Abstract
Because of the availability of disease and genetic models, the mouse has become a valuable species for auditory neuroscience that will facilitate long-term goals of understanding neuronal mechanisms underlying the perception and processing of sounds. The goal of this study was to define the basic sound-evoked response properties of single neurons in the mouse dorsal cochlear nucleus (DCN). Neurons producing complex spikes were distinguished as cartwheel cells (CWCs), and other neurons were classified according to the response map scheme previously developed in DCN. Similar to observations in other rodent species, neurons of the mouse DCN exhibit relatively little sound-driven inhibition. As a result, type III was the most commonly observed response. Our findings are generally consistent with the model of DCN function that has been developed in the cat and the gerbil, suggesting that this in vivo mouse preparation will be a useful tool for future studies of auditory physiology.
Keywords: in vivo preparation, extracellular spike, auditory physiology
the mammalian cochlear nucleus is the central termination for the auditory nerve and can be divided into subsections based on cell morphology, connectivity, and function. The dorsal portion of the cochlear nucleus (DCN) is particularly interesting because it has a complex cytoarchitecture and because it receives a second stream of excitatory input from nonauditory and feedback auditory sources (Brawer et al. 1974; Cant and Morest 1984; Lorente de No 1981; Mugnaini et al. 1980; Osen 1969; Ryugo et al. 1981). One of the proposed roles of the DCN is that it participates in localization of sound sources by detecting spectral cues generated by the pinna. This hypothesis is supported by behavioral experiments in which cats show a deficit in their ability to detect the elevation of a sound source after DCN lesion (May 2000; Sutherland et al. 1998a, 1998b). For a more comprehensive review of DCN physiology, the reader is directed to one of the many overviews of DCN (e.g., Nelken and Young 1996; Oertel and Young 2004; Young and Davis 2002).
Our motivation was to develop a mouse in vivo preparation that would be suitable for future studies of DCN utilizing whole cell patch-clamp recordings and two-photon imaging. Mice are small and easily handled, and disease and genetic models are readily available. Mice also provide an opportunity to conduct parallel studies using brain slice preparations. Portfors and colleagues (Portfors and Roberts 2007; Roberts and Portfors 2008) have described single-neuron activity in the awake mouse preparation, and Luo et al. (2009) studied the tonotopic organization of cochlear nucleus in anesthetized mice. For our experiments, however, we required that the preparation allow direct visualization of the surface of DCN. It is possible to remove the overlying cerebellum in anesthetized mice; however, the same procedure may present complications in an awake preparation. Additionally, we wanted to avoid the potentially confounding effects of anesthesia on spontaneous and sound-evoked neuronal responses (see, e.g., Anderson and Young 2004; Evans and Nelson 1973; Young and Brownell 1976). In light of these considerations, we elected to pursue a decerebrate preparation, which has been widely used for DCN studies in the cat and the gerbil (e.g., Davis et al. 1996a; Davis and Voigt 1997; Davis and Young 2000; Ding and Voigt 1997; Shofner and Young 1985; Spirou et al. 1999; Voigt and Young 1980; Young 1980).
In this report, we describe the surgical requirements for a decerebrate mouse preparation and present an overview of single-neuron responses to sound stimuli. Single neurons were classified according to the response map scheme described in rodents (Davis et al. 1996a). Mouse DCN was largely comparable to both the decerebrate gerbil and awake mouse DCN models in that putative principal neurons showed decreased sound-evoked inhibition compared with the cat. Also, we observed relatively few type II responses (n = 1), typically associated with tuberculoventral neurons. Features of the neuronal population are detailed for use as a baseline for future studies.
METHODS
Animal care and surgery.
Adult male CBA/J mice (8–12 wk of age) from Harlan Labs were used. All experiments were conducted in accordance with procedures approved by the National Institutes of Health Animal Care and Use Committee. At the beginning of each experiment, a mouse (∼30 g) was anesthetized by intraperitoneal injection of 100 mg/kg ketamine and 10 mg/kg xylazine. Ophthalmic ointment was applied to prevent corneal drying, and a 1-ml injection of normal saline was administered subcutaneously to provide hydration. We made a midline incision ∼1 cm long on the crown of the skull with a no. 10 scalpel and reflected the skin to expose bregma and lambda. Auditory cortex was exposed with a dental drill fitted with a 1-mm-diameter spherical bit, and the animal was decerebrated by aspirating a complete cut, separating forebrain from thalamus. To expose the DCN, the musculature overlying the interparietal and occipital bones was reflected and bilateral holes were drilled to expose caudal cerebellum. The dorsal aspect of DCN was visualized by aspirating the overlying cerebellum and could be observed as a raised area of yellow-white tissue adhered to the brain stem. DCN was bounded rostrally by the cerebellar peduncle and by the brain stem on its medial and caudal sides. After surgery, at least 30 min was allowed for the animal to recover from anesthesia.
It was critical to remove symmetrical portions of cerebellum. After recovery from anesthesia, if cerebellar lesions were not symmetrical the animals could show coordinated movement of their tail and hind paws that suggested a righting motion or possibly vertigo. These movements did not appear to be driven by pain. If an animal in this state were removed from the stereotaxic instrument, it would persist in twirling on its long axis while making no attempt to flee or cower.
During the recording session, the animal's pinnae were left intact, in a natural orientation, and its head was held horizontally with a head post secured to the frontal bones. Animals sat unrestrained on a pedestal with their body supported on an ∼45° incline. Core temperature was maintained at 36–38°C with a feedback-controlled heating pad. Decerebrate animals exhibited spinal reflexes (e.g., paw withdrawal) but did not display responses suggesting pain from the surgical site or other manipulations (e.g., wincing, whisking, squeaking, or elevated respiratory rate).
Single-neuron recording.
Recordings were made with glass pipettes filled with neurobiotin in either 1 M sodium chloride or 2 M potassium acetate solution (10- to 100-MΩ resistance), although some data were collected with commercially purchased tungsten electrodes (A-M Systems, Sequim, WA). Electrodes were placed on the exposed surface of DCN and advanced at 25–35° from vertical and laterally with respect to the sagittal plane. This approach roughly parallels the cellular lamina organized along the long (strial) axis of DCN in mice as determined by gross dissections that were performed with the animal's head secured to the head bar. The first electrode each day was low impedance (5–20 MΩ) and used to map the frequency response of the electrode track.
The electrode signal was amplified (3,000–20,000 times), low-pass filtered at 5 kHz, and digitized at ∼12 kHz. For online spike detection, the amplifier signal was high-pass filtered at 10 Hz. “Spikes” or extracellular action potentials were detected when the electrode signal passed the variable threshold in the specified direction. A variable time-out was used to reduce accidental spike detection.
Acoustic stimulation.
A double-walled sound chamber (Industrial Acoustics, Bronx, NY) served as both the surgical platform and the recording chamber. The interior of the chamber was covered with 2-in. acoustic foam from Acoustical Solutions (Richmond, VA). The stereotaxic apparatus was positioned atop a translatable metal plate near the geometric center of the chamber. Free field sound was presented via a Tucker-Davis Technologies (TDT, Alachua, FL) ES1 electrostatic speaker positioned ∼5 cm directly in front of the animal. During the experiment, a microphone was positioned ∼2 cm above and behind the animal's ipsilateral pinna. The electrostatic speaker was housed inside a Faraday cage to reduce electrode noise, but no other objects were placed in the area between the speaker and the animal's ears.
Acoustic and current presentations were generated on a TDT RX6 processor. Sound was sampled and recorded at a 200-kHz sample rate. Stimuli were 200-ms-long presentations of pure tones or broadband noise with 10-ms ramps at the beginning and end of the presentation and were presented with a 200-ms delay for calculation of spontaneous firing rates. Broadband noise presentations were generated de novo and band-pass filtered between 5 and 100 kHz. Tones were presented with a random starting phase.
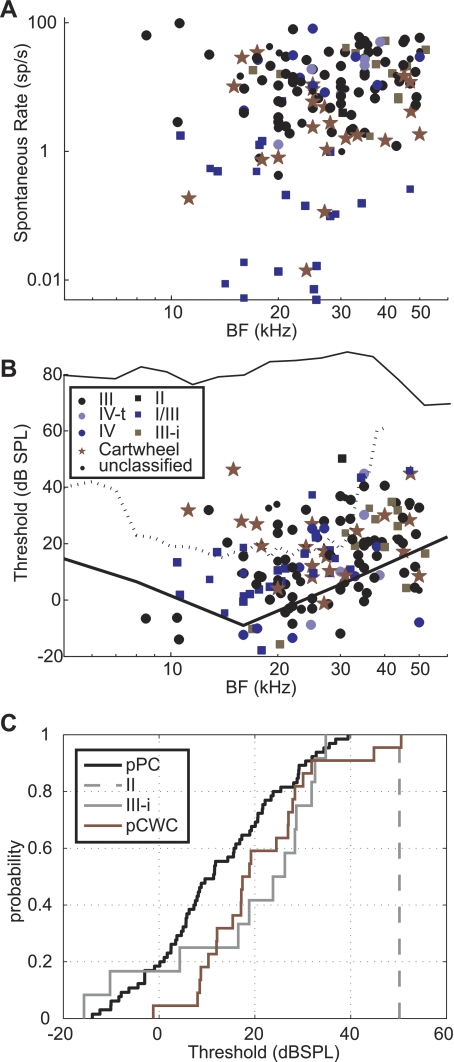
Calibrations were taken at the end of each experiment with the animal and all other objects in place. The microphone tip was positioned so that it displaced the animal's ipsilateral pinna (usually the right). Output of the speaker was relatively flat (80 dB SPL ± 10 dB) until 60 kHz and then dropped >20 dB above 70 kHz (see Fig. 6B). No attempt was made in this study to correct for the frequency response of speaker output. Most of the data in this paper are presented using sound level units of decibel attenuation (referenced to the maximum output of the speaker). Single-neuron thresholds are noted with dB sound pressure level (SPL) for the figures and discussions that consider the population as a whole.
Fig. 6.
Population data. Each neuron is represented by a single data point. A: spontaneous rates as a function of BF. B: single-neuron thresholds as a function of BF. An example speaker calibration (dB SPL) is plotted as the black line at top of B. Auditory nerve thresholds (Taberner and Liberman 2005) are plotted as a dotted line. Behavioral thresholds (Heffner and Masterton 1980) are plotted as a solid line. C: thresholds plotted as cumulative histograms. The thresholds of putative cartwheel cells (pCWC) were significantly higher than the putative principal neurons (pPC, Mann-Whitney U; P < 0.05). However, the thresholds of type III-i neurons were not.
Labeling.
To verify the location of a recording site, neurobiotin was ejected iontophoretically from the recording electrode with current pulses of 1–5 nA at 1 Hz for 10–50 min with 50% duty cycle (Pinault 1996). After this procedure, the animal was allowed to survive for at least 1 h, reanesthetized with 100 mg/kg ketamine and 10 mg/kg xylazine, exsanguinated via cardiac perfusion with phosphate-buffered saline (PBS), and fixed with a solution of 4% paraformaldehyde in PBS. Brains were processed with standard procedures for horseradish peroxidase (HRP) or Alexa Fluor 488 visualization.
Principal component analysis.
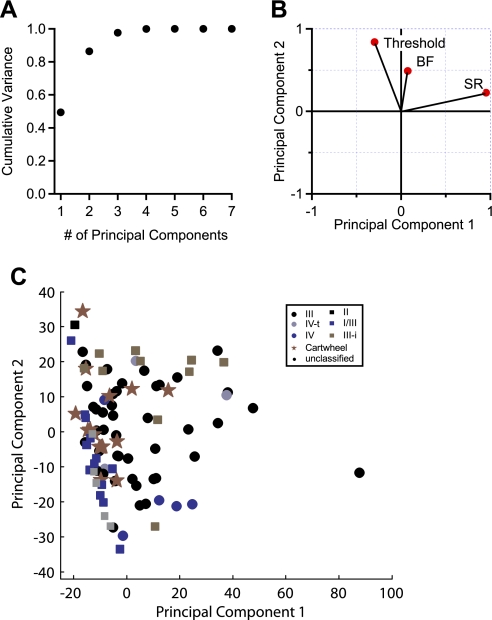
Principal component analysis (PCA) was performed with Matlab on a data set consisting of 96 units for which we obtained the following parameters: best frequency (BF), threshold, normalized nonmonotonic slope of BF rate-level function (Spirou et al. 1999), relative noise response (Spirou et al. 1999), mean spontaneous rate, and action potential width at half-maximum. Eigenvectors and eigenvalues were computed for the resulting covariance matrix. The eigenvalues indicate the variance contributed by each principal component, and the coefficients of the corresponding eigenvector indicate the relative influence of the experimentally measured parameters on the respective principal component.
RESULTS
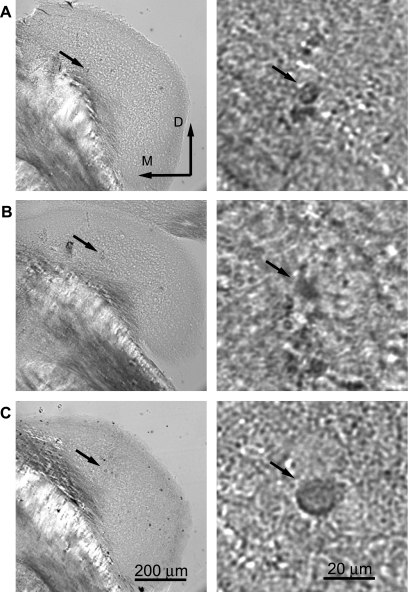
We report 163 single-neuron data sets from 68 mice. Three lines of evidence indicate that the neurons were located within the DCN. First, with most electrodes it was possible to monitor population responses in the region surrounding the electrode tip by presenting tones at that region's BF. The best stimulus frequency began at a high frequency and declined as the electrode tip advanced. This confirms that the electrode track was oriented along the long (tonotopic) axis of DCN (Kiang et al. 1975; Luo et al. 2009; Spirou et al. 1993). Depending on the angle, at the ventral end of an electrode track the electrode could either touch bone or enter a region of brain that did not respond well to sound. When this point was reached, the electrode was withdrawn and the ventral border of DCN was noted. To minimize the possibility of recording from the ventral cochlear nucleus (VCN), we limited recordings to the dorsal-most portions of any track and avoided recording below any abrupt frequency shift or where there was a different organization of frequencies. These features have been shown to occur when the electrode moves from the DCN to the VCN (Rose et al. 1960). As a result of these practices, our recordings were restricted to the top 350–400 μm of any track (referenced to the surface of DCN). Finally, in 26 of 34 labeling attempts we recovered filled somas corresponding to the location of the electrode tip during current injections. Under confocal microscopy, a lesion was visible adjacent to the filled neuron(s). In all cases, the lesion was located in either the middle or deep layers of DCN, based on gross morphology (Fig. 1) and orientation of the tissue referenced to brain stem and the small cell regions. Figure 1 shows the site of three single neurons visualized with HRP. Since dendritic morphology was not visualized, the targeted neurons cannot be unequivocally identified; however, the recording locations can be confidently placed in DCN.
Fig. 1.
Recording sites were marked by iontophoresis of neurobiotin from the recording pipette. Tissue was processed with standard protocols using either horseradish peroxidase (HRP) or avidin Alexa Fluor. Neurons could not be identified unequivocally since the dendrites were not labeled; however, the location in dorsal cochlear nucleus is clear. Right: same tissue at higher magnification. A: neuron that produced a type III-i response [best frequency (BF) = 45 kHz, threshold = 28 dB SPL]. B: type III (BF = 34 kHz, threshold = 13 dB SPL). C: type III (BF = 24 kHz, threshold = 32 dB SPL).
Physiological responses of single units.
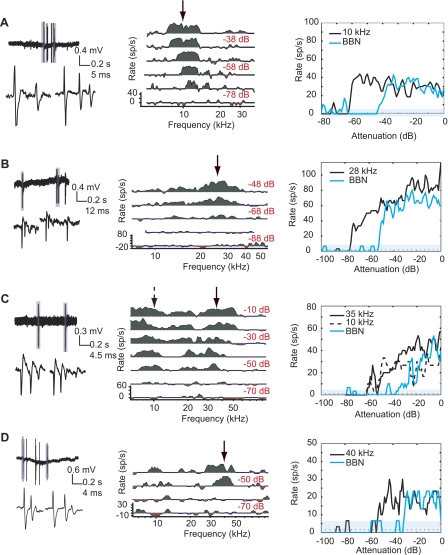
In DCN, cartwheel cells (CWCs) are readily identifiable as the only neurons that produce complex spikes (Davis et al. 1996a; Davis and Young 1997; Ding and Voigt 1997; Manis et al. 1994; Parham and Kim 1995; Portfors and Roberts 2007; Zhang and Oertel 1993a; although see Ding et al. 1999). Complex spikes are a subset of bursts, where bursts are a group of closely spaced spikes of set interstimulus interval (ISI) < 3.5 ms. A total of 29 neurons produced burst-type activity, 22 of which were characterized as complex spikes. Extracellular complex spikes appeared as closely spaced action potentials that successively widened and decreased in amplitude (Parham and Kim 1995; Fig. 2). It is possible for CWCs to be in a state in which only simple spikes are produced (Kim and Trussell 2007). However, in no case did we observe a neuron switch to complex spikes after an extended bout of simple spikes or vice versa. Rather, all putative CWCs showed a combination of complex and simple spikes throughout the duration of recording. As expected, CWC response to sound was generally weakly driven and disorganized and showed longer latency than non-CWCs (although see Portfors and Roberts 2007). CWCs also had low spontaneous rates (6 ± 9 sp/s) and relatively high thresholds (21 ± 12 dB SPL). Two putative CWCs showed very sharp tuning (e.g., Fig. 2A). Both of these neurons showed exceptionally long latencies (∼100 and 250 ms). Since the stimulus periods were constant (200 ms), the second of these neurons could also be interpreted as being an offset neuron with ∼50-ms delay.
Fig. 2.
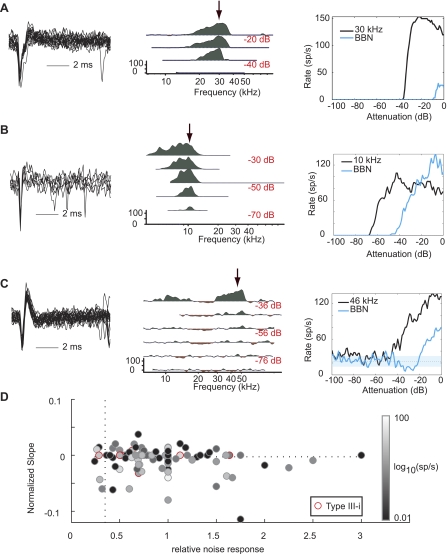
Representative data from 4 cartwheel cells. Left: 1-s trace (top) and close-up of complex spike morphology (bottom). Center: that neuron's response map. Frequencies are spaced 1/40th of an octave apart, and all curves are smoothed with a 3-point triangular filter. Response map curves are centered on mean spontaneous rate. Green areas show excitation >1 SD above and red areas show suppression >1 SD below mean spontaneous rate. Response strength is measured during the period 210–410 ms, with the exception of A, where the response was measured from 310–510 ms. Arrow indicates frequencies represented at right. Right: plot of the neuron's response to tones and broadband noise (BBN) at a range of sound levels separated by 1 dB. In rate-level functions, the mean spontaneous rate is plotted as a dashed line surrounded by a cyan region indicating ±1 SD. The goal is to collect the response to BF tones; however, BF of cartwheel cells can be difficult to determine during the experiment. A: BF = 11.2 kHz, threshold = 32 dB SPL. B: BF = 28 kHz, threshold = 10 dB SPL. C: BF = 25 kHz, threshold = 12 dB SPL. D: BF = 40 kHz, threshold = 30 dB SPL.
During recording sessions, spikes were superimposed to visualize their morphology and verify the isolation of each neuron. This type of plot is shown in subsequent figures; however, in the case of CWCs we show single traces of complex spike morphology to illustrate the similarity between our data and previously published extracellular traces (e.g., Parham and Kim 1995; Portfors and Roberts 2007). Action potential waveforms were quantified based on their half-maximum width and were significantly wider in CWCs than simple spikes in other cell types (Mann-Whitney U, P < 0.01; Table 1).
Table 1.
Summary of response map types in mouse dorsal cochlear nucleus
| Response Map | N | Threshold, dB SPL | Spontaneous Rate, sp/s | Half-Max Width, s (× 10−4) | Response Latency, ms |
|---|---|---|---|---|---|
| III | 72 | 13.2 ± 13.3 | 18.9 ± 20.3 | 5.3 ± 2.6 | 16 ± 5 [n = 37] |
| IV-t | 4 | 13.7 ± 11.1 | 20.4 ± 21.8 | 4.2 ± 0.9 | 16 ± 2 [3] |
| IV | 11 | 3.1 ± 15.3 | 24.1 ± 21.3 | 5.7 ± 1.9 | 18 ± 4 [3] |
| I/III | 29 | 11.4 ± 14.0 | 0.5 ± 1.2 | 4.4 ± 1.3 | 19 ± 8 [14] |
| III-i | 13 | 18.4 ± 16.2 | 22.7 ± 14.8 | 4.9 ± 1.2 | 15 ± 3 [10] |
| II | 1 | 50 | 0 | 5.7 ± 0 | |
| Complex spiking | 22 | 21 ± 12 | 6 ± 9 | 6.3 ± 2.7 | 24 ± 10 [20] |
| Unclassified | 11 | 25.0 ± 11.8 |
Unit classification.
The response map classification system was initially established by Evans and Nelson (1973) to group physiological responses in the cat. Since then, criteria for the different classes have been elaborated, refined (Shofner and Young 1985; Spirou et al. 1999; Voigt and Young 1980; Voigt and Young 1988), and extended by Voigt and colleagues to include gerbils (Davis et al. 1996a; Davis and Voigt 1997). Also, response classes have been associated with the major DCN neuron types via experimental evidence and conceptual models linking the anatomical and physiological observations. These classes are most useful when they are linked to a single neuronal generator, thus allowing an in vivo researcher to reasonably identify the neuron type online. In the cat, principal neurons are strongly associated with response type IV (Hancock and Voigt 2002a; Rhode et al. 1983; Young 1980), although in gerbils the majority of fusiform cells give type III responses (Ding et al. 1999). Tuberculoventral neurons have been associated with response type II in both species (Davis and Voigt 1997; Rhode 1999; Spirou et al. 1999; Voigt and Young 1980).
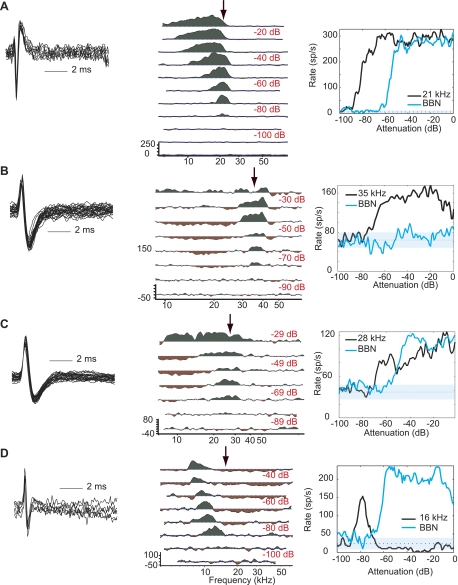
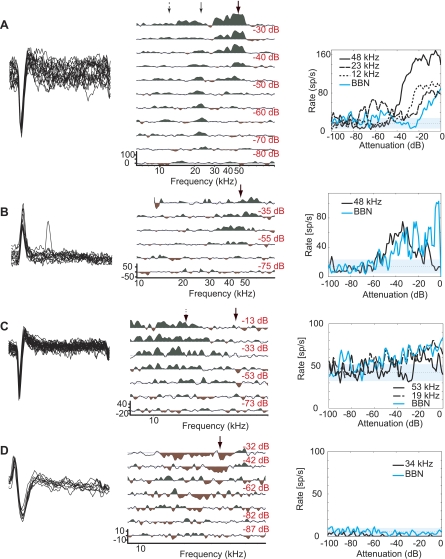
As an initial approach to characterizing in vivo responses in the mouse DCN, we classified units according to the response map scheme. In our experiments, type III was the most commonly encountered response class, accounting for 44% of our identified population (Fig. 3). In contrast, type IV, the most commonly observed response type in the cat, accounted for only 7% of the responses in our population (Fig. 3D). Putative principal neuron types generally include types III, IV-t, and IV. Response type III shows little or no inhibition at BF, which translates to a monotonic rate-level function (Fig. 3A); however, these units can exhibit strong inhibition at nearby frequencies (Fig. 3, B and C). Both types IV and IV-t are identified by a nonmonotonic response to BF tones, showing strong excitation at low sound levels followed by inhibition at higher levels. In the case of response type IV, the inhibition at high levels is complete (Fig. 3D). Type IV-t responses are characterized by BF tone-driven rates that drop below half of their maximal driven rates within 35 dB of the peak (Ding and Voigt 1997) and comprised 2% of the units encountered in mouse DCN.
Fig. 3.
Representative type III and IV responses. Left: superimposed spikes from a 1-s period to illustrate spike morphology and isolation. Center: the neuron's response map plotted as in Fig. 2, B–D. Right: plot of the neuron's rate-level function. A: type III response (BF = 21 kHz, threshold = −4 dB SPL). B: type III response (BF = 36 kHz, threshold = 21 dB SPL). C: type III response (BF = 28 kHz, threshold = 16 dB SPL). D: type IV response (BF = 16 kHz, threshold = −12 dB SPL).
Type II responses are characterized by low spontaneous activity (<2.5 sp/s), excitatory response to BF tones, and little to no noise response. Response type II is strongly associated with tuberculoventral neurons and can be further defined as having a maximal noise response less than 35% of the tone response and a nonmonotonic tone response with normalized slope less than −0.0025. Although these criteria do not provide definitive identification of tuberculoventral neurons, they provide a conservative definition that efficiently distinguishes this cell type in the cat (Spirou et al. 1999). We examined all neurons according to these criteria and found that nonspontaneously active neurons were homogeneous with spontaneously active neurons (Fig. 4D). Although three neurons met the aforementioned criteria for type II, only one of these was not spontaneously active (Fig. 4A). The remaining nonspontaneous responses were placed in class I/III, a category that is generally associated with other generators (e.g., Fig. 4B).
Fig. 4.
Representative putative tuberculoventral neurons. Panels are arranged as in Figs. 2 and 3. A: type II response (BF = 30 kHz, threshold = 50 dB SPL). B: type I/III response (10 kHz, 13 dB SPL). C: type III-i response (BF = 17 kHz, threshold = −10 dB SPL). D: relative noise response is reported for all neurons with complete BF tone and noise rate level functions as (max of noise function/max of tone function). Normalized slope is reported for all neurons as the slope of a line fitted above the first inflection point in the BF tone rate-level function provided that the region being measured was relatively flat for >20 dB (i.e., not a dip in the function). Of the 3 neurons that met the criteria for type II (lower left quadrant), only 1 was not spontaneously active (<2.5 sp/s; A). Type III-i neurons (red outline) were otherwise indistinguishable from the population as a whole.
Response type III-i, a subclass of response type III, has also been associated with tuberculoventral neurons in the gerbil (Davis et al. 1996a; Davis and Voigt 1997), although this association can be ambiguous (Ding et al. 1999). Type III-i is distinguished from the larger set of type III responses by inhibition to midlevel noise stimuli (Davis et al. 1996a; Davis and Voigt 1997). In contrast to type II, type III-i neurons in both gerbil and mouse are spontaneously active. In the mouse, type III-i neurons had a mean spontaneous rate of 22 ± 15 sp/s and accounted for 8% of the population (e.g., Fig. 4C).
Finally, 11 neurons could not be assigned to the existing response map classes (Fig. 5). These neurons were generally responsive to sound but showed weakly driven rates and no clear best frequency. These neurons shared many response map features with CWCs but did not produce complex spikes. These were all well-isolated neurons with complete data sets and clearly located within DCN.
Fig. 5.
Responses not assigned to response map classes. Eleven neurons did not fit well into response map classes. A–D: examples of weakly tuned or unusual responses. A: this neuron had multiple excitatory regions. This neuron was also classified as “broad.” B–D: most of the neurons not placed in response map classes were weakly responsive to sound.
Population distribution.
The population divided into response map types is summarized in Table 1 and Fig. 6. BFs of the population ranged from 8 to 52 kHz, which is within the auditory range of the mouse (Heffner and Masterton 1980) and was most likely limited, especially at the high-frequency end, by the capabilities of our speaker system. Response map classes were distributed evenly across the observed frequency range. Spontaneous rates ranged from 0 to 98.3 sp/s (mean = 15.4 ± 18.7 sp/s), with 73% of the population showing spontaneous levels below 20 sp/s (Fig. 6A). As expected, types II and I/III had significantly lower spontaneous rates than the putative principal neurons (Mann-Whitney U, P < 0.01). CWCs also had significantly lower spontaneous rates (P < 0.01).
Threshold was reported as the lowest level of pure tone stimulus that elicited excitation or suppression of neuronal activity >1 SD from spontaneous levels (Fig. 6B). Because of the rarity of on-BF inhibition, in practice, inhibitory responses were not often used to determine threshold. Threshold values follow the expected frequency contour in that there is a central region of lowest thresholds between ∼12 and 24 kHz (Heffner and Masterton 1980; Taberner and Liberman 2005). In the cat, tuberculoventral neurons exhibit a higher threshold than their principal neuron counterparts (Spirou et al. 1999; Voigt and Young 1980). This feature is important for conceptual models that address the low-level excitatory responses observed in type IV responses obtained from principal neurons (Nelken and Young 1996; Oertel and Young 2004; Young and Davis 2002). The thresholds of putative CWCs and the sole type II neuron were higher than presumed principal neurons (Mann-Whitney U, P < 0.05; Fig. 6C). Although there was a trend toward higher thresholds in type III-i versus type III units, it did not reach statistical significance (Mann-Whitney U, P > 0.05).
Finally, we used PCA as an unbiased classification scheme to test whether the population would break into natural divisions based on their response features. We analyzed 96 units for which we had complete data sets (described in methods). The first two principal components accounted for >86% of variance in the data (Fig. 7A). The first principal component had a large weighting from the spontaneous firing rate, and the second principal component had large weightings for threshold and BF (Fig. 7B). When the data are projected onto the axes of principal components 1 and 2, some trends are apparent (Fig. 7C). For instance, units classified as type III-i have large values for principal component 2, and units classified as I/III have low values for principal component 1. By definition, type I/III units have low spontaneous activity, so it is intuitive that these units are associated with principal component 1. Also, it is apparent in our data set (Fig. 6B) that most type III-i units have BFs above 30 kHz and thresholds above ∼20 dB SPL, consistent with the weightings of principal component 2. However, CWCs and type III responses were widely scattered on the principal axes, obscuring any discrete clustering. Several additional approaches were taken in performing PCA, including standardization of the data set (Johnson and Wichern 1992), inclusion or exclusion of CWCs, and inclusion or exclusion of the parameters that are predicted to be unbiased according to unit type (such as BF). Although these modifications altered the weightings of the parameters in the resulting principal component vectors, they did not affect the overall finding that the response parameters used for PCA did not produce clustering or efficiently separate the response types observed in mouse DCN.
Fig. 7.
Principal component analysis (PCA) of response parameters. PCA was performed with 7 parameters (see methods) obtained in 96 units. A: principal components 1 and 2 accounted for 86.3% of the population variance. B: 3 parameters with the largest weightings for principal components 1 and 2 projected onto the principal axes. C: scatterplot of data projected onto the axes of principal components 1 and 2. Plot symbols indicate the type assignments of each unit.
DISCUSSION
In this report, we characterized the sound-evoked response properties of single neurons in unanesthetized mouse DCN. The data were from well-isolated DCN neurons and serve as a baseline for further studies that take advantage of important properties of this model species. Neurons were categorized according to the response map scheme, which associates response maps with their neuronal generators (Davis et al. 1996a; Davis and Voigt 1997; Hancock and Voigt 2002a, 2002b; Voigt and Young 1980; Young 1980;). Similar to that in the gerbil, mouse DCN exhibited reduced or altered tuberculoventral inhibition compared with the best-studied model, the cat. This was demonstrated by the low rate of observation of certain principal neuron response types (e.g., types IV-t and IV) or the response types that have been associated with tuberculoventral neurons themselves (types II and III-i). Table 2 compares the distribution of different response classes in the present study with previous studies in awake mouse (Roberts and Portfors 2008), decerebrate gerbil (Davis et al. 1996a), decerebrate cat (Shofner and Young 1985), and anesthetized cat (Joris 1998).
Table 2.
Comparison of response map types in different species
| Putative Generator | Response Map | % Population (decerebrate mouse) | % Population (awake mouse) | % Population (gerbil) | % Population (decerebrate cat) | % Population (anesthetized cat) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Principal neurons (fusiform or giant) | III | 47 | 57 | 38 | 38 | 44 | 59 | 24 | 58 | 43 | 84 |
| IV-t | 3 | 5 | 4 | ||||||||
| IV | 7 | 5 | 31 | 41 | |||||||
| IV-i | 6 | ||||||||||
| Unidentified or mixed | Inhibited | 19 | 12 | 17 | 14 | 23 | 23 | ||||
| I/III | 19 | 5 | 14 | 23 | 23 | ||||||
| Tuberculoventral neurons | III-i | 9 | 10 | 18 | 19 | 26 | 19 | 16 | |||
| II | 1 | 18 | 8 | 19 | 16 | ||||||
| Cartwheel cells | Complex spiking | 14 | 14 | 27 | 27 | 0 | 0 | 0 | |||
Our observations of reduced or altered tuberculoventral inhibition was somewhat surprising given that these neurons have been identified in mouse DCN by electrophysiology (Zhang and Oertel 1993b) and anatomical methods (Ryugo and Willard 1985; Wickesberg et al. 1991). One possible explanation is that we undersampled deep DCN, the region where tuberculoventral neurons are located. However, our anatomical reconstructions confirm that at least some electrode tracks were in the region containing tuberculoventral neurons (Fig. 1). Another possibility is that it may be difficult to record from tuberculoventral neurons with glass electrodes and therefore the composition of our population was influenced by electrode preference. A more likely hypothesis, however, was proposed by Davis and Voigt (Davis et al. 1996a) as an explanation for the identification of type III-i responses as tuberculoventral neurons. Voigt and colleagues have proposed that the balance of excitation and inhibition in the DCN circuit might be different from that in the cat. As a result, tuberculoventral neurons may be spontaneously active and have response maps more similar to type III. A prediction from having spontaneously active inhibitory neurons was that principal neuron types would have lower spontaneous rates, although this was not evident in their data. Concurrent with this latter prediction, the aggregated spontaneous rate for principal neuron response types in mouse DCN (∼21 sp/s) was low relative to cat (∼35 sp/s; Ma and Young 2006) or gerbil (∼35 sp/s; Davis et al. 1996a). Finally, since tuberculoventral neurons in this hypothetical mechanism would be responsive to noise, an additional prediction is that the principal neurons or neurons receiving tuberculoventral input could have decreased noise response (e.g., Fig. 4).
Studies in cats have led to the hypothesis that DCN plays a role in spectral processing, specifically regarding sound localization because of the ability of principal neurons to detect direction-dependent spectral notches generated by the pinnae (Oertel and Young 2004; Rice et al. 1992). In principle, a similar role for DCN is possible in the mouse, although this has not been tested and behavioral evidence suggests that localization is not as well-developed in mice as in cats (Heffner and Heffner 1988; Heffner and Masterton 1980; Lauer et al. 2011). Another role for spectral processing, given the evidence that the mouse is a highly vocal species (e.g., Grimsley et al. 2011; Kalcounis-Rueppell et al. 2010; Kikusui et al. 2011; Shepard and Liu 2011), may be processing the acoustic spectrum of vocalizations or in suppressing background noise. On the basis of similarities between the circuitry of the apical layer of DCN and that of cerebellar cortex and the electrosensory lobe of mormyrid fish, it has also been proposed that the DCN contributes to sensory filtering, enabling an animal to predict or suppress responses to self-generated noises generated by chewing or vocalization (Davis et al. 1996b; Koehler et al. 2011; Nelken and Young 1996; Requarth and Sawtell 2011; Shore 2005; Young et al. 1995). Mice are distinct from cats and gerbils in that their hearing range is primarily ultrasonic and exhibit high response thresholds for sounds <1 kHz (Davis et al. 1996a; Ehret 1974; Neff and Hind 1955; West 1985). It is possible that the DCN may have distinct functional roles in species with ultrasonic hearing ranges.
Our data raise several questions, specifically regarding the range of responses generated by principal and tuberculoventral neurons in mouse DCN. Whereas principal and tuberculoventral neurons in the cat generally exhibit strongly contrasting properties that make them straightforward to distinguish in vivo (i.e., type II and type IV), our results suggest that these two neuron classes exhibit less distinct physiological traits in mice. We therefore used PCA to test whether we could distinguish two or more groups of physiological responses. Concurrent with the physiological findings, however, the population of single-unit responses in mouse DCN showed a high degree of overlap given the response map parameters. Going forward, the association between response classes and specific neuron types should be addressed in studies that combine electrophysiological characterization of sound-evoked responses with anatomical reconstruction of recorded neurons (Ding et al. 1999; Hancock and Voigt 2002a, 2000b) or antidromic stimulation (Young 1980). Another promising direction will be intracellular recordings, which simplify anatomical identification of recorded neurons and enable measurement of parameters that are not obtainable with extracellular recordings, like input resistance and the presence of specific intrinsic membrane conductances. For example, brief hyperpolarization of a fusiform cell relieves inactivation of the A-type potassium current, thereby increasing first-spike latency or first interspike interval (Kanold and Manis 1999). This conductance distinguishes fusiform cells from tuberculoventral cells (Zhang and Oertel 1993b) and provides a straightforward method for distinguishing these classes of neurons during an intracellular in vivo recording. Many hypothesis-driven studies of DCN will target the responses of a particular neuron type, so it is important to determine cellular properties that facilitate discrimination of the response properties of different classes of neurons in the mouse DCN.
GRANTS
Funding for this research was provided by the National Institute on Deafness and Other Communication Disorders Intramural Research Program.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: W.-L.D.M. and S.D.B. conception and design of research; W.-L.D.M. performed experiments; W.-L.D.M. and S.D.B. analyzed data; W.-L.D.M. and S.D.B. interpreted results of experiments; W.-L.D.M. and S.D.B. prepared figures; W.-L.D.M. and S.D.B. drafted manuscript; W.-L.D.M. and S.D.B. edited and revised manuscript; W.-L.D.M. and S.D.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank David Axe and Mark Hanus for technical support, and Dr. Eric Young, who provided critical comments on this manuscript.
REFERENCES
- Adams JC. Ascending projections to the inferior colliculus. J Comp Neurol 183: 519–538, 1979 [DOI] [PubMed] [Google Scholar]
- Anderson MJ, Young ED. Isoflurane/N2O anesthesia suppresses narrowband but not wideband inhibition in dorsal cochlear nucleus. Hear Res 188: 29–41, 2004 [DOI] [PubMed] [Google Scholar]
- Brawer JR, Morest DK, Kane EC. The neuronal architecture of the cochlear nucleus in cat. J Comp Neurol 155: 251–300, 1974 [DOI] [PubMed] [Google Scholar]
- Cant NB, Morest DK. The structural basis for stimulus coding on the cochlear nucleus of the cat. In: Hearing Science: Recent Advances, edited by Berlin CI. San Diego, CA: College Hill, 1984 [Google Scholar]
- Cao XJ, Oertel DO. The magnitudes of hyperpolarization-activated and low-voltage-activated potassium currents co-vary in neurons of the ventral cochlear nucleus. J Neurophysiol 106: 630–640, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Cain D, Jen P. Sound pressure transformation at the pinna of Mus domesticus. J Exp Biol 198: 2007–2023, 1995 [DOI] [PubMed] [Google Scholar]
- Davis KA. Contralateral effects and binaural interactions in dorsal cochlear nucleus. J Assoc Res Otolaryngol 6: 280–296, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KA, Ding J, Benson TE, Voigt HF. Response properties of units in the dorsal cochlear nucleus of unanesthetized decerebrate gerbil. J Neurophysiol 75: 1411–1431, 1996a [DOI] [PubMed] [Google Scholar]
- Davis KA, Miller RL, Young ED. Effects of somatosensory and parallel-fiber stimulation on neurons in the dorsal cochlear nucleus. J Neurophysiol 76: 3012–3024, 1996b [DOI] [PubMed] [Google Scholar]
- Davis KA, Voigt HF. Evidence of stimulus-dependent correlated activity in the dorsal cochlear nucleus of decerebrate gerbil. J Neurophysiol 78: 229–247, 1997 [DOI] [PubMed] [Google Scholar]
- Davis KA, Young ED. Granule cell activation of complex-spiking neurons in dorsal cochlear nucleus. J Neurosci 17: 6798–5806, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KA, Young ED. Pharmacological evidence of inhibitory and disinhibitory neuronal circuits in dorsal cochlear nucleus. J Neurophysiol 83: 926–940, 2000 [DOI] [PubMed] [Google Scholar]
- Ding J, Voigt HF. Intracellular response properties of units in the dorsal cochlear nucleus of unanesthetized decerebrate gerbil. J Neurophysiol 77: 2549–2572, 1997 [DOI] [PubMed] [Google Scholar]
- Ding J, Benson TE, Voigt HF. Acoustic and current-pulse responses of identified neurons in the dorsal cochlear nucleus of unanesthetized, decerebrate gerbils. J Neurophysiol 82: 3434–3457, 1999 [DOI] [PubMed] [Google Scholar]
- Ehret G. Age-dependent hearing loss in normal hearing mice. Naturwissenschaften 61: 506–507, 1974 [DOI] [PubMed] [Google Scholar]
- Evans EF. The frequency response and other properties of single fibres in the guinea pig cochlear nerve. J Physiol 226: 263–287, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans EF, Nelson PG. The responses of single neurones in the cochlear nucleus of the cat as a function of their location and the anaesthetic state. Exp Brain Res 17: 402–427, 1973 [DOI] [PubMed] [Google Scholar]
- Grimsley JM, Monaghan JJ, Wenstrup JJ. Development of social vocalizations in mice. PLoS One 6: e17460, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock KE, Voigt HF. Intracellularly labeled fusiform cells in dorsal cochlear nucleus of the gerbil. I. Physiological response properties. J Neurophysiol 87: 2505–2519, 2002a [DOI] [PubMed] [Google Scholar]
- Hancock KE, Voigt HF. Intracellularly labeled fusiform cells in dorsal cochlear nucleus of the gerbil. II. Comparison of physiology and anatomy. J Neurophysiol 87: 2520–2530, 2002b [DOI] [PubMed] [Google Scholar]
- Heffner RS, Heffner HE. Sound localization acuity in the cat: effect of azimuth, signal duration and test procedure. Hear Res 36: 221–232, 1988 [DOI] [PubMed] [Google Scholar]
- Heffner HE, Masterton B. Hearing in glires: domestic rabbit, cotton rat, house mouse and kangaroo rat. J Acoust Soc Am 68: 1584–1599, 1980 [Google Scholar]
- Johnson RA, Wichern DW. Applied Multivariate Statistical Analysis. Englewood Cliffs, NJ: Prentice Hall, 1992 [Google Scholar]
- Joris P. Response classes in the dorsal cochlear nucleus and its output tract in the chloralose-anesthetized cat. J Neurosci 18: 3955–3966, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalcounis-Rueppell MC, Petric R, Briggs JR, Carney C, Marshall MM, Willse JT, Rueppell O, Ribble DO, Crossland JP. Differences in ultrasonic vocalizations between wild and laboratory California mice (Peromyscus californicus). PLoS One 5: e9705, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane EC. Synaptic organization in the dorsal cochlear nucleus of the cat: a light and electron microscopic study. J Comp Neurol 155: 301–329, 1974 [DOI] [PubMed] [Google Scholar]
- Kanold PO, Manis PB. Transient potassium currents regulate the discharge patterns of dorsal cochlear nucleus pyramidal cells. J Neurophysiol 19: 2195–2208, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiang NY, Godfrey DA, Norris BE, Moxon SE. A block model of the cat cochlear nucleus. J Comp Neurol 162: 221–245, 1975 [DOI] [PubMed] [Google Scholar]
- Kikusui T, Nakanishi K, Nakagawa R, Nagasawa M, Mogi K, Okanoya K. Cross fostering experiments suggest that mice songs are innate. PLoS One 6: e17721, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Trussell LO. Ion channels generating complex spikes in cartwheel cells of the dorsal cochlear nucleus. J Neurophysiol 97: 1705–1725, 2007 [DOI] [PubMed] [Google Scholar]
- Koehler SD, Pradhan S, Manis PB, Shore SE. Somatosensory inputs modify auditory spike timing in dorsal cochlear nucleus principal cells. Eur J Neurosci 33: 409–420, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer AM, Slee SJ, May BJ. Acoustic basis of directional selectivity in laboratory mice. J Assoc Res Otolaryngol 12: 633–645, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman MC, Dodds LW. Single-neuron labeling and chronic cochlear pathology. III. Stereocilia damage and alterations of threshold tuning curves. Hear Res 16: 55–74, 1984 [DOI] [PubMed] [Google Scholar]
- Lorente de No R. The Primary Acoustic Nuclei. New York: Raven, 1981 [Google Scholar]
- Luo F, Wang Q, Farid N, Liu X, Yan J. Three-dimensional tonotopic organization of the C57 mouse cochlear nucleus. Hear Res 257: 75–82, 2009 [DOI] [PubMed] [Google Scholar]
- Ma WL, Young ED. Dorsal cochlear nucleus response properties following acoustic trauma: response maps and spontaneous activity. Hear Res 216–217: 176–188, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manis PB, Spirou GA, Wright DD, Paydar S, Ryugo DK. Physiology and morphology of complex spiking neurons in the guinea pig dorsal cochlear nucleus. J Comp Neurol 348: 261–276, 1994 [DOI] [PubMed] [Google Scholar]
- May BJ. Role of the dorsal cochlear nucleus in the sound localization behavior of cats. Hear Res 148: 74–87, 2000 [DOI] [PubMed] [Google Scholar]
- Mugnaini E, Warr WB, Osen KK. Distribution and light microscopic findings of granule cells in the cochlear nuclei of cat, rat and mouse. J Comp Neurol 191: 581–606, 1980 [DOI] [PubMed] [Google Scholar]
- Neff WD, Hind JE. Auditory thresholds of the cat. J Acoust Soc Am 27: 480–483, 1955 [Google Scholar]
- Nelken I, Young ED. Why do cats need a dorsal cochlear nucleus? J Basic Clin Physiol Pharmacol 7: 199–220, 1996 [DOI] [PubMed] [Google Scholar]
- Oertel D, Young ED. What's a cerebellar circuit doing in the auditory system? Trends Neurosci 27: 104–110, 2004 [DOI] [PubMed] [Google Scholar]
- Oliver DL. Dorsal cochlear nucleus projections to the inferior colliculus in the cat: a light and electron microscopic study. J Comp Neurol 223: 155–172, 1984 [DOI] [PubMed] [Google Scholar]
- Osen KK. Cytoarchitecture of the cochlear nuclei in the cat. J Comp Neurol 136: 454–483, 1969 [DOI] [PubMed] [Google Scholar]
- Parham K, Kim DO. Spontaneous and sound-evoked discharge characteristics of complex-spiking neurons in the dorsal cochlear nucleus of the unanesthetized decerebrate cat. J Neurophysiol 73: 550–561, 1995 [DOI] [PubMed] [Google Scholar]
- Pinault D. A novel single-cell staining procedure performed in vivo under electrophysiological control: morpho-functional features of juxtacellularly labeled thalamic cells and other central neurons with biocytin or Neurobiotin. J Neurosci Methods 65: 113–136, 1996 [DOI] [PubMed] [Google Scholar]
- Portfors C, Roberts PD. Temporal and frequency characteristics of cartwheel cells in the dorsal cochlear nucleus of the awake mouse. J Neurophysiol 98: 744–756, 2007 [DOI] [PubMed] [Google Scholar]
- Requarth T, Sawtell NB. Neural mechanisms for filtering self-generated sensory signals in cerebellum-like circuits. Curr Opin Neurobiol 21: 602–608, 2011 [DOI] [PubMed] [Google Scholar]
- Rhode WS. Vertical cell responses to sound in cat dorsal cochlear nucleus. J Neurophysiol 82: 1019–1032, 1999 [DOI] [PubMed] [Google Scholar]
- Rhode WS, Oertel D, Smith PH. Physiological response properties of cells labeled intracellularly with horseradish peroxidase in cat dorsal cochlear nucleus. J Comp Neurol 213: 426–447, 1983 [DOI] [PubMed] [Google Scholar]
- Rice JJ, May BJ, Spirou GA, Young ED. Pinna-based spectral cues for sound localization in cat. Hear Res 58: 132–152, 1992 [DOI] [PubMed] [Google Scholar]
- Roberts PD, Portfors CV. Design principles of sensory processing in cerebellum-like structures. Early stage processing of electrosensory and auditory objects. Biol Cybern 98: 491–507, 2008 [DOI] [PubMed] [Google Scholar]
- Rose JE, Galambos R, Hughes JR. Organization of frequency sensitive neurons in the cochlear nuclear complex of the cat. In: Neural Mechanisms of the Auditory and Vestibular Systems, edited by Rasmussen GL, Windle WF. Springfield, IL: Thomas, 1960 [Google Scholar]
- Rousseeuw PJ. Silhouettes: a graphical aid to the interpretation and validation of cluster analysis. J Comput Appl Math 20: 53–65, 1981 [Google Scholar]
- Ryugo DK, Willard FH, Fekete DM. Differential afferent projections to the inferior colliculus from the cochlear nucleus in the albino mouse. Brain Res 210: 342–349, 1981 [DOI] [PubMed] [Google Scholar]
- Ryugo DK, Willard FH. The dorsal cochlear nucleus of the mouse: a light microscopic analysis of neurons that project to the inferior colliculus. J Comp Neurol 242: 381–396, 1985 [DOI] [PubMed] [Google Scholar]
- Shepard KN, Liu RC. Experience restores innate female preference for male ultrasonic vocalizations. Genes Brain Behav 10: 28–34, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shofner WP, Young ED. Excitatory/inhibitory response types in the cochlear nucleus: relationships to discharge patterns and responses to electrical stimulation of the auditory nerve. J Neurophysiol 54: 917–939, 1985 [DOI] [PubMed] [Google Scholar]
- Shore SE. Multisensory integration in the dorsal cochlear nucleus: unit responses to acoustic and trigeminal ganglion stimulation. Eur J Neurosci 21: 3334–3348, 2005 [DOI] [PubMed] [Google Scholar]
- Spirou GA, Davis KA, Nelken I, Young ED. Spectral integration by type II interneurons in dorsal cochlear nucleus. J Neurophysiol 82:648–663, 1999 [DOI] [PubMed] [Google Scholar]
- Spirou GA, May BJ, Wright DD, Ryugo DK. Frequency organization of the dorsal cochlear nucleus in cats. J Comp Neurol 329: 36–52, 1993 [DOI] [PubMed] [Google Scholar]
- Sutherland DP, Masterton RB, Glendenning KK. Role of acoustic striae in hearing: reflexive responses to elevated sound-sources. Behav Brain Res 97: 1–12, 1998a [DOI] [PubMed] [Google Scholar]
- Sutherland DP, Glendenning KK, Masterton RB. Role of acoustic striae in hearing: discrimination of sound-source elevation. Hear Res 120: 86–108, 1998b [DOI] [PubMed] [Google Scholar]
- Taberner AM, Liberman C. Response properties of single auditory nerve fibers in the mouse. J Neurophysiol 93: 557–569, 2005 [DOI] [PubMed] [Google Scholar]
- Voigt HF, Young ED. Evidence of inhibitory interactions between neurons in dorsal cochlear nucleus. J Neurophysiol 44: 76–96, 1980 [DOI] [PubMed] [Google Scholar]
- Voigt HF, Young ED. Neural correlations in the dorsal cochlear nucleus: pairs of units with similar response properties. J Neurophysiol 59: 1014–1032, 1988 [DOI] [PubMed] [Google Scholar]
- West CD. The relationship of the spiral turns of the cochlea and the length of the basilar membrane to the range of audible frequencies in ground dwelling mammals. J Acoust Soc Am 77: 1091–1101, 1985 [DOI] [PubMed] [Google Scholar]
- Wickesberg RE, Whitlon D, Oertel D. Tuberculoventral neurons project to the multipolar cell area but not to the octopus cell area of the posteroventral cochlear nucleus. J Comp Neurol 313: 457–468, 1991 [DOI] [PubMed] [Google Scholar]
- Young ED. Identification of response properties of ascending axons from dorsal cochlear nucleus. Brain Res 200: 23–37, 1980 [DOI] [PubMed] [Google Scholar]
- Young ED, Brownell WE. Responses to tones and noise of single cells in dorsal cochlear nucleus of unanesthetized cats. J Neurophysiol 39: 282–300, 1976 [DOI] [PubMed] [Google Scholar]
- Young ED, Davis KA. Circuitry and function of the dorsal cochlear nucleus. In: Springer Handbook of Auditory Research Vol. 15: Integrative Functions in the Mammalian Auditory Pathway, edited by Oertel D, Fay RR, Popper AN. New York: Springer, 2002, chapter 5 [Google Scholar]
- Young ED, Nelken I, Conley RA. Somatosensory effects on neurons in dorsal cochlear nucleus. J Neurophysiol 73: 743–765, 1995 [DOI] [PubMed] [Google Scholar]
- Zhang S, Oertel D. Cartwheel and superficial stellate cells of the dorsal cochlear nucleus: intracellular recordings in slices. J Neurophysiol 69: 1384–1397, 1993a [DOI] [PubMed] [Google Scholar]
- Zhang S, Oertel D. Tuberculoventral cells of the dorsal cochlear nucleus in mice: intracellular recordings in slices. J Neurophysiol 69: 1409–1421, 1993b [DOI] [PubMed] [Google Scholar]