Abstract
Although a multitude of factors that influence skeletal muscle blood flow have been extensively investigated, the influence of muscle length on limb blood flow has received little attention. Thus the purpose of this investigation was to determine if cyclic changes in muscle length influence resting blood flow. Nine healthy men (28 ± 4 yr of age) underwent a passive knee extension protocol during which the subjects' knee joint was passively extended and flexed through 100–180° knee joint angle at a rate of 1 cycle per 30 s. Femoral blood flow, cardiac output (CO), heart rate (HR), stroke volume (SV), and mean arterial pressure (MAP) were continuously recorded during the entire protocol. These measurements revealed that slow passive changes in knee joint angle did not have a significant influence on HR, SV, MAP, or CO; however, net femoral blood flow demonstrated a curvilinear increase with knee joint angle (r2 = 0.98) such that blood flow increased by ∼90% (125 ml/min) across the 80° range of motion. This net change in blood flow was due to a constant antegrade blood flow across knee joint angle and negative relationship between retrograde blood flow and knee joint angle (r2 = 0.98). Thus, despite the absence of central hemodynamic changes and local metabolic factors, blood flow to the leg was altered by changes in muscle length. Therefore, when designing research protocols, researchers need to be cognizant of the fact that joint angle, and ultimately muscle length, influence limb blood flow.
Keywords: skeletal muscle, hyperemia
understanding the regulation of skeletal muscle blood flow has a wide range of implications from metabolic disease to exercise tolerance and sports performance. Thus elucidating the mechanisms responsible for movement-induced hyperemia has been a major research focus. It is now recognized that blood flow through a vascular bed is dictated by the arterial-venous pressure difference and vascular conductance. Many variables regulate blood pressure and vascular conductance, such as cardiac output modulated by afferent feedback (1, 2, 15), sympathetic nerve activity (7, 8), blood flow-induced alterations in vessel tone (19, 27), and vessel sensitivity to vasoactive substances and metabolites (9). However, in addition to these more commonly reported factors, skeletal muscle length-dependent changes in the tortuosity of the capillary network may also influence blood flow (17, 21, 26, 29, 31). As all previous research in this area used animal models, there is little information regarding how changes in muscle length across a physiological range of motion influence human muscle blood flow.
Skeletal muscle capillaries are well secured to surrounding tissue, thus muscle lengthening and shortening results in stretching and compressing the vasculature, which alters resistance and subsequently alters blood flow through this network. Poole et al. (29) reported that capillary tortuosity decreased with increases in sarcomere length up to 2.6 μm, at which point further increases in sarcomere length reduced mean capillary diameter. As a result of these changes in capillary geometry, blood flow decreased by 40%. Thus it is likely that as the muscle is stretched the initial decrease in tortuosity increases vascular conductance, while further stretching of the muscle and narrowing of the vessels results in decreased vascular conductance. There is also evidence that sympathetic nerve activity and vasomotor tone are influenced by muscle length such that increasing muscle length results in increased sympathetic tone (35). Additionally, rapid changes in muscle length can increase mechanoreceptor afferent feedback to the cardiovascular control center, increasing heart rate and ultimately cardiac output (14, 22, 23, 33, 34). Thus, although changes in muscle length may alter blood flow through multiple mechanisms, using very slow passive muscle movement, below that which would stimulate the mechanoreceptors, may allow an examination of the influence of changes in muscle length on blood flow in humans without the increase in cardiac output that is observed at faster rates of movement.
Thus, using a reductionist approach, the purpose of this investigation was to determine if slow changes in muscle length in humans would 1) elicit a cardiovascular response and 2) influence peripheral skeletal muscle blood flow. Specifically, we aimed to determine if femoral blood flow is affected by slow passive extension and flexion of the knee joint. In regards to this model we presume that changes in the length of the knee extensors would predominate over the competing effects of the simultaneous and opposite changes in knee flexors owing to the 2.5-fold greater mass of the knee extensors relative to the flexors (3, 5) and the greater length changes the extensors undergo during knee extension/flexion compared with the flexors (13). Thus we hypothesized that during this slow passive movement femoral blood flow will increase as knee joint angle increases, a surrogate for muscle length, despite an unchanged cardiac output.
METHODS
Subjects and General Procedures
Nine healthy men (28 ± 4 yr, 178 ± 8 cm, 87 ± 21 kg) participated in the current study. The protocol was approved by the Institutional Review Boards of the University of Utah and the Salt Lake City Veterans Affairs Medical Center, and written informed consent was obtained from all subjects prior to their inclusion in the study. All studies were performed in a thermoneutral environment (22°C). Subjects refrained from exercise for 24 h before data collection.
Passive Knee Extension and Flexion
Prior to the start of data collection, subjects lay supine for 20 min. During this period subjects were equipped with ECG electrodes and a finometer finger pressure cuff. In addition, the subjects were outfitted with a knee brace equipped with a position sensor that supplied real-time knee angle feedback that ensured a consistent range of motion during slow passive leg movement and served as a surrogate measure of thigh muscle length changes. One minute before the start of each slow passive exercise protocol, a pneumatic cuff, placed distal to the knee on the passively moved leg, was inflated to 250 mmHg, eliminating blood flow to the lower leg. The cuff, which remained inflated throughout the entire protocol, eliminated fluctuations in blood flow to the lower leg as a consequence of partial occlusion of the popliteal artery during knee flexion as well as movement-related changes in gravitational and centrifugal forces. The protocol itself consisted of five cycles of slow passive knee extension/flexion (30 s per flexion/extension cycle) through the range of motion defined by 100 and 180° knee joint angle (full knee extension = 180°). Prior to the start and throughout the protocol subjects were encouraged to remain passive and resist any urge to assist with leg movement. In the rare instance that a subject assisted with or resisted the movement, the protocol was terminated and repeated after 10 min of recovery.
Measurements
Femoral blood flow.
Measurements of femoral arterial blood velocity and vessel diameter were taken in the passively moved leg distal to the inguinal ligament and proximal to the bifurcation of the superficial and deep femoral artery with a Logic 7 ultrasound system (General Electric Medical Systems, Milwaukee, WI). The Logic 7 was equipped with a linear array transducer operating at an imaging frequency of 14 MHz. Vessel diameter was determined at a perpendicular angle along the central axis of the scanned area. Blood velocity was obtained using the same transducers with a Doppler frequency of 5 MHz. All blood velocity measurements were obtained with the probe appropriately positioned to maintain an insonation angle of 60° or less. The sample volume was maximized according to vessel size and was centered within the vessel based on real-time ultrasound visualization. Arterial diameter was measured and angle corrected, and intensity weighted mean velocity (Vmean) values were then calculated using commercially available software (Logic 7). By using arterial diameter and Vmean, blood flow in the femoral artery was calculated as: blood flow = Vmeanπ(vessel diameter/2)2 × 60, where blood flow is in milliliters per minute.
Central variables.
HR, SV, CO, and MAP were determined with a Finometer (Finapres Medical Systems BV, Amsterdam, the Netherlands). SV was calculated using the Modelflow method (6, 11, 32), which includes age, sex, height, and weight in its algorithm (Beatscope version 1.1; Finapres Medical Systems BV). CO was then calculated as the product of HR and SV. Vascular conductance within the passively moved leg was calculated as leg blood flow/MAP (ml·min−1·mmHg−1).
Knee joint angle.
During each protocol, knee joint angle of the passive leg was continuously recorded using a Vishay Spectrol 360 degree Smart Position Sensor (Vashay Intertechnology, Malvern, PA) mounted on a BREG X2K knee brace (BREG, Vista, CA) worn by the subjects.
Data acquisition.
Throughout each protocol, signals reflecting HR, SV, CO, MAP, and knee joint angle underwent analog to digital conversion and were simultaneously acquired (200 Hz) using commercially available AcqKnowledge data-acquisition software (Biopac Systems, Goleta, CA). In addition, the antegrade and retrograde audio signals from the Doppler ultrasound system were acquired (10,000 Hz) to serve as a qualitative indicator of blood velocity changes and to ensure accurate temporal alignment of blood velocity measurements obtained from the Doppler system and other variables collected (i.e., HR, SV, CO, MAP, as well as the knee joint angle).
Data Analysis
All central variables (HR, SV, CO, MAP) and femoral blood flow were recorded on a beat by beat basis. These variables were initially partitioned into three ranges of knee joint angle (lower 15%, middle 15%, and upper 15% range). Repeated-measures ANOVA was performed on HR, SV, CO, MAP, net blood flow, antegrade blood flow, retrograde blood flow, and conductance across these three ranges of joint angle. Post hoc comparisons (with a Bonferoni adjustment) were used to determine where differences existed if the overall ANOVA was significant. Regression analyses were performed on a group and individual basis for net blood flow, antegrade blood flow, retrograde blood flow, as well as the change in blood flow relative to blood flow at 100° that was averaged into 5° increments across all five passive knee extension/flexion cycles. Initially the regression analysis was run on the average data for all subjects. If this analysis yielded a significant linear relationship, then a linear analysis was also applied to all the individual data sets for that variable; however, if this analysis revealed a quadratic relationship, as indicated by a statistically significant squared term, then a quadratic analysis was also applied to the individual data sets for that variable. The alpha level for all comparisons was 0.05, and all data are presented as mean ± SD.
RESULTS
Central Variables
Change in knee joint angle during slow cyclic knee extension and flexion did not have a significant effect on HR, SV, or CO (Table 1). MAP, although not statistically significant, tended to be greater as knee angle decreased (P = 0.06; Table 1).
Table 1.
HR, SV, MAP, and CO across three ranges of knee joint angle
| Lower 15% | Middle 15% | Upper 15% | |
|---|---|---|---|
| HR, beats/min | 58 ± 7 | 59 ± 8 | 59 ± 7 |
| SV, ml | 111 ± 20 | 111 ± 21 | 111 ± 21 |
| MAP, mmHg | 88 ± 9 | 87 ± 9 | 86 ± 8 |
| CO, l/min | 6.5 ± 1.7 | 6.5 ± 1.7 | 6.6 ± 1.7 |
| Conductance, ml · min−1 · mmHg−1 | 2.4 ± 0.8† | 3.2 ± 1.1* | 3.6 ± 1.1*† |
Values are means ± SD. HR, heart rate; SV, stroke volume; MAP, mean arterial pressure; CO, cardiac output.
Significant difference from the lower 15% range of knee joint.
Significant difference from the middle 15% range of knee joint.
Blood Flow and Vascular Conductance During Slow Passive Movement
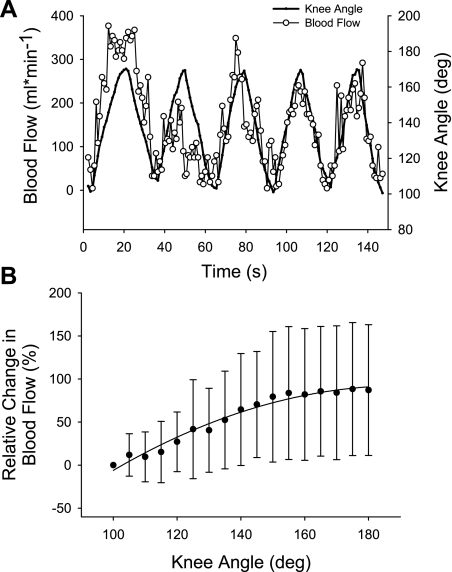
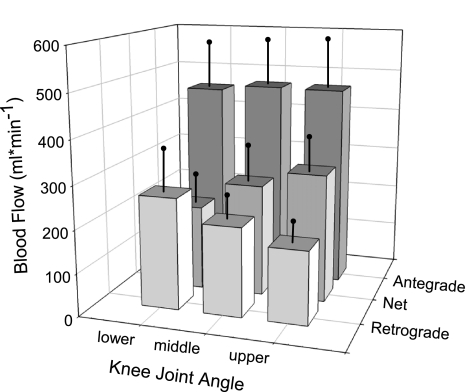
Relative femoral blood flow and knee joint angle during slow knee flexion/extension from one representative subject is presented in Fig. 1A. Relative to blood flow at the lower range of knee joint angle, blood flow increased by ∼32% and ∼51% at the middle and upper range of knee joint angles, respectively (Fig. 2). Retrograde blood flow decreased across all three ranges of knee joint angle (Fig. 2). Specifically, retrograde blood flow decreased by 20% when the knee was passively extended from the lower to the middle range of knee joint angle and by an additional 15% as it continued through to the upper range of knee joint angle. Unlike net and retrograde blood flow, there was no difference in antegrade blood flow across the three ranges of knee joint angle (P = 0.48). In addition, conductance increased by ∼30% as the knee was extended from the lower to middle range of knee joint angle and by an additional 16% as it continued through to the upper range of knee joint angle (Table 1).
Fig. 1.
Knee joint angle and blood flow during slow passive limb movement. A: second-by-second knee joint angle and femoral blood flow for 1 subject. B: average relative change in blood flow across the 80° range of motion used in this study (r2 = 0.97).
Fig. 2.
Changes in femoral blood flow across 3 levels of knee joint angle. Across all 3 ranges of knee joint angle net blood flow and retrograde blood flow were significantly different from each other. There was no difference across the 3 levels of antegrade blood flow.
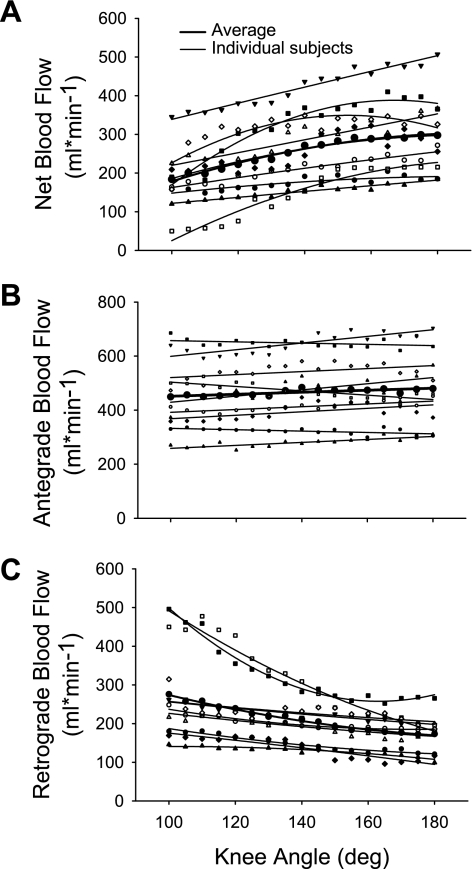
Regression analysis revealed that there was a quadratic relationship between net femoral blood flow and knee joint angle: femoral blood flow = −234 + 6 (k) − 0.0144(k)2; r2 = 0.98 where k = knee joint angle (Fig. 3A). This equation reveals an increase of 125.4 ml/min across the entire range of motion used in this investigation (from 100 to 180°) or an average of 1.6 ml/min/°. The average r2 for all nine individual regression equations equaled 0.83 ± 0.13 (range: 0.61 to 0.98). Expressing the change in net blood flow at each 5° knee joint angle, relative to blood flow at 100° knee joint angle, also revealed a significant quadratic relationship: relative change in blood flow = −342 + 4.55 (k) + −0.012 (k)2 (r2 = 0.97; Fig. 1B). Thus blood flow increased by 90% as the knee was extended from 100 to 180° (note: this difference is larger than that described with the repeated-measures analysis because it focuses on the extreme joint angles, which are not averaged into the three knee joint ranges, thus maximizing the effect size).
Fig. 3.
Net, antegrade, and retrograde blood flow across knee joint angle. Quadratic relationships with knee joint angle were significant for the net blood flow (A) and retrograde blood flow (C) while a linear relationship existed between knee joint angle and antegrade blood flow (B).
Antegrade and retrograde blood flow regression analysis partially agreed with the repeated-measures analysis of the three ranges of knee joint angle (lower, middle, and upper). Specifically, when all individual antegrade data were combined into one regression, a significant positive relationship still existed [r2 = 0.62 and slope = 0.37 ml/min/°]. However, individual significant positive linear relationships between antegrade blood flow and knee joint angle were only evident in 5 of the 9 subjects with an average r2 = 0.54 ± 0.18 and average slope = 0.74 ± 0.35 ml/min/° (Fig. 3). Of the remaining four, two individual antegrade regressions were not significant, whereas two others revealed a significant negative relationship with an average r2 = 0.61 ± 0.05 and average slope of −0.52 ± 0.39 ml/min/°. In regards to retrograde blood flow, when all individual data were combined into one regression, a significant quadratic relationship was evident (r2 = 0.98). Individual quadratic regression analyses for retrograde blood flow were more consistent than the anterograde blood flow (average r2 = 0.82 and a range of 0.55 to 0.98; Fig. 3C).
DISCUSSION
Although a multitude of factors that influence skeletal muscle blood flow have been extensively investigated, the influence of muscle length on blood flow in humans has received little attention. Thus in the present study we sought to determine the influence of slow changes in knee joint angle, a surrogate for quadriceps muscle length, on femoral blood flow. The major finding of this study was that, at rest, femoral blood flow increased by ∼90% as knee joint angle increased from 100 to 180°. As central parameters including CO, MAP, SV, and HR did not change and the cuff occlusion below the knee eliminated the influence of both gravitational forces and partial popliteal artery occlusion from influencing blood flow, the change in femoral artery blood flow most likely resulted from peripheral factors, including changes in capillary tortuosity and decreased diameter associated with the changes in muscle length. These results are not only important for the understanding of blood flow distribution, but also have important implications for the methodological approach employed by investigators measuring resting blood flow in human limbs.
Blood Flow and Joint Angle
The data acquired during this investigation reveal that femoral artery blood flow is clearly altered by knee joint angle. As illustrated in Fig. 1A, during five cycles of slow passive knee extension/flexion the second-by-second changes in blood flow directly reflect changes in knee joint angle. Two subsequent analyses were performed on these data. Initially both central and peripheral variables collected during the slow passive movement were divided into three ranges: upper, middle, and lower 15% of the knee joint angle. This analytical approach revealed that femoral artery blood flow increased as the knee was extended from the lower to the middle and upper range of knee joint angle (Fig. 2) and that factors underlying this response are peripheral in nature as passive knee extension did not increase HR, SV, or CO (Table 1). Although not statistically significant the tendency for MAP to decrease as the knee joint angle was increased contributed to the parallel increase in vascular conductance (Table 1). Additionally, the decrease in retrograde blood flow at greater knee joint angles can be interpreted to indicate that the microvascular network was capable of accepting more blood as the knee joint was extended (Fig. 2). In contrast, the constancy of the antegrade blood flow, perhaps predominantly determined by the capacitance of the large arteries, was unaffected by joint angle (Fig. 2).
Regression analysis, which includes data throughout the entire range of motion, also lead to the conclusion that femoral artery blood flow at rest was, at least partially, dictated by knee joint angle (Figs. 1B and 3). On the basis of the quadratic relationship between net femoral blood flow and knee joint angle femoral blood flow would be expected to increase by ∼125 ml/min (90%) across the 80° range of motion used in the current investigation. The changes in conductance within the vascular network are most likely due to muscle length-dependent changes in capillary tortuosity and vessel diameter. Specifically, Mathieu-Costello (21) determined that muscle sarcomere length, not fiber type or muscle architecture, determines the degree of vessel tortuosity. At short muscle lengths, the capillaries are highly tortuous and as muscle length increases the vessels become aligned with the longitudinal axis of the muscle fiber and tortuosity decreases. Further lengthening of the muscle results in narrowing of the capillary diameter (24, 28). Although we did not observe a clear local maximum blood flow, as would be expected based on Mathieu-Costello's data, the documented relationship between knee joint angle and blood flow was indeed curvilinear in nature (Figs. 1 and 3). If, however, the quadriceps were allowed to become shorter than the length at a knee angle of 180°, we would hypothesize that the increase in vessel tortuosity would have a greater influence at such short lengths and ultimately additionally impede blood flow. Although, to some extent, the current data, collected in vivo in humans, support these prior morphometric measurements in rats, further comparison would not be appropriate due to the dissimilarities in species, type of muscle, muscle architecture, and range of motion used in prior studies and the present investigation.
Poole et al. (29) used an in situ technique to determine whether these muscle length-dependent changes in capillary alignment and diameter are associated with changes in red blood cell flow dynamics. They concluded that in rat spinotrapezius muscle blood flow decreased by 40% as the muscle length increased across the largest range of motion. More recently, Mizuno et al. (25) reported a greater pressor response during submaximal isometric knee extension at 90° degrees compared with 130° (25). Although not measured in their investigation, they attributed the greater pressor response to reduced skeletal muscle perfusion at 90°. Mizuno et al. (25) concluded that the decreased perfusion resulted in a reduction in tissue oxygenation and subsequent afferent feedback to the cardiovascular centers. The data from this investigation, albeit in relaxed muscle, support their conclusion as muscle perfusion was drastically reduced with greater flexion of the knee joint. In addition to the muscle length changes in capillary diameter, it seems likely that stretch-induced increases in intramuscular pressure may also impinge on arterioles and venules (4, 16, 30). Thus, as the knee joint flexes from 180 to 90°, the lengthening of the quadriceps muscle group increases vascular resistance and subsequently decreases net femoral blood flow. Experimentally, such muscle length-dependent changes in skeletal muscle blood flow could have a significant impact on the dispersion of blood flow due, for example, to the infusion of a vasoactive substance or the magnitude of increase in blood flow if pre- and postassessments are not made at the same joint angle.
Perivascular Sympathetic Nerve Stimulation and Muscle Length
To elucidate additional mechanisms that may be responsible for the change in blood flow with muscle length, Welsh and Segal (35) investigated whether sympathetic nerve activity coordinates muscle length-dependent changes in hamster retractor muscle blood flow. Similarly to the previously mentioned investigations, their data also revealed that passive lengthening of the muscle appeared to result in actual vasoconstriction and reduction in blood flow by 50–60%. The infusion of nitroprusside attenuated the vasoconstriction induced by muscle lengthening, suggesting that factors other than muscular pressure and vessel lengthening result in the narrowing of the vessels. Pharmacological intervention with tetrodotoxin (TTX) infusion, a specific inhibitor of the fast voltage-sensitive sodium channels that underlie action potentials, also attenuated lengthening-induced vasoconstriction while neuroblockade with TTX proximal to the muscle did not influence the vasomotor response. Welsh and Segal (35) concluded that the increase in vascular resistance with muscle lengthening arises primarily from the activation of perivascular sympathetic nerves, resulting in norepinephrine release and vasoconstriction that is local in nature and does not involve afferent feedback or the cardiovascular control center. More recently, the same laboratory also revealed that muscle lengthening results in reduced conduction of vasodilation resulting from muscle length-dependent sensitivity of cell-to-cell signaling along the resistance vasculature (12). Thus it is also likely that activation of the perivascular sympathetic nerves and sensitivity of cell-to-cell signaling during knee flexion also contributed to increased resistance and decreased blood flow to the limb in the current investigation.
Muscle Length in the Knee Flexors and Knee Extensors
Limb blood flow was measured with the Doppler probe placed proximal to the bifurcation of the superficial and deep femoral artery. This placement results in quantification of blood flow to both the knee extensors as well as the knee flexors. It can thus be argued that during this cyclic passive movement the change in muscle length of the knee extensors are opposite to that of the knee flexors, thus we cannot be sure that the increase in femoral blood flow resulted from the shortening of the knee extensors or lengthening of the knee flexors. However, the knee extensors muscle volume is ∼2.5 times greater than the knee flexors (3, 5, 10, 20) and the relative change in muscle length across the range of motion used in this investigation is greater in the knee extensors compared with the knee flexors (13). Taken together the knee extensors likely had a greater impact on femoral blood flow compared with the knee flexors. Furthermore, previous investigations using isolated muscle support the notion that it is in fact the shortening of a muscle that results in the increase in blood flow (18, 29).
It can also be argued that any change in blood flow in the knee extensors would be offset by a concomitant, but opposite change in blood flow to the knee flexors resulting in no change in blood flow observed in the common femoral artery. Obviously, in the current scenario, this was not the case and again we contend the knee extensors have a much larger weighting in terms of blood flow distribution compared with the knee flexors based on size and current excursion. However, the existence of at least a partial offset by these agonists likely resulted in an underestimation of the change in blood flow directed toward the knee extensor muscles as a consequence of muscle length. Measuring the blood flowing toward a single muscle or muscle group would therefore likely lead to a more dramatic result than those observed in the current study. However, this having been said, we are unaware of a vessel that does not feed an agonist/antagonist muscle pair, and blood flow can accurately be measured across a full range of motion using a Doppler ultrasound. Hence, this appears to be an unavoidable limitation of such an in vivo human experiment.
Summary
In conclusion, in the presence of constant MAP and CO, femoral blood flow was significantly altered by passive changes in knee joint angle. Specifically, during slow passive limb movement femoral blood flow decreased with knee flexion. The curvilinear relationship between femoral blood flow and increasing knee joint angle is most likely due to an increase in vessel tortuosity at the shorter muscle lengths, despite increased vessel diameter associated with reduced mechanical stretch of the quadriceps. In addition, perivascular sympathetic nerve stimulation with increasing muscle length may also contribute to the decreased blood flow with knee flexion. These data not only highlight additional factors that influence peripheral blood flow in humans at rest, but also emphasize the need for investigators, when designing research protocols, to be cognizant of the fact that joint angle, and ultimately muscle length, influence limb blood flow.
GRANTS
Financial support for this work by Department of VA Rehab R&D CDA2 E7560W and National Institutes of Health Grant POI HL-091830.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: J.M., S.J.I., and R.S.R. conception and design of research; J.M., S.J.I., and R.S.R. performed experiments; J.M. and R.S.R. analyzed data; J.M. and R.S.R. interpreted results of experiments; J.M. prepared figures; J.M. and S.J.I. drafted manuscript; J.M., S.J.I., and R.S.R. edited and revised manuscript; J.M., S.J.I., and R.S.R. approved final version of manuscript.
REFERENCES
- 1. Adreani CM, Hill JM, Kaufman MP. Responses of group III and IV muscle afferents to dynamic exercise. J Appl Physiol 82: 1811–1817, 1997 [DOI] [PubMed] [Google Scholar]
- 2. Adreani CM, Kaufman MP. Effect of arterial occlusion on responses of group III and IV afferents to dynamic exercise. J Appl Physiol 84: 1827–1833, 1998 [DOI] [PubMed] [Google Scholar]
- 3. Akima H, Kawakami Y, Kubo K, Sekiguchi C, Ohshima H, Miyamoto A, Fukunaga T. Effect of short-duration spaceflight on thigh and leg muscle volume. Med Sci Sports Exerc 32: 1743–1747, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Alamaki A, Hakkinen A, Malkia E, Ylinen J. Muscle tone in different joint positions and at submaximal isometric torque levels. Physiol Meas 28: 793–802, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Belavy DL, Miokovic T, Rittweger J, Felsenberg D. Estimation of changes in volume of individual lower-limb muscles using magnetic resonance imaging (during bed-rest). Physiol Meas 32: 35–50, 2010 [DOI] [PubMed] [Google Scholar]
- 6. Bogert LW, van Lieshout JJ. Non-invasive pulsatile arterial pressure and stroke volume changes from the human finger. Exp Physiol 90: 437–446, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Buckwalter JB, Mueller PJ, Clifford PS. Autonomic control of skeletal muscle vasodilation during exercise. J Appl Physiol 83: 2037–2042, 1997 [DOI] [PubMed] [Google Scholar]
- 8. Buckwalter JB, Mueller PJ, Clifford PS. Sympathetic vasoconstriction in active skeletal muscles during dynamic exercise. J Appl Physiol 83: 1575–1580, 1997 [DOI] [PubMed] [Google Scholar]
- 9. Clifford PS, Hellsten Y. Vasodilatory mechanisms in contracting skeletal muscle. J Appl Physiol 97: 393–403, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Cotofana S, Hudelmaier M, Wirth W, Himmer M, Ring-Dimitriou S, Sanger AM, Eckstein F. Correlation between single-slice muscle anatomical cross-sectional area and muscle volume in thigh extensors, flexors and adductors of perimenopausal women. Eur J Appl Physiol 110: 91–97, 2010 [DOI] [PubMed] [Google Scholar]
- 11. De Wilde RB, Geerts BF, Cui J, van den Berg PC, Jansen JR. Performance of three minimally invasive cardiac output monitoring systems. Anaesthesia 64: 762–769, 2009 [DOI] [PubMed] [Google Scholar]
- 12. Haug SJ, Welsh DG, Segal SS. Sympathetic nerves inhibit conducted vasodilatation along feed arteries during passive stretch of hamster skeletal muscle. J Physiol 552: 273–282, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hawkins D, Hull ML. A method for determining lower extremity muscle-tendon lengths during flexion/extension movements. J Biomech 23: 487–494, 1990 [DOI] [PubMed] [Google Scholar]
- 14. Hayman MA, Nativi JN, Stehlik J, McDaniel J, Fjeldstad AS, Ives SJ, Walter Wray D, Bader F, Gilbert EM, Richardson RS. Understanding exercise-induced hyperemia: central and peripheral hemodynamic responses to passive limb movement in heart transplant recipients. Am J Physiol Heart Circ Physiol 299: H1653–H1659, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Herr MD, Imadojemu V, Kunselman AR, Sinoway LI. Characteristics of the muscle mechanoreflex during quadriceps contractions in humans. J Appl Physiol 86: 767–772, 1999 [DOI] [PubMed] [Google Scholar]
- 16. Hussain SN, Magder S. Diaphragmatic intramuscular pressure in relation to tension, shortening, and blood flow. J Appl Physiol 71: 159–167, 1991 [DOI] [PubMed] [Google Scholar]
- 17. Kindig CA, Poole DC. Effects of skeletal muscle sarcomere length on in vivo capillary distensibility. Microvasc Res 57: 144–152, 1999 [DOI] [PubMed] [Google Scholar]
- 18. Kindig CA, Poole DC. Sarcomere length-induced alterations of capillary hemodynamics in rat spinotrapezius muscle: vasoactive vs passive control. Microvasc Res 61: 64–74, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Kooijman M, Thijssen DH, de Groot PC, Bleeker MW, van Kuppevelt HJ, Green DJ, Rongen GA, Smits P, Hopman MT. Flow-mediated dilatation in the superficial femoral artery is nitric oxide mediated in humans. J Physiol 586: 1137–1145, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Macaluso A, Nimmo MA, Foster JE, Cockburn M, McMillan NC, De Vito G. Contractile muscle volume and agonist-antagonist coactivation account for differences in torque between young and older women. Muscle Nerve 25: 858–863, 2002 [DOI] [PubMed] [Google Scholar]
- 21. Mathieu-Costello O. Muscle capillary tortuosity in high altitude mice depends on sarcomere length. Respir Physiol 76: 289–302, 1989 [DOI] [PubMed] [Google Scholar]
- 22. McDaniel J, Fjeldstad AS, Ives S, Hayman M, Kithas P, Richardson RS. Central and peripheral contributors to skeletal muscle hyperemia: response to passive limb movement. J Appl Physiol 108: 76–84, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McDaniel J, Hayman MA, Ives S, Fjeldstad AS, Trinity JD, Wray DW, Richardson RS. Attenuated exercise induced hyperaemia with age: mechanistic insight from passive limb movement. J Physiol 588: 4507–4517, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mermod L, Hoppeler H, Kayar SR, Straub R, Weibel ER. Variability of fiber size, capillary density and capillary length related to horse muscle fixation procedures. Acta Anat (Basel) 133: 89–95, 1988 [DOI] [PubMed] [Google Scholar]
- 25. Mizuno M, Tokizawa K, Muraoka I. Changes in perfusion related to muscle length affect the pressor response to isometric muscle contraction. Adv Exp Med Biol 662: 371–377, 2010 [DOI] [PubMed] [Google Scholar]
- 26. Nakao M, Segal SS. Muscle length alters geometry of arterioles and venules in hamster retractor. Am J Physiol Heart Circ Physiol 268: H336–H344, 1995 [DOI] [PubMed] [Google Scholar]
- 27. Pohl U, Holtz J, Busse R, Bassenge E. Crucial role of endothelium in the vasodilator response to increased flow in vivo. Hypertension 8: 37–44, 1986 [DOI] [PubMed] [Google Scholar]
- 28. Poole DC, Mathieu-Costello O. Capillary and fiber geometry in rat diaphragm perfusion fixed in situ at different sarcomere lengths. J Appl Physiol 73: 151–159, 1992 [DOI] [PubMed] [Google Scholar]
- 29. Poole DC, Musch TI, Kindig CA. In vivo microvascular structural and functional consequences of muscle length changes. Am J Physiol Heart Circ Physiol 272: H2107–H2114, 1997 [DOI] [PubMed] [Google Scholar]
- 30. Saltin B, Radegran G, Koskolou MD, Roach RC. Skeletal muscle blood flow in humans and its regulation during exercise. Acta Physiol Scand 162: 421–436, 1998 [DOI] [PubMed] [Google Scholar]
- 31. Segal SS. Integration of blood flow control to skeletal muscle: key role of feed arteries. Acta Physiol Scand 168: 511–518, 2000 [DOI] [PubMed] [Google Scholar]
- 32. Sugawara J, Tanabe T, Miyachi M, Yamamoto K, Takahashi K, Iemitsu M, Otsuki T, Homma S, Maeda S, Ajisaka R, Matsuda M. Non-invasive assessment of cardiac output during exercise in healthy young humans: comparison between Modelflow method and Doppler echocardiography method. Acta Physiol Scand 179: 361–366, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Trinity JD, Amann M, McDaniel J, Fjeldstad AS, Barrett-O'Keefe Z, Runnels S, Morgan DE, Wray DW, Richardson RS. Limb movement-induced hyperemia has a central hemodynamic component: evidence from a neural blockade study. Am J Physiol Heart Circ Physiol 299: H1693–H1700, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Trinity JD, McDaniel J, Venturelli M, Fjeldstad AS, Ives SJ, Witman MA, Barrett-O'Keefe Z, Amann M, Wray DW, Richardson RS. Impact of body position on central and peripheral hemodynamic contributions to movement-induced hyperemia: implications for rehabilitative medicine. Am J Physiol Heart Circ Physiol 300: H1885–H1891, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Welsh DG, Segal SS. Muscle length directs sympathetic nerve activity and vasomotor tone in resistance vessels of hamster retractor. Circ Res 79: 551–559, 1996 [DOI] [PubMed] [Google Scholar]