Abstract
ATP signaling to neurons and glia in the nervous system occurs via activation of both P2Y and P2X receptors. Here, we investigated the effects of P2Y1 receptor stimulation in developing striatal medium-sized neurons using patch-clamp recordings from acute brain slices of 7- and 28-day-old rats. Application of the selective P2Y1 receptor agonist 2-(Methylthio) ADP trisodium salt (2-MeSADP; 250 nM) increased outward K+ currents evoked by a ramp depolarization protocol in voltage-clamp recordings. This effect was observed in 59 out of 82 cells (72%) and was blocked completely by the P2Y1 antagonist, 2′-deoxy-N6-methyl adenosine 3′,5′-diphosphate. The averaged 2-MeSADP-sensitive conductance was fitted by the sum of a linear conductance and a Boltzmann relation, giving one-half activation voltage of −14.2 mV and an equivalent charge of 2.91. The 2MeSADP-mediated effect was sensitive to submillimolar concentrations of tetraethylammonium (TEA; 200 μM), to 200 nM iberiotoxin and to 100 nM apamin, suggesting the involvement of both big and small potassium (BK and SK, respectively) calcium-activated channels. In current-clamp experiments, 2-MeSADP decreased depolarization-evoked action potential (AP) firing in all 26 cells investigated, and this effect was reversed by TEA and by apamin but not by iberiotoxin. We conclude that the stimulation of P2Y1 receptors in developing striatal neurons leads to activation of calcium-activated potassium channels [IK(Ca)] of both BK and SK subtypes, the latter responsible for decreasing the frequency of AP firing in response to current injection. Therefore, P2Y1 signaling leading to activation of IK(Ca) may be important in regulating the activity of medium-sized neurons in the striatum.
Keywords: ATP, medium-spiny neuron, striatum, potassium channel
purinergic p2 receptor signaling has been investigated extensively in the nervous system, both in neuron-to-glia communication (see, e.g., Abbrachio et al. 2009; Fields and Burnstock 2006; Pocock and Kettenmann 2007) and in synaptic transmission (Edwards et al. 1992; Nieber et al. 1997; Pankratov et al. 1998, 2007).
The influence of P2 receptors on neuron and glial differentiation and development is of particular interest (Arthur et al. 2005, 2006; Mishra et al. 2006; Neary and Zimmermann 2009; Stevens and Fields 2000; Weissman et al. 2004), and we have therefore investigated the effect of P2Y receptor activation in developing neurons of the rat striatum. There is also growing interest in P2Y receptors as therapeutic targets (Jacobson and Boeynaems 2010), adding to the need to understand the physiological effects of P2Y receptor activation and block.
Purinergic P2 receptors are divided into P2X cation channels and P2Y metabotropic receptors (Abbracchio and Burnstock 1994; Burnstock 2007, 2008; Inoue et al. 1996; North 2002; Ralevic and Burnstock 1998). P2Y receptors are G-protein coupled (Abbracchio et al. 2006; von Kugelgen 2006). Eight subtypes (P2Y1,2,4,6,11,12,13,14) are known (Abbracchio et al. 2006; Alexander et al. 2007; Burnstock 2007, 2008) with P2Y1,2,4,6,11 coupling via Gq/11 proteins to stimulate PLC, resulting in hydrolysis of membrane phosphatidylinositol 4,5-bisphosphate to generate inositol trisphosphate and diacylglycerol and mobilization of intracellular Ca2+. P2Y12,13,14 activate Gi proteins to inhibit adenylate cyclase (Burnstock 2007; Fredholm et al. 2003; Paredes-Gamero et al. 2006; Sanches et al. 2002; von Kugelgen 2006).
P2Y1 receptors are widely distributed in the nervous system, and both mRNA (Moore et al. 2001) and protein expression (Amadio et al. 2007; Franke et al. 2003; Moore et al. 2000) have been demonstrated in human and rat striatum. However, there is conflicting evidence about their functional role in this brain region. For example, Ikeuchi and Nishizaki (1995) demonstrated that in primary cultures of striatal neurons (from 1-day-old rats), ATP activated a sustained outward potassium current. In contrast, Scheibler et al. (2004) reported a lack of functional effects after either P2X or P2Y stimulation in medium-spiny neurons of 5- to 26-day-old rat striatal slices, despite observing P2X2 and P2Y1 protein expression by immunehistochemistry in roughly 50% of medium-spiny neurons and cholinergic interneurons (Scheibler et al. 2004).
It has been proposed that the absence or the presence of a P2Y1-mediated response in striatal neurons might be due to variations in postnatal development (Rubini et al. 2006). Medium-spiny neurons undergo profound electrophysiological changes during the first few postnatal weeks, reaching a fully developed firing activity after the 3rd postnatal wk (Tepper et al. 1998; Tepper and Trent 1993).
In the present work, we investigated the effect of P2Y1 receptor activation in medium-sized neurons in acute striatal slices from neonatal and young (1- and 4-wk-old) rats. Our results show that the selective P2Y1 agonist, 2-(Methylthio) ADP trisodium salt (2-MeSADP), can activate Ca2+-sensitive K+ channel currents recorded during voltage-ramp depolarizations and regulate the action potential (AP) firing rate in current-clamp recordings.
METHODS
Solutions.
A modified Krebs solution with the following composition was used for slicing (in mM): sucrose 206 (instead of NaCl because of better preservation of slice cells), KCl 2.5, CaCl2 1.0, MgCl2 1, NaH2PO4 1.25, NaHCO3 26, glucose 25, pH 7.4, when bubbled with 95% O2 and 5% CO2.. An artificial cerebrospinal fluid with the following composition was used for the recording solution (in mM): NaCl 125, KCl 2.5, CaCl2 1, MgCl2 1, NaH2PO4 1.25, NaHCO3 26, glucose 25, pH 7.4. When recording voltage-dependent calcium currents, TTX (500 nM) was used, BaCl (5 mM) was substituted for CaCl, and tetraethylammonium (TEA) chloride (5 mM) was added to block potassium currents. The slicing and recording solutions were gassed continuously with a mixture of O2 (95%) and CO2 (5%). In some experiments, TTX (100 nM) was added to the external recording solution to block voltage-gated Na+ currents.
To approximate physiological conditions, patch pipettes were filled with a potassium gluconate (KGlu) pipette solution. The composition of the pipette solution was (in mM): KGlu 140, EGTA 0.2, HEPES 10, NaCl 10, MgCl2 1, adjusted to pH 7.4, with potassium hydroxide. A relatively low concentration of intracellular calcium buffer (EGTA) was used to avoid disrupting calcium signaling in response to P2Y receptor activation. This contrasts with a previous study of P2 receptors in striatal cells, where a high concentration of EGTA (11 mM) was used (Scheibler et al. 2004). Membrane voltages given in the results are not corrected for the liquid junction potential (−8 mV) of the pipette solution. This solution was stored at −20°C in 1 ml aliquots. ATP 2 mM and GTP 0.5 mM were added daily to each aliquot and the solution stored on ice during use. The pipette solution used when recording voltage-dependent calcium currents contained (in mM): CsCl 40, N-methyl-d-glucamine (NMDG) 140, EGTA 10, HEPES 10, NaCl 10, MgCl2 1, ATP 2 mM, and GTP 0.5 mM, at pH 7.4. To observe cell morphology, in some experiments, the pipette solution also contained 1 mg/ml Lucifer yellow.
Drugs and chemicals.
NaCl, NaOH, NaH2PO4, NaHCO3, KCl, CaCl2, MgCl2, sucrose, CsCl, NMDG, and glucose were purchased from BDH Laboratory Supplies (Poole, England). HEPES, EGTA, ATP, GTP, 2-MeSADP, 2′-deoxy-N6-methyl adenosine 3′,5′-diphosphate (MRS 2179), Lucifer yellow dipotassium salt, iberiotoxin, and apamin were purchased from Sigma (St. Louis, MO). TTX was purchased from Ascent Scientific (Bristol, UK) or Alomone Labs (Jerusalem, Israel).
The concentration of the P2Y1-selective agonist 2-MeSADP (250 nM) was chosen on the basis of ligand-binding studies from Gao et al. (2004), who reported an inhibition constant value of 57 nM in astrocytoma cells. The Hill-Langmuir equation was used to calculate the agonist concentration necessary to obtain 80% occupancy of P2Y1 receptors. The concentration of the P2Y1-selective antagonist MRS 2179 (15 μM) was chosen on the basis of the dissociation constant = 177 nM, reported by Moro et al. (1998), to obtain a 95% block of P2Y1 receptors in the presence of 250 nM 2-MeSADP. Apamin and iberiotoxin concentrations were chosen on the basis of previous work, showing a maximal block of big and small potassium (BK and SK, respectively) calcium-activated channels [IK(Ca)] at 10 nM for both toxins in rat striatum (Bargas et al. 1999; Nisenbaum et al. 1996). Slices were superfused (1 ml/min in a bath volume of 0.3 ml) in the recording chamber by a three-way, gravity-fed system. In each slice, only one whole-cell recording was made in the presence of 250 nM 2-MeSADP.
Brain slice preparation.
Striatum slices were prepared as described elsewhere (Edwards et al. 1989). All experimental procedures were approved following ethical review by the UK Home Office and carried out in accordance with the UK Animals (Scientific Procedures) Act, 1986. Every effort was made to minimize animal suffering and the number of animals used. Seven-day-old male Sprague-Dawley rats were killed humanely by decapitation, whereas 28-day-old rats were deeply anesthetized with isofluorane before decapitation. The brain was removed from the skull and submerged in ice-cold slicing solution, continuously bubbled with a mixture of O2 (95%) and CO2 (5%). Thick, horizontal striatal brain slices (300 μm) were cut with a vibroslicer (DTK 1000, Dosaka, Kyoto, Japan) and transferred into a 100-ml incubation chamber (Edwards and Konnerth 1992) containing external recording solution, continuously bubbled with a mixture of O2 (95%) and CO2 (5%). Slices were incubated at room temperature (20–24°C) for a period that ranged from 45 min to 8 h before experimenting.
The cell bodies of individual neurons in brain slices were visualised under Nomarski differential interference contrast optics (Edwards et al. 1989). Striatal neurons suitable for patch clamping were identified by their location, size, and morphology. Neuron morphology was examined in a proportion of experiments by including the fluorescent dye Lucifer yellow in the pipette solution to visualize the shape of the cell and dendritic tree during the experiment (Fig. 1A). These neurons were, on average, 14.8 ± 1.2 μm in diameter, and had between two and five dendrites (mean of 2.8 ± 0.29), which ranged from 34 to 180 μm in length with a first branch point at 19.8 ± 4.9 μm from the cell body. As illustrated in Fig. 1B, 2-MeSADP produced a decrease in firing rate in these cells when equivalent depolarizing current pulses were applied from a resting potential of −70 mV. Cell capacitance averaged 19.2 ± 0.98 pF. As medium-spiny neurons comprise ∼90% of striatal neurons (Jain et al. 2001; Kawaguchi 1995; Rymar et al. 2004) and are ∼15 μm in diameter, the neurons in this study are likely to be medium-spiny neurons based on their size and electrophysiological properties (Blomeley et al. 2009; Bracci and Panzeri 2006; Calabresi et al. 1987; Kawaguchi 1992, 1993; Kawaguchi et al. 1989).
Fig. 1.
Illustration of the action of 2-(Methylthio) ADP trisodium salt (2-MeSADP) in neurons filled with the fluorescent dye, Lucifer yellow. A: differential interference contrast image of a striatal slice from a P7 rat during whole-cell recording where the pipette solution contains Lucifer yellow. Also evident in this image is a band of axons (bracketed) typical of the striatal slice at this stage of development. On the left of the figure (arrowed) is the patch pipette attached to the cell imaged using fluorescence optics on the right of the figure (original scale bars, 30 μm). B: 2-MeSADP (250 nM) application to the cell imaged in A during current clamp during depolarizing current steps shows a characteristic reduction in firing frequency from 16 Hz in control to 12 Hz in the presence of the P2Y1 agonist. The voltage response to 2 current steps of 10 pA and 80 pA is shown for control and in the presence of 2-MeSADP. Between current pulses, a small, hyperpolarizing current was applied to maintain the resting membrane potential close to −70 mV.
Whole-cell recordings.
Patch pipettes for whole-cell recordings were made from thick-walled borosilicate glass capillaries (GC150F-7.5, Harvard Apparatus, Holliston, MA) of final resistance of 6–8 MΩ. For current-clamp recordings, pipettes were coated with silicone resin (Sylgard 184, Dow Corning, Midland, MI) to reduce the pipette capacitance. To reduce the voltage error due to the series resistance (Rs) existing in whole-cell voltage and current-clamp experiments, >75% of the Rs was compensated before starting recordings.
Voltage-clamp experiments.
Whole-cell currents were recorded using an Axopatch 200B amplifier and evoked by voltage ramps applied and recorded using the program WinWCP (available from Strathclyde Electrophysiology Software, Glasgow, UK, at http://spider.science.strath.ac.uk/PhysPharm/showPage.php?page Name=software_ses). Currents were amplified and filtered at 2 kHz (8-pole Bessel) and digitized at 20 kHz using an analog-to-digital converter (CED micro 1401, Cambridge Electronic Design, UK). Four successive voltage ramps (from −100 mV to +40 mV; 1.8-s duration) were applied to the cell at 20-s intervals (using WinWCP; available from Strathclyde Electrophysiology Software). All traces shown are the average of four consecutive ramp episodes. When the ramps recorded in control conditions were sufficiently stable (<10% variation) the P2Y1 agonist 2-MeSADP (250 nM) was superfused. Further voltage-ramp responses were aquired 3 and 8 min after drug application. In some experiments, potassium channel blockers (TEA 200 μM, iberiotoxin 200 nM, apamin 100 nM) were added to the superfusion before or in the presence of 2-MeSADP to evaluate whether they were able to block the effect observed with the P2Y1 agonist. At the end of the treatment, all drugs were washed out for at least 5 min, and further voltage-ramp currents were then recorded. Voltage-dependent calcium currents were evoked using 100 ms voltage steps from a holding potential of −90 mV to test potentials at 10-mV increments from −80 mV to +30 mV. The average current was calculated from four steps to each test voltage with linear leakage and capacitance currents subtracted using a P/4 protocol. Current-voltage (I-V) relationships were analyzed in Excel or Prism 4.00 software after each recording.
Current-clamp experiments.
Current-clamp recordings were performed in a TTX-free extracellular solution, and when necessary, a small negative or positive current injection (5–10 pA) was used to obtain a resting membrane potential of −70 mV. Ten steps of positive current injection (1.2-s duration; 10-pA increments) were applied in control condition, at 20-s intervals. The amplitude of the first step (varying from 2 to 50 pA) was chosen on the basis of each single cell recorded to obtain both subthreshold and suprathreshold responses.
Current-clamp recordings were filtered at 10 kHz and digitized at 35 kHz using the program WinWCP. For each cell following control recordings and 5 min application of 2-MeSADP, the same stimulation protocol was applied to the cell again and in some cells, repeated after superfusion with different potassium channel blockers. In a separate group of cells, the effect of potassium channel blockers was tested following control recordings and prior to application of 2-MeSADP. The AP frequency was measured as the number of APs/s. The rate of AP depolarization and hyperpolarization was measured as the first derivative of membrane potential over time (mV/ms). AP threshold was defined as the point at which the derivative was ≥30 mV/ms. AP amplitude was calculated as the difference between the peak reached by the overshoot and the resting potential. The AP width was measured as the difference between the time of reaching the threshold during the rising phase and the time when the repolarizing potential crossed the threshold value again. The medium afterhyperpolarization potential (mAHP) was measured as the difference between the minimum potential reached after the AP peak and the voltage reached by the step stimulation. No slow AHP (sAHP) after the end of the step was observed in any cell investigated. The AP latency was determined as the time between the stimulus onset and when the first AP threshold was reached. Interspike intervals (ISIs) were measured at the AP peak. Spike-frequency adaptation during repetitive firing was quantified by dividing the first ISI by the second ISI. Values ∼1 show no spike-frequency adaptation.
Statistics.
Averaged data in the text are reported as mean ± SE. Student's paired t-test was used for statistical comparisons between data obtained from the same cell before and after any treatment. For multiple comparisons, one-way ANOVA with Tukey's multiple comparisons test was used (Graphpad Prism Software, La Jolla, CA). Statistical significance was set at P < 0.05.
RESULTS
P2Y1 receptor activation increases outward currents elicited by voltage-ramp depolarizations in medium-sized striatal neurons.
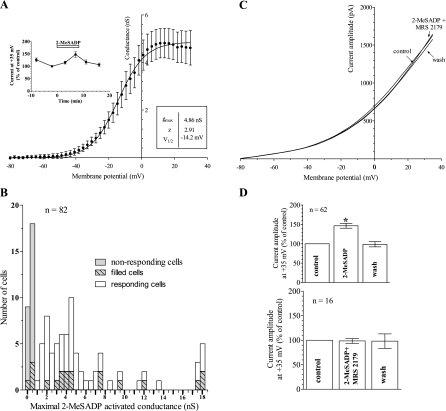
To investigate purinergic modulation of the electrical activity of striatal neurons, we recorded whole-cell currents evoked by voltage-ramp depolarization in medium-sized neurons of rat striatal slices in the absence and in the presence of the P2Y1-selective agonist 2-MeSADP. In slices from both 7- and 28-day-old rats, 2-MeSADP was found to increase the outward current in response to a voltage-ramp depolarization (Fig. 2). Initially, we measured the amplitude of voltage-ramp currents evoked before, during, and after 8 min application of 250 nM 2-MeSADP, a selective P2Y1 agonist. As shown in Fig. 2A, the current recorded in control conditions shows little inward component, as expected in the presence of the Na+-channel blocker TTX (100 nM) and in accordance with previous observations demonstrating that in medium-spiny neurons, voltage-dependent Ca2+ currents do not normally contribute to steady-state, whole-cell currents (Bargas et al. 1999; Hoehn et al. 1993). As shown in Fig. 2A, voltage ramps under control conditions were dominated by outward potassium currents. After 5 min superfusion of 250 nM 2-MeSADP, the outward current elicited by the ramp depolarization was markedly increased (to 159 ± 18% of control), and the effect was reversed after drug removal. It should be noted that the large amplitude of the outward current at positive voltages means that the Rs error will tend to result in underestimation of the magnitude of the P2Y potentiation. Figure 2B shows the 2MeSADP-activated current obtained by subtraction of the ramp current recorded in control from the current in the presence of the P2Y1 agonist. Figure 2B(i) shows the time course of current changes at +35 mV recorded before, during, and after 2MeSADP application. The maximal effect was reached after 8 min of drug superfusion and is reversed after a few minutes of washout. Figure 2C shows the 2-MeSADP-sensitive conductance calculated, assuming a potassium reversal potential of −101 mV. The curve obtained can be fitted by the sum of a small, linear conductance and a Boltzmann relation for voltage-dependent membrane conductances
where gmin and gmax are the minimum and maximum value for the voltage-dependent conductance; V1/2 is the membrane potential at which one-half of gmax is reached; z is the equivalent charge; and R, T, and F are the gas constant, the absolute temperature, and the Faraday constant, respectively. The values obtained from fitting the 2-MeSADP-activated conductance in this cell were: V1/2 = −15.2 mV, z = 2.31, gmin = 0.76 nS, and gmax = 10.7 nS.
Fig. 2.
2-MeSADP activates an outward current during voltage ramps in medium-sized neurons of rat striatal slices. A(i): the inset figure shows the ramp protocol of a typical experiment. The whole-cell outward current activated by the ramp protocol increased after 5 min application of 250 nM 2-MeSADP. In this cell, the effect of 2-MeSADP reversed after 20 min of drug removal (wash). (ii): example of an equivalent experiment from 28-day-old rat striatum. B(i): the 2-MeSADP-sensitive current recorded in the cell shown in A(i) is obtained by subtraction of the control trace from the ramp current recorded in the presence of the P2Y agonist. Inset: time course of current amplitude at +35 mV activated by the ramp protocol before, during, and after 2-MeSADP application. (ii): 2-MeSADP-sensitive current recorded in the cell shown in A(ii). C(i) and (ii): 2-MeSADP-activated conductance in the same cells as shown in A(i) and (ii). Solid lines show the fit of the data from −80 mV to +35 mV to the sum of a linear minimum conductance (gmin) and a Boltzmann relation giving estimates of the maximum conductance (gmax), 1/2 maximal voltage (V1/2), and equivalent charge (z).
In slices from 28-day-old rats, the outward current was also potentiated by 2-MeSADP [Fig. 2A(ii)]. In these cells 2MeSADP significantly increased (by 23.7 ± 5.9%) the outward current at +35 mV by 1,233 ± 363 pA (P < 0.05, paired t-test; n = 8).
Figure 3A shows the average conductance activated by 2-MeSADP in 62 cells investigated. The data points were fitted with the sum of a small, linear conductance and Boltzmann relation giving the parameters. Figure 3A shows the time course of the average value of current amplitude elicited by the ramp protocol at a membrane potential of +35 mV in 19 cells, where 2-MeSADP was applied and then washed off the slice without further treatment. After 8 min application of the P2Y1 agonist, the current was increased significantly relative to control (P = 0.0005, Student's paired t-test). Figure 3B illustrates the population distribution of the maximal increase in membrane conductance recorded in the presence of 2MeSADP in all of the cells investigated. It should be noted that 23 out of a total of 82 cells investigated (28%) did not show an increase in conductance (<0.5 nS) during 10 min superfusion with 2-MeSADP. Figure 3B shows the distribution of conductance increase observed with 2-MeSADP in cells that were imaged during the experiment by filling the cell with Lucifer yellow via the patch pipette. No significant difference was observed in the response of cells imaged in this way compared with the population as a whole.
Fig. 3.
Outward current activation by 2-MeSADP is blocked by the selective P2Y1 antagonist 2′-deoxy-N6-methyl adenosine 3′,5′-diphosphate (MRS 2179). A: the averaged conductance (±SE) normalized to the value at 0 mV obtained in 43 cells investigated. The gmax was 3.95 ± 0.69 nS. The solid line shows the fitted curve obtained by the combination of a linear function and the Boltzmann equation with parameter values shown in the bottom inset. Top inset: the average current amplitude elicited at +35 mV normalized to the control value as a function of time. A significant increase was observed in the presence of 2-MeSADP. B: population graph showing the distribution of responding and nonresponding cells in relation to the maximal value measured for the P2Y1-activated conductance. Hatched bars show the response of cells that were imaged using cell loading with Lucifer yellow during recording. The distribution of conductance increase in response to 2-MeSADP in Lucifer yellow-filled cells was similar to the overall distribution. C: block of the 2-MeSADP effect by the P2Y1 antagonist, MRS 2179. In a typical experiment, no difference was found in the ramp current in the absence or in the presence of 2-MeSADP (250 nM) and MRS 2179 (15 μM). D: normalized current amplitudes at +35 mV in experiments with 2-MeSADP alone (top panel) or during the coapplication of the P2Y1 agonist with 15 μM MRS 2179 (bottom panel). *P = 0.0065, Student's paired t-test.
In a separate group of experiments, we recorded the ramp-evoked current before, during, and after coapplication of 250 nM 2-MeSADP and 15 μM MRS 2179, a P2Y1-selective antagonist. Figure 3C shows the recording from a representative cell in which no changes in the outward current elicited by the voltage ramp were observed during the coapplication of the two drugs. Figure 3D shows that no significant difference was found between the current amplitude measured at +35 mV in control conditions and during the coapplication of 2-MeSADP and MRS 2179 (P > 0.05, Student's paired t-test; n = 16) compared with an average of 38 ± 7.6% increase in current evoked by 2-MeSADP alone. These data indicate that the increase in the outward current amplitude recorded in the presence of 2-MeSADP is due to P2Y1 receptor activation.
Characterization of the P2Y1-activated conductance in medium-sized striatal neurons.
To investigate which type(s) of K+ conductance are activated by the P2Y1 receptor agonist, we applied three different kinds of K-channel blockers. Since only 70% of cells showed a response to the P2Y1 agonist, the K+-channel blocker was applied by superfusion after a clear response to 2-MeSADP was observed.
TEA was applied at a concentration (200 μM) that is relatively selective for blocking the BK channels. As shown in Fig. 4A, the application of 200 μM TEA blocked the P2Y1-activated current and a proportion of the control whole-cell current. The normalized current change in seven cells is shown in Fig. 4A. The amplitude of currents elicited at +35 mV by the ramp was increased significantly by application of 2-MeSADP, and this effect is blocked in the presence of TEA. The average of the TEA-sensitive conductance (obtained by subtraction of the ramp current recorded in the presence of 2-MeSADP + TEA from the ramp current recorded in the presence of 2-MeSADP) obtained in the seven cells investigated is shown in Fig. 5A and was well fitted by a Boltzmann relation with a gmax value of 3.84 nS, a z value of 1.95, and a V1/2 value of 0.39 mV. It is particularly noticeable that the TEA-sensitive conductance is more prominent at negative potentials and has a shallow voltage sensitivity (z = 1.95) compared with conductances blocked by the more selective blockers, iberiotoxin and apamin. TEA also blocked a proportion of the control ramp conductance.
Fig. 4.
Effect of different potassium channel blockers on the 2-MeSADP-activated current. A: ramp currents from a single experiment where 2-MeSADP application (250 nM) was followed by perfusion of 2-MeSADP + 200 μM tetraethylammonium (TEA). The current increase observed in the presence of the P2Y1 agonist was blocked completely by TEA. Inset: average current amplitudes recorded at +35 mV in each cell of this experimental group. *P < 0.05 from the control (Ctrl) group; **P < 0.05 from the 2-MeSADP group, Student's paired t-test. B: a similar protocol was performed in the presence of 200 nM iberiotoxin (IbTX) and (C) in the presence of 100 nM apamin. D: when 200 μM TEA was applied before the P2Y1 agonist, no increase in a ramp-evoked outward current was observed. TEA applied alone significantly reduced the total outward current evoked by the voltage ramp (P = 0.0197, Student's paired t-test). E: preapplication of apamin + iberiotoxin slightly but not significantly reduced the whole-cell current by 11.7 ± 5.4%. In the presence of apamin + iberiotoxin, 2-MeSADP did not evoke a significant change in the whole-cell current. F: following application of 2-MeSADP, sequential coapplication of apamin and the combination of apamin + iberiotoxin partially and then completely reversed the effect of 2-MeSADP (**P < 0.05 from the 2-MeSADP group).
Fig. 5.
Averaged TEA-, iberiotoxin-, and apamin-sensitive conductances compared with the P2Y1-activated conductance. Filled circles represent the averaged P2Y1-activated conductance in 43 cells investigated. A: solid lines show curves obtained by fitting the sum of a linear function and the Bolzmann equation (with parameters as given; see Table 1). Filled squares show the averaged TEA-sensitive conductance in the presence of 2-MeSADP. Filled triangles show the iberiotoxin-sensitive conductance. B: filled diamonds indicate the apamin-sensitive conductance.
Since TEA could also decrease the currents recorded in the presence of 2-MeSADP by blocking K+ currents already active in control conditions, we performed a series of experiments in which we superfused TEA alone for 5 min before applying 2-MeSADP. In six out of eight cells, an increase in conductance elicited by the P2Y1 agonist was not observed, indicating that the action of 2-MeSADP was largely due to TEA-sensitive ion channels (Fig. 4D). Application of TEA significantly reduced (P = 0.0197, Student's paired t-test; n = 8) the current amplitude measured at +35 mV in control cells; in fact, it was also able to reduce the current amplitude below the control level in the group where it was applied after 2-MeSADP superfusion (Fig. 4A; P = 0.034, Student's paired t-test; n = 7). In the remaining two cells, a small increase of outward current was still observed in the presence of TEA (not shown), suggesting that K+ channels other than BK could also be involved in the 2-MeSADP-mediated effect.
The selective blocker of BK channels, iberiotoxin (200 nM), was applied after the 2-MeSADP-induced effect was observed in a separate set of experiments. When a significant increase of the current amplitude was elicited by the P2Y1 agonist, the application of iberiotoxin partly blocked the effect in 12 cells (Fig. 4B) and in one cell, showed no obvious effect. The normalized current change is shown in Fig. 4B, and the average of the iberiotoxin-sensitive conductance is shown in Fig. 5A.
To better investigate the nature of these non-BK-mediated effects of 2-MeSADP, apamin (100 nM), a selective blocker of SK channels (Fig. 4C) was applied after the 2-MeSADP-mediated effect was elicited. As shown in Fig. 4C, the application of apamin (100 nM) partially reversed the effect elicited by the P2Y1 agonist in nine out of 11 cells investigated. The average change in the apamin-sensitive current is shown in Fig. 4C, and the average of the apamin-sensitive conductances is shown in Fig. 5B. As illustrated for the 2-MeSADP-activated conductance [Fig. 2C(i) and (ii)], this curve has a small linear component at negative potentials (<−30 mV). For this reason, we fitted the curve with the sum of a linear equation (to describe the nonvoltage-dependent membrane conductance at potentials ≤−30 mV) and a Boltzmann relation. A summary of the parameters obtained by fitting the voltage-conductance relations sensitive to each potassium channel blocker investigated is presented in Table 1.
Table 1.
Fitted parameters for averaged conductance-voltage relations
| 2-MeSADP | 2-MeSADP | 2-MeSADP | 2-MeSADP | |
|---|---|---|---|---|
| +TEA | +IbTX | +Apamin | ||
| gmin (nS) | 0.02 | 0.08 | 0.01 | 0.02 |
| gmax (nS) | 4.86 | 3.84 | 4.17 | 2.41 |
| z | 2.91 | 1.95 | 2.9 | 4.41 |
| V1/2 (mV) | −14.2 | 0.39 | −1.07 | −14.7 |
Values for maximum value for the voltage-dependent conductance (gmax), equivalent charge (z), and membrane potential at which 1/2 of gmax is reached (V1/2 ) obtained by fitting the 2-(Methylthio) ADP trisodium salt (2-MeSADP)-activated conductance (n = 62), tetraethylammonium (TEA)-sensitive conductance (n = 8), iberiotoxin (IbTX)-sensitive conductance (n = 12), and apamin-sensitive conductance (n = 11). gmin, minimum value for the voltage-dependent conductance.
To investigate the proportion of the control whole-cell current attributable to IK(Ca), a separate group of experiments was performed, in which apamin and iberiotoxin were applied for 5 min prior to application of 2-MeSADP. The results of these experiments are summarized in Fig. 4E. Application of apamin and iberiotoxin together did not significantly change the whole-cell current recorded at +35 mV (11.8 ± 5.4% decrease; n = 7 cells), and coapplication of 2-MeSADP then caused no significant change in the whole-cell current. Coapplication of apamin and iberiotoxin was also tested in the presence of 2-MeSADP following previous application of apamin and 2-MeSADP (Fig. 4F). The addition of iberiotoxin was found to block the apamin-insensitive, 2-MeSADP-evoked current. These results suggest that the majority of the 2-MeSADP-evoked current is carried by BK and SK potassium channels.
To determine if part of the effect of 2-MeSADP on calcium-sensitive potassium currents is due to modulation of voltage-dependent calcium currents, recordings were made of calcium currents under conditions where potassium currents and voltage-dependent sodium currents were blocked and using 5 mM barium as the charge carrier (Fig. 6). Whereas other G-protein-coupled receptors, such as muscarinic M1 receptors and dopamine D2 receptors (Olson et al. 2005), have been shown to inhibit calcium currents in striatal neurons, 2-MeSADP (250 nM) had no significant effect. The peak of the I-V relationship occurs approximately −10 mV, where the mean currents were: control, −400 ± 42.6 pA; 2-MeSADP, −302 ± 46.9 pA (n = 15). The shape of the I-V relation and magnitude of the calcium current were similar to previous measurements made with barium as the charge carrier in striatal neurons (Howe and Surmeier 1995; Olson et al. 2005).
Fig. 6.
Voltage-dependent calcium currents are unaffected by 2-MeSADP. A: example of averaged and leak-subtracted currents shown for voltage steps from −90 mV to −10 mV in control and in the presence of 250 nM 2-MeSADP. B: current-voltage relationships for the currents recorded as in A. There was no significant effect of 2-MeSADP on the mean current recorded at each voltage (P > 0.05; n = 15).
2-MeSADP decreases AP firing rate.
To gain insight into possible physiological effects of P2Y1 receptor activation in striatal neurons, we investigated the effects of 2-MeSADP (250 nM) on the firing pattern of these cells during current-clamp recordings.
Ten incremental current injection steps (1.2-s duration; 10-pA increments) from subthreshold to suprathreshold potentials were performed from a resting potential of −70 mV to establish the threshold value for spiking activity in each cell investigated. The amplitude of the initial current injection step was chosen on the basis of the electrophysiological characteristics of each cell recorded and varied from 2 to 50 pA.
Figure 7A shows example recordings of the response of a neuron to a single current injection step of 30 pA in control in the presence of 2-MeSADP and in the presence of the SK channel-blocking drug apamin. 2-MeSADP reduced the frequency of APs, and this effect was reversed by apamin. Increasing suprathreshold current injections caused these neurons to fire with increasing frequency (Fig. 7B), and little spike-frequency adaptation was observed during each current pulse (see Table 2), as previously described for mediumspiny neurons (Calabresi et al. 1987; Kawaguchi 1992, 1993; Kawaguchi et al. 1989). A mAHP was usually evident after each AP. 2-MeSADP did not significantly increase the mAHP. No sAHP was observed at the end of the depolarizing step, as expected for striatal neurons (Kawaguchi 1992, 1993), particularly during the 1st postnatal wk (Tepper et al. 1998; Tepper and Trent 1993). The current-clamp parameters measured in 26 cells are shown in Table 2 (measured at an average voltage of −40.7 mV).
Fig. 7.
2-MeSADP application reduces action potential (AP) firing of medium-spiny neurons. A: traces recorded in current clamp in the same cell stimulated by suprathreshold (30 pA) current injections in control conditions (left panel) and after 5 min application of 250 nM 2-MeSADP (middle panel) and following coapplication of apamin (right panel). In the presence of the selective P2Y1 (P2) agonist, the spike frequency is greatly reduced, and this effect was reversed by apamin. B: effect of 2-MeSADP on AP frequency as a function of the voltage obtained during the current-clamp step (left panel), effect of apamin (Apa) in the presence of 2-MeSADP (middle panel), and the effect of iberiotoxin in the presence of 2-MeSADP (right panel). Note that iberiotoxin did not modify the firing frequency recorded in the presence of 2-MeSADP, except at the most depolarized potential. C: a significant increase in AP latency (left panel), interspike interval (ISI) first and second AP (middle panel), and ISI second and third AP (right panel) was found in the presence of 2-MeSADP (at an average step membrane potential of −36.1 mV), and these effects were reversed by TEA and apamin but not by iberiotoxin. *P < 0.05 from the control group; **P < 0.05 from the 2-MeSADP group.
Table 2.
Current-clamp parameters in the absence and in the presence of 2-MeSADP
| Control | 2-MeSADP | |
|---|---|---|
| First AP amplitude (mV) | 107 ± 2.42 | 108 ± 2.30 |
| AP threshold | −33.5 ± 0.70 | −34.8 ± 0.78 |
| AP depolarization rate (mV/ms) | 133 ± 3.53 | 129 ± 4.16 |
| AP hyperpolarization rate (mV/ms) | −92.1 ± 3.69 | −85.5 ± 3.98 |
| AP width (ms) | 3.16 ± 0.12 | 3.29 ± 0.12 |
| AP latency (ms) | 79.4 ± 11.7 | 124 ± 26.4 |
| mAHP amplitude (mV) | 9.32 ± 0.58 | 10.21 ± 0.74 |
| ISI first-second (ms) | 75.9 ± 8.86 | 116 ± 13.8* |
| ISI second-third (ms) | 74.2 ± 14.4 | 124 ± 19.3* |
| Spike-frequency adaptation | 1.15 ± 0.07 | 1.05 ± 0.08 |
| AP frequency (Hz) | 15.4 ± 1.25 | 9.69 ± 1.01* |
The values of different current-clamp parameters, recorded in 26 medium-spiny neurons, are expressed as mean ± SE and show the average of values obtained by suprathreshold current injection for steps to an average voltage of −40.7 mV.
P < 0.05 from control values, Student's paired t-test. AP, action potential; mAHP, medium afterhyperpolarization potential; ISI, interspike interval.
After a 5- to 8-min application of 2-MeSADP (250 nM), a 31.8 ± 4.9% decrease in AP frequency (Fig. 7B) was observed (P < 0.001, paired t-test; n = 26 cells). This was reversed by apamin (Fig. 7B) but was unaffected by iberiotoxin (Fig. 7B). At more depolarized step potentials, iberiotoxin decreased AP frequency and also decreased the total number of APs observed during the step. This is consistent with previous data demonstrating that during large current injections, BK channel activation facilitates high-frequency firing by speeding the recovery from inactivation of voltage-dependent sodium channels (Gu et al. 2007). We also recorded an increase in AP latency (Fig. 7C; P < 0.05) and ISI between the first and second AP (Fig. 7C; P < 0.01), as well as the ISI between the second and third AP (Fig. 7C; P < 0.01) in the presence of 2-MeSADP. All of these effects were reversed by apamin but not by iberiotoxin (Fig. 7C). Ca2+-dependent K+ conductances, mainly SK channels, are crucial factors in determining firing frequency and adaptation in medium-spiny neurons (Pineda et al. 1992); so, these results are in agreement with the purinergic-mediated activation of Ca2+-dependent K+ currents observed during our voltage-ramp experiments. In contrast, other current-clamp parameters (i.e., AP threshold, amplitude, and duration) were not affected significantly by 2-MeSADP (Table 2). Surprisingly, the mAHP amplitude, known to depend on SK channel activation, was not increased significantly by the P2Y1 agonist.
In a group of four cells, where block by TEA was investigated in current-clamp mode, 2-MeSADP application produced a 43.7 ± 6.4% decrease in AP frequency, and when this was followed by coapplication of the P2Y1 agonist + TEA (200 μM), the AP frequency was increased to 26.3 ± 9.1% less than control. Surprisingly, TEA also reversed the effects of P2Y1 receptor activation on AP latency and ISI (Fig. 7C).
DISCUSSION
In the present work, we have found that the activation of P2Y1 receptors by a selective agonist increases outward potassium currents in 70% of medium-sized neurons in striatal slices from 7-day-old rats, and this effect is blocked by the P2Y1 antagonist MRS 2179. We conclude that this is a potassium current, based on the sensitivity to potassium channel blockers and that it is mainly carried by apamin-sensitive SK channels and iberiotoxin-sensitive BK channels. In separate experiments, we found no evidence for modulation of voltage-sensitive calcium currents by the P2Y1 agonist, and so, the most likely explanation for these results is that P2Y1 receptor activation increases calcium-sensitive potassium currents by increasing intracellular calcium.
There is clear evidence that P2Y purinergic receptors are expressed in the striatum. Both mRNA for P2Y1,2,4,6,11 (Moore et al. 2001) and P2Y1 protein expression (Amadio et al. 2007; Franke et al. 2003; Moore et al. 2000; Scheibler et al. 2004) have been found in human and rat striatum. However, our results showing increased Ca2+-activated potassium current in response to P2Y1 receptor activation contrast with those of Scheibler et al. (2004), who in an extensive study, found no electrophysiological response (using mainly 10- to 14-day-old rats). One possible explanation for this is the difference in the strength of calcium buffering used in the pipette solutions. Whereas Scheibler et al. (2004) used a high buffering capacity (11 mM EGTA), in this study, we used lower calcium buffering (0.2 mM EGTA) to avoid interfering with calcium signaling in response to P2Y1 receptor activation.
A relatively high percentage (∼30%) of neurons did not respond to P2Y1 agonist application with an increase in outward current. This variability in response may be due to the different cell populations of striatal neurons presenting different expression patterns of purinergic receptors at this developmental stage. For example, medium-spiny neurons can be divided into substance-P and enkephalin-expressing neurons on the basis of different staining for these two histological markers (Gerfen et al. 1988; Izzo et al. 1987), and these neurons correspond to direct and indirect pathway neurons of the basal ganglia (Bolam et al. 2000). It may be that these two different subpopulations of cells (not distinguishable in our experimental conditions) express distinct patterns of P2 purinergic receptors, as for example, medium-spiny neurons also selectively express M4 muscarinic receptors in the direct pathway and D2 dopamine receptors in the indirect pathway (Kreitzer and Malenka 2007). This hypothesis is consistent with the results from Scheibler and colleagues (2004), showing that ∼50% of striatal medium-spiny neurons from 10- to 14-day-old rats expresses the P2Y1 purinergic receptor.
The V½ activation potential of the 2-MeSADPactivated conductance is approximately −15 mV, and the gmax is reached at about +20 mV. These characteristics are typical of Ca2+-activated K+ channels, since their activity is partly dependent on calcium entry via voltage-dependent calcium channels, which would be expected to reach a maximum of ∼0 mV (in our experiments, the peak of the barium current I-V relation was at −10 mV). 2-MeSADP, however, had no significant effect on voltage-dependent calcium channel currents in these cells (Fig. 6). P2 receptor activation is generally linked to a rise in intracellular Ca2+ (Kawano et al. 2006; Rubini et al. 2006; Scemes et al. 2003), whereas P2Y1 signaling can itself be voltage dependent (Gurung et al. 2008; Martinez-Pinna et al. 2005). The contributions of these different factors to the voltage dependence of the 2-MeSADP-activated conductance are therefore difficult to predict.
Both BK and SK channels are widely distributed in the mammalian brain (Faber and Sah 2003; Salkoff et al. 2006; Stocker and Pedarzani 2000; Stocker 2004). In striatal neurons, BK and SK channels have been histochemically and functionally described (Hopf et al. 2010; Knaus et al. 1996; Pineda et al. 1992) and have been shown to carry >50% of outward currents in acutely dissociated medium-spiny neurons (Bargas et al. 1999). BK channels are known to be voltage sensitive, with both intracellular calcium rise and membrane depolarization enhancing the channel open probability (Bargas et al. 1999; Horrigan and Aldrich 2002; Rothberg and Magleby 2000). In contrast, SK channels are voltage independent.
These observations support the conclusions of the present study, where BK and SK channels were identified using selective blockers. BK channels are the most likely K+ channels blocked by submillimolar concentrations of TEA (Faber and Sah 2003) or by iberiotoxin (except for BK channels containing the β4 subunit; see Faber and Sah 2003; Meera et al. 2000) but not by apamin, whereas mammalian SK channels are very sensitive to block by apamin, with IC50 values found in the pM range (Castle and Haylett 1987; Kohler et al. 1996). In the present study, voltage-clamp experiments show that TEA (at submillimolar concentrations), iberiotoxin, or apamin was able to significantly block the current activated following 2-MeSADP application, confirming the involvement of both BK and SK channels in the P2Y1-mediated effect. Surprisingly, the effect of TEA was greater than that observed with iberiotoxin or apamin, although the concentrations of both toxins (iberiotoxin: 200 nM; apamin: 100 nM) should be largely saturating (complete blockade at 10 nM for both toxins has been demonstrated in striatal neurons; Bargas et al. 1999; Nisenbaum et al. 1996). It is also clear that the application of 200 μM TEA after the P2Y1 agonist caused a significant reduction of the total outward current, even compared with control values (Fig. 4A), whereas iberiotoxin or apamin partially but not completely reversed the effect of 2-MeSADP (Fig. 4, B and C). In addition, whereas the combined application of apamin and iberiotoxin (Fig. 4E) did not significantly decrease the whole-cell current below control (11.8 ± 5.4% decrease), TEA was able to significantly (22 ± 7.4%) reduce the whole-cell current in the absence of the P2Y1 agonist (Fig. 4D). This may be due to additional effects of TEA on some other potassium channels, for example, of the KCNQ type (Kv7.x) not blocked by iberiotoxin or apamin but blocked by low TEA concentrations (Faber and Sah 2003; Hadley et al. 2000; Robbins 2001).
As shown in Fig. 4B, at positive potentials, the whole-cell conductance was increased by 53 ± 12% in the presence of 2-MeSADP, and this was reduced to 17 ± 11% above control in 2-MeSADP + iberiotoxin; so, in the presence of 2MeSADP, the iberiotoxin-sensitive conductance (gmax = 4.2 nS; Table 1) was ∼36% of the total whole-cell conductance (at +35 mV) in those experiments. In the presence of 2-MeSADP, the apamin-sensitive conductance (gmax = 2.4 nS) was ∼20% of the total. We would therefore expect ∼6.6 nS (55%) of conductance to be blocked by the combination of iberiotoxin + apamin, but in fact, in those experiments (perhaps due to variation in 2-MeSADP effect between cells), the whole-cell conductance was reduced ∼12% below the control value: a total of ∼5 nS of block. These results suggest that on average, 2-MeSADP increases the whole-cell conductance by ∼4 nS to a total of ∼12 nS, with ∼4.2 nS (36%) of this conductance attributable to BK channels and ∼2.4 nS (20%) attributable to SK channels. These results suggest that P2Y receptor activation increases three- to fourfold the SK and BK conductance in these striatal neurons.
The voltage dependence of K+ channel blocker-sensitive conductances found in the present work was well described by a Boltzmann relationship. This result was expected for TEA and iberiotoxin-sensitive conductances if these reflect the blockade of voltage-dependent BK channels. The apamin-sensitive conductance shown in Fig. 5B had a small linear I-V component at potentials negative to −40 mV but also a large voltage-sensitive component at higher potentials, which can be fitted by a Boltzmann relationship. This may reflect the voltage sensitivity of calcium influx through voltage-activated calcium channels combined with the steep cooperativity of the calcium dependence of SK channel activation. Likewise, during current-clamp experiments, although the plateau potential during the step is in the range −60 mV to −30 mV, APs during the plateau will also generate significant calcium influx. In cholinergic interneurons of rat striatum, it has been demonstrated that SK channels are activated by calcium influx from N-type calcium channels, as well as by the classical calcium release from intracellular stores (Goldberg et al. 2009; Goldberg and Wilson 2005).
In control current-clamp experiments, we recorded the typical electrophysiological characteristics previously described for small striatal neurons (Kawaguchi 1992; Kawaguchi 1993; Tepper et al. 1993): a resting membrane potential approximately −80 mV, little spike frequency adaptation, a sAHP, high threshold for AP firing, and no visible sAHP after the current step (see Table 2). We found that 2-MeSADP caused a significant reduction of firing frequency during positive current injection, as well as an increase in AP latency at more depolarized step potentials (Fig. 7C and Table 2) and ISIs compared with control values. These data are consistent with the idea of SK channel activation by the P2Y1 agonist. It has been shown that the frequency of AP firing and long-term depression in striatal medium-spiny neurons is finely tuned by SK channels (Hopf et al. 2010). In contrast, BK channels are mostly implicated in shaping AP repolarization, since they are characterized by fast activation and inactivation kinetics. In fact, iberiotoxin was unable to reverse the decrease of AP frequency caused by P2Y1 receptor activation, confirming that under the conditions of these experiments, BK channels are not involved in determining spike frequency adaptation. Surprisingly, submillimolar concentrations of TEA (that are not expected to block SK channels) markedly reversed the P2Y1-mediated effect, causing an increase in firing frequency, even compared with control conditions. It could be suggested that TEA is blocking distinct potassium currents tonically activated in these neurons but not related to the P2Y1 effect. From these data, we conclude that in current-clamp mode (where membrane potentials range mainly between −70 and −30 mV), SK channel activation elicited by the P2Y1 agonist predominates over BK channel-mediated actions in determining the AP firing rate. The lack of increase in AHP amplitude (Table 2) during 2-MeSADP application is surprising if we expect that P2Y1 receptor activation results in an increased SK current. A possible explanation may be that a long-lasting hyperpolarization after thalamic or cortical stimulation in vivo is recorded in medium-spiny neurons only after the 3rd postnatal wk (Tepper et al. 1998; Tepper and Trent 1993), when the electrophysiological characteristics of these cells reach their mature phenotype. It could be argued that the lack of AHP increase in our experiments reflects some immature characteristic of this cell type.
In vivo (but not in vitro) medium-spiny neurons show spontaneous oscillations of membrane potential in the subthreshold range. Calcium transients resulting from synaptic activation of N-methyl-d-aspartate receptors or from voltage-dependent calcium channels may also regulate IK(Ca) during the up state, and SK channel activity, in particular, may contribute to regulating synaptic plasticity in the developing striatum (Faber et al. 2005; Hopf et al. 2010; Ngo-Ahn et al. 2005). On the basis of data obtained in the present work, we hypothesize that P2Y1 receptor activation, by increasing the activity of SK and BK channels, may influence the pattern of activity during the up state in neonatal animals and moderate the firing activity of striatal neurons in vivo in the young animal. In this area of the nervous system, the source of ATP that could mediate P2Y receptor activation is not yet known, although we could speculate that it may be coreleased with transmitter glutamate (Pankratov et al. 2007; Potter and White 1980) or released in response to astrocyte depolarization (Verkhratsky and Krishtal 2009).
Finally, although we also observed that 2-MeSADP produces a similar increase in outward current in 28-day-old rats, there is considerable evidence showing that the electrophysiological characteristics of medium-spiny neurons are different between 7-day-old and adult rats (Sharpe and Tepper 1998; Tepper et al. 1998; Tepper and Trent 1993). In these experiments, we identify the main effect of P2Y1 receptor activation during early developmental stages of postnatal life as an increase in IK(Ca). P2 receptor activation is also associated with increased proliferation and migration of neuronal (Hogg et al. 2004; Ryu et al. 2003; Scemes et al. 2003) and glial (Agresti et al. 2005; Weissman et al. 2004) progenitor cells. It may also be the case that P2Y receptor activation influences the development and maturation of the striatal neuronal network during the first few postnatal weeks.
GRANTS
This work was supported by FIRB 2003 Project M.RBNE30YA3L009, Italian Ministry of Education, University and Research, and by the Wellcome Trust and the Biotechnology and Biological Sciences Research Council.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: E.C., F.P., and A.J.G. conception and design of research; E.C. and A.J.G. performed experiments; E.C. and A.J.G. analyzed data; E.C., F.P., and A.J.G. interpreted results of experiments; E.C. and A.J.G. prepared figures; E.C. and A.J.G. drafted manuscript; F.P. and A.J.G. edited and revised manuscript; A.J.G. approved final version of manuscript.
NOTE ADDED IN PROOF
Recently, two important papers have been published that are very relevant to our topic:
Dehorter N, Michel FJ, Marissal T, Rotrou Y, Martrot B, Lopez C, Humphries MD, Hammond C. Onset of pup locomotion coincides with loss of NR2C/D-mediated cortico-striatal EPSCs and dampening of striatal network immature activity. Front Cell Neurosci 5: 24, 2011.
Schicker KW, Chandaka GK, Geier P, Kubista H, Boehm S. P2Y1 receptors mediate an activation of neuronal calcium-dependent K+ channels. J Physiol 588: 3713–3725, 2010.
REFERENCES
- Abbracchio MP, Burnstock G. Purinoceptors: are there families of P2X and P2Y purinoceptors? Pharmacol Ther 64: 445–475 1994 [DOI] [PubMed] [Google Scholar]
- Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, Weisman GA. International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev 58: 281–341, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbracchio MP, Burnstock G, Verkhratsky A, Zimmermann H. Purinergic signalling in the nervous system: an overview. Trends Neurosci 32: 19–29, 2009 [DOI] [PubMed] [Google Scholar]
- Agresti C, Meomartini ME, Amadio S, Ambrosini E, Volonte C, Aloisi F, Visentin S. ATP regulates oligodendrocyte progenitor migration, proliferation, and differentiation: involvement of metabotropic P2 receptors. Brain Res Brain Res Rev 48: 157–165, 2005 [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 2nd edition (2007 revision). Br J Pharmacol 150, Suppl 1: S1–S168, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amadio S, Montilli C, Picconi B, Calabresi P, Volonté C. Mapping P2X and P2Y receptor proteins in striatum and substantia nigra: an immunohistological study. Purinergic Signal 3: 389–398, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur DB, Akassoglou K, Insel PA. P2Y2 receptor activates nerve growth factor/TrkA signaling to enhance neuronal differentiation. Proc Natl Acad Sci USA 102: 19138–19143, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur DB, Georgi S, Akassoglou K, Insel PA. Inhibition of apoptosis by P2Y2 receptor activation: novel pathways for neuronal survival. J Neurosci 26: 3798–3804, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargas J, Ayala GX, Vilchis C, Pineda JC, Galarraga E. Ca2+-activated outward currents in neostriatal neurons. Neuroscience 88: 479–488, 1999 [DOI] [PubMed] [Google Scholar]
- Blomeley CP, Kehoe LA, Bracci E. Substance P mediates excitatory interactions between striatal projection neurons. J Neurosci 29: 4953–4963, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolam JP, Hanley JJ, Booth PA, Bevan MD. Synaptic organisation of the basal ganglia. J Anat 196: 527–542, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracci E, Panzeri S. Excitatory GABAergic effects in striatal projection neurons. J Neurophysiol 95: 1285–1290, 2006 [DOI] [PubMed] [Google Scholar]
- Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev 87: 659–797, 2007 [DOI] [PubMed] [Google Scholar]
- Burnstock G. Purinergic signalling and disorders of the central nervous system. Nat Rev Drug Discov 7: 575–590, 2008 [DOI] [PubMed] [Google Scholar]
- Calabresi P, Misgeld U, Dodt HU. Intrinsic membrane properties of neostriatal neurons can account for their low level of spontaneous activity. Neuroscience 20: 293–303, 1987 [DOI] [PubMed] [Google Scholar]
- Castle NS, Haylett DG. Effects of apamin, quinine and neuromuscular blockers on calcium-activated potassium channels of guinea-pig hepatocytes. J Physiol 358: 373–394, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards FA, Gibb AJ, Colquhoun D. ATP receptor-mediated synaptic currents in the central nervous system. Nature 359: 144–147, 1992 [DOI] [PubMed] [Google Scholar]
- Edwards FA, Konnerth A. Patch-clamping cells in sliced tissue preparations. Methods Enzymol 207: 208–222, 1992 [DOI] [PubMed] [Google Scholar]
- Edwards FA, Konnerth A, Sakmann B, Takahashi T. A thin slice preparation for patch clamp recordings from neurones of the mammalian central nervous system. Pflugers Arch 414: 600–612, 1989 [DOI] [PubMed] [Google Scholar]
- Faber ES, Delaney AJ, Sah P. SK channels regulate excitatory synaptic transmission and plasticity in the lateral amygdala. Nat Neurosci 8: 635–641, 2005 [DOI] [PubMed] [Google Scholar]
- Faber ES, Sah P. Calcium-activated potassium channels: multiple contributions to neuronal function. Neuroscientist 9: 181–194, 2003 [DOI] [PubMed] [Google Scholar]
- Fields RD, Burnstock G. Purinergic signalling in neuron-glia interactions. Nat Rev Neurosci 7: 423–436, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke H, Kittner H, Grosche J, Illes P. Enhanced P2Y1 receptor expression in the brain after sensitisation with d-amphetamine. Psychopharmacology (Berl) 167: 187–194, 2003 [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Assender JW, Irenius E, Kodama N, Saito N. Synergistic effects of adenosine A1 and P2Y receptor stimulation on calcium mobilization and PKC translocation in DDT1 MF-2 cells. Cell Mol Neurobiol 23: 379–400, 2003 [DOI] [PubMed] [Google Scholar]
- Gao ZG, Mamedova L, Tchilibon S, Gross AS, Jacobson KA. 2,2′-Pyridylisatogen tosylate antagonizes P2Y1 receptor signaling without affecting nucleotide binding. Biochem Pharmacol 68: 231–237, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Young WSIII. Distribution of striatonigral and striatopallidal peptidergic neurons in both patch and matrix compartments: an in situ hybridization histochemistry and fluorescent retrograde tracing study. Brain Res 460: 161–167, 1988 [DOI] [PubMed] [Google Scholar]
- Goldberg JA, Teagarden MA, Foehring RC, Wilson CJ. Nonequilibrium calcium dynamics regulate the autonomous firing pattern of rat striatal cholinergic interneurons. J Neurosci 29: 8396–8407, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JA, Wilson CJ. Control of spontaneous firing patterns by the selective coupling of calcium currents to calcium-activated potassium currents in striatal cholinergic interneurons. J Neurosci 25: 10230–10238, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu N, Vervaeke K, Storm JF. BK potassium channels facilitate high-frequency firing and cause early spike frequency adaptation in rat CA1 hippocampal pyramidal cells. J Physiol 580: 859–882, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurung IS, Martinez-Pinna J, Mahaut-Smith M. Novel consequences of voltage-dependence to G-protein-coupled P2Y1 receptors. Br J Pharmacol 154: 882–889, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley JK, Noda M, Selyanko AA, Wood IC, Abogadie FC, Brown DA. Differential tetraethylammonium sensitivity of KCNQ1–4 potassium channels. Br J Pharmacol 129: 413–415, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehn K, Watson TW, MacVicar BA. Multiple types of calcium channels in acutely isolated rat neostriatal neurons. J Neurosci 13: 1244–1257, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg RC, Chipperfield H, Whyte KA, Stafford MR, Hansen MA, Cool SM, Nurcombe V, Adams DJ. Functional maturation of isolated neural progenitor cells from the adult rat hippocampus. Eur J Neurosci 19: 2410–2420, 2004 [DOI] [PubMed] [Google Scholar]
- Hopf FW, Seif T, Mohamedi ML, Chen BT, Bonci A. The small conductance calcium-activated potassium channel is a key modulator of firing and long-term depression in the dorsal striatum. Eur J Neurosci 1–14, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horrigan FT, Aldrich RW. Coupling between voltage sensor activation, Ca2+ binding and channel opening in large conductance (BK) potassium channels. J Gen Physiol 120: 267–305, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe AR, Surmeier DJ. Muscarinic receptors modulate N-, P-, and L-type Ca2+ currents in rat striatal neurons through parallel pathways. J Neurosci 15: 458–469, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeuchi Y, Nishizaki T. ATP-evoked potassium currents in rat striatal neurons are mediated by a P2 purinergic receptor. Neurosci Lett 190: 89–92, 1995 [DOI] [PubMed] [Google Scholar]
- Inoue K, Koizumi S, Ueno S. Implication of ATP receptors in brain functions. Prog Neurobiol 50: 483–492, 1996 [DOI] [PubMed] [Google Scholar]
- Izzo PN, Graybiel AM, Bolam JP. Characterization of substance P- and [Met]enkephalin-immunoreactive neurons in the caudate nucleus of cat and ferret by a single section Golgi procedure. Neuroscience 20: 577–587, 1987 [DOI] [PubMed] [Google Scholar]
- Jacobson KA, Boeynaems JM. P2Y nucleotide receptors: promise of therapeutic applications. Drug Discov Today 15: 570–578, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain M, Armstrong RJ, Barker RA, Rosser AE. Cellular and molecular aspects of striatal development. Brain Res Bull 55: 533–540, 2001 [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y. Large aspiny cells in the matrix of the rat neostriatum in vitro: physiological identification, relation to the compartments and excitatory postsynaptic currents. J Neurophysiol 67: 1669–1682, 1992 [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y. Physiological, morphological, and histochemical characterization of three classes of interneurons in rat neostriatum. J Neurosci 13: 4908–4923, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Wilson CJ, Augood SJ, Emson PC. Striatal interneurones: chemical, physiological and morphological characterization. Trends Neurosci 18: 527–535, 1995 [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Wilson CJ, Emson PC. Intracellular recording of identified neostriatal patch and matrix spiny cells in a slice preparation preserving cortical inputs. J Neurophysiol 62: 1052–1068, 1989 [DOI] [PubMed] [Google Scholar]
- Kawano S, Otsu K, Kuruma A, Shoji S, Yanagida E, Muto Y, Yoshikawa F, Hirayama Y, Mikoshiba K, Furuichi T. ATP autocrine/paracrine signaling induces calcium oscillations and NFAT activation in human mesenchymal stem cells. Cell Calcium 39: 313–324, 2006 [DOI] [PubMed] [Google Scholar]
- Knaus HG, Schwarzer C, Koch RO, Eberhart A, Kaczorowski GJ, Glossmann H, Wunder F, Pongs O, Garcia ML, Sperk G. Distribution of high-conductance Ca2+-activated K+ channels in rat brain: targeting to axons and nerve terminals. J Neurosci 16: 955–963, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler M, Hirschberg B, Bond CT, Kinzie JM, Marrion NV, Maylie J, Adelman JP. Small-conductance, calcium-activated potassium channels from mammalian brain. Science 273: 1709–1714, 1996 [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Endocannabinoid-mediated rescue of striatal LTD and motor deficits in Parkinson's disease models. Nature 445: 643–647, 2007 [DOI] [PubMed] [Google Scholar]
- Martinez-Pinna J, Gurung IS, Vial C, Leon C, Gachet C, Evans RJ, Mahaut-Smith MP. Direct voltage control of signalling via P2Y1 and other Gαq-coupled receptors. J Biol Chem 280: 1490–1498, 2005 [DOI] [PubMed] [Google Scholar]
- Meera P, Wallner M, Toro L. A neuronal beta subunit (KCNMB4) makes the large conductance, voltage- and Ca2+-activated K+ channel resistant to charybdotoxin and iberiotoxin. Proc Natl Acad Sci USA 97: 5562–5567, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra SK, Braun N, Shukla V, Fullgrabe M, Schomerus C, Korf HW, Gachet C, Ikehara Y, Sevigny J, Robson SC, Zimmermann H. Extracellular nucleotide signaling in adult neural stem cells: synergism with growth factor-mediated cellular proliferation. Development 133: 675–684, 2006 [DOI] [PubMed] [Google Scholar]
- Moore DJ, Chambers J, Waldvogel H, Faull R, Emson P. Regional and cellular distribution of the P2Y(1) purinergic receptor in the human brain: striking neuronal localisation. J Comp Neurol 421: 374–384, 2000 [DOI] [PubMed] [Google Scholar]
- Moore DJ, Chambers JK, Wahlin JP, Tan KB, Moore GB, Jenkins O, Emson PC, Murdock PR. Expression pattern of human P2Y receptor subtypes: a quantitative reverse transcription-polymerase chain reaction study. Biochim Biophys Acta 1521: 107–119, 2001 [DOI] [PubMed] [Google Scholar]
- Moro S, Guo D, Camaioni E, Boyer JL, Harden TK, Jacobson KA. Human P2Y1 receptor: molecular modeling and site-directed mutagenesis as tools to identify agonist and antagonist recognition sites. J Med Chem 41: 1456–1466, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neary JT, Zimmermann H. Trophic functions of nucleotides in the central nervous system. Trends Neurosci 32: 189–198, 2009 [DOI] [PubMed] [Google Scholar]
- Ngo-Anh TJ, Bloodgood BL, Lin M, Sabatini BL, Maylie J, Adelman JP. SK channels and NMDA receptors form a Ca2+-mediated feedback loop in dendritic spines. Nat Neurosci 8: 642–649, 2005 [DOI] [PubMed] [Google Scholar]
- Nieber K, Poelchen W, Illes P. Role of ATP in fast excitatory synaptic potentials in locus coeruleus neurones of the rat. Br J Pharmacol 122: 423–430, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisenbaum ES, Wilson CJ, Foehring RC, Surmeier DJ. Isolation and characterization of a persistent potassium current in neostriatal neurons. J Neurophysiol 76: 1180–1194, 1996 [DOI] [PubMed] [Google Scholar]
- North RA. Molecular physiology of P2X receptors. Physiol Rev 82: 1013–1067, 2002 [DOI] [PubMed] [Google Scholar]
- Olson PA, Tkatch T, Hernandez-Lopez S, Ulrich S, Ilijic E, Mugnaini E, Zhang H, Bezprozvanny I, Surmeier DJ. G-protein-coupled receptor modulation of striatal CaV1.3 L-type Ca2+ channels is dependent on a Shank-binding domain. J Neurosci 25: 1050–1062, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankratov Y, Castro E, Miras-Portugal MT, Krishtal O. A purinergic component of the excitatory postsynaptic current mediated by P2X receptors in the CA1 neurons of the rat hippocampus. Eur J Neurosci 10: 3898–3902, 1998 [DOI] [PubMed] [Google Scholar]
- Pankratov Y, Lalo U, Verkhratsky A, North RA. Quantal release of ATP in mouse cortex. J Gen Physiol 129: 257–265, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes-Gamero EJ, Craveiro RB, Pesquero JB, Franca JP, Oshiro ME, Ferreira AT. Activation of P2Y1 receptor triggers two calcium signaling pathways in bone marrow erythroblasts. Eur J Pharmacol 534: 30–38, 2006 [DOI] [PubMed] [Google Scholar]
- Pineda JC, Galarraga E, Bargas J, Cristancho M, Aceves J. Charybdotoxin and apamin sensitivity of the calcium-dependent repolarization and the afterhyperpolarization in neostriatal neurons. J Neurophysiol 68: 287–294, 1992 [DOI] [PubMed] [Google Scholar]
- Pocock JM, Kettenmann H. Neurotransmitter receptors on microglia. Trends Neurosci 30: 527–535, 2007 [DOI] [PubMed] [Google Scholar]
- Potter P, White TD. Release of adenosine 5′-triphosphate from synaptosomes from different regions of rat brain. Neuroscience 5: 1351–1356, 1980 [DOI] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev 50: 413–492, 1998 [PubMed] [Google Scholar]
- Robbins J. KCNQ potassium channels: physiology, pathophysiology, and pharmacology. Pharmacol Ther 90: 1–19, 2001 [DOI] [PubMed] [Google Scholar]
- Rothberg BS, Magleby KL. Voltage and Ca2+ activation of single large-conductance Ca2+-activated K+ channels described by a two-tiered allosteric gating mechanism. J Gen Physiol 116: 75–99, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubini P, Pinkwart C, Franke H, Gerevich Z, Norenberg W, Illes P. Regulation of intracellular Ca2+ by P2Y1 receptors may depend on the developmental stage of cultured rat striatal neurons. J Cell Physiol 209: 81–93, 2006 [DOI] [PubMed] [Google Scholar]
- Rymar VV, Sasseville R, Luk KC, Sadikot AF. Neurogenesis and stereological morphometry of calretinin-immunoreactive GABAergic interneurons of the neostriatum. J Comp Neurol 469: 325–339, 2004 [DOI] [PubMed] [Google Scholar]
- Ryu JK, Choi HB, Hatori K, Heisel RL, Pelech SL, McLarnon JG, Kim SU. Adenosine triphosphate induces proliferation of human neural stem cells: role of calcium and p70 ribosomal protein S6 kinase. J Neurosci Res 72: 352–362, 2003 [DOI] [PubMed] [Google Scholar]
- Salkoff L, Butler A, Ferreira G, Santi C, Wei A. High-conductance potassium channels of the SLO family. Nat Rev Neurosci 7: 921–931, 2006 [DOI] [PubMed] [Google Scholar]
- Sanches G, de Alencar LS, Ventura AL. ATP induces proliferation of retinal cells in culture via activation of PKC and extracellular signal-regulated kinase cascade. Int J Dev Neurosci 20: 21–27, 2002 [DOI] [PubMed] [Google Scholar]
- Scemes E, Duval N, Meda P. Reduced expression of P2Y1 receptors in connexin43-null mice alters calcium signaling and migration of neural progenitor cells. J Neurosci 23: 11444–11452, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibler P, Pesic M, Franke H, Reinhardt R, Wirkner K, Illes P, Norenberg W. P2X2 and P2Y1 immunofluorescence in rat neostriatal medium-spiny projection neurones and cholinergic interneurones is not linked to respective purinergic receptor function. Br J Pharmacol 143: 119–131, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe NA, Tepper JM. Postnatal development of excitatory synaptic input to the rat neostriatum: an electron microscopic study. Neuroscience 84: 1163–1175, 1998 [DOI] [PubMed] [Google Scholar]
- Stevens B, Fields RD. Response of Schwann cells to action potentials in development. Science 287: 2267–2271, 2000 [DOI] [PubMed] [Google Scholar]
- Stocker M. Ca2+-activated K+ channels: molecular determinants and function of the SK family. Nat Rev Neurosci 5: 758–770, 2004 [DOI] [PubMed] [Google Scholar]
- Stocker M, Pedarzani P. Differential distribution of three Ca(2+)-activated K(+) channel subunits, SK1, SK2, and SK3, in the adult rat central nervous system. Mol Cell Neurosci 15: 476–493, 2000 [DOI] [PubMed] [Google Scholar]
- Tepper JM, Sharpe NA, Koos TZ, Trent F. Postnatal development of the rat neostriatum: electrophysiological, light- and electron-microscopic studies. Dev Neurosci 20: 125–145, 1998 [DOI] [PubMed] [Google Scholar]
- Tepper JM, Trent F. In vivo studies of the postnatal development of rat neostriatal neurons. Prog Brain Res 99: 35–50, 1993 [DOI] [PubMed] [Google Scholar]
- Verkhratsky Krishtal A, O. Adenosine triphosphate (ATP) as a neurotransmitter. In: Encyclopedia of Neuroscience, edited by Squire LR. Oxford, UK: Academic Press, 2009, p. 115–123 [Google Scholar]
- von Kugelgen I. Pharmacological profiles of cloned mammalian P2Y-receptor subtypes. Pharmacol Ther 110: 415–432, 2006 [DOI] [PubMed] [Google Scholar]
- Weissman TA, Riquelme PA, Ivic L, Flint AC, Kriegstein AR. Calcium waves propagate through radial glial cells and modulate proliferation in the developing neocortex. Neuron 43: 647–661, 2004 [DOI] [PubMed] [Google Scholar]