Abstract
The developmental plasticity of excitatory synapses is well established, particularly as a function of age. If similar principles apply to inhibitory synapses, then we would expect manipulations during juvenile development to produce a greater effect and experience-dependent changes to persist into adulthood. In this study, we first characterized the maturation of cortical inhibitory synapse function from just before the onset of hearing through adulthood. We then examined the long-term effects of developmental conductive hearing loss (CHL). Whole cell recordings from gerbil thalamocortical brain slices revealed a significant decrease in the decay time of inhibitory currents during the first 3 mo of normal development. When assessed in adults, developmental CHL led to an enduring decrease of inhibitory synaptic strength, whereas the maturation of synaptic decay time was only delayed. Early CHL also depressed the maximum discharge rate of fast-spiking, but not low-threshold-spiking, inhibitory interneurons. We then asked whether adult onset CHL had a similar effect, but neither inhibitory current amplitude nor decay time was altered. Thus inhibitory synapse function displays a protracted development during which deficits can be induced by juvenile, but not adult, hearing loss. These long-lasting changes to inhibitory function may contribute to the auditory processing deficits associated with early hearing loss.
Keywords: auditory cortex, conductive hearing loss, development, fast-spiking interneuron, γ-aminobutyric acid A receptor
a general theory of neural development holds that early sensory experience can permanently alter the functional properties of synapses. Consistent with this idea, a broad range of auditory cortical inhibitory synaptic properties are disrupted immediately following developmental hearing loss (Kotak et al. 2005, 2008; Takesian et al. 2010; Xu et al. 2010). Inhibitory synapse maturation plays a significant role in regulating the plasticity of cortical excitatory circuits (Fagiolini and Hensch 2000; Fagiolini et al. 2004; Hensch 2005; Hensch and Stryker 2004; Hensch et al. 1998; Huang et al. 1999; Iwai et al. 2003; Katagiri et al. 2007; Southwell et al. 2010; Sugiyama et al. 2008; Yazaki-Sugiyama et al. 2009), yet information on the age dependence of inhibitory plasticity is limited. If inhibitory synapse function does display age-dependent plasticity, then it should fail to mature properly following developmental conductive hearing loss (CHL) but remain relatively unaffected by adult hearing loss. Furthermore, if inhibitory dysfunction is causally related to adult perceptual deficits, then aberrant properties should persist into adulthood. Therefore, we asked whether the identical hearing loss manipulation produced the same outcome when carried out in juvenile and adult animals, and whether the developmental effect was transient or permanent.
Immediately after the onset of hearing, both subcortical and cortical circuits are vulnerable to perturbations of the sensory environment, such as chronic deprivation or continuous stimulation (Sanes and Bao 2009). At the cellular level, deprivation leads to a net increase in the excitability of both midbrain and cortical neurons that is due, in part, to a decline in the magnitude of inhibitory synaptic events (Kotak et al. 2005, 2008; Takesian et al. 2010; Vale and Sanes 2000). Although adult hearing loss is thought to affect inhibitory synapse function, this has only been measured indirectly (Bledsoe et al. 1995; Burianova et al. 2009; Caspary et al. 1990, 1995, 1999; Ling et al. 2005; Rajan 1998, 2001; Raza et al. 1994). Here, we were particularly interested in the effects of a less severe form of auditory deprivation, one in which animals hear, albeit with higher thresholds, and the relative impact of this deprivation in juvenile and adult animals.
The effects of sensory deprivation on synaptic properties are extensive, yet the assessment age is generally restricted to a narrow time window during juvenile development. At least one study in the visual cortex has demonstrated that inhibitory synapse strength remains diminished in adult animals raised with visual deprivation (Morales et al. 2002). In the auditory system, recordings from the cochlear nucleus of mice with a congenital form of progressive hearing loss show that changes to excitatory synapses and membrane properties are present in young adults (Wang and Manis 2005, 2006). However, some cellular effects, such as changes in expression of glutamic acid decarboxylase (GAD) or Fos, occur only transiently following deprivation (Mossop et al. 2000; Sun et al. 2009). Direct measures of inhibitory synapse function in adults following a developmental manipulation have not been made.
In this report, we describe the long-term alterations to inhibitory synapses in auditory cortex that are associated with moderate developmental CHL. First, we show that CHL induced during early life profoundly affects inhibitory synapses recorded in juvenile animals and that these effects persist into adulthood. We then show that CHL induced in adulthood does not alter inhibitory synapses, suggesting that at least one form of inhibitory plasticity in auditory cortex is restricted to a developmental period.
MATERIALS AND METHODS
Experimental animals.
Gerbils (Meriones unguiculatus) aged postnatal day (P) 8–210 born from breeding pairs (Charles River Laboratories) were used. Animal care, maintenance, and surgeries were in accordance with the guidelines and rules of the Institutional Animal Care and Use Committee, New York University (NYU), approved by the Office of Laboratory Animal Welfare, Office of Extramural Research, U.S. National Institutes of Health (NIH; Bethesda, MD). The data are drawn from voltage-clamp recordings of 173 pyramidal cells from 55 animals and from current-clamp recordings of 50 interneurons from 14 animals.
Conductive hearing loss.
CHL was induced by tympanic membrane puncture and malleus extirpation using procedures similar to those described previously (Tucci et al. 1999; Xu et al. 2007). This form of CHL elevates auditory thresholds by about 35–45 dB and is thought to be a reasonable model for childhood hearing loss associated with certain cases of otitis media (Tucci et al. 1999; Whitton and Polley 2011). Gerbil pups were anesthetized with halogenated ethyl methyl ether methoxyflurane (Metofane), and anesthetic induction occurred within 10 min, which was confirmed by a complete elimination of responses to nociceptive stimuli. A postauricular incision was made, the tympanic membrane was punctured, and the malleus was removed with forceps. The postauricular wound was closed with cyanoacrylate glue, and the procedure was repeated on the other side. The success of each surgery was confirmed anatomically after the brain slice was prepared to ensure that the stapes was preserved during malleus removal.
Thalamocortical brain slice preparation.
Thalamocortical brain slices (500 μm) were generated from gerbils as described previously (Kotak et al. 2005). The brain was sectioned perihorizontally to preserve the ventral medial geniculate (MGv) and its ascending pathways to the auditory cortex (Cruikshank et al. 2002). To account for developmental changes in the relative location of the auditory cortex, thalamocortical slices were cut at a modified 25° angle in adult animals. In some experiments using P180–P210 animals, thinner slices (200 μm) were used to allow for the visualization of inhibitory interneurons under infrared differential interface contrast (IR-DIC). The slices were incubated in artificial cerebral spinal fluid (ACSF) at 32°C for 30 min, then at room temperature for 60 min, and were transferred to a recording chamber continuously superfused (3 ml/min) with ACSF at 32°C. The ACSF contained (in mM) 125 NaCl, 4 KCl, 1.2 KH2PO4, 1.3 MgSO4, 24 NaHCO3, 15 glucose, 2.4 CaCl2, and 0.4 l-ascorbic acid (pH 7.3 when bubbled with 95%O2-5%CO2). Before each recording, the auditory cortex was identified in all 500-μm thalamocortical slices by extracellular field responses to MGv stimulation.
Whole cell recordings.
To assess synaptic inhibition, whole cell recordings (PC-501A; Warner Instruments, Hamden, CT) were obtained from pyramidal neurons in cortical layers (L) 2/3. Recording electrodes were fabricated from borosilicate glass microcapillaries (outer diameter, 1.5 mm) with a micropipette puller (model P-97; Sutter Instruments, Novato, CA). The internal solution contained (in mM) 100 KCl, 40 K-gluconate, 8 NaCl, 10 HEPES, 2 MgCl2, 0.1 EGTA, 2 adenosine 5′-triphosphate disodium salt (ATP), 0.3 guanosine 5′-triphosphate sodium salt (GTP), and 5 lidocaine derivative QX-314 (pH 7.2 with KOH). This internal solution contained a high chloride concentration to obtain inward inhibitory postsynaptic currents (IPSCs) at a holding potential of −60 mV. The tip resistance of the patch electrode filled with internal solution was 5–10 MΩ. Access resistances were 15–30 MΩ and were compensated by about 70%. Neurons visually identified as pyramidal cell bodies under IR-DIC were selected. It is not possible to characterize cells based on discharge properties because intracellular QX-314 blocks sodium channels. However, after breaking into the cell, it was possible to immediately obtain a resting potential (<50 mV) and an overshooting action potential. IPSCs were recorded in the presence of the ionotropic glutamate receptor blockers 6,7-dinitroquinoxaline-2,3-dione (DNQX; 20 μM; Sigma, St. Louis, MO) and 2-amino-5-phosphopentanoate (AP-5; 50 μM; Tocris Cookson, Ballwin, MO), added to the superfusing ACSF. The drugs were applied for 15 min before IPSCs were recorded. Spontaneous (s) IPSCs were recorded by acquiring 5–10 sweeps of 30-s duration for each cell. To assess evoked IPSCs, synaptic responses were elicited with electrical stimuli delivered via a stimulus isolator (model BSI-9501; Dagan, Minneapolis, MN) to a bipolar stimulating electrode (MX211EWDS2; Matrix Microelectrode) placed on cortical L4. All stimuli were 100 μs in duration. Incremental stimulus intensities were delivered at 0.05 Hz until an evoked IPSC was discernible from failures (Kotak et al. 2005, 2008). Minimum-evoked (me-) IPSCs were then collected using a stimulus intensity that evoked about 50% failures. The average stimulation current required to elicit a me-IPSC was 14.7 ± 1.4 μA. There was no significant difference in the stimulation current required between groups. In addition, maximum-evoked (max-) IPSCs were examined by increasing the stimulus amplitude (0 to 100 μA, 10-μA increments, 0.05 Hz). Max-IPSCs were calculated as the mean maximum amplitude obtained from five sweeps. For me-IPSC, max-IPSC, and sIPSC recordings, the presynaptic population of interneurons that contributes to the IPSCs is unknown. However, to verify that the recorded IPSCs were mediated by GABAA receptors, gabazine (1 μM; Tocris Biosciences) was applied at the end of some experiments (n = 3).
Interneuron recordings.
Interneuron recordings from the supragranular auditory cortex were performed as described previously (Takesian et al. 2010). The current-clamp internal solution contained (in mM) 5 KCl, 127.5 K-gluconate, 10 HEPES, 2 MgCl2, 0.6 EGTA, 2 ATP, 0.3 GTP, and 5 phosphocreatine (pH 7.2 with KOH).
Fast-spiking (FS) interneurons were targeted based on the soma shape under IR-DIC and identified by their spiking responses to current injections. FS cells were distinguished physiologically by their characteristic narrow spike, deep afterhyperpolarization (AHP), and high discharge (Connors and Gutnick 1990; Markram et al. 2004; Metherate and Aramakis 1999). The FS basket cell anatomy was confirmed in a subset of recorded neurons (Markram et al. 2004). Low-threshold-spiking (LTS) cells were distinguished from FS cells by their broader spike half-widths, decreased AHP amplitudes, and pronounced spike adaptation (Gibson et al. 1999; Xiang et al. 1998). The LTS anatomy, including an ovoid cell body and a vertically oriented, bitufted dendritic morphology, was confirmed in a subset of recorded neurons (Gibson et al. 1999; Reyes et al. 1998; Xiang et al. 1998). Passive and intrinsic firing properties were evaluated on the basis of responses to current injection (1,500 ms). To determine spike threshold, incremental current intensities (1,500 ms, 10-pA steps) were delivered at 0.2 Hz until a spike was evoked.
Data acquisition and analysis.
Data were acquired at a sampling rate of 10 kHz using a custom-designed IGOR (version 4.08; WaveMetrics, Lake Oswego, OR) macro on a Macintosh platform (Apple, Cupertino, CA). A second IGOR macro was used for offline analysis. For sIPSC and me-IPSC analyses of amplitude or duration, summated IPSCs were excluded. sIPSC amplitudes were based on measurements from an average of 550 events per cell. Amplitudes were determined from the peak of the sIPSC or me-IPSC to baseline. me-IPSC amplitudes were measured from a baseline averaged for 5 ms before the stimulus onset. sIPSC amplitudes were measured from local baselines continuously identified during the 30-s traces using slope thresholds. An 8-pA amplitude threshold was used to detect sIPSCs from the baseline noise. sIPSC decay time constants were measured from single exponential fits of individual sIPSCs and were excluded if a subsequent IPSC occurred within 250 ms or if the reduced χ2 of the fit was >7.5. These parameters produced IPSC decay fits that were not contaminated by subsequent events. To exclude the effects of amplitude on sIPSC kinetics, kinetics were only measured from sIPSCs with amplitudes between 20 and 80 pA (Kotak et al. 2008). sIPSC kinetics were based on measurements from all sIPSCs that met these criteria, yielding an average of about 50 IPSCs per cell. sIPSC charge transfer was calculated as the mean integrated area under each sIPSC. Only sIPSCs with amplitudes above 20 pA were used for this analysis.
To measure interneuron firing properties, the maximum firing frequency was calculated as the maximum rate when current steps from threshold to 800 pA were applied in increments of 50 pA (0.1 Hz, 1,500 ms). Frequency-intensity curves were calculated as the mean firing frequency of cells that had reached spike threshold. For the calculation of maximum firing rates and frequency-intensity curves, only FS cells with firing rates >100 Hz were used. One FS cell each from control and CHL animals were excluded that fired <100 Hz. Their exclusion did not affect the significance of the difference in firing rates between FS cells from control and CHL animals. Spike adaptation was calculated as the average of the last two interspike intervals (ISIs) divided by the average of the first two ISIs in response to a depolarizing step that evoked firing rates of ∼50 Hz.
All data are means ± SE. Statistical tests were performed using statistical software (JMP; SAS Institute, Cary, NC). These include Levene's test for equal variance, ANOVA or Student's t-test for data distributed normally (t), and Kruskal-Wallis or Wilcoxon's nonparametric tests for data not distributed normally (χ2). Traces shown are individual traces from single neurons. Stimulus artifacts were decreased.
RESULTS
Development of cortical inhibitory synapses.
To facilitate the interpretation of experience-dependent changes, the normal development of inhibitory synaptic properties was first examined. In principle, deprivation can prevent or delay normal maturation, induce a relapse to an earlier state, or initiate a degenerative response. Therefore, inhibitory synaptic properties in auditory cortex were first evaluated at several developmental periods: before hearing onset (P8–P11), following hearing onset (P17–P22), before sexual maturity, which occurs at ∼P70 in gerbils (P30–P60), and at two adult ages (P90–P110, P180–P210). sIPSCs were recorded in L2/3 pyramidal cells in the presence of ionotropic glutamate receptor antagonists (Fig. 1A).
Fig. 1.
Development of cortical inhibitory synapses. A: spontaneous inhibitory postsynaptic currents (sIPSCs; 30 s) recorded in layer (L) 2/3 pyramidal cells in auditory cortex from animals age postnatal day (P) 8 to P186 [holding potential (Vhold) = −60 mV]. B: single sIPSCs from traces shown at left with an exponential decay fit overlay (gray). Scale bar, 10 pA. C: bar graphs (means ± SE) and distributions of average amplitude and decay time constants (tau) from all recorded cells across postnatal age groups (P8–P11, n = 13; P17–P22, n = 14; P30–P60, n = 11; P90–P110, n = 29; P180–P210, n = 26). *P < 0.05; **P < 0.01.
As shown in Fig. 1C, there was a modest change in amplitude across the age groups examined [sIPSC amplitude (pA): P8–P11: 30.4 ± 3.0, n = 13; P17–P22: 29.0 ± 4.0, n = 14; P30–P60: 25.4 ± 2.8, n = 11; P90–P110: 27.2 ± 2.7, n = 29; P180–P210: 34.5 ± 2.5, n = 26; χ2 = 11.5, degrees of freedom (df) = 4, P = 0.02] that occurred after P90–P110 (P17–P22 vs. P180–P210, χ2 = 4.8, df = 1, P = 0.03; P30–P60 vs. P180–P210, χ2 = 7.1, df = 1, P = 0.008; P90–P110 vs. P180–P210, χ2 = 8.0, df = 1, P = 0.005). Furthermore, the sIPSC decay kinetics, measured from single exponential fits (Fig. 1B), displayed a significant reduction throughout the examined age range [Fig. 1C; sIPSC decay constant (ms): P8–P11: 25.3 ± 1.4, n = 13; P17–P22: 19.0 ± 1.6, n = 13; P30–P60: 17.3 ± 1.3, n = 11; P90–P110: 11.7 ± 1.1, n = 27; P180–P210: 11.6 ± 0.6, n = 24; χ2 = 42.5, df = 4, P < 0.0001]. However, there was no significant change in sIPSC frequency across development [sIPSC frequency (Hz): P8–P11: 1.9 ± 0.3, n = 13; P17–P22: 3.5 ± 0.8, n = 14; P30–P60: 2.6 ± 0.8, n = 11; P90–P110: 4.3 ± 0.8, n = 29; P180–P210: 3.6 ± 0.4, n = 26; χ2 = 5.7, df = 4, P = 0.22]. Finally, the mean sIPSC charge transfer also showed a significant decrease from the juvenile to adult age range [sIPSC charge transfer (fC): P17–P22: 993 ± 101, n = 14; P180–P210: 665 ± 51, n = 14; t = 2.9, P = 0.007]. Thus, for the parameters examined, several properties of cortical inhibitory synapses were found to display a protracted period of maturation, into early adulthood.
CHL disrupts inhibitory synapse function.
To assess the effects of moderate CHL, the malleus was removed bilaterally in gerbils aged P10, elevating auditory thresholds by about 35–45 dB (Tucci et al. 1999; Xu et al. 2007). Animals were then reared for about a week after hearing onset (P17–P22) and compared with age-matched controls reared with normal auditory experience. As shown in Fig. 2A, neurons recorded from animals with CHL displayed significantly smaller sIPSCs compared with age-matched controls [sIPSC amplitude (pA): control: 29.0 ± 4.0, n = 14; CHL: 18.7 ± 2.4, n = 10; χ2 = −4.4, P = 0.04]. However, there was no significant change in the mean sIPSC frequency [sIPSC frequency (Hz): control: 3.5 ± 0.8 Hz, n = 14; CHL: 4.2 ± 0.6 Hz, n = 10; χ2 = 1.7, P = 0.20]. To determine whether CHL alters the kinetics of sIPSCs, ∼50 events from each neuron were fitted with a single exponential (see materials and methods). Figure 2B shows that the mean decay time constant was longer in CHL neurons compared with controls [sIPSC decay constant (ms): control: 19.0 ± 1.6, n = 13; CHL: 26.1 ± 3.5, n = 9; χ2 = 3.6; P = 0.05].
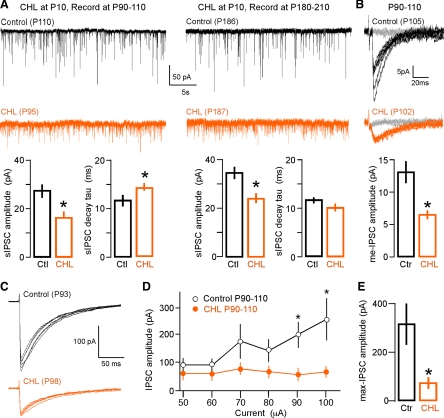
Fig. 2.
Early conductive hearing loss (CHL) alters inhibitory synapses. A: sIPSCs are smaller following CHL induced at P10. Top, sIPSCs (30 s) recorded in L2/3 cortical pyramidal cells from control and CHL animals (Vhold = −60 mV). Bottom, bar graph (means ± SE) summarizing mean sIPSC amplitudes (control, n = 14; CHL, n = 10). *P < 0.05. DNQX, 6,7-dinitroquinoxaline-2,3-dione; AP-5, 2-amino-5-phosphopentanoate; Ctl, control. B: sIPSCs display slower decay kinetics after CHL induced at P10. Top, representative single sIPSCs recorded in L2/3 cortical pyramidal cells from control and CHL animals (Vhold = −60 mV) with single exponential overlay (gray). Bottom, bar graph (means ± SE) summarizing mean sIPSC decay time constants (control, n = 13; CHL,: n = 9). *P = 0.05. C: minimum-evoked IPSCs (me-IPSCs) are smaller following CHL. Top, representative me-IPSCs evoked by intracortical stimulation (arrowheads) and recorded in L2/3 cortical pyramidal cells from control and CHL animals (Vhold = −60 mV). Gray traces indicate failures. Bottom, bar graph (means ± SE) summarizing mean me-IPSC amplitudes (control,: n = 9; CHL,: n = 8). *P < 0.01.
Finally, to determine whether CHL affects the strength of putative unitary inhibitory connections to L2/3 pyramidal neurons, IPSCs were elicited by minimum stimulation to L4. Figure 2C shows that me-IPSCs recorded in slices from CHL animals were significantly smaller than those recorded in controls [me-IPSC amplitude (pA): control: 12.1 ± 1.3, n = 9; CHL: 5.3 ± 0.5, n = 8; χ2 = 8.9, P = 0.003]. However, there was no significant change in the me-IPSC decay time constant [me-IPSC decay constant (ms): control: 32.1 ± 3.5, n = 9; CHL: 38.0 ± 10.1, n = 8; t = 0.6, P = 0.57].
CHL-induced changes in inhibition persist into adulthood.
The effect of sensory deprivation on synaptic function is typically examined at 1–2 wk after the manipulation, but it has yet to be determined whether early hearing loss induces long-term changes in synaptic inhibition. If inhibitory synaptic deficits persist past the juvenile stage and into adulthood, these effects would be potential candidates to explain the adult perceptual deficits associated with early hearing loss (Whitton and Polley 2011). Therefore, we sought to determine whether CHL-induced changes at inhibitory synapses persist into adulthood.
CHL was induced at P10, and animals were reared past sexual maturation. Pyramidal neurons were recorded at either of two ages (P90–P110 and P180–P210) in CHL and age-matched control animals. Figure 3A shows that sIPSC amplitude remained significantly smaller in CHL neurons compared with controls when recorded at P90–P110 (control: 27.2 ± 2.7 pA, n = 29; CHL: 16.5 ± 2.1 pA, n = 11; χ2 = 8.2, P = 0.004) or at P180–P210 (control: 34.5 ± 2.5 pA, n = 26; CHL: 24.1 ± 1.9 pA, n = 39; χ2 = 14.8, P = 0.0001). A significant increase in mean sIPSC frequency emerged in CHL neurons recorded at P180–P210 (P90–P110: control: 4.3 ± 0.8 Hz, n = 29; CHL: 3.1 ± 1.4 Hz, n = 11; χ2 = 0.55, P = 0.46; P180–P210: control: 3.6 ± 0.4 Hz, n = 26; CHL: 6.0 ± 0.5 Hz, n = 39; χ2 = 9.5, P = 0.002). Finally, the mean sIPSC decay time constant remained longer in CHL neurons recorded at P90–P110 compared with age-matched controls (control: 11.7 ± 1.1 ms, n = 27; CHL: 14.4 ± 0.7 ms, n = 9; t = 2.1; P = 0.046) but did not show a significant difference in CHL neurons recorded at P180–P210 (control: 11.6 ± 0.6 ms, n = 24; CHL: 10.2 ± 0.7 ms, n = 37; t = −1.4; P = 0.16).
Fig. 3.
Long-term impact of CHL on adult inhibition. A: sIPSCs recorded in neurons from adult animals (P90–P110, P180–P210) are smaller following CHL induced at P10. Top, sIPSCs (30 s) recorded in adult L2/3 cortical pyramidal cells (Vhold = −60 mV). Bottom, bar graphs (means ± SE) summarizing mean sIPSC amplitudes and decay time constants (control P90–P110, n = 29; CHL P90–P110, n = 11; control P180–P210, n = 26; CHL P180–P210, n = 39). *P < 0.05. B: me-IPSCs recorded in adult neurons (P90–P110) are smaller following CHL. Top, representative me-IPSCs recorded in L2/3 cortical pyramidal cells from adult control and CHL animals (Vhold = −60 mV). Bottom, bar graph (means ± SE) summarizing mean me-IPSC amplitudes (control, n = 13; CHL,: n = 6). *P < 0.05. C: representative IPSCs recorded in L2/3 cortical pyramidal cells from adult control and CHL animals (Vhold = −60 mV) in response to increasing stimulus current amplitude (50–100 μA, 10-μA steps). D: IPSC amplitude in response to increasing stimulus current amplitude (means ± SE; control, n = 12; CHL, n = 6). *P < 0.05. E: maximum-evoked IPSCs (max-IPSCs) recorded in adult neurons (P90–P110) are smaller following CHL. Bar graph (means ± SE) summarizes mean max-IPSC amplitudes (control, n = 12; CHL, n = 6). *P < 0.05.
Comparison of the adult evoked IPSCs also confirmed a long-term reduction in IPSC strength. Figure 3B shows that putative unitary inhibitory connections to pyramidal cells were significantly reduced in CHL neurons recorded at P90–P110 [me-IPSC amplitude (pA): control: 13.0 ± 1.7, n = 13; CHL: 6.4 ± 0.7, n = 6; χ2 = 4.8, P = 0.028]. Furthermore, me-IPSC decay time constants were longer in CHL neurons [me-IPSC decay constant (ms): control: 14.6 ± 1.7, n = 13; CHL: 29.7 ± 5.7, n = 6; χ2 = 6.9, P = 0.009]. Similarly, max-IPSCs were significantly smaller in CHL animals compared with controls [Fig. 3, C–E; max-IPSC amplitude (pA): control: 315 ± 83, n = 12; CHL: 73 ± 22, n = 6; χ2 = 4.2, P = 0.04] and showed prolonged decay time constants [max-IPSC decay constant (ms); control: 24.0 ± 4.7, n = 12; CHL: 51.3 ± 12.5, n = 6; χ2 = 5.1, P = 0.02].
Since inhibitory synapses display long-lasting effects of early CHL, we asked whether the cellular intrinsic properties of inhibitory interneurons that formed these synapses were also altered. To examine the long-term effects of developmental CHL on adult interneuron intrinsic properties, current-clamp recordings were obtained from two major interneuron subtypes, FS and LTS cells, from adult control animals (P180–P210) and age-matched animals with CHL induced at P10. Intrinsic firing properties were evaluated on the basis of suprathreshold responses to current injection (1,500 ms). The results showed that CHL had a significant impact on the firing properties of FS, but not LTS, interneurons. As shown in Fig. 4, the average firing rates in response to increasing current injections were significantly reduced in FS, but not LTS, cells from animals with long-term CHL. CHL resulted in a significant decrease in the maximum spike rate evoked by up to 800-pA current injection in FS cells (control: 235 ± 24 Hz, n = 10; CHL: 141 ± 20 Hz, n = 7; t = −2.9, P = 0.01), but not in LTS cells (control: 49 ± 10 Hz, n = 13; CHL: 47 ± 5 Hz, n = 20; χ2 = 0.20, P = 0.66). The decreased FS cell firing rates were associated with an increase in spike adaptation following CHL, calculated here as the average of the last two ISIs divided by the average of the first two ISIs (control: 1.4 ± 0.2, n = 10; CHL: 3.6 ± 0.7, n = 7, χ2 = 6.94, P = 0.008). However, CHL did not produce significant effects on the passive membrane properties of FS or LTS interneurons, including resting membrane potential, input resistance, and membrane time constant (Table 1).
Fig. 4.
Early CHL alters the firing properties of adult fast-spiking (FS) interneurons. A: representative spiking responses of FS and low-threshold-spiking (LTS) interneurons to suprathreshold current injection (1,500 ms) recorded from adult (P180–P210) control and CHL animals. FS interneurons displayed characteristic high-frequency spiking. Note the lower firing rate and pronounced spike adaptation in the FS cell from the CHL animal. LTS interneurons showed characteristic spike adaptation. B: firing rate as a function of current injection amplitude for FS and LTS cells (means ± SE). CHL significantly decreased FS cell firing rates but did not affect LTS cell firing (FS control, n = 10; FS CHL, n = 7; LTS control, n = 13; LTS CHL, n = 20). *P < 0.05.
Table 1.
Effects of early CHL on adult FS and LTS cell intrinsic properties
| FS control | FS CHL | LTS Control | LTS CHL | |
|---|---|---|---|---|
| n | 10 | 7 | 13 | 20 |
| RMP,mV | −59 ± 2 | −57 ± 1 | −59 ± 2 | −59 ± 1 |
| Rin, MΩ | 142 ± 20 | 211 ± 69 | 151 ± 10 | 175 ± 19 |
| τ, ms | 4.8 ± 0.5 | 5.2 ± 1.2 | 9.7 ± 1.3 | 10.3 ± 1.1 |
| I thresh, pA | 123 ± 22 | 206 ± 76 | 105 ± 15 | 93 ± 16 |
| V thresh, mV | −40 ± 2 | −35 ± 2 | −43 ± 2 | −43 ± 2 |
| AP width, ms | 0.30 ± 0.02 | 0.34 ± 0.04 | 0.74 ± 0.03 | 0.81 ± 0.04 |
| AHP, mv | −19 ± 1 | −19 ± 1 | −14 ± 1 | −14 ± 1 |
| AP amp, mV | 87 ± 5 | 98 ± 2 | 104 ± 2 | 107 ± 2 |
| Adaptation ratio | 1.4 ± 0.2 | 3.6 ± 0.7† | 5.3 ± 1.1 | 8.1 ± 2.2 |
| Max spike rate, Hz | 235 ± 23 | 141 ± 20* | 49 ± 10 | 47 ± 5 |
Values are means ± SE (n = no. of cells sampled) of intrinsic properties of fast-spiking (FS) and low-threshold-spiking (LTS) interneurons from adult [postnatal day (P)180–P200] control and conductive hearing loss (CHL) animals.
P < 0.05;
P < 0.01. RMP, resting membrane potential; Rin, input resistance; τ, membrane time constant; I thresh, current threshold required to elicit a spike; V thresh, voltage threshold to spike; AP width, action potential half-width; AP amp, action potential amplitude; adaptation ratio, average of last 2 interspike-intervals (ISI) divided by average of first 2 ISIs; Max spike rate, maximum spike rate evoked by ≤800-pA current injection.
Adult CHL does not affect inhibition.
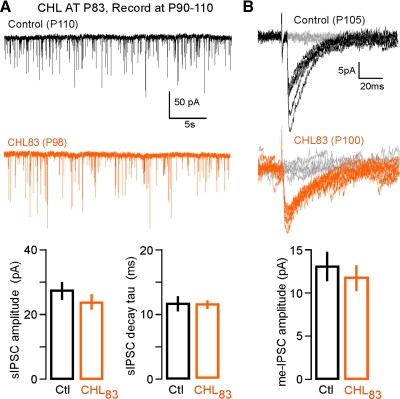
To determine whether inhibitory synapses are equivalently vulnerable to deprivation in adulthood, CHL was induced after sexual maturation (P83). As with the younger experimental group, animals were reared for about a week (P90–P110) and compared with age-matched controls. Following adult CHL, there was not a significant change in sIPSC amplitude, frequency, or decay constant compared with controls [Fig. 5A; sIPSC amplitude (pA): control: 27.2 ± 2.7, n = 29; CHL83: 24.0 ± 2.1, n = 20; χ2 = 0.17, P = 0.68; sIPSC frequency (Hz): control: 4.3 ± 0.8, n = 29; CHL83: 4.0 ± 0.9, n = 20; χ2 = 0.002, P = 0.97; sIPSC decay constant (ms): control: 11.7 ± 1.1, n = 27; CHL83: 11.5 ± 0.6, n = 16; χ2 = 1.2, P = 0.27]. Furthermore, adult CHL did not induce a significant change in me-IPSC amplitude or decay time constant [Fig. 5B; me-IPSC amplitude (pA): control: 13.0 ± 1.7, n = 13; CHL83: 11.7 ± 1.5, n = 16; t = −0.6, P = 0.56; me-IPSC decay constant (ms): control: 14.6 ± 1.7, n = 13; CHL83: 18.7 ± 1.3, n = 16; t = 1.9, P = 0.07].
Fig. 5.
Adult CHL does not alter inhibitory synapses. A: sIPSCs showed no significant change following CHL induced during adulthood at P83 (CHL83). Top, sIPSCs (30 s) recorded in L2/3 cortical pyramidal cells from adult animals (P90–P110; Vhold = −60 mV). Bottom, bar graphs (means ± SEM) summarizing the mean sIPSC amplitudes and decay time constants (control, n = 29; CHL83, n = 20). B: me-IPSCs showed no significant change in amplitude following CHL induced during adulthood. Top: representative me-IPSCs recorded in L2/3 cortical pyramidal cells from adult animals (P90–P110; Vhold = −60 mV). Bottom, bar graph (means ± SE) summarizing mean me-IPSC amplitudes (control, n = 13; CHL83, n = 16).
DISCUSSION
When animals are raised in a degraded environment, central nervous system (CNS) function can become disrupted. Depending on the manipulation, these effects are less prominent or absent when adult animals are subjected to the same environment (Harris and Rubel, 2006; Hensch 2005; Hooks and Chen 2007; Lewis and Maurer 2005; Knudsen 2004; Sanes and Bao 2009; Sharma et al. 2009; Woolsey 1990). In the auditory system, developmental hearing loss results in a profound reduction in the magnitude of inhibitory synaptic responses. If this dysfunction persists into adulthood, then it may contribute to diminished auditory behavioral performance (Whitton and Polley 2011). In this report, we present evidence that moderate hearing loss induced near the onset of hearing does result in enduring changes to cortical GABAergic inhibition. However, when induced in adulthood, inhibitory function was unaffected.
Inhibitory functional deficits persist to adulthood.
Conductive hearing loss, which produces a moderate increase in the threshold for hearing, was shown to impact cortical inhibitory synapse function when induced early in development (Fig. 2) The 35% decrease in sIPSC amplitude following CHL is consistent with the 30% decrease in the amplitude of miniature (m) IPSCs following developmental deprivation in the visual cortex (Maffei et al. 2010). Furthermore, the decrease in sIPSC and me-IPSC amplitudes following CHL is comparable in magnitude to that elicited by the most severe manipulation: sensorineural hearing loss (SNHL) (Kotak et al. 2008). This is consistent with recent findings that CHL and SNHL produce comparable effects on synaptic and intrinsic temporal features of L2/3 pyramidal cells (Takesian et al. 2010; Xu et al. 2007) as well as a similar reduction of long-term inhibitory potentiation (Xu et al. 2010).
The early CHL-induced reduction in inhibitory synaptic strength was as prominent in adult animals as it was within a week of the manipulation (Fig. 3). This was not entirely expected, because some previous reports have shown that hearing loss can result in transient adjustments in neural function (Mossop et al. 2000; Sun et al. 2009). In contrast, our results indicate that the CHL-induced decrease in inhibitory current amplitude did not recover in adults after more than 6 mo. This is consistent with the long-term change in 2-deoxyglucose uptake in the auditory brain stem and midbrain following unilateral CHL (Tucci et al. 1999) and the persistent (>6 mo) enhancement of ipsilaterally evoked responses in the inferior colliculus following unilateral neonatal cochlear ablation (Kitzes 1984; Kitzes and Semple 1985).
The long-term effect of early hearing loss is not uniform across all inhibitory synaptic properties. The kinetic properties of inhibitory synapses eventually recover to control levels over 6 mo. By comparing the inhibitory properties of control neurons from before hearing onset to adulthood (Fig. 1) with neurons from CHL animals (Fig. 3), we could establish that CHL delays the maturation of IPSC kinetics. In normal animals, IPSC kinetics show a protracted development during postnatal life: the IPSC decay time constant continues to decline for several months, corresponding to a significant decrease in IPSC charge transfer across development. This is consistent with longer duration inhibitory postsynaptic potentials (IPSPs) in kittens compared with adult cats (Purpura et al. 1965, 1968) and with a prolonged maturation of IPSP decay time in the primate prefrontal cortex (Hashimoto et al. 2009).
Both pre- and postsynaptic changes may underlie the developmental and CHL-induced effects on inhibitory function. For example, the maturation of sIPSCs in the primate prefrontal cortex corresponds with the emergence of the adult GABAA receptor subunit composition (Hashimoto et al. 2009). Similarly, in rat, the expression of GABAA receptor subunits undergoes dramatic changes during postnatal development (Laurie et al. 1992), and hippocampal inhibitory currents display age-dependent changes in responses to GABAAergic modulatory agents, extending into adulthood (Cohen et al. 1995). Finally, both pre- and postsynaptic markers of human GABAergic synaptic function show prolonged developmental trajectories that extend into adulthood (Pinto et al. 2010). Therefore, it is plausible that developmental CHL may produce a long delay in the expression of the mature GABAA receptor subunits that confer the fast activation and deactivation kinetics (Bosman et al. 2005; Ducić et al. 1995; Gingrich et al. 1995; Tia et al. 1996). This is consistent with previous observations from SNHL animals in which slower IPSC kinetics are associated with a failure or delay in the emergence of functional α1- and β2/3-subunits (Kotak et al. 2008; Sarro et al. 2008). Similar observations have been made in the visual cortex following developmental monocular deprivation (Maffei et al. 2010). In addition to postsynaptic alterations in GABAA receptor subunit composition, CHL may induce parallel presynaptic changes. This is supported by our present finding that long-term CHL leads to an increase in sIPSC frequency. Furthermore, our previous study showed that early CHL prevents a developmental shift from short-term depression to facilitation of IPSCs, suggesting that presynaptic release properties are affected (Takesian et al. 2010).
The effect of developmental CHL on IPSC amplitude and kinetics is most likely to occur at the inhibitory synapses formed by FS interneurons. We have previously reported that FS-evoked IPSCs display a threefold decrease in amplitude and slower kinetics after developmental SNHL; in contrast, this does not occur at LTS synapses (Takesian et al. 2010). Our present results further reveal that early hearing loss influences FS interneuron discharge properties into adulthood, but not those of LTS cells (Fig. 4). CHL permanently diminishes the maximum discharge rate of FS cells, presumably by disrupting developmental mechanisms that have been characterized previously (Itami et al. 2007; Okaty et al. 2009). For example, CHL may prevent the developmental upregulation of potassium channels Kv3.1b/Kv3.2 (Grabert and Wahle 2008) that endow normal adult FS cells with the capability to fire at high frequencies with little spike frequency adaptation (Du et al. 1996; Erisir et al. 1999; Okaty et al. 2009; Tansey et al. 2002). This is consistent with disrupted maturation of parvalbumin-positive inhibitory cells in noise-exposed regions of the auditory cortex (de Villers et al. 2008). Our results also agree with previous reports from auditory, visual, and somatosensory cortices, each of which demonstrates that FS and non-FS interneurons display a differential effect of developmental manipulations (Bartley et al. 2008; Maffei et al. 2004; Sun 2009; Takesian et al. 2010). The cumulative effects of decreased FS cell firing and diminished inhibitory synaptic strength are expected to result in less FS cell stimulus-evoked inhibition following CHL. Such a deficit may affect auditory tuning and temporal response properties that are thought to rely on strong FS cell feed-forward inhibition (Oswald et al. 2006).
Age-dependent influence of CHL on inhibitory synapse function.
Although the decline of cortical plasticity with age has been explored at the cellular level, previous studies generally focused on the developmental properties of excitatory afferents, particularly in the visual pathway (Hensch 2005; Hooks and Chen 2007). Since developmental hearing loss has a profound impact on inhibitory synapse development, we have proposed that this effect may explain some of the associated perceptual deficits (Sanes et al. 2009; Takesian et al. 2009). However, if similar changes occur following adult hearing loss, then this idea would be less compelling. In the present study, we found that CHL had a significant effect on inhibitory synapse function when induced during early life, whereas the same manipulation in adulthood had no effect. This suggests that there exists a developmental period during which CHL is maximally effective. Future studies that systematically vary the manipulation age across development will be instrumental in identifying the period of maximum sensitivity (i.e., critical or sensitive period) to this particular manipulation. Our results are consistent with several studies in visual cortex showing that the effects of deprivation on inhibitory function are age dependent (He et al. 2006; Maffei et al. 2010; Morales et al. 2002; Yazaki-Sugiyama et al. 2009).
The extent and type of inhibitory plasticity likely depend on several parameters, including the type of hearing loss, age of onset, magnitude, and duration (Sanes et al. 2009; Takesian et al. 2009). Inhibitory plasticity clearly occurs after early life. For example, it has been shown that adult cortical GABAergic neurons continue to undergo dendritic remodeling, suggesting that they remain plastic (Lee et al. 2006). Furthermore, adult deprivation can decrease GABAergic markers, although this may be a transient effect (Massie et al. 2003). Similarly, aging is associated with a decrease in GABAergic transmission, and this has been proposed to reflect a loss of hearing (presbycusis) analogous to changes induced by developmental hearing loss (Caspary et al. 2008). However, age-related hearing loss is likely to involve a prolonged degeneration of hair cell function, in contrast to CHL, and this could induce changes to the adult CNS. Changes in GABAergic inhibition may also lead to auditory cortex suppression in adults following passive exposure to band-limited tone pip ensembles (Noreña et al. 2006; Pienkowski et al. 2011). Our study suggests that, at a minimum, cortical inhibitory plasticity occurs over a slower time scale in the adult compared with the developing animal.
Implications for auditory dysfunction after early CHL.
Many previous reports have demonstrated that the early acoustic environment influences central coding properties and maps (Chang and Merzenich 2003; Chang et al. 2005; Kandler et al. 2009; Knudsen et al. 1984a, 1984b; Poon et al. 1990; Razak et al. 2008; Sanes and Constantine-Paton 1985; Zhang et al. 2002; Zhou et al. 2008). When the identical manipulation is performed at different times during development, age-dependent effects are often observed, indicating a critical period (de Villers-Sidani et al. 2007; Insanally et al. 2009). This principle appears to extend to hearing loss (Kral et al. 2002). For example, ear-plugging mice at P17–P21 leads to a dramatic increase in sensitivity to sound, but the same manipulation at P42–P46 does not induce this sensitivity (McGinn and Henry 1975). Similarly, monaural CHL shifts cortical tonotopic maps and augments responses from the unaffected ear, but only when it is induced at an early age (Popescu and Polley 2010).
CHL-induced alterations in central response properties in developing animals may underlie the impairments in sound localization (Clements and Kelly 1978) and binaural masking (Moore et al. 2003) that persist even after CHL is reversed. In humans, children with a history of severe CHL resulting from ear infections are at risk for receptive language deficits that can persist even after normal audiometric hearing is restored (Whitton and Polley 2011). We suggest that a persistent change in inhibitory synaptic strength may be involved in such long-term deficits associated with early CHL. Understanding the precise synaptic mechanisms underlying these changes will be instrumental in identifying targets to recover inhibitory function in adulthood.
GRANTS
This work was supported by National Institute of Deafness and Other Communications Disorders Grants DC011284 (to D. H. Sanes and V. C. Kotak) and DC008920 (to A. E. Takesian).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.E.T., V.C.K., and D.H.S. conception and design of research; A.E.T. performed experiments; A.E.T. analyzed data; A.E.T., V.C.K., and D.H.S. interpreted results of experiments; A.E.T. prepared figures; A.E.T., V.C.K., and D.H.S. drafted manuscript; A.E.T., V.C.K., and D.H.S. edited and revised manuscript; A.E.T., V.C.K., and D.H.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Chiye Aoki, Dr. Adam Carter, Dr. Barry Connors, and Dr. Alex Reyes for helpful comments on the thesis chapter from which this manuscript was derived.
REFERENCES
- Bartley AF, Huang ZJ, Huber KM, Gibson JR. Differential activity-dependent, homeostatic plasticity of two neocortical inhibitory circuits. J Neurophysiol 100: 1983–1994, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bledsoe SC, Jr, Nagase S, Miller JM, Altschuler RA. Deafness-induced plasticity in the mature central auditory system. Neuroreport 7: 225–229, 1995 [PubMed] [Google Scholar]
- Bosman LW, Heinen K, Spijker S, Brussaard AB. Mice lacking the major adult GABAA receptor subtype have normal number of synapses, but retain juvenile IPSC kinetics until adulthood. J Neurophysiol 94: 338–346, 2005 [DOI] [PubMed] [Google Scholar]
- Burianova J, Ouda L, Profant O, Syka J. Age-related changes in GAD levels in the central auditory system of the rat. Exp Gerontol 44: 161–169, 2009 [DOI] [PubMed] [Google Scholar]
- Caspary DM, Holder TM, Hughes LF, Milbrandt JC, McKernan RM, Naritoku DK. Age-related changes in GABAA receptor subunit composition and function in rat auditory system. Neuroscience 93: 307–312, 1999 [DOI] [PubMed] [Google Scholar]
- Caspary DM, Ling L, Turner JG, Hughes LF. Inhibitory neurotransmission, plasticity and aging in the mammalian central auditory system. J Exp Biol 211: 1781–1791, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspary DM, Milbrandt JC, Helfert RH. Central auditory aging: GABA changes in the inferior colliculus. Exp Gerontol 30: 349–360, 1995 [DOI] [PubMed] [Google Scholar]
- Caspary DM, Raza A, Lawhorn Armour BA, Pippin J, Arnerić SP. Immunocytochemical and neurochemical evidence for age-related loss of GABA in the inferior colliculus: implications for neural presbycusis. J Neurosci 10: 2363–2672, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang EF, Bao S, Imaizumi K, Schreiner CE, Merzenich MM. Development of spectral and temporal response selectivity in the auditory cortex. Proc Natl Acad Sci USA 102: 16460–16465, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang EF, Merzenich MM. Environmental noise retards auditory cortical development. Science 300: 498–502, 2003 [DOI] [PubMed] [Google Scholar]
- Clements M, Kelly JB. Auditory spatial responses of young guinea pigs (Cavia porcellus) during and after ear blocking. J Comp Physiol Psychol 92: 33–44, 1978 [DOI] [PubMed] [Google Scholar]
- Cohen AS, Lin DD, Coulter DA. Protracted postnatal development of inhibitory synaptic transmission in rat hippocampal area CA1 neurons. J Neurophysiol 84: 2465–2476, 1995 [DOI] [PubMed] [Google Scholar]
- Connors BW, Gutnick MJ. Intrinsic firing patterns of diverse neocortical neurons. Trends Neurosci 13: 365–366, 1990 [DOI] [PubMed] [Google Scholar]
- Cruikshank SJ, Rose HJ, Metherate R. Auditory thalamocortical synaptic transmission in vitro. J Neurophysiol 87: 361–384, 2002 [DOI] [PubMed] [Google Scholar]
- de Villers-Sidani E, Chang EF, Bao S, Merzenich MM. Critical period window for spectral tuning defined in the primary auditory cortex (A1) in the rat. J Neurosci 27: 180–189, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Villers-Sidani E, Simpson KL, Lu YF, Lin RCS, Merzenich MM. Manipulating critical period closure across different sectors of the primary auditory cortex. Nat Neurosci 11: 957–965, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Zhang L, Weiser M, Rudy B, McBain CJ. Developmental expression and functional characterization of the potassium-channel subunit Kv3.1b in parvalbumin-containing interneurons of the rat hippocampus. J Neurosci 16: 506–518, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducić I, Caruncho HJ, Zhu WJ, Vicini S, Costa E. γ-Aminobutyric acid gating of Cl− channels in recombinant GABAA receptors. J Pharmacol Exp Ther 272: 438–445, 1995 [PubMed] [Google Scholar]
- Erisir A, Lau D, Rudy B, Leonard CS. Function of specific K+ channels in sustained high-frequency firing of fast-spiking neocortical interneurons. J Neurophysiol 82: 2476–2489, 1999 [DOI] [PubMed] [Google Scholar]
- Fagiolini M, Fritschy JM, Löw K, Möhler H, Rudolph U, Hensch TK. Specific GABAA circuits for visual cortical plasticity. Science 303: 1681–1683, 2004 [DOI] [PubMed] [Google Scholar]
- Fagiolini M, Hensch TK. Inhibitory threshold for critical-period activation in primary visual cortex. Nature 404: 183–186, 2000 [DOI] [PubMed] [Google Scholar]
- Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled inhibitory neurons in neocortex. Nature 402: 75–79, 1999 [DOI] [PubMed] [Google Scholar]
- Gingrich KJ, Roberts WA, Kass RS. Dependence of the GABAA receptor gating kinetics on the alpha-subunit isoform: implications for structure-function and synaptic transmission. J Physiol 489: 529–543, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabert J, Wahle P. Neuronal activity and TrkB ligands influence Kv3.1b and Kv3.2 expression in developing cortical interneurons. Neuroscience 156: 618–629, 2008 [DOI] [PubMed] [Google Scholar]
- Harris JA, Rubel EW. Afferent regulation of neuron number in the cochlear nucleus: cellular and molecular analyses of a critical period. Hear Res 216–217: , 2006127–137 [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Nguyen QL, Rotaru D, Keenan T, Arion D, Beneyto M, Gonzalez-Burgos G, Lewis DA. Protracted developmental trajectories of GABAA receptor α1 and α2 subunit expression in primate prefrontal cortex. Biol Psychiatry 65: 1015–1023, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He HY, Hodos W, Quinlan EM. Visual deprivation reactivates rapid ocular dominance plasticity in adult visual cortex. J Neurosci 26: 2951–2955, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci 6: 877–888, 2005 [DOI] [PubMed] [Google Scholar]
- Hensch TK, Fagiolini M, Mataga N, Stryker MP, Baekkeskov S, Kash SF. Local GABA circuit control of experience-dependent plasticity in developing visual cortex. Science 282: 1504–1508, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensch TK, Stryker MP. Columnar architecture sculpted by GABA circuits in developing cat visual cortex. Science 303: 1678–1681, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooks BM, Chen C. Critical periods in the visual system: changing views for a model of experience-dependent plasticity. Neuron 56: 312–326, 2007 [DOI] [PubMed] [Google Scholar]
- Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF, Maffei L, Tonegawa S. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell 98: 739–755, 1999 [DOI] [PubMed] [Google Scholar]
- Insanally MN, Köver H, Kim H, Bao S. Feature-dependent sensitive periods in the development of complex sound representation. J Neurosci 29: 5456–5462, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itami C, Kimura F, Nakamura S. Brain-derived neurotrophic factor regulates the maturation of layer 4 fast-spiking cells after the second postnatal week in the barrel cortex. J Neurosci 27: 2241–2252, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai Y, Fagiolini M, Obata K, Hensch TK. Rapid critical period induction by tonic inhibition in visual cortex. J Neurosci 23: 6695–6702, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandler K, Clause A, Noh J. Tonotopic reorganization of developing auditory brainstem circuits. Nat Neurosci 12: 711–717, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri H, Fagiolini M, Hensch TK. Optimization of somatic inhibition at critical period onset in mouse visual cortex. Neuron 53: 805–812, 2007 [DOI] [PubMed] [Google Scholar]
- Kitzes LM, Semple MN. Single-unit responses in the inferior colliculus: effects of neonatal unilateral cochlear ablation. J Neurophysiol 53: 1483–1500, 1985 [DOI] [PubMed] [Google Scholar]
- Kitzes LM. Some physiological consequences of neonatal cochlear destruction in the inferior colliculus of the gerbil, Meriones unguiculatus. Brain Res 306: 291–298, 1984 [DOI] [PubMed] [Google Scholar]
- Knudsen EI. Sensitive periods in the development of the brain and behavior. J Cogn Neurosci 16: 1412–1425, 2004 [DOI] [PubMed] [Google Scholar]
- Knudsen EI, Esterly SD, Knudsen PF. Monaural occlusion alters sound localization during a sensitive period in the barn owl. J Neurosci 4: 1001–1011, 1984a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen EI, Knudsen PF, Esterly SD. A critical period for the recovery of sound localization accuracy following monaural occlusion in the barn owl. J Neurosci 4: 1012–1020, 1984b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotak VC, Fujisawa S, Lee FA, Karthikeyan O, Aoki C, Sanes DH. Hearing loss raises excitability in the auditory cortex. J Neurosci 25: 3908–3918, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotak VC, Takesian AE, Sanes DH. Hearing loss prevents the maturation of GABAergic transmission in the auditory cortex. Cereb Cortex 18: 2098–2108, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kral A, Hartmann R, Tillein J, Heid S, Klinke R. Hearing after congenital deafness: central auditory plasticity and sensory deprivation. Cereb Cortex 12: 797–807, 2002 [DOI] [PubMed] [Google Scholar]
- Laurie DJ, Wisden W, Seeburg PH. The distribution of thirteen GABAA receptor subunit mRNAs in the rat brain. III. Embryonic and postnatal development. J Neurosci 12: 4151–4172, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WC, Huang H, Feng G, Sanes JR, Brown EN, So PT, Nedivi E. Dynamic remodeling of dendritic arbors in GABAergic interneurons of adult visual cortex. PLoS Biol 4: e29, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis TL, Maurer D. Multiple sensitive periods in human visual development: evidence from visually deprived children. Dev Psychobiol 46: 163–183, 2005 [DOI] [PubMed] [Google Scholar]
- Ling LL, Hughes LF, Caspary DM. Age-related loss of the GABA synthetic enzyme glutamic acid decarboxylase in rat primary auditory cortex. Neuroscience 132: 1103–1113, 2005 [DOI] [PubMed] [Google Scholar]
- Maffei A, Lambo ME, Turrigiano GG. Critical period for inhibitory plasticity in rodent binocular V1. J Neurosci 30: 3304–3309, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei A, Nelson SB, Turrigiano GG. Selective reconfiguration of layer 4 visual cortical circuitry by visual deprivation. Nat Neurosci 7: 1353–1359, 2004 [DOI] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci 5: 793–807, 2004 [DOI] [PubMed] [Google Scholar]
- Massie A, Cnops L, Smolders I, Van Damme K, Vandenbussche E, Vandesande F, Eysel UT, Arckens L. Extracellular GABA concentrations in area 17 of cat visual cortex during topographic map reorganization following binocular central retinal lesioning. Brain Res 976: 100–108, 2003 [DOI] [PubMed] [Google Scholar]
- McGinn MD, Henry KR. Acute versus chronic acoustic deprivation: effects on auditory evoked potentials and seizures in mice. Dev Psychobiol 8: 223–232, 1975 [DOI] [PubMed] [Google Scholar]
- Metherate R, Aramakis VB. Intrinsic electrophysiology of neurons in thalamorecipient layers of developing rat auditory cortex. Brain Res Dev 115: 131–44, 1999 [DOI] [PubMed] [Google Scholar]
- Moore DR, Hartley DE, Hogan SC. Effects of otitis media with effusion (OME) on central auditory function. Int J Pediatr Otorhinolaryngol 67, Suppl 1: S63–S67, 2003 [DOI] [PubMed] [Google Scholar]
- Morales B, Choi Y, Kirkwood A. Dark rearing alters the development of GABAergic transmission in visual cortex. J Neurosci 22: 8084–8090, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossop JE, Wilson MJ, Caspary DM, Moore DR. Down-regulation of inhibition following unilateral deafening. Hear Res 147: 183–187, 2000 [DOI] [PubMed] [Google Scholar]
- Noreña AJ, Gourévitch B, Aizawa N, Eggermont JJ. Spectrally enhanced acoustic environment disrupts frequency representation in the cat auditory cortex. Nat Neurosci 9: 932–939, 2006 [DOI] [PubMed] [Google Scholar]
- Okaty BW, Miller MN, Sugino K, Hempel CM, Nelson SB. Transcriptional and electrophysiological maturation of neocortical fast-spiking GABAergic interneurons. J Neurosci 29: 7040–7052, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald AM, Schiff ML, Reyes AD. Synaptic mechanisms underlying auditory processing. Curr Opin Neurobiol 16: 371–376, 2006 [DOI] [PubMed] [Google Scholar]
- Pienkowski M, Munguia R, Eggermont JJ. Passive exposure of adult cats to bandlimited tone pip ensembles or noise leads to long-term response suppression in auditory cortex. Hear Res 277: 117–126, 2011 [DOI] [PubMed] [Google Scholar]
- Pinto JG, Hornby KR, Jones DG, Murphy KM. Developmental changes in GABAergic mechanisms in human visual cortex across the lifespan. Front Cell Neurosci 4: 16, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon PW, Chen XY, Hwang JC. Altered sensitivities of auditory neurons in the rat midbrain following early postnatal exposure to patterned sounds. Brain Res 524: 327–330, 1990 [DOI] [PubMed] [Google Scholar]
- Popescu MV, Polley DB. Monaural deprivation disrupts development of binaural selectivity in auditory midbrain and cortex. Neuron 65: 718–731, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purpura DP, Prelevic S, Santini M. Postsynaptic potentials and spike variations in the feline hippocampus during postnatal ontogenesis. Exp Neurol 22: 408–422, 1968 [DOI] [PubMed] [Google Scholar]
- Purpura DP, Shofer RJ, Scarff T. Properties of synaptic activities and spike potentials of neurons in immature neocortex. J Neurophysiol 28: 925–942, 1965 [DOI] [PubMed] [Google Scholar]
- Rajan R. Plasticity of excitation and inhibition in the receptive field of primary auditory cortical neurons after limited receptor organ damage. Cereb Cortex 11: 171–182, 2001 [DOI] [PubMed] [Google Scholar]
- Rajan R. Receptor organ damage causes loss of cortical surround inhibition without topographic map plasticity. Nat Neurosci 1: 38–43, 1998 [DOI] [PubMed] [Google Scholar]
- Raza A, Milbrandt JC, Arneric SP, Caspary DM. Age-related changes in brainstem auditory neurotransmitters: measures of GABA and acetylcholine function. Hear Res 77: 221–230, 1994 [DOI] [PubMed] [Google Scholar]
- Razak KA, Richardson MD, Fuzessery ZM. Experience is required for the maintenance and refinement of FM sweep selectivity in the developing auditory cortex. Proc Natl Acad Sci USA 105: 4465–4470, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes A, Lujan R, Rozov A, Burnashev N, Somogyi P, Sakmann B. Target-cell-specific facilitation and depression in neocortical circuits. Nat Neurosci 1: 279–285, 1998 [DOI] [PubMed] [Google Scholar]
- Sanes DH, Bao S. Tuning up the developing auditory CNS. Curr Opin Neurobiol 19: 188–199, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes DH, Constantine-Paton M. The sharpening of frequency tuning curves requires patterned activity during development in the mouse, Mus musculus. J Neurosci 5: 1152–1166, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes DH, Sarro EC, Takesian AE, Aoki C, Kotak VC. Regulation of inhibitory synapse function in the developing auditory CNS. In: Developmental Plasticity of Inhibitory Circuitry, edited by Pallas SL. New York: Springer, 2009, p. 43–70 [Google Scholar]
- Sarro EC, Kotak VC, Sanes DH, Aoki C. Hearing loss alters the subcellular distribution of presynaptic GAD and postsynaptic GABA-A receptors in the auditory cortex. Cereb Cortex 18: 2855–2867, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Nash AA, Dorman M. Cortical development, plasticity and re-organization in children with cochlear implants. J Commun Disord 42: 272–279, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwell DG, Froemke RC, Alvarez-Buylla A, Stryker MP, Gandhi SP. Cortical plasticity induced by inhibitory neuron transplantation. Science 327: 1145–1148, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama S, Di Nardo AA, Aizawa S, Matsuo I, Volovitch M, Prochiantz A, Hensch TK. Experience-dependent transfer of Otx2 homeoprotein into the visual cortex activates postnatal plasticity. Cell 134: 508–520, 2008 [DOI] [PubMed] [Google Scholar]
- Sun QQ. Experience-dependent intrinsic plasticity in interneurons of barrel cortex layer IV. J Neurophysiol 102: 2955–2973, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Guo YP, Shum DKY, Chan YS, He J. Time course of cortically induced fos expression in auditory thalamus and midbrain after bilateral cochlear ablation. Neuroscience 160: 186–197, 2009 [DOI] [PubMed] [Google Scholar]
- Takesian AE, Kotak VC, Sanes DH. Developmental hearing loss disrupts synaptic inhibition: implications for auditory processing. Future Neurol 4: 331–349, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takesian AE, Kotak VC, Sanes DH. Presynaptic GABAB receptors regulate experience-dependent development of inhibitory short-term plasticity. J Neurosci 30: 2716–2727, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tansey EP, Chow A, Rudy B, McBain CJ. Developmental expression of potassium-channel subunit Kv3.2 within subpopulations of mouse hippocampal inhibitory interneurons. Hippocampus 12: 137–148, 2002 [DOI] [PubMed] [Google Scholar]
- Tia S, Wang JF, Kotchabhakdi N, Vicini S. Distinct deactivation and desensitization kinetics of recombinant GABAA receptors. Neuropharmacology 35: 1375–1382, 1996 [DOI] [PubMed] [Google Scholar]
- Tucci DL, Cant NB, Durham D. Conductive hearing loss results in a decrease in central auditory system activity in the young gerbil. Laryngoscope 109: 1359–1371, 1999 [DOI] [PubMed] [Google Scholar]
- Vale C, Sanes DH. Afferent regulation of inhibitory synaptic transmission in the developing auditory midbrain. J Neurosci 20: 1912–1921, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Manis PB. Synaptic transmission at the cochlear nucleus endbulb synapse during age-related hearing loss in mice. J Neurophysiol 94: 1814–1824, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Manis PB. Temporal coding by cochlear nucleus bushy cells in DBA/2J mice with early onset hearing loss. J Assoc Res Otolaryngol 7: 412–424, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitton JP, Polley DB. Evaluating the perceptual and pathophysiological consequences of auditory deprivation in early postnatal life: a comparison of basic and clinical studies. J Assoc Res Otolaryngol 12: 535–547, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolsey TA. Peripheral alteration and somatosensory development. In: Development of Sensory Systems in Mammals, edited by Coleman EJ. New York: Wiley, 1990, p. 461–516 [Google Scholar]
- Xiang Z, Huguenard JR, Prince DA. Cholinergic switching within neocortical inhibitory networks. Science 281: 985–988, 1998 [DOI] [PubMed] [Google Scholar]
- Xu H, Kotak VC, Sanes DH. Conductive hearing loss disrupts synaptic and spike adaptation in developing auditory cortex. J Neurosci 27: 9417–9426, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Kotak VC, Sanes DH. Normal hearing is required for the emergence of long-lasting inhibitory potentiation in cortex. J Neurosci 30: 331–341, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazaki-Sugiyama Y, Kang S, Cåteau H, Fukai T, Hensch TK. Bidirectional plasticity in fast-spiking GABA circuits by visual experience. Nature 462: 218–221, 2009 [DOI] [PubMed] [Google Scholar]
- Zhang LI, Bao S, Merzenich MM. Disruption of primary auditory cortex by synchronous auditory inputs during a critical period. Proc Natl Acad Sci USA 99: 2309–2314, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Nagarajan N, Mossop BJ, Merzenich MM. Influences of un-modulated acoustic inputs on functional maturation and critical-period plasticity of the primary auditory cortex. Neuroscience 154: 390–396, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]