Abstract
Motoneuron discharge patterns reflect the interaction of synaptic inputs with intrinsic conductances. Recent work has focused on the contribution of conductances mediating persistent inward currents (PICs), which amplify and prolong the effects of synaptic inputs on motoneuron discharge. Certain features of human motor unit discharge are thought to reflect a relatively stereotyped activation of PICs by excitatory synaptic inputs; these features include rate saturation and de-recruitment at a lower level of net excitation than that required for recruitment. However, PIC activation is also influenced by the pattern and spatial distribution of inhibitory inputs that are activated concurrently with excitatory inputs. To estimate the potential contributions of PIC activation and synaptic input patterns to motor unit discharge patterns, we examined the responses of a set of cable motoneuron models to different patterns of excitatory and inhibitory inputs. The models were first tuned to approximate the current- and voltage-clamp responses of low- and medium-threshold spinal motoneurons studied in decerebrate cats and then driven with different patterns of excitatory and inhibitory inputs. The responses of the models to excitatory inputs reproduced a number of features of human motor unit discharge. However, the pattern of rate modulation was strongly influenced by the temporal and spatial pattern of concurrent inhibitory inputs. Thus, even though PIC activation is likely to exert a strong influence on firing rate modulation, PIC activation in combination with different patterns of excitatory and inhibitory synaptic inputs can produce a wide variety of motor unit discharge patterns.
Keywords: persistent inward current, rate modulation, synaptic amplification
the discharge patterns of motoneurons are shaped not only by the pattern of synaptic input that they receive but also by their own intrinsic properties. Recent work has focused on the contribution of persistent inward currents (PICs) mediated primarily by voltage-dependent channels on motoneuron dendrites, which can act to amplify and prolong the effects of synaptic inputs (Heckman et al. 2008a; Hultborn et al. 2004). Although these currents were identified and described in motoneurons in reduced animal preparations, they have also been proposed to contribute to certain features of human motor unit discharge (Heckman et al. 2008b; Hornby et al. 2002). For example, during slowly increasing voluntary contractions, motor unit discharge rate will often show a rapid increase after recruitment, followed by a much lower rate of rise with increasing force (De Luca et al. 1982; Kiehn and Eken 1997; Monster and Chan 1977; Mottram et al. 2009). The initial, rapid rate increase is thought to reflect increasing PIC activation, whereas the slow rate of rise is thought to reflect PIC saturation (Heckman et al. 2008b; Hornby et al. 2002). PIC activation will also allow motoneurons to continue to discharge at lower levels of net excitatory drive than those required to first activate the neuron (Gorassini et al. 2002).
Since PICs are under neuromodulatory control (Heckman et al. 2008a, 2008b; Hultborn et al. 2004), changes in motor unit discharge patterns resulting from central nervous system (CNS) injury or pharmacological treatments may reflect changes in PIC threshold or amplitude (ElBasiouny et al. 2010; Gorassini et al. 2004; Udina et al. 2010). However, changes in the pattern of synaptic drive to motoneurons may also contribute to changes in motor unit activation (Mottram et al. 2009, 2010).
The contribution of PICs to motor unit rate modulation and recruitment is likely to depend on the mixture of excitatory and inhibitory synaptic currents contributing to the net synaptic drive to the motoneuron. High-conductance states due to concurrent excitatory and inhibitory inputs that vary in parallel [“balanced inhibition and excitation” (Berg et al. 2007)] have been shown to reduce the influence of PICs on motoneuron firing patterns (Alaburda et al. 2005). Alternatively, reciprocal activation of excitatory and inhibitory synaptic inputs can lead to larger variations in PICs than produced with excitatory input alone (Heckman et al. 2008a; Hyngstrom et al. 2007).
The aim of the present study was to investigate the potential contributions of PIC activation and synaptic input patterns to motor unit recruitment and rate modulation based on simulations with a set of cable models of motoneurons. The size and intrinsic properties of the models were systematically varied to replicate the range of properties seen in low- to medium-threshold (presumably type S and FR) motoneurons recorded in decerebrate cats, except that afterhyperpolarization (AHP) durations were increased to reproduce the lower firing rates in human motor units. (This required a compensatory decrease in the PIC activation threshold.) We focused on low- and medium-threshold motoneurons because the associated motor units are mostly likely to be sampled during slow, submaximal, voluntary contractions in humans. Every member of this “pool” was driven with a noisy, excitatory conductance input that was uniformly distributed across the soma and dendrite. The mean value and variance of excitatory conductance increased linearly for 10 s and then decreased linearly back to its initial level. On subsequent simulation runs, three different patterns of inhibition were combined with this excitatory command: 1) a constant level of background inhibition, 2) an inhibitory input that varied inversely with the excitatory command [“push-pull inhibition” (Johnson and Heckman 2010)], and 3) an inhibitory input that rose and fell in parallel with the excitatory input [“balanced inhibition” (Berg et al. 2007), referred to here as “proportional inhibition”]. The inhibitory inputs were either distributed uniformly across the soma and dendrite or concentrated on the soma and proximal portion of the dendrite.
Our results indicate that all of the models in the pool responded to the different synaptic inputs in a qualitatively similar way and that the responses reproduced a number of features of human motor unit discharge, including rate saturation and de-recruitment at a lower level of net excitation than that required for recruitment. As discussed above, these features are thought to reflect the influence of PIC activation. However, the pattern of rate modulation was strongly influenced by the temporal and spatial pattern of concurrent inhibitory inputs. Together, these results indicate that even though PIC activation is likely to exert a strong influence on firing rate modulation, PIC activation in combination with different patterns of excitatory and inhibitory synaptic inputs can produce a wide variety of patterns of motor unit rate modulation.
METHODS
Model structure and passive properties.
All models were run in NEURON 7.0 (Hines 1989). A single motoneuron model consisted of an initial segment, axon hillock, soma, and dendritic cable. The initial segment consisted of 5 compartments of equal diameter, the axon hillock of 11 compartments whose diameters increased linearly from the end attached to the initial segment to that attached to the soma, a single soma compartment, and 25 dendritic compartments whose diameters first increased with distance from the soma over the first 20% of its length and then decreased linearly to zero over the remainder of its length. The pool of motoneurons consisted of 10 models with different geometrical and membrane properties. The lengths and diameters of all compartments varied linearly across the pool so that total surface area varied from 407,897 to 646,848 μm2. This range is slightly larger than that reported for a small sample of type-identified S and FR motoneurons from the cat medial gastrocnemius muscle (Cullheim et al. 1987). The axial resistivity and specific capacitance were set at 70 Ω·cm and 1 μF/cm2 for all models. The specific membrane resistivity was specified as in the step model of Fleshman et al. (1988), i.e., low specific membrane resistivity for the somatic compartment and high and constant specific membrane resistivity for the dendritic compartments. Both the somatic and dendritic specific membrane resistivities varied linearly across the pool and were highest in the smallest motoneurons. The combination of size differences and differences in specific membrane resistivity (along with different HCN channel densities, see below) produced a range of cell input conductances (estimated from the slope of the current-voltage relation near the resting potential) from 0.4 to 1.48 μS (input resistances from 0.68 to 2.47 MΩ), which covers the range for all but the highest conductance motoneurons recorded in the decerebrate cat (Lee and Heckman 1999). The ranges of anatomical and passive membrane properties are shown in Table 1.
Table 1.
Membrane resistivity and model geometry
| Property | Smallest Motoneuron | Largest Motoneuron |
|---|---|---|
| Soma length, μm | 45 | 65 |
| Soma diameter, μm | 45 | 65 |
| Dendrite length, μm | 5,800 | 7,200 |
| Initial dendrite diameter, μm | 35 | 45 |
| Peak dendrite diameter, μm | 37 | 47 |
| Initial segment diameter, μm | 3.4 | 4.4 |
| Axon hillock end diameter, μm | 10 | 15 |
| Rms, kΩ/cm2 | 0.8 | 0.2 |
| Rmd, kΩ/cm2 | 20,000 | 10,000 |
Rms and Rmd, specific resistivities of the soma and dendrite, respectively.
Active membrane properties.
The models included most of the active conductances known or hypothesized to exist in mammalian motoneurons. In some cases, different conductances with similar functional effects were represented as a single conductance for simplicity. For example, although spike repolarization may depend on a mixture of voltage-dependent potassium (K) conductances (Viana et al. 1993), we used a single delayed rectifier type of K+ conductance. Similarly, although calcium (Ca) entry through two different types of Ca channels is thought to activate Ca-activated K channels responsible for the medium-duration AHP following motoneuron spikes (Umemiya and Berger 1994), we used a single Ca channel model. We incorporated three different broad functional types of active conductances into our models: 1) action potential (AP)- and AHP-generating conductances, 2) conductances controlling synaptic amplification and dendritic plateau generation, and 3) conductances contributing to resting membrane potential and neuronal input conductance. Some conductances could contribute to more than one function.
Five conductances contributed to the generation of APs and AHPs. The upswing of the AP was initiated by the influx of sodium (Na) through voltage-dependent Na channels that consisted of both a transient and persistent component. Although the same channels are likely to contribute to both the transient and persistent components (Crill 1996), accurate representation of both of these components requires a multistate kinetics model (Kuo and Bean 1994), so we opted to use separate models for each component, adapted from those used by Royeck et al. (2008). Both the transient and persistent components were subject to slow inactivation as described by Fleidervish et al. (1996). A single voltage-dependent K conductance described by Kuo et al. (2006) contributed to spike repolarization, along with inactivation of the transient Na current. AHPs were produced by a Ca-activated K conductance whose relative activation was a function of local Ca concentration, which in turn reflected Ca influx during the spike and diffusion away from the channel site. Ca influx during the AP was mediated by a rapidly activating, noninactivating, high-threshold Ca conductance.
Synaptic amplification and dendritic plateau generation depended on the relative activation of a low-threshold Ca conductance, a Ca-activated K conductance, the Na and K channels responsible for spike generation (present at a much lower concentration on the dendrite, see below), and an HCN conductance mediating a hyperpolarization-activated mixed cation current. Although other models of synaptic amplification and dendritic plateau generation in motoneurons have focused exclusively on the role of low-threshold Ca channels [Cav1.3 type (e.g., Bui et al. 2006; Elbasiouny et al. 2006, 2005)], there is a variety of evidence suggesting the presence of other types of channels on motoneuron dendrites. Fast amplification of synaptic input transients during somatic voltage clamp (Jones and Lee 2006) is consistent with the presence of dendritic Na channels, since Cav1.3 channels are thought to exhibit slow kinetics in situ (Heckman et al. 2005; Powers and Binder 2001). Internal K channel blockers increase the amplitude and prolong the time course of distally generated synaptic potentials, suggesting that the amplifying action of persistent Na may be opposed by dendritic K channels with relatively fast kinetics (Clements et al. 1986). Recent evidence has shown that Cav1 channels in the dendrites slowly activate a Ca-activated K conductance, which acts to limit plateau duration (Li and Bennett 2007). Finally, there is both theoretical and anatomical evidence for dendritic HCN channels mediating a hyperpolarization-activated mixed cation current (Manuel et al. 2007; Zhao et al. 2010).
Although the input conductance and resting potential were largely determined by the model size and leak conductance (the reciprocal of specific membrane resistivity), the HCN-mediated current was also activated around the resting potential, depolarizing the resting potential and lowering input conductance. The persistent component of the Na current was minimally activated at the resting potential but also led to a slight depolarization and a decrease in input conductance.
The equations describing the steady-state activation and kinetics of the active conductions are provided in the Appendix.
Distribution of active conductances.
There is relatively little direct information regarding the spatial distribution of ion channels in motoneurons, but certain inferences about distribution can be drawn based on the electrophysiological behavior of motoneurons together with what is known about ion channel distribution in other cell types. Action potentials in motoneurons are initiated in a portion of the axon adjacent to the soma (Coombs et al. 1957), most likely the initial segment, as is the case in other types of neurons (Bean 2007). The lower threshold of the initial segment is thought to reflect a number of factors, including a high density of Na channels together with a lower threshold for their activation (Royeck et al. 2008). Although there may be a gradation of Na channel density and subunit composition from the distal end of the initial segment through the axon hillock (Lorincz and Nusser 2008), for simplicity we made Na channel density high and constant across the initial segment and axon hillock compartments and made the half-activation threshold for both the transient and persistent components 8 mV hyperpolarized to those of Na channels on the soma and dendrites. The Na channel density on the initial segment and axon hillock increased linearly from small to large motoneurons to ensure robust back-propagation in the face of the increased electrical load provided by the larger soma and dendrite. The Na density was lower on the soma and first dendritic compartment (from 12.5 to 17 times less than that of the axon) and lowest on the remainder of the dendrite (1.7% of the somatic density). The density of the persistent Na component was 2% of that of the transient component for the axon and distal dendrite and 1% for the soma and proximal dendrite. The density of the delayed rectifier K channels followed the same pattern as that of the Na channels: high on the axon, lower on the soma and proximal dendrite, and lowest on the remainder of the dendrite.
The amplitude of AHPs in motoneurons is sensitive to somatic membrane potential (Coombs et al. 1955), suggesting a proximal location, and a recent immunocytochemical study reported clusters of the channels thought to mediate the AHP (SK channels) on the soma and proximal dendrites of motoneurons (Deng et al. 2007). Accordingly, we placed the Ca-activated K channels and the Ca channels providing Ca for activating these K channels on the soma and most proximal dendritic compartment. AHP duration varies inversely with motoneuron size (Powers and Binder 2001). The physiological basis of this variation is not known. For simplicity, we changed AHP duration by changing the time constant of Ca decay.
Ca-activated K channels are also activated by Cav1 channels thought to be located more distally in the dendrite, but these do not contribute to the AHP, since Cav1 blockers do not affect the AHP (Li and Bennett 2007; Viana et al. 1993). These more distal K channels are thought to limit the size and duration of the dendritic plateau mediated by distal Cav1 channels (Li and Bennett 2007). The spatial distribution of these dendritic Cav1 channels is not precisely known, but limited immunohistochemical data in cat spinal motoneurons (Ballou et al. 2006), together with simulations with compartmental models based on reconstructed cat spinal motoneurons (Bui et al. 2006; Elbasiouny et al. 2005), suggests a mid-dendritic location. In our standard motoneuron pool, Cav1 channels were only present on the dendrite, where they were uniformly distributed along part of each model's dendritic cable from 32 to 56% of its total physical length. Ca-activated K channels were also uniformly distributed along this length of the cable. Recent simulation work has suggested that the physical distance between the soma and the Cav1 channels increases with motoneuron size (Grande et al. 2007). This systematic shift holds for our pool, as well, since the length of the dendritic cable varied systematically across the pool.
The motoneuron's response to a step of injected current shows a “sag” in voltage (Ito and Oshima 1965) that is thought to reflect the activation of a hyperpolarization-activated mixed cation current mediated by HCN-type channels. Manuel et al. (2007) suggested, based on a cable motoneuron model, that the response of motoneurons to injected current was best explained by the presence of HCN channels on both the soma and dendrites, and a recent immunohistochemical study supports such a distribution (Zhao et al. 2010). In our models, HCN channels were uniformly distributed at the same density on the soma and dendrite, and this density increased linearly across the pool.
The spatial distribution of ion channels for the lowest threshold and highest threshold motoneuron are given in Table 2. For the rest of the pool, channel density varied linearly between the values given for the lowest and highest threshold cell.
Table 2.
Active conductances
| Property | Smallest Motoneuron | Largest Motoneuron |
|---|---|---|
| Axon conductances, mS/cm2 | ||
| Na | 550 | 750 |
| Nap | 11 | 15 |
| Kdr | 400 | 400 |
| Soma conductances, mS/cm2 | ||
| Na | 44 | 44 |
| Nap | 0.44 | 0.44 |
| Kdr | 70 | 70 |
| H | 0.09 | 0.175 |
| KCa | 7.6 | 7.6 |
| Dendrite conductances, mS/cm2 | ||
| Na | 0.75 | 0.75 |
| Nap | 0.015 | 0.015 |
| Kdr | 0.33 | 0.33 |
| H | 0.09 | 0.175 |
| KCa | 0.04 | 0.04 |
| Cav1.3 | 0.16 | 0.16 |
Axon conductances are those of the initial segment and axon hillock, which have the same conductance densities. Na, transient sodium conductance; NaP, persistent sodium conductance; Kdr, delayed rectifier potassium conductance; H, HCN-mediated mixed cation conductance; KCa, calcium-activated potassium conductance; Cav1.3, low-threshold calcium conductance. Na, NaP, and Kdr conductance densities on the most proximal segment of the dendrite are the same as those on the soma; densities on the rest of the dendrite are shown. KCa density on the most proximal dendritic segment is one-half of that of the soma. Dendrite KCa and Cav1.3 densities shown are on a segment of the dendrite from 0.32 to 0.56 of the length away from the soma.
Simulation protocols.
All of the models in the pool were driven by three types of simulated inputs: 1) somatically injected current, 2) a somatic voltage-clamp command, and 3) a noisy, excitatory conductance command that was applied either alone or in combination with different patterns of inhibitory input. The current- and voltage-clamp commands were triangular waveforms similar to those used experimentally in cat spinal motoneurons (Lee and Heckman 1998a, 1998b, 1999). The current-clamp command rose linearly over 5 s at a rate of 6 nA/s and then decreased linearly at the same rate. The initial current value increased with cell size and started at −15 nA for the smallest cell and 10 nA for the largest cell, ensuring a wide range of firing rate modulation for cells with different thresholds. The voltage-clamp command started at −70 mV, rose for 5 s at 6 mV/s, and then returned to −70 mV at the same rate. The excitatory and inhibitory conductance commands were based on the fluctuating synaptic conductance model described by Destexhe et al. (2001). Each conductance is filtered noise with a truncated Gaussian distribution (negative values are not allowed) specified by a mean value, standard deviation, and filtering time constant (0.5 ms for the excitatory conductance and 2 ms for the inhibitory conductance). For the present study, we modified this model so that instead of being a point conductance command (such as that applied in a dynamic clamp experiment), it was specified as a conductance density. In addition, the mean and standard deviation of each conductance could vary as a function of time. The excitatory conductance command was specified by a triangular command that rose and fell over 20 s. The inhibitory command was either zero, a constant value, decreased as the excitatory command was increasing and increased as the excitatory command decreased (push-pull inhibition), or rose and fell in parallel with the excitatory command (proportional inhibition). The inhibitory conductance was either uniformly distributed or concentrated on the soma and the first 20% of the length of the dendrite.
Quantitative analysis.
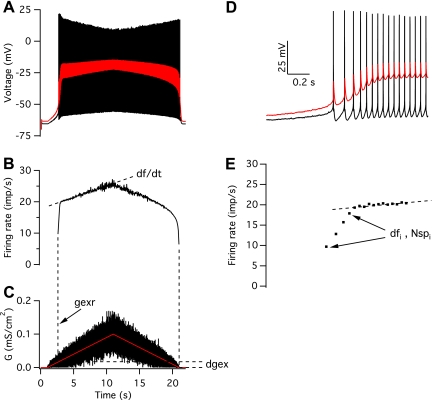
The discharge pattern of each motoneuron in response to a given synaptic command was described using five different outcome measures. As illustrated in Fig. 3, in response to an increasing excitatory synaptic command, motoneuron firing rate typically showed two linear phases of increase: an initial rapid increase, followed by a shallower increase. These two phases were separated by first fitting a line to the shallower increase in firing rate, extrapolating this line back to the onset of discharge, and defining the breakpoint between the two phases as the last of three interspike intervals whose instantaneous rate was less than the extrapolated rate by more than 1 imp/s. The initial rapid firing rate increase was quantified by the difference in firing rate between its onset and offset (dfi) and the number of spikes in this initial phase (Nspi). The subsequent shallower increase in firing rate was characterized in terms of its slope (df/dt). The other two outcome measures are gexr, which is the mean level of excitatory drive at recruitment, expressed as a percentage of the maximum value and dgex, which is the percent difference in mean excitatory drive between recruitment and de-recruitment.
Fig. 3.
Response of a medium-threshold motoneuron to a triangular, noisy, excitatory conductance waveform. A: membrane voltage at the soma (black) and the middle of the dendrite (red). B: instantaneous firing rate. C: excitatory synaptic conductance (G). The red trace shows the mean conductance level. The graph plots the conductance density, which was uniform on the soma and dendritic compartments. D and E: membrane potential (D) and instantaneous firing rate (E) at the beginning of the response, shown on an expanded time scale. See Quantitative analysis for definitions.
We used a two-way ANOVA to quantify the effects of different spatiotemporal patterns of inhibition on these outcome measures. One factor was temporal pattern, which included four conditions: excitation alone, excitation plus constant inhibition, excitation plus push-pull inhibition, and excitation plus proportional inhibition. The other factor was the spatial pattern of inhibition: uniform vs. proximal. We used the responses of the 10 motoneurons in our population to provide a distribution of values for each outcome measure for each spatiotemporal pattern of inhibition. For each outcome measure, we also compared each of the distribution of values for a given spatiotemporal pattern against the other patterns using multiple t-tests with the Bonferroni correction for multiple comparisons.
RESULTS
Current-clamp and voltage-clamp behavior.
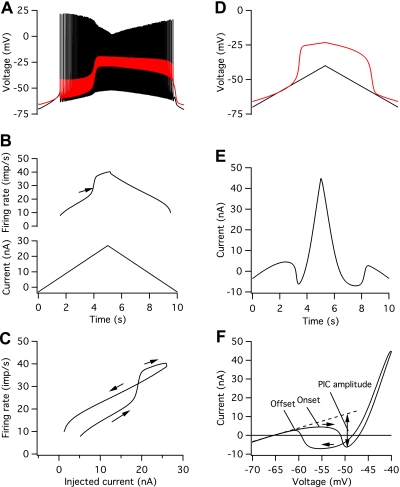
The model parameters were tuned so that both the frequency-current (F–I) and current-voltage (I–V) relations exhibited counterclockwise hysteresis, i.e., firing rates and inward currents are generally greater on the descending limb of the current/voltage command, and the thresholds for discharge and PIC activation are lower on the descending limb of the command. Figure 1 illustrates this behavior for a medium-threshold motoneuron in the pool. Figure 1A shows the voltage recorded at the soma and in the middle of the dendrite in response to a triangular injected-current waveform (Fig. 1B, bottom). A dendritic plateau depolarization is initiated near the peak of the ascending portion of the current-clamp command, and this leads to an acceleration in firing rate (arrow in Fig. 1B, top) that lasts throughout the rising phase of the dendritic plateau. After the plateau has reached a steady level, firing rate changes at a lower rate but remains higher over much of the descending limb of the current-clamp command than at equal levels of injected current during the ascending limb. Firing stops at a lower level of injected current than was required to initiate discharge and occurs at the offset of the dendritic plateau. These features are shown more clearly in Fig. 1C, which plots the relations between firing rate and current as current increases (rightward arrows) and decreases (leftward arrow).
Fig. 1.
Frequency-current (F–I) and I–V relations for a model of a medium-threshold motoneuron. A: somatic (black) and mid-dendritic (red) voltage in response to the triangular injected current command shown in the bottom panel of B. B: time course of instantaneous discharge rate (top) and injected current (bottom). C: F–I relation. Arrows indicate firing rates during the ascending and descending portions of the injected current command. D: somatic (black) and mid-dendritic (red) voltage in response to a somatic voltage-clamp command from −70 to −40 mV and back. E: voltage-clamp current vs. time. F: I–V relation. Horizontal arrows indicate current recorded during the ascending and descending portions of the voltage-clamp command. PIC, persistent inward current.
The relation between current and voltage during a triangular somatic voltage-clamp command exhibits a similar type of hysteresis that is related to the onset and termination of a dendritic plateau depolarization (Fig. 1, D–F). Figure 1D shows that a dendritic plateau depolarization is initiated when the somatic voltage reaches about −54 mV. This leads to inward current flow measured at the soma that then reverses to outward current flow at higher levels of depolarization (Fig. 1E). A similar sequence is observed on the descending limb, except that the inward current turns off at a more hyperpolarized voltage than was required to initiate the inward current. Figure 1F illustrates the I–V relation for the ascending (rightward arrow) and descending (leftward arrow) portions of the voltage-clamp command. The onset of the PIC is taken as the first zero-slope point on the ascending I–V relation, and the offset is taken as the second zero-slope point on the descending I–V relation (Lee and Heckman 1998a, 1999). PIC amplitude is measured as the peak inward current on the ascending limb after the current due to the leak conductance is subtracted [dashed line in Fig. 1F (Lee and Heckman 1998a, 1999)], and total PIC is 18.1 nA in this model.
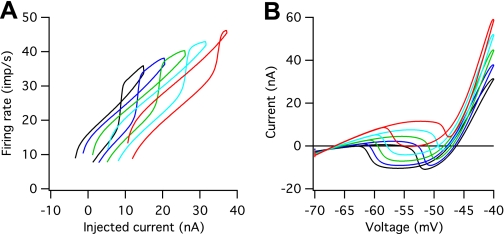
Systematic differences in size, specific membrane resistivity, and AHP duration in our pool of models led to systematic differences in F–I and I–V behavior that are illustrated in Fig. 2. Our standard pool of motoneurons had 10 models, but for clarity we illustrate the behavior of just 5 of these: the mid-threshold model shown in Fig. 1 (green trace in Fig. 2) and two higher and two lower threshold motoneuron models. Figure 2A shows that variations in size and membrane resistivity led to systematic differences in current threshold for repetitive discharge: the smallest motoneuron model had a threshold of 1.3 nA and the largest a threshold of 14.1 nA. These differences are reflected in shifts in the F–I relations along the current axis. In all cases, the models' F–I relations exhibited firing rate acceleration on the ascending limb of the current command and counterclockwise hysteresis; discharge stopped at a lower level of injected current than was required to initiate discharge. However, the amount of hysteresis was less in the higher threshold models, and the difference between the current required to initiate discharge and the current at which firing rate acceleration occurred was greater in the higher threshold models.
Fig. 2.
F–I and I–V relations for the model pool. The pool consists of 10 motoneuron models, but for clarity only the response of every other model is shown. A: F–I relations. B: I–V relations.
Figure 2B shows that the input conductance, estimated from the slope of the I–V relation near the resting potential, increased from a value of 0.4 μS for the smallest motoneuron model to 1.5 μS for the largest motoneuron model, which covers most of the range observed experimentally (Lee and Heckman 1998a, 1999). The high-threshold, high-input conductance motoneuron models exhibited a higher threshold for PIC onset than lower threshold models, and all models showed a lower threshold for PIC offset than onset, also consistent with experimental observations (Lee and Heckman 1998a, 1999). Total PIC amplitudes increased slightly with increasing input conductance from a value of 15.9 nA for the lowest conductance model to 21.5 nA for the highest conductance model. These values are within the range reported in the decerebrate cat without the addition of exogenous monoamines but fall closer to the mean values reported for the decerebrate cat after intrathecal administration of methoxamine (see Fig. 4 in Lee and Heckman 1999).
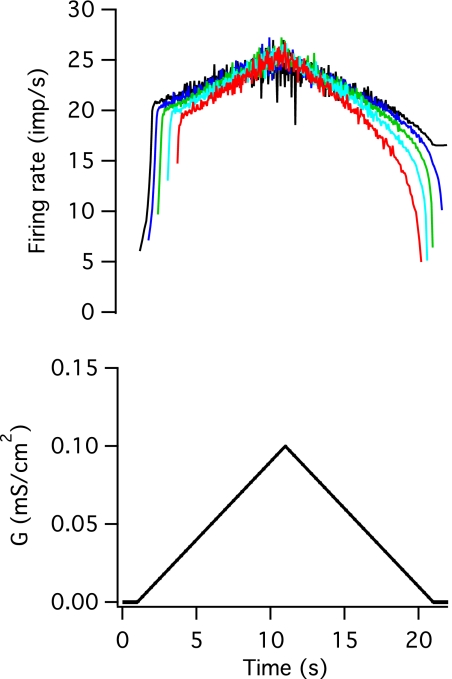
Fig. 4.
Response of the motoneuron pool to an excitatory conductance command. Top: instantaneous firing rates of 5 members of the pool. Bottom: mean excitatory conductance command.
Our models are thus able to reproduce many aspects of the F–I and I–V relations observed experimentally. Our goal was to produce a pool of models that reproduced a range of motoneuron behaviors that depended only on variations in motoneuron properties for which there is reasonable evidence, i.e., size, specific membrane resistivity, HCN channel density [given the range of sag responses in motoneurons (e.g., Manuel et al. 2007)], and AHP duration (Powers and Binder 2001). Although there are some features of the experimental data that are not captured by our models (see below and discussion), we believe that they nonetheless provide a reasonable basis for investigating the potential relations between intrinsic motoneuron properties, synaptic input patterns, and discharge patterns in human motor units. Discrepancies between simulated and experimental behavior and potential changes in model parameters to reduce these discrepancies are considered in the discussion.
Responses to noisy, excitatory conductance commands.
In contrast to the response to somatic current injection, where PIC activation occurs well after motoneuron recruitment, excitatory synaptic input led to the much earlier activation of PICs and the consequent generation of dendritic plateaus, in keeping with previous simulation and experimental studies (e.g., Bennett et al. 1998; Bui et al. 2006; Elbasiouny et al. 2006; Lee et al. 2003). This presumably reflects the fact that synaptic input directly depolarizes the areas of dendritic membrane where the channels mediating PICs are located. Figure 3 shows the response of a medium-threshold motoneuron model to a noisy, excitatory conductance command (Fig. 3C; red trace shows the mean conductance level). There is a rapid increase in firing rate immediately after recruitment (Fig. 3B), associated with the onset of a dendritic plateau (red trace in Fig. 3A). Once the dendritic plateau reaches a relatively steady level, the firing rate increases much more slowly with increasing excitation. Decreasing excitation leads to a slow decline in firing rate until just before de-recruitment, when the rate drops more rapidly. Figure 3, D and E, shows firing rate acceleration and plateau onset on an expanded time scale. As was the case for injected current, discharge stops at a lower level of excitatory conductance than was required to initiate discharge: for this model and input, discharge started when the mean level of excitatory conductance was 16% of its maximal value and stopped when the conductance returned to zero.
Figure 4 shows the firing rate responses of five motoneuron models to the same excitatory conductance command. For clarity, in this and all other figures, only the time-varying mean level of conductance is illustrated (Fig. 4, bottom). In all cases, Gaussian distributed noise was superimposed on this mean level, and the standard deviation of the noise increased in parallel with the mean conductance level. The different threshold models exhibited qualitatively similar responses to the input. All of the models were de-recruited at a lower level of excitatory conductance than was required to recruit the model (the lowest threshold model continued to discharge after the excitatory conductance returned to zero). Not surprisingly, models that had higher thresholds to current injection also showed later recruitment and earlier de-recruitment in response to the excitatory conductance input. All of the models showed an initial steep acceleration in firing rate following recruitment, followed by a much slower increase; however, the period of steeply rising firing rate was longer and the change in firing rate was greater in the lower threshold models. The lowest unit had 8 spikes in the initial phase of firing rate increase, and the firing rate increased from 6.1 to 20.0 imp/s during this phase, whereas this phase lasted for only 3 spikes in the highest threshold unit, and the firing rate changed from 16.1 to 18.0 imp/s. The firing rates of the later recruited units were less than or equal to those of the earlier recruited units throughout the entire trial; a similar behavior has been reported for human motor units in some muscles (e.g., De Luca et al. 1982), but not others (e.g., Oya et al. 2009).
Effects of concurrent inhibitory input.
We examined the effects of superimposing three different patterns of noisy, inhibitory input on the standard excitatory conductance waveform: 1) a constant mean level of inhibition, 2) inhibition whose mean level varied inversely with that of the excitatory input (push-pull inhibition), and 3) inhibition whose mean level varied in parallel with that of the excitatory input (proportional inhibition). For each temporal pattern of inhibitory input, we examined the effects of two different spatial distributions of inhibitory input: a uniform distribution on the soma and dendrite, and a proximal distribution in which the same total amount of inhibitory input was concentrated on the soma and the proximal 20% of the dendrite.
Table 3 provides the mean and standard deviation of the 5 primary outcome measures (see methods) for our population of 10 motoneurons for excitation alone and for the 6 different spatiotemporal patterns of superimposed inhibitory input. The last three columns show the significance values based on a two-way ANOVA of the effects of spatial and temporal patterns of inhibition and their interaction on each of the outcome measures. The following sections illustrate and describe the effects of each pattern of inhibition in more detail.
Table 3.
Distribution of 5 quantitative measures of discharge behavior for the population of 10 motoneurons driven by excitation alone and excitation in combination with 6 different spatiotemporal patterns of inhibition
| Constant Inhibition |
Push-Pull Inhibition |
Proportional Inhibition |
Significance |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Excitation Alone | Uniform | Proximal | Uniform | Proximal | Uniform | Proximal | Temporal | Spatial | Interaction | |
| gexr, % | 17.98 ± 9.69 | 25.63 ± 10.53 | 23.3 ± 9.38 | 41.83 ± 8.8 | 34.8 ± 7.28 | 43.97 ± 22.11 | 29.86 ± 11.69 | P < 0.001 | P < 0.05 | NS |
| Nspi | 4.5 ± 2.37 | 6.5 ± 3.87 | 3.1 ± 2.02 | 9.7 ± 3.74 | 1.2 ± 0.42 | 11.2 ± 7.77 | 1.9 ± 1.29 | NS | P < 0.001 | P < 0.001 |
| df/dt, imp·s−1·s−1 | 0.74 ± 0.17 | 0.79 ± 0.18 | 0.76 ± 0.2 | 1.62 ± 0.23 | 1.8 ± 0.41 | 0.48 ± 0.39 | −0.28 ± 0.13 | P < 0.001 | P < 0.05 | P < 0.001 |
| dfi, imp/s | 8.6 ± 4.21 | 8.52 ± 4.4 | 6.19 ± 3.77 | 8.72 ± 3.17 | 2.16 ± 1.72 | 5.66 ± 3.81 | 2.75 ± 4.19 | P < 0.01 | P < 0.01 | NS |
| dgex, % | 18.3 ± 2.86 | 11.69 ± 4.31 | 19.2 ± 3.0 | 3.21 ± 3.0 | 11.97 ± 7.75 | 5.39 ± 9.94 | 16.73 ± 18.4 | P < 0.001 | P < 0.001 | NS |
Gexr, percentage of maximum excitatory drive at recruitment; Nspi, the number of spikes in the initial rapid phase of firing rate increase; df/dt, slope of second phase of firing rate increase; dfi, difference in firing rate between the beginning and end of the initial phase of firing rate increase; dgex, percent difference in excitatory drive at recruitment and de-recruitment. Significance values are the results of a 2-way ANOVA of the significance of temporal and spatial patterns of inhibition on each outcome measure along with the interaction of spatial and temporal factors. NS, no significant difference.
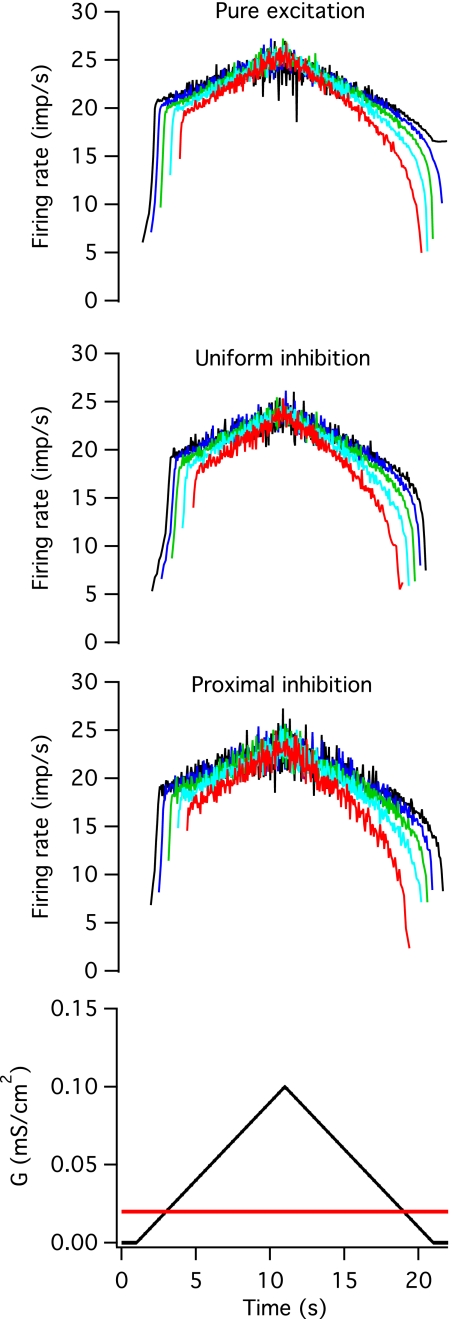
Effects of constant inhibition.
The time course of firing rate modulation in response to an excitatory input was qualitatively similar in the presence and absence of a constant inhibitory input, as shown in Fig. 5. The top panel in Figs. 5, 6, and 7 shows the responses to pure excitation for comparison, and the next two panels show the firing rate modulation with uniformly and proximally distributed inhibition. The bottom panel shows the time course of the mean level of excitatory (black) and inhibitory (red) conductance. In the presence of inhibition, all of the motoneuron models fired at lower rates and were recruited at higher levels of the excitatory command (gexr), but the range of gexr values was similar to those seen with excitation alone. However, the average gexr values were not significantly different from excitation alone (excitation alone: 17.98 ± 9.69; uniform constant inhibition: 25.63 ± 10.53; proximal constant inhibition: 23.30 ± 9.38). The difference between recruitment and de-recruitment thresholds (dgex) was significantly reduced for uniformly distributed inhibition (excitation alone: 18.30 ± 2.86; constant uniform inhibition: 11.69 ± 4.31, P < 0.05), and this distribution of constant inhibition also prevented the lowest threshold units from self-sustained firing after the excitatory conductance had returned to zero. In the presence of uniformly distributed inhibition, the period of rapid rate acceleration occurred over more intervals than in the absence of inhibition. The opposite trends were seen in the presence of proximal inhibition, although neither of these differences was statistically significant. As described below, the dependence of firing rate profiles on the spatial distribution of inhibitory input is more marked in the case of push-pull and balanced inhibition.
Fig. 5.
Constant inhibition. A constant-background, noisy inhibitory conductance, was added to the noisy, excitatory conductance waveform. The average value of each waveform is shown in the bottom panel (black for excitatory and red for inhibitory). The top panel shows instantaneous firing rates in 5 different models in response to pure excitation for comparison, and the middle panels shows responses when the inhibitory conductance was distributed uniformly across the soma and dendrites (uniform inhibition) or when it was concentrated on the soma and the first 20% of the dendritic cable (proximal inhibition).
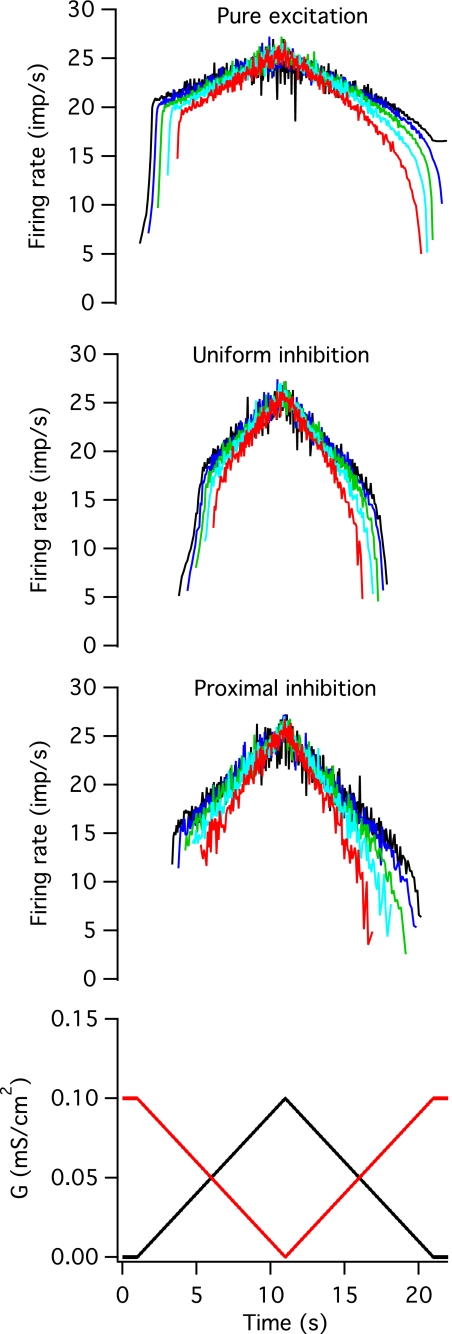
Fig. 6.
Push-pull inhibition. A time-varying, noisy inhibitory conductance whose mean value varied inversely with that of the mean excitatory conductance level was added to the noisy, excitatory conductance waveform. The average value of each waveform is shown in the bottom panel (black for excitatory and red for inhibitory). The top panel shows instantaneous firing rates in 5 different models in response to pure excitation for comparison, and the middle panels shows responses when the inhibitory conductance was distributed uniformly across the soma and dendrites or when it was concentrated on the soma and the first 20% of the dendritic cable.
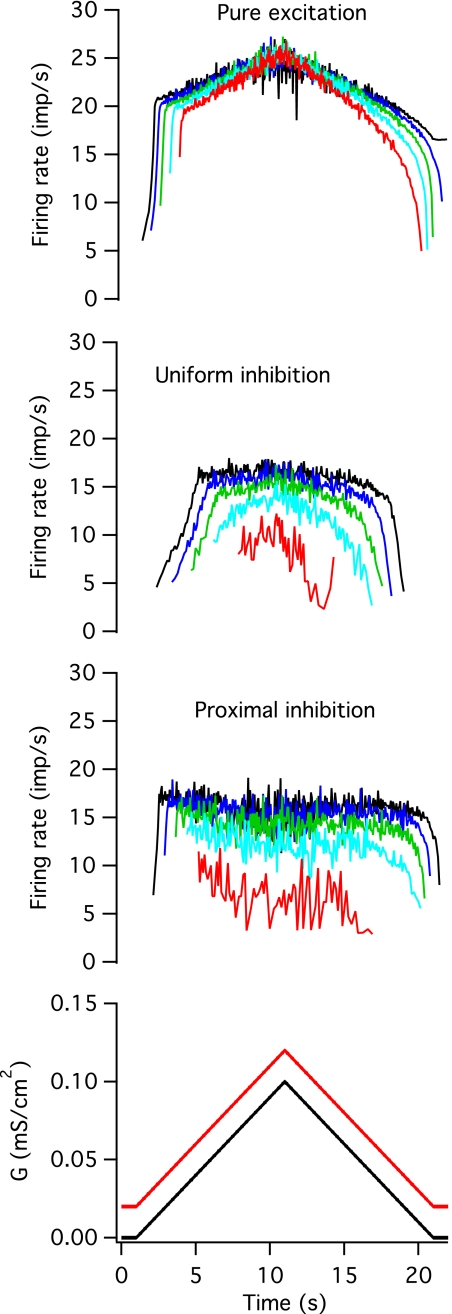
Fig. 7.
Proportional inhibition. A time-varying, noisy inhibitory conductance whose mean value changed in parallel with that of the mean excitatory conductance was added to the noisy, excitatory conductance waveform. The average value of each waveform is shown in the bottom panel (black for excitatory and red for inhibitory). The top panel shows instantaneous firing rates in 5 different models in response to pure excitation for comparison, and the middle panels shows responses when the inhibitory conductance was distributed uniformly across the soma and dendrites or when it was concentrated on the soma and the first 20% of the dendritic cable.
Effects of push-pull inhibition.
The effects of inhibitory input that varied inversely with the excitatory input (push-pull inhibition) were qualitatively different depending on the spatial distribution of inhibition. Figure 6 shows time course of firing rate modulation in response to excitation alone (top panel) and combined with either uniformly distributed (second panel) or proximally distributed (third panel) inhibition. As was observed for constant inhibition, gexr values were increased in the presence of push-pull inhibition. The levels of excitatory conductance at recruitment were highest for uniformly distributed inhibitory input but were significantly greater than excitation alone for both spatial patterns of inhibition (uniform push-pull inhibition: 41.83 ± 8.8, P < 0.001; proximal push-pull inhibition: 34.8 ± 7.28, P < 0.001). The difference in recruitment and de-recruitment thresholds (dgex) was significantly reduced by uniform, but not by proximally distributed, inhibition (uniform push-pull inhibition: 3.21 ± 3.0, P < 0.001; proximal push-pull inhibition: 11.97 ± 7.75). The time course of firing rate modulation was qualitatively different depending on whether inhibition was distributed uniformly or confined to the soma and proximal dendrite. In the presence of uniformly distributed inhibition, the period of rapid rate acceleration occurred over more intervals than in the absence of inhibition (9.7 ± 3.74 vs. 6.5 ± 3.87, P < 0.05), but the time course of firing rate modulation was qualitatively similar to that seen with excitation alone; firing rate modulation exhibited a nonlinear time course on both the ascending and descending portion of the excitatory command. In contrast, in the presence of proximally distributed push-pull inhibition, the period of initial rate acceleration was essentially eliminated (Nspi = 1.2 ± 0.42, P < 0.001) and firing rate changed in a fairly linear fashion for both increasing and decreasing excitation. Finally, for both spatial distributions of push-pull inhibition, the slope of the second (or only) linear phase of firing rate increase (df/dt) was significantly higher than for excitation alone (uniform: 1.62 ± 0.23 imp·s−1·s−1, P < 0.001; proximal: 1.8 ± 0.41 imp·s−1·s−1, P < 0.001).
Effects of proportional inhibition.
Proportional inhibition acted to clamp the firing rate at a nearly constant level after an initial period of increasing firing rate. As was observed for the other two patterns of inhibition, units were recruited later and de-recruited earlier in the presence of proportional inhibition, and these differences were greater for uniformly distributed inhibitory input. The range of gexr values was increased compared with pure excitation, particularly for uniformly distributed inhibition (uniform: 43.97 ± 22.11, P < 0.001; proximal: 29.86 ± 11.69, P < 0.01). As was the case for proportional inhibition, the difference in recruitment and de-recruitment thresholds was significantly reduced in the case of uniformly distributed inhibition (dgex = 5.39 ± 9.94, P < 0.001), but not proximal inhibition (dgex = 16.73 ± 18.4). The effects of proportional inhibition on the time course of firing rate also depended upon its spatial distribution. For uniformly-distributed inhibition (Fig. 7, second panel), there was a steep rise in firing rate before it reached a steady-state level, but this initial rise was slower and lasted longer than in the absence of inhibition (Nspi = 11.2 ± 7.77 vs. 4.5 ± 2.37, P < 0.01). For proximally-distributed inhibition (third panel), the initial steep increase in firing rate lasted at most only a few intervals (Nspi = 1.9 ± 1.29, P < 0.001), and was completely absent in the later recruited units. The subsequent period of firing rate increase (df/dt) was much shallower than for excitation alone, particularly for proximal inhibition (uniform: 0.48 ± 0.39 imp/s/s, P < 0.05; proximal: −0.28 ± 0.13 imp/s/s, P < 0.001). For proximal inhibition, the later recruited units actually exhibited a slight decline in firing rate over the course of the trial, even when the excitatory conductance was increasing.
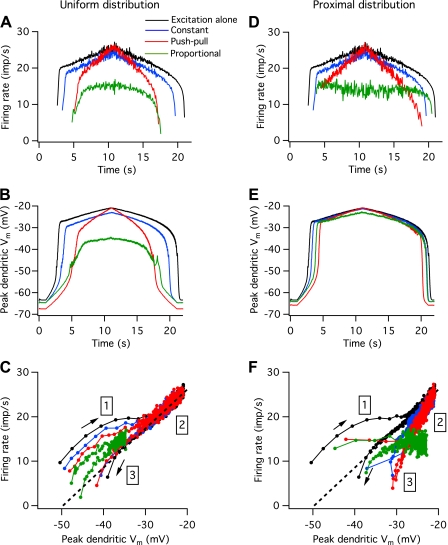
Relation of firing rate modulation to dendritic plateau generation.
Figure 8, A and D, shows the effects of different patterns and spatial distributions of inhibitory input on the discharge of a medium-threshold motoneuron. The black traces show the response to excitation alone, and the remaining traces show the effects of a constant level of inhibition (blue traces), push-pull inhibition (red traces), and proportional inhibition (green traces). For a uniform spatial distribution of inhibition (Fig. 8A), the initial firing rate was lower and the duration of the initial rapid rise in firing rate was longer for all patterns of inhibition, whereas the opposite effect occurred with proximal inhibition (Fig. 8D). Subsequent changes in firing rate were similar for the two spatial patterns of inhibition. For constant inhibition, the rise and fall of firing rate with increasing and decreasing excitation was parallel to that seen with excitation alone. Push-pull inhibition produced a steeper change in firing rate, whereas proportional inhibition produced a much shallower change in firing rate, particularly for proximal inhibition.
Fig. 8.
Dendritic plateau activation during different patterns of synaptic input. A: instantaneous firing rate vs. time for a medium-threshold motoneuron in response to excitation alone (black trace) and to excitation plus uniformly distributed inhibition of a constant mean level (blue trace), push-pull inhibition (red trace), and proportional inhibition (green trace). B: peak dendritic voltage (Vm) for excitation and different patterns of inhibition. C: firing rate vs. peak dendritic voltage. D–F: same as A–C, only for proximal inhibition. Different regions of the rate vs. dendritic voltage relations in C and F are region 1, during the initial activation of the plateau; region 2, during the period when the plateau depolarization is fairly stable; and region 3, during plateau deactivation. The dashed black line in C and F is the best linear fit to the firing rate vs. dendritic depolarization relation over region 2, for excitation alone.
The different patterns of rate modulation observed with uniformly distributed vs. proximal inhibition reflect the degree of coupling between the region of plateau generation on the dendrite and the region of spike and AHP generation on the axon, soma, and proximal dendrite. Increasing excitatory drive leads to increasing activation of Cav1.3 channels on the dendrite, leading to increasing dendritic depolarization and an increase in the current delivered to the spike-generating region. When inhibitory input is uniformly distributed, it acts on both the plateau-generating region and on more proximal and distal regions of the dendrite. As a result, changes in the peak depolarization in the dendrite and firing rate are more tightly linked than in the case of proximal inhibition. In the latter case, inhibitory inputs decrease the coupling between the plateau- and spike-generating regions.
Once the dendritic plateau is generated, the peak depolarization along the dendrite occurs at the distal end of the region containing Cav1.3 channels. Figure 8, B and E, shows the low-pass filtered (15 Hz) time course of peak dendritic depolarization for excitation alone and in the presence of different patterns of uniformly distributed (B) or proximally distributed inhibition (E). For a uniform distribution of inhibition, the time course of peak dendritic depolarization is similar to that of instantaneous firing rate. The rapid generation of the dendritic plateau depolarization after the motoneuron begins to fire is associated with a rapid increase in firing rate until the plateau approaches its final level at which Cav1.3 channels are almost fully activated, except in the case of proportional inhibition. Shallower increases and decreases in firing rate occur with relatively little change in plateau depolarization until de-recruitment, when both firing rate and dendritic depolarization decrease rapidly.
The relation between instantaneous firing rate and dendritic depolarization for uniformly distributed inhibition is plotted in Fig. 8C. In the absence of inhibition (black trace), firing rate increases rapidly with dendritic depolarization after the motoneuron is initially recruited (region 1) but then increases more slowly near the end of the period of rapid dendritic depolarization. Over most of the trial (region 2), firing rate shows a linear relation to changes in dendritic depolarization (the dashed black line shows the best linear fit to this portion of the relation) until just before de-recruitment (region 3), when firing rate falls off more steeply with decreasing dendritic depolarization. Both constant inhibition (blue) and push-pull inhibition (red) are associated with firing rate vs. dendritic depolarization relations that closely parallel those observed with excitation alone, except that firing rates in region 2 are slightly higher for a given amount of dendritic depolarization than those for excitation alone. Proportional inhibition is associated with a similar relation, except both firing rate and dendritic depolarization are much lower than that seen for excitation alone.
For proximal inhibition, the relation between dendritic depolarization and firing rates is very different from that seen for excitation alone. Despite the fact that the patterns of firing rate modulation look very different for different patterns of inhibition (Fig. 8D), the profiles of dendritic depolarization are very similar, differing only in their onset and offset times (Fig. 8E). For proportional and push-pull inhibition, the initial steep increase in rate is nearly absent so that the initial large change in dendritic depolarization occurs with little or no change in firing rate (Fig. 8F, region 1). In the case of proportional inhibition, firing rate is nearly constant for the entire trial, except for a decrease over the last few intervals before de-recruitment (region 3). In the case of push-pull inhibition, nearly all of the firing rate modulation occurs over a region when dendritic depolarization is changing relatively little (region 2). For all types of proximal inhibition, firing rates are lower for a given amount of dendritic depolarization than for excitation alone (i.e., the points lie below the dashed black line in Fig. 8F).
The two different spatial distributions of inhibition also had different effects of firing rate hysteresis (i.e., the difference in excitatory drive at recruitment and de-recruitment). In the absence of inhibition, excitatory drive was on average 18.3 ± 2.9% higher at recruitment than at de-recruitment across all units. This mean difference was still positive but significantly smaller (see above) in the presence of all types of uniform inhibition (11.7 ± 4.3% for constant inhibition, 3.2 ± 3.0% for push-pull inhibition, 7.7 + 7.3% for proportional inhibition). In contrast, none of the forms of proximal inhibition produced a significant change in firing rate hysteresis.
Dependence of firing rate modulation on intrinsic motoneuron properties.
Although we did not systematically explore the relation of intrinsic motoneuron properties to firing rate modulation, we did examine patterns of firing rate modulation for two different modifications of the basic parameter set. In the first modification, we eliminated Na and delayed rectifier K channels on all of the dendrite except for the most proximal component and made compensatory changes in the density of Cav1.3 channels (increased by 10%) and the dendritic Ca-activated K channels activated by these Cav1.3 channels (decreased by 50%) so that the F–I and I–V relations were very similar to those the standard population. In the second modification, we reduced the density of Cav1.3 channels on the standard model by 20%. This led to PIC amplitudes that were closer to the mean values observed in the decerebrate cat without methoxamine (Lee and Heckman 1999) and F–I relations that lacked hysteresis and firing rate acceleration except for the lowest threshold motoneurons. The first modification led to patterns of firing rate modulation that were virtually indistinguishable from those of the basic model (data not shown), suggesting that model parameter sets that lead to similar F–I and I–V behavior will produce similar behaviors in response to different patterns of synaptic input. Not surprisingly, the second modification did produce clear differences in the responses to synaptic inputs, as described below.
Figure 9 compares the responses of the standard medium-threshold motoneuron (black traces) with those of a model with a 20% reduction in Cav1.3 channel density (red traces) to the excitatory input in combination with the six different types of inhibition: constant, push-pull, and proportional inhibition with a uniform spatial distribution (A–C) and a proximal distribution (D–F). (The responses to excitation alone are not illustrated, but the differences between the responses of the 2 models are similar to those shown for constant inhibition.) For all patterns and distributions of inhibition, recruitment occurred at about the same time in the original and reduced Cav1.3 model, but the initial firing rate at recruitment was lower in the reduced Cav1.3 model, and the period over which firing rate increased rapidly was longer, except in the case of proportional inhibition with a uniform distribution (Fig. 9C). In contrast to the standard model, the reduced Cav1.3 model exhibited a fairly linear increase and decrease in firing rate, which reflected a linear and graded increase and decrease in Cav1.3 activation between recruitment and de-recruitment (not shown). De-recruitment occurred earlier in all cases for the reduced Cav1.3 model so that the difference between mean excitatory drive at recruitment and de-recruitment (dgex) was less than in the standard model. The average values of dgex were significantly lower (P < 0.05) for the population of low Cav1.3 models than for the standard population for all conditions of synaptic input, except for uniformly distributed balanced inhibition, where the mean value of dgex was lower, but the difference did not reach statistical significance due to the large range of values for the standard model (standard: 5.39 ± 9.94; low Ca: −1.10 ± 1.20). These results are consistent with the idea that indirect measures of the difference between excitatory drive at recruitment and de-recruitment reflect the contribution of PIC to motor unit excitation (Gorassini et al. 2002). In many cases, particularly in the presence of uniformly distributed inhibition, the difference in excitatory drive at recruitment and de-recruitment was near zero or even negative for the reduced Cav1.3 models, despite the fact that Cav1.3 channels were clearly activated and thus contributed to firing rate modulation. This suggests that the absence of counterclockwise hysteresis in the conductance vs. firing rate relation does not necessarily indicate the absence of PIC.
Fig. 9.
Effects of a 20% reduction Cav1.3 density on the behavior of a medium-threshold motoneuron. Responses of the standard model are shown in black, whereas those of the reduced Cav1.3 model are shown in red. A: responses to constant inhibition with a uniform spatial distribution. B: responses to push-pull inhibition with a uniform spatial distribution. C: responses to proportional inhibition with a uniform spatial distribution. D–F: same as A–C but with a proximal distribution of inhibition.
Taken together, these simulation results suggest that changes in neuromodulatory drive to motoneurons [producing changes in PIC amplitude (Heckman et al. 2008a, 2008b)] together with different patterns of ionotropic drive can produce a wide variety of patterns of motoneuron firing rate modulation. They also point out the importance of the spatial distribution of inhibition for determining both firing rate patterns and the link between firing rate modulation and dendritic PIC activation. In the discussion, we consider the extent to which these conclusions are based on the particular features of our models and how the realism of the models might be improved based on constraints provided by additional experimental data.
DISCUSSION
The results of our simulations show that a set of motoneuron models that reproduces many features of the response of cat motoneurons to injected current and voltage-clamp commands can also replicate certain features of human motor unit discharge when the models are driven by noisy synaptic inputs. Our results indicate that all of the models in the pool responded to the different synaptic inputs in a qualitatively similar way: pure excitatory inputs produced a rapid increase in firing rate reflecting rapid activation of PIC shortly after recruitment, followed by a much shallower increase in firing rate with increasing excitatory drive. Decreasing excitation led to a slow, linear decrease in firing rate, followed by a steeper decline just before de-recruitment. All of the units were de-recruited at a lower level of net excitatory drive than that required for recruitment. Constant background inhibition led to lower firing rates, later recruitment, and earlier de-recruitment, but the pattern of firing rate modulation was qualitatively similar to that seen with excitation alone. Uniform proportional inhibition markedly reduced or eliminated rate modulation following the initial rate acceleration, whereas uniform push-pull inhibition had the opposite effect. Proximal proportional inhibition eliminated the initial increase in firing rate in most of the units, and firing rate either remained flat or declined over time after recruitment. Proximal push-pull inhibition also eliminated the initial rapid increase in firing rate and led to remarkably linear changes in firing rate on both the increasing and decreasing portions of the excitatory conductance command.
The biphasic increase in firing rate as the excitatory input increases is consistent with patterns of rate modulation observed in human motor units during slowly increasing isometric contractions (e.g., Kiehn and Eken 1997; Monster and Chan 1977; Mottram et al. 2009; Person and Kudina 1972; Udina et al. 2010). However, the nonlinear decrease in firing rate during decreasing excitation as shown in Fig. 3 is not typically seen in human motor units during slow decreases in muscle force; human motor units typically exhibit a linear decline in firing rate (Kiehn and Eken 1997; Mottram et al. 2009; Person and Kudina 1972; Udina et al. 2010), although motor units in the first dorsal interosseous muscle can exhibit nonlinear decreases in rate when they are activated in their nonpreferred directions (Suresh N, personal communication). It is possible that slow changes in AHP duration (Wienecke et al. 2009) or in spike threshold that are not well captured by our models may account for this discrepancy. Another possibility is that decreases in isometric force reflect decreasing excitation in combination with increasing (push-pull) inhibition, since push-pull inhibition led to fairly linear decreases in firing rate, particularly when inhibition had a proximal distribution (Fig. 6, third panel).
Our pool of motoneuron models also captured much of the behavior observed experimentally in cat spinal motoneurons in response to somatic current-clamp and voltage-clamp commands. However, there are some aspects of the experimental data that are not well represented by our models. As mentioned above, the models were designed to replicate the lower discharge rates seen in human motor units compared with cat spinal motoneurons. For this reason, the AHP durations in our models are longer than those observed in cat spinal motoneurons. These longer AHP durations tend to clamp the somatic membrane potential at a lower mean value during repetitive discharge, which would also tend to keep the dendritic membrane potentials at lower values. To allow the development of a dendritic plateau depolarization at these lower, more hyperpolarized voltages, we lowered the threshold for Cav1 channel activation. As a result, PIC onset voltages in our models ranged from −59 mV for the lowest conductance model to −51 mV for the highest conductance model compared with average experimental values ranging from −52 to −42 mV for motoneurons with comparable input conductances (see Fig. 5B in Lee and Heckman 1999). In addition, the amount of hysteresis in PIC activation observed experimentally (the difference between the onset voltage on the ascending voltage-clamp command and offset voltage on the descending command, see Fig. 1F) is systematically smaller in high- than in low-conductance cells (see Fig. 6 in Lee and Heckman 1999). In our model pool, PIC hysteresis values were within the experimental range but did not show this downward trend with increasing input conductance. The decreased hysteresis in high-threshold motoneurons is likely to reflect an increased slow inactivation of PIC currents or increased slow activation of outward currents. For example, it is possible that high-threshold motoneurons have a higher proportion of Na-mediated PIC that is subject to slow inactivation.
The morphology of our models represents a compromise between even simpler two-compartment models (Booth et al. 1997; Kim and Jones 2011; Kurian et al. 2011), and models based on fully reconstructed dendritic trees (Bui et al. 2006; Elbasiouny et al. 2006). Many of the behaviors produced in our models simply depend on an electrical separation between spike- and plateau-generating regions and can be reproduced by two-compartment models with an appropriate value of coupling conductance between the two compartments. However, the shunting effects of proximal inhibition cannot be replicated in two-compartment models, unless the value of the coupling conductance is allowed to vary with the amount of inhibitory input. More complex models based on full reconstructions of dendritic trees would allow for greater range of motoneuron responses to synaptic input, since they can represent multiple regions of PIC activation with different values of electrical coupling to the soma (e.g., Carlin et al. 2009) or even segregation of regions of PIC generation based on Na and Ca channels (Shapiro and Lee 2007). However, it is likely that the basic effects produced by different patterns of synaptic inhibition would be similar in these more complex models; for example, the firing rate acceleration on recruitment and shunting effect of proximal inhibition is similar in these more complex models (Bui et al. 2008; Elbasiouny et al. 2006).
Although the quantitative features of the simulated motor unit firing rates certainly depend on the model parameters we have chosen, the qualitative features of the models' responses to different synaptic inputs (e.g., the bilinear increases in firing rate and the onset-offset hysteresis) are likely to be obtained in any set of models that is also capable of reproducing the basic aspects of the responses of cat spinal motoneurons somatic current- and voltage-clamp commands. If we can assume that human motoneurons share these behaviors and their basic underlying mechanisms, these simulations indicate that different patterns of inhibitory input can produce quite flexible control of motor unit discharge, despite the relatively all-or-none activation of dendritic PIC channels.
Our simulation results suggest that there are certain features of human motor discharge patterns that are indicative of a PIC contribution to firing rate modulation, but that the absence of these features does not necessarily indicate the absence of a PIC contribution. For example, a rapid initial increase in firing rate followed by a much shallower increase in firing rate is consistent with rapid PIC activation shortly after recruitment, followed by PIC saturation. However, a number of factors including proximal inhibitory input, a lowered PIC threshold, or an increased spike threshold could lead to rapid PIC activation before recruitment, eliminating the initial rapid increase in firing rate. Similarly, recruitment at a higher level of excitatory drive than that present at de-recruitment [as judged by the firing rate of a concurrently active unit (Gorassini et al. 2002)] indicates a PIC contribution, but as shown by our simulations with a reduced Cav1.3 density, the absence of this onset-offset hysteresis does not necessarily indicate the absence of a PIC contribution (Fuglevand et al. 2006). Variations in patterns of synaptic input to motoneurons could contribute to the variations in firing rate measures of PIC observed experimentally (Stephenson and Maluf 2011).
Different patterns of rate modulation in a given motor unit during different tasks might indicate different strategies for controlling motor output; steeper rate modulation might indicate the use of push-pull inhibition, whereas shallower rate modulation could reflect proportional inhibition. Finally, although levels of PIC expression are known to change following spinal cord injury (ElBasiouny et al. 2010; Li et al. 2004), disruptions in synaptic control are also likely to contribute to abnormal motor unit discharge patterns seen after CNS injury. For example, high levels of proportional inhibition might underlie the extremely low or even negative rate modulation seen in many motor units in stroke patients (Mottram et al. 2009).
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grants R01 NS062200 and R01 NS071951.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.K.P., S.M.E., W.Z.R., and C.J.H. conception and design of research; R.K.P. performed experiments; R.K.P. and S.M.E. analyzed data; R.K.P., S.M.E., W.Z.R., and C.J.H. interpreted results of experiments; R.K.P. prepared figures; R.K.P. drafted manuscript; R.K.P., S.M.E., W.Z.R., and C.J.H. edited and revised manuscript; R.K.P., S.M.E., W.Z.R., and C.J.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Robert H. Lee and Nina L. Suresh for helpful suggestions for the simulations and Dr. Edmund Ballou for programming assistance.
APPENDIX
The membrane potential in each compartment of our models was calculated using the following differential equation governing the balance of currents flowing across the membrane capacitance (Cm), the leak conductance (IL), to adjacent compartments (Icoupling) and other ionic conductances (Iionic), together with synaptic (Isyn) and injected current (Iinj):
The ionic currents included voltage-activated Na, K, and Ca currents, HCN-mediated mixed cation currents (H currents), and Ca-activated K currents. Gating of voltage- and Ca-sensitive channels mediating these currents was described as follows:
where p represents the fraction of open channels, p∞ is the equilibrium state, and τp is the time constant for approaching the equilibrium state.
Na currents.
The transient Na current was simulated using a model similar to that used by Royeck et al. (2008) and originally described by Migliore et al. (1999):
where gNaTmax is the maximum conductance, m, h, and s are the gating variables for activation, fast inactivation, and slow inactivation, respectively, and VNa (+50 mV) is the equilibrium potential for Na. The equations describing activation (αm) and deactivation (βm) are
where if τm < 0.2 ms, then τm = 0.02 ms.
The time constant for activation (τm) was originally described based on recordings at 24°C and was reduced by a factor Q(T) = 2.46, based on a Q10 of 2 and a temperature of 37°C. The parameter ΔV1/2 was used to shift the midpoints of the activation curve and fast inactivation curves and had a value of −3 mV for the axonal and initial segment Na channels and 5 mV for the other Na channels.
Fast inactivation was described by the following equations:
where if τh < 0.5 ms, then τh = 0.5 ms.
Slow inactivation was simulated as described by Fleidervish et al. (1996), and the minimum value of the slow inactivation gate was set to 0.4:
The persistent Na current depended on an activation gate and the same slow inactivation gate as used for the transient Na current:
K currents.
The models contained two types of K currents, a voltage-sensitive delayed rectifier K current and a Ca-activated K current. The delayed rectifier current was governed by the following equations:
with VK = −77 mV.
where n is the delayed rectifier activation variable.
The equations governing the Ca-activated K conductance (KCa) were similar to those used by Sah (1992), except that Ca dynamics were represented by a simple, first-order process representing influx through Ca channels and decay with a single time constant:
where τCa is the time constant of decay, iCa is Ca current density, F is Faraday's constant, and d is the depth of the inner shell around the KCa channels. This depth was given a small value (0.1 μm) for the KCa channels governing the AHP, to allow for a relatively fast activation of these channels by Ca influx during APs, whereas it was given a large value (200 μm) for the KCa channels colocalized with the Cav1.3 channels to produce the relatively slow activation of this current seen experimentally (Li and Bennett 2007). The time constant of decay for this distal KCa channel was also given a relatively large value to produce a slow decay (120 ms), whereas the values of τCa for the KCa channel mediating the AHP varied from 70 to 40 ms for the smallest and largest motoneurons to produce a range of AHP durations.
The relation between Ca concentration and the activation of KCa channels was described by the following equations:
Ca currents.
Both the high-threshold Ca current providing the Ca for KCa channel mediating the AHP and the low-threshold Cav1.3 current were simulated using a sigmoidal steady-state activation curve and a voltage-independent time constant:
The Ca equilibrium potential (VCa) was set to +80 mV. The half-activation voltage (V1/2) was −30 mV for the high-threshold Ca channel and −43 mV for the low-threshold Cav1.3 channel. The activation slope (Vs) was 4 mV for the high-threshold channel and 6 mV for the low-threshold channel, and the time constants were 1 and 60 ms, respectively.
HCN current.
The hyperpolarization-activated mixed cation current was also simulated using a sigmoidal steady-state activation curve and a voltage-independent time constant of 50 ms:
The equilibrium potential for the mixed cation current (Vh) was set to −38.9 mV. The relatively depolarized value for the half-activation threshold (−75 mV) reflects the depolarizing shift expected with tonic neuromodulatory drive (Larkman and Kelly 1992).
Synaptic currents.
The synaptic currents were generated by noisy excitatory and inhibitory conductances as described by Destexhe et al. (2001):
where Ve = 0 mV and Vi = −75 mV are the excitatory and inhibitory synaptic reversal potentials and ge(t) and gi(t) are the time-varying excitatory and inhibitory conductances. The mean levels of excitatory and inhibitory conductances are given by ge0 and gi0, their standard deviations by σe and σi, and their filtering time constants by τe and τi. χ1(t) and χ2(t) are two independent random Gaussian noise processes with a mean of 0 and a standard deviation of 1.
NEURON files for generating a model motoneuron's response to current-clamp, voltage-clamp, and synaptic conductance commands will be made available at http://senselab.med.yale.edu/modeldb.
REFERENCES
- Alaburda A, Russo R, MacAulay N, Hounsgaard J. Periodic high-conductance states in spinal neurons during scratch-like network activity in adult turtles. J Neurosci 25: 6316–6321, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballou EW, Smith WB, Anelli R, Heckman CJ. Measuring dendritic distribution of membrane proteins. J Neurosci Methods 156: 257–266, 2006 [DOI] [PubMed] [Google Scholar]
- Bean BP. The action potential in mammalian central neurons. Nat Rev Neurosci 8: 451–465, 2007 [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Hultborn H, Fedirchuk B, Gorassini M. Synaptic activation of plateaus in hindlimb motoneurons of decerebrate cats. J Neurophysiol 80: 2023–2037, 1998 [DOI] [PubMed] [Google Scholar]
- Berg RW, Alaburda A, Hounsgaard J. Balanced inhibition and excitation drive spike activity in spinal half-centers. Science 315: 390–393, 2007 [DOI] [PubMed] [Google Scholar]
- Booth V, Rinzel J, Kiehn O. Compartmental model of vertebrate motoneurons for Ca2+-dependent spiking and plateau potentials under pharmacological treatment. J Neurophysiol 78: 3371–3385, 1997 [DOI] [PubMed] [Google Scholar]
- Bui TV, Grande G, Rose PK. Relative location of inhibitory synapses and persistent inward currents determines the magnitude and mode of synaptic amplification in motoneurons. J Neurophysiol 99: 583–594, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui TV, Ter-Mikaelian M, Bedrossian D, Rose PK. Computational estimation of the distribution of L-type Ca2+ channels in motoneurons based on variable threshold of activation of persistent inward currents. J Neurophysiol 95: 225–241, 2006 [DOI] [PubMed] [Google Scholar]
- Carlin KP, Bui TV, Dai Y, Brownstone RM. Staircase currents in motoneurons: insight into the spatial arrangement of calcium channels in the dendritic tree. J Neurosci 29: 5343–5353, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements JD, Nelson PG, Redman SJ. Intracellular tetraethylammonium ions enhance group Ia excitatory post-synaptic potentials evoked in cat motoneurones. J Physiol 377: 267–282, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs JS, Curtis DR, Eccles JC. The generation of impulses in motoneurones. J Physiol 139: 232–249, 1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs JS, Eccles JC, Fatt P. The electrical properties of the motoneurone membrane. J Physiol 130: 291–325, 1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crill WE. Persistent sodium current in mammalian central neurons. Annu Rev Physiol 58: 349–362, 1996 [DOI] [PubMed] [Google Scholar]
- Cullheim S, Fleshman JW, Glenn LL, Burke RE. Membrane area and dendritic structure in type-identified triceps surae alpha motoneurons. J Comp Neurol 255: 68–81, 1987 [DOI] [PubMed] [Google Scholar]
- De Luca CJ, LeFever RS, McCue MP, Xenakis AP. Behaviour of human motor units in different muscles during linearly varying contractions. J Physiol 329: 113–128, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Z, Romer SH, Cope TC, Goss L, Nardelli P, Bullinger K, Fyffe REW. Expression of small conductance Ca2+-activated potassium (SK) channels in mammalian spinal motoneurons. Soc Neurosci Abstr 408.417, 2007 [Google Scholar]
- Destexhe A, Rudolph M, Fellous JM, Sejnowski TJ. Fluctuating synaptic conductances recreate in vivo-like activity in neocortical neurons. Neuroscience 107: 13–24, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbasiouny SM, Bennett DJ, Mushahwar VK. Simulation of Ca2+ persistent inward currents in spinal motoneurones: mode of activation and integration of synaptic inputs. J Physiol 570: 355–374, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbasiouny SM, Bennett DJ, Mushahwar VK. Simulation of dendritic CaV1.3 channels in cat lumbar motoneurons: spatial distribution. J Neurophysiol 94: 3961–3974, 2005 [DOI] [PubMed] [Google Scholar]
- ElBasiouny SM, Schuster JE, Heckman CJ. Persistent inward currents in spinal motoneurons: important for normal function but potentially harmful after spinal cord injury and in amyotrophic lateral sclerosis. Clin Neurophysiol 121: 1669–1679, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleidervish IA, Friedman A, Gutnick MJ. Slow inactivation of Na+ current and slow cumulative spike adaptation in mouse and guinea-pig neocortical neurones in slices. J Physiol 493: 83–97, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleshman JW, Segev I, Burke RB. Electrotonic architecture of type-identified alpha-motoneurons in the cat spinal cord. J Neurophysiol 60: 60–85, 1988 [DOI] [PubMed] [Google Scholar]
- Fuglevand AJ, Dutoit AP, Johns RK, Keen DA. Evaluation of plateau-potential-mediated ‘warm up’ in human motor units. J Physiol 571: 683–693, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorassini M, Yang JF, Siu M, Bennett DJ. Intrinsic activation of human motoneurons: possible contribution to motor unit excitation. J Neurophysiol 87: 1850–1858, 2002 [DOI] [PubMed] [Google Scholar]
- Gorassini MA, Knash ME, Harvey PJ, Bennett DJ, Yang JF. Role of motoneurons in the generation of muscle spasms after spinal cord injury. Brain 127: 2247–2258, 2004 [DOI] [PubMed] [Google Scholar]
- Grande G, Bui TV, Rose PK. Estimates of the location of L-type Ca2+ channels in motoneurons of different sizes: a computational study. J Neurophysiol 97: 4023–4035, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman CJ, Gorassini MA, Bennett DJ. Persistent inward currents in motoneuron dendrites: implications for motor output. Muscle Nerve 31: 135–156, 2005 [DOI] [PubMed] [Google Scholar]
- Heckman CJ, Hyngstrom AS, Johnson MD. Active properties of motoneurone dendrites: diffuse descending neuromodulation, focused local inhibition. J Physiol 586: 1225–1231, 2008a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman CJ, Johnson M, Mottram C, Schuster J. Persistent inward currents in spinal motoneurons and their influence on human motoneuron firing patterns. Neuroscientist 14: 264–275, 2008b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines M. A program for simulation of nerve equations with branching geometries. Int J Biomed Comput 24: 55–68, 1989 [DOI] [PubMed] [Google Scholar]
- Hornby TG, McDonagh JC, Reinking RM, Stuart DG. Motoneurons: a preferred firing range across vertebrate species? Muscle Nerve 25: 632–648, 2002 [DOI] [PubMed] [Google Scholar]
- Hultborn H, Brownstone RM, Toth TI, Gossard JP. Key mechanisms for setting the input-output gain across the motoneuron pool. Prog Brain Res 143: 77–95, 2004 [DOI] [PubMed] [Google Scholar]
- Hyngstrom AS, Johnson MD, Miller JF, Heckman CJ. Intrinsic electrical properties of spinal motoneurons vary with joint angle. Nat Neurosci 10: 363–369, 2007 [DOI] [PubMed] [Google Scholar]
- Ito M, Oshima T. Electrical behavior of the motoneurone membrane during intracellularly applied current steps. J Physiol 180: 607–635, 1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MD, Heckman CJ. Interactions between focused synaptic inputs and diffuse neuromodulation in the spinal cord. Ann NY Acad Sci 1198: 35–41, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SM, Lee RH. Fast amplification of dynamic synaptic inputs in spinal motoneurons in vivo. J Neurophysiol 96: 2200–2206, 2006 [DOI] [PubMed] [Google Scholar]
- Kiehn O, Eken T. Prolonged firing in motor units: evidence of plateau potentials in human motoneurons? J Neurophysiol 78: 3061–3068, 1997 [DOI] [PubMed] [Google Scholar]
- Kim H, Jones KE. Asymmetric electrotonic coupling between the soma and dendrites alters the bistable firing behaviour of reduced models. J Comput Neurosci 30: 659–674, 2011 [DOI] [PubMed] [Google Scholar]
- Kuo C, Bean BP. Na+ channels must deactivate to recover from inactivation. Neuron 12: 819–829, 1994 [DOI] [PubMed] [Google Scholar]
- Kuo JJ, Lee RH, Zhang L, Heckman CJ. Essential role of the persistent sodium current in spike initiation during slowly rising inputs in mouse spinal neurones. J Physiol 574: 819–834, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurian M, Crook SM, Jung R. Motoneuron model of self-sustained firing after spinal cord injury. J Comput Neurosci 31: 625–645, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkman PM, Kelly JS. Ionic mechanisms mediating 5-hydroxytryptamine- and noradrenaline-evoked depolarization of adult rat facial motoneurones. J Physiol 456: 473–490, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Bistability in spinal motoneurons in vivo: systematic variations in persistent inward currents. J Neurophysiol 80: 583–593, 1998a [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Bistability in spinal motoneurons in vivo: systematic variations in rhythmic firing patterns. J Neurophysiol 80: 572–582, 1998b [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Enhancement of bistability in spinal motoneurons in vivo by the noradrenergic α1 agonist methoxamine. J Neurophysiol 81: 2164–2174, 1999 [DOI] [PubMed] [Google Scholar]
- Lee RH, Kuo JJ, Jiang MC, Heckman CJ. Influence of active dendritic currents on input-output processing in spinal motoneurons in vivo. J Neurophysiol 89: 27–39, 2003 [DOI] [PubMed] [Google Scholar]
- Li X, Bennett DJ. Apamin-sensitive calcium-activated potassium currents (SK) are activated by persistent calcium currents in rat motoneurons. J Neurophysiol 97: 3314–3330, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Gorassini MA, Bennett DJ. Role of persistent sodium and calcium currents in motoneuron firing and spasticity in chronic spinal rats. J Neurophysiol 91: 767–783, 2004 [DOI] [PubMed] [Google Scholar]
- Lorincz A, Nusser Z. Cell-type-dependent molecular composition of the axon initial segment. J Neurosci 28: 14329–14340, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuel M, Meunier C, Donnet M, Zytnicki D. Resonant or not, two amplification modes of proprioceptive inputs by persistent inward currents in spinal motoneurons. J Neurosci 27: 12977–12988, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliore M, Hoffman DA, Magee JC, Johnston D. Role of an A-type K+ conductance in the back-propagation of action potentials in the dendrites of hippocampal pyramidal neurons. J Comput Neurosci 7: 5–15, 1999 [DOI] [PubMed] [Google Scholar]
- Monster AW, Chan H. Isometric force production by motor units of extensor digitorum communis muscle in man. J Neurophysiol 40: 1432–1443, 1977 [DOI] [PubMed] [Google Scholar]
- Mottram CJ, Suresh NL, Heckman CJ, Gorassini MA, Rymer WZ. Origins of abnormal excitability in biceps brachii motoneurons of spastic-paretic stroke survivors. J Neurophysiol 102: 2026–2038, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottram CJ, Wallace CL, Chikando CN, Rymer WZ. Origins of spontaneous firing of motor units in the spastic-paretic biceps brachii muscle of stroke survivors. J Neurophysiol 104: 3168–3179, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oya T, Riek S, Cresswell AG. Recruitment and rate coding organisation for soleus motor units across entire range of voluntary isometric plantar flexions. J Physiol 587: 4737–4748, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Person RS, Kudina LP. Discharge frequency and discharge pattern of human motor units during voluntary contraction of muscle. Electroencephalogr Clin Neurophysiol 32: 471–483, 1972 [DOI] [PubMed] [Google Scholar]
- Powers RK, Binder MD. Input-output functions of mammalian motoneurons. Rev Physiol Biochem Pharmacol 143: 137–263, 2001 [DOI] [PubMed] [Google Scholar]
- Royeck M, Horstmann MT, Remy S, Reitze M, Yaari Y, Beck H. Role of axonal NaV1.6 sodium channels in action potential initiation of CA1 pyramidal neurons. J Neurophysiol 100: 2361–2380, 2008 [DOI] [PubMed] [Google Scholar]
- Sah P. Role of calcium influx and buffering in the kinetics of Ca2+-activated K+ current in rat vagal motoneurons. J Neurophysiol 68: 2237–2247, 1992 [DOI] [PubMed] [Google Scholar]
- Shapiro NP, Lee RH. Synaptic amplification versus bistability in motoneuron dendritic processing: a top-down modeling approach. J Neurophysiol 97: 3948–3960, 2007 [DOI] [PubMed] [Google Scholar]
- Stephenson JL, Maluf KS. Dependence of the paired motor unit analysis on motor unit discharge characteristics in the human tibialis anterior muscle. J Neurosci Methods 198: 84–92, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udina E, D'Amico J, Bergquist AJ, Gorassini MA. Amphetamine increases persistent inward currents in human motoneurons estimated from paired motor-unit activity. J Neurophysiol 103: 1295–1303, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemiya M, Berger AJ. Properties and function of low- and high-voltage-activated Ca2+ channels in hypoglossal motoneurons. J Neurosci 14: 5652–5660, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viana F, Bayliss DA, Berger AJ. Multiple potassium conductances and their role in action potential repolarization and repetitive firing behavior of neonatal rat hypoglossal motoneurons. J Neurophysiol 69: 2150–2163, 1993 [DOI] [PubMed] [Google Scholar]
- Wienecke J, Zhang M, Hultborn H. A prolongation of the postspike afterhyperpolarization following spike trains can partly explain the lower firing rates at derecruitment than those at recruitment. J Neurophysiol 102: 3698–3710, 2009 [DOI] [PubMed] [Google Scholar]
- Zhao EY, Neuber-Hess M, Fenrich KK, Maratta R, Rose PK. Mapping the distribution of HCN1 channels on neck motoneurons. Soc Neurosci Abstr 685.–611, 2010 [Google Scholar]