Abstract
This study reports the findings of two classes of corneal afferents excited by drying of the cornea (dry responses) in isoflurane-anesthetized rats: cold-sensitive (CS; 87%) and cold-insensitive (CI; 13%) neurons. Compared with CI neurons, CS neurons showed significantly higher firing rates over warmer corneal temperatures (∼31–15°C) and greater responses to menthol, drying, and wetting of the cornea but lower responses when hyperosmolar solutions were applied to the ocular surface. We proposed that the dry responses of these corneal afferents derive from cooling and an increased osmolarity of the ocular surface, leading to the production of basal tears. An ocular application of the transient receptor potential channel TRPM8 antagonist BCTC (20 μM) decreased the dry responses by ∼45–80% but failed to completely block them, whereas the TRPA1 antagonist HC030031 did not influence the responses to drying of the cornea or hyperosmolar tears. Furthermore, the responses produced by cold stimulation of the cornea accounted for only 28% of the dry responses. These results support the view that the stimulus for basal tearing (corneal dryness) derives partly from cooling of the cornea that activates TRPM8 channels but that non-TRPM8 channels also contribute significantly to the dry responses and to basal tearing. Finally, we hypothesized that activation of TRPM8 by cooling in CS corneal afferents not only gives rise to the sensation of ocular coolness but also to the “wetness” perception (Thunberg's illusion), whereas a precise role of the CI afferents in basal tearing and other ocular dryness-related functions such as eye blink and the “dryness” sensation remain to be elucidated.
Keywords: dry eye, transient receptor potentials ion channels, temperature sensitivity
millions of americans suffer from dry eye disease (DED), especially women and aged populations (Dogru and Tsubota 2011). DED is a chronic ocular disorder characterized by dryness, discomfort, and a burning sensation in the eye. DED is thought to result from a disturbance of the lacrimal functional units (LFUs), which comprise sensory afferents and secretory efferents as well as their satellite tissues such as the cornea, conjunctiva, and lacrimal and meibomiean glands (Definition and Classification Subcommittee of the International Dry Eye Workshop 2007). Normally, LFUs together maintain a healthy tear film to protect the ocular surface and provide optical acuity. A dysfunction of any one component of these LFUs (such as the corneal nerves) is presumed to result in DED. We recently reported (Hirata and Meng 2009, 2010) that a special type of corneal afferent is strongly excited by the physiological stimuli considered to be important in basal tear production (e.g., drying and gentle cooling of the cornea, as well as evaporation and hyperosmolar tears covering the anterior eye). The data therefore suggest that these primary afferent neurons are involved in basal tear production and constitute the afferent limb of the lacrimation reflex (a component of the LFU). Also, because these neurons are such an exquisite sensor of cooling and menthol applied to the cornea, we hypothesize that a member of transient receptor potential (TRP) channels, TRPM8, must reside in these nerve endings to detect ocular cooling.
Parra et al. (2010) recently reported that nerve terminal impulses recorded from the cold-sensitive neurons in the mouse-isolated cornea showed cooling and menthol sensitivities similar to the afferents we identified as the afferent limb of the lacrimation reflex (Hirata and Meng 2010). This study also reported that the responses to these ocular stimuli were largely absent in TRPM8-knockout (KO) mice and could be reduced by the TRPM8 blocker N-(4-tert-butylphenyl)-4-(3-chloropyridin-2-yl)piperazine-1-carboxamide (BCTC) in normal animals. Furthermore, the basal tearing measured at the normal corneal temperature (30.6°C) was significantly diminished in TRPM8-KO mice compared with wild-type (WT) mice. These data support the hypothesis that TRPM8 mediates the detection of small temperature fluctuations at the corneal surface and that its activation by temperature changes provides the necessary stimulus for basal tearing. However, it is not known if the responses to other physiological ocular stimuli (such as drying and hyperosmolar stimuli that occur during the evaporation of tears and presumably trigger basal tearing) are also mediated by TRPM8. We previously proposed (Hirata and Meng 2010) that the responses to drying of the cornea result from cooling and hyperosmolar stimulations of the ocular surface, since the process of evaporation (i.e., drying of the cornea) should result in gentle cooling and hyperosmolar stimulations at the corneal surface. Thus one goal of the present study was to assess the contribution of TRPM8 to the dry responses by attempting to block them with an ocular application of the TRPM8 antagonist BCTC. Also, because it was reported recently that human embryonic kidney cells expressing TRPA1 were activated by hypertonic saline (Zhang et al. 2008), we sought to determine if the responses to drying of the cornea and hyperosmolar tears are also mediated by TRPA1. Our initial goals to pharmacologically characterize the cold-sensitive (CS) corneal afferents have led serendipitously to one of our novel findings of the CI neurons in the present study.
MATERIALS AND METHODS
Surgery and recordings.
Under 2.5% isoflurane (in 100% oxygen) anesthesia, the femoral vein and artery of male Sprague-Dawley rats (350–570 g) were catheterized, respectively, for fluid injections and mean arterial pressure (MAP) recordings. The animal was placed in a stereotaxic instrument that held its head firmly with mouth and ear bars, and the tracheal tube was connected to a ventilator after tracheostomy. A partial craniotomy was performed to expose the brain overlying the left trigeminal ganglion (TG). The animals were artificially respired (rodent ventilator model 55-3438; Harvard Apparatus, South Natick, MA) and the end-tidal CO2 was monitored with a CO2 analyzer (4–5%; CWE, Ardmore, PA). The core temperature was maintained at 37–38°C with a feedback-controlled regulator (FHC, Bowdoin, ME). Just before the recordings, the isoflurane concentration was decreased to and maintained at 1.5% throughout the experiment. After a check for noxious stimulation-evoked withdrawal reflexes, pancuronium bromide (0.6 mg·kg−1·h−1) was infused continuously during electrophysiological recordings. A tungsten microelectrode (5–9 MΩ; FHC) was lowered into the left TG to search for a spontaneously active neuron. After an amplification and discrimination with template matching software (CED, Cambridge, UK), the neural spike outputs, MAP, and the temperatures during the corneal thermal stimulation were acquired and analyzed using CED Power 1401 and Spike2, version 5.21 (Cambridge, UK). Neurons that responded to room temperature (RT) saline solution applied to the eye (cooling stimulus, 21–23°C) with a brief (duration <4 s) burst of activity were isolated. Receptive fields (RFs) were identified on the cornea with an ice-cooled dental metal probe (tip diameter ∼1 mm). At the end of the experiment, each animal was euthanized with Euthasol (200 mg/kg ip). The experimental protocol was approved by the Thomas Jefferson University Institutional Animal Care and Use Committee.
Dry and wet corneal stimulation.
After the RF was located, the discharge rate of each unit was recorded during two conditions of corneal fluid status. The wet cornea condition (5 min) occurred when the cornea was moistened with 100–200 μl of rat artificial tears (ATs) via a sterile pipette dropped into a plastic well that enclosed an entire anterior eye. The dry cornea condition (1–2 min) occurred after the well was detached from the eye and the excess ATs were removed with a piece of filter paper. There were two types of stimulation protocols: short (5 min) drug application and prolonged (1 h) drug application. In the short experiments (see Figs. 5 and 7A), each stimulus pair (wet and dry stimulus) was presented three times before the antagonists or their vehicles were applied. Antagonists were left on the cornea for 5 min and then removed with a filter paper to provide the dry stimulus for 1–2 min. After washing with ATs (∼5 ml), the same stimulus pairs were again applied three times to determine the recovery from the drug effects. In the prolonged experiments (see Figs. 6A and 7C), each stimulus pair was applied three times before the drug, and then the ocular surface was moistened with the drug for 5 min. The drug was then removed to provide the dry stimulus for 1–2 min, which was followed by reapplication of the drug (instead of washing the drug) for 15 min (i.e., total duration of 20 min of drug application). Subsequently, the drug was reapplied two more times, each lasting 20 min and interspersed with 1–2 min of dry stimuli. The experimental room was air-conditioned with a heating, ventilation, and air conditioning (HVAC) system at ∼21°C and maintained at ∼40% humidity throughout the experiment.
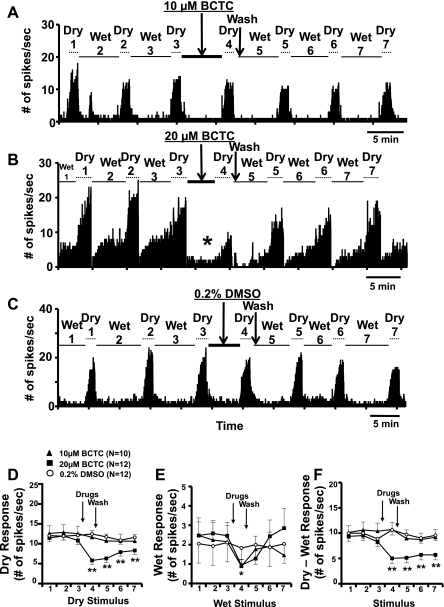
Fig. 5.
A–C: PSTHs in response to series of dry and wet stimuli before and after short applications (5 min) of the N-(4-tert-butylphenyl)-4-(3-chloropyridin-2-yl)piperazine-1-carboxamide (BCTC) and the vehicle solutions from 3 CS corneal afferents. Note the decrease in wet response after 20 μM BCTC (asterisk in B). The brief increases in spike activity at the beginning and end of dry stimuli due to artifacts and wetting of the cornea (cold stimulation, see materials and methods), which were present in original data, were removed for clarity in these PSTHs. D–F: average dry, wet, and “dry-wet” responses before and after BCTC and vehicle (DMSO). Numbers on x-axis (stimulus number) represent the repeated stimuli shown in A–C. *P < 0.05; **P < 0.01 vs. stimulus 3.
Fig. 7.
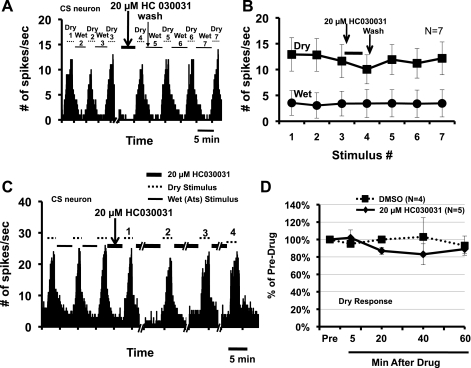
A: PSTH of a CS neuron in response to series of wet and dry stimuli before and after short ocular application of the TRPA1 antagonist 20 μM HC030031. B: average dry (squares) and wet responses (circles) before and after HC030031 (6 CS neurons and 1 CI neuron). C: PSTH of a CS neuron in response to series of wet and dry stimuli during the repeated, prolonged ocular application of 20 μM HC030031. Stimuli 1, 2, 3, and 4 indicated above the dotted lines are, respectively, the dry stimuli presented after 5, 20, 40, and 60 min of HC030031. D: average dry responses to prolonged HC030031 (4 CS neurons and 1 CI neuron) and vehicle (DMSO; 3 CS neurons and 1 CI neuron) applications.
Fig. 6.
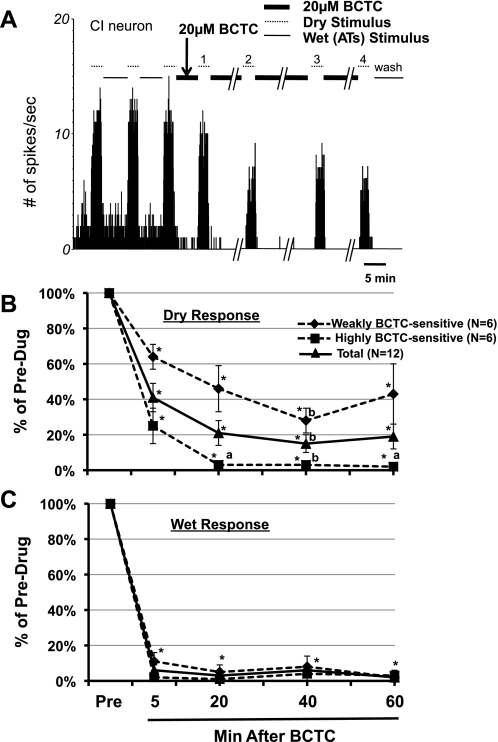
A: PSTH of a CI afferent in response to dry and wet stimuli during the repeated, prolonged BCTC applications. After 3 pre-BCTC control stimuli, BCTC (thick lines) was presented for a total duration of 1 h except when the dry stimuli (dotted lines) were applied. There was no washout throughout the test series until after the last dry stimulus (stimulus 4). Stimuli 1, 2, 3, and 4 indicated above the dotted lines are, respectively, the dry stimuli presented after 5, 20, 40, and 60 min of BCTC. B and C: %changes in dry (B) and responses wet (C) after prolonged BCTC applications. *P < 0.0001 vs. predrug control response (to 3rd dry or wet stimuli). aP < 0.01; bP < 0.05 vs. dry response (stimulus 1) after 5 min of BCTC.
Chemical stimulation.
Chemical stimuli (50 μM menthol, 585 mOsm mannitol) were applied to the ocular surface in 100- to 200-μl solutions dropped into the plastic well with a sterile pipette. These were left on the cornea for 30–40 s. The chemical solutions were then washed by flushing the eye with copious amounts of ATs (∼5 ml). Ten minutes later, the ATs were removed with a filter paper to be replaced with the antagonist to bathe the anterior eye for 5 min. The antagonist solutions were then removed and replaced with the cocktail solutions of antagonist and chemical stimuli for 30–40 s. Fifteen minutes after the cocktail solution was washed, the chemical stimuli without antagonists were again applied into the well to assess the recovery from the antagonist (see Fig. 8A). The concentrations of chemical stimuli were chosen because they represented the middle range of dose-response functions previously observed (Hirata and Meng 2010). Menthol (Sigma-Aldrich) was dissolved in 40% ethanol to make a 10 mM stock solution, which was then diluted to the required concentrations with ATs on the day of the experiment. The solutions of mannitol (Sigma-Aldridge) were prepared by dissolving them in ATs, and their osmolarities were measured with an osmometer (μ-OSMETTE; Precision System, Natick, MA). The composition of ATs (in mM) was 106.5 NaCl, 26.1 NaHCO3, 18.7 KCl, 1.0 MgCl2, 0.5 NaH2PO4, 1.1 CaCl2, and 10 HEPES, pH 7.45 (Kessler et al. 1995).
Fig. 8.
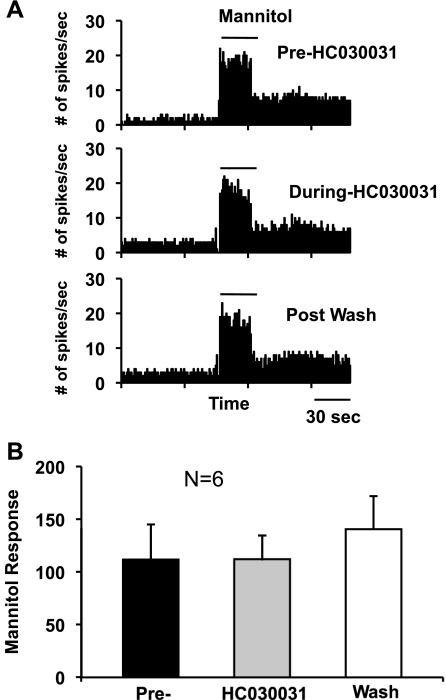
A: PSTHs from another CS neuron in responses to hyperosmolar stimuli (585 mOsm mannitol) before (pre), during, and after (postwash) HC030031. Artifacts and the responses to cooling when mannitol was applied and washed (see materials and methods) were removed for clarity. B: average graphs for the mannitol responses before, during, and after HC030031 (6 CS neurons).
Thermal stimulation.
Temperature stimuli were applied to the ocular surface via fluids that bathed the anterior eye. The temperatures of the fluids were regulated by a Peltier-based device (temperature controller; Warner Instruments, Hamden, CT) placed between the reservoir and the plastic well that enclosed the anterior eye. The fluids descended from the reservoir (60-ml syringe) by gravity via polyethylene tubing through the temperature controller down into the plastic well and escaped from the well into the collecting flask situated underneath the animal. Because the flow rates of the fluids depended on gravity and the temperature changes depended on the flow rates, the fluids filled the reservoir at the same height each time the temperature changes were initiated to ensure the same rates of flow. In the steady-state (SS) temperature series, cooling stimuli were presented to the cornea beginning with a 31°C adapting temperature, followed by consecutive 2°C incremental decreases in temperature to 10–11°C, followed in some experiments by a final temperature of 6–7°C, with each temperature lasting for 60 s (see Fig. 1). The rates of temperature change were, on average, 0.15°C/s (range 0.13–0.18°C/s).
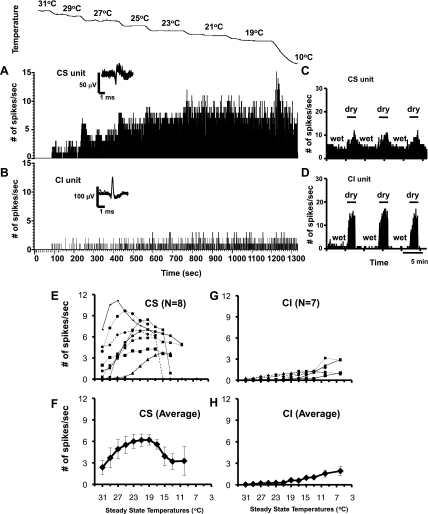
Fig. 1.
A–D: peristimulus time histograms (PSTHs) showing responses to steady-state (SS) temperatures (A and B) and to dry and wet stimuli (C and D) applied to the cornea in cold-sensitive (CS) and cold-insensitive (CI) corneal afferents. CS and CI units were recorded simultaneously from the same microelectrode but were discriminated by the shape of the waveforms (insets above PSTHs, 10 superimposed spikes) using software. The records in A and C were from 1 unit; those in B and D were from another unit. The timescale in B applies also to A. E–H: individual (E and G) and averaged (F and H) SS temperature-response profiles of CS and CI corneal afferents. Average graph values are means ± SE.
Drugs.
The drugs were the TRPM8 antagonist BCTC (Biomol International, Plymouth Meeting, PA) and the TRPA1 antagonist HC030031 (Tocris Bioscience). BCTC and HC030031 were dissolved in 100% dimethyl sulfoxide (DMSO) to make 10 mM stock solutions, aliquoted, and kept in a −20°C freezer until the day of the experiment. A 10 mM solution was diluted to the desired concentration with ATs on the day of the experiment. Normally, one antagonist was applied to the ocular surface per neuron, and only one neuron was studied per animal, unless two simultaneously recorded neurons were encountered in an animal (Fig. 1, A and B). When two antagonists or two concentrations of an antagonist were used per unit, at least 1 h elapsed between drug applications, since the drug effects faded away within 1 h (see for example Fig. 5). Capsaicin (Sigma) was dissolved in ethanol and Tween 80 to prepare a 10 mM solution and then diluted to the desired concentration with ATs.
Data analysis.
Neural discharges were analyzed based on 1-s bins acquired with Spike2 software. The discharge rates (no. of spikes/s) during dry corneal conditions (dry responses) were based on the averages of the last 30 s of a 1- to 2-min dry cornea period. This period was chosen because our previous studies (Hirata and Meng 2010) showed that the dry responses after the ATs were removed (start of the dry period) gradually increased over ∼30 s to a more stable level thereafter. The discharge rates during wet cornea conditions (wet responses) were based on the averages of 30 s immediately preceding the beginning of the dry periods. The chemical stimuli (menthol, mannitol) were kept at RT (21–23°C, which was colder than the average corneal temperature of the rats of 31–33°C). Therefore, when applied to the ocular surface, these stimuli evoked responses attributable to both “cold” and chemical stimulation of the ocular surface. Thus the evoked responses to chemical stimuli in this study were defined as the total number of spikes during the stimulus minus the activity produced by the vehicle (ATs or antagonist solutions). The activity during the SS cooling series (Fig. 1) was calculated based on the last 30 s of each of the 1-min temperature stimuli. This period was chosen because it represented the SS discharge rates after the dynamic responses to temperature changes subside. Statistical analyses for the effects of the drugs (antagonists) on neural discharges were performed with ANOVA (GraphPad Prism5) with or without repeated measures. Post hoc analyses were done with a Bonferroni multiple comparison test for individual comparisons. We also used t-tests to evaluate two-sample populations.
RESULTS
CS vs. CI corneal afferents.
We found a total of 103 corneal afferents from 91 animals that were excited by drying of the cornea. Of these, 90 neurons (87%) responded to ocular application of the RT saline solutions (21–23°C), and their RFs could be easily identified as restricted to the cornea with an ice-cold metal probe; they were designated as CS neurons. In addition, the present study found 13 neurons (13%) that were unresponsive to the RT saline eye drops used for the initial attempt to identify a unit (see materials and methods), and their RFs were difficult to identify due to their diminished or missing sensitivities to the ice-cold metal probe used to locate them on the cornea; they were classified as CI neurons. However, in 3 of these neurons, the RFs could be located within the corneal-limbus border with an ice-cold probe, whereas the other 10 could not be driven by this stimulus. The CI neurons were found because of their high discharge rates during the dry corneal conditions and near silence during the wetting of the cornea.
To assess possible functional significance of CS and CI afferents, we recorded their responses to different ocular stimuli as shown in Figs. 1–3. Figure 1 illustrates the sharply contrasting response profiles to temperature stimuli and dry and wet stimuli applied to the ocular surface. Figure 1, A and B, shows the peristimulus time histograms (PSTHs) of responses to 2°C step cooling in one CS and one CI afferent. This CS neuron displayed dynamic and static responses observed in a typical innocuous cold thermoreceptor (Hensel 1974), whereas the CI neuron showed very little dynamic or static discharge throughout the temperature range (31–10°C). Figure 1, C and D, illustrates the differences in their responses to drying and wetting of the cornea. During the wet cornea condition, there was considerable discharge in this CS neuron, but only a trickling of the firing was seen in the CI neuron. The average and individual temperature responses for 8 CS and 7 CI neurons (3 of these responded to the ice-cold probe) are shown in Fig. 1, E–H. Unlike the CS neurons, none of the CI neurons displayed noticeable activity (∼1 spikes/s) until the temperatures reached extreme cold (<10–13°C). These temperatures were well below that of the RT saline solutions (21–23°C) and therefore could not excite CI afferents, as described above.
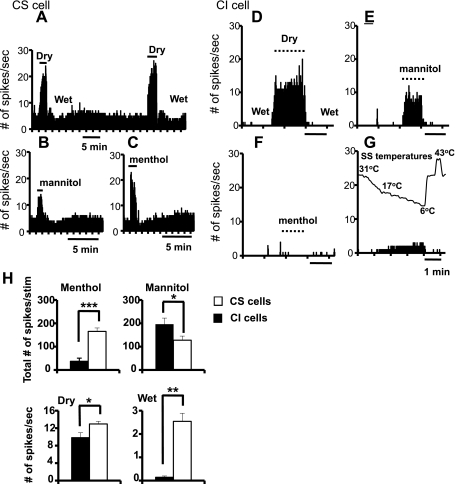
Fig. 2.
PSTHs of a CS (A–C) and a CI corneal afferent (D–G) in response to a variety of ocular stimuli. Notice that there was little or no response to menthol and temperatures, whereas the responses to dry and hyperosmolar stimuli were substantial. H: average graphs showing the differences in response to 4 types of ocular stimuli in CS and CI corneal afferents. The sample sizes for CI and CS units, respectively, were 6 and 25 for menthol (50 μM), 6 and 15 for mannitol (585 mOsm), and 10 and 60 for dry and for wet responses. *P < 0.05; **P < 0.01; ***P < 0.001.
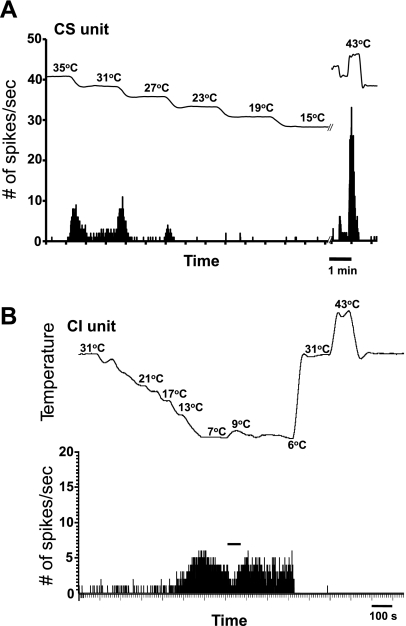
Fig. 3.
A: PSTH of a “dynamic response only” CS corneal afferent in response to 4°C step cooling of the cornea from 35°C to 15°C. Note the exaggerated response to heat. B: PSTH of a CI neuron in response to cooling and heating applied to the cornea. This corneal afferent was virtually unresponsive to cooling stimuli until the temperatures were approximately <13°C. Notice the inhibition of activity by warming from 7°C to 9°C (indicated by solid horizontal line).
Figure 2 shows further the comparisons between CI and CS neurons in their responses to four types of ocular stimuli. The average graphs shown in Fig. 2H demonstrate that the responses to menthol were considerably weaker for the CI neurons than for the CS neurons (P = 0.0003, 2-tailed t-test): 31.75 ± 21.27 spikes/stimulus for CI neurons (n = 6) vs. 169.71 ± 17.41 spikes/stimulus for CS neurons (n = 25). By contrast, the responses to mannitol (the hyperosmolar stimulus) were marginally greater for the CI neurons than for the CS neurons (P = 0.0480, 2-tailed t-test): 227.67 ± 28.59 spikes/stimulus for CI neurons (n = 6) vs. 124.5 ± 17.24 spikes/stimulus for CS neurons (n = 15). Furthermore, the dry response was slightly larger for the CS neurons than for the CI neurons (P = 0.0461, 2-tailed t-test): 12.97 ± 0.60 spikes/s for CS neurons (n = 60) vs. 9.83 ± 1.13 spikes/s for CI neurons (n = 10), whereas the response to the wet stimulus was much greater for the CS neurons than for the CI neurons (P = 0.0064, 2-tailed t-test): 2.54 ± 0.35 spikes/s for CS neurons (n = 60) vs. 0.14 ± 0.06 spikes/s for CI neurons (n = 10).
There were also differences in their responses to heat (∼43°C): all 6 CS units responded (“paradoxical” responses) (Long 1977; Parra et al. 2010), but none of 5 CI units had responses to this stimulus. The examples are shown in Fig. 3. Interestingly, despite the relative insensitivity to cold stimuli among the CI neurons depicted in Fig. 1, their response to warming was similar to that of CS neurons: it inhibited the firing (Fig. 3B). Also similar to the CS neurons reported previously (Hirata and Meng 2010), the CI neurons did not exhibit a mechanical sensitivity. Moreover, 6 CS neurons and 1 CI neuron were tested with 10 μM capsaicin: 5 CS neurons and 1 CI neuron did not respond to capsaicin, whereas 1 CS neuron showed responses to capsaicin (data not shown). In addition, as reported earlier (Hirata and Meng 2010), one CS unit showed a “dynamic response only” profile to the temperature stimuli with little or no SS discharge (Fig. 3A). This unit showed disproportionately large responses to the 43°C heat stimulus compared with the cooling responses (Fig. 3A), but capsaicin was not tested. Furthermore, the patterns of discharge were not affected by various stimuli or antagonists (Fig. 2, A–G) except for the bursting patterns occasionally observed during cold stimulation in some CS neurons as reported earlier (Hirata and Meng 2010). These observations indicate that the CS neurons, whose response characteristics are consistent with those of classic innocuous “cold” thermoreceptors, are better suited for small temperature discrimination, perhaps utilizing TRPM8 as a molecular sensor, than CI neurons.
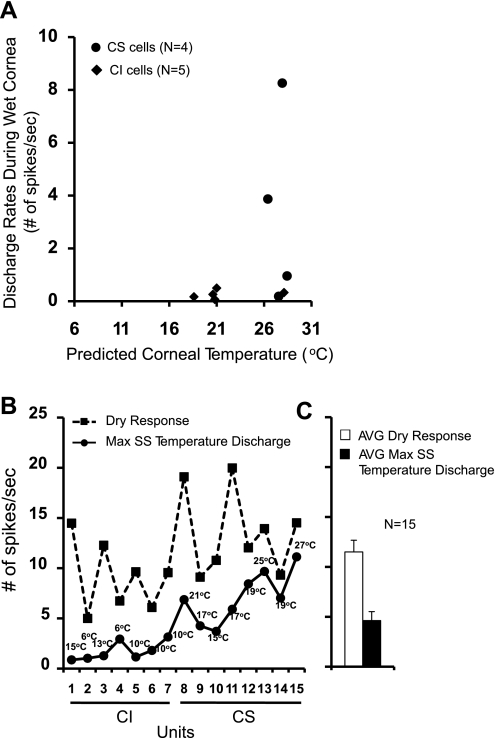
Predicting the corneal temperatures during wet and dry cornea conditions.
To determine if the corneal temperatures are the exclusive determinant of the dry and the wet responses in the CS and CI afferents, we predicted the corneal temperatures during wet or dry corneal conditions by comparing for each unit the average 30-s discharge rates during the SS temperature stimuli to the wet or dry responses. If the dry or wet responses were driven by the temperature fluctuations of the corneal surface alone during this period, the discharge rates of the dry or wet responses should match those produced by temperature stimulation of the cornea. For example, comparison of the discharge rates between Fig. 1, A and C, indicates that the discharge rate during the wet corneal condition (3.73 spikes/s) was similar to the rate during 27°C SS temperature (2.94 spikes/s) for this CS unit: the exact bath temperature at which the wet response (3.73 spikes/s) would have been observed was extrapolated to be 26.3°C. In this manner, the corneal temperatures during the wet and dry corneal conditions could be predicted in 9 of 15 units in response to wet conditions (Fig. 4A) and in none of 15 afferents in response to dry conditions (Fig. 4B). Figure 4A shows that the predicted corneal temperatures during the wet cornea conditions appear to cluster around 18–21°C and 26–28°C. However, the exact corneal temperatures could not be determined for 2 CI units because the same discharge rates during the wet cornea (0 spikes/s) were observed at temperatures between 31 and 21°C. Also, the corneal temperatures could not be established in 4 CS afferents because their rates during the wet cornea states were much higher than those observed at any SS temperature tested. This was also the reason for all 15 units (8 CS and 7 CI neurons) whose corneal temperatures during the dry cornea conditions could not be predicted (Fig. 4B): the dry responses of all 15 units were considerably higher than the discharge rates to SS temperature stimuli. Figure 4B also shows that the optimum temperatures that produced maximum discharges were below 15°C for all CI neurons and above 15°C for all CS neurons, justifying the partition of these neurons into two classes. The average rates for the responses to temperature and dry stimuli are shown in Fig. 4C (11.5 ± 1.17 spikes/s for the dry response and 4.62 ± 0.90 spikes/s for the SS temperature response). The SS temperature response accounted for only 28% of the dry response. In addition, one CS unit, which displayed only the dynamic responses to temperature changes, had a substantial dry response (10.97 spikes/s) but little or no SS discharge rate at any temperature (Fig. 3A). These results indicate that although the wet responses may be accounted for by the corneal temperature fluctuations in the majority of the afferents, the dry responses could not have been produced solely by the temperature stimulation of the cornea. The additional mechanisms would have to be invoked to explain the totality of the dry responses.
Fig. 4.
A: relationship between the discharge rates during the wet cornea (wet responses) and the predicted corneal temperatures, which would have produced the wet responses. The corneal temperature during the wet condition was predicted for each neuron from the data shown in Fig. 1, E and G. B: relationship between maximum SS temperature responses and the dry responses for 7 CI and 8 CS corneal afferents. CI and CS units are numbered as indicated on the x-axis. The temperatures shown above circles indicate the temperatures at which the maximum discharge rates were observed. C: average dry and SS temperature responses of 7 CI and 8 CS neurons taken from B. The vertical scale in B applies also to C. Max, maximum.
Effects of BCTC on dry and wet responses.
To ascertain the molecular mechanisms underlying the dry and wet responses of CS and CI neurons, we applied the TRPM8 antagonist BCTC to the ocular surface to attempt to block the responses to drying and wetting of the cornea. Figure 5, A–C, shows the examples from three CS corneal afferents in responses to a series of drying and wetting of the cornea before and after a short (5 min) application of BCTC and its vehicle, DMSO. As shown, 20 μM but not 10 μM BCTC was able to decrease significantly the responses to both drying (dry response: P < 0.0001, 1-way ANOVA) and wetting (wet response: P < 0.0035, 1-way ANOVA) of the cornea. The average reductions by 20 μM BCTC were 45% (Fig. 5D) from the predrug control responses to the 3rd dry stimulus (range 14–104%) and 54% (Fig. 5E) from the responses to the 3rd wet stimulus (range 0–123%). The average activities recovered to 74% of the predrug level for the dry response and 95% for the wet response within 15 min after the wash. Although neither 10 μM BCTC nor 0.2% DMSO attenuated the dry responses significantly (P > 0.05, 1-way ANOVA), the wet responses were decreased greatly by 10 μM BCTC (P < 0.0180, 1-way ANOVA), as shown in Fig. 5E (compare the responses to wet stimulus 3 vs. 4 in Fig. 5B). When the wet responses were taken into account in calculating the evoked responses to drying of the cornea (i.e., subtracting the wet from the dry responses), the overall effects of BCTC were similar (Fig. 5F). There was one CI unit in each of the 10 and 20 μM BCTC groups, whereas no CI unit was in the DMSO group. The effects of BCTC on dry responses in these CI units were similar to the those in CS units (−29% with 20 μM and −14% with 10 μM BCTC), and the statistical results were the same whether the CI neurons were included or not.
To assess the degree to which the poor drug access might have contributed to the failure of short (5 min) BCTC application to completely abolish the dry responses, we applied and reapplied 20 μM BCTC for a total duration of 1 h in an effort to block the dry responses in 12 separate afferents. We divided these neurons into two types depending on whether the dry responses were above (weakly BCTC sensitive) or below (highly BCTC sensitive) 20% of the predrug level after 20-min BCTC application. Figure 6 shows that the prolonged application of BCTC did produce progressive decreases in dry responses over 5–60 min in all neurons, but a substantial portion (43% of the predrug level) still remained in one-half of the afferents tested (weakly BCTC sensitive) after 1 h. Interestingly, of the six weakly BCTC-sensitive neurons, three were CI afferents. Figure 6B shows that the average dry responses after 5, 20, 40, and 60 min of BCTC applications were, respectively, 45%, 24%, 16%, and 22% of the predrug level [P < 0.0001 vs. predrug dry responses (3rd dry response in Fig. 6A), 1-way ANOVA with repeated measure]. Figure 6B also demonstrates that the average dry responses after 20 and 60 min of BCTC application were not different from the dry responses after 5 min of BCTC application. By contrast, Fig. 6C shows that the wet response was virtually silenced by 20 μM BCTC in all neurons even after 5 min of application and remained quiet over 20–60 min of BCTC presence. The average wet responses after 5, 20, 40, and 60 min of BCTC applications were, respectively, 6%, 3%, 6%, and 2% of the predrug level (P < 0.0001 vs. predrug dry responses, 1-way ANOVA with repeated measure). The results from short and long BCTC application demonstrate that TRPM8 channel activation may not explain all of the dry response magnitudes, whereas the wet responses presumably are mediated entirely by the TRPM8.
Effects of HC030031 on dry and mannitol responses.
To determine if additional mechanisms besides TRPM8 might be responsible for the production of the dry and wet responses, we applied a specific TRPA1 antagonist, HC030031, to the ocular surface to attempt to block these responses. Figure 7 shows that both short (5 min, Fig. 7, A and B) and long (1 h, Fig. 7, C and D) application of HC030031 had no effect on the responses to drying or wetting of the cornea (P > 0.05, 1-way ANOVA with repeated measure). For the 5-min application, the average decreases in dry and wet responses (to 4th stimuli) by HC030031 were 13% and 11% from the control (to 3rd stimulus), respectively. For a long application, although there was a small progressive decline in the dry responses after a 20- to 60-min application (11–17% decreases), this was not statistically significant compared with either the predrug level (P > 0.05, 1-way ANOVA with repeated measure) or the DMSO control group (P > 0.05, 2-way ANOVA with repeated measure). Similar comparisons for the wet responses showed that HC030031 was also without a significant effect (P > 0.05, 1- or 2-way ANOVA with repeated measure; data not shown).
In addition, because we hypothesized previously (Hirata and Meng 2010) that the dry responses might derive partly from the hyperosmolar stimulation of the cornea, we tested the responses to mannitol application before and after HC030031. Figure 8 demonstrates that HC030031 had no significant influence on the responses to hyperosmolar stimuli (585 mOsm mannitol: P = 0.3952, 1-way ANOVA with repeated measure). The average response magnitudes to hyperosmolar stimuli before, during, and after HC030031 (washout) were 111.7 ± 33.3, 112 ± 22.6, and 241.8 ± 85.2 spikes/stimulus, respectively.
DISCUSSION
The present study found that the corneal afferents that detect dryness of the cornea could be divided into two types based on their temperature sensitivities: CS and CI neurons. Although the biophysical and electrophysiological properties of CS and CI had been described previously for the cultured TG cell population (Viana et al. 2002), to our knowledge the present study is the first to report the presence of CI neurons among the corneal afferents. These two classes of neurons in the present study were distinguished by their ability (CS) or inability (CI) to respond clearly to RT (21–23°C) saline drop and an ice-cold probe. The CI neurons in our study are very similar to the CI neurons defined by Viana et al. (2002) in not responding to a ramp cooling to ∼19°C or to menthol, although their later study (Madrid et al. 2009) demonstrated that some of these CI neurons became CS when an extreme cooling stimulus down to ∼8°C was employed.
Previously, we reported that a special class of corneal afferents was excited by drying of the cornea as well as by other types of ocular stimuli thought to be essential for production of basal tears, such as gentle cooling of the cornea, evaporation, and hyperosmolarity of the tears (Hirata and Meng 2010). These response characteristics and their additional sensitivities to menthol are all consistent with those of classic innocuous “cold” thermoreceptors found in the orofacial skin (Hensel 1974; Poulos and Lende 1970) and fall into the present classification of CS afferents. Initially, we (Hirata and Meng 2009, 2010) and others (Parra et al. 2010) proposed that activation of a mild temperature sensor by cooling in the CS neurons, particularly the TRPM8 channels, is crucial to the production of basal tears. However, the finding of CI afferents in the present study that were also robustly activated by drying of the cornea but displayed little or no sensitivity to normal ambient temperatures indicates that a fluctuation of the corneal temperature may not be the sole stimulus for evoking basal tears. This conclusion is supported by our observation that a maximum firing of a neuron at its optimum SS corneal temperature fell far short of the discharge rates evoked by drying of the cornea (Fig. 4). Had the corneal temperatures been the only determinant of the dry responses, the discharge rates produced by these two stimuli would have been identical. The SS temperature responses averaged over the 30-s period were chosen for comparison with the dry responses over the same period for each neuron partly because the amounts of basal tears are typically measured during 30 s to 2 min of ocular surface exposures in animals (Barabino and Dana 2004).
Furthermore, our assertion that the cooling stimulus may only be a part of the stimulus that drives these innocuous “cold” thermoreceptors to drying of the cornea is also consistent with our observation that an application of the saturating concentration of BCTC (20 μM) failed to completely abolish the neural excitation produced by drying of the cornea (Figs. 5 and 6). The blocking ability of BCTC on the cooling responses has not been consistent, however. Previous studies reported that 10 μM BCTC did not reduce the cold-evoked responses of the majority of the guinea pig corneal nerve endings, although it completely blocked the cold-evoked responses of TG neurons (Madrid et al. 2006). However, in approximately one-third of the corneal nerve endings in that study, BCTC produced a small but statistically significant decrease in cold-evoked discharges. In our study, a substantial portion (∼40%) of the dry responses of some CS and all CI neurons (weakly BCTC-sensitive afferents) remained even after prolonged application of BCTC (Fig. 6), whereas other neurons (highly BCTC-sensitive afferents) were affected greatly by a 1-h BCTC application. It has been argued that this variability might be the result of a poor permeability of the compound across the corneal epithelia (Parra et al. 2010) or variable depths of the receptors at the nerve endings (Parra et al. 2010). Thus the possibility that an incomplete effect of BCTC on the dry response in our study may be due to a drug access problem cannot be excluded. However, it is important to note that in the present study, the wet responses were nearly completely blocked by BCTC after a short application (Fig. 6), suggesting that the drug might have effectively permeated to the receptor sites. Use of a more specific TRPM8 antagonist or TRPM8-deficient transgenic mice might resolve these issues in the future.
Previously, we (Hirata and Meng 2010) proposed that drying of the cornea leads to gentle cooling and an ensuing increase in osmolarity of the tears as the tears evaporate from the corneal surface, and that the response to cooling of the cornea is mediated by TRPM8 channel activation and the response to hyperosmolar stimulus by TRPA1 channel activation. Although our result of BCTC attenuating the dry response by ∼45–80% is consistent with TRPM8-mediated processes, the effect of HC030031 on the dry response failed to support the latter hypothesis (Fig. 8). Some evidence indicates that this hyperosmolar tear-induced activation of the corneal afferents may be based on the TRPV1 receptors and not on TRPA1. The arginine- vasopressin-containing neurons in the hypothalamus are sensitive to an increase in osmolarity of extracellular fluid (330–360 mOsm), presumably to regulate body fluids in animals (Oliet and Bourque 1993, 1996). Activation of these osmosensory neurons to osmotic stimuli was absent in TRPV1-KO mice (Ciura and Bourque 2006; Sharif Naeini et al. 2006). Also, an increased afferent renal nerve activity produced by hypertonic saline (600 mOsm) was blocked by a TRPV1 antagonist, capsazepine, when perfused into the renal pelvis (Zhu et al. 2007). However, BCTC is also an effective blocker of TRPV1 channels (Valenzano et al. 2003) and should have produced some effect on the dry response. Thus the mechanisms underlying the responses to hyperosmolar stimulus are yet to be elucidated.
A revelation of the mechanisms underlying the dry response may come from the studies of CI afferents, which we were not able to investigate in any significant manner in the present study. For example, we found that the CI neurons were much more resistant to the TRPM8 antagonist (Fig. 6B) than CS neurons, suggesting that the greater portion of the dry responses in the CI neurons may be mediated by non-TRPM8 (hyperosmolar stimulus based) mechanisms than the low-threshold CS afferents. This view is consistent with our present observation that CI neurons were more strongly activated by a hyperosmolar stimulus than CS neurons (Fig. 2H). Evaporation during ocular dryness is expected to lead to local spots of hyperosmolarity (Kimball et al. 2010; King-Smith et al. 2008), which can then become a source of ocular irritation and discomfort (Wolkoff 2010). These non-TRPM8 mechanisms may play a significant role in the ocular sensations encountered in dry eye (DE) patients. It is entirely possible that CI (and not CS) neurons may become a major player in the processing of the dryness sensation under pathological conditions such as inflammation or in the aged population. It has been known for some time that mechanically insensitive cutaneous afferents become mechanically sensitive under inflammatory conditions (Andrew and Greenspan 1999; Davis et al. 1993) or develop spontaneous activity in neuropathic states in humans (Orstavik et al. 2003). Another important function for the CI neurons may be the mediation of tearing under extremely cold environment, since these neurons do not become active until the ambient temperature reaches below ∼10–13°C (Fig. 3B).
Although it is almost universally accepted that activity of the CS neurons, especially of those that had been classified as low-threshold CS neurons (Madrid et al. 2009), leads to a sensation of gentle cooling via a TRPM8 channel activation (Bautista et al. 2007; Colburn et al. 2007; Dhaka et al. 2007; McKemy et al. 2002), there may be an additional intriguing possibility that the temperature sensation carried by CS neurons may in fact be interpreted also as an ocular “wetness.” Over one hundred years ago, Thunberg (1905) described a sensation of a “liquid” when the forehead is stimulated with a cold substance for an interval of 20 s (Bershansky 1923; Carnahan et al. 2010). Similarly, cold tears on the ocular surface caused by evaporation could feel “wet.” The ocular wetness sensation, however, is rarely reported in normal humans, perhaps because the sensation has adapted due to a continual presence of the basal tears. The converse (dryness), however, appears to be a very powerful sensation and is one of the major complaints among DE patients (Begley et al. 2003). It is possible that a lack of tears in DE patients, and therefore a lack of the wetness sensation, can be perceived as “dry” and that an increased activity of the CS afferents may serve as a neural substrate for the “dryness” sensation (Belmonte and Gallar 2011).
In conclusion, the present study found two populations of corneal afferents that were strongly excited by drying of the cornea. The responses of these afferents to ocular dryness are likely mediated by both TRPM8 and non-TRPM8 mechanisms. Furthermore, the CI neurons were more strongly activated by hyperosmolar tears than the CS neurons, whereas the CS neurons were much more vigorously excited by cooling and menthol than CI neurons. Because these two stimuli (cooling and increased osmotic pressure) are intimately involved in the process of drying and both CI and CS neurons respond to drying of the cornea, we hypothesize that both afferents are germane to the ocular dryness-related functions such as tearing, dryness sensation, and eye blink. Unraveling of the mechanisms underlying these functions is an important step toward understanding the cellular and molecular processes that culminate in the dry eye disease.
GRANTS
This work was supported by the Thomas Jefferson University Pilot Research Award and National Eye Institute Grant EY020667.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: H.H. conception and design of research; H.H. performed experiments; H.H. analyzed data; H.H. interpreted results of experiments; H.H. prepared figures; H.H. drafted manuscript; H.H. and M.L.O. edited and revised manuscript; H.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Manuel Cavarrubias for reading the manuscript and Dr. Richard Horn for loaning us the temperature controller.
REFERENCES
- The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye Workshop (2007). Ocul Surf 5: 75–92, 2007 [DOI] [PubMed] [Google Scholar]
- Andrew D, Greenspan JD. Mechanical and heat sensitization of cutaneous nociceptors after peripheral inflammation in the rat. J Neurophysiol 82: 2649–2656, 1999 [DOI] [PubMed] [Google Scholar]
- Barabino S, Dana MR. Animal models of dry eye: a critical assessment of opportunities and limitations. Invest Ophthalmol Vis Sci 45: 1641–1646, 2004 [DOI] [PubMed] [Google Scholar]
- Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, Jordt SE, Julius D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 448: 204–208, 2007 [DOI] [PubMed] [Google Scholar]
- Begley CG, Chalmers RL, Abetz L, Venkataraman K, Mertzanis P, Caffery BA, Snyder C, Edrington T, Nelson D, Simpson T. The relationship between habitual patient-reported symptoms and clinical signs among patients with dry eye of varying severity. Invest Ophthalmol Vis Sci 44: 4753–4761, 2003 [DOI] [PubMed] [Google Scholar]
- Belmonte C, Gallar J. Cold thermoreceptors, unexpected players in tear production and ocular dryness sensations. Invest Ophthalmol Vis Sci 52: 3888–3892, 2011 [DOI] [PubMed] [Google Scholar]
- Bershansky L. Thunberg's illusion. Am J Psychol 34: 291–295, 1923 [Google Scholar]
- Carnahan H, Dubrowski A, Grierson LE. Temperature influences both haptic perception and force production when grasping. Int J Ind Ergon 40: 55–58, 2010 [Google Scholar]
- Ciura S, Bourque CW. Transient receptor potential vanilloid 1 is required for intrinsic osmoreception in organum vasculosum lamina terminalis neurons and for normal thirst responses to systemic hyperosmolality. J Neurosci 26: 9069–9075, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colburn RW, Lubin ML, Stone DJ, Jr, Wang Y, Lawrence D, D'Andrea MR, Brandt MR, Liu Y, Flores CM, Qin N. Attenuated cold sensitivity in TRPM8 null mice. Neuron 54: 379–386, 2007 [DOI] [PubMed] [Google Scholar]
- Davis KD, Meyer RA, Campbell JN. Chemosensitivity and sensitization of nociceptive afferents that innervate the hairy skin of monkey. J Neurophysiol 69: 1071–1081, 1993 [DOI] [PubMed] [Google Scholar]
- Dhaka A, Murray AN, Mathur J, Earley TJ, Petrus MJ, Patapoutian A. TRPM8 is required for cold sensation in mice. Neuron 54: 371–378, 2007 [DOI] [PubMed] [Google Scholar]
- Dogru M, Tsubota K. Pharmacotherapy of dry eye. Expert Opin Pharmacother 12: 325–334, 2011 [DOI] [PubMed] [Google Scholar]
- Hensel H. Thermoreceptors. Annu Rev Physiol 36: 233–249, 1974 [DOI] [PubMed] [Google Scholar]
- Hirata H, Meng ID. A special type of primary afferent sensory neurons plays a critical role in tear production: implications for dry eye disease. Invest Ophthalmol Vis Sci 50: ARVO E-Abstract 1172, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata H, Meng ID. Cold-sensitive corneal afferents respond to a variety of ocular stimuli central to tear production: implications for dry eye disease. Invest Ophthalmol Vis Sci 51: 3969–3976, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler TL, Mercer HJ, Zieske JD, McCarthy DM, Dartt DA. Stimulation of goblet cell mucous secretion by activation of nerves in rat conjunctiva. Curr Eye Res 14: 985–992, 1995 [DOI] [PubMed] [Google Scholar]
- Kimball SH, King-Smith PE, Nichols JJ. Evidence for the major contribution of evaporation to tear film thinning between blinks. Invest Ophthalmol Vis Sci 51: 6294–6297, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King-Smith PE, Nichols JJ, Nichols KK, Fink BA, Braun RJ. Contributions of evaporation and other mechanisms to tear film thinning and break-up. Optom Vis Sci 85: 623–630, 2008 [DOI] [PubMed] [Google Scholar]
- Long RR. Sensitivity of cutaneous cold fibers to noxious heat: paradoxical cold discharge. J Neurophysiol 40: 489–502, 1977 [DOI] [PubMed] [Google Scholar]
- Madrid R, de la Pena E, Donovan-Rodriguez T, Belmonte C, Viana F. Variable threshold of trigeminal cold-thermosensitive neurons is determined by a balance between TRPM8 and Kv1 potassium channels. J Neurosci 29: 3120–3131, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrid R, Donovan-Rodriguez T, Meseguer V, Acosta MC, Belmonte C, Viana F. Contribution of TRPM8 channels to cold transduction in primary sensory neurons and peripheral nerve terminals. J Neurosci 26: 12512–12525, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 416: 52–58, 2002 [DOI] [PubMed] [Google Scholar]
- Oliet SH, Bourque CW. Gadolinium uncouples mechanical detection and osmoreceptor potential in supraoptic neurons. Neuron 16: 175–181, 1996 [DOI] [PubMed] [Google Scholar]
- Oliet SH, Bourque CW. Mechanosensitive channels transduce osmosensitivity in supraoptic neurons. Nature 364: 341–343, 1993 [DOI] [PubMed] [Google Scholar]
- Orstavik K, Weidner C, Schmidt R, Schmelz M, Hilliges M, Jorum E, Handwerker H, Torebjork E. Pathological C-fibres in patients with a chronic painful condition. Brain 126: 567–578, 2003 [DOI] [PubMed] [Google Scholar]
- Parra A, Madrid R, Echevarria D, del Olmo S, Morenilla-Palao C, Acosta MC, Gallar J, Dhaka A, Viana F, Belmonte C. Ocular surface wetness is regulated by TRPM8-dependent cold thermoreceptors of the cornea. Nat Med 16: 1396–1399, 2010 [DOI] [PubMed] [Google Scholar]
- Poulos DA, Lende RA. Response of trigeminal ganglion neurons to thermal stimulation of oral-facial regions. I. Steady-state response. J Neurophysiol 33: 508–517, 1970 [DOI] [PubMed] [Google Scholar]
- Sharif Naeini R, Witty MF, Seguela P, Bourque CW. An N-terminal variant of Trpv1 channel is required for osmosensory transduction. Nat Neurosci 9: 93–98, 2006 [DOI] [PubMed] [Google Scholar]
- Thunberg T. Die Apperzeptionszeiten der Hautempfindungen. In: Handbuch der Physiologie des Menschen, edited by Nagel WA. Berlin: Braunschweig, 1905, vol. 3, p. 708–711 [Google Scholar]
- Valenzano KJ, Grant ER, Wu G, Hachicha M, Schmid L, Tafesse L, Sun Q, Rotshteyn Y, Francis J, Limberis J, Malik S, Whittemore ER, Hodges D. N-(4-tertiarybutylphenyl)-4-(3-chloropyridin-2-yl)tetrahydropyrazine-1(2H)-carbox-amide (BCTC), a novel, orally effective vanilloid receptor 1 antagonist with analgesic properties: I. in vitro characterization and pharmacokinetic properties. J Pharmacol Exp Ther 306: 377–386, 2003 [DOI] [PubMed] [Google Scholar]
- Viana F, de la Pena E, Belmonte C. Specificity of cold thermotransduction is determined by differential ionic channel expression. Nat Neurosci 5: 254–260, 2002 [DOI] [PubMed] [Google Scholar]
- Wolkoff P. Ocular discomfort by environmental and personal risk factors altering the precorneal tear film. Toxicol Lett 199: 203–212, 2010 [DOI] [PubMed] [Google Scholar]
- Zhang XF, Chen J, Faltynek CR, Moreland RB, Neelands TR. Transient receptor potential A1 mediates an osmotically activated ion channel. Eur J Neurosci 27: 605–611, 2008 [DOI] [PubMed] [Google Scholar]
- Zhu Y, Xie C, Wang DH. TRPV1-mediated diuresis and natriuresis induced by hypertonic saline perfusion of the renal pelvis. Am J Nephrol 27: 530–537, 2007 [DOI] [PubMed] [Google Scholar]