Abstract
Sleep is associated with marked alterations in ventilatory control that lead to perturbations in respiratory timing, breathing pattern, ventilation, pharyngeal collapsibility, and sleep-related breathing disorders (SRBD). Mouse models offer powerful insight into the pathogenesis of SRBD; however, methods for obtaining the full complement of continuous, high-fidelity respiratory, electroencephalographic (EEG), and electromyographic (EMG) signals in unrestrained mice during sleep and wake have not been developed. We adapted whole body plethysmography to record EEG, EMG, and respiratory signals continuously in unrestrained, unanesthetized mice. Whole body plethysmography tidal volume and airflow signals and a novel noninvasive surrogate for respiratory effort (respiratory movement signal) were validated against simultaneously measured gold standard signals. Compared with the gold standard, we validated 1) tidal volume (correlation, R2 = 0.87, P < 0.001; and agreement within 1%, P < 0.001); 2) inspiratory airflow (correlation, R2 = 0.92, P < 0.001; agreement within 4%, P < 0.001); 3) expiratory airflow (correlation, R2 = 0.83, P < 0.001); and 4) respiratory movement signal (correlation, R2 = 0.79–0.84, P < 0.001). The expiratory airflow signal, however, demonstrated a decrease in amplitude compared with the gold standard. Integrating respiratory and EEG/EMG signals, we fully characterized sleep and breathing patterns in conscious, unrestrained mice and demonstrated inspiratory flow limitation in a New Zealand Obese mouse. Our approach will facilitate studies of SRBD mechanisms in inbred mouse strains and offer a powerful platform to investigate the effects of environmental and pharmacological exposures on breathing disturbances during sleep and wakefulness.
Keywords: open system, tidal volume, airflow, respiratory effort signal, polysomnography, inspiratory flow limitation, upper airway
sleep is associated with marked alterations in ventilatory control that lead to perturbations in breathing patterns, frank periods of hypoventilation, and upper airway obstruction (35, 43). In susceptible individuals, these changes precipitate a spectrum of sleep-related breathing disorders (SRBD) including obstructive sleep apnea (OSA) and hypoventilation syndromes (35, 43). SRBD are highly prevalent in Western society and have been linked to numerous clinical sequelae, including stroke, hypertension, diabetes, and frank respiratory failure (13, 25, 30, 31, 38, 47). In humans, the polysomnogram, which encompasses respiratory (tidal volume, airflow, and effort) and electroencephalographic (EEG)/electromyographic (EMG) signals, is a pivotal tool for characterizing SRBD and sleep-related respiratory disturbances, including periodic apneas and hypopneas and alterations in gas exchange, tidal volume, and airflow.
Animal models can offer powerful insight into the pathogenesis of SRBD. Investigators have demonstrated recurrent central and obstructive apnea episodes with continuous recordings of airflow and respiratory effort in several large-animal models (7, 17, 22, 29). Nevertheless, these studies have been hindered by difficulty in procuring, instrumenting, and acclimatizing the animals to polysomnographic recordings. Mice and rats have also been utilized to characterize ventilatory control and respiratory instability during sleep and wakefulness based on measures of tidal volume, respiratory rate, and minute ventilation (12, 15, 24, 32, 39). Mice are ideally suited for the study of SRBD mechanisms, since a wide variety of inbred strains are available, body weight and environment can be easily manipulated, and large numbers of animals can be rapidly characterized. In addition, obese strains susceptible to upper airway obstruction and SRDB have been identified (4, 5, 32, 36). Nevertheless, measures of tidal airflow and respiratory effort have been lacking, limiting the ability to detect upper airway obstruction during sleep. In addition, methods for obtaining the full complement of continuous, high-fidelity tidal volume, tidal airflow, respiratory effort, and EEG/EMG signals in mice have not been developed.
Whole body plethysmography (WBP) has been utilized to record respiration during sleep and wake in unrestrained, freely moving mice (12, 15, 27, 28, 32, 40). This approach provides an indirect measure of tidal volume, which is directly proportional to the cyclic chamber pressure signal produced during respiration in a sealed chamber (9). While the overall accuracy of WBP-derived tidal volume measurement (compared with direct pneumotachography) has been confirmed, several key limitations limit the use of WBP for continuously monitoring respiratory signals during sleep and wakefulness, as follows (2, 6, 9, 33, 45). First, traditional WBP methods provide intermittent high-fidelity tidal volume measurement, but techniques for recording a continuous, accurate tidal volume signal have not been validated in commercial chambers (6, 26, 45). Second, the indirectly measured WBP tidal airflow signal has not been validated. Third, methods for assessing respiratory effort noninvasively have not been developed or validated.
The major purpose of this study was to develop and validate an approach for making continuous high-fidelity respiratory recordings during sleep and wakefulness in mice. Specifically, we addressed the inherent limitations of standard WBP to enable the continuous recording of validated measures of tidal volume, tidal airflow, and respiratory effort surrogate in an unrestrained, unanesthetized mouse. The addition of standard EEG and EMG recording technology allowed us to fully characterize respiratory patterns during sleep and wakefulness and demonstrate the development of dynamic upper airway obstruction [inspiratory flow limitation (IFL)] during sleep in a susceptible, obese murine strain.
MATERIALS AND METHODS
Animals
Eight male C57BL/6J mice, weighing 25–30 g (age 10–12 wk), and one New Zealand Obese (NZO) mouse, weighing 41.3 g (age 12 wk) from Jackson Laboratory (Bar Harbor, ME) were used in the study. The study was approved by the Johns Hopkins University Animal Care and Use Committee (ACUC). For all surgical procedures, anesthesia was induced using isoflurane (1–2%) administered through a chamber and face mask and maintained using intraperitoneal injection of ketamine (100 mg/kg), xylazine (20 mg/kg), and acepromazine (3 mg/kg), as per ACUC protocol. At the completion of experiments, animals were euthanized with pentobarbital sodium (60 mg ip).
Surgical Procedures
EEG/EMG headmount placement.
In an anesthetized mouse, EEG and EMG electrodes were implanted with an EEG/EMG Headmount (no. 8201, Pinnacle Technology, Lawrence, KS). A longitudinal incision was made over the skull, and the underlying fascia was gently cleared from the skull surface. The headmount was secured with glue (Loctite Precision Super Glue) to the skull directly over top of the bregma, and four holes were bored through the skull (at the site of preexisting holes in the headmount base) in the left and right frontal regions and left and right parietal regions. Four silver electrodes 0.1 mm (no. 8209, Pinnacle Technology) anterior and 0.12 mm (no. 8212, Pinnacle Technology) posterior were screwed through the holes and secured with silver conductive epoxy (no. 8331, MG Chemicals) to provide unipolar conductive EEG electrodes. The headmount EMG leads were tunneled subcutaneously and placed over the nuchal muscles posterior to the skull. Fingernail acrylic was used to cover and insulate the electrodes. The incision was sutured, and analgesia was administered per ACUC protocol. Each mouse was observed and allowed 3 days to recover before use in experiments.
Endotracheal intubation.
In an anesthetized mouse, an incision was made over the anterior neck, and the trachea was exposed via blunt dissection. Intubation was performed with an 18-gauge angiocatheter (Cathalon, no. 4454). The trachea was tied off with 6.0 nylon suture to prevent air leakage around the catheter.
General Setup, Signal Acquisition, and Processing
WBP tidal volume and airflow signals.
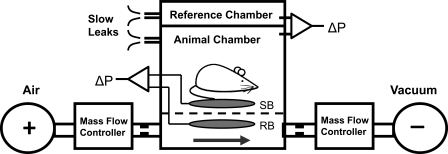
The WBP (mouse whole body plethysmograph, Buxco, Wilmington, NC; modified by Emka Technologies to increase the diameter of the top port to allow for passage of EEG/EMG leads) consisted of a sealed animal chamber, a reference chamber, and a platform to support the mouse (Fig. 1). The chamber is equipped with two ports (pneumotachographs) on the upper surface and one large side port and three small side ports at the base, which we utilized to customize our system and optimize its performance (see below).The Drorbaugh and Fenn equation was used to calculate the WBP tidal volume signal from the WBP chamber pressure signal (9). Application of this formula required the measurement of the following variables during each WBP recording session: mouse rectal temperature, chamber temperature, room temperature and relative humidity, and chamber gas constant, which was calculated by utilizing a known volume injection and the resultant WBP pressure deflection. During full polysomnographic recording sessions (see below), the chamber was humidified to 90% relative humidity, and the mouse was allowed 45 min to acclimate to the chamber before recordings were initiated. All signals were digitized at 1,000 Hz (sampling frequency per channel) and recorded in LabChart 7 Pro (version 7.2).
Fig. 1.
Modified whole body plethysmography (WBP) open-system schematic. Positive and negative pressure sources were utilized in series with mass flow controllers and high-resistance elements to generate a continuous bias flow through the animal chamber while maintaining a sufficiently high time constant. The reference chamber served to filter ambient noise from the pressure signal. Slow leaks present on both chambers allowed for equilibration with atmospheric pressure. The sensor bladder (SB) transduced the mechanical pressure changes associated with mouse breathing, while the reference bladder (RB) signal allowed for cancellation of the contaminating chamber pressure signal via the differential pressure transducer. +, Pressurized air source; −, vacuum source; narrowed tubes, high resistance elements; arrow, bias flow; dashed line, platform; ΔP, differential pressure transducer.
WBP pressure was recorded with a differential pressure transducer (no. 3613, Emka Technologies, Falls Church, VA). The differential pressure transducer was calibrated with a manometer (no. 840085, Sper Scientific) against known pressures. The transducer was activated by, and output to, an amplifier (no. 7DAF, Grass Technologies, West Warwick, RI). A 0.5-Hz high-pass filter was applied to the signal to remove low-frequency fluctuations around the baseline.
A first-order derivative (dV/dt) was applied to the WBP tidal volume signal to yield a WBP tidal airflow signal. The derivative was calculated with a 25-point window, which acted as a low-pass filter and served to optimize the signal-to-noise ratio of the WBP flow signal. Increasing the window width reduced noise, but resulted in attenuation of the high-frequency component (peak) of expiratory flow, while decreasing the window width resulted in increased noise. Optimization of window width was performed by increasing the window width until the WBP flow signal had a satisfactory visual and graphic (R2 > 0.8) correlation with the tracheal flow signal.
WBP respiratory movement signal (surrogate for respiratory effort).
Air bladders (infant blood pressure cuff, Critikon no. 2128; foreshortened to 7.5 cm × 4.0 cm with adhesive tape) were placed above (sensor bladder) and below (reference bladder) the rigid WBP platform (Fig. 1), such that they were completely isolated from each other. When the mouse lay on the platform, the mechanical displacement of its torso during breathing was transduced by the upper sensor bladder (situated between the platform and mouse). We recognized, however, that this sensor bladder was contained within the WBP and would also transduce tidal fluctuations in chamber pressure. Although the tidal fluctuations in reference bladder were identical to those produced in the sensor bladder, it did not record the mouse's respiratory motion, because it was spatially isolated from the mouse. We, therefore, compared signals from the sensor and reference bladders with a differential pressure transducer, which subtracted the chamber pressure fluctuations in the reference bladder from the composite (chamber and motion pressure fluctuations) in sensor bladder signal. The approach effectively removed the contaminating tidal fluctuations in chamber pressure from the respiratory effort signal in the sensor bladder. The air bladders were injected with 0.5 ml of air and connected (through the lateral base ports in the WBP chamber) to a calibrated differential pressure transducer (no. DP 45-26, Validyne Engineering, Northridge, CA), which output to an amplifier (no. 7DAF, Grass Technologies). A band-pass filter was applied from 0.5 to 10 Hz to optimize the signal-to-noise ratio of the differential pressure signal.
EEG/EMG signals.
Headmount leads were connected to preamplifier, which was attached to a preamplifier analog adapter (nos. 8201, 8202, 8242, Pinnacle Technology) and output to an amplifier (no. 7DAF, Grass Technologies).
Pneumotachographic respiratory signals.
The mouse endotracheal tube was connected to a calibrated pneumotachograph (resistance = 0.40 cmH2O·s·ml−1). The pneumotachograph was connected to a differential pressure transducer (no. MP 45-14-871, Validyne Engineering, Northridge, CA), output to an amplifier (no. 7DAF, Grass Technologies). The tracheal flow was integrated to yield a tidal volume signal.
Methodological Background and Approach
Full polysomnography requires the continuous recording of high-fidelity measures of tidal volume, tidal airflow, and respiratory effort, along with EEG and EMG signals. Standard WBP provides for intermittent, rather than continuous, high-fidelity respiratory recordings in a sealed chamber (closed system) (2, 6, 9, 26, 33, 45). To prevent CO2 from accumulating in a sealed chamber, airflow (bias flow) must be periodically applied to the chamber via inlet and outlet ports (open system), which results in the loss of data and may disrupt sleep. To make continuous recordings in an open system, substantial chamber modifications are required to prevent pressure signal attenuation during animal respiration (2, 6, 45). As previously described, the bias flow inlet and outlet ports must be adjusted so that the time constant of the chamber (to a step change in pressure) is sufficiently long compared with the inspiratory time of the animal to prevent pressure leakage during respiration (6, 8, 18, 44).
Historically, both Pappenheimer (34) and Jacky (18) devised strategies to apply an open system to WBP. The Pappenheimer method relied on high-resistance inlet and outlet ports in series with positive and negative pressure sources. This technique was adapted by Seifert et al. (44), who incorporated a computer feedback system to precisely balance the inflow and outflow to maintain atmospheric pressure within the chamber. The Jacky method was somewhat simpler and utilized a low-resistance inlet attached to a long tube, thus eliminating the need for pressurized air sources (18). The inertance of the air column present in the tube served to “seal” the chamber and prevent pressure signal attenuation. Both methods require substantial testing and validation and were specifically adapted to chambers of variable size and design, making it difficult to replicate the chamber time constant and inertance.
A respiratory effort signal is also required for classifying sleep-disordered breathing events as central or obstructive. To monitor respiratory effort during stationary periods of sleep and quiet wakefulness, we developed a noninvasive, unrestrictive method of detecting respiratory movement in WBP chamber based on transducing the mechanical displacement of the chest and abdomen through an air bladder on which the mouse lay. We recognized, however, that the air bladder could also sense respiratory fluctuations in WBP pressure (barometric contamination), which ranged from 0.01 to 0.03 cmH2O, and could potentially overwhelm the effort signal, which produced pressure fluctuations during tidal breathing of 0.001 to 0.01 cmH2O. We, therefore, devised a method to eliminate barometric contamination. Thus, to obtain a full complement of continuous polysomnographic signals in a mouse, it was necessary to devise an open-system methodology, validate WBP measures of tidal volume and airflow, and develop and validate a novel, noninvasive, unrestrictive surrogate measure of respiratory effort.
Experimental Protocols and Validation Studies
Open system setup.
Building on the Seifert method, we applied a bias flow through the chamber.1 High-resistance elements were interposed at the chamber's inflow and outflow ports so as to increase the time constant of the chamber to approximately 10 times that of the mouse's inspiratory time, thereby allowing for continuous, unattenuated signal recordings in an open system (8, 44). The time constant of our open-system WBP chamber before modification was <0.1 s. We modified our WBP chamber to achieve a targeted time constant of 1.5 s (10 times the upper limit of a mouse's inspiratory time of ∼0.15 s). High-resistance elements (needles) were placed at the bias flow inlet (Becton Dickinson, 25 g, 1.5 cm) and outlet (Becton Dickinson, 27 g, 1.0 cm) to achieve this time constant.
We then determined that a bias flow of 150 ml/min was adequate to maintain a level of CO2 <1% (mean 0.4%, range 0.2–0.9%). This flow was maintained by applying pressurized air and a vacuum source across the inlet and outlet of the chamber, respectively (Fig. 1). Minor fluctuations in inflow and outflow rates, however, led to transient deviations in chamber pressure from atmospheric. To prevent such deviations, air delivery and removal were precisely regulated with mass flow controllers (MC-1SLPM-D/5M, Alicat Scientific, Tucson, AZ; inlet setting: mass flow = 0.152, outlet setting: mass flow = 0.152). The mass flow controllers allowed for the inlet and outlet bias flows to be precisely matched by mass rather than volume, which changes across the high-resistance elements. Minor pressure fluctuations were further minimized by implementing a slow leak through a needle (Becton Dickinson, 25 g, 1.5 cm) connected to another chamber port to allow the chamber pressure to equilibrate with atmospheric pressure. After implementing the high-resistance elements and slow leak, the final time constant of our chamber was 1.6 s in duration. [Please note that the two upper surface ports (pneumotachographs) of the Buxco chamber were fitted with the bias flow outlet and slow leak needles; one of the base side ports was fitted with the bias flow inlet needle.]
To cancel out ambient barometric pressure changes and noise, the WBP animal chamber was referenced to a reference chamber with a similar time constant using a differential pressure transducer (8). Because we increased the time constant of our animal chamber, we also added a high-resistance element in the form of a needle (Becton Dickinson, 27 g, 1.0 cm) to the reference chamber slow leak port to match the time constants of the two chambers. To check the overall resilience of our system to changes in ambient pressure, we created local atmospheric disturbances (e.g., opening and closing the laboratory door) and noted no deflection in the pressure signal.
Open-system validation protocol.
To compare our measurements of chamber pressure in the open-system chamber to gold standard closed-system (sealed) chamber measurements, we connected a small-mammal mechanical ventilator (HSE Mouse Ventilator, type 845) to the WBP to produce a cyclic pressure signal. The WBP pressure signal fluctuations were recorded serially in both the open- and closed-system configurations over ventilator frequencies ranging from 80 to 400 cycles/min, which encompass the upper and lower limits of mouse respiratory rates and inspiratory times (during both normal breathing and hypoxic and hypercapneic challenges) (14, 32, 46). Pressure measurements in the open and closed system were compared to quantify any signal attenuation in the open system. At frequencies of 80–400 cycles/min, we demonstrated a flat frequency response in the open system that remained within 1% of that in the closed system, thus indicating no significant attenuation of the WBP pressure signal in the open system over the full range of potential mouse respiratory rates.
WBP tidal volume and airflow signal validation protocol.
We validated WBP measures of tidal volume and airflow in our system against gold standard pneumotachographic measurements of these variables in four mice. The recordings from the pneumotach and WBP were made simultaneously. Each mouse was anesthetized, intubated, and placed on a heating pad to maintain normal body temperature (to preserve the temperature gradient between the mouse and room air and thus maintain the respiratory-related changes in pressure). The endotracheal tube was connected to a calibrated pneumotachograph and differential pressure transducer (no. MP 45-14-871, Validyne Engineering, Northridge, CA). The mouse and pneumotachograph were then placed in the open-system WBP chamber (with bias flow applied). Simultaneous respiratory recordings were obtained from the pneumotachograph and WBP chamber for multiple 15- to 20-s periods, such that WBP and pneumotachographic measure of tidal volume and airflow could be compared on the same breaths. The mouse was taken out of the chamber and placed on the heating pad between recording periods. The mouse rectal temperature was taken before and after each recording period (differential ranged from 0 to 1.0°C), and the average of these two numbers was used for calculating the WBP tidal volume. WBP measurements of calculated tidal volume and derived flow were compared with pneumotachographic measurement flow and tidal volume. The mouse was euthanized after the study.
Respiratory movement signal-barometric contamination cancellation protocol.
A sensor air bladder and reference air bladder were placed in an open-system WBP, as described above (Fig. 1). A small-mammal mechanical ventilator was attached to the WBP to simulate a cyclic pressure signal similar to that produced by a breathing mouse. The relative strength of the contaminating signal was checked both with and without a euthanized mouse placed on the sensor bladder to account for the potential impact of differences in the unstressed volume of the balloon on the effort signal. When the differential pressure transducer was referenced to atmospheric pressure, the mechanical ventilator produced a contaminating signal of ∼0.3 cmH2O. When the differential pressure transducer was reconnected to the reference bladder, the contaminating signal was not detectable, whether or not the euthanized mouse was placed on the sensor cuff.
Respiratory movement signal-validation protocol.
We validated our respiratory movement signal against a gold standard pneumotachographic measure of tracheal pressure in three anesthetized mice. Each mouse was intubated and placed on the sensor air bladder (outside of the WBP chamber). The endotracheal tube was connected to a pneumotachograph and calibrated pressure transducer (no. P23, Gould Statham, Bayamon, PR). To obtain a wide range of tracheal pressures, dead space (45-cm tube, volume = 2.8 cm3) were added to the breathing circuit for multiple 10- to 15-s periods in each mouse. As CO2 accumulated in the breathing circuit, respiratory effort increased, and the differential bladder pressure was compared with the tracheal pressure signal. Mouse position and orientation on the air bladder were also varied with each trial to investigate a possible positional component to performance.
WBP full polysomnographic study protocol.
The tidal volume, airflow, respiratory movement, and EEG/EMG signals were acquired simultaneously and recorded continuously in an unrestrained C57BL/6J or NZO mouse in the open WBP system with a bias flow of 150 ml/min. After an equilibration period of 45 min, 2 h of recording were collected from 2 PM to 4 PM. Tidal volume, respiratory rate, and minute ventilation were measured during stable periods of wakefulness, non-rapid eye movement (NREM) and rapid eye movement (REM) sleep.
Data Analysis
Values in the results section are reported as means and SDs. Correlation analysis was used to compare experimental measures (e.g., WBP tidal volume) to gold standard measures (e.g., tracheal tidal volume) in validation protocols. In addition, Bland-Altman analysis was utilized to quantify bias and limits of agreement (LOA). As previously described, a repeated-measures ANOVA was utilized to account for multiple measurements made within a single subject (3). A P value of <0.05 was accepted as the threshold for inferring statistical significance. Ninety-five percent LOA were calculated as ±1.96 × the SD.
RESULTS
WBP Tidal Volume Signal Validation
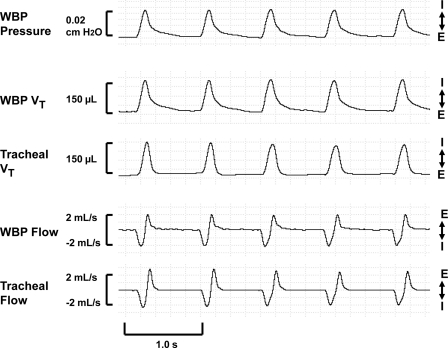
In Fig. 2, a representative recording of a WBP tidal volume and airflow signal validation trial shows that the indirectly measured WBP tidal volume signal was similar in amplitude and morphology to the simultaneously obtained gold standard tracheal tidal volume signal during the inspiratory limb. The expiratory limb demonstrated a gradual roll-off or shoulder immediately before returning to baseline, consistent with previous reports (11, 19, 37).2
Fig. 2.
Representative recording of a tidal volume (Vt) and airflow validation study recording in a single, anesthetized mouse. WBP Vt was similar in amplitude and waveform morphology to tracheal Vt during the inspiratory limb and demonstrated a gradual roll-off or shoulder before returning to baseline in the expiratory limb. Compared with tracheal airflow, WBP inspiratory flow (I) showed a similar amplitude and morphology, while expiratory flow (E) demonstrated an attenuation in signal amplitude but similar morphology. Signals (from top to bottom) include WBP pressure, WBP Vt, tracheal Vt, WBP airflow, and tracheal airflow.
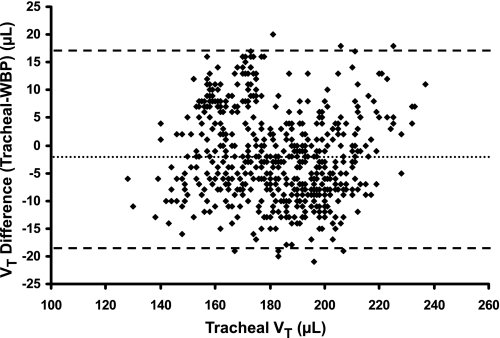
In Fig. 3, a Bland-Altman plot was used to compare WBP tidal volume (for all four mice) to tracheal tidal volume. Tracheal tidal volume was plotted on the X-axis (rather than mean tidal volume) as the gold standard (20). The mean difference of the tidal volume signals (−1.80 μl) represented only 1% of the mean tracheal tidal volume, indicating minimal systematic bias in the WBP signal. The 95% LOA were also narrow (±17.50 μl around the mean difference), which represented ∼10% of mean tracheal tidal volume. No skew was apparent in mean tidal volume difference as a function of change in tracheal tidal volume over a wide range of tidal volumes. Bland-Altman analysis of the individual mouse trials (Table 1, top; plots not shown) revealed minimal bias, no skew, and within-mouse LOA of ±10.88 μl (SD 5.55 μl, P < 0.001), which were even more narrow than those for the combined data. The correlation coefficient (R2) between tracheal tidal volume and WBP tidal volume of the combined data was 0.87 (P < 0.001).
Fig. 3.
Bland-Altman plot of Vt difference (tracheal Vt − WBP Vt) vs. gold standard tracheal Vt in four mice. The Y-axis represents the difference between the tracheal and plethysmographic Vt values. The dotted line delineates the mean Vt difference, which is −1.80 μl. The limits of agreement lie at 15.7 and −19.3 μl (±17.5 μl) (SD 8.9 μl, P < 0.001). There was no skew as a function of increasing or decreasing tracheal Vt.
Table 1.
Tidal volume and flow validation study data
| Mouse | No. of Breaths | Mean Vt, μl | Mean Difference, μl | SD, μl | Mean Difference 95% CI | Relative Mean Difference, % | CV, % |
|---|---|---|---|---|---|---|---|
| Vt | |||||||
| 1 | 142 | 193.2 | 0.0 | 7.2 | −1.2–1.2 | 0.0 | 3.7 |
| 2 | 189 | 180.4 | −6.2 | 4.8 | −6.9–−5.5 | −3.5 | 2.7 |
| 3 | 131 | 163.8 | 7.9 | 4.8 | 7.1–8.8 | 4.8 | 3.0 |
| 4 | 102 | 195.9 | −8.6 | 4.4 | −9.5–−7.7 | −4.4 | 2.3 |
| Total | 564 | 182.6 | −1.8 | 8.9 | −2.5–−1.1 | −1.0 | 4.9 |
| Phase | No. of Breaths (5 Per Mouse) | Mean Flow, μl/s | Mean Difference, μl/s | SD, μl/s | Mean Difference | Relative Mean | CV, % |
|---|---|---|---|---|---|---|---|
| Tidal airflow | |||||||
| Inspiration | 20 | 845.1 | −40.5 | 264.9 | −49.5–−31.5 | 3.7 | 24.4 |
| Expiration | 20 | −761.8 | −201.7 | 698.5 | −219.4–−184.0 | −30.9 | 107.0 |
Tidal airflow data set consists of 5 consecutive breaths from 4 individual mice (20 breaths total). Top: mean Vt, mean tracheal tidal volume; mean difference, mean tracheal Vt minus mean whole body plethysmography Vt; SD, SD of mean difference; relative mean difference, mean difference/mean tracheal tidal volume; CV, coefficient of variation = mean difference/mean tracheal Vt. Bottom: mean flow, mean tracheal airflow; mean difference, mean tracheal airflow minus mean whole body plethysmography airflow; relative mean difference, mean difference/mean tracheal airflow; CV, coefficient of variation = mean difference/mean tracheal airflow.
WBP Tidal Airflow Signal Validation
Figure 2 shows a recording of the WBP airflow signal adjacent to the simultaneously obtained gold standard tracheal airflow signal. Compared with the tracheal flow signal, the WBP inspiratory flow waveform was similar in amplitude and morphology, while expiratory flow showed an attenuation in signal amplitude but similar morphology.3
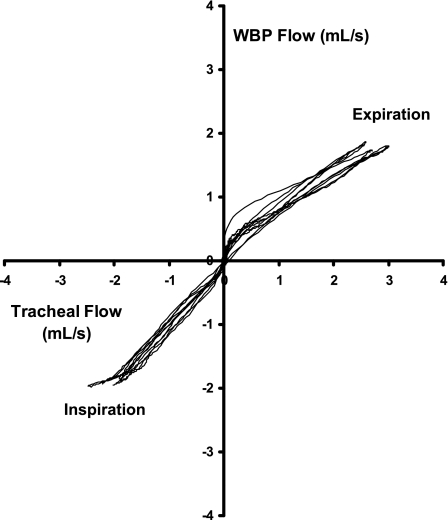
In Fig. 4, comparison of the WBP and tracheal flow signals for the five representative breaths illustrated in the recording example in Fig. 2 revealed a high degree of correlation throughout the respiratory cycle between the two (R2 = 0.97 for inspiration, R2 = 0.96 for expiration, P < 0.001 for both). Specifically, the WBP and tracheal airflow signals tracked one another along the line of identity throughout inspiration (see bottom left quadrant, Fig. 4). In contrast, while WBP expiratory flow correlated well with tracheal flow, attenuation of the WBP flow signal was evident as tracheal expiratory flow increased (see skew in curve at higher tracheal flow levels, right top quadrant, Fig. 4).
Fig. 4.
Identity plot of WBP airflow vs. gold standard tracheal flow in a single mouse (from Fig. 2). The X-axis represents tracheal airflow, and the Y-axis represents WBP airflow. Positive flow indicates expiration, and negative flow indicates inspiration. Good correlation was seen across both the inspiratory (R2 = 0.97, P < 0.001) and expiratory (R2 = 0.96, P < 0.001) phases. Inspiratory flow demonstrated good agreement between WBP and tracheal values, while expiratory flow showed attenuation of the WBP flow relative to the tracheal flow signal.
In Table 1, separate Bland-Altman analyses appear for inspiratory and expiratory flow data encompassing four mice (five breaths each). Inspiratory data showed good agreement between WBP inspiratory flow and the gold standard (relative mean difference = 3.7%). Expiratory phase data demonstrated significant attenuation of WBP expiratory flow compared with tracheal flow (relative mean difference = −30.9%). Bland-Altman analyses (plots not shown) of our flow data yielded LOA of ±519.2 μl (SD 264.9, P < 0.001) for inspiratory flows and ±1,369.1 μl (SD 698.5, P < 0.001) for expiratory flows. The correlation coefficients (R2) of the inspiratory and expiratory phases of the combined data were 0.92 (P < 0.001) and 0.83 (P < 0.001), respectively.
Respiratory Movement Signal Validation
In Fig. 5, representative recordings of noninvasive respiratory movement (air bladder pressure) and gold standard tracheal pressure illustrate the response of these signals to CO2 rebreathing when dead space was added to the breathing circuit of an anesthetized mouse. As shown, the air bladder pressure swings tracked those in the tracheal pressure as effort progressively increased (Fig. 5, top). Expanded views at low (Fig. 5, bottom left) and high (bottom right) demonstrate that excursions in the air bladder pressure paralleled those in the tracheal pressure signal.
Fig. 5.
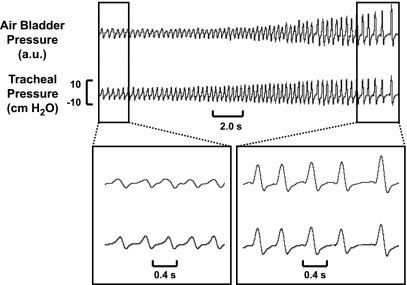
Representative recording of respiratory movement signal validation study. The top signal represents air bladder pressure (novel respiratory effort signal), and the bottom signal represents tracheal pressure (gold standard respiratory effort signal). During the rebreathing trial, both tracheal pressure and air bladder pressure increased in parallel (top panel). Expanded recordings at low (bottom left panel) and high (bottom right panel) effort further demonstrate that the two signals paralleled one another over a wide range of effort. au, Arbitrary units.
To further investigate the correlation between these two signals, we compared peak tidal swings (amplitudes) in air bladder pressure and tracheal pressure in each of three mice for a total of 17 rebreathing trials and 908 breaths. In these mice, mean correlation coefficients (R2) were 0.79 (0.60–0.86), 0.84 (0.76–0.93), and 0.84 (0.81–0.92) (all P values < 0.001), demonstrating a high degree of correlation in the amplitude of the respiratory movement signal and tracheal pressure signal across multiple trials. We did not detect any influence of mouse position or orientation on the air bladder on signal performance, as reflected by the high level of correlation between signals.
To further characterize the performance characteristics of the air bladder respiratory movement signal, we correlated within-breath excursions of the air bladder pressure signal and gold standard tracheal pressure signal over five breaths in each mouse during the rebreathing trials. Correlation coefficients (R2) in the three mice were 0.96, 0.86, and 0.93 (all P values < 0.001), indicating that the air bladder-transduced respiratory effort signal correlated closely with tracheal pressure over the full waveform of each breath.
Full Polysomnographic Recordings
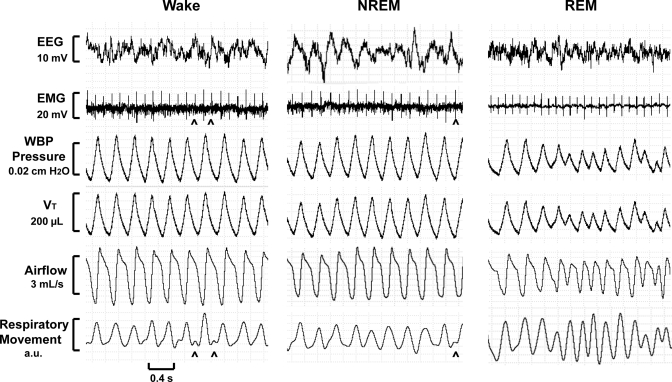
Figure 6 shows recording segments from a 2-h full polysomnographic study from one mouse. Quiet wakefulness was characterized by a respiratory pattern that was regular in amplitude and timing. Mean tidal volume, respiratory rate, and minute ventilation were 240 μl (range = 220–255 μl), 217 breaths/min, and 52.1 ml/min, respectively. NREM sleep demonstrated a regular respiratory pattern with a mean tidal volume, respiratory rate, and minute ventilation of 217 μl (range = 198–230 μl), 211 breaths/min, and 45.8 ml/min, respectively. REM sleep was characterized by an irregular breathing pattern with highly variable tidal volumes. Mean tidal volume, respiratory rate, and minute ventilation were 125 μl (range = 66–196 μl), 289 breaths/min, and 36.1 ml/min, respectively. REM sleep demonstrated a period of decreasing tidal volumes with simultaneously increasing respiratory movement, which may indicate an increase in airway resistance during that period.
Fig. 6.
Recording sections of a WBP full polysomnographic study demonstrating respiratory waveforms during quiet wakefulness (left), non-rapid eye movement (NREM) sleep (middle), and rapid eye movement (REM) sleep (right) in one mouse. Signals (from top to bottom) include electroencephalographic (EEG) signal, nuchal electromyographic (EMG) signal, WBP chamber pressure, WBP Vt, WBP airflow, and respiratory movement (surrogate for respiratory effort). Intermittent cardiac artifact (carets) can be seen in the EMG and respiratory movement channels.
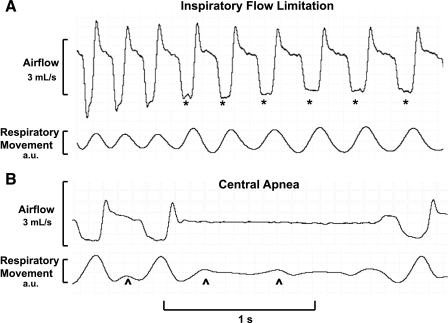
During a 2-h full polysomnographic study in a single NZO mouse, we identified several sleep-related disturbances in airflow (see representative examples seen in Fig. 7). Figure 7A shows a period of progressive decreases in inspiratory airflow. In the first several breaths, a somewhat rounded inspiratory flow contour gave way to broader plateaus in early inspiration on subsequent breaths. Inspiratory airflow plateaued at a maximal level (maximal inspiratory airflow), despite increasing inspiratory respiratory movement (see asterisks), suggesting the presence of IFL. These breaths also exhibited other characteristics of IFL, including reductions in maximal inspiratory airflow, increased respiratory movement swings, increased inspiratory time, and negative effort dependence. In contrast, flow and effort signal fluctuations ceased during a 1.4-s central apnea (see Fig. 7B).
Fig. 7.
Recording examples from a WBP study in an New Zealand Obese mouse during sleep. A: a period of progressive decrease in inspiratory airflow. The inspiratory flow contour exhibited progressively broader midinspiratory plateaus (see asterisks), despite increasing inspiratory respiratory movement, consistent with the development of inspiratory flow limitation (IFL). Additional characteristics of IFL include increased respiratory movement swings (compared with earlier breaths), a reduction in maximal inspiratory airflow, an increase in inspiratory time, and negative effort dependence (which can be seen on the first, third, and fifth flow-limited breaths). B: a 1.4-s central apnea characterized by the absence of respiratory flow and movement. Intermittent cardiac artifact (see carets) can be seen in the effort signal.
DISCUSSION
We have fully developed a system for obtaining high-fidelity, continuous respiratory recordings in an undisturbed mouse during sleep and wake and used this system to demonstrate IFL in an NZO mouse. Implementation of this system required novel application of open-system methodology to a commercial chamber, validation of respiratory signals, development and validation of a noninvasive surrogate respiratory effort signal, and signal integration into full polysomnographic pilot recordings.
Open-system Application
A high-fidelity, open-system WBP chamber is integral for monitoring respiration during continuous sleep-wake recordings; however, all previous methodologies have employed custom-designed chambers. Novel modifications to the Seifert system allowed us to maintain atmospheric pressure within the chamber and obviated the need for implementing a separate computer-feedback system to control chamber pressure. Our open system demonstrated a flat frequency response to a cyclic pressure signal applied over a wide frequency range, spanning the upper and lower limits of mouse respiratory rate and inspiratory time (see materials and methods, Open-system validation protocol). We were thus able to make continuous, accurate respiratory signal measurements in a commercial WBP chamber with off-the-shelf components.
WBP Tidal Volume and Airflow Validation
Accurate measurement of tidal volume and airflow are crucial for characterizing respiratory patterns during sleep and wakefulness. In contrast to past WBP tidal volume validation studies, we utilized a commercially available WBP chamber. The LOA of our Bland-Altman tidal volume plot are approximately ±10% (of mean tracheal tidal volume) for our combined data and approximately ±6% for individual mice, indicating that our technique is able to reliably detect tidal volume changes of 10% between mice and 6% within an individual mouse. In prior studies, investigators have documented 20–50% changes in tidal volume across a range of sleep-wake states, weight, and murine strains (12, 15, 32). The accuracy of the plethysmographic airflow signal has never been previously validated. We demonstrated a WBP flow signal that shows excellent correlation and good agreement with the gold standard during the inspiratory phase and excellent correlation but amplitude attenuation during the expiratory phase. Thus our system offers adequate power to detect relatively small changes in tidal volume and accurately describes the inspiratory airflow contour and amplitude.
Respiratory Movement Signal Validation
A respiratory effort signal is integral in characterizing disturbances in tidal airflow and detecting airflow obstruction. In large mammals, noninvasive effort monitoring has been accomplished with piezoelectric vests and belts and impedance plethysmography, which are ill suited for rodents due to size constraints and intolerance to wearing jackets and bands (10, 17, 22, 29). In anesthetized and immobilized rats, use of invasive pressure manometry and subcutaneously tunneled EMG leads has been reported (1, 23, 41); however, these approaches add substantial cost and burden and are not well validated in unrestrained, undisturbed mice. To address these concerns, we utilized a novel dual air bladder system for noninvasively sensing respiratory movement as a surrogate for respiratory effort. Changes in bladder pressure demonstrated a high correlation with tracheal pressure swings during CO2 rebreathing experiments. In addition, the air bladder signal demonstrated a high intrabreath correlation with the gold standard tracheal pressure, thus offering a reliable surrogate for respiratory effort and the ability to detect effort responses to respiratory loads.
Mouse Polysomnography and IFL
Using this system, we detected sleep-related changes in ventilatory patterns (Fig. 6) and disturbances in respiratory airflow (Fig. 7). Of note, airflow and respiratory movement signals were crucial in detecting IFL, which is characterized by increasing respiratory effort during a plateau in inspiratory airflow, in an obese mouse strain previously considered susceptible to upper airway obstruction (4, 5). These flow-limited breaths were characterized by a decrease in inspiratory flow, an increase in inspiratory time, an increase in respiratory movement, and negative effort dependence, all of which are consistent with the development of dynamic upper airway obstruction (42). Previous studies have suggested that the most likely site of extrathoracic obstruction in the mouse is the pharynx (4, 5, 21, 36). IFL in a sleeping mouse is a novel, potentially intriguing finding and suggests that this strain might serve as mouse model of OSA. While central apneas have been reported in both mice and rats, a rodent model of OSA has never been reported (15, 24, 40, 41).
Limitations
Several limitations should be considered in interpreting and applying our findings. First, as mentioned above, while our system was tuned to transduce the inspiratory flow signal accurately, significant amplitude attenuation of the expiratory flow signal was observed. Nevertheless, greater accuracy of the expiratory flow signal could be implemented by incorporating a correction factor, increasing the signal-to-noise ratio for the underlying chamber pressure, or signal averaging the expiratory signal over multiple breaths. Second, we acknowledge that our noninvasive respiratory movement signal provides a relative measure of respiratory effort, the amplitude of which varies with mouse position on the air bladder. As such, signal changes can be compared during stationary periods (i.e., sleep or quiet wakefulness), but cannot be compared across periods in which the mouse has shifted position. In addition, movement artifacts contaminate both the respiratory effort signal and the WBP tidal volume signal, precluding any meaningful measurements during active wakefulness. Third, full polysomnography usually includes oxyhemoglobin saturation monitoring, which we have not yet incorporated into WBP systems such as ours. Fourth, while this system was developed solely to studying mice, it can be used for rat plethysmography with some modifications. A larger commercial chamber is necessary, and this chamber must be fitted with the proper needle resistances to achieve an adequate time constant and bias flow, as described in materials and methods.
Implications and Conclusions
In summary, we have developed an easily replicable system for continuously recording a full complement of polysomnographic signals in unanesthetized, unrestrained mice. This testing platform will facilitate studies examining the interrelationships between sleep-wake state and respiratory control in several ways. First, continuous recordings can be acquired to characterize inherent biological variability in respiratory patterns across sleep-wake states and elucidate the interplay between sleep-related respiratory disturbances and sleep architecture. Second, a noninvasive surrogate for respiratory effort will enhance our ability to characterize sleep-disordered breathing episodes as either central or obstructive, and allows us to detect IFL. Third, our open system can be used to quantify effects of gas challenges (e.g., hypoxia, hypercapnia, asphyxia) and environmental exposures (e.g., diet-induced obesity) on sleep and breathing patterns. Fourth, inbred murine strains, which exhibit alterations in lung mechanics and/or ventilatory control, can be studied to implicate underlying genetic susceptibility to sleep-disordered breathing and respiratory insufficiency. We report a novel IFL phenotype in one such strain, the NZO mouse. These investigative strategies will enhance our ability to build murine models of SRBD and respiratory failure.
GRANTS
This study was supported by National Institutes of Health Grants HL50381, HL37379, and T32 90030860.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.B.H., M.P., W.C.H., and A.R.S. conception and design of research; A.B.H. performed experiments; A.B.H., J.P.K., P.L.S., H.S., and A.R.S. analyzed data; A.B.H., J.P.K., P.L.S., H.S., and A.R.S. interpreted results of experiments; A.B.H. prepared figures; A.B.H. drafted manuscript; A.B.H., J.P.K., P.L.S., H.S., M.P., R.A.R., W.C.H., and A.R.S. edited and revised manuscript; A.B.H., J.P.K., P.L.S., H.S., M.P., R.A.R., W.C.H., and A.R.S. approved final version of manuscript.
Footnotes
Of note, we had also attempted to apply the Jacky open-system method to our chamber (18). While this system resulted in a largely unattenuated pressure wave, the waveform contained a small, unexplained negative pressure deflection, which had been previously documented (16). Due to concern over this possible artifact, we chose to proceed with adapting the Seifert methodology.
While the expiratory limb of our Vt signal shows a gradual roll-off or shoulder before returning to baseline (compared with the gold standard tracheal signal), this shoulder does not confound tidal volume measurements, because tidal volume is calculated as the difference between signal peak and baseline (45). Investigators have previously documented this shoulder in the WBP expiratory waveform, which has been attributed to a reduced rate of cooling of the expiratory airstream (11, 19, 37).
Selective expiratory attenuation resulted from implementing a wide window for differentiating the tidal volume signal, which acted as a low-pass filter, which preferentially dampens the high-frequency component of the expiratory flow waveform. This effect may also be partially due to the roll-off in the expiratory limb of the tidal volume curve discussed above.
REFERENCES
- 1. Alexandrova NP, Donina ZA, Danilova GA. Effect of central hypervolemia on respiratory function. J Physiol Pharmacol 58, Suppl 5: 9–15, 2007 [PubMed] [Google Scholar]
- 2. Baekey DM, Feng P, Decker MJ, Strohl KP. Breathing and sleep: measurement methods, genetic influences, and developmental impacts. ILAR J 50: 248–261, 2009 [DOI] [PubMed] [Google Scholar]
- 3. Bland JM, Altman DG. Agreement between methods of measurement with multiple observations per individual. J Biopharm Stat 17: 571–582, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Brennick MJ, Kuna ST, Pickup S, Cater J, Schwab RJ. Respiratory modulation of the pharyngeal airway in lean and obese mice. Respir Physiol Neurobiol 175: 296–302, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brennick MJ, Pack AI, Ko K, Kim E, Pickup S, Maislin G, Schwab RJ. Altered upper airway and soft tissue structures in the New Zealand Obese mouse. Am J Respir Crit Care Med 179: 158–169, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carley DW, Radulovacki M. Sleep-Related Breathing Disorders: Experimental Models and Therapeutic Potential. New York: Dekker, 2003, p. 43–48 [Google Scholar]
- 7. Chow CM, Xi L, Smith CA, Saupe KW, Dempsey JA. A volume-dependent apneic threshold during NREM sleep in the dog. J Appl Physiol 76: 2315–2325, 1994 [DOI] [PubMed] [Google Scholar]
- 8. Coates AL, Peslin R, Rodenstein D, Stocks J. Measurement of lung volumes by plethysmography. Eur Respir J 10: 1415–1427, 1997 [DOI] [PubMed] [Google Scholar]
- 9. Drorbaugh JE, Fenn WO. A barometric method for measuring ventilation in newborn infants. Pediatrics 16: 81–87, 1955 [PubMed] [Google Scholar]
- 10. Fiz JA, Jané R, Torres A, Morera J, Galdiz B, Gea J, Grassino A. Non-invasive monitoring of diaphragmatic timing by means of surface contact sensors: an experimental study in dogs. BMC Pulm Med 4: 8, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fleming PJ, Levine MR, Goncalves AL, Woollard S. Barometric plethysmograph: advantages and limitations in recording infant respiration. J Appl Physiol 55: 1924–1931, 1983 [DOI] [PubMed] [Google Scholar]
- 12. Friedman L, Haines A, Klann K, Gallaugher L, Salibra L, Han F, Strohl KP. Ventilatory behavior during sleep among A/J and C57BL/6J mouse strains. J Appl Physiol 97: 1787–1795, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Goldstein LB, Adams R, Alberts MJ, Appel LJ, Brass LM, Bushnell CD, Culebras A, Degraba TJ, Gorelick PB, Guyton JR, Hart RG, Howard G, Kelly-Hayes M, Nixon JV, Sacco RL. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council. Stroke 37: 1583–1633, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Han F, Subramanian S, Dick TE, Dreshaj IA, Strohl KP. Ventilatory behavior after hypoxia in C57BL/6J and A/J mice. J Appl Physiol 91: 1962–1970, 2001 [DOI] [PubMed] [Google Scholar]
- 15. Han F, Subramanian S, Price ER, Nadeau J, Strohl KP. Periodic breathing in the mouse. J Appl Physiol 92: 1133–1140, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Harris MB, Milsom WK. The influence of NMDA receptor-mediated processes on breathing pattern in ground squirrels. Respir Physiol 125: 181–197, 2001 [DOI] [PubMed] [Google Scholar]
- 17. Hendricks JC, Kline LR, Kovalski RJ, O'Brien JA, Morrison AR, Pack AI. The English bulldog: a natural model of sleep-disordered breathing. J Appl Physiol 63: 1344–1350, 1987 [DOI] [PubMed] [Google Scholar]
- 18. Jacky JP. A plethysmograph for long-term measurements of ventilation in unrestrained animals. J Appl Physiol 45: 644–647, 1978 [DOI] [PubMed] [Google Scholar]
- 19. Jacky JP. Barometric measurement of tidal volume: effects of pattern and nasal temperature. J Appl Physiol 49: 319–325, 1980 [DOI] [PubMed] [Google Scholar]
- 20. Krouwer JS. Why Bland-Altman plots should use X, not (Y+X)/2 when X is a reference method. Stat Med 27: 778–780, 2008 [DOI] [PubMed] [Google Scholar]
- 21. Liu A, Pichard L, Schneider H, Patil SP, Smith PL, Polotsky V, Schwartz AR. Neuromechanical control of the isolated upper airway of mice. J Appl Physiol 105: 1237–1245, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lonergan RP, 3rd, Ware JC, Atkinson RL, Winter WC, Suratt PM. Sleep apnea in obese miniature pigs. J Appl Physiol 84: 531–536, 1998 [DOI] [PubMed] [Google Scholar]
- 23. Megirian D, Ryan AT, Sherrey JH. An electrophysiological analysis of sleep and respiration of rats breathing different gas mixtures: diaphragmatic muscle function. Electroencephalogr Clin Neurophysiol 50: 303–313, 1980 [DOI] [PubMed] [Google Scholar]
- 24. Mendelson WB, Martin JV, Perlis M, Giesen H, Wagner R, Rapoport SI. Periodic cessation of respiratory effort during sleep in adult rats. Physiol Behav 43: 229–234, 1988 [DOI] [PubMed] [Google Scholar]
- 25. Mokhlesi B, Tulaimat A, Faibussowitsch I, Wang Y, Evans AT. Obesity hypoventilation syndrome: prevalence and predictors in patients with obstructive sleep apnea. Sleep Breath 11: 117–124, 1998 [DOI] [PubMed] [Google Scholar]
- 26. Mortola JP, Frappell PB. On the barometric method for measurements of ventilation, and its use in small animals. Can J Physiol Pharmacol 76: 937–944, 1998 [DOI] [PubMed] [Google Scholar]
- 27. Nakamura A, Fukuda Y, Kuwaki T. Sleep apnea and effect of chemostimulation on breathing instability in mice. J Appl Physiol 94: 525–532, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Nakamura A, Zhang W, Yanagisawa M, Fukuda Y, Kuwaki T. Vigilance state-dependent attenuation of hypercapnic chemoreflex and exaggerated sleep apnea in orexin knockout mice. J Appl Physiol 102: 241–248, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Neuzeret PC, Gormand F, Reix P, Parrot S, Sastre JP, Buda C, Guidon G, Sakai K, Lin JS. A new animal model of obstructive sleep apnea responding to continuous positive airway pressure. Sleep 34: 541–548, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, D'Agostino RB, Newman AB, Lebowitz MD, Pickering TG. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. JAMA 283: 1829–1836, 2000 [DOI] [PubMed] [Google Scholar]
- 31. Nowbar S, Burkart KM, Gonzales R, Fedorowicz A, Gozansky WS, Gaudio JC, Taylor MR, Zwillich CW. Obesity-associated hypoventilation in hospitalized patients: prevalence, effects, and outcome. Am J Med 116: 1–7, 2004 [DOI] [PubMed] [Google Scholar]
- 32. O'Donnell CP, Schaub CD, Haines AS, Berkowitz DE, Tankersley CG, Schwartz AR, Smith PL. Leptin prevents respiratory depression in obesity. Am J Respir Crit Care Med 159: 1477–1484, 1999 [DOI] [PubMed] [Google Scholar]
- 33. Onodera M, Kuwaki T, Kumada M, Masuda Y. Determination of ventilatory volume in mice by whole body plethysmography. Jpn J Physiol 47: 317–326, 1997 [DOI] [PubMed] [Google Scholar]
- 34. Pappenheimer JR. Sleep and respiration of rats during hypoxia. J Physiol 266: 191–207, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Piper AJ, Grunstein RR. Big breathing: the complex interaction of obesity, hypoventilation, weight loss, and respiratory function. J Appl Physiol 108: 199–205, 2010 [DOI] [PubMed] [Google Scholar]
- 36. Polotsky M, Elsayed-Ahmed AS, Pichard LE, Richardson RA, Smith PL, Schneider H, Kirkness JP, Polotsky VY, Schwartz AR. Effect of age and weight on upper airway function in a mouse model. J Appl Physiol 111: 696–703, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Polotsky VY, Wilson JA, Smaldone MC, Haines AS, Hurn PD, Tankersley CG, Smith PL, Schwartz AR, O'Donnell CP. Female gender exacerbates respiratory depression in leptin-deficient obesity. Am J Respir Crit Care Med 164: 1470–1475, 2001 [DOI] [PubMed] [Google Scholar]
- 38. Punjabi NM, Sorkin JD, Katzel LI, Goldberg AP, Schwartz AR, Smith PL. Sleep-disordered breathing and insulin resistance in middle-aged and overweight men. Am J Respir Crit Care Med 165: 677–682, 2002 [DOI] [PubMed] [Google Scholar]
- 39. Radulovacki M, Trbovic SM, Carley DW. Acute hypotension reduces sleep apneas in Zucker lean and Zucer obese rats. Sleep 19: 767–773, 1996 [DOI] [PubMed] [Google Scholar]
- 40. Real C, Popa D, Seif I, Callebert J, Launay JM, Adrien J, Escourrou P. Sleep apneas are increased in mice lacking monoamine oxidase A. Sleep 30: 1295–1302, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sato T, Saito H, Seto K, Takatsuji H. Sleep apneas and cardiac arrhythmias in freely moving rats. Am J Physiol Regul Integr Comp Physiol 259: R282–R287, 1990 [DOI] [PubMed] [Google Scholar]
- 42. Schwartz AR, Smith PL, Wise RA, Bankman I, Permutt S. Effect of positive nasal pressure on upper airway pressure-flow relationships. J Appl Physiol 66: 1626–1634, 1989 [DOI] [PubMed] [Google Scholar]
- 43. Schwartz AR, Patil SP, Squier S, Schneider H, Kirkness JP, Smith PL. Obesity and upper airway control during sleep. J Appl Physiol 108: 430–435, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Seifert EL, Knowles J, Mortola JP. Continuous circadian measurements of ventilation in behaving adult rats. Respir Physiol 120: 179–183, 2000 [DOI] [PubMed] [Google Scholar]
- 45. Stephenson R, Gucciardi EJ. Theoretical and practical considerations in the application of whole body plethysmography to sleep research. Eur J Appl Physiol 87: 207–219, 2002 [DOI] [PubMed] [Google Scholar]
- 46. Trapp S, Tucker SJ, Gourine AV. Respiratory responses to hypercapnia and hypoxia in mice with genetic ablation of Kir5.1 (Kcnj16). Exp Physiol 96: 451–459, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 328: 1230–1235, 1993 [DOI] [PubMed] [Google Scholar]