Abstract
The inferior colliculus (IC) is thought to have two main subdivisions, a central region that forms an important stop on the ascending auditory pathway and a surrounding shell region that may play a more modulatory role. In this study, we investigated whether eye position affects activity in both the central and shell regions. Accordingly, we mapped the location of eye position-sensitive neurons in six monkeys making spontaneous eye movements by sampling multiunit activity at regularly spaced intervals throughout the IC. We used a functional map based on auditory response patterns to estimate the anatomical location of recordings, in conjunction with structural MRI and histology. We found eye position-sensitive sites throughout the IC, including at 27% of sites in tonotopically organized recording penetrations (putatively the central nucleus). Recordings from surrounding tissue showed a larger proportion of sites indicating an influence of eye position (33–43%). When present, the magnitude of the change in activity due to eye position was often comparable to that seen for sound frequency. Our results indicate that the primary ascending auditory pathway is influenced by the position of the eyes. Because eye position is essential for visual-auditory integration, our findings suggest that computations underlying visual-auditory integration begin early in the ascending auditory pathway.
Keywords: multisensory, reference frame, primate, Macaca mulatta
the inferior colliculus (IC) is the principal auditory nucleus of the mammalian midbrain (for review, see Winer and Schreiner 2005). Despite occupying a pivotal position in the ascending auditory system, IC neurons are modulated by more than just acoustic stimuli. Several nonauditory factors such as visual stimuli, tactile stimuli, the position of the eyes, and impending reward also have a role in shaping neural activity (Aitkin et al. 1981; Bergan and Knudsen 2009; Groh et al. 2001; Gutfreund et al. 2002; Mascetti and Strozzi 1988; Metzger et al. 2006; Porter et al. 2006, 2007; Zwiers et al. 2004).
Insight into how these signals affect auditory processing as a whole can be gained by identifying precisely where in the IC they are found. Different subregions of the IC have different input and output connectivity patterns, with the central nucleus situated on the primary ascending pathway projecting to the ventral medial geniculate body of the thalamus (Winer and Schreiner 2005). The surrounding shell regions also project to the thalamus but more heavily target medial and dorsal regions of the medial geniculate (Wenstrup 2005) along a proposed modulatory pathway in the auditory system (Lee and Sherman 2010).
The differential connectivity of the IC led us to investigate whether eye position signals are 1) present in both the central and peripheral areas and/or 2) differentially distributed in these two areas. Information about eye position is thought to be a necessary component of visual-auditory integration in the spatial domain. Sound location is computed in a head-centered frame of reference, whereas visual stimulus location is detected with respect to the retina. A representation of the orientation of the eyes with respect to the head is essential for reconciling the disparity between these two reference frames. If eye position information is present in the central nucleus of the IC, then this essential element for visual-auditory coordination is present in the primary ascending route of the auditory pathway. In contrast, if eye position sensitivity is limited to the more peripheral regions of the IC, this would suggest that such visual-auditory coordination might be handled by more specialized circuitry. Somatosensory inputs to the IC show precisely this sort of pattern: they are found in the lateral nucleus of the IC (Aitkin et al. 1978, 1981), a shell region, but are not seen in the central nucleus.
To identify the location of eye position-sensitive neurons in the IC, we used a functional map defined on the basis of auditory responses. Electrophysiological studies in macaques are typically conducted in a small number of animals with many recordings taken from each subject over an extended duration. Small changes in chronically placed electrode guiding apparatuses are inevitable, and this makes the precise histological reconstruction of more than a few recording locations virtually impossible, particularly with recordings from deep structures such as the IC. Functional maps provide an indirect estimate of location, but one that is centered on the recording electrode rather than the apparatus used to position it.
We previously defined a functional map by recording auditory responses throughout the midbrain of six macaques (Bulkin and Groh 2011). We sampled the activity of small clusters of neurons while varying recording locations in systematic fashion, forming a three-dimensional grid of recordings. We then identified distinct regions based on responses to auditory stimuli. In this article we report the effects of eye position on the neural responses we mapped, using the topography of auditory responses to interpret the results.
We recorded the orientation of the eyes while subjects moved them freely and spontaneously. Using uncontrolled eye movements allowed us to collect a large amount of data rapidly, because we were not restricted by performance on a fixation task. This was necessary since we mapped the responses of up to 20 recording sites within an individual recording session.
We found that eye position exerts an influence throughout the IC, but to varying degrees. Eye position modulation was present in 27% of recordings along tonotopic penetrations through IC, which putatively traversed the central nucleus. The prevalence of eye position sensitivity was higher in more peripheral regions (33 and 43% for “frequency-tuned” and “frequency-untuned” penetrations). When present, the effect of eye position can be powerful, on par with effects of changes in auditory frequency. The results suggest that eye position modulates the main ascending auditory signal, perhaps via the more strongly affected peripheral regions of the IC.
METHODS
Surgical preparation and recording procedures.
Three male and three female rhesus monkeys participated in the experiments. All procedures were approved by the Institutional Animal Care and Use Committee at Dartmouth College and Duke University and were conducted in accordance with the principles of laboratory animal care of the National Institutes of Health (Publication 86-23, Revised 1985). Additional details of these experiments are available in Bulkin and Groh (2011).
Surgical procedures were performed using isoflurane anesthesia and aseptic techniques as well as postoperative analgesia. The monkeys underwent an initial surgery to implant a head post for restraining the head and a scleral eye coil for monitoring eye position (Judge et al. 1980; Robinson 1963). After recovery, an additional surgery was performed to make a craniotomy and to implant a recording cylinder positioned to allow electrodes to approach the IC at an angle ∼30 deg from vertical in the coronal plane. The chamber contained a fixed grid of holes (Crist Instruments, Gaithersburg, MD) aligned such that electrode penetrations could be made in 1-mm increments in the anterior-posterior and (tilted) medial-lateral dimensions. Recordings were made using tungsten microelectrodes (1–3 MΩ; FHC, Bowdoin, ME). Multiunit clusters were selected using a window discriminator (monkeys A and W: Plexon, Dallas, TX; monkeys E, M, C, and X: Bak Electronics, Germantown, MD), and spike times were stored for off-line analysis. The location of the IC was determined using an anatomical MRI scan in which the recording chamber and plastic grid could be visualized, and was verified using physiological responses in all monkeys as well as by histological analysis in two monkeys. Electrodes were lowered along the dorsolateral-ventromedial axis of the IC (established by the placement of the recording cylinder), and recordings were taken from multiunit clusters every 0.5 mm along the trajectory of the penetration.
Stimulus presentation.
Experiments were conducted in complete darkness in a single-walled IAC sound isolation booth. Echo-absorbent material lined the walls and ceiling (3-in. Sonex Painted One acoustic foam), as well as the floor (carpet). Auditory stimuli consisted of tones of 16 frequencies ranging from 0.4 to 12 kHz (approximately one-quarter octave increments), as well as broadband noise (spectrum ranging from 0.5 to 18 kHz). Sounds were generally presented using loudspeakers (Audax model TWO25V2 or Bose Acoustimas cube speakers) located 90 deg contralateral to the recording chamber and 57 in. from the subject's head. Sound levels were calibrated to 50 ± 1 dB using a sound meter (Brüel & Kjær model 2237 with model 4137 condenser microphone; A-weighted) placed at the position that the monkey's head would occupy in the experiment.
Eye position was monitored throughout the experiment, and the monkey was woken up if drifting eye movements characteristic of sleep were observed. As described in more detail in Data analysis: eye position, any trials in which the eyes moved by more than 2 deg during the relevant analysis periods were excluded from analysis. In monkeys A, W, E, and M, an unrelated nonauditory task was run at some recording sites as part of a separate experiment, not described in this report. These trials were run in separate blocks after auditory and spontaneous eye position data were collected at a given depth.
Data analysis: inclusion of recording sites and responses to sound frequency.
Data were analyzed off-line to determine which sites along a penetration showed auditory responses. The times of action potentials were binned in 1-ms windows aligned on stimulus onset to form a peristimulus time histogram (PSTH). The PSTH was then smoothed using a 5-ms moving average. A site was marked as auditory if the smoothed PSTH exceeded 3 standard deviations (SD) above baseline for 10 consecutive milliseconds in a 50-ms window following stimulus onset. We further restricted analysis to penetrations that contained 3 or more responsive sites and excluded responsive sites that were more than 1.5 mm shallower or deeper than anatomical estimates gathered from MRI. Occasionally, we repeated recording penetration locations to verify that results were reproducible across sessions. We restricted the analysis of population properties to unique recording locations, selecting the penetration that showed the clearest auditory responses.
We tested a subset of sites, notably those in the rostral-most penetrations in monkey A, with microstimulation to rule out the possibility that they were in the superior colliculus (SC). The SC is an oculomotor structure rostral and dorsal to the IC, and it exhibits auditory responsiveness when animals are engaged in auditory saccade tasks (Jay and Sparks 1984; Populin et al. 2004). The SC's auditory activity is quite weak when the animal is not engaged in such tasks (Jay and Sparks 1987a, 1987b). Occasionally, sites with weak auditory responses in a region coinciding with SC were observed, but these were generally excluded from our analysis either because firing rate did not exceed the threshold for inclusion or because recording locations were outside of the region established in MRI. Microstimulation confirmed our criteria were adequate: saccades were commonly evoked dorsal to the IC but were only elicited at 2 of 51 tested sites included in the present data set. Since microstimulation was not conducted in all monkeys, we relied on the MRI and auditory responsiveness criteria for site inclusion, which is why these two sites were included in the data set.
Frequency tuning at each site was characterized by counting spikes in a 200-ms window following sound onset and comparing the sum to a 200-ms baseline period before the sound. This response was computed for each of the different stimulus frequencies and then fitted by a Gaussian function relating response to log(frequency). The best frequency (BF) was labeled as the frequency corresponding to the peak of the Gaussian curve, provided the Gaussian successfully described the data (F-test, P < 0.05). Penetrations were classified as frequency-tuned if three or more sites were fit by Gaussian functions. Penetrations with fewer sites were classified as frequency-untuned. Such penetrations could include either sites not very responsive to tones or sites responsive to tones but insensitive to their frequency.
Frequency-tuned penetrations were tested for the presence of a tonotopic progression by relating the BF to depth with a linear regression, using a Monte Carlo simulation to estimate confidence intervals. Tonotopic penetrations were those in which there was a statistically significant relationship between BF and depth. A thorough report of these methods has been described elsewhere (Bulkin and Groh 2011).
Data analysis: eye position.
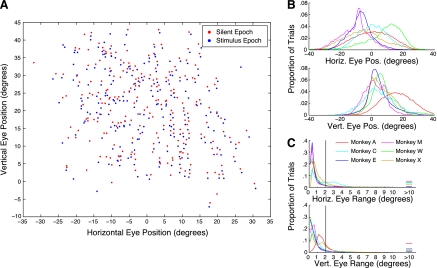
Monkeys moved their eyes spontaneously in the dark. The orientation of the eyes was recorded throughout the trial. We probed for effects of eye position in two periods: during a 200-ms silent period preceding stimulus onset and during a 200-ms period in which a sound was being presented. Figure 1A shows the location of spontaneous fixations for these two periods recorded from an example recording site. For each trial, the mean horizontal and vertical eye positions in the silent epoch (red points) and the stimulus epoch (blue points) are plotted. Only trials in which the eyes moved by <2 deg horizontally or vertically are displayed; those epochs in which the eyes moved above this threshold were not considered for further analysis. In this example, the monkey's fixations spanned a range of about ±20 deg in the horizontal domain and spanned about −10 to +30 deg in the vertical domain. The upward bias was likely due to a tendency for the eyes to drift upward in the dark. Figure 1B shows that the distribution of eye positions in this sample was typical across the different animals and experimental sessions. Figure 1C shows a histogram of how much the eyes moved over the course of the baseline epoch, including trials in which the eyes moved beyond the 2-deg threshold (vertical line; 30% of trials eliminated).
Fig. 1.
Characteristics of spontaneous eye movement in the dark. A: mean eye position during silent (red) and stimulus (blue) epochs recorded over the course of a sample session from a single site. Only data from those trials in which movement did not exceed a 2-deg threshold are displayed (see methods). B: average eye positions analyzed during the silent period across all recordings for each monkey. Horizontal eye position (top) was distributed fairly normally, although there was some variation across subjects. Monkeys more often directed gaze at points above the vertical meridian (bottom). C: distributions of the total movement magnitude over all trials during the silent period. The threshold for including trials is indicated. Results for spontaneous eye positions measured during auditory stimuli were the same (data not shown). Horiz., horizontal; vert., vertical; eye pos., eye position.
Eye position was coded as the average horizontal and vertical eye position separately over each of the two 200-ms tested epochs. To determine whether and how neural activity was related to eye position, we performed a multiple linear regression:
| (1) |
where “response” corresponds to the neural activity in either the baseline or the stimulus epochs, and the coefficients c capture its relationship to horizontal and vertical eye position. If this regression was not flat (i.e., if either or both of the coefficients were nonzero with the use of 95% confidence intervals), the site was marked as sensitive to eye position. Similar techniques have been used previously to probe for eye position sensitivity (Porter et al. 2006; Zwiers et al. 2004). We also tried a related analysis involving a two-part linear regression split at an inflection point, similar to that used by Wang et al. (2007), but did not observe an improvement in the fits sufficient to justify the larger number of parameters (i.e., the inflection point and 2 slopes in each dimension).
The regression analysis of Eq. 1 was repeated for the silent and stimulus epochs. When probing for eye position effects in either of the two tested epochs (i.e., any effect of eye position), we used a Bonferroni-corrected α-value to estimate confidence intervals of the coefficients in the regression.
To describe the strength of eye position effects on neural response, we calculated a depth of modulation metric for firing rate changes related to both eye position and auditory frequency. For these analyses, we were interested in determining the amount of modulation in firing that occurred for eye position or sound frequency values that spanned the range tested or normally encountered by the animal. For sound frequency, neurons show Gaussian tuning, so the amplitude of the Gaussian served as a logical measure of response modulation. Only statistically significant Gaussian fits were included in this analysis. We also excluded 31 of 451 sites for which the peak height of the Gaussian curve was more than 1 SD above the best response (the response in the condition evoking the largest increase in firing rate).
The relationship between neural activity and eye position is more commonly monotonic (Porter et al. 2006). We measured the response modulation as the difference in the linear regression evaluated at ±20 deg (40-deg range). We chose this orbital range because it corresponds to the “commonly used” oculomotor range. Although the eyes can move out to ±40 deg, an 80-deg range, movements beyond ±20 deg are often accompanied by head movements (Freedman and Sparks 1997). Indeed, as shown in Fig. 1, the spontaneous eye position range of the monkeys was typically 40 deg. Only sites with statistically significant linear regression fits for eye position were included for this analysis.
RESULTS
We found neurons sensitive to eye position in each of the 6 monkeys we tested, at a total of 34% of the sites tested, ranging from 21 to 55% in individual monkeys (Table 1). Eye position effects were found both during periods of silence and during auditory stimulation, with many recordings showing responses in both analyzed epochs: 44% of sites showing an effect in either period (Bonferroni corrected) showed an effect in both. The majority of sites that showed an effect of eye position were modulated by both horizontal and vertical components (i.e., nonzero terms in both the horizontal and vertical coefficients of the regression ∼69%).
Table 1.
Proportion of eye position sensitivity across recordings
| Monkey |
|||||||
|---|---|---|---|---|---|---|---|
| A | W | E | M | C | X | Total | |
| Sites tested (unique penetrations) | 202 | 97 | 49 | 42 | 53 | 77 | 520 |
| EP effect (silent period) | 55 (27%) | 33 (34%) | 25 (51%) | 24 (57%) | 13 (25%) | 10 (13%) | 160 (31%) |
| EP effect (stimulus period) | 61 (30%) | 27 (28%) | 14 (29%) | 16 (38%) | 11 (21%) | 13 (17%) | 142 (27%) |
| EP effect (either period)* | 61 (30%) | 36 (37%) | 24 (49%) | 23 (55%) | 15 (28%) | 16 (21%) | 175 (34%) |
Values are the number of tested sites in each monkey and the number of sites in which multiple regressions fit to the data were not flat (i.e., 95% confidence intervals of slopes not including 0). Only uniquely sampled penetrations are included; in cases where a location was sampled more than once, the session showing the clearest auditory response pattern was used. The number of eye position (EP)-sensitive sites is noted for 2 tested epochs: a silent period and a stimulus period (during the presence of a sound). For combined tests across both epochs, a Bonferroni-corrected P value is used to estimate 95% confidence intervals across 2 tests.
Bonferroni-corrected P = 0.0253, corresponding to P < 0.05 across tests.
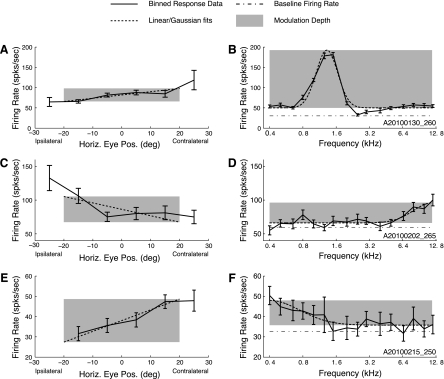
Figure 2 shows examples of the effects of eye position for three recording sites. The eye position effects are portrayed at left (A, C, and E) and auditory frequency tuning is shown at right (B, D, and F). Multiunit activity at the sites in A and E showed increasing activity for contralateral eye positions, whereas multiunit activity in C showed an increase in activity for ipsilateral eye positions. Frequency tuning for these sites showed mid-, high-, and low-frequency tuning (B, D, and F). The example data shown in A and B were taken from a site along a penetration that indicated a tonotopic progression of sound tuning as the electrode passed through the IC; data displayed in C–F were taken from sites along penetrations that showed reliable tuning to sound frequency but no tonotopic organization.
Fig. 2.
Example fits of horizontal eye position and sound frequency to firing rate. A, C, and E: binned firing rates across spontaneously varying horizontal eye positions (solid line) for 3 example recording sites. Error bars indicate SE. Overlaid on each plot is the horizontal component of the regression (dotted line), evaluated at the mean of the orthogonal (i.e., vertical) component. B, D, and F: auditory data from the same sites. The frequency of each tested stimulus is plotted against the firing rate (solid line). Error bars indicate SE. Overlaid on each plot is the Gaussian fit to the frequency-response curve (dotted line). The shaded regions indicate the measured effect size for eye position and frequency. (For display purposes for this analysis only, the eye position effect size was calculated for the horizontal dimension only; for all other analyses, both horizontal and vertical components were incorporated.) Over the tested range, the site depicted in A and B has a larger effect of sound frequency than eye position, whereas the site depicted in C and D has similar effect strengths in both dimensions, and the site depicted in E and F shows a greater effect of eye position than sound frequency.
Location of eye position-sensitive neurons.
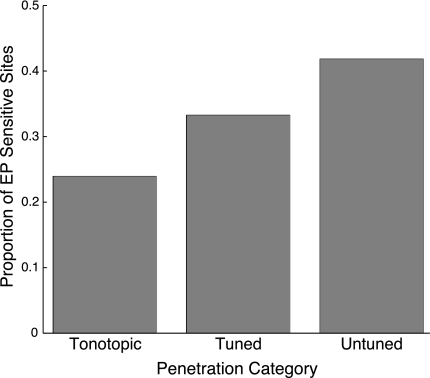
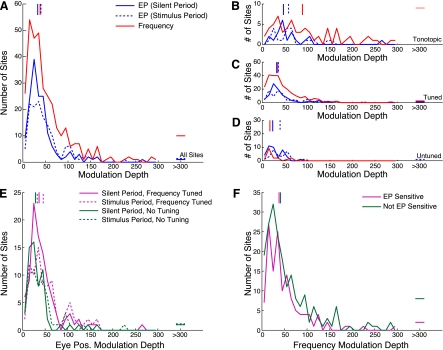
Eye position sensitivity was present in each of three functional classes of auditory penetration type: frequency-tuned but not tonotopic, tonotopic, and frequency-untuned. As noted above, the examples shown in Fig. 2 came from tonotopic (A and B) and frequency-tuned penetration types (C–F). Figure 3 shows the proportion of recordings sensitive to eye position in each of these categories (i.e., significantly nonzero terms in a multiple linear regression). All categories showed much greater than chance prevalence of eye position sensitivity. Tonotopic penetrations contained the smallest proportion of recordings showing eye position sensitivity (∼24%), responses in frequency-tuned (but not tonotopic) penetrations were more likely to show effects of eye position (∼33%), and frequency-untuned penetrations included the largest proportion of recordings in which activity varied with eye position (∼42%). The differences in proportions were statistically significant when all penetrations were included [χ2 = 9.05, degrees of freedom (df) = 2, P < 0.05] but did not quite reach significance when only unique penetrations were included, although the overall proportions were similar (tonotopic ∼27%, frequency-tuned ∼33%, frequency-untuned ∼43%; χ2 = 5.16, df = 2, P ≈ 0.08).
Fig. 3.
Extent of eye position sensitivity by functionally defined region in the inferior colliculus (IC). Bars indicate the proportion of eye position-sensitive recordings within each category. Tonotopic penetrations were those that showed an increase of best frequency as the electrode advanced along a dorsolateral-to-ventromedial trajectory. Frequency-tuned penetrations contained recordings that had well-defined best frequencies but no systematic change with depth. Frequency-untuned penetrations did not show tuning at more than 2 sites. The locations of these penetration categories are indicated in Fig. 4. EP, eye position.
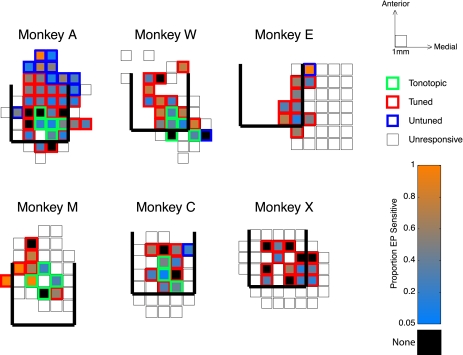
Other than the breakdown into tonotopic/frequency-tuned/frequency-untuned categories, we did not see any obvious patterns to the distribution of eye position sensitivity as a function of location in the IC. Figure 4 shows the proportion of eye position-sensitive sites in each penetration for each monkey. The locations of tonotopic, frequency-tuned, and frequency-untuned penetrations are indicated with green, red, and blue squares, respectively. The color that the squares are filled with marks the proportion of sites showing a significant effect of eye position, with warmer colors indicating a higher proportion of eye position-sensitive sites and cooler colors indicating a lower proportion. Locations in which we saw no eye position effect beyond chance (i.e., <5% of sites showing an effect) are marked with black-filled squares, and locations that were probed but did not show any auditory response are indicated with open squares. Estimates of the posterior, medial, and lateral boundaries of the IC, determined from MRI, are displayed with thick black lines (the anterior boundary is not readily evident on MRI). Although a range of sensitivity was seen in all monkeys, no consistent pattern can be seen in the location of penetrations showing no influence of eye position (or those showing dramatic influence of eye position). Thus, although many sites showed no effect of eye position, those that did were spread throughout the recordings.
Fig. 4.
Distribution of eye position sensitivity across recording penetrations. Maps of penetrations in each subject show the average eye position sensitivity in each location we sampled, averaged over the depth of recordings. Warmer fill colors indicate a higher proportion of sensitive sites; cooler colors indicate more sparse sensitivity. Locations in which no eye position sensitivity was found (i.e., <5% of sampled sites) are filled with black. The outlines of the squares indicate how the location was categorized based on auditory responses. Thick black lines mark posterior, medial, and lateral borders of the IC estimated from MRI. In cases where a location was sampled more than once, the average of the sensitivity across penetrations is shown.
The maps in Fig. 4 show results collapsed across multiple recording depths. Because the IC is a three-dimensional structure, it is important to ask whether the shallower recordings (corresponding to more dorsolateral recording locations; see methods) or deeper recordings showed differing sensitivity to eye position. Figure 5 shows the proportion of sites showing eye position effects by the depth of recordings. Depths were aligned on the first auditory response in each penetration, providing a depth relative to the electrode's entry into the IC. Eye position sensitivity was generally observed at all depths in all penetration categories, with the deepest frequency-untuned sites showing the highest prevalence. Because the plot is aligned on the first auditory recording and the length of a penetration was variable, the number of sampled points decreases with depth, making proportion estimates less reliable at the deepest locations tested.
Fig. 5.
Changes in eye position sensitivity over depth of recordings. Bars indicate the proportion of eye position sensitivity at each recording depth, aligned on the first auditory-responsive recording in the penetration. Shading of the bars indicates the classification of auditory response. Numbers at top indicate how many sites were tested for each depth/category. Depth wrt. IC entry, depth with respect to IC entry.
Tonotopic penetrations likely passed through the shell region (i.e., the dorsal cortex of the IC) before entering the central nucleus. Thus not every site along a tonotopic penetration is likely to be located within the central nucleus. Because the length of tonotopic penetrations can vary depending on whether the penetration passes through the center of the ICC, and because the thickness of the overlying shell is not precisely established, it would be difficult to apply a fixed depth criterion for entry/exit into the central nucleus. Instead, a more reliable marker for central nucleus sites is the combination of tonotopy and high BF: sites with high BFs were found almost exclusively at the deeper sites along tonotopic penetrations (Bulkin and Groh 2011). Accordingly, we assessed the proportion of eye position sensitivity in sites with BFs above 1 kHz from tonotopic penetrations. Thirteen of 50 sites with BFs >1 kHz were eye position sensitive (26%), similar to the 19 of 66 (28.8%) sites with BFs ≤ 1 kHz. This analysis confirms that eye position sensitivity is found within the central nucleus.
Population characteristics: effect size and bias in favor of contralateral eye positions.
To investigate the strength of eye position effects across the population of IC recording sites, we constructed a measure of eye position modulation depth and compared it with a similar metric calculated on the basis of differential auditory frequency responses (see methods). Analysis of eye position effect sizes were derived from the slopes of the regression plane, and analysis of frequency effect strengths were based on the amplitude of the Gaussian fits to responses to tones of different frequencies. The example shown in Fig. 2, A and B, has a larger modulation following changes in sound frequency (over the range of stimuli tested) than following changes in eye position (over a range of ±20 deg). In contrast, the site displayed in Fig. 2, C and D, shows a similar strength of effect of eye position and auditory frequency, and the example shown in Fig. 2, E and F, exhibits a larger effect of eye position than sound frequency. Indeed, over the entire population, modulation depth to eye position and sound frequency were largely similar (t-test, P ≈ 0.37). Figure 6A shows the distribution of modulation depths to these two factors throughout the population. The strength of eye position effects was similar in the two epochs we tested (t-test, P ≈ 0.27; blue solid and dotted traces) and largely overlapped with the measure of frequency modulation depth. Eye position effects were also alike in sites that showed frequency tuning compared with those that did not (t-test, P ≈ 0.23; Fig. 6E), and sound frequency modulation depths were not different in sites that showed an effect of eye position compared with those that did not (t-test, P ≈ 0.11; Fig. 6F).
Fig. 6.
Distribution of eye position and sound frequency modulation depths. A: distributions of eye position modulation depths (blue lines) and frequency modulation depths (red line) throughout the population. The strength of eye position effects was similar in the 2 epochs tested (broken and solid blue lines), and eye position showed a similar modulation over the tested range as sound frequency. Horizontal lines show outliers with modulation depths outside of the plotted range. Vertical lines show the medians of the distributions. B, C, and D: distributions in 3 regions that were functionally defined on the basis of auditory responses (tonotopic, frequency-tuned, and frequency-untuned, respectively). Frequency effects were more powerful in responses gathered from sites along penetrations showing tonotopicity (1-way ANOVA, P < 0.0001). Eye position effects showed a similar strength in all regions (1-way ANOVA, P ≈ 0.22). E: distributions of eye position modulation depth for sites with (magenta lines) and without (green lines) frequency tuning. Broken lines show effects measured in the stimulus epoch, and solid lines show effects measured in the silent epoch. F: distributions of frequency modulation depth in sites that showed eye position sensitivity (magenta line) and those that did not (green line). No differences were found between eye position modulation strength in frequency-tuned and frequency-untuned sites (t-test, P ≈ 0.23) or in sound frequency modulation depth in sites that showed eye position sensitivity vs. sites that did not (t-test, P ≈ 0.11).
These similarities held in each of the functional subregions we identified. Figure 6, B, C, and D, shows distributions of modulation depth restricted to tonotopic, frequency-tuned, and frequency-untuned penetrations, respectively. Along tonotopic penetrations, the effect of sound frequency was often stronger than in frequency-tuned or frequency-untuned penetrations (1-way ANOVA, P < 0.0001), consistent with previous reports (Aitkin et al. 1994). In contrast, the effect of eye position showed a similar strength across functionally defined regions in the IC (1-way ANOVA, P ≈ 0.22).
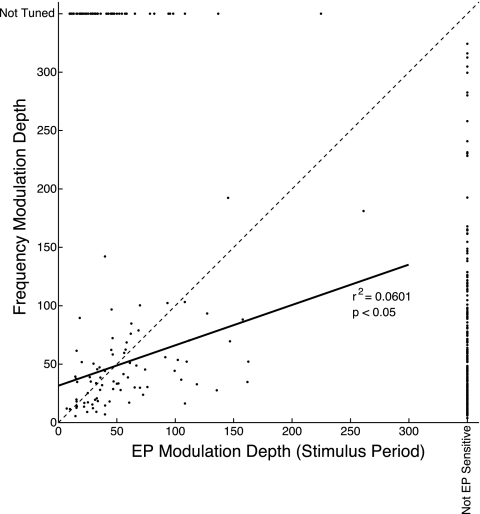
Recording sites that showed significant effects of both eye position and sound frequency indicated a relationship between their modulation depths (Fig. 7). Those sites that showed a large increase in firing rate across the range of tested frequencies also generally showed a large span of firing rates across eye positions. The slope of a regression through these points was significantly different from 0 (P < 0.05).
Fig. 7.
Within-site comparison of eye position and sound frequency effect sizes. The modulation depths of eye position and frequency effects are plotted against each other for sites in which both effects were found. The magnitude of the eye position effect was calculated based on data from the stimulus epoch (the effects in baseline and stimulus epochs were highly similar; see Fig. 6 and text). The solid line shows a linear regression fit to the data. Sites that contained one effect but not the other are portrayed at the far right and top of the axis (distributions of these data are shown in Fig. 6, E and F). The broken line indicates unity.
Eye position sensitivity showed a distribution of coefficient values in the horizontal and vertical aspects of the regression, with a bias toward increasing activity for contralateral eye positions. Figure 8 shows values of the horizontal and vertical coefficients in eye position-sensitive recordings. Activity more often increased with more contralateral eye positions (i.e., horizontal slopes are skewed toward positive numbers) for both of the temporal periods we examined (Wilcoxon signed rank test, P < 0.05). We did not observe this effect when we limited analysis to tonotopic penetrations, although the sample size was far more limited and a weak effect may have been undetectable. Vertical eye position terms were more normally distributed and did not favor positive (upward) or negative (downward) slopes, although this result should be interpreted with caution because the uncontrolled eye position showed less spontaneous variation in elevation than in azimuth (Fig. 1B). Note, however, that because the slope is used, an offset in the center (from the meridian) of the distribution of vertical eye positions does not affect this result.
Fig. 8.
Coefficient values for horizontal and vertical components of multiple regressions. The slope of horizontal and vertical coefficients of eye position-sensitive sites are plotted, along with histograms summarizing the total number of points in each dimension. Positive values of the horizontal coefficients indicate regressions in which firing rate increased with more contralateral locations, and positive values of vertical coefficients indicate regressions in which firing rate increased with elevation. Data are plotted separately for results based on the 2 epochs analyzed. The distribution of horizontal coefficients is biased in favor of increasing activity for more contralateral eye positions (Wilcoxon signed rank test, P < 0.05 for both tested epochs), whereas there was no significant bias in the vertical dimension (Wilcoxon signed rank test, P > 0.05).
DISCUSSION
We mapped the location of eye position-sensitive neurons in the IC, systematically sampling a three-dimensional grid throughout the auditory midbrain of six rhesus macaques. We found effects of eye position throughout our recordings, indicating that no part of the IC is devoid of this nonauditory signal. Overall, the proportion of eye position sensitivity we found (21–49%) was similar to previous single-unit studies in which the structure was not mapped (24–41%) (Groh et al. 2001; Porter et al. 2006; Zwiers et al. 2004). This was the case even though 1) the current study measured multiunit activity, which could have obscured eye position sensitivity if present in only a subset of the component units; and 2) we relied on the animal's spontaneous behavior rather than performance of an eye movement task, a limitation for expedience, but one that also could have hindered detection of eye position sensitivity.
The proportion of eye position-sensitive sites was not constant across recordings. We found a relationship between eye position sensitivity and the location of recordings based on a functional map defined using auditory tuning properties. Recordings in tonotopic penetrations, likely corresponding to the central nucleus of the IC, showed a lower proportion of sites with eye position sensitivity than recordings in nontonotopic penetrations, corresponding to the shell regions of the IC. The greatest proportion of eye position sensitivity was seen in frequency-untuned penetrations, found in the periphery of our sample and perhaps arising from pericollicular tissue.
Interestingly, when an eye position effect was present, the estimated change in firing rate over a 40-deg ocular range was similar to the span of responses to the tested frequencies in frequency-sensitive sites. In tonotopic distributions, modulation based on sound frequency was more powerful than elsewhere in the IC, but eye position modulation showed a similar effect size in each of the functionally defined regions we tested. Obviously, the modulation indexes we chose are somewhat arbitrary, and different metrics would give somewhat different outcomes. Nevertheless, this finding illustrates the eye position signal is not a minor or negligible determinant of neural firing in the IC.
Eye position sensitivity and multisensory integration in the auditory pathway.
Our results reveal that the eye position signal is not confined to shell areas of the IC but modulates neurons in the central nucleus, providing an influence on the main ascending auditory stream. The pattern in auditory cortex is similar: eye position signals are present in both core and belt (Fu et al. 2004; Maier and Groh 2010; Werner-Reiss et al. 2003). This result contrasts with previous findings regarding somatosensory signals in the IC, which seem to be absent in the central nucleus (Aitkin et al. 1978, 1981). Similarly overt somatosensory responses appear limited to the belt region of auditory cortex (Schroeder et al. 2001). However, recent work has uncovered somatosensory modulation of auditory responses in the cortical core region via changes in the phase of ongoing subthreshold oscillatory activity (Lakatos et al. 2007). Such effects have not been investigated at the level of the IC. Interestingly, both somatosensory and eye position effects in auditory cortex are most prominent in the cortical supragranular layers (Fu et al. 2004; Schroeder et al. 2001). This is particularly noteworthy because calbindin-reactive (or “matrix”) cells found in the thalamus, hypothesized to carry modulatory signals originating from the nontonotopic regions of IC, also project more heavily to the supragranular layers of the cortex [although they diffusely target core and belt regions (Jones 1998, 2001)].
Together, these findings suggest a new look at how the senses might be integrated in the brain. The dogma that the senses are processed separately and then integrated has been called into question with both anatomical and physiological demonstrations of multisensory processes in primary (previously presumed unisensory) areas (for review, see Ghazanfar and Schroeder 2006; Bulkin and Groh 2006; and Schroeder and Foxe 2005). The present report suggests that the computations underlying sensory integration are found even earlier, in the subcortical auditory processing stream.
From a behavioral point of view, the idea that sensory channels influence each other early is not surprising. Information from auditory and visual environments do not merely sum to provide multiple maps of the environment. One modality can dramatically influence the other. Estimates of the location of sound sources are reliably biased in the direction of a task-irrelevant displaced visual cue in humans and in monkeys (Knudsen and Knudsen 1985, 1989; Kopco et al. 2009; Recanzone 1998, 2009; Woods and Recanzone 2004). Participants in such ventriloquism experiments are subject to a direct effect of visual information on auditory percepts. Given that the computations of interaural timing and level differences underlying auditory localization occur subcortically (for review, see Popper and Fay 2005), visual influences on auditory localization likely also occur subcortically. Indeed, long-term plastic changes in the auditory space map of barn owls driven by a displaced visual environment have been localized to anatomical changes within the IC [notably in connections from the central nucleus to the surrounding shell (DeBello et al. 2001; Feldman and Knudsen 1997)]. In primates and other species with mobile eyes, the computation to reconcile visual and auditory spatial information when the eyes are not directed centrally necessarily requires input regarding the position of the eyes (Groh and Sparks 1992; for review, see Groh and Werner-Reiss 2002).
Another example of visual information exerting an influence on auditory perception is the McGurk effect (McGurk and Macdonald 1976). In this illusion, when subjects are presented with a video of a speaker pronouncing a syllable (“ga”) matched with an auditory presentation of a different syllable (“ba”), they hear something that is a mix of the two (“da”). A recent case report of a patient with a unilateral IC lesion showed that the effect was reduced when stimuli were presented in the contralateral hemifield (Champoux et al. 2006). These data directly implicate the IC in audiovisual integration.
Whether or not visual signals influence processing in the central nucleus remains an open question. Neurons in the monkey IC show overt visual and oculomotor responses (Porter et al. 2007), although the location of responses has not yet been mapped. In a noncentral region of the barn owl IC, visual stimuli can modulate responses to accompanying auditory stimuli, although they rarely elicit responses independently (Bergan and Knudsen 2009) except with accompanying pharmacological manipulation of the SC (Gutfreund et al. 2002). Anatomical studies have revealed multiple visually sensitive regions that project to shell regions of the IC, including connections from visual and parietal cortices (Coleman and Clerici 1987; Cooper and Young 1976; Druga et al. 1997), the retina (Herbin et al. 1994; Itaya and Van Hoesen 1982; Yamauchi and Yamadori 1982), and the SC (Adams 1980; Coleman and Clerici 1987; Covey et al. 1987). Whether such inputs also project to the central nucleus is less clear, but the signals could conceivably reach that region through less direct routes involving connections within the auditory pathway (e.g., Coleman and Clerici 1987). It is also possible that visual information does not directly reach the central nucleus, but rather that eye position modulation facilitates visual attention to sounds, perhaps by influencing the excitability of individual neurons to auditory input (e.g., Rajkai et al. 2008). Indeed, this provides a mechanism for visual information to guide auditory processing in the absence of neurons directly responsive to both modalities.
Eye position sensitivity relationship to anatomical connectivity patterns.
Our results show differential eye position sensitivity among the central and peripheral regions of the IC. Although the primary ascending signal in the central nucleus is clearly influenced by the position of the eyes, the prevalence of eye position sensitivity was higher in surrounding tissue than in the central nucleus. The connections of neurons in the central nucleus and its surrounding tissue to thalamus and ultimately to cortex differ in a number of ways. Whereas the central nucleus provides the majority of input ascending to the ventral medial geniculate body [the principal ascending auditory nucleus of the thalamus (Winer and Schreiner 2005)], the surrounding regions primarily project to the medial and dorsal regions of the thalamus (Wenstrup 2005). Connections arriving in a segregated fashion in the thalamus from the IC appear to continue to be segregated as signals ascend from thalamus to cortex. Immunoreactivity studies have described a distinction between calbindin-reactive “matrix” cells that receive input from the shell of the IC and project widely in auditory cortex, and parvalbumin-reactive “core” cells that receive input from the central nucleus and show more focused projections to auditory cortex (Jones 1998, 2001). Within cortex, the laminar distribution of these inputs also differs, with matrix inputs targeting the superficial layers where eye position sensitivity has been reported (Fu et al. 2004) and core inputs targeting the middle layers. Finally, descending projections from auditory cortex are also differentially distributed in the IC, with the highest concentration in the shell regions and lower concentrations in the central nucleus (Andersen et al. 1980; Diamond et al. 1969; Druga et al. 1997; Druga and Syka 1984a, 1984b; Coleman and Clerici 1987; Saldaña et al. 1996; Schofield, 2009; Winer et al. 2002).
A recent model based on synaptic differences of glutamatergic tectothalamic connections has suggested that projections from the central nucleus to the thalamus show properties of “driver” neurons (Lee and Sherman 2008, 2010; Sherman and Guillery 1998). These neurons are implicated in the main transmission of auditory information: they show larger excitatory postsynaptic potentials (EPSPs), thicker axons, and more dense terminal arbors, and they target dendrites more proximally to the cell body. Projections from noncentral IC to thalamus exhibit properties of “modulator” neurons, showing the opposite pattern. These connections seem less likely to be involved in the rapid transmission of primary auditory information but can produce prolonged modulation on the auditory signal. Our finding that eye position influences were more prevalent in recordings outside of the central nucleus is consistent with this model. Relatedly, we have shown previously that eye position signals at the level of the IC show evidence of modulating the auditory responses of neurons: the magnitude of the eye position sensitivity depends on the vigor of the auditory responses (Maier and Groh 2010). The presence of differential eye position sensitivity in the IC suggests that thalamic cells should show modulation based on eye position, with the ventral regions perhaps showing fewer sensitive sites than medial and dorsal regions.
Sources of eye position input to the IC.
Identifying the origin of the eye position signal found in the IC is challenging. Inferring connectivity patterns from the latency of this signal is difficult because postural signals like the orientation of the eyes are always present, unlike external stimuli, which can appear and disappear. Furthermore, the eye position signal may derive from a copy of the motor command to move the eyes and thus could lead the change in eye position in time.
The IC receives input from a number of eye position-sensitive regions, including the SC (Adams 1980; Coleman and Clerici 1987; Covey et al. 1987), parietal cortex (Druga et al. 1997), and the fastigial nucleus in the cerebellum (Carpenter 1959; Earle and Matzke 1974). The IC also receives input from the trigeminal nucleus, both directly (Zhou and Shore 2006) and via the cochlear nucleus (Shore et al. 2000). Eye position sensitivity also has been identified in both the core and belt regions of the auditory cortex (Fu et al. 2004; Maier and Groh 2010; Werner-Reiss et al. 2003), which contain descending projections to the IC (Andersen et al. 1980; Diamond et al. 1969; Druga et al. 1997; Druga and Syka 1984a, 1984b; Coleman and Clerici 1987; Saldaña et al. 1996; Schofield 2009; Winer et al. 2002). Indeed, the eye position signal may not meet the auditory system at the level of the IC. Instead, the modulation we observed might reach the IC through other auditory areas. The auditory nuclei of the brain stem have not yet been probed for effects of eye position. Our present findings suggest that these regions should be tested.
GRANTS
This research was supported by the National Institutes of Health Grants EY016478 (to J. M. Groh) and DC010294 (to D. A. Bulkin).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: D.A.B. and J.M.G. conception and design of research; D.A.B. performed experiments; D.A.B. analyzed data; D.A.B. and J.M.G. interpreted results of experiments; D.A.B. prepared figures; D.A.B. drafted manuscript; D.A.B. and J.M.G. edited and revised manuscript; D.A.B. and J.M.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Nate Greene, Vanessa Kennedy, Uri Werner-Reiss, and Nick Del Grosso for collecting some of the data for these experiments, and we are grateful to Abigail Underhill, Tom Heil, and Jessi Cruger for technical support.
REFERENCES
- Adams JC. Crossed and descending projections to the inferior colliculus. Neurosci Lett 19: 1–5, 1980 [DOI] [PubMed] [Google Scholar]
- Aitkin LM, Dickhaus H, Schult W, Zimmermann M. External nucleus of inferior colliculus: auditory and spinal somatosensory afferents and their interactions. J Neurophysiol 41: 837–847, 1978 [DOI] [PubMed] [Google Scholar]
- Aitkin LM, Kenyon CE, Philpott P. The representation of the auditory and somatosensory systems in the external nucleus of the cat inferior colliculus. J Comp Neurol 196: 25–40, 1981 [DOI] [PubMed] [Google Scholar]
- Aitkin L, Tran L, Syka J. he responses of neurons in subdivisions of the inferior colliculus of cats to tonal, noise, and vocal stimuli. Exp Brain Res 98: 53–64, 1994 [DOI] [PubMed] [Google Scholar]
- Andersen RA, Snyder RL, Merzenich MM. The topographic organization of corticocollicular projections from physiologically identified loci in the AI, AII, and anterior auditory cortical fields of the cat. J Comp Neurol 191: 479–494, 1980 [DOI] [PubMed] [Google Scholar]
- Bergan JF, Knudsen EI. Visual modulation of auditory responses in the owl inferior colliculus. J Neurophysiol 101: 2924–2933, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulkin DA, Groh JM. Seeing sounds: visual and auditory interactions in the brain. Curr Opin Neurobiol 16: 415–419, 2006 [DOI] [PubMed] [Google Scholar]
- Bulkin DA, Groh JM. Systematic mapping of the monkey inferior colliculus reveals enhanced low frequency sound representation. J Neurophysiol 105: 1785–1797, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter MB. Lesions of the fastigial nuclei in the rhesus monkey. Am J Anat 104: 1–33, 1959 [DOI] [PubMed] [Google Scholar]
- Champoux F, Tremblay C, Mercier C, Lassonde M, Lepore F, Gagne JP, Theoret H. A role for the inferior colliculus in multisensory speech integration. Neuroreport 17: 1607–1610, 2006 [DOI] [PubMed] [Google Scholar]
- Coleman JR, Clerici WJ. Sources of projections to subdivisions of the inferior colliculus in the rat. J Comp Neurol 262: 215–226, 1987 [DOI] [PubMed] [Google Scholar]
- Cooper MH, Young PA. Cortical projections to the inferior colliculus of the cat. Exp Neurol 51: 488–502, 1976 [DOI] [PubMed] [Google Scholar]
- Covey E, Hall WC, Kobler JB. Subcortical connections of the superior colliculus in the mustache bat, Pteronotus parnellii. J Comp Neurol 263: 179–197, 1987 [DOI] [PubMed] [Google Scholar]
- DeBello WM, Feldman DE, Knudsen EI. Adaptive axonal remodeling in the midbrain auditory space map. J Neurosci 21: 3161–3174, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond IT, Jones EG, Powell TP. The projection of the auditory cortex upon the diencephalon and brain stem in the cat. Brain Res 15: 305–340, 1969 [DOI] [PubMed] [Google Scholar]
- Druga R, Syka J. Ascending and descending projections to the inferior colliculus in the rat. Physiol Bohemoslov 33: 31–42, 1984a [PubMed] [Google Scholar]
- Druga R, Syka J. Neocortical projections to the inferior colliculus in the rat. (An experimental study using anterograde degeneration techniques). Physiol Bohemoslov 33: 251–253, 1984b [PubMed] [Google Scholar]
- Druga R, Syka J, Rajkowska G. Projections of auditory cortex onto the inferior colliculus in the rat. Physiol Res 46: 215–222, 1997 [PubMed] [Google Scholar]
- Earle AM, Matzke HA. Efferent fibers of the deep cerebellar nuclei in hedgehogs. J Comp Neurol 154: 117–131, 1974 [DOI] [PubMed] [Google Scholar]
- Feldman DE, Knudsen EI. An anatomical basis for visual calibration of the auditory space map in the barn owl's midbrain. J Neurosci 17: 6820–6837, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman EG, Sparks DL. Eye-head coordination during head-unrestrained gaze shifts in rhesus monkeys. J Neurophysiol 77: 2328–2348, 1997 [DOI] [PubMed] [Google Scholar]
- Fu KM, Shah AS, O'Connell MN, McGinnis T, Eckholdt H, Lakatos P, Smiley J, Schroeder CE. Timing and laminar profile of eye-position effects on auditory responses in primate auditory cortex. J Neurophysiol 92: 3522–3531, 2004 [DOI] [PubMed] [Google Scholar]
- Ghazanfar AA, Schroeder CE. Is neocortex essentially multisensory? Trends Cogn Sci 10: 278–285, 2006 [DOI] [PubMed] [Google Scholar]
- Groh JM, Sparks DL. Two models for transforming auditory signals from head-centered to eye-centered coordinates. Biol Cybern 67: 291–302, 1992 [DOI] [PubMed] [Google Scholar]
- Groh JM, Trause AS, Underhill AM, Clark KR, Inati S. Eye position influences auditory responses in primate inferior colliculus. Neuron 29: 509–518, 2001 [DOI] [PubMed] [Google Scholar]
- Groh JM, Werner-Reiss U. Visual and auditory integration. In: Encyclopedia of the Human Brain, edited by Ramachandran VS. San Diego, CA: Academic, 2002, p. 739–752 [Google Scholar]
- Gutfreund Y, Zheng W, Knudsen EI. Gated visual input to the central auditory system. Science 297: 1556–1559, 2002 [DOI] [PubMed] [Google Scholar]
- Herbin M, Reperant J, Cooper HM. Visual system of the fossorial mole-lemmings, Ellobius talpinus and Ellobius lutescens. J Comp Neurol 346: 253–275, 1994 [DOI] [PubMed] [Google Scholar]
- Itaya SK, Van Hoesen GW. Retinal innervation of the inferior colliculus in rat and monkey. Brain Res 233: 45–52, 1982 [DOI] [PubMed] [Google Scholar]
- Jay MF, Sparks DL. Auditory receptive fields in primate superior colliculus shift with changes in eye position. Nature 309: 345–347, 1984 [DOI] [PubMed] [Google Scholar]
- Jay MF, Sparks DL. Sensorimotor integration in the primate superior colliculus. I. Motor convergence. J Neurophysiol 57: 22–34, 1987a [DOI] [PubMed] [Google Scholar]
- Jay MF, Sparks DL. Sensorimotor integration in the primate superior colliculus. II. Coordinates of auditory signals. J Neurophysiol 57: 35–55, 1987b [DOI] [PubMed] [Google Scholar]
- Jones EG. Viewpoint: the core and matrix of thalamic organization. Neuroscience 85: 331–345, 1998 [DOI] [PubMed] [Google Scholar]
- Jones EG. The thalamic matrix and thalamocortical synchrony. Trends Neurosci 24: 595–601, 2001 [DOI] [PubMed] [Google Scholar]
- Judge SJ, Richmond BJ, Chu FC. Implantation of magnetic search coils for measurement of eye position: an improved method. Vision Res 20: 535–538, 1980 [DOI] [PubMed] [Google Scholar]
- Knudsen EI, Knudsen PF. Vision guides the adjustment of auditory localization in young barn owls. Science 230: 545–548, 1985 [DOI] [PubMed] [Google Scholar]
- Knudsen EI, Knudsen PF. Visuomotor adaptation to displacing prisms by adult, and baby barn owls. J Neurosci 9: 3297–3305, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopco N, Lin IF, Shinn-Cunningham BG, Groh JM. Reference frame of the ventriloquism aftereffect. J Neurosci 29: 13809–13814, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatos P, Chen CM, O'Connell MN, Mills A, Schroeder CE. Neuronal oscillations and multisensory interaction in primary auditory cortex. Neuron 53: 279–292, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, Sherman SM. Synaptic properties of thalamic and intracortical inputs to layer 4 of the first- and higher-order cortical areas in the auditory and somatosensory systems. J Neurophysiol 100: 317–326, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC, Sherman SM. Drivers and modulators in the central auditory pathways. Front Neurosci 4: 79, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier JX, Groh JM. Comparison of gain-like properties of eye position signals in inferior colliculus versus auditory cortex of primates. Front Integr Neurosci 4: 121, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascetti GG, Strozzi L. Visual cells in the inferior colliculus of the cat. Brain Res 442: 387–390, 1988 [DOI] [PubMed] [Google Scholar]
- McGurk H, Macdonald J. Hearing lips and seeing voices. Nature 264: 746–748, 1976 [DOI] [PubMed] [Google Scholar]
- Metzger RR, Greene NT, Porter KK, Groh JM. Effects of reward and behavioral context on neural activity in the primate inferior colliculus. J Neurosci 26: 7468–7476, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popper AN, Fay RR. Sound Source Localization. New York: Springer, 2005 [Google Scholar]
- Populin LC, Tollin DJ, Yin TC. Effect of eye position on saccades and neuronal responses to acoustic stimuli in the superior colliculus of the behaving cat. J Neurophysiol 92: 2151–2167, 2004 [DOI] [PubMed] [Google Scholar]
- Porter KK, Metzger RR, Groh JM. Representation of eye position in primate inferior colliculus. J Neurophysiol 95: 1826–1842, 2006 [DOI] [PubMed] [Google Scholar]
- Porter KK, Metzger RR, Groh JM. Visual- and saccade-related signals in the primate inferior colliculus. Proc Natl Acad Sci USA 104: 17855–17860, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkai C, Lakatos P, Chen CM, Pincze Z, Karmos G, Schroeder CE. Transient cortical excitation at the onset of visual fixation. Cereb Cortex 18: 200–209, 2008 [DOI] [PubMed] [Google Scholar]
- Recanzone GH. Rapidly induced auditory plasticity: the ventriloquism aftereffect. Proc Natl Acad Sci USA 95: 869–875, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recanzone GH. Interactions of auditory and visual stimuli in space and time. Hear Res 258: 89–99, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DA. A method of measuring eye movement using a scleral search coil in a magnetic field. IEEE Trans Biomed Eng 10: 137–145, 1963 [DOI] [PubMed] [Google Scholar]
- Saldaña E, Feliciano M, Mugnaini E. Distribution of descending projections from primary auditory neocortex to inferior colliculus mimics the topography of intracollicular projections. J Comp Neurol 371: 15–40, 1996 [DOI] [PubMed] [Google Scholar]
- Schofield BR. Projections to the inferior colliculus from layer VI cells of auditory cortex. Neuroscience 159: 246–258, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder CE, Foxe J. Multisensory contributions to low-level ‘unisensory’ processing. Curr Opin Neurobiol 15: 454–458, 2005 [DOI] [PubMed] [Google Scholar]
- Schroeder CE, Lindsley RW, Specht C, Marcovici A, Smiley JF, Javitt DC. Somatosensory input to auditory association cortex in the macaque monkey. J Neurophysiol 85: 1322–1327, 2001 [DOI] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. On the actions that one nerve cell can have on another: distinguishing “drivers” from “modulators”. Proc Natl Acad Sci USA 95: 7121–7126, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore SE, Vass Z, Wys NL, Altschuler RA. Trigeminal ganglion innervates the auditory brainstem. J Comp Neurol 419: 271–285, 2000 [DOI] [PubMed] [Google Scholar]
- Wang X, Zhang M, Cohen IS, Goldberg ME. The proprioceptive representation of eye position in monkey primary somatosensory cortex. Nat Neurosci 10: 640–646, 2007 [DOI] [PubMed] [Google Scholar]
- Wenstrup JJ. The tectothalamic system. In: The Inferior Colliculus, edited by Winer JA, Schreiner CE. New York: Springer, 2005, p. 200–230 [Google Scholar]
- Werner-Reiss U, Kelly KA, Trause AS, Underhill AM, Groh JM. Eye position affects activity in primary auditory cortex of primates. Curr Biol 13: 554–562, 2003 [DOI] [PubMed] [Google Scholar]
- Winer JA, Chernock ML, Larue DT, Cheung SW. Descending projections to the inferior colliculus from the posterior thalamus and the auditory cortex in rat, cat, and monkey. Hear Res 168: 181–195, 2002 [DOI] [PubMed] [Google Scholar]
- Winer JA, Schreiner CE. The central auditory system: a functional analysis. In: The Inferior Colliculus, edited by Winer JA, Schreiner CE. New York: Springer, 2005, p. 1–68 [Google Scholar]
- Woods TM, Recanzone GH. Visually induced plasticity of auditory spatial perception in macaques. Curr Biol 14: 1559–1564, 2004 [DOI] [PubMed] [Google Scholar]
- Yamauchi K, Yamadori T. Retinal projection to the inferior colliculus in the rat. Acta Anat (Basel) 114: 355–360, 1982 [DOI] [PubMed] [Google Scholar]
- Zhou J, Shore S. Convergence of spinal trigeminal and cochlear nucleus projections in the inferior colliculus of the guinea pig. J Comp Neurol 495: 100–112, 2006 [DOI] [PubMed] [Google Scholar]
- Zwiers MP, Versnel H, Van Opstal AJ. Involvement of monkey inferior colliculus in spatial hearing. J Neurosci 24: 4145–4156, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]