± 0.116 | 0.853 ± 0.112 | <0.001 | ||
| ITBMD, g/cm2 | Dominant | 42 | 1.229 ± 0.146 | 1.257 ± 0.143 | 0.003 | 0.14 |
| Nondominant | 300 | 1.240 ± 0.157 | 1.259 ± 0.151 | <0.001 | ||
| WTBMD, g/cm2 | Dominant | 42 | 0.877 ± 0.149 | 0.910 ± 0.140 | <0.001 | 0.09 |
| Nondominant | 300 | 0.888 ± 0.149 | 0.911 ± 0.148 | <0.001 | ||
| PFBMD, g/cm2 | Dominant | 42 | 1.777 ± 0.165 | 1.799 ± 0.162 | 0.004 | 0.83 |
| Nondominant | 298 | 1.778 ± 0.183 | 1.801 ± 0.175 | <0.001 | ||
| QUS measurements | ||||||
| BUA, dB/MHz | Dominant | 36 | 90.56 ± 16.81 | 90.97 ± 13.65 | 0.75 | 0.14 |
| Nondominant | 236 | 87.28 ± 13.73 | 90.10 ± 13.69 | <0.001 | ||
| VOS, ms−1 | Dominant | 36 | 1,669.75 ± 33.82 | 1,668.61 ± 34.66 | 0.83 | 0.67 |
| Nondominant | 236 | 1,661.57 ± 34.93 | 1,662.41 ± 33.55 | 0.59 | ||
| MRI volume measurements | ||||||
| PV, mm3 | Dominant | 330 | 26,184.47 ± 3,017.5 | 26,399.09 ± 3,140.7 | <0.001 | 0.69 |
| Nondominant | 330 | 25,761.11 ± 3,127.5 | 25,962.30 ± 3,135.7 | <0.001 | ||
| EV, mm3 | Dominant | 330 | 6,056.43 ± 1,790.0 | 6,052.45 ± 1,739.9 | 0.92 | 0.91 |
| Nondominant | 330 | 5,991.80 ± 1,801.1 | 5,977.54 ± 1,743.7 | 0.88 | ||
| CV, mm3 | Dominant | 330 | 20,128.04 ± 2,644.0 | 20,346.63 ± 2,459.9 | <0.001 | 0.92 |
| Nondominant | 330 | 19,769.30 ± 2,398.4 | 19,984.76 ± 2,581.9 | <0.001 |
Values are means ± SD; n, no. of measurements.
For this analysis, the few (n = 14) individuals who had leg codominance were excluded. Data are summarized in Tables 4 and 5. With regards to DXA and QUS measurements for which the left leg was scanned, the measurements were assigned as “nondominant” for the individuals who were right-leg-dominant, and vice versa. For the MRI measures, where both legs were scanned, paired measurements were available and assigned according to each subject's dominance.
At baseline (Table 4), no differences were found between dominant and nondominant measurements for all DXA and QUS measures (P > 0.20). For MRI measurements, however, the dominant femur had significantly higher PV (P < 0.001), CV (P < 0.001), and EV (P = 0.001). The same held true among those for whom paired training data were available: dominant and nondominant DXA (P ≥ 0.6) and QUS measures (P > 0.19) did not differ, while the dominant femur still had significantly higher PV (P < 0.001), CV (P < 0.001), and EV (P = 0.045).
Dominance was unrelated to the magnitude of training response for all variables (P > 0.06) (Table 5). Specifically, all DXA variables increased significantly (P ≤ 0.006). No significant changes were seen in VOS (P ≥ 0.59), while a significant increase was seen in BUA for those in whom the scanned left leg was their nondominant limb (P < 0.001), but not for the smaller group (n = 36) who were left-leg dominant (P = 0.75). Significant increases were seen in both PV and CV on both dominant and nondominant legs (P < 0.001), with no change in EV (P = 0.92 and P = 0.72, respectively) (Table 5).
DISCUSSION
Using three phenotyping modalities, we have characterized the lower limb skeletal response to 12 wk of uniform exercise training in young Caucasian men. Substantial increases in BMD (of 0.85–2.93% in all areas of the femur assessed) are shown, for the first time, to occur over a short time frame such as this, but also accompanied by parallel changes in femoral bone morphology. Bone deposition is shown to be largely cortical.
These findings are of likely relevance to a wider population and especially to athletes. However, they may also find especial resonance for military recruits, among whom rapid bone remodeling seems associated with bone injury. Asymptomatic bone injury (56) and symptomatic stress fracture (44) are both commonplace. Furthermore, the geometric factors we describe (and their responses to training) may play an important role in the etiology of (or prevention from) such injury (27).
Changes in BMD With Training
In our study, THBMD rose by 1.89 ± 0.15% (mean of individual changes), from 1.083 ± 0.007 to 1.102 ± 0.007 g/cm2, with mean rises of 0.85, 1.98, 1.76, 1.36, and 2.93% seen for FNBMD, TRBMD, ITBMD, PFBMD, and WTBMD, respectively, findings in keeping with those of others. Thus tibial diaphyseal BMD increases with 14–15 wk of military training (15, 66), and femoral BMD with 16 wk of training in middle-aged and elderly men (79), 12 mo of high-impact exercise in premenopausal women (39, 107), and >30 mo in young male hockey players (87). We have extended such observations, showing that BMD responds to exercise in an even shorter time frame of 12 wk. Such data add granularity to those reported by Lester and colleagues (69), who studied the response of 56 young women (mean age 20.3 yr) undertaking one of three 8-wk programs of physical training. Increases in biomarkers of bone formation in those undertaking exercise with an aerobic component (whether alone or combined with resistance training) occurred in association with small changes in DXA- and pQCT-derived volumetric and areal distal tibial BMD. Levels of tartrate-resistant acid phosphatase (a biomarker of bone resorption) fell in all exercising groups (69).
The pattern of change in BMD is also consistent with existing published data, albeit those resulting from much longer training periods. Thus we observed the greatest change in BMD to be in Ward's triangle. In a group of young men, 6 mo of physical training induced changes of 1.4% for TRBMD, 2.4% for FNBMD, and 3.0% for WTBMD (93). Greater increases in WTBMD are also seen in older women (57) in response to resistance and impact exercise. WTBMD is not a true anatomic area, but is generated by the DXA scan software as the area having the lowest BMD in the femoral head. Being an area of high turnover trabecular bone, it may well be more responsive to the osteogenic stimulus.
Changes in QUS With Training
That BMD is only able to predict 66–74% of the variation in bone strength (2) is reflected in the considerable overlap in BMD values between women with and without fractures (16). In this regard, QUS may be additionally informative, providing information on other characteristics of bone, such as porosity, connectivity, and anisotropy, that are distinct from BMD (32).
We showed BUA to rise with training (from 88.10 ± 14.92 to 90.60 ± 13.93 dB/MHz, P < 0.001), a finding in keeping with the higher BUA values observed in 45- to 74-yr-old men participating in >2 h/wk of high-impact activity compared with the sedentary (47), and in pubertal female gymnasts than in controls (65). They are also in keeping with studies of young male army recruits (25), postmenopausal (4) and premenopausal women (39), peripubertal girls (64), and young men (18) over training periods of 10 wk and 6, 12, and 18 mo, respectively. Meanwhile, BUA increased over 6 mo to a similar degree in Finnish male army recruits as it did in controls (112), suggesting that BUA can continue to increase with growth (albeit over a much longer time frame than that of our study). Caution must thus be exercised in attributing the changes in BUA that we observed to the training stimulus alone. Nonetheless, the fact that these changes occurred in the context of changes in macroscopic form and bone density and are in keeping with both animal data and past human studies does offer some degree of confidence that the training stimulus was indeed the main driver of change.
While VOS is elevated in physically active boys (5), professional footballers (50), and pubertal female gymnasts (65) compared with controls, our failure to identify a training-related rise in VOS (1,662.6 ± 2.00 vs. 1,663.5 ± 1.92 ms−1; +0.06 ± 0.09%, P = 0.58) is not unusual (4, 18, 39). Training-related increases were, however, identified in one study of peripubertal girls (64), while a small study (n = 26) of UK army recruits undergoing 10 wk of training identified an associated significant decrease in mean VOS of 1.7% (25). Such findings are somewhat contradictory: a “stronger” bone would be expected to attenuate sound waves better (and thus have a higher BUA), but also allow sound to pass through it faster (with a higher VOS). The authors speculated that this finding was due to increases in trabecular separation due to microfracture and resultant loss of trabeculae (24). Alternatively, training intensity may have differed from our study, as might the pretraining habitual fitness of the subjects. This might be of importance if, as has been speculated, VOS initially falls in response to exercise, followed by a rise after continued activity (25).
Changes in Bone Volume With Training
Only one other study has examined changes in bone morphology at the proximal femur over a similar period in human subjects (20). Although small and not primarily intended to characterize the response of femoral cortical bone to training, the study did provide evidence that bone geometry can acutely change in response to exercise, even within a short period. We have confirmed and extended these observations. Both left and right femoral bone volumes increased with training: CV by 1.09 ± 0.20 and 0.71 ± 0.20%, and PV by 0.78 ± 0.16 and 0.59 ± 0.13%, respectively. EV did not change significantly (0.44 ± 0.51 and 1.13 ± 0.59%, respectively). This is the first study to show increases in femoral CV in such a short training period (12 wk), but also to show that such short-term changes predominantly reflect differences in periosteal rather than endosteal bone. Such increases in periosteal bone deposition as we have identified would greatly enhance bone strength beyond that associated with simple mineralization (17, 96, 105).
Studies comparing the playing and nonplaying arms of athletes (35, 51), the bones of athletes and controls (36), and those engaged in different sporting activities (19) or experiencing different training volumes (73) support such a role for mechanical loading in modulating gross bone geometry. In adult long-term tennis players, cortical bone volume is increased in the dominant arm (3, 21, 35, 58), seemingly as a result of both periosteal expansion and endocortical contraction (34, 51). In athletes of mixed sex and sporting discipline, tibial diaphyseal cortical CSA was increased compared with controls, due to increases in periosteal, but not endocortical, circumference (114). Similarly, a population study of 1,068 young men showed increased physical activity to be associated with increased tibial and radial cortical bone size through periosteal apposition (72), while young female athletes/triathletes had higher cortical CSA and smaller medullary CSA than those engaged in non-weight-bearing sports (such as swimmers) (22).
The differential responses of the periosteal and endosteal surfaces may be both age (92) and site dependent (95, 97): prepubertal racquet-sport athletes demonstrate preferential periosteal new bone deposition in their playing arms (8, 58), while prepubertal boys undergoing 8 mo of weight-bearing exercise showed increased femoral midshaft cortical thickness due to a decrease in endocortical diameter, with no periosteal expansion (13). The arms of postpubertal players respond through medullary contraction (8). In postpubertal female tennis players, increases in midhumeral cortical area seemed to result from periosteal expansion alone, while at the distal humerus, medullary contraction contributed more (8). This may be due to differences in the maturation between proximal and distal parts of a limb (7, 8) and to differential loading of forces at different parts of a limb (51).
Differences by Limb Dominance
Baseline DXA measures of BMD were similar in the dominant and nondominant legs (Table 4).
Because the upper limb does not bear the weight of the body, loading is strongly influenced by limb dominance. Therefore, the dominant arm of tennis (34, 36, 54, 58) and baseball players shows an increase in DXA-derived areal BMD (77). However, volumetric BMD (detected by QCT) tends to show no increase (3) or a marginal increase (21), suggesting that loading induces changes in the geometry of the upper limb bones, but not necessarily in cortical volumetric density (3, 35, 58). Meanwhile, the mode of loading may accentuate asymmetry; greater DXA-derived BMD measurements for the right arm of volleyball, basketball, and soccer players have been observed compared with swimmers (63), with an increase in BMC among volleyball players compared with sedentary control (1). Findings in the lower limb of nonathletic individuals are less consistent: some reveal no difference by limb dominance (22, 80, 119), while DXA-derived BMD has been reported to be higher in the nondominant leg of volleyball players (63) and football and tennis players (80, 88). In a study of 106 athletes, racquet players (and to a lesser extent, rowers) showed greater BMD on the dominant upper limb, but no differences between lower limbs in any discipline (83).
Just as for DXA, baseline QUS measures were similar in the dominant and nondominant legs (Table 4), a finding in agreement with others (120). Where differences are identified, they are often small (33, 46). This might not be unexpected: while a one-sided preference for specific kicking and mobilization tasks is common, there is often little or no preference for stabilization tasks (e.g., one-leg stance, clearing an obstacle) (104), while the mobilizing leg will often depend on the nondominant leg for support. Furthermore, the presence of asymmetry may depend on the activity status of the subjects: BUA values are higher in the heels of football players than in nonathletic controls, with significant side differences found only in the nonathletic subject (103). Asymmetry may also increase with age (75) and (as for DXA) may be site dependent: in healthy children, calcaneal asymmetry (75) is not reflected in hand proximal phalanges (6) or midshaft tibia (68). Where identified, VOS tends to be higher on the nondominant side (80, 88).
However, all MRI-derived bone volumes were greater in the dominant than nondominant femur (PV: 25,991.49 ± 3,207.8 vs. 25,572.68 ± 3,176.6 mm3, P < 0.001; CV: 19,928.22 ± 2,616.6 vs. 19,589.56 ± 2,598.1 mm3, P < 0.001; EV: 6,063.33 ± 1,875.19 vs. 5,983.12 ± 1,814.0 mm3, P = 0.001). This phenomenon may be more evident because of the age of our subjects. Tennis training that begins in adulthood induces an increase in bone mass without change in width (3, 36). Those who tend to have played from their youth increase their total CSA and cortical wall thickness without a change in volumetric or trabecular bone density (35). This age-differential effect of mechanical loading of bone has also been found for the humerus, where increased loading has effects on both the periosteal and endocortical surfaces, with the relevant contribution of each surface to the increase in size being site specific (8). Another study of 94 men (range 18–28 yr) showed side-to-side differences in BMD and CW (but not periosteal area) at the tibial diaphysis, with higher values in the nondominant side (i.e., the limb supporting body weight rather than that involved with manipulation and dexterity) in both the athletic and control group (100). Assuming that the dominant leg is exposed to greater loading over a long period, the results from this study suggest that, in the long-term (in the proximal femur of young Caucasian men at least), this increased loading leads to an increase in CV, by relatively greater periosteal expansion than endocortical apposition and without any change in BMD measured either using quantitative computerized tomography (volumetric BMD) or DXA (area-derived BMD).
The magnitude of training response was independent of limb dominance for all variables. It is plausible that the military training program provided a similar osteogenic stimulus in both limbs. Alternatively, given that MRI data did differ at baseline, it is possible that the mechanisms involved in the long-term regulation of bone geometry may differ from those regulating the response to short-term loading stimuli.
Comparable data are sparse. After 14 wk of strenuous physical training in young men, one study showed greater increases in BMC in the dominant leg compared with the nondominant (76), while, in contrast, a 3-yr longitudinal study showed similar changes in both dominant and nondominant humerii and femurs of young athletes (87).
Study Limitations
It is possible that increases in skeletal geometry may have reflected continued growth in some individuals, and a radiological marker of skeletal maturity may have contributed to the interpretation of geometric change. Furthermore, while the identification of a comparable sedentary control group of sufficient scale would have proved a challenge, this would have helped in differentiating the contribution of such effects.
Second, more frequent observation might have yielded greater granularity of data. Thus an initial fall of VOS as an adaptive response to exercise may be followed by a rise after continued activity (25). However, logistic limitations did not allow interim measures in our study. In addition, data on nutritional aspects before training were unavailable. Nor can one ensure that diet was constant for each individual during training. However, while insufficient energy intake can be detrimental to bone health (40), our training regime is not associated with a significant energy deficit (90). Nonetheless, information on intake or current status of calcium, vitamin D, other trace nutrients, protein, and fat may have shown interaction with baseline measures or the response to training (38).
Third, only the lower limb was studied, and changes here may not represent those in other load-bearing skeletal structures such as the spine. Indeed, the geometric response to increased mechanical load does seem site dependent (8, 89), and 15 wk of military training have been associated with increase in tibial diaphyseal BMD and a concurrent decrease at the lumbar spine (15).
Fourth, a study such as this does not permit quantification of the precise pattern and magnitude of skeletal loading. Direct measurement of bone strains is invasive (45), while indirect measurement of ground reaction forces is not practicable in studies like ours, as it is limited in time and place. Accelerometer-based data can be used as surrogates for bone strain (39). Such devices have been used to investigate the skeletal response to exercise (39, 48, 106, 108–110). However, strain relating to muscle forces on bone is not quantified. Furthermore, most devices only measure the vertical component of acceleration, and impacts from atypical loading directions strongly influence femoral neck strength (84, 85). The applicability and validity of using such an approach in recruit training thus remains unvalidated.
Fifth, leg dominance was recorded by asking the recruits whether they were right, left, or both footed, with regards to which leg they would normally use to kick a ball. A formal assessment of dominance was not possible due to time constraints. Appropriate methodologies are also debated: some argue that the stance leg should be deemed dominant, while others believe it should be the kicking leg. Previous results have shown little difference between subjective leg dominance based on kicking and functional dominance (41).
Sixth, biochemical markers of bone turnover were not measured. However, the relationship between markers and actual phenotypic change (for instance, in BMD) is generally weak (71, 78) and may be weaker still in a younger male population (28, 71). Their correlation with change in BMD may be modest, with a high variation in the identified statistical significance and polarity of correlation (29, 71). Markers are also influenced by longer term factors (such as season) and also change dramatically in the very short term, being subject to diurnal variation and the influence of exercise and bed rest (31, 71, 82). Indeed, markers of bone turnover rise within 30 min of acute exercise, with the observed changes exceeding those that could be explained by hemoconcentration (14, 113). Temporal variations in access to the army recruits sampled would thus have proven a major confounder, worsened by the unavoidable variation in the nature, intensity, timing, and duration of exercise taken immediately before study.
In addition, alternative modes of phenotyping might be applied in future studies. Sensitivities relating to x-radiation exposure led us to favor the use of a mobile MRI scanner for the assessment of bone macro-geometry. However, the use of pQCT allows assessment of bone geometry and also of true volumetric (and trabecular and cortical) BMD, and indexes of stress-strain. Future application of such technology may thus offer advantages.
Finally, care should be taken in the extrapolation of our findings to women and to those of different age. The skeletal response to exercise may be greater in the young (53, 117) and may even differ between middle-aged and elderly men (79). Bone characteristics and their response to exercise are also sex dependent (9, 27). Although no sex differences have been shown in the BMD response to training in controlled human studies (93), both animal (101, 113) and human studies (26) have shown significant differences in bone turnover.
In conclusion, we have reported the first large-scale study to investigate the skeletal changes to training using three different modalities (DXA, QUS, and MRI) in young Caucasian men. Femoral geometry differs by limb dominance at baseline and responds to training primarily by an increase in periosteal bone deposition. Hip and femoral BMD, and calcaneal BUA, all increased substantially in only 12 wk. Such data offer insight into the speed and nature of the skeletal response to exercise and lend further support to the encouragement to exercise as a means of improving bone health in the young.
GRANTS
The Lichfield Bone study (and its sister “LARGE Heart” study) were primarily funded by a British Heart Foundation Project Grant (PG/02/021) and an unconditional educational grant from Aventis UK. Funding for the musculoskeletal component of this study was provided by Research Into Ageing, National Osteoporosis Society, Wishbone Orthopaedic Trust, Dupuy, and the Fares Haddad Research Fund. J. R. Payne (PG/02/021) and S. E. Humphries (RG95007 and PG2008/08) were funded by the British Heart Foundation, which also provides core funding for the Centre for Cardiovascular Genetics. L. E. James and K. I. Eleftheriou were funded by Research into Ageing Fellowships. D. J. Pennell was supported by the UK NIHR Cardiovascular Biomedical Research Unit of Royal Brompton and Harefield NHS Foundation Trust and Imperial College. The study was also supported by CORDA The Heart Charity.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: K.I.E., L.E.J., J.R.P., and S.E.H. performed experiments; K.I.E., J.S.R., A.K., L.E.J., J.R.P., F.D., and D.J.P. analyzed data; K.I.E., J.S.R., A.K., L.E.J., J.R.P., and F.D. interpreted results of experiments; K.I.E., J.S.R., and F.D. prepared figures; K.I.E., J.S.R., J.R.S., and Z.A.P. drafted manuscript; K.I.E., J.S.R., A.K., L.E.J., J.R.P., J.R.S., Z.A.P., D.J.P., M.L., M.W., S.E.H., F.S.H., and H.E.M. approved final version of manuscript; J.S.R., J.R.S., Z.A.P., D.J.P., M.L., M.W., S.E.H., F.S.H., and H.E.M. edited and revised manuscript; L.E.J., J.R.P., D.J.P., M.W., S.E.H., F.S.H., and H.E.M. conception and design of research.
ACKNOWLEDGMENTS
The authors thank the army recruit volunteers at Lichfield, and the staff at the Army Training Regiment Lichfield for hospitality and cooperation. We especially recognize the staff of the Medical Reception Station and Officer's Mess and the commanding officer Lt. Col. Nicholas Chapman. The authors also thank Alliance Medical Limited, which provided the mobile scanner.
REFERENCES
- 1. Alfredson H, Nordstrom P, Pietila T, Lorentzon R. Long-term loading and regional bone mass of the arm in female volleyball players. Calcif Tissue Int 62: 303–308, 1998 [DOI] [PubMed] [Google Scholar]
- 2. Ammann P, Rizzoli R. Bone strength and its determinants. Osteoporos Int 3: S13–S18, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Ashizawa N, Nonaka K, Michikami S, Mizuki T, Amagai H, Tokuyama K, Suzuki M. Tomographical description of tennis-loaded radius: reciprocal relation between bone size and volumetric BMD. J Appl Physiol 86: 1347–1351, 1999 [DOI] [PubMed] [Google Scholar]
- 4. Ay A, Yurtkuran M. Influence of aquatic and weight-bearing exercises on quantitative ultrasound variables in postmenopausal women. Am J Phys Med Rehabil 84: 52–61, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Babaroutsi E, Magkos F, Manios Y, Sidossis LS. Body mass index, calcium intake, and physical activity affect calcaneal ultrasound in healthy Greek males in an age-dependent and parameter-specific manner. J Bone Miner Metab 23: 157–166, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Baroncelli GI, Federico G, Bertelloni S, de Terlizzi F, Cadossi R, Saggese G. Bone quality assessment by quantitative ultrasound of proximal phalanxes of the hand in healthy subjects aged 3–21 years. Pediatr Res 49: 713–718, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Bass S, Delmas PD, Pearce G, Hendrich E, Tabensky A, Seeman E. The differing tempo of growth in bone size, mass, and density in girls is region-specific. J Clin Invest 104: 795–804, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bass SL, Saxon L, Daly RM, Turner CH, Robling AG, Seeman E, Stuckey S. The effect of mechanical loading on the size and shape of bone in pre-, peri-, and postpubertal girls: a study in tennis players. J Bone Miner Res 17: 2274–2280, 2002 [DOI] [PubMed] [Google Scholar]
- 9. Beck TJ, Ruff CB, Shaffer RA, Betsinger K, Trone DW, Brodine SK. Stress fracture in military recruits: gender differences in muscle and bone susceptibility factors. Bone 27: 437–444, 2000 [DOI] [PubMed] [Google Scholar]
- 10. Bergman BP, Miller SA. Equal opportunities, equal risks? Overuse injuries in female military recruits. J Public Health Med 23: 35–39, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Bertelsen PK, Clement JG, Thomas CD. A morphometric study of the cortex of the human femur from early childhood to advanced old age. Forensic Sci Int 74: 63–77, 1995 [DOI] [PubMed] [Google Scholar]
- 12. Blake GM, Gluer CC, Fogelman I. Bone densitometry: current status and future prospects. Br J Radiol 70: S177–S186, 1997 [DOI] [PubMed] [Google Scholar]
- 13. Bradney M, Pearce G, Naughton G, Sullivan C, Bass S, Beck T, Carlson J, Seeman E. Moderate exercise during growth in prepubertal boys: changes in bone mass, size, volumetric density, and bone strength: a controlled prospective study. J Bone Miner Res 13: 1814–1821, 1998 [DOI] [PubMed] [Google Scholar]
- 14. Brahm H, Strom H, Piehl-Aulin K, Mallmin H, Ljunghall S. Bone metabolism in endurance trained athletes: a comparison to population-based controls based on DXA, SXA, quantitative ultrasound, and biochemical markers. Calcif Tissue Int 61: 448–454, 1997 [DOI] [PubMed] [Google Scholar]
- 15. Casez JP, Fischer S, Stussi E, Stalder H, Gerber A, Delmas PD, Colombo JP, Jaeger P. Bone mass at lumbar spine and tibia in young males–impact of physical fitness, exercise, and anthropometric parameters: a prospective study in a cohort of military recruits. Bone 17: 211–219, 1995 [DOI] [PubMed] [Google Scholar]
- 16. Cummings SR, Black DM, Nevitt MC, Browner WS, Cauley JA, Genant HK, Mascioli SR, Scott JC, Seeley DG, Steiger P, et al. Appendicular bone density and age predict hip fracture in women. The Study of Osteoporotic Fractures Research Group. JAMA 263: 665–668, 1990 [PubMed] [Google Scholar]
- 17. Currey JD. Bone strength: what are we trying to measure? Calcif Tissue Int 68: 205–210, 2001 [DOI] [PubMed] [Google Scholar]
- 18. Daly RM, Rich PA, Klein R, Bass S. Effects of high-impact exercise on ultrasonic and biochemical indices of skeletal status: a prospective study in young male gymnasts. J Bone Miner Res 14: 1222–1230, 1999 [DOI] [PubMed] [Google Scholar]
- 19. Daly RM, Saxon L, Turner CH, Robling AG, Bass SL. The relationship between muscle size and bone geometry during growth and in response to exercise. Bone 34: 281–287, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Dhamrait SS, James L, Brull DJ, Myerson S, Hawe E, Pennell DJ, World M, Humphries SE, Haddad F, Montgomery HE. Cortical bone resorption during exercise is interleukin-6 genotype-dependent. Eur J Appl Physiol 89: 21–25, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Ducher G, Courteix D, Meme S, Magni C, Viala JF, Benhamou CL. Bone geometry in response to long-term tennis playing and its relationship with muscle volume: a quantitative magnetic resonance imaging study in tennis players. Bone 37: 457–466, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Duncan CS, Blimkie CJ, Kemp A, Higgs W, Cowell CT, Woodhead H, Briody JN, Howman-Giles R. Mid-femur geometry and biomechanical properties in 15- to 18-yr-old female athletes. Med Sci Sports Exerc 34: 673–681, 2002 [DOI] [PubMed] [Google Scholar]
- 23. El Maghraoui A, Roux C. DXA scanning in clinical practice. QJM 101: 605–617, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Etherington J, Keeling J, Bramley R, Swaminathan R, McCurdie I. The effects of 10 wk military training on heel ultrasound attenuation and bone turnover. Calcif Tissue Int 64: 389–393, 1999 [DOI] [PubMed] [Google Scholar]
- 25. Etherington J, Keeling J, Bramley R, Swaminathan R, McCurdie I, Spector TD. The effects of 10 weeks military training on heel ultrasound and bone turnover. Calcif Tissue Int 64: 389–393, 1999 [DOI] [PubMed] [Google Scholar]
- 26. Evans RK, Antczak AJ, Lester M, Yanovich R, Israeli E, Moran DS. Effects of a 4-month recruit training program on markers of bone metabolism. Med Sci Sports Exerc 40: S660–S670, 2008 [DOI] [PubMed] [Google Scholar]
- 27. Evans RK, Negus C, Antczak AJ, Yanovich R, Israeli E, Moran DS. Sex differences in parameters of bone strength in new recruits: beyond bone density. Med Sci Sports Exerc 40: S645–S653, 2008 [DOI] [PubMed] [Google Scholar]
- 28. Garnero P, Sornay-Rendu E, Chapuy MC, Delmas PD. Increased bone turnover in late postmenopausal women is a major determinant of osteoporosis. J Bone Miner Res 11: 337–349, 1996 [DOI] [PubMed] [Google Scholar]
- 29. Garnero P, Sornay-Rendu E, Duboeuf F, Delmas PD. Markers of bone turnover predict postmenopausal forearm bone loss over 4 years: the OFELY study. J Bone Miner Res 14: 1614–1621, 1999 [DOI] [PubMed] [Google Scholar]
- 30. Gemmell IM. Injuries among female army recruits: a conflict of legislation. J R Soc Med 95: 23–27, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ginty F, Flynn A, Cashman K. Inter and intra-individual variations in urinary excretion of pyridinium crosslinks of collagen in healthy young adults. Eur J Clin Nutr 52: 71–73, 1998 [DOI] [PubMed] [Google Scholar]
- 32. Gluer CC, Wu CY, Jergas M, Goldstein SA, Genant HK. Three quantitative ultrasound parameters reflect bone structure. Calcif Tissue Int 55: 46–52, 1994 [DOI] [PubMed] [Google Scholar]
- 33. Graafmans WC, Bouter LM, Lips P. The influence of physical activity and fractures on ultrasound parameters in elderly people. Osteoporos Int 8: 449–454, 1998 [DOI] [PubMed] [Google Scholar]
- 34. Haapasalo H, Kannus P, Sievanen H, Pasanen M, Uusi-Rasi K, Heinonen A, Oja P, Vuori I. Effect of long-term unilateral activity on bone mineral density of female junior tennis players. J Bone Miner Res 13: 310–319, 1998 [DOI] [PubMed] [Google Scholar]
- 35. Haapasalo H, Kontulainen S, Sievanen H, Kannus P, Jarvinen M, Vuori I. Exercise-induced bone gain is due to enlargement in bone size without a change in volumetric bone density: a peripheral quantitative computed tomography study of the upper arms of male tennis players. Bone 27: 351–357, 2000 [DOI] [PubMed] [Google Scholar]
- 36. Haapasalo H, Sievanen H, Kannus P, Heinonen A, Oja P, Vuori I. Dimensions and estimated mechanical characteristics of the humerus after long-term tennis loading. J Bone Miner Res 11: 864–872, 1996 [DOI] [PubMed] [Google Scholar]
- 37. Hans D, Schott AM, Chapuy MC, Benamar M, Kotzki PD, Cormier C, Pouilles JM, Meunier PJ. Ultrasound measurements on the os calcis in a prospective multicenter study. Calcif Tissue Int 55: 94–99, 1994 [DOI] [PubMed] [Google Scholar]
- 38. Heaney RP, Abrams S, Dawson-Hughes B, Looker A, Marcus R, Matkovic V, Weaver C. Peak bone mass. Osteoporos Int 11: 985–1009, 2000 [DOI] [PubMed] [Google Scholar]
- 39. Heikkinen R, Vihriala E, Vainionpaa A, Korpelainen R, Jamsa T. Acceleration slope of exercise-induced impacts is a determinant of changes in bone density. J Biomech 40: 2967–2974, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Hind K, Burrows M. Weight-bearing exercise and bone mineral accrual in children and adolescents: a review of controlled trials. Bone 40: 14–27, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Hoffman M, Schrader J, Applegate T, Koceja D. Unilateral postural control of the functionally dominant and nondominant extremities of healthy subjects. J Athl Train 33: 319–322, 1998 [PMC free article] [PubMed] [Google Scholar]
- 42. Hogler W, Blimkie CJ, Cowell CT, Kemp AF, Briody J, Wiebe P, Farpour-Lambert N, Duncan CS, Woodhead HJ. A comparison of bone geometry and cortical density at the mid-femur between prepuberty and young adulthood using magnetic resonance imaging. Bone 33: 771–778, 2003 [DOI] [PubMed] [Google Scholar]
- 43. Hong J, Hipp JA, Mulkern RV, Jaramillo D, Snyder BD. Magnetic resonance imaging measurements of bone density and cross-sectional geometry. Calcif Tissue Int 66: 74–78, 2000 [DOI] [PubMed] [Google Scholar]
- 44. Hong SH, Chu IT. Stress fracture of the proximal fibula in military recruits. Clin Orthop Surg 1: 161–164, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hoshaw SJ, Fyhrie DP, Takano Y, Burr DB, Milgrom C. A method suitable for in vivo measurement of bone strain in humans. J Biomech 30: 521–524, 1997 [DOI] [PubMed] [Google Scholar]
- 46. Howard GM, Nguyen TV, Pocock NA, Kelly PJ, Eisman JA. Influence of handedness on calcaneal ultrasound: implications for assessment of osteoporosis and study design. Osteoporos Int 7: 190–194, 1997 [DOI] [PubMed] [Google Scholar]
- 47. Jakes RW, Khaw K, Day NE, Bingham S, Welch A, Oakes S, Luben R, Dalzell N, Reeve J, Wareham NJ. Patterns of physical activity and ultrasound attenuation by heel bone among Norfolk cohort of European Prospective Investigation of Cancer (EPIC Norfolk): population based study. BMJ 322: 140, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jamsa T, Vainionpaa A, Korpelainen R, Vihriala E, Leppaluoto J. Effect of daily physical activity on proximal femur. Clin Biomech (Bristol, Avon) 21: 1–7, 2006 [DOI] [PubMed] [Google Scholar]
- 49. Jergas M, Gluer CC. Assessment of fracture risk by bone density measurements. Semin Nucl Med 27: 261–275, 1997 [DOI] [PubMed] [Google Scholar]
- 50. Jergas M, Uffmann M, Wittenberg R, Muller P, Koster O. [Ultrasonic velocity measurements at weight-bearing and non-weight-bearing sites of the peripheral skeleton. The effect of physical activity in soccer players.] Rofo 157: 420–424, 1992 [DOI] [PubMed] [Google Scholar]
- 51. Jones HH, Priest JD, Hayes WC, Tichenor CC, Nagel DA. Humeral hypertrophy in response to exercise. J Bone Joint Surg Am 59: 204–208, 1977 [PubMed] [Google Scholar]
- 52. Joo YI, Sone T, Fukunaga M, Lim SG, Onodera S. Effects of endurance exercise on three-dimensional trabecular bone microarchitecture in young growing rats. Bone 33: 485–493, 2003 [DOI] [PubMed] [Google Scholar]
- 53. Kannus P, Haapasalo H, Sankelo M, Sievanen H, Pasanen M, Heinonen A, Oja P, Vuori I. Effect of starting age of physical activity on bone mass in the dominant arm of tennis and squash players. Ann Intern Med 123: 27–31, 1995 [DOI] [PubMed] [Google Scholar]
- 54. Kannus P, Haapasalo H, Sievanen H, Oja P, Vuori I. The site-specific effects of long-term unilateral activity on bone mineral density and content. Bone 15: 279–284, 1994 [DOI] [PubMed] [Google Scholar]
- 55. Kaptoge S, da Silva JA, Brixen K, Reid DM, Kroger H, Nielsen TL, Andersen M, Hagen C, Lorenc R, Boonen S, de Vernejoul MC, Stepan JJ, Adams J, Kaufman JM, Reeve J. Geographical variation in DXA bone mineral density in young European men and women. Results from the Network in Europe on Male Osteoporosis (NEMO) study. Bone 43: 332–339, 2008 [DOI] [PubMed] [Google Scholar]
- 56. Kiuru MJ, Niva M, Reponen A, Pihlajamaki HK. Bone stress injuries in asymptomatic elite recruits: a clinical and magnetic resonance imaging study. Am J Sports Med 33: 272–276, 2005 [DOI] [PubMed] [Google Scholar]
- 57. Kohrt WM, Ehsani AA, Birge SJ., Jr Effects of exercise involving predominantly either joint-reaction or ground-reaction forces on bone mineral density in older women. J Bone Miner Res 12: 1253–1261, 1997 [DOI] [PubMed] [Google Scholar]
- 58. Kontulainen S, Sievanen H, Kannus P, Pasanen M, Vuori I. Effect of long-term impact-loading on mass, size, and estimated strength of humerus and radius of female racquet-sports players: a peripheral quantitative computed tomography study between young and old starters and controls. J Bone Miner Res 18: 352–359, 2003 [DOI] [PubMed] [Google Scholar]
- 59. Kroger H, Vainio P, Nieminen J, Kotaniemi A. Comparison of different models for interpreting bone mineral density measurements using DXA and MRI technology. Bone 17: 157–159, 1995 [DOI] [PubMed] [Google Scholar]
- 60. Langton CM, Langton DK. Male and female normative data for ultrasound measurement of the calcaneus within the UK adult population. Br J Radiol 70: 580–585, 1997 [DOI] [PubMed] [Google Scholar]
- 61. Langton CM, Palmer SB, Porter RW. The measurement of broadband ultrasonic attenuation in cancellous bone. Eng Med 13: 89–91, 1984 [DOI] [PubMed] [Google Scholar]
- 62. Laskey MA, Flaxman ME, Barber RW, Trafford S, Hayball MP, Lyttle KD, Crisp AJ, Compston JE. Comparative performance in vitro and in vivo of Lunar DPX and Hologic QDR-1000 dual energy X-ray absorptiometers. Br J Radiol 64: 1023–1029, 1991 [DOI] [PubMed] [Google Scholar]
- 63. Lee EJ, Long KA, Risser WL, Poindexter HB, Gibbons WE, Goldzieher J. Variations in bone status of contralateral and regional sites in young athletic women. Med Sci Sports Exerc 27: 1354–1361, 1995 [PubMed] [Google Scholar]
- 64. Lehtonen-Veromaa M, Mottonen T, Kautiainen H, Heinonen OJ, Viikari J. Influence of physical activity and cessation of training on calcaneal quantitative ultrasound measurements in peripubertal girls: a 1-year prospective study. Calcif Tissue Int 68: 146–150, 2001 [DOI] [PubMed] [Google Scholar]
- 65. Lehtonen-Veromaa M, Mottonen T, Nuotio I, Heinonen OJ, Viikari J. Influence of physical activity on ultrasound and dual-energy X-ray absorptiometry bone measurements in peripubertal girls: a cross-sectional study. Calcif Tissue Int 66: 248–254, 2000 [DOI] [PubMed] [Google Scholar]
- 66. Leichter I, Simkin A, Margulies JY, Bivas A, Steinberg R, Giladi M, Milgrom C. Gain in mass density of bone following strenuous physical activity. J Orthop Res 7: 86–90, 1989 [DOI] [PubMed] [Google Scholar]
- 67. Leonard CM, Roza MA, Barr RD, Webber CE. Reproducibility of DXA measurements of bone mineral density and body composition in children. Pediatr Radiol 39: 148–154, 2009 [DOI] [PubMed] [Google Scholar]
- 68. Lequin MH, van Rijn RR, Robben SG, Hop WC, Dijkhuis S, Fijten MM, Meijer LA, van Kuijk C. Evaluation of short-term precision for tibial ultrasonometry. Calcif Tissue Int 64: 24–27, 1999 [DOI] [PubMed] [Google Scholar]
- 69. Lester ME, Urso ML, Evans RK, Pierce JR, Spiering BA, Maresh CM, Hatfield DL, Kraemer WJ, Nindl BC. Influence of exercise mode and osteogenic index on bone biomarker responses during short-term physical training. Bone 45: 768–776, 2009 [DOI] [PubMed] [Google Scholar]
- 70. Lewiecki EM, Watts NB, McClung MR, Petak SM, Bachrach LK, Shepherd JA, Downs RW., Jr Official positions of the international society for clinical densitometry. J Clin Endocrinol Metab 89: 3651–3655, 2004 [DOI] [PubMed] [Google Scholar]
- 71. Looker AC, Bauer DC, Chesnut CH, 3rd, Gundberg CM, Hochberg MC, Klee G, Kleerekoper M, Watts NB, Bell NH. Clinical use of biochemical markers of bone remodeling: current status and future directions. Osteoporos Int 11: 467–480, 2000 [DOI] [PubMed] [Google Scholar]
- 72. Lorentzon M, Mellstrom D, Ohlsson C. Association of amount of physical activity with cortical bone size and trabecular volumetric BMD in young adult men: the GOOD study. J Bone Miner Res 20: 1936–1943, 2005 [DOI] [PubMed] [Google Scholar]
- 73. MacDougall JD, Webber CE, Martin J, Ormerod S, Chesley A, Younglai EV, Gordon CL, Blimkie CJ. Relationship among running mileage, bone density, and serum testosterone in male runners. J Appl Physiol 73: 1165–1170, 1992 [DOI] [PubMed] [Google Scholar]
- 74. Macleod MA, Houston AS, Sanders L, Anagnostopoulos C. Incidence of trauma related stress fractures and shin splints in male and female army recruits: retrospective case study. BMJ 318: 29, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Magkos F, Manios Y, Babaroutsi E, Sidossis LS. Contralateral differences in quantitative ultrasound of the heel: the importance of side in clinical practice. Osteoporos Int 16: 879–886, 2005 [DOI] [PubMed] [Google Scholar]
- 76. Margulies JY, Simkin A, Leichter I, Bivas A, Steinberg R, Giladi M, Stein M, Kashtan H, Milgrom C. Effect of intense physical activity on the bone-mineral content in the lower limbs of young adults. J Bone Joint Surg Am 68: 1090–1093, 1986 [PubMed] [Google Scholar]
- 77. McClanahan BS, Harmon-Clayton K, Ward KD, Klesges RC, Vukadinovich CM, Cantler ED. Side-to-side comparisons of bone mineral density in upper and lower limbs of collegiate athletes. J Strength Cond Res 16: 586–590, 2002 [PMC free article] [PubMed] [Google Scholar]
- 78. Melton LJ, 3rd, Khosla S, Atkinson EJ, O'Fallon WM, Riggs BL. Relationship of bone turnover to bone density and fractures. J Bone Miner Res 12: 1083–1091, 1997 [DOI] [PubMed] [Google Scholar]
- 79. Menkes A, Mazel S, Redmond RA, Koffler K, Libanati CR, Gundberg CM, Zizic TM, Hagberg JM, Pratley RE, Hurley BF. Strength training increases regional bone mineral density and bone remodeling in middle-aged and older men. J Appl Physiol 74: 2478–2484, 1993 [DOI] [PubMed] [Google Scholar]
- 80. Meszaros S, Ferencz V, Csupor E, Mester A, Hosszu E, Toth E, Horvath C. Comparison of the femoral neck bone density, quantitative ultrasound and bone density of the heel between dominant and non-dominant side. Eur J Radiol 60: 293–298, 2006 [DOI] [PubMed] [Google Scholar]
- 81. Modlesky CM, Lewis RD, Yetman KA, Rose B, Rosskopf LB, Snow TK, Sparling PB. Comparison of body composition and bone mineral measurements from two DXA instruments in young men. Am J Clin Nutr 64: 669–676, 1996 [DOI] [PubMed] [Google Scholar]
- 82. Morris HA, Wishart JM, Horowitz M, Need AG, Nordin BE. The reproducibility of bone-related biochemical variables in post-menopausal women. Ann Clin Biochem 27: 562–568, 1990 [DOI] [PubMed] [Google Scholar]
- 83. Nevill AM, Holder RL, Stewart AD. Modeling elite male athletes' peripheral bone mass, assessed using regional dual x-ray absorptiometry. Bone 32: 62–68, 2003 [DOI] [PubMed] [Google Scholar]
- 84. Nikander R, Kannus P, Dastidar P, Hannula M, Harrison L, Cervinka T, Narra NG, Aktour R, Arola T, Eskola H, Soimakallio S, Heinonen A, Hyttinen J, Sievanen H. Targeted exercises against hip fragility. Osteoporos Int 20: 1321–1328, 2009 [DOI] [PubMed] [Google Scholar]
- 85. Nikander R, Sievanen H, Heinonen A, Kannus P. Femoral neck structure in adult female athletes subjected to different loading modalities. J Bone Miner Res 20: 520–528, 2005 [DOI] [PubMed] [Google Scholar]
- 86. Njeh CF, Kuo CW, Langton CM, Atrah HI, Boivin CM. Prediction of human femoral bone strength using ultrasound velocity and BMD: an in vitro study. Osteoporos Int 7: 471–477, 1997 [DOI] [PubMed] [Google Scholar]
- 87. Nordstrom A, Olsson T, Nordstrom P. Bone gained from physical activity and lost through detraining: a longitudinal study in young males. Osteoporos Int 16: 835–841, 2005 [DOI] [PubMed] [Google Scholar]
- 88. Oral A, Yaliman A, Sindel D. Differences between the right and the left foot in calcaneal quantitative ultrasound measurements. Eur Radiol 14: 1427–1431, 2004 [DOI] [PubMed] [Google Scholar]
- 89. Plochocki JH, Rivera JP, Zhang C, Ebba SA. Bone modeling response to voluntary exercise in the hindlimb of mice. J Morphol 269: 313–318, 2008 [DOI] [PubMed] [Google Scholar]
- 90. Rayson MWD. Energy Expenditure During CMS. Surrey, UK: Optimal Performance, 2001 [Google Scholar]
- 91. Rayson MWDM, Blacker S. The Physical Demands of CMS: An Ergonomic Assessment. Bristol, UK: Optimal Performance, 2002 [Google Scholar]
- 92. Ruff CB, Walker A, Trinkaus E. Postcranial robusticity in Homo. III. Ontogeny. Am J Phys Anthropol 93: 35–54, 1994 [DOI] [PubMed] [Google Scholar]
- 93. Ryan AS, Ivey FM, Hurlbut DE, Martel GF, Lemmer JT, Sorkin JD, Metter EJ, Fleg JL, Hurley BF. Regional bone mineral density after resistive training in young and older men and women. Scand J Med Sci Sports 14: 16–23, 2004 [DOI] [PubMed] [Google Scholar]
- 94. Sainz J, Van Tornout JM, Loro ML, Sayre J, Roe TF, Gilsanz V. Vitamin D-receptor gene polymorphisms and bone density in prepubertal American girls of Mexican descent. N Engl J Med 337: 77–82, 1997 [DOI] [PubMed] [Google Scholar]
- 95. Seeman E. An exercise in geometry. J Bone Miner Res 17: 373–380, 2002 [DOI] [PubMed] [Google Scholar]
- 96. Seeman E. Periosteal bone formation–a neglected determinant of bone strength. N Engl J Med 349: 320–323, 2003 [DOI] [PubMed] [Google Scholar]
- 97. Seeman E, Duan Y, Fong C, Edmonds J. Fracture site-specific deficits in bone size and volumetric density in men with spine or hip fractures. J Bone Miner Res 16: 120–127, 2001 [DOI] [PubMed] [Google Scholar]
- 98. Sievanen H, Koskue V, Rauhio A, Kannus P, Heinonen A, Vuori I. Peripheral quantitative computed tomography in human long bones: evaluation of in vitro and in vivo precision. J Bone Miner Res 13: 871–882, 1998 [DOI] [PubMed] [Google Scholar]
- 99. Skerry TM, Suva LJ. Investigation of the regulation of bone mass by mechanical loading: from quantitative cytochemistry to gene array. Cell Biochem Funct 21: 223–229, 2003 [DOI] [PubMed] [Google Scholar]
- 100. Sone T, Imai Y, Joo YI, Onodera S, Tomomitsu T, Fukunaga M. Side-to-side differences in cortical bone mineral density of tibiae in young male athletes. Bone 38: 708–713, 2006 [DOI] [PubMed] [Google Scholar]
- 101. Sugiyama T, Saxon LK, Zaman G, Moustafa A, Sunters A, Price JS, Lanyon LE. Mechanical loading enhances the anabolic effects of intermittent parathyroid hormone (1–34) on trabecular and cortical bone in mice. Bone 43: 238–248, 2008 [DOI] [PubMed] [Google Scholar]
- 102. Svendsen OL, Marslew U, Hassager C, Christiansen C. Measurements of bone mineral density of the proximal femur by two commercially available dual energy X-ray absorptiometric systems. Eur J Nucl Med 19: 41–46, 1992 [DOI] [PubMed] [Google Scholar]
- 103. Tarakci D, Oral A. How do contralateral calcaneal quantitative ultrasound measurements in male professional football (soccer) players reflect the effects of high-impact physical activity on bone? J Sports Med Phys Fitness 49: 78–84, 2009 [PubMed] [Google Scholar]
- 104. Teixeira MC, Teixeira LA. Leg preference and interlateral performance asymmetry in soccer player children. Dev Psychobiol 50: 799–806, 2008 [DOI] [PubMed] [Google Scholar]
- 105. Turner CH, Burr DB. Basic biomechanical measurements of bone: a tutorial. Bone 14: 595–608, 1993 [DOI] [PubMed] [Google Scholar]
- 106. Vainionpaa A, Korpelainen R, Kaikkonen H, Knip M, Leppaluoto J, Jamsa T. Effect of impact exercise on physical performance and cardiovascular risk factors. Med Sci Sports Exerc 39: 756–763, 2007 [DOI] [PubMed] [Google Scholar]
- 107. Vainionpaa A, Korpelainen R, Leppaluoto J, Jamsa T. Effects of high-impact exercise on bone mineral density: a randomized controlled trial in premenopausal women. Osteoporos Int 16: 191–197, 2005 [DOI] [PubMed] [Google Scholar]
- 108. Vainionpaa A, Korpelainen R, Sievanen H, Vihriala E, Leppaluoto J, Jamsa T. Effect of impact exercise and its intensity on bone geometry at weight-bearing tibia and femur. Bone 40: 604–611, 2007 [DOI] [PubMed] [Google Scholar]
- 109. Vainionpaa A, Korpelainen R, Vaananen HK, Haapalahti J, Jamsa T, Leppaluoto J. Effect of impact exercise on bone metabolism. Osteoporos Int 20: 1725–1733, 2009 [DOI] [PubMed] [Google Scholar]
- 110. Vainionpaa A, Korpelainen R, Vihriala E, Rinta-Paavola A, Leppaluoto J, Jamsa T. Intensity of exercise is associated with bone density change in premenopausal women. Osteoporos Int 17: 455–463, 2006 [DOI] [PubMed] [Google Scholar]
- 111. Valimaki MJ, Karkkainen M, Lamberg-Allardt C, Laitinen K, Alhava E, Heikkinen J, Impivaara O, Makela P, Palmgren J, Seppanen R, et al. Exercise, smoking, and calcium intake during adolescence and early adulthood as determinants of peak bone mass. Cardiovascular Risk in Young Finns Study Group. BMJ 309: 230–235, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Valimaki VV, Loyttyniemi E, Valimaki MJ. Quantitative ultrasound variables of the heel in Finnish men aged 18–20 yr: predictors, relationship to bone mineral content, and changes during military service. Osteoporos Int 17: 1763–1771, 2006 [DOI] [PubMed] [Google Scholar]
- 113. Wallace JD, Cuneo RC, Lundberg PA, Rosen T, Jorgensen JO, Longobardi S, Keay N, Sacca L, Christiansen JS, Bengtsson BA, Sonksen PH. Responses of markers of bone and collagen turnover to exercise, growth hormone (GH) administration, and GH withdrawal in trained adult males. J Clin Endocrinol Metab 85: 124–133, 2000 [DOI] [PubMed] [Google Scholar]
- 114. Wilks DC, Winwood K, Gilliver SF, Kwiet A, Chatfield M, Michaelis I, Sun LW, Ferretti JL, Sargeant AJ, Felsenberg D, Rittweger J. Bone mass and geometry of the tibia and the radius of master sprinters, middle and long distance runners, race-walkers and sedentary control participants: a pQCT study. Bone 45: 91–97, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Williams AG, Rayson MP, Jones DA. Effects of basic training on material handling ability and physical fitness of British Army recruits. Ergonomics 42: 1114–1124, 1999 [DOI] [PubMed] [Google Scholar]
- 116. Williams AG, Rayson MP, Jones DA. Resistance training and the enhancement of the gains in material-handling ability and physical fitness of British Army recruits during basic training. Ergonomics 45: 267–279, 2002 [DOI] [PubMed] [Google Scholar]
- 117. Wolff I, van Croonenborg JJ, Kemper HC, Kostense PJ, Twisk JW. The effect of exercise training programs on bone mass: a meta-analysis of published controlled trials in pre- and postmenopausal women. Osteoporos Int 9: 1–12, 1999 [DOI] [PubMed] [Google Scholar]
- 118. Woodhead HJ, Kemp AF, Blimkie CJR, Briody JN, Duncan CS, Thompson M, Lam A, Howman-Giles R, Cowell CT. Measurement of midfemoral shaft geometry: repeatability and accuracy using magnetic resonance imaging and dual-energy X-ray absorptiometry. J Bone Miner Res 16: 2251–2259, 2001 [DOI] [PubMed] [Google Scholar]
- 119. Yang RS, Tsai KS, Liu TK, Chieng PU, Dorey FJ. Risk of underestimation of the bone mineral density of proximal femur. Bone 20: 295–300, 1997 [DOI] [PubMed] [Google Scholar]
- 120. Yung PS, Lai YM, Tung PY, Tsui HT, Wong CK, Hung VW, Qin L. Effects of weight bearing and non-weight bearing exercises on bone properties using calcaneal quantitative ultrasound. Br J Sports Med 39: 547–551, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Zochling J, Nguyen TV, March LM, Sambrook PN. Quantitative ultrasound measurements of bone: measurement error, discordance, and their effects on longitudinal studies. Osteoporos Int 15: 619–624, 2004 [DOI] [PubMed] [Google Scholar]