Abstract
How the brain processes signals in the presence of noise impacts much of behavioral neuroscience. Thresholds provide one way to assay noise. While perceptual thresholds have been widely investigated, vestibuloocular reflex (VOR) thresholds have seldom been studied and VOR threshold dynamics have never, to our knowledge, been reported. Therefore, we assessed VOR thresholds as a function of frequency. Specifically, we measured horizontal VOR thresholds evoked by yaw rotation in rhesus monkeys, using standard signal detection approaches like those used in earlier human vestibular perceptual threshold studies. We measured VOR thresholds ranging between 0.21 and 0.76°/s; the VOR thresholds increased slightly with frequency across the measured frequency range (0.2–3 Hz). These results do not mimic the frequency response of human perceptual thresholds that have been shown to increase substantially as frequency decreases below 0.5 Hz. These reported VOR threshold findings could indicate a qualitative difference between vestibular responses of humans and nonhuman primates, but a more likely explanation is an additional dynamic neural mechanism that does not influence the VOR but, rather, influences perceptual thresholds via a decision-making process included in direction recognition tasks.
Keywords: vestibular, semicircular canals, signal detection theory
thresholds are important because they provide an indication of performance limits and provide an assay of physiological noise. Human perceptual thresholds have been widely studied during whole-body motions (e.g., Barnett-Cowan and Harris 2009; Benson et al. 1986, 1989; Carpenter-Smith et al. 1995; Clark and Stewart 1968; De Vrijer et al. 2008; Doty 1969; Grabherr et al. 2008; MacNeilage et al. 2010; Mah et al. 1989; Mallery et al. 2010; Soyka et al. 2011; Zupan and Merfeld 2008), but we are aware of only one study that has reported vestibuloocular reflex (VOR) thresholds (Seemungal et al. 2004). This study reported VOR thresholds (0.5°/s/s) in humans that were significantly less than perceptual thresholds (1.2°/s/s) measured in the same subjects, but the broadband angular velocity ramp stimuli used do not allow an assessment of threshold dynamics (e.g., thresholds as a function of frequency). Therefore, our goals were, for the first time, 1) to assess VOR thresholds with detection theory methods that mimic those used to record human perceptual thresholds and 2) to measure VOR thresholds as a function of frequency.
Before proceeding, it is important to define precisely what we mean by threshold. We do not use the term “threshold” in the sense of an absolute (e.g., mechanical) limit below which detection is impossible (e.g., Mergner et al. 1995). Rather, we define “threshold” using the standard signal detection theory definition. Specifically, we define threshold as the level at which a signal becomes recognized relative to noise (see, e.g., Green and Swets 1966; Macmillan and Creelman 2005; Merfeld 2011). For perceptual thresholds, noise includes transduction noise and neural noise and may also include noise applied intentionally or incidentally via the stimuli. For VOR thresholds, noise additionally includes oculomotor noise.
Earlier studies have examined the frequency dependence of human vestibular perceptual thresholds. These studies have shown that human yaw angular velocity perceptual thresholds are roughly constant between ∼0.5 and 5.0 Hz (Grabherr et al. 2008) but increase substantially as frequency decreases from 0.5 to 0.05 Hz (Benson et al. 1989; Grabherr et al. 2008). The cause of this threshold pattern as a function of frequency is unclear. One potential explanation for this pattern of human perceptual thresholds is that vestibular noise (e.g., transduction noise, central or peripheral neural noise, and/or mechanical vibration of the vestibular periphery) increases at lower stimulus frequencies. One way to test this hypothesis is to measure VOR thresholds to see whether they demonstrate a similar increase at frequencies below 0.5 Hz.
Alternatively, one might hypothesize that human perceptual yaw rotation threshold dynamics result from vestibular noise that is independent of stimulation frequency with the perceptual signal high-pass filtered (Grabherr et al. 2008), since the threshold dynamics can be modeled with a high-pass filter having a cutoff frequency of 0.2 Hz. This cutoff frequency is nearly an order of magnitude greater than the cutoff frequency of the semicircular canal afferent measured in squirrel monkeys (∼0.03 Hz; see, e.g., Fernandez and Goldberg 1971). Furthermore, these perceptual threshold dynamics do not match high-pass VOR dynamics (Cohen et al. 1981; Dimitri et al. 2002; Leigh and Zee 2006; Peterka et al. 1990a; Raphan et al. 1979) that demonstrate “velocity storage”—an effect that has been modeled in several different ways (Merfeld et al. 1993; Raphan et al. 1977; Robinson 1977) that each yield a VOR system having high-pass characteristics with a cutoff frequency of ∼0.01 Hz. Finally, these threshold dynamics also do not match the dynamics of human yaw rotation perception measured by magnitude estimation (Bertolini et al. 2011; Bronstein et al. 2008; Okada et al. 1999; Sinha et al. 2008), which have been hypothesized to result from the same velocity storage mechanisms as the VOR.
These various differences raise several interesting questions. Do VOR threshold dynamics match perceptual threshold dynamics? If so, this would be consistent with the hypothesis that the magnitude of the peripheral vestibular noise changes with stimulus frequency. Alternatively, are VOR threshold dynamics over the measureable frequency range (∼0.2–3 Hz) roughly constant—matching VOR dynamics over this frequency range (e.g., Peterka et al. 1990b)? These questions highlight the importance of studying threshold dynamics. Specifically, we hypothesized that VOR thresholds are relatively constant across a broad range of frequencies—consistent with measurements of VOR dynamics at suprathreshold levels (e.g., Peterka et al. 1990b). To test this hypothesis, we measured VOR thresholds in three monkeys. Testing was conducted at five frequencies—0.2, 0.3, 1, 2, and 3 Hz—with the exact same motion device and motion trajectories as an earlier human perception study (Grabherr et al. 2008).
MATERIALS AND METHODS
We designed our study—both data collection and data analysis—to mimic the methods used in published perceptual threshold studies as closely as possible. Specifically, we designed a VOR direction recognition task to mimic a previously utilized yaw rotation direction recognition task (Benson et al. 1989; Grabherr et al. 2008). Furthermore, single cycles of sinusoidal acceleration, which have long been used for vestibular threshold research (e.g., Benson et al. 1986, 1989; Grabherr et al. 2008), were utilized as the motion stimuli. Finally, VOR threshold analysis was intentionally designed to mimic vestibular perceptual threshold analysis as closely as possible and was tailored to these single-cycle sinusoidal acceleration motion stimuli. Specifically, we designed an analysis that made one direction recognition decision (was the VOR to the left or right?) per trial to mimic the direction recognition task in which subjects report whether they rotated to the left or right after each trial.
Monkeys were never tested more than once each day. Test sessions lasted between 30 and 90 min. Session duration was primarily determined by monkey cooperation; the number of test sessions was determined by monkey cooperation and data yields. Monkeys were tested 24 (S1), 41 (S2), and 39 (S3) times. Table 1 summarizes the total number of trials.
Table 1.
Number of trials accepted and total number of trials for different operators, subjects, and frequencies
|
Naccepted/Ntotal |
|||||
|---|---|---|---|---|---|
| 0.2 Hz | 0.3 Hz | 1 Hz | 2 Hz | 3 Hz | |
| O2 (less conservative) | |||||
| S1 | 494/1,254 | 448/1,135 | 718/1,158 | ||
| S2 | 480/842 | 515/609 | 376/535 | 426/573 | 451/512 |
| S3 | 338/1,000 | 290/508 | 341/531 | 308/442 | 390/468 |
| O1 (conservative) | |||||
| S1 | 307/1,254 | 287/1,135 | 414/1,158 | ||
| S2 | 273/609 | 326/535 | 459/573 | 388/512 | |
| S3 | 294/531 | 307/442 | 386/468 | ||
Number of trials accepted (Naccepted) and total number of trials (Ntotal) are shown for different operators (O1, O2), subjects (S1, S2, S3), and frequencies. Empty cells indicate conditions with too few trials (<200 accepted trials) for analysis.
Animals.
Three adult monkeys (Macaca mulatta) were tested while seated in a custom-made chair placed in a Robinson-style magnetic coil frame. Horizontal and vertical eye positions were measured with surgically implanted search coils that were low-pass filtered with a cutoff frequency of 50 Hz and sampled at 600 Hz; eye position sign was defined as positive to the left (horizontal) and down (vertical). When a stationary calibration coil, like that implanted on the eye, was placed in the field, the resultant horizontal eye position signal had an average standard deviation of 0.002°. The chair was positioned so that the head was centered relative to the earth-vertical rotation axis. The head was pitched forward 17° to position the horizontal canals near the earth-horizontal.
Surgeries were performed under general anesthesia with sterile procedures. In one surgery a head bolt was attached to immobilize the head during training and testing. In a second surgery, a two-turn 17-mm coil was inserted under the conjunctiva in the frontal plane in one eye (Judge et al. 1980). All experiments were approved by the Massachusetts Eye and Ear Infirmary Institutional Animal Care and Use Committee (IACUC) and were consistent with USDA guidelines.
The monkeys were trained to fixate a stationary dim target on a monitor; the dim target was located at the midline 40 cm in front of the eyes and at eye level. The target was extinguished, and yaw rotation was initiated 20 ms after the animal fixated the target for between 300 and 500 ms.
Motion stimuli.
Monkeys were upright and rotated about a head-centered earth-vertical axis with single cycles of sinusoidal acceleration like those used in previous studies (Benson et al. 1986, 1989; Grabherr et al. 2008; Zupan and Merfeld 2008). The equations defining the angular acceleration (α), velocity (ω), and displacement (Δθ) are:
| (1) |
| (2) |
| (3) |
where αp is the peak acceleration and f the frequency (0.2, 0.3, 1, 2, and 3 Hz). The acceleration was bidirectional with zero mean. Since motion began at zero velocity, integration yields an oscillatory velocity and displacement that are both unidirectional (see Fig. 1 for examples). The magnitude of the peak acceleration αp, peak velocity ωp, and displacement Δθ are related as αp/πf = ωp = 2fΔθ.
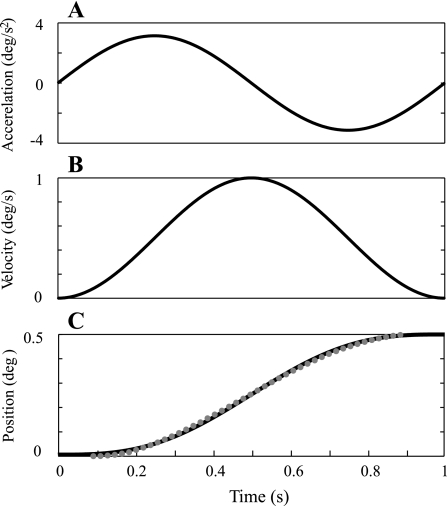
Fig. 1.
Example stimuli at 1 Hz. Black traces show theoretical motion trajectories. Traces show single cycle of sinusoidal angular acceleration (A), which is bidirectional, resulting angular velocity (B), which is unidirectional, and angular displacement (C), which is unidirectional. C also shows an actual position trajectory recorded at 60 Hz, which is the data rate for the Moog platform, on a single trial (gray dots) overlaid on theoretic displacement (black).
We initially tested all three monkeys at 1, 2, and 3 Hz. After seeing the general similarity of these VOR thresholds to average human perceptual thresholds, we decided that testing at lower frequencies was warranted. At low frequencies, fewer and fewer trials were accepted because of the presence of blinks and saccades. Nonetheless, we desired to test at the lowest frequency possible because the increase in human thresholds with decreasing frequency would be expected to maximize any difference between perceptual and VOR thresholds at lower frequencies. Therefore, we performed additional tests at 0.2 and 0.3 Hz, which were the lowest frequencies at which thresholds could be measured in monkeys. In fact, at these frequencies only two of the three monkeys maintained eye position long enough to yield VOR threshold measurements.
Stimuli order was randomized by a MATLAB (v. 7.7) computer program using a random number generator (“rand”). For the majority of conditions, stimuli amplitudes were ±4, ±2, ±1, ±0.75, ±0.50, ±0.25, and 0°/s. (For initial tests, stimuli of ±20, ±10, and ±5°/s were used to help establish that these stimuli were well above threshold, but these were not utilized for most conditions.)
Data analysis.
Like earlier similar analyses of eye movement thresholds (e.g., Rasche and Gegenfurtner 2009; Stone and Krauzlis 2003), data analysis was automated with operator intervention limited to trials that had saccades, blinks, etc. For each trial, an automated MATLAB program plotted the eye position data before, during, and after the motion and then found the average eye position just before the motion and subtracted this from the average position just after the motion to calculate the eye displacement.
It is important to emphasize that this analysis accounts for any and all potential transients (e.g., jerk, acceleration, and/or velocity transients). Specifically, because the change in eye position is the integral of eye velocity (which, in turn, is the integral of acceleration, which, in turn, is the integral of jerk…), this difference of end point minus starting point includes all that happens between the beginning and the end of the trial, including any jerk or acceleration or velocity transients. We considered differentiating the eye position data and determining whether the resultant slow-phase eye velocity is leftward or rightward. However, the optimal strategy for determining if a noisy velocity signal is rightward or leftward is to calculate the average velocity in the given time interval and determine the sign of this average velocity. But the sign of the average velocity is, by definition, the same as the sign of the eye displacement. Therefore, calculating the change in position is the optimal strategy for determining if a noisy velocity signal is, on average, rightward or leftward. In fact, similar approaches have been used for smooth pursuit analysis. For example, Stone and Krauzlis (2003) differentiated the eye position signal to obtain eye velocity but then used a boxcar filter on their direction signal to calculate the average direction, which is analogous to average velocity for our application.
To focus the analysis only upon the VOR, we needed to identify trials that had saccades, blinks, or other artifacts. Whether subjective or objective approaches are used, the overriding concern is that results not be prejudiced by the chosen method. Objective algorithms have been used for similar experiments (e.g., Stone and Krauzlis 2003) where there was little chance of more than one saccade in the relative short period of interest (350 ms), but even for this approach, a subjective trial-by-trial inspection was used to remove ∼10% of the trials. For comparison, some of our trials took 5 s, and it was common for several blinks/saccades to occur in that interval, especially for stimuli that evoked nystagmus.
There have been many attempts to develop automated saccade/blink detection algorithms using a wide variety of approaches (e.g., Allum et al. 1975; Anzaldi and Mira 1975; Baloh et al. 1980; Juhola 1988; Shelhamer and Zalewski 2001; Wall and Black 1981). No consensus exists regarding a generally accepted algorithm (or even a generally accepted approach) for detecting saccades and blinks. In fact, it has even been shown that human experts do not always agree on fast phase detection (Gentles and Barber 1973). Furthermore, even if such a consensus existed, that would still leave ambiguity in how to deal with a detected event, which is especially difficult when the signal is near the level of the noise, as it was in this study. For example, should one extrapolate (or interpolate) across the detected event? Should this extrapolation (interpolation) be done in position or velocity? How would one go about defining the beginning and end of the event, which becomes particularly problematic for small saccades like those measured near threshold? In the presence of such imperfections, any algorithm could eventually lead to the criticism that the results were prejudiced by the algorithm.
Therefore, to eliminate such prejudice, we instead chose the standard technique of having trained operators perform blind data analysis. Specifically, to minimize the possibility of prejudice, the operators were blind to whether the rotation was to the left or to the right during analysis. (Recall that whether VOR is to the left or right is the characteristic of interest.) More specifically, while viewing a graph nearly identical to Fig. 2, the operators were given a set of fixed options—discard the trial, accept the trial, or calculate a different eye position change by marking regions representative of the change in eye position. If the trial were not discarded, the change in eye position was converted to a binary code (1 for rightward; 0 for leftward). While this is more time consuming than a totally automated analysis, we can be sure that this blind analysis by trained operators is unprejudiced; the same cannot be said for any totally automated analysis.
Fig. 2.
Black and white example of display provided to operator for data analysis. To demonstrate the analysis in full detail, we intentionally show replicas of the plots observed by the operator during analysis. Because the goal is primarily to show the display observed during analysis, minimal “touching-up” has been performed for publication purposes. A and B: horizontal eye position. C and D: vertical eye position. E and F: rotation position, including the mirror image of position profile. The 2 columns show data from the same trial but with different time scales. The right column focuses exclusively on actual motion. The left column shows eye position for 0.5 s before and 0.3 s after motion. Gray bars show the time period used for automatic displacement calculations. The upward sloping dashed line in A shows the horizontal eye position change automatically calculated by the program. The downward sloping gray line in A shows an operator-selected eye position change that eliminated the influence of the fast phase. The dashed vertical lines in C and E show the beginning and end of the trial. Operators saw this information on a color LCD display.
To help evaluate the validity of this approach, we instructed two operators to use different criteria. This allows us to evaluate the maximal size of the effect that subjective operator decisions might have on the resultant analysis. Operator 1 (O1) was instructed to be ultraconservative and to keep only those trials that included no observable artifacts (e.g., no nystagmus). Operator 2 (O2) was instructed to use judgment to determine the direction of the eye movement. This was important because nystagmus was often evoked, especially at low frequencies. O1 discarded such trials. O2 recognized nystagmus and determined the direction of slow eye movement. Not surprisingly, O2 generally accepted more trials than O1 (see Table 1 for precise numbers for each operator at each frequency.) As will be shown, the results for the two quite different analyses were similar. Therefore, we present both data sets but focus almost exclusively on the less conservative analysis. We emphasize that even O2, who was encouraged to use judgment, was always blind to the direction of actual motion during analysis. In discussion, we compare the findings from the two analyses.
To fit the data, we took the same standard approach used previously for similar vestibular threshold studies (Zupan and Merfeld 2008). A Gaussian cumulative distribution function was fit to the data with a generalized linear model (GLM) and a probit link function. This fit yields a “maximum likelihood” model fit. The data in this case were two vectors—a vector of peak angular velocity amplitudes (ω) and a VOR response vector (y) indicating whether the VOR was to the left (0) or right (1) for each angular velocity. The function call in MATLAB was b=glmfit(ω,y,‘binomial’,‘link’,‘probit’) using the statistics toolbox (v. 7.0), where b is a two-element vector. It can be shown (e.g., Dobson and Barnett 2008) that the elements of b can be related to an underlying Gaussian probability distribution, with a “bias” (μ), which represents an offset from zero (Merfeld 2011), μ = −b(1)/b(2), and “sigma” (σ), which represents the standard deviation of the noise, σ = 1/b(2).
Since data analysis involved the manual review of thousands of trials, we looked for potential mistakes (e.g., an operator missed the presence of a small, but influential, saccade), using a simple statistical outlier approach. A jackknife method (Tukey 1958) was used to identify the biggest outliers for each fit. If there were N trials being fit, the fit was run N + 1 times—once with all trials and once with each trial removed. The maximum change in the deviance, returned by MATLAB's glmfit, identified the biggest potential outlier. The first and last authors manually reviewed potential outliers identified by this algorithm. On average, this outlier detection process changed the direction of ∼1 out of 1,500 accepted trials.
Human perceptual data calculations.
Human yaw rotation perceptual thresholds (Grabherr et al. 2008) were measured with a 3-down/1-up paradigm that yields 79.4% correct at threshold (Leek 2001); these perceptual thresholds correspond to 0.82 times the noise standard deviation (T79.4% = 0.82σ) (Merfeld 2011). Since the VOR thresholds reported here are reported as one-sigma thresholds (Tσ), which simply equals the estimated noise standard deviation (σ), we scaled the previously published human thresholds (Grabherr et al. 2008) by dividing by 0.82.
In addition, the term σ′(1 + f′/f), where σ′ and f′ are fitted parameters and f represents the stimulus frequency, was fit to the human perceptual threshold data—using MATLAB's “fmincon” from the optimization toolbox (v. 4.1) to minimize the mean square error between the data and fitted curve.
To determine error bars for these published human perceptual data, we used standard delete-one jackknife resampling (Tukey 1958). Specifically, one by one, each of the data points (one datum per subject per frequency) was deleted and the remaining data fit using the term from the previous paragraph. Fits were performed after taking the logarithm, because of the lognormal nature of human thresholds (Benson et al. 1989; Grabherr et al. 2008). The lognormal distribution was confirmed by determining that the residuals were not significantly different from a lognormal distribution. The mean and error bars for these curve fits were transformed back to physical units for presentation.
Statistics.
Despite the species difference, a simple comparison between rhesus VOR and human perceptual threshold data seems warranted to help determine whether the same threshold pattern as a function of frequency was evident in these two data sets. A two-sided two-sample t-test was used to compare the measured monkey VOR thresholds to human perceptual thresholds at each of several frequencies. As above, because of the lognormal distribution of human perceptual thresholds, we took the log of the data at each frequency before performing the t-test.
At 0.3 Hz human perceptual threshold data (Grabherr et al. 2008) were not available, so we used the value of each human subject's curve fit at 0.3 Hz, σ′(1 + f′/0.3), as the best available estimate of each subject's threshold at 0.3 Hz. We validated this estimate, using data at 0.2 Hz, by comparing the t-statistic calculated directly from the measured 0.2 Hz threshold data, which yielded a t-statistic t = 5.00, to that calculated with the curve fit data for each subject, which yielded a t-statistic t = 6.84. The two values were close, although the t-statistic for the curve fit was greater—consistent with variability being reduced because of smoothing associated with influence of data at nearby frequencies.
RESULTS
Example horizontal VOR time traces for stimuli of varying magnitude are shown in Fig. 3. The eye movement direction for a large motion (e.g., Fig, 3A) is easy to determine. Given the presence of physiological noise, the eye movement direction for small motions (e.g., Fig. 3C) is more difficult to determine, which leads to the use of the standard signal detection methods. Specifically, the direction (i.e., +/−) of the eye movement for each accepted trial was determined as described in methods.
Fig. 3.
A–C: example horizontal eye trajectories for 1-s-duration (1 Hz) stimuli, with decreasing motion amplitude from left to right (D–F). Note that the y-axis for the eye position signals (A–C) changes to help highlight changes in eye position.
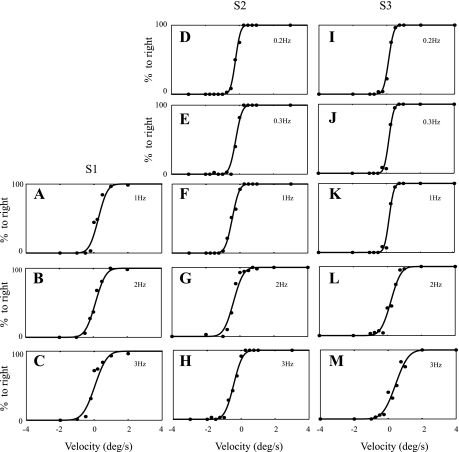
We then calculated the percentage of eye movements to the right at each stimulus level as shown in Fig. 4 for each monkey at each frequency. Note that for large positive (leftward) yaw velocities, the monkey's eye rotated in the compensatory rightward direction for 100% of the trials. Similarly, for large negative (rightward) yaw velocities, the monkey's eye rotated in the compensatory leftward direction for 100% of the trials (0% rightward). In between, the percentage of eye rotations to the right increased as stimulus amplitude increased. All three monkeys adequately maintained gaze at 1, 2, and 3 Hz. One of the three monkeys did not adequately maintain gaze in the dark at 0.2 and 0.3 Hz.
Fig. 4.
Percent eye rotation to the right is plotted vs. peak velocity amplitude for each monkey at each frequency. Data from S1 (A–C), S2 (D–H), and S3 (I–M), are shown at left, center, and right, respectively. Each row shows data acquired at a different frequency (top row, 0.2 Hz; 2nd row, 0.3 Hz; 3rd row, 1 Hz; 4th row, 2 Hz; bottom row, 3 Hz). The maximum likelihood fit of a cumulative Gaussian is shown as the solid curve for each example. Fitted parameters yielding fits shown are provided in Table 2.
Fits of a cumulative Gaussian to each data set as analyzed by the less conservative analysis (O2) are shown in Fig. 4. Each fit demonstrates a reasonable match to the data. For example, Fig. 4I shows the data for S3 at 0.2 Hz. For this condition, the data, representing 338 trials, were fit with a bias of 0.11°/s and a noise standard deviation of 0.23°/s, which we refer to as the “one-sigma threshold” (Tσ).
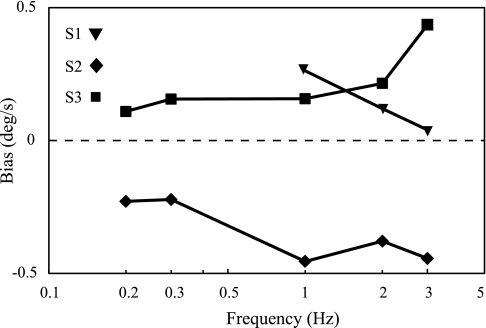
These data fits yielded relatively small fit biases, which are summarized in Table 2 and Fig. 5. Two animals consistently had a small positive (leftward) fit bias across all frequencies, while the third animal had a small consistent negative (rightward) fit bias. These small fit biases were always consistent in direction for each animal across all frequencies.
Table 2.
Fitted VOR thresholds and fitted bias values for different operators, subjects, and frequencies
| 0.2 Hz | 0.3 Hz | 1Hz | 2 Hz | 3Hz | |
|---|---|---|---|---|---|
| O2 (less conservative) | |||||
| S1 | |||||
| Tσ, °/s | 0.42 (±0.043) | 0.47 (±0.050) | 0.56 (±0.049) | ||
| Bias, °/s | 0.262 | 0.120 | 0.038 | ||
| S2 | |||||
| Tσ, °/s | 0.23 (±0.032) | 0.29 (±0.032) | 0.32 (±0.041) | 0.45 (±0.045) | 0.41 (±0.043) |
| Bias, °/s | −0.229 | −0.222 | −0.455 | −0.379 | −0.444 |
| S3 | |||||
| Tσ, °/s | 0.23 (±0.0329) | 0.22 (±0.035) | 0.23 (±0.032) | 0.49 (±0.051) | 0.59 (±0.059) |
| Bias, °/s | 0.109 | 0.156 | 0.157 | 0.215 | 0.436 |
| O1 (conservative) | |||||
| S1 | |||||
| Tσ, °/s | 0.59 (±0.070) | 0.61 (±0.030) | 0.76 (±0.082) | ||
| Bias, °/s | 0.148 | 0.056 | −0.198 | ||
| S2 | |||||
| Tσ, °/s | 0.34 (±0.042) | 0.35 (±0.042) | 0.62 (±0.066) | 0.60 (±0.060) | |
| Bias, °/s | −0.250 | −0.650 | −0.907 | −0.945 | |
| S3 | |||||
| Tσ, °/s | 0.33 (±0.038) | 0.68 (±0.720) | 0.69 (±0.068) | ||
| Bias, °/s | 0.144 | 0.120 | 0.353 |
Fitted vestibuloocular reflex (VOR) thresholds (Tσ) (±SD in parentheses) as well as fitted bias values are shown for different operators (O1, O2), subjects (S1, S2, S3), and frequencies. Empty cells indicate conditions with too few trials (<200 accepted trials) for analysis.
Fig. 5.
Vestibuloocular reflex (VOR) fitted biases as a function of frequency. Fitted biases were always the same sign for each of 3 monkeys across frequency. There was some evidence that the fit bias varied somewhat over time, but our study design does not allow a quantitative analysis of such an effect. Fit biases shown are from the less conservative analysis.
This fit bias was not an analysis artifact; evidence of the underlying vestibular bias could be observed by simply measuring (or observing) the eye movement in the dark in the absence of motion. Specifically, all three animals exhibited a very slow nystagmic eye movement at rest in the dark. (Recall that zero velocity trials were regularly and randomly distributed throughout the experiment.) Two of the animals demonstrated slow phases to the left (positive), while the third animal demonstrated slow phases to the right (negative). Small spontaneous nystagmus like this is common in rhesus monkeys in the dark, and certainly contributes to—and possibly causes—the observed fit bias.
For 11 of 13 fits, the magnitude of the fitted bias was less than the standard deviation of the noise (e.g., 1-sigma threshold). For the remaining two fits, the magnitude of the fitted bias was slightly greater than the standard deviation of the noise.
Table 2 also summarizes the fitted thresholds for the ultraconservative analysis. We only show numerical values when >200 trials were accepted. As shown in Table 2, thresholds from the ultraconservative analysis and the conservative analysis were similar when an adequate number of trials were available, although the conservative analysis consistently yielded a somewhat lower threshold than the ultraconservative analysis.
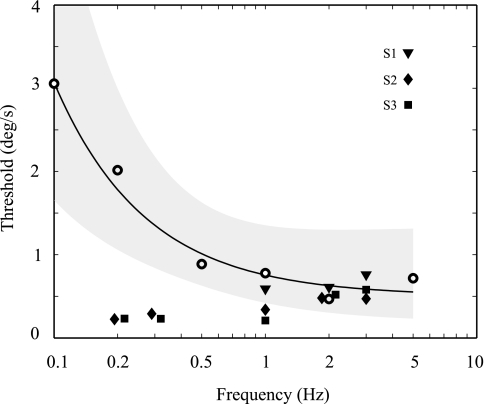
Figure 6 plots the one-sigma threshold obtained by using the less conservative analysis for all animals and frequencies. These data are plotted alongside human perceptual threshold data for yaw rotation obtained on the same motion device with the same motion stimuli (Grabherr et al. 2008). The VOR thresholds increase slightly as frequency increases—opposite the trend observed for perceptual thresholds. The VOR and perceptual thresholds overlap at 2 Hz but clearly diverge at lower frequencies. Specifically, the VOR and perceptual thresholds were not significantly different from one another at 1 and 2 Hz (P = 0.12 and P = 0.74, respectively, dof = 8) but were significantly different from one another at 0.2 Hz (P = 0.0002, dof = 7) and 0.3 Hz (P = 0.00001, dof = 7).
Fig. 6.
VOR “one-sigma” thresholds (Tσ) compared with human perceptual thresholds. Filled symbols show the VOR thresholds for each of the 3 monkeys plotted vs. frequency. The frequency for some data points is offset slightly for clarity. For context, the open symbols show average human perceptual thresholds (Grabherr et al. 2008) converted to one-sigma thresholds. The solid line shows the least-squares fit to the human threshold data, with the shaded area indicating 1 standard deviation from the mean. Because of lognormal distribution of human thresholds, standard deviations are not symmetrical on plot. VOR thresholds shown are from the less conservative analysis.
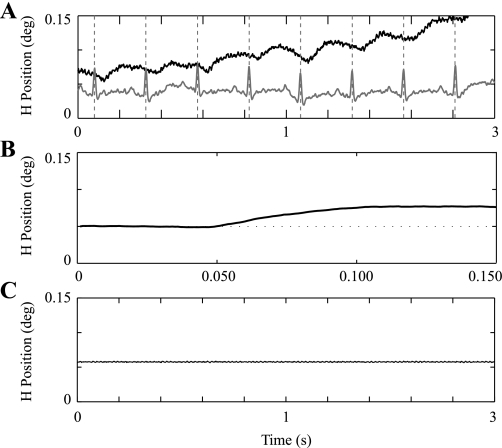
On ∼50% of the trials, we recorded oscillatory eye movements. Figure 7A shows example eye signals that demonstrate oscillations at a frequency around 3 Hz. Similar responses were intermittently recorded for all three animals. It is interesting to note that the frequency of the eye response is near the rhesus monkey heartbeat frequency. In fact, when synchronized to the peak of an electrocardiogram signal, a consistent eye response can be observed (Fig. 7B).
Fig. 7.
Eye oscillations. A: time traces of horizontal eye position (black) and cardiac potential (gray) show a small eye oscillation that appears synchronized with cardiac potential. Such oscillations were observed in all 3 animals and were observed on roughly half of the trials. The oscillations typically had a frequency between 2 and 4 Hz and were only observed when the small eye displacement traces were expanded for near-threshold eye motion. B: example of average eye oscillations synchronized with the peak of an electrocardiogram signal acquired simultaneously. Note that B has a very different time scale from A. C: example time trace with stationary calibration coil to demonstrate relative size of electronic coil measurement noise.
DISCUSSION
Brief overview of primary findings.
We found that rhesus monkey horizontal VOR direction recognition thresholds were relatively constant over a 10-fold change in frequency—slightly increasing with yaw rotation frequency. This is our primary phenomenological finding and matches our hypothesis that the VOR thresholds would be relatively constant across a broad range of frequencies—consistent with measurements of VOR dynamics at suprathreshold levels (e.g., Paige 1983; Peterka et al. 1990b).
This threshold pattern as a function of frequency is qualitatively different from that observed for human yaw rotation perceptual direction recognition thresholds, whereby the human perceptual thresholds increased substantially for frequencies below 0.5 Hz. Since the exact same motion device and motion trajectories were used for the human perceptual threshold study (Grabherr et al. 2008) and the monkey VOR threshold study, this difference is unlikely to be due to some subtle motion dependent effect (e.g., vibration, jerk, etc.) that escaped our observation.
Cross-species comparison considerations.
Our VOR threshold and perceptual threshold comparisons come with the obvious caveat that the VOR thresholds are measured in rhesus monkeys while the perceptual thresholds were measured in humans. Such cross-species comparisons must always be made cautiously, especially since quantitative differences between vestibular responses between human and nonhuman primates (e.g., Merfeld and Zupan 2002)—and even within individual members of a single species (e.g., Peterka et al. 1990a, 1990b)—can be substantive. One of our earlier papers discussed this issue at length for canal-otolith integration (Merfeld and Zupan 2002). In this earlier paper, we provided a thorough analysis, including system dynamic modeling, and concluded that the available data showed no qualitative differences between human vestibular responses and those in nonhuman primates. This implies that the qualitative difference we report in the present study is more likely due to a qualitative difference in vestibular perception and the VOR than to a qualitative difference between monkey and human responses.
We briefly explain why we performed these studies with nonhuman primates. The primary reason is that nonphysiological artifacts (nonphysiological “noise”) are smaller in nonhuman primates. Specifically, implanted head bolts yield much smaller head-motion artifacts than are possible with humans. Furthermore, surgically implanted eye coils yield lower measurement noise than available video methods or the motion artifacts associated with human eye coils embedded in contact lenses (see, e.g., Bergamin et al. 2004). Such noise/artifacts can often be ignored because the applied motion is much greater than the artifact, but this may not be warranted for small threshold-level motions.
Data fits.
We took great care to be certain that our analysis was not prejudiced. Specifically, to evaluate the issue of prejudicial analysis, we trained a second operator to analyze all of the data independently with an ultraconservative approach. We found that the two analyses yielded similar results, although the ultraconservative analysis yielded threshold estimates that were on average ∼20% greater. This difference can probably be explained by the fact that the ultraconservative analysis accepted fewer trials at high stimulus levels, because nystagmus intervened more often for large than for small trials. Therefore, the conservative fits were less constrained at these higher amplitudes, which could lead to slightly higher threshold estimates. In any event, the two dramatically different analyses provide rough estimates of the upper and lower bounds for the actual threshold values. We believe that the actual thresholds probably fall nearer the less conservative analysis, but the difference between the two, ∼20%, is small, especially compared with human perceptual thresholds, which vary across a population of subjects by a factor of 10 (Benson et al. 1989).
VOR threshold fit biases.
The fact that the fit biases were consistent for each animal across frequency (Fig. 5), and relatively consistent across time, suggests that such fit biases have a physiological basis. For example, an imbalance between the left and right labyrinths could cause a vestibular bias, which would then yield a VOR in the opposite direction (e.g., a leftward vestibular bias would cause a rightward VOR). In fact, when patients lose one of their two vestibular labyrinths, extreme vestibular biases become evident (see, e.g., Böhmer 1996; Fisch 1973). While speculative, similar vestibular biases may impact perceptual thresholds as well, but this cannot be proven with these VOR data. Certainly the potential presence of such vestibular biases has been noted in earlier perceptual threshold studies (Grabherr et al. 2008).
What does a positive VOR threshold bias imply? A negative (rightward) vestibular bias yields a positive (leftward) VOR at rest, which yields a positive (leftward) VOR fit bias. The fact that a rightward vestibular bias yields a leftward fit bias seems straightforward, since the direction of the VOR opposes (“compensates for”) the motion direction. In contrast, psychometric bias may seem less intuitive. Negative (rightward) vestibular bias yields a negative (rightward) sensation of rotation at rest. This, in turn, induces a positive (leftward) psychometric fit bias [see Merfeld (2011) for details]. In summary, the measurement of a leftward psychometric fit bias and/or a leftward VOR fit bias suggests a rightward vestibular bias.
The magnitude of the VOR threshold biases measured in this study were never much greater than the one-sigma VOR thresholds assayed in this study; this suggests that physiological calibration, which could act to eliminate vestibular bias, might be limited by the presence/magnitude of physiological noise observed on the VOR responses and indicated by the measured VOR threshold.
VOR and perceptual thresholds diverge below 0.5 Hz.
As shown in Fig. 6, there was a clear divergence of the perceptual and VOR thresholds, which was predominantly caused by a substantial increase in human yaw rotation thresholds, at frequencies below 0.5 Hz (Benson et al. 1989; Grabherr et al. 2008). While we cannot rule out a species effect, we think that such differences between vestibular perception and vestibular action (e.g., the VOR) suggest that some neural processing affects vestibular perception—specifically perceptual decision making—with little or no effect on the VOR (Merfeld et al. 2005a, 2005b).
The fact that VOR thresholds do not increase with decreasing frequency—in fact, demonstrating a small trend in the opposite direction—is inconsistent with the hypothesis that peripheral vestibular noise increases for lower-frequency stimuli. In fact, the apparent qualitative difference between VOR threshold dynamics and perceptual threshold dynamics is inconsistent with any hypothesis that posits an explanatory mechanism prior to the divergence of VOR pathways from perceptual pathways.
In addition, it is worth noting that this finding is consistent with—and even helps explain—the earlier report (Seemungal et al. 2004) that VOR thresholds were significantly less than perceptual thresholds. Specifically, since VOR thresholds are lower than perceptual thresholds at low frequencies and are similar at high frequencies, the velocity ramps used by Seemungal et al., which have substantial low-frequency components, will lead to lower VOR thresholds than perceptual thresholds.
It has been suggested that the low-frequency perceptual threshold increase is caused by some form of high-pass filtering (Grabherr et al. 2008) of the rotational signal. The absence of any indication that high-pass filtering influences VOR responses near threshold shows that VOR responses are not similarly filtered even for small near-threshold stimuli. Therefore, any such filtering must occur after the VOR pathways diverge from the thalamocortical pathways associated with perception.
The VOR thresholds we measured at 0.2 and 0.3 Hz were a little lower than those measured at 2 and 3 Hz. One explanation may be found in regular afferent recordings that demonstrate a similar small increase in thresholds with increasing frequency (Sadeghi et al. 2007). Another explanation might be found in our measurement of a small eye oscillation—having a magnitude of ∼0.05°—at a frequency around 3 Hz (Fig. 7). Such eye oscillations were intermittently observed in all three monkeys and would act to increase the measured VOR threshold in frequency regions near the eye oscillation frequency. Recall that the average VOR threshold at 3 Hz was ∼0.5°/s. The rotational displacement (Δθ = ωp/2f) at this threshold level was ∼0.08°, which is about the magnitude of the oscillatory eye response. Our experiments do not allow us to determine whether this oscillatory eye response is predominantly due to a sensory or motor influence. While not central to this study, it is interesting to note that the frequency of the measured eye oscillation is near the frequency of the cardiac cycle in rhesus monkeys (∼200 beats/min on average) and appears synchronized with the cardiac cycle (Fig. 7B).
Regardless of cause, this eye oscillation impacts VOR threshold measurements. From the viewpoint of VOR thresholds, this contributes a source of noise concentrated around 2–3 Hz, which contributes to the somewhat higher VOR thresholds we report at 2 and 3 Hz.
VOR and perceptual thresholds overlap above 1 Hz.
Perceptual thresholds appear to be influenced by a high-pass filter that does not affect VOR thresholds. However, vestibular perception and action could still share common pathways, common noise, and common calculations. In fact, as one specific example, the overlap of VOR and perceptual thresholds at high frequencies is consistent with the hypothesis that VOR thresholds are predominantly due to noise that is common to the motor and perceptual pathways—presumably transduction noise prior to divergence of the VOR and thalamocortical pathways. An alternate hypothesis is that the incremental motor noise accumulated along the motor pathways is the same as the incremental sensory noise accumulated along the perceptual pathways.
Our data cannot definitively address this issue. Furthermore, any such comparisons are speculative, because the VOR thresholds reported here were measured in rhesus monkeys and compared with human perceptual direction recognition thresholds. This cross-species comparison was made because available perceptual data in rhesus monkeys are limited. It has been reported that translation thresholds in rhesus monkeys in the dark (average of 0.004 G, range 0.002–0.006, N = 4) during a heading direction recognition task (Gu et al. 2007) are indistinguishable from human direction recognition thresholds (average of 0.006 G, range 0.002–0.014, N = 24) for interaural motion detection (Benson et al. 1986). Therefore, while available rhesus vestibular threshold data are not extensive, the available evidence does not demonstrate a substantial difference between rhesus and human perceptual thresholds.
Signal divergence.
We now compare VOR threshold dynamics to both perceptual threshold dynamics and the dynamics of perceived rotation measured by magnitude estimation. The location of the mechanisms that cause VOR threshold dynamics to differ from perceptual threshold dynamics and to differ from the dynamics of perceived rotation assayed via magnitude estimation is unknown. Studies suggest that position vestibular pause (PVP) neurons project to the oculomotor nuclei while vestibular only (VO) neurons project to vestibular thalamus (Cullen and Roy 2004; Marlinski and McCrea 2009; McCrea et al. 1987; Scudder and Fuchs 1992), but that still leaves a huge knowledge gap. Since so little is known about vestibular thalamocortical projections, we provide a simple framework based on speculations given the available information. Recall that Grabherr et al. (2008) noted that the time constant associated with perceptual thresholds (∼0.7 s) was substantially lower than the decay time constant of the semicircular canals (∼5 s). This, in turn, is substantially less than the decay time constant (∼15 s) of human yaw rotation sensations (Bertolini et al. 2011; Bronstein et al. 2008; Okada et al. 1999; Sinha et al. 2008).
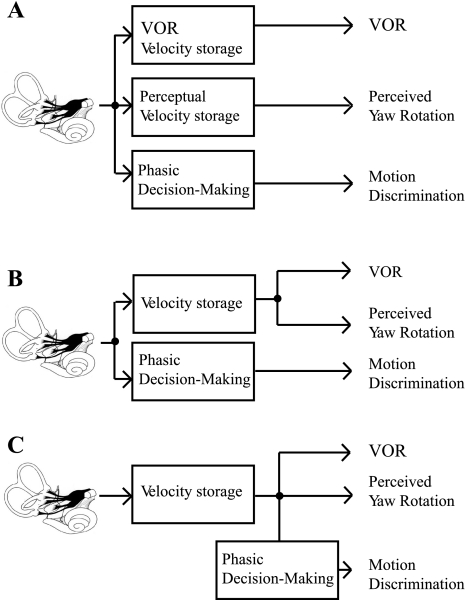
Obviously, since the VOR, perceptual thresholds, and perceived rotation measured by magnitude estimation are qualitatively different responses evoked by motion, the neural pathways must diverge, but where? At one extreme (Fig. 8, A and B), this divergence could occur as early as the vestibular nuclei (or even the vestibular periphery). Because of a dearth of information, there is little evidence for or against perceptual discrimination pathways that parallel the motion perception pathways. If such parallel perceptual pathways exist, the increase in perceptual thresholds at lower frequencies might be consistent with thresholds in VO neurons, which also show a tendency to increase at lower frequencies (Massot et al. 2011).
Fig. 8.
Conceptual pathways showing possible divergence of VOR and perceptual pathways. A: parallel pathways could begin as early as the vestibular periphery or vestibular nuclei. B: perceptual motion discrimination pathways could diverge early, with yaw motion sensation and VOR pathways diverging after common velocity storage calculations. C: signals for motion discrimination pathways could diverge from neurons carrying yaw rotation sensation after velocity storage contributions.
At the other extreme (Fig. 8C), the perceptual pathways could lead to yaw rotation sensations that demonstrate velocity storage yielding a time constant for rotation sensations between 10 and 30 s (Bertolini et al. 2011). Then, the direction recognition/discrimination pathways that lead to binary decisions regarding motion direction could access the neurons carrying this velocity storage signal and, as part of the binary decision-making process, high-pass filter this signal. Such phasic filtering would be consistent with what is sometimes called neural adaptation—phasic neural responses to constant or low-frequency stimulation. There is some evidence for such phasic high-pass filtering as part of visual decision-making processes (Aston-Jones and Cohen 2005a, 2005b).
Other considerations.
It has been suggested that the canonical variable for rotation thresholds is angular velocity—as opposed to angular displacement or angular acceleration. This is primarily based upon two facts: 1) the rotation velocity at perceptual threshold is roughly constant at physiological frequencies in the one-decade range between 0.5 and 5 Hz (Grabherr et al. 2008) and 2) time-to-detect tasks using velocity ramps demonstrate a nearly constant angular velocity at threshold (Clark and Stewart 1962). As noted in methods the eye displacement analysis we used is the optimal strategy for determining whether a noisy velocity signal is, on average, rightward or leftward. This does not lead to the conclusion that the nervous system necessarily applies this strategy for perceptual tasks but simply provides a theoretical rationale for our VOR threshold analyses.
Taking this one step further, we consider the behavioral implications of a hypothetical process that integrates a noisy perceptual velocity signal to obtain a perceptual displacement signal (or average velocity signal, which has the same sign). Such an integration of velocity noise could help explain the existence of the neural high-pass filter that has been hypothesized (Grabherr et al. 2008) to filter the perceptual angular velocity threshold signal plus noise but does not affect the VOR. Specifically, consider when there is no underlying motion signal (i.e., no motion) that the integration of zero-mean Gaussian white noise1 yields Brownian noise (Brown and Hwang 1992), which is also called a random walk. Brownian noise is a zero-mean signal that has an average displacement magnitude (i.e., standard deviation) that grows unbounded with time (Brown and Hwang 1992), which is a form of low-frequency drift. One way for perceptual discrimination/detection/recognition processes to manage this drift is to high-pass filter the signal, thereby eliminating low-frequency drift. Given that eye position is regularly reset by saccades, microsaccades, and/or fast phases, an analogous high-pass filter would not be required to reset oculomotor position.
Comparison to smooth pursuit.
Our findings appear qualitatively consistent with some earlier smooth pursuit findings that suggested that sensory noise is a substantive contributor to smooth pursuit noise (Kowler and McKee 1987; Osborne et al. 2005, 2007; Rasche and Gegenfurtner 2009; Stone and Krauzlis 2003; Watamaniuk and Heinen 1999). Just as smooth pursuit provides an easily measureable signal that has been reported to reveal characteristics associated with sensory processing noise associated with vision (e.g., Beutter and Stone 2000; Osborne et al. 2007), the VOR may eventually provide a readily measureable signal that reveals characteristics of vestibular sensory noise, though this remains to be shown. At the same time, the difference that we measured between VOR and perceptual thresholds below 1 Hz highlights that any such comparison must be done carefully. For example, ignoring dynamics—like the perceptual threshold dynamics reported previously (e.g., Grabherr et al. 2008) or the low-pass characteristics of the oculomotor plant (e.g., Robinson 1964)—can yield erroneous conclusions. Similar cautions almost certainly apply to other sensorimotor systems.
GRANTS
This work was supported by National Institute on Deafness and Other Communication Disorders Grant R01-DC-04158.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: C.H., R.F.L., and D.M.M. conception and design of research; C.H. and D.M.M. performed experiments; C.H. and D.M.M. analyzed data; C.H., R.F.L., and D.M.M. interpreted results of experiments; C.H. and D.M.M. prepared figures; C.H., R.F.L., and D.M.M. drafted manuscript; C.H., R.F.L., and D.M.M. edited and revised manuscript; C.H., R.F.L., and D.M.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank S. Fukuda for all her data collection and data analysis efforts and M. Lankow for administrative assistance. We thank Dr. Faisal Karmali and Koeun Lim for scientific conversations, assistance, and comments on a preliminary manuscript. We thank Dr. Michael Saginaw for his work that reduced coil signal noise substantially—making the study more straightforward.
Footnotes
The assumption of Gaussian white noise is not crucial to the general point but is a necessary assumption for the output of the integral to be Brownian noise.
REFERENCES
- Allum JH, Tole JR, Weiss AD. MITNYS-II—a digital program for on-line analysis of nystagmus. IEEE Trans Biomed Eng 22: 196–202, 1975 [DOI] [PubMed] [Google Scholar]
- Anzaldi E, Mira E. An interactive program for the analysis of ENG tracings. Acta Otolaryngol 80: 120–127, 1975 [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. Adaptive gain and the role of the locus coeruleus-norepinephrine system in optimal performance. J Comp Neurol 493: 99–110, 2005a [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci 28: 403–450, 2005b [DOI] [PubMed] [Google Scholar]
- Baloh RW, Langhofer L, Honrubia V, Yee RD. On-line analysis of eye movements using a digital computer. Aviat Space Environ Med 51: 563–567, 1980 [PubMed] [Google Scholar]
- Barnett-Cowan M, Harris LR. Perceived timing of vestibular stimulation relative to touch, light and sound. Exp Brain Res 198: 221–231, 2009 [DOI] [PubMed] [Google Scholar]
- Benson A, Hutt E, Brown S. Thresholds for the perception of whole body angular movement about a vertical axis. Aviat Space Environ Med 60: 205–213, 1989 [PubMed] [Google Scholar]
- Benson AJ, Spencer MB, Stott JR. Thresholds for the detection of the direction of whole-body, linear movement in the horizontal plane. Aviat Space Environ Med 57: 1088–1096, 1986 [PubMed] [Google Scholar]
- Bergamin O, Ramat S, Straumann D, Zee DS. Influence of orientation of exiting wire of search coil annulus on torsion after saccades. Invest Ophthalmol Vis Sci 45: 131–137, 2004 [DOI] [PubMed] [Google Scholar]
- Bertolini G, Ramat S, Laurens J, Bockisch CJ, Marti S, Straumann D, Palla A. Velocity storage contribution to vestibular self-motion perception in healthy human subjects. J Neurophysiol 105: 209–223, 2011 [DOI] [PubMed] [Google Scholar]
- Beutter BR, Stone LS. Motion coherence affects human perception and pursuit similarly. Vis Neurosci 17: 139–153, 2000 [DOI] [PubMed] [Google Scholar]
- Böhmer A. Acute unilateral peripheral vestibulopathy. In: Disorders of the Vestibular System, edited by Baloh R, Halmagyi GM. Oxford, UK: Oxford Univ. Press, 1996, p. 318–327 [Google Scholar]
- Bronstein AM, Grunfeld EA, Faldon M, Okada T. Reduced self-motion perception in patients with midline cerebellar lesions. Neuroreport 19: 691–693, 2008 [DOI] [PubMed] [Google Scholar]
- Brown RG, Hwang P. Introduction to Random Signals and Applied Kalman Filtering. New York: Wiley, 1992 [Google Scholar]
- Carpenter-Smith TR, Futamura RG, Parker DE. Inertial acceleration as a measure of linear vection: an alternative to magnitude estimation. Percept Psychophys 57: 35–42, 1995 [DOI] [PubMed] [Google Scholar]
- Clark B, Stewart JD. Comparison of three methods to determine thresholds for perception of angular acceleration. Am J Psychol 81: 207–216, 1968 [PubMed] [Google Scholar]
- Clark B, Stewart JD. Perception of angular acceleration about the yaw axis of a flight simulator. Thresholds and reaction latency for research pilots. Aerospace Med 33: 1426–1432, 1962 [PubMed] [Google Scholar]
- Cohen B, Henn V, Raphan T, Dennett D. Velocity storage, nystagmus, and visual-vestibular interactions in humans. Ann NY Acad Sci 374: 421–433, 1981 [DOI] [PubMed] [Google Scholar]
- Cullen KE, Roy JE. Signal processing in the vestibular system during active versus passive head movements. J Neurophysiol 91: 1919–1933, 2004 [DOI] [PubMed] [Google Scholar]
- De Vrijer M, Medendorp WP, Van Gisbergen JA. Shared computational mechanism for tilt compensation accounts for biased verticality percepts in motion and pattern vision. J Neurophysiol 99: 915–930, 2008 [DOI] [PubMed] [Google Scholar]
- Dimitri PS, Wall IC, Rauch SD. Multivariate vestibular testing: thresholds for bilateral Meniere's disease and aminoglycoside ototoxicity. J Vestib Res 11: 391–404, 2002 [PubMed] [Google Scholar]
- Dobson A, Barnett A. An Introduction to Generalized Linear Models (3rd ed.). Boca Raton, FL: Chapman and Hall/CRC, 2008, p. 320 [Google Scholar]
- Doty RL. Effect of duration of stimulus presentation on the angular acceleration threshold. J Exp Psychol 80: 317–321, 1969 [DOI] [PubMed] [Google Scholar]
- Fernandez C, Goldberg J. Physiology of peripheral neurons innervating semicircular canals of the squirrel monkey. II. Response to sinusoidal stimulation and dynamics of peripheral vestibular system. J Neurophysiol 34: 661–675, 1971 [DOI] [PubMed] [Google Scholar]
- Fisch U. The vestibular response following unilateral vestibular neurectomy. Acta Otolaryngol 76: 229–238, 1973 [DOI] [PubMed] [Google Scholar]
- Gentles W, Barber HO. Computer and human variation in the measurement of post caloric nystagmus. Int J Equilib Res 3: 173–182, 1973 [PubMed] [Google Scholar]
- Grabherr L, Nicoucar K, Mast FW, Merfeld DM. Vestibular thresholds for yaw rotation about an earth-vertical axis as a function of frequency. Exp Brain Res 186: 677–681, 2008 [DOI] [PubMed] [Google Scholar]
- Green D, Swets J. Signal Detection Theory and Psychophysics. New York: Wiley, 1966 [Google Scholar]
- Gu Y, DeAngelis GC, Angelaki DE. A functional link between area MSTd and heading perception based on vestibular signals. Nat Neurosci 10: 1038–1047, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judge SJ, Richmond BJ, Chu FC. Implantation of magnetic search coils for measurement of eye position: an improved method. Vision Res 20: 535–538, 1980 [DOI] [PubMed] [Google Scholar]
- Juhola M. Detection of nystagmus eye movements using a recursive digital filter. IEEE Trans Biomed Eng 35: 389–395, 1988 [DOI] [PubMed] [Google Scholar]
- Kowler E, McKee SP. Sensitivity of smooth eye movement to small differences in target velocity. Vision Res 27: 993–1015, 1987 [DOI] [PubMed] [Google Scholar]
- Leek MR. Adaptive procedures in psychophysical research. Percept Psychophys 63: 1279–1292, 2001 [DOI] [PubMed] [Google Scholar]
- Leigh RJ, Zee D. Neurology of Eye Movements. New York: Oxford Univ. Press, 2006 [Google Scholar]
- Macmillan NA, Creelman CD. Detection Theory: A User's Guide. Mahwah, NJ: Erlbaum, 2005 [Google Scholar]
- MacNeilage PR, Banks MS, DeAngelis GC, Angelaki DE. Vestibular heading discrimination and sensitivity to linear acceleration in head and world coordinates. J Neurosci 30: 9084–9094, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mah RW, Young LR, Steele CR, Schubert ED. Threshold perception of whole-body motion to linear sinusoidal stimulation. In: AlAA Flight Simulation Technologies Conference and Exhibit Boston, MA, 1989 [Google Scholar]
- Mallery RM, Olomu OU, Uchanski RM, Militchin VA, Hullar TE. Human discrimination of rotational velocities. Exp Brain Res 204: 11–20, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlinski V, McCrea RA. Self-motion signals in vestibular nuclei neurons projecting to the thalamus in the alert squirrel monkey. J Neurophysiol 101: 1730–1741, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massot C, Chacron MJ, Cullen KE. Information transmission and detection thresholds in the vestibular nuclei: single neurons vs. population encoding. J Neurophysiol 105: 1798–1814, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrea RA, Strassman A, May E, Highstein SM. Anatomical and physiological characteristics of vestibular neurons mediating the horizontal vestibulo-ocular reflex of the squirrel monkey. J Comp Neurol 264: 547–570, 1987 [DOI] [PubMed] [Google Scholar]
- Merfeld DM. Signal detection theory and vestibular thresholds. I. Basic theory and practical considerations. Exp Brain Res 210: 389–405, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merfeld DM, Park S, Gianna-Poulin C, Black FO, Wood S. Vestibular perception and action employ qualitatively different mechanisms. I. Frequency response of VOR and perceptual responses during translation and tilt. J Neurophysiol 94: 186–198, 2005a [DOI] [PubMed] [Google Scholar]
- Merfeld DM, Park S, Gianna-Poulin C, Black FO, Wood S. Vestibular perception and action employ qualitatively different mechanisms. II. VOR and perceptual responses during combined tilt and translation. J Neurophysiol 94: 199–205, 2005b [DOI] [PubMed] [Google Scholar]
- Merfeld DM, Young L, Oman C, Shelhamer M. A multi-dimensional model of the effect of gravity on the spatial orientation of the monkey. J Vestib Res 3: 141–161, 1993 [PubMed] [Google Scholar]
- Merfeld DM, Zupan LH. Neural processing of gravitoinertial cues in humans. III. Modeling tilt and translation responses. J Neurophysiol 87: 819–833, 2002 [DOI] [PubMed] [Google Scholar]
- Mergner T, Schweigart G, Kolev O, Hlavacka F, Becker W. Visual-vestibular interaction for human ego-motion perception. In: Multisensory Control of Posture, edited by Mergner T, Hlavacka F. New York: Plenum, 1995, p. 157–167 [Google Scholar]
- Okada T, Grunfeld E, Shallo-Hoffmann J, Bronstein AM. Vestibular perception of angular velocity in normal subjects and in patients with congenital nystagmus. Brain 122: 1293–1303, 1999 [DOI] [PubMed] [Google Scholar]
- Osborne LC, Hohl SS, Bialek W, Lisberger SG. Time course of precision in smooth-pursuit eye movements of monkeys. J Neurosci 27: 2987–2998, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne LC, Lisberger SG, Bialek W. A sensory source for motor variation. Nature 437: 412–416, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paige GD. Vestibuloocular reflex and its interactions with visual following mechanisms in the squirrel monkey. I. Response characteristics in normal animals. J Neurophysiol 49: 134–151, 1983 [DOI] [PubMed] [Google Scholar]
- Peterka R, Black FO, Schoenhoff M. Age-related changes in human vestibulo-ocular and optokinetic reflexes: pseudorandom rotation tests. J Vestib Res 1: 61–71, 1990a [PubMed] [Google Scholar]
- Peterka R, Black FO, Schoenhoff M. Age-related changes in human vestibulo-ocular reflexes: sinusoidal rotation and caloric tests. J Vestib Res 1: 49–59, 1990b [PubMed] [Google Scholar]
- Raphan T, Matsuo V, Cohen B. Velocity storage in the vestibulo-ocular reflex arc (VOR). Exp Brain Res 35: 229–248, 1979 [DOI] [PubMed] [Google Scholar]
- Raphan T, Matsuo V, Cohen B. A velocity storage mechanism responsible for optokinetic nystagmus (OKN), optokinetic after-nystagmus (OKAN) and vestibular nystagmus. In: Control of Gaze by Brain Stem Neurons, Developments in Neuroscience, edited by Baker R, Berthoz A. Amsterdam: Elsevier/North Holland Biomedical, 1977, p. 37–47 [Google Scholar]
- Rasche C, Gegenfurtner KR. Precision of speed discrimination and smooth pursuit eye movements. Vision Res 49: 514–523, 2009 [DOI] [PubMed] [Google Scholar]
- Robinson D. The mechanics of human saccadic eye movement. J Physiol 174: 245–264, 1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson D. Vestibular and optokinetic symbiosis: an example of explaining by modelling. In: Control of Gaze by Brain Stem Neurons, Developments in Neuroscience, edited by Baker R, Berthoz A. Amsterdam: Elsevier/North-Holland Biomedical, 1977, p. 49–58 [Google Scholar]
- Sadeghi SG, Chacron MJ, Taylor MC, Cullen KE. Neural variability, detection thresholds, and information transmission in the vestibular system. J Neurosci 27: 771–781, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scudder CA, Fuchs AF. Physiological and behavioral identification of vestibular nucleus neurons mediating the horizontal vestibuloocular reflex in trained rhesus monkeys. J Neurophysiol 68: 244–264, 1992 [DOI] [PubMed] [Google Scholar]
- Seemungal BM, Gunaratne IA, Fleming IO, Gresty MA, Bronstein AM. Perceptual and nystagmic thresholds of vestibular function in yaw. J Vestib Res 14: 461–466, 2004 [PubMed] [Google Scholar]
- Shelhamer M, Zalewski S. A new application for time-delay reconstruction: detection of fast phase eye movements. Phys Lett A 291: 349–354, 2001 [Google Scholar]
- Sinha N, Zaher N, Shaikh AG, Lasker AG, Zee DS, Tarnutzer AA. Perception of self motion during and after passive rotation of the body around an earth-vertical axis. Prog Brain Res 171: 277–281, 2008 [DOI] [PubMed] [Google Scholar]
- Soyka F, Robuffo Giordano P, Beykirch K, Bulthoff HH. Predicting direction detection thresholds for arbitrary translational acceleration profiles in the horizontal plane. Exp Brain Res 209: 95–107, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone LS, Krauzlis RJ. Shared motion signals for human perceptual decisions and oculomotor actions. J Vis 3: 725–736, 2003 [DOI] [PubMed] [Google Scholar]
- Tukey JW. Bias and confidence in not quite large samples. Ann Math Sci 29: 614, 1958 [Google Scholar]
- Wall C, 3rd, Black FO. Algorithms for the clinical analysis of nystagmus eye movements. IEEE Trans Biomed Eng 28: 638–646, 1981 [DOI] [PubMed] [Google Scholar]
- Watamaniuk SN, Heinen SJ. Human smooth pursuit direction discrimination. Vision Res 39: 59–70, 1999 [DOI] [PubMed] [Google Scholar]
- Zupan LH, Merfeld DM. Interaural self-motion linear velocity thresholds are shifted by roll vection. Exp Brain Res 191: 505–511, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]