Abstract
To understand how activity in mammalian neural circuits controls behavior, the mouse is a promising model system due to the convergence of genetic, optical, and physiological methods. The ability to control and quantify behavior precisely is also essential for these studies. We developed an operant visual detection paradigm to make visual psychophysical measurements: head-fixed mice make responses by pressing a lever. We designed this task to permit neurophysiological studies of behavior in cerebral cortex, where activity is variable from trial to trial and neurons encode many types of information simultaneously. To study neural responses in the face of this complexity, we trained mice to do a task where they perform hundreds of trials daily and perceptual thresholds can be measured. We used this task to measure both visual acuity and the minimum detectable contrast in behaving mice. We found that the mouse contrast response function is similar in shape to other species. They can detect low-contrast stimuli, with a peak contrast threshold of 2%, equivalent to ∼15° eccentric in human vision. Mouse acuity is modest, with an upper limit near 0.5 cycles/°, consistent with prior data.
Keywords: acuity, psychophysics, vision
the availability of genetic, optical, and physiological techniques makes the mouse a useful model for studying mammalian brain circuits. A full understanding of brain function requires linking knowledge about function in these circuits to behavior.
Although rodents have been used for many years in behavioral studies, understanding how neural activity controls behavior places specific requirements on a behavioral paradigm. Many neurons in the brain are variable from trial to trial, and individual neurons can be modulated by sensory, motor, and cognitive effects (Boudreau et al. 2006; Otazu et al. 2009; Spitzer et al. 1988; Tolhurst et al. 1983), necessitating repeated behavioral measurements and precise tracking of sensory and motor state. Also, it is preferable for animals to behave while in a static position to permit control of visual stimuli as well as facilitating optical manipulations such as in vivo microscopy. Finally, we wish to monitor whether performance is limited by perception or other task demands, in contrast to possible fluctuations in motivation or cognitive state. Psychophysical measurements provide a powerful way to do this. Therefore, we developed a behavioral task in which 1) animals perform hundreds of trials daily, 2) perform behaviors while sitting stably in the apparatus, and 3) respond reliably near perceptual thresholds so that psychophysical measurements can be made.
In this behavioral paradigm, animals report when a visual stimulus changes. We use the paradigm to measure fundamental limits of the mouse visual system by asking them to detect stimuli of varying contrast and spatial frequency.
Acuity and contrast thresholds have been previously measured in mice physiologically and behaviorally. We focus here on photopic measurements, as prior studies have worked mainly in the photopic range, although scotopic conditions are also likely to be important to a nocturnal animal like the mouse. Most methods agree that maximum photopic acuity is approximately 0.5–0.6 cycles per degree (cpd), whereas measurements of contrast sensitivity vary.
Two established behavioral methods rely on visual reflexes: optomotor studies (Abdeljalil et al. 2005; Prusky et al. 2004) measure motion of the head or body in response to full-field motion, and optokinetic studies (Sinex et al. 1979; van Alphen et al. 2009) measure motion of the eyes. Operant studies have used mazes (Gianfranceschi et al. 1999) and water mazes (Prusky et al. 2000). The optokinetic, optomotor, and operant studies have all yielded acuity thresholds at 0.4–0.6 cpd. Data from nearly all of these studies show that animals can detect at best a minimum full-field contrast of 5–15%. The exception is the recent work of van Alphen (2009), which shows that stimuli of ∼1% contrast can produce optokinetic eye movements.
Physiologically, visual evoked potentials have been used to measure contrast thresholds and acuity. These are often measured from the scalp or skull above V1 (Fischer et al. 2007; Lickey et al. 2004; Ridder and Nusinowitz 2006) but can also be measured using local field potential recordings from penetrating electrodes (Porciatti et al. 1999). These studies also estimate maximum acuity of 0.5–0.6 cpd and contrast thresholds near 5%.
The density of retinal ganglion cells (RGCs) and the magnification of the retinal image limit maximum acuity. Consequently, higher acuity is seen in animals with larger eyes and higher peak density of RGCs, such as primates (human and macaques: 40–60 cpd; Merigan and Katz 1990; Thibos et al. 1987) and cats (6–10 cpd). Both primates and cats have a region of their retinas where ganglion cells are concentrated: primates have a fovea where single cones map to single RGCs, and cats have an elongated area centralis/visual streak (Hughes 1977) with a density of RGCs 50-fold greater than at the periphery (Stone 1965, 1978). In contrast, ganglion cell density in mice varies only by a factor of 2–4 between the central retina and periphery (Jeon et al. 1998; Salinas-Navarro et al. 2009).
Thus mouse acuity is lower than most other mammalian species and is likely to be limited by the size of the image projected onto the retina and the density of retinal cells. However, do mouse contrast thresholds differ from other species? If the mouse central retina is like the peripheral retina of other species, contrast thresholds might be similar. Humans and macaques have a minimum detectable contrast threshold near 0.5% (Barten 1999; van Nes et al. 1967) and cats ∼1% (Blake et al. 1974). At 10–15° eccentricity in the macaque, where RGC density matches that of the mouse, contrast thresholds are still ∼2% (Virsu and Rovamo 1979).
Even though RGC density and optomotor tasks predict low-contrast thresholds, operant studies in the mouse find contrast thresholds to be 5% or above, including a water maze study that showed a peak contrast threshold of 15% (Prusky and Douglas 2004) In the rat, with its similar retina, operant studies have also found contrast thresholds of ∼15% (Keller et al. 2000; Meier et al. 2011). In addition, a recent study of mice (Busse et al. 2011) used an operant task with a nose-poke response to measure contrast thresholds at an optimal spatial frequency (0.13 cpd). They obtained a somewhat worse threshold than previous studies, slightly above 20%.
A key advantage of psychophysical measurements is that deviations in behavior due to attentional or motivational fluctuations can be detected and animals trained to prevent these lapses. Therefore, we developed a behavioral paradigm to make visual psychophysical measurements in the mouse. We used this paradigm to measure mouse maximum acuity and minimum contrast threshold. Our data on maximum acuity agree well with past measurements. However, we find a minimum contrast threshold of ∼2%, much better than previously measured behaviorally. This is consistent with the idea that the mouse retina has photopic contrast sensitivity similar to the peripheral retina of other species.
METHODS
Animals and behavior.
All animal procedures were approved by the Institutional Animal Care and Use Committee at Harvard Medical School and conform to National Institutes of Health guidelines. To begin training, a custom-made titanium or stainless steel head holder was implanted on the skull in an aseptic surgery. After recovery, animals (n = 3, 30–90 days postnatal age; C57BL/6-BALB/c hybrids, see below) were trained to perform the visual change detection task via operant conditioning with positive reinforcement. During training, mice received fluid only as reinforcement or via supplement when total reinforcement volume was below a minimum of 25 ml·kg−1·day−1. Animals received water rewards (1–2 μl per reward once performance was stable; larger rewards were used during early training). Animals received ≥25 ml/kg and often more on nontraining days and were typically stable at 80–85% of their free-feeding weight during training periods. We did not track eye position as we wished to estimate peak acuity and used large stimuli that filled the monitor (69 × 81° of visual angle). Animals had to respond within a reaction time interval of 600–700 ms; nearly all responses occurred within 500 ms (e.g., Figs. 2 and 3), so the exact length of the reaction time window was not important for our measurements.
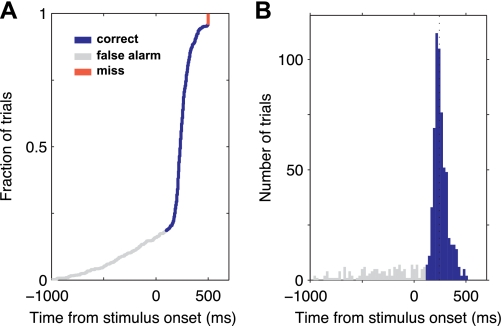
Fig. 2.
Animals perform the task consistently. Reaction times at 100% contrast: example of 1 animal's performance over 1 session at 100% contrast at the end of training to respond to visual stimuli but before training on multiple contrasts. A: cumulative distribution function of reaction times (measured relative to the onset of the visual stimulus). B: histogram of the same data. Median reaction time: 244 ms. Animal made 596 correct responses out of 768 total trials (78%) during a period of 62 min.
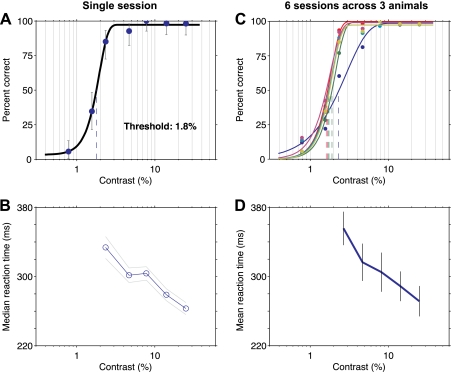
Fig. 3.
Mice are reliable psychophysical observers. A: psychometric function for 1 animal for 1 behavioral session. Grating spatial frequency: 0.09 cycles per degree (cpd). Measured threshold is 1.8% contrast. Error bars: 95% confidence intervals (for a binomial using the Clopper-Pearson method). Percentage correct is calculated over correct response and missed trials only. False alarms are ignored (see methods); as they come before the stimulus onset, they are not responses to the visual stimulus and must be the same over all contrast conditions. In addition, the spurious correct rate due to false alarm guesses is calculated and subtracted (methods). B: median reaction times for the same session (error bars: SE over trials). Reaction times were not computed for the 2 lowest contrasts as too few correct responses were made. Reaction times are slower for lower contrasts, as seen in human vision (Manahilov et al. 2003). C: data for 6 different sessions of similar spatial frequency (green and blue lines: 0.12 cpd; yellow line: 0.09 cpd; remainder: 0.1 cpd). Thresholds range from 1.7 to 2.3%. One curve has a slightly shallower slope, and this has little effect on the threshold; the contrast levels we chose were selected to provide good estimates of threshold and not slope. Yellow: same data as in A. D: reaction times for sessions shown in C. Blue line, mean across sessions; gray bars, SE over sessions.
The 3 animals used in this study were the 1st 3 head-fixed mice we trained to perform this task. The training began by teaching animals to hold the lever until a high-contrast visual stimulus appeared. There was no explicit habituation to handling; we implanted a titanium or steel plate for head fixation, and after ∼2 wk of recovery, head-fixed shaping began. This took a mean of 21 sessions (range 18–25). Next, the visual stimulus onset time was increased to the maximum (2 s) over a mean of 9 sessions (range 7–11). For unrelated reasons, these animals then stopped training for 8 wk. On restart, it took a mean of 32 sessions (25–45) for animals to perform the task stably near threshold with a range of stimulus contrasts. Total training days: mean 61, range 54–74. This time is long for typical mouse behavioral experiments but similar to the amount of time required to train a nonhuman primate on a near-threshold lever press detection task.
We have since trained more animals on similar tasks to perform neurophysiological recordings. Based on all of the mice we have trained on a visual detection task (n = 21), approximately half (n = 12) learned the task fully and performed at a high level. Of the rest, n = 3 failed to behave consistently, and we stopped training them within 10 sessions, n = 2 were withdrawn for unrelated reasons, and n = 4 learned the task but performed at a slightly lower level so we stopped training them to focus on other animals. The example animal shown in Supplemental Video S1 (available in the data supplement online at the Journal of Neurophysiology web site) is from a later set of animals trained to do the 100% contrast detection task (as in Fig. 2) only because the video was made several months after the threshold data here were collected. The video captures the essential features of the contrast threshold detection task.
The animals are 1st-generation offspring of female BALB/c and male C57BL/6 mice (Charles River Laboratories). We used this cross to facilitate breeding and secondarily to work behaviorally with vigorous hybrid animals. However, our data combined with past work (e.g., Prusky et al. 2004; van Alphen et al. 2009) show that these offspring have visual acuity similar to their C57BL/6 parents. Although some mouse strains carry alleles resulting in retinal degeneration in adulthood, neither parental strain does (Chang et al. 2002), and they have normal adult retinas (Hawes et al. 1999). The offspring animals are agouti-colored with pigmented retinas. Visual behavioral performance was stable for several months in the adult hybrid animals.
Visual stimulation.
Visual stimuli were displayed on a γ-corrected liquid crystal display (LCD) display (Dell P190S). Background brightness was 60 cd/m2. We measured the time to transition between background and the 100% contrast visual stimulus as <10 ms. At this brightness, pixel intensity oscillates at 200 Hz as the backlight is pulse-width modulated at that frequency. This is far above mouse flicker-fusion frequency as measured with ERG (Tanimoto et al. 2009). Regardless, for physiological experiments, this oscillation can be eliminated by setting the display to maximum brightness. Tests with a cathode ray tube (CRT) video display were made using a Viewsonic P95f+ running at 120 Hz. Monitors were linearized by measuring intensity for each gray level (Eye-One Display 2; X-Rite). The monitors were placed 18–30 cm from the eye; most measurements were made at 26-cm distance.
Threshold calculation.
To calculate threshold contrast, we first removed false alarm trials. Responses that occurred within 100 ms after stimulus onset were not counted as correct as they were too early to have been responses to the stimulus. Spurious correct responses due to false alarm guesses occurring during the response period were subtracted. We estimated the false alarm rate over a time window 100 ms before to 100 ms after the stimulus and removed a proportional number of correct trials based on the length of the reaction time interval. The range of trials removed over the six example sessions in Fig. 3 was 7.5–18.9%. Response rate data (Figs. 2, 4A, and 6) are data before this correction was applied; y-axes of psychometric curves (Fig. 3, A and B, and Fig. 4C) are data after correction. We then fit a Weibull function (Quick 1974). Threshold was estimated as the midpoint between the upper and lower saturation limits of the Weibull.
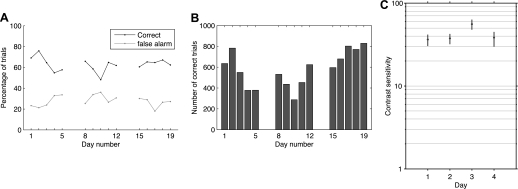
Fig. 4.
Performance is reliable over days. A and B: data from 1 animal over 3 wk, just before introducing different contrast levels to train to respond near threshold. A: percentage correct and percentage false alarm responses. B: number of correct trials per daily session. C: measured contrast sensitivity for the same animal at the completion of training to respond near threshold, 14 training sessions after data in A and B. Sensitivity (100 divided by threshold contrast in percent) measured as in Fig. 3A. Error bars are 99% confidence intervals computed via bootstrap.
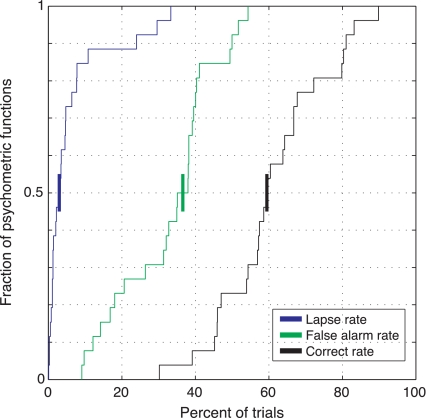
Fig. 6.
Mice perform this task with few lapses, high correct rates, and moderate numbers of false alarms. We plot cumulative distributions of response rates for all experimental sessions (same sessions as in Fig. 5). Median response rates (shown as heavy bars intersecting y = 0.5): 3% lapse, 37% false alarm, and 60% correct. During a small number of sessions (3, 1 each at 0.09, 0.04, and 0.1 cpd), lapse rates were >20%; no notable fluctuations in threshold were seen.
RESULTS
Behavioral task: visual change detection.
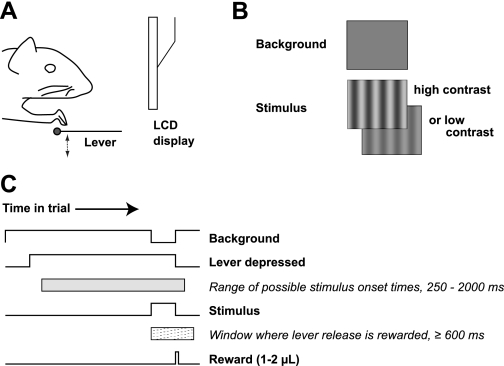
We trained mice to make operant responses by manipulating a lever while their heads are fixed. Animals sit in a tube that supports their weight. A surgically implanted titanium headpost is held by a clamp above the tube. A lightweight lever attached to a microswitch is placed in front of the tube so that animals can rest one forepaw on the lever (shown schematically in Fig. 1A). The entire behavioral apparatus fits in a sound-attenuating box 71 cm on a side, which allows us to train several animals simultaneously in identical behavioral devices.
Fig. 1.
Behavioral task. Mice perform a visual change detection task by manipulating a lever while their heads are held fixed. A: schematic; animals sit on all fours in front of a liquid crystal display (LCD) where visual stimuli are presented; the lever is positioned below 1 forepaw. Approximately 20 mN (∼2 gram-force) are required to depress the lever. B: visual stimuli are vertically oriented sinusoidal gratings of varying contrast and spatial frequency. The background is a spatially constant gray. C: time course of a single trial. Animals initiate the trial by depressing the lever; the stimulus is displayed at a random time, and animals must report detecting the stimulus by releasing the lever. Correct responses within a reaction time window of 600 or 700 ms (no difference in behavior was observed; see methods) after stimulus onset are rewarded with a water drop.
The paradigm we used to measure acuity and contrast thresholds is a visual change detection task (Fig. 1, B and C). A neutral gray background stimulus is displayed on an LCD video display (mean luminance 60 cd/m2, 1,280 × 1,024 pixels, 72 × 58°, 100-Hz frame rate) at all times. Animals press the lever to initiate the trial. After a variable period (250–2,000 ms), the sinusoidal luminance pattern appears, and animals must respond to its onset by releasing the lever. Animals earn a water reward for each correct response (see methods). The random onset time of the stimulus ensures animals perform the task by responding to the stimulus onset. In addition, the animal must respond in a short time window (600–700 ms) after the stimulus onset.
Behavioral responses from an example session using 100% contrast stimuli are shown in Fig. 2, where 78% of trials were correct. The tight reaction time distribution confirms that animals did not use a response strategy based on timing, but instead made consistent responses to the stimulus (Fig. 2, A and B). Early releases (false alarms, gray in Fig. 2, A and B) are distributed roughly equally in time before stimulus onset, and failures to respond (red) were rare.
Psychophysical measurement of contrast threshold.
We used this task to measure animals' contrast thresholds as a function of the spatial frequency of the stimulus. Using sinusoidal gratings of various spatial frequencies is an effective way to characterize the response of the visual system. This linear systems approach allows the response at a single frequency to be used to predict subjects' spatial visual performance for more complex images. For this reason, it is a classic approach in vision research (reviewed in Thibos 1998).
By varying the contrast of a single grating, we could measure the animal's contrast threshold at the spatial frequency of the grating (Fig. 3). The resulting psychometric functions are well-described by a sigmoidal function. If animals are limited by perceptual factors, we might expect reaction time to increase as stimulus contrast decreases, as performance can be improved for low contrasts with longer viewing times (Manahilov et al. 2003). We observed this sort of temporal summation effect; reaction times are shortest for the highest contrast stimuli (Fig. 3B).
Animals' performance is reliable from session to session.
We set out to develop a behavioral paradigm in which psychophysical measurements could be made, and animals perform hundreds of trials in a given experimental session. This visual detection task meets these requirements. Mice perform reliably across sessions (Fig. 4). Correct and false alarm rates are stable, as shown by example data from a representative animal in Fig. 4A. This animal performed hundreds of correct trials each day over a 3-wk period (mean 583, SD 166, 5 training days/wk). Psychophysical estimates of contrast threshold are also stable (Fig. 4C).
Contrast thresholds are reliable across animals.
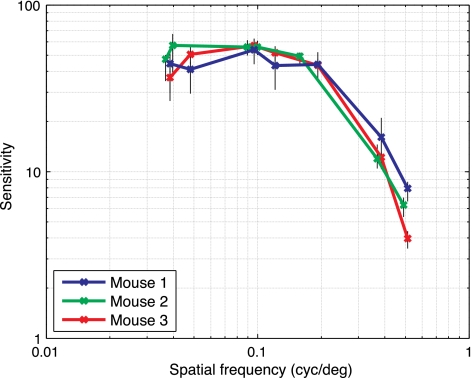
We used the visual detection task to measure the contrast response function (Campbell and Robson 1968). We obtained contrast thresholds across a range of spatial frequencies (Fig. 5; n = 3 animals). The resulting contrast response functions are consistent across animals. The minimum detectable contrast level was ∼2% (sensitivity = 50), at a spatial frequency of ∼0.1 cpd.
Fig. 5.
Contrast thresholds for 3 animals are shown as a function of grating spatial frequency. Y-axis: contrast sensitivity (100 divided by threshold contrast in percent). Thresholds are similar across animals, and contrast required to detect the stimulus rises (sensitivity decreases) as spatial frequency increases. Black vertical error bars show 99% confidence intervals around each threshold measurement (computed via bootstrap). cyc/deg, cpd.
We used an LCD monitor to collect these data, but CRT monitors are better characterized for visual measurements and have artifacts that are better understood. Even though we linearized the luminance steps of the LCD, we wished to completely rule out any artifacts that might result from potential temporal “overshoot” or spatial dithering by internal processing in the LCD. Therefore, we made measurements with a CRT at a single spatial frequency (0.1 cpd), near the contrast threshold peak. At that spatial frequency, animals' contrast sensitivity with an LCD was 54 (mean; SD 4.6; n = 3 animals; data same as in Fig. 5). Their contrast sensitivity with the CRT was very similar: 44, SD 6.4 (n = 7 measurements from 2 animals), confirming that the LCD monitor accurately replicated the desired contrast steps.
Animals showed low lapse rates (Fig. 6). A nonzero lapse rate would appear in Fig. 3, A and C, as an upper asymptote below 100%, but in this task animals typically made correct responses for the highest contrast stimuli. Across all trials, the median proportion of correct responses was ∼60%. Animals showed moderate false alarm rates (i.e., lever releases before the stimulus appeared). These did not affect our threshold measurements, as false alarms are ignored when computing the psychometric function. Furthermore, we corrected for spurious correct rates due to guesses that occurred while the stimulus was displayed (see methods; Macmillan and Creelman 2005). False alarms increased during training when low-contrast stimuli were added to the 100% contrast stimuli that were used during initial training (data not shown). This is a rational strategy; decision criteria should decrease when less-detectable stimuli are used, resulting in more false alarms. Although it should be possible to train animals to reduce early responses (by manipulating task timing or reward schedule as a function of stimulus onset time), because it does not affect threshold measurements we decided not to train animals to suppress false alarms.
DISCUSSION
We have measured mouse visual contrast thresholds under photopic conditions. We use a novel operant lever-pressing task that permits precise behavioral measurements of contrast sensitivity.
Psychophysical paradigm.
One goal of this work was to develop a behavioral paradigm that could be used to study how neuronal circuits relate to behavior in mice. What features are important in such a paradigm?
First, it is important for animals to perform many repeated trials within individual experimental sessions. This is because the activity of neurons in most central structures, including the cerebral cortex, basal ganglia, and thalamus, is both variable from trial to trial and can be affected by cognitive phenomena including arousal, degree of engagement, and task difficulty (Boudreau et al. 2006; Otazu et al. 2009; Spitzer et al. 1988; Tolhurst et al. 1983). Having many trials within each session makes it possible to obtain enough data from individual neurons to characterize their properties precisely. Moreover, cortical neurons often encode multiple task variables, and each variable might cause only a small change in the activity of the neuron. As a simple example of the former, V1 neurons can encode direction, disparity, eye preference, retinotopic location, and size, among other properties. Performing many trials allows the contribution of these different factors to be separated. Finally, small changes in the activity of single neurons have been suggested to have major effects at the population level (Cohen and Maunsell 2009; London et al. 2010), and studying these small changes in the presence of neuronal variability requires averaging over many trials.
Another goal was to have animals perform well while head-fixed. We chose to train animals to respond by manipulating a lever. Other response modalities have been used with head-fixed mice (e.g., Andermann et al. 2010; Dombeck et al. 2007; O'Connor et al. 2010). Selection of the best response type for a particular experiment depends on many things, including the numbers of trials produced and animals' speed of learning. An important difference may be brain stability, which is needed for in vivo microscopy. Licking has been shown to produce larger displacements of the brain, especially in the dorsal/ventral direction, than paw motions (Andermann et al. 2010), and thus lever tasks may be more suitable for, e.g., two-photon microscopy, although direct comparisons have not yet been made.
A final goal was to control the animals' behavior to obtain measurements limited by perceptual factors. When animals are performing near threshold, fluctuations in motivation, attention, or arousal can be detected in increased error rates or other variations in psychometric functions. We have trained animals to perform consistently to reduce these fluctuations (Fig. 4). Also, a benefit of making psychometric measurements is that changes in internal state can be detected. Animals perform this task while they rest stably in the behavioral apparatus, facilitating optical and electrophysiological recording and manipulations.
Choice of paradigm: yes-no and forced-choice tasks.
We used a “yes-no” task to measure thresholds. When the stimulus appeared, animals either released the lever to report its presence or made a “no” response by holding the lever. Another possibility is a two-alternative forced-choice (2AFC) task, where two stimuli are presented at different locations or times and animals must choose one of the two options. The most important reason we chose a detection task is because we thought it easier to train animals to perform with a single lever. An additional minor factor is that a 2AFC task typically requires more trials to measure thresholds because the chance performance level of 50% compresses the response range. On the other hand, thresholds measured with 2AFC tasks are often a factor of ∼2 better than yes-no tasks. In a 2AFC task, animals compare two stimuli separated by only a short time, whereas in a yes-no task, animals must choose a criterion level based on the signal and noise distributions when the stimulus is present and absent. The difference in thresholds may be due to criterion deviating slightly from optimal in yes-no tasks due to the longer time intervals over which subjects sample the stimuli (Macmillan and Creelman 2005). If we had used a 2AFC task, we might thus measure better contrast thresholds. Matching tasks, such as what Virsu and Rovamo (1979) used in human subjects, also require comparisons over short time intervals and may be more similar to a 2AFC task.
Our measurements could have been degraded if animals used a nonoptimal decision strategy. Deviations from optimal behavior fall into two major categories: fluctuations in internal decision criterion or fluctuations in attentional or motivational state (failing to pay attention or choosing to try hard on some trials and not others). Both deviations would produce changes in threshold estimates from day to day, but our threshold estimates were repeatable both within the same animal and across animals (Figs. 3–5). Substantial day-to-day threshold fluctuations were seen by Busse et al. (2011) in a contrast detection task and might have been caused by variance in motivation, which would explain why their threshold estimate (∼20% at 0.13 cpd) was much higher than ours (∼2% at 0.13 cpd). Although the 2AFC design used by Busse et al. (2011) can provide better thresholds, it does not prevent attentional or motivational changes from degrading measurements. The low lapse rates and relatively stable thresholds that we measured suggest that our animals were well-motivated and that the thresholds we measured accurately reflect behavioral abilities for these mice.
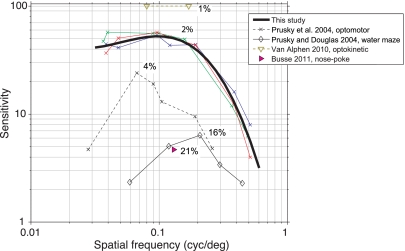
Thresholds for detecting the onset of a stimulus and for discriminating the features of a stimulus can differ. Thibos and colleagues (1996) have studied this extensively in humans. When human observers are asked to report the onset of a visual grating, in the periphery they can detect the presence of high-spatial-frequency gratings for which orientation they cannot reliably report (but see Virsu and Rovamo 1979). This difference likely arises from aliasing of the image on the RGC mosaic. In principle, aliasing could allow mice to detect slightly higher spatial frequency stimuli than they could identify, as the optics of the mouse eye do not limit acuity up to 1.0 cpd (Geng et al. 2011). However, reported aliasing effects occur in the human periphery only at high spatial frequencies and at contrasts above ∼15% (Fig. 6 of Thibos et al. 1996). We avoided these effects by measuring contrast sensitivity only to 0.5 cpd (Fig. 5), which resulted in thresholds near 15% and better. Because the slope of the contrast sensitivity function at high frequencies (e.g., Fig. 7, heavy black line) involves contrasts >15% and could be sensitive to aliasing, we do not attempt to assign a precise maximum acuity, however, such an estimate would be near 0.6 cpd (Fig. 7).
Fig. 7.
A summary of previous behavioral measurements of contrast sensitivity using both reflexive and operant measures. X-axis: spatial frequency. Y-axis: contrast sensitivity (reciprocal of threshold); peak contrast threshold for each data set is noted in black. Colored lines: replotting of data in Fig. 5 with same conventions. Thick black line: best-fit interpolating cubic spline to data from Fig. 5. Black dashed line: optomotor measurements adapted from adult data in Fig. 4 of Prusky et al. (2004). Thin black line: operant measurements using a water maze task (Prusky and Douglas 2004). Magenta triangle: operant measurement using a head-free nose-poke 2-alternative forced-choice (2AFC) task from Busse et al. (2011). Brown: optokinetic measurements from Fig. 3 of van Alphen et al. (2009). We plot the 2 points that produced an eye movement gain significantly different from 0. This may differ slightly from psychometric threshold because contrasts between 1 and 5% were not tested and any movement at all is considered above threshold (instead of 50% or higher as in a psychometric function; see discussion).
Comparison with previous contrast measurements.
To compare our data with previous measurements of contrast threshold, we fit a cubic spline to extract a single contrast threshold as a function of spatial frequency (Fig. 7) because our animals' thresholds were measured at slightly different spatial frequencies. The most complete previous measurements of contrast sensitivity in the mouse have been obtained in an optomotor task (Prusky et al. 2004) and a water maze task (Prusky and Douglas 2004). In our operant paradigm, contrast thresholds are significantly better, for both peak acuity (0.05–0.1 cpd) and higher spatial frequencies near the limit of mouse acuity. This might be due to increased sensitivity that comes from an operant task instead of a reflexive behavior. For example, reflexive responses may use a single finely tuned circuit (perhaps involving, e.g., direction-selective RGCs or the superior colliculus). In an operant task, animals are motivated to use sensory information from any neural system to earn a reward.
Other factors affect the comparison of our contrast thresholds with prior results. First, we used full-field stimuli. Spatial summation is expected to make thresholds for large stimuli lower than thresholds for smaller stimuli (Virsu and Rovamo 1979; Watson 1992), and smaller stimuli could explain why some measurements in rats found contrast thresholds ∼15% (Keller et al. 2000; Meier et al. 2011). Correspondingly, van Alphen et al. (2009) might have found lower contrast thresholds than ours because their stimuli filled the entire visual field. Second, although our minimum contrast thresholds are within a factor of two of those measured with optokinetic (eye movement) responses (van Alphen et al. 2010), it is difficult to construct contrast sensitivity functions from their data because they did not search for minimal detectable contrasts or spatial frequencies. Perceptual learning was unlikely to affect our thresholds, as the spatial frequency of the stimulus was most often changed from one session to the next (cf. Merigan and Katz 1990). It is also important to consider the method used to calculate threshold when comparing between studies. We used the 50% point of the psychometric function, i.e., the midpoint between the upper and lower saturation points. Animals perform above chance below this threshold: for example, in Fig. 3, the 50% threshold is 2.6%, but animals perform above chance for a contrast of 1.5%. Most previous work in rodents, including the optokinetic study of van Alphen et al. (2010), has used the minimal threshold, making our measurements conservative compared with the optokinetic measurements.
The most important factor likely to differentiate our results, showing a peak contrast threshold of 2%, from previous operant studies that obtained thresholds of 15% or higher (Busse et al. 2011; Prusky and Douglas 2004; Prusky et al. 2004) is our control over animals' motivational state. First, because we measured a psychometric function in each session, we could use the lapse rate to detect decreased motivation, and the majority of our lapse rates were near zero (Fig. 6). Also, our measurements are highly reliable, both from day to day for the same animal (Figs. 3 and 4) and across animals at similar spatial frequencies (Fig. 5). If our animals had deviated from optimal perceptual behavior, we would expect to see fluctuating performance or a higher lapse rate. Thus, in this task, mice are likely to be performing very near their perceptual limit. Because behavior is limited by perception, and not internal state changes like attention or motivation, the task is suitable for studies of neural activity in the mouse visual system.
Conclusion.
These data show that mice have good photopic contrast sensitivity, similar to that at ∼15° eccentricity in the human. We have also demonstrated that psychophysical methods can be applied in the mouse to measure quantities that characterize their perceptual systems. This psychophysical paradigm is a useful tool for linking structure and function of neural circuits in the mouse to behavior.
GRANTS
This work was supported by the Howard Hughes Medical Institute.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
M.H.H. and J.H.R.M. conception and design of research; M.H.H. and L.A.C. performed experiments; M.H.H. analyzed data; M.H.H. and J.H.R.M. interpreted results of experiments; M.H.H. prepared figures; M.H.H. drafted manuscript; M.H.H. and J.H.R.M. edited and revised manuscript; M.H.H., L.A.C., and J.H.R.M. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
We thank R. H. Masland, M. A. Andermann, D. A. Ruff, and J. E. Dowling for useful comments on the manuscript. A. Thavikulwat, S. Sleboda, and T. Wang helped with training and contributed to development of training protocols.
REFERENCES
- Abdeljalil J, Hamid M, Abdel-Mouttalib O, Stéphane R, Raymond R, Johan A, José S, Pierre C, Serge P. The optomotor response: a robust first-line visual screening method for mice. Vision Res 45: 1439–1446, 2005 [DOI] [PubMed] [Google Scholar]
- Andermann ML, Kerlin AM, Reid RC. Chronic cellular imaging of mouse visual cortex during operant behavior and passive viewing. Front Cell Neurosci 4: 3, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barten PJ. Contrast Sensitivity of the Human Eye and its Effects on Image Quality. Bellingham, WA: SPIE Optical Engineering Press, 1999 [Google Scholar]
- Blake R, Cool SJ, Crawford ML. Visual resolution in the cat. Vision Res 14: 1211–1217, 1974 [DOI] [PubMed] [Google Scholar]
- Boudreau CE, Williford TH, Maunsell JH. Effects of task difficulty and target likelihood in area V4 of macaque monkeys. J Neurophysiol 96: 2377–2387, 2006 [DOI] [PubMed] [Google Scholar]
- Busse L, Ayaz A, Dhruv N, Katzner S, Saleem AB, Schölvinck ML, Zaharia AD, Carandini M. The detection of visual contrast in the behaving mouse. J Neurosci 31: 11351–11361, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell FW, Robson JG. Application of Fourier analysis to the visibility of gratings. J Physiol 197: 551–566, 1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang B, Hawes NL, Hurd RE, Davisson MT, Nusinowitz S, Heckenlively JR. Retinal degeneration mutants in the mouse. Vision Res 42: 517–525, 2002 [DOI] [PubMed] [Google Scholar]
- Cohen MR, Maunsell JH. Attention improves performance primarily by reducing interneuronal correlations. Nat Neurosci 12: 1594–1600, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombeck DA, Khabbaz AN, Collman F, Adelman TL, Tank DW. Imaging large-scale neural activity with cellular resolution in awake, mobile mice. Neuron 56: 43–57, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer QS, Graves A, Evans S, Lickey ME, Pham TA. Monocular deprivation in adult mice alters visual acuity and single-unit activity. Learn Mem 14: 277–286, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng Y, Schery LA, Sharma R, Dubra A, Ahmad K, Libby RT, Williams DR. Optical properties of the mouse eye. Biomed Opt Express 2: 717–738, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianfranceschi L, Fiorentini A, Maffei L. Behavioural visual acuity of wild type and bcl2 transgenic mouse. Vision Res 39: 569–574, 1999 [DOI] [PubMed] [Google Scholar]
- Hawes NL, Smith RS, Chang B, Davisson M, Heckenlively JR, John SW. Mouse fundus photography and angiography: a catalogue of normal and mutant phenotypes. Mol Vis 5: 22, 1999 [PubMed] [Google Scholar]
- Hughes A. The topography of vision in mammals of contrasting lifestyle: comparative optics and retinal organization. In: Handbook of Sensory Physiology, edited by Crescitelli F. Berlin: Springer-Verlag, 1977, vol. 7, p. 613–756 [Google Scholar]
- Jeon CJ, Strettoi E, Masland RH. The major cell populations of the mouse retina. J Neurosci 18: 8936–8946, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller J, Strasburger H, Cerutti DT, Sabel BA. Assessing spatial vision - automated measurement of the contrast-sensitivity function in the hooded rat. J Neurosci Methods 97: 103–110, 2000 [DOI] [PubMed] [Google Scholar]
- Lickey ME, Pham TA, Gordon B. Swept contrast visual evoked potentials and their plasticity following monocular deprivation in mice. Vision Res 44: 3381–3387, 2004 [DOI] [PubMed] [Google Scholar]
- London M, Roth A, Beeren L, Häusser M, Latham PE. Sensitivity to perturbations in vivo implies high noise and suggests rate coding in cortex. Nature 466: 123–127, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macmillan NA, Creelman CD. Detection Theory: A Users' Guide (2nd ed). London: Lawrence Ehrlbaum Associates, 2005 [Google Scholar]
- Manahilov V, Calvert J, Simpson WA. Temporal properties of the visual responses to luminance and contrast modulated noise. Vision Res 43: 1855–1867, 2003 [DOI] [PubMed] [Google Scholar]
- Meier P, Flister E, Reinagel P. Collinear features impair visual detection by rats. J Vis 11: 22, 2011 [DOI] [PubMed] [Google Scholar]
- Merigan WH, Katz LM. Spatial resolution across the macaque retina. Vision Res 30: 985–991, 1990 [DOI] [PubMed] [Google Scholar]
- O'Connor DH, Peron SP, Huber D, Svoboda K. Neural activity in barrel cortex underlying vibrissa-based object localization in mice. Neuron 67: 1048–1061, 2010 [DOI] [PubMed] [Google Scholar]
- Otazu GH, Tai LH, Yang Y, Zador AM. Engaging in an auditory task suppresses responses in auditory cortex. Nat Neurosci 12: 646–654, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porciatti V, Pizzorusso T, Maffei L. The visual physiology of the wild type mouse determined with pattern VEPs. Vision Res 39: 3071–3081, 1999 [DOI] [PubMed] [Google Scholar]
- Prusky GT, Alam NM, Beekman S, Douglas RM. Rapid quantification of adult and developing mouse spatial vision using a virtual optomotor system. Invest Ophthalmol Vis Sci 45: 4611–4616, 2004 [DOI] [PubMed] [Google Scholar]
- Prusky GT, Douglas RM. Characterization of mouse cortical spatial vision. Vision Res 44: 3411–3418, 2004 [DOI] [PubMed] [Google Scholar]
- Prusky GT, West PW, Douglas RM. Behavioral assessment of visual acuity in mice and rats. Vision Res 40: 2201–2209, 2000 [DOI] [PubMed] [Google Scholar]
- Quick RF., Jr A vector-magnitude model of contrast detection. Kybernetik 16: 65–67, 1974 [DOI] [PubMed] [Google Scholar]
- Ridder WH, Nusinowitz S. The visual evoked potential in the mouse–origins and response characteristics. Vision Res 46: 902–913, 2006 [DOI] [PubMed] [Google Scholar]
- Salinas-Navarro M, Jiménez-López M, Valiente-Soriano FJ, Alarcón-Martínez L, Avilés-Trigueros M, Mayor S, Holmes T, Lund RD, Villegas-Pérez MP, Vidal-Sanz M. Retinal ganglion cell population in adult albino and pigmented mice: a computerized analysis of the entire population and its spatial distribution. Vision Res 49: 637–647, 2009 [DOI] [PubMed] [Google Scholar]
- Sinex DG, Burdette LJ, Pearlman AL. A psychophysical investigation of spatial vision in the normal and reeler mutant mouse. Vision Res 19: 853–857, 1979 [DOI] [PubMed] [Google Scholar]
- Spitzer H, Desimone R, Moran J. Increased attention enhances both behavioral and neuronal performance. Science 240: 338–340, 1988 [DOI] [PubMed] [Google Scholar]
- Stone J. A quantitative analysis of the distribution of ganglion cells in the cat's retina. J Comp Neurol 124: 337–352, 1965 [DOI] [PubMed] [Google Scholar]
- Stone J. The number and distribution of ganglion cells in the cat's retina. J Comp Neurol 180: 753–771, 1978 [DOI] [PubMed] [Google Scholar]
- Tanimoto N, Muehlfriedel RL, Fischer MD, Fahl E, Humphries P, Biel M, Seeliger MW. Vision tests in the mouse: functional phenotyping with electroretinography. Front Biosci 14: 2730–2737, 2009 [DOI] [PubMed] [Google Scholar]
- Thibos LN. Acuity perimetry and the sampling theory of visual resolution. Optom Vis Sci 75: 399–406, 1998 [DOI] [PubMed] [Google Scholar]
- Thibos LN, Cheney FE, Walsh DJ. Retinal limits to the detection and resolution of gratings. J Opt Soc Am A 4: 1524–1529, 1987 [DOI] [PubMed] [Google Scholar]
- Thibos LN, Still DL, Bradley A. Characterization of spatial aliasing and contrast sensitivity in peripheral vision. Vision Res 36: 249–258, 1996 [DOI] [PubMed] [Google Scholar]
- Tolhurst DJ, Movshon JA, Dean AF. The statistical reliability of signals in single neurons in cat and monkey visual cortex. Vision Res 23: 775–785, 1983 [DOI] [PubMed] [Google Scholar]
- van Alphen B, Winkelman BH, Frens MA. Age- and sex-related differences in contrast sensitivity in C57BL/6 mice. Invest Ophthalmol Vis Sci 50: 2451–2458, 2009 [DOI] [PubMed] [Google Scholar]
- van Alphen B, Winkelman BH, Frens MA. Three-dimensional optokinetic eye movements in the C57BL/6J mouse. Invest Ophthalmol Vis Sci 51: 623–630, 2010 [DOI] [PubMed] [Google Scholar]
- van Nes FL, Koenderink JJ, Nas H, Bouman MA. Spatiotemporal modulation transfer in the human eye. J Opt Soc Am 57: 1082–1088, 1967 [DOI] [PubMed] [Google Scholar]
- Virsu V, Rovamo J. Visual resolution, contrast sensitivity, and the cortical magnification factor. Exp Brain Res 37: 475–494, 1979 [DOI] [PubMed] [Google Scholar]
- Watson AB. Transfer of contrast sensitivity in linear visual networks. Vis Neurosci 8: 65–76, 1992 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.